Abstract
Bacterial sepsis is a leading cause of mortality among febrile patients in low- and middle-income countries, but blood culture services are not widely available. Consequently, empiric antimicrobial management of suspected bloodstream infection is based on generic guidelines that are rarely informed by local data on etiology and patterns of antimicrobial resistance. To evaluate the cost-effectiveness of surveillance for bloodstream infections to inform empiric management of suspected sepsis in low-resource areas, we compared costs and outcomes of generic antimicrobial management with management informed by local data on etiology and patterns of antimicrobial resistance. We applied a decision tree model to a hypothetical population of febrile patients presenting at the district hospital level in Africa. We found that the evidence-based regimen saved 534 more lives per 100,000 patients at an additional cost of $25.35 per patient, resulting in an incremental cost-effectiveness ratio of $4,739. This ratio compares favorably to standard cost-effectiveness thresholds, but should ultimately be compared with other policy-relevant alternatives to determine whether routine surveillance for bloodstream infections is a cost-effective strategy in the African context.
Introduction
Sepsis due to bacterial bloodstream infection is a serious and life threatening condition that is common among hospitalized patients in the tropics.1,2 Timely and appropriate antimicrobial therapy can be lifesaving.3 There is considerable geographic and temporal variation in causes of bloodstream infection and in antimicrobial susceptibility patterns of isolated organisms.1,2 Consequently, a robust understanding of locally important causes of bloodstream infection and patterns of antimicrobial resistance among bacteria isolated from the bloodstream is essential to form empiric treatment strategies for patients with suspected sepsis.
Blood cultures lack in sensitivity, and the time from collecting the sample to detection means that the results cannot inform the initial management of seriously ill patients. However, aggregate blood culture data from a health-care facility provides invaluable information on the local epidemiology of bloodstream infections for the selection of empiric antimicrobial therapy for sepsis. Yet clinical microbiology services are limited in many low-resource areas and blood culture services are often absent.4,5 Consequently, antimicrobial therapy for sepsis in much of the developing world is based on international guidelines, such as the Integrated Management of Adolescent and Adult Illness (IMAI),6,7 without adaptation to locally important infections, risking mismatching of pathogens and treatment.
We sought to understand the costs and benefits of routine surveillance for bloodstream infections among hospitalized patients in low-resource settings. We were interested in the cost, as well as the impacts on treatment costs and patient survival, of having data to adapt empiric antimicrobial therapy to the local epidemiology of bloodstream infections.
Materials and Methods
Overview.
To evaluate the cost-effectiveness of surveillance for bloodstream infections to inform empiric management of suspected sepsis in low-resource settings, we compared costs and outcomes of generic antimicrobial management with management informed by local data on etiology and patterns of antimicrobial resistance. Using susceptibility patterns from bloodstream infections in Africa, we assessed a number of scenarios, varying the mix of pathogens and antimicrobial susceptibility patterns across different regions. We then investigated the effect on mortality for appropriately and inappropriately treated sepsis on cost-effectiveness. For each scenario, we calculated survival per 100,000 patients and the cost per patient for both the generic and evidence-based antimicrobial regimens, as well as the incremental cost-effectiveness ratio (ICER), or the marginal cost per additional life saved, of surveillance for sepsis. The ICER is commonly used to provide a practical approach to decision making regarding health interventions. Although ultimately it is preferable to compare ICERs to the policy relevant alternatives,8 we measured cost-effectiveness against an ICER threshold of $5,000 per additional life saved based on World Health Organization (WHO) threshold values developed for the “choosing interventions that are cost effective” (CHOICE) project for intervention cost-effectiveness in the Afro D region.9 The WHO threshold values are based on a maximum cost per disability-adjusted life year averted, not lives saved, using a threshold of between one to three times the Gross Domestic Product per capita by region. Thus this threshold is extremely conservative.
Base case.
Table 1 describes the probability input parameters used in our model. Our population of interest was febrile patients presenting at the district hospital level in a low-resource setting. The prevalence of sepsis among patients admitted to district hospitals was assumed to be 13.4%, based on a systematic review of community-acquired bloodstream infections in Africa.2 For the performance of clinical assessment of sepsis, we used data from a 2003 study on neonates presenting with illness to health facilities in four countries (Ethiopia, The Gambia, Papua New Guinea, and The Philippines).10 Using a rule requiring observation of at least one of nine clinical signs of severe illness including sepsis, the authors found a diagnostic performance of clinical assessment of 83% sensitivity and 62% specificity.
Table 1.
Model variables: base-case and best- and worst-case probabilities
| Input variable | Base case, % | Worst case, % | Best case, % | References |
|---|---|---|---|---|
| Prevalence of sepsis | 13.4 | – | – | 2 |
| Clinical assessment of sepsis test performance | ||||
| Sensitivity | 83 | – | – | 10 |
| Specificity | 62 | – | – | |
| Probability organisms are susceptible to antimicrobials | 2 | |||
| Generic antimicrobials | ||||
| North Africa | 45 | 8 | 52 | |
| West and central Africa | 22 | 13 | 45 | |
| Southern Africa | 24 | 16 | 56 | |
| East Africa | 29 | 14 | 48 | |
| All of Africa | 27 | 12 | 36 | |
| Hypothetical 1* | 7 | 0 | 10 | |
| Hypothetical 2† | 25 | 5 | 30 | |
| Evidence-based antimicrobials | ||||
| North Africa | 56 | 56 | 58 | |
| West and central Africa | 56 | 45 | 57 | |
| Southern Africa | 62 | 44 | 62 | |
| East Africa | 47 | 38 | 49 | |
| All of Africa | 43 | 42 | 44 | |
| Hypothetical 1* | 35 | 35 | 35 | |
| Hypothetical 2† | 34 | 34 | 35 | |
| Probability of survival | 11–15 | |||
| Appropriate antimicrobials | 80 | 32 | 90 | |
| Inappropriate antimicrobials | 50 | 25 | 85 | |
| Difference in survival (base-case and extremes)‡ | 30 | 65 | 53 | |
| Test false positive | 100 | – | – | Estimate |
| Test true negative | 100 | – | – | Estimate |
| Test false negative | 50 | – | – | Estimate |
Hypothetical scenario 1: based on all of Africa prevalence values. Reconfigured such that all Salmonella enterica and Streptococcus pneumoniae isolates are resistant to ampicillin.
Hypothetical scenario 2: based on north Africa prevalence values. Reconfigured such that Salmonella enterica and Brucella spp. prevalence values are exchanged (note that it is assumed that Salmonella serotype Typhi remains predominant Salmonella enterica serovar).
Differences in survival estimated based on differences in ranges reported in the literature. Worst-case difference in survival shows the difference between best-case probability of survival using appropriate antimicrobials and worst-case probability of survival using inappropriate antimicrobials. Best-case difference in survival shows the difference between best-case probability of survival using inappropriate antimicrobials and worst-case probability of survival using appropriate antimicrobials.
Patient survival.
Our outcome of interest was patient survival. A base-case survival of 80% for individuals who test true positive was calculated based on published data.11–15 We used the same sources to determine the survival for individuals who test false negative and estimated a base-case survival of 50% based on the range of values reported in the literature. We assumed that true positive test cases were treated with appropriate antimicrobials and that treatment was inappropriately withheld among those with false-negative tests. For individuals who were negative, regardless of whether they test false positive or true negative, we assumed a case fatality ratio of zero. The studies that informed our survival estimates reported survival data for appropriately and inappropriately treated patients infected with a range of pathogens. Because robust case fatality ratio data were not available by pathogen, we adopted a single case fatality ratio across all pathogens.
Antimicrobial therapy.
Antimicrobial treatment recommendations in the IMAI district clinician manual (2011) were used to inform the generic and evidence-based antimicrobial therapy scenarios.7 It was assumed generic antimicrobial therapy would consist of ampicillin and gentamicin, whereas ceftriaxone was selected as an example of an evidence-based antimicrobial therapy.
Antimicrobial susceptibility patterns.
To reflect the different etiology and susceptibility patterns present in different regions of Africa, the probability that a febrile patient presenting with sepsis would be infected with an organism susceptible to either the generic (ampicillin and gentamicin) or the evidence-based (ceftriaxone) antimicrobial regimens was derived from susceptibility and prevalence data reported in the systematic review of community-acquired bloodstream infections in Africa.2 Estimates of the proportion of isolates susceptible to the antimicrobial regimen were calculated based on the three most common isolates identified in blood cultures in the north, west and central, southern and eastern regions of Africa over the period 1984–2006.2 In addition, the four most prevalent pathogens from across Africa were used to estimate susceptibility patterns for the entire African region.
The proportion of organisms susceptible to a given antimicrobial was based on reported antimicrobial susceptibilities of bloodstream isolates in Africa, by region. Because the generic antimicrobial regimen used both ampicillin and gentamicin, susceptibility probabilities were estimated based on the component antimicrobial that was more effective against a given organism. The base-case scenarios were calculated by multiplying the proportion of positive blood cultures attributable to each of the top three (or, in the case of Africa as a whole, four) organisms in each region by the proportion of those organisms found to be susceptible to either the generic or evidence-based antimicrobial treatment regimens (Table 2). Thus, the probability that an antimicrobial was appropriate captured both the prevalence of the most common bacterial isolates in a given region and the susceptibility patterns of those organisms based on data from across Africa. Worst- and best-case scenarios were calculated based on the reported lower and upper ranges of the proportion of isolates susceptible for each organism.2 All isolates not accounted for in the top three most common isolates were assumed to be resistant to treatment with either generic or evidence-based antimicrobials. In addition, it was assumed that all Salmonella enterica and Staphylococcus aureus isolates were resistant to gentamicin and that all Brucella spp. isolates were resistant to both the generic and evidence-based antimicrobial regimens. In the west and central, southern and eastern African regions Salmonella enterica serotype Enteritidis susceptibilities were used as a proxy for non-typhoidal Salmonella.
Table 2.
Base-case probability that a patient presenting with sepsis is infected with an organism susceptible to the antimicrobial regimen
| Proportion of total isolates | Predominant serotype | Proportion of predominant subtype | Probability of susceptibility | ||||
|---|---|---|---|---|---|---|---|
| Ampicillin | Gentamicin | “Generic” regimen | Evidence-based (ceftriaxone) | ||||
| North Africa | |||||||
| Salmonella enterica | 0.499 | Serotype Typhi | 0.99 | 0.440 | 0.000 | 0.440 | 0.484 |
| Brucella spp. | 0.269 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Staphylococcus aureus | 0.077 | – | – | 0.007 | 0.000 | 0.007 | 0.077 |
| Other | 0.155 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Total | 1.000 | – | – | 0.447 | 0.000 | 0.447 | 0.561 |
| West and central Africa | |||||||
| Salmonella enterica | 0.208 | Non-typhoidal | 0.87 | 0.030 | 0.000 | 0.030 | 0.203 |
| Streptococcus pneumoniae | 0.189 | – | – | 0.171 | 0.000 | 0.171 | 0.189 |
| Staphylococcus aureus | 0.172 | – | – | 0.016 | 0.000 | 0.016 | 0.172 |
| Other | 0.431 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Total | 1.000 | – | – | 0.216 | 0.000 | 0.216 | 0.564 |
| Southern Africa | |||||||
| Salmonella enterica | 0.290 | Non-typhoidal | 0.97 | 0.016 | 0.000 | 0.016 | 0.283 |
| Streptococcus pneumoniae | 0.240 | – | – | 0.217 | 0.000 | 0.217 | 0.240 |
| Staphylococcus aureus | 0.094 | – | – | 0.009 | 0.000 | 0.009 | 0.094 |
| Other | 0.376 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Total | 1.000 | – | – | 0.241 | 0.000 | 0.241 | 0.617 |
| East Africa | |||||||
| Streptococcus pneumoniae | 0.212 | – | – | 0.191 | 0.000 | 0.191 | 0.205 |
| Salmonella enterica | 0.178 | Non-typhoidal | 0.88 | 0.024 | 0.000 | 0.024 | 0.174 |
| Escherichia coli | 0.095 | – | – | 0.008 | 0.076 | 0.076 | 0.095 |
| Other | 0.515 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Total | 1.000 | – | – | 0.224 | 0.076 | 0.291 | 0.474 |
| All of Africa | |||||||
| Salmonella enterica | 0.291 | Serotype Typhimurium | 0.081 | 0.001 | 0.000 | 0.001 | 0.023 |
| – | Serotype Enteritidis | 0.041 | 0.004 | 0.000 | 0.004 | 0.012 | |
| – | Serotype Typhi | 0.099 | 0.026 | 0.000 | 0.026 | 0.028 | |
| – | All other | 0.069 | 0.007 | 0.000 | 0.007 | 0.020 | |
| – | Total | – | – | – | 0.037 | 0.083 | |
| Streptococcus pneumoniae | 0.183 | – | – | 0.165 | 0.000 | 0.165 | 0.183 |
| Staphylococcus aureus | 0.095 | – | – | 0.009 | 0.000 | 0.009 | 0.095 |
| Escherichia coli | 0.073 | – | – | 0.006 | 0.059 | 0.059 | 0.073 |
| Other | 0.358 | – | – | 0.000 | 0.000 | 0.000 | 0.000 |
| Total | 1.000 | – | – | – | – | 0.270 | 0.434 |
Based on data sourced from Reddy and others (2010).2
Two additional hypothetical scenarios were developed to test the effect of potential changes in resistance and prevalence. Salmonella enterica and Streptococcus pneumoniae are a leading cause of community-acquired bloodstream infection in Africa.2 Since antimicrobial resistance, including to ampicillin, is increasing among both pathogens and ampicillin has been a widely recommended component of antimicrobial management of severe febrile illness, the first hypothetical scenario examined the cost-effectiveness of blood culture surveillance using prevalence values for the entire African continent but reconfigured such that all Salmonella enterica and Streptococcus pneumoniae were resistant to ampicillin. In areas where brucellosis is endemic, such as parts of north Africa, Brucella spp. is often a leading cause of community-acquired bloodstream infection.2 However, the antimicrobial management of brucellosis differs substantially from regimens widely recommended for the antimicrobial management of severe febrile illness. Therefore, the second hypothetical scenario examined the cost-effectiveness of blood culture surveillance in a setting where brucellosis was common and where empiric antimicrobial management regimens might otherwise be poorly matched with infecting organisms.
Costs.
Table 3 describes the cost inputs in the model. Our cost data relied largely on data from a clinical laboratory operating to Good Clinical Laboratory Practices standards in northern Tanzania. All costs are expressed in 2011 U.S. dollars ($). For the clinical assessment, we assumed that labor is the only cost, and base our value on the cost per hour for a laboratory technician performing an human immunodeficiency virus (HIV) point of care test ($6.10/hour) (JA Crump, personal communication, 2014). Assuming that clinical assessment takes 10 minutes, labor costs for the clinical assessment were set at $1.02 per case, consistent with costs reported in earlier studies.16
Table 3.
Costs used in the model*
| Cost variable | References | |
|---|---|---|
| IMAI Assessment, $ per test | ||
| Labor | 1.02 | J. A. Crump, pers. comm., 2014,16 |
| Total | 1.02 | |
| Laboratory assessment for sepsis | ||
| Negative blood culture, $ per test | J. A. Crump, pers. comm., 2014 | |
| Reagents and Supplies | 6.20 | |
| Labor | 2.27 | |
| Indirect costs | 1.79 | |
| Equipment | 1.15 | |
| Total cost negative blood culture | 11.41 | |
| Positive blood culture, $ per test | J. A. Crump, pers. comm., 2014 | |
| Reagents and supplies | 10.26 | |
| Labor | 6.85 | |
| Indirect costs | 1.79 | |
| Equipment | 1.15 | |
| Subtotal | 20.05 | |
| ID and susceptibilities, $ per test | J. A. Crump, pers. comm., 2014 | |
| Reagents and supplies | 44.02 | |
| Labor | 6.10 | |
| Indirect costs | 1.79 | |
| Subtotal | 51.91 | |
| Total cost positive blood culture | 71.96 | |
| Cost of antimicrobial treatment, $ per case | 7,17 | |
| Generic antimicrobials | ||
| Emergency dose | 0.59 | |
| Standard treatment | 12.63 | |
| Total cost generic antimicrobials | 13.22 | |
| Evidence-based antimicrobials, $ per case | ||
| Emergency dose | 4.41 | |
| Standard treatment | 22.05 | |
| Total cost evidence-based antimicrobials | 26.46 | |
IMAI = Integrated Management of Adolescent and Adult Illness.
Costs adjusted to 2011 U.S. dollars ($) values using gross domestic product (GDP) deflator.
The costs of laboratory assessment for sepsis were based on the costs associated with a single blood culture. To assess the value of bloodstream infection surveillance for patients with fever, it was assumed all patients would have a blood culture taken, irrespective of whether they were diagnosed as having sepsis using clinical assessment protocols. Where patients were incorrectly diagnosed as having sepsis (a false positive), it was assumed blood cultures would remain negative, giving a cost of $11.41. Where patients were correctly diagnosed (a true positive), it was assumed additional costs associated with processing a positive blood culture would be incurred, for a cost of $20.05. The costs of carrying out identification and susceptibility testing were valued at $51.91. In total, the cost associated with a positive blood culture was $71.96. Patients incorrectly diagnosed as not having sepsis (a false negative) also incurred the costs of a positive blood culture, whereas those correctly diagnosed as not having sepsis (a true negative) incurred the costs of a negative blood culture.
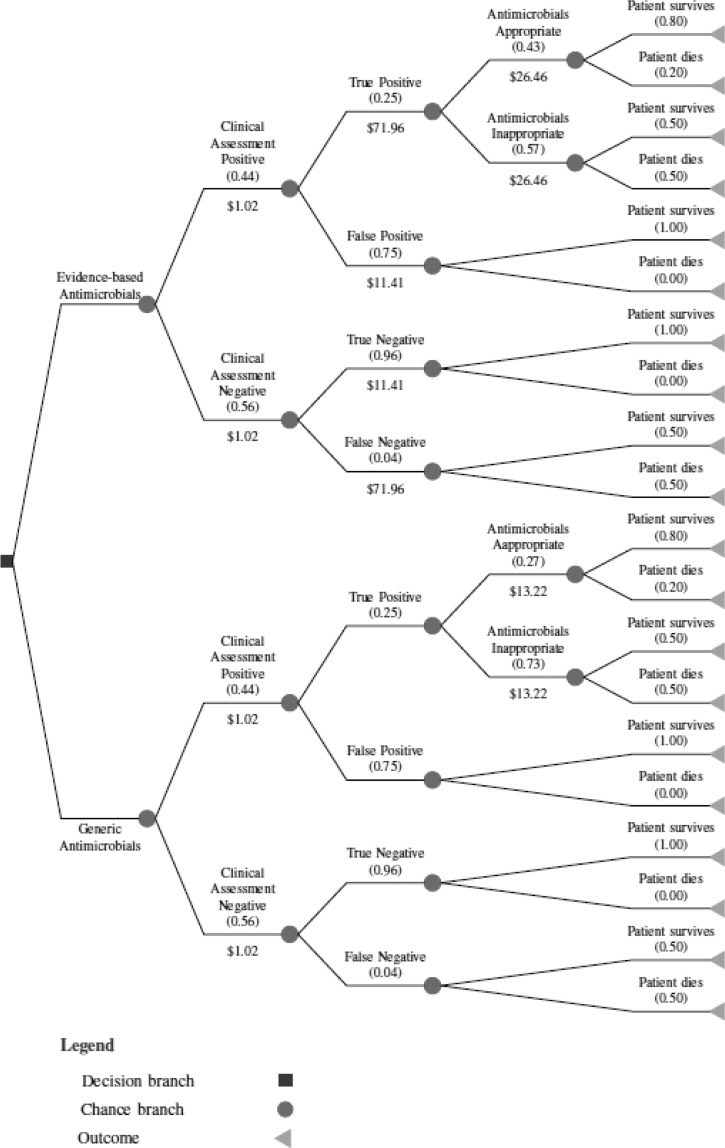
Based on IMAI guidelines that patients diagnosed with sepsis receive 6 days of antimicrobial therapy, consisting of an emergency dose (one day) and 5 days of standard treatment, the cost of treatment with generic antimicrobials was valued at $13.22 compared with $26.46 for evidence-based therapy.7,17 Figure 1 illustrates the decision tree used, pre-filled with data from the base-case scenario for the all Africa case.
Figure 1.
Decision tree representing probabilities, costs, and outcomes for a febrile patient presenting at a district hospital in a low-resource setting and receiving either generic or evidence-based antimicrobial treatment using base case all of Africa data. Cost figures represent the total costs of tests and antimicrobial treatment. Probabilities of an event occurring are shown in in brackets. Probabilities are rounded to two decimal places.
Sensitivity analysis.
We conducted sensitivity analysis across three dimensions. First, we assessed the effect of varying susceptibility patterns using the best- and worst-case susceptibility patterns in different regions of Africa, based on the upper and lower ranges of susceptibility reported by Reddy and others (2010).2 Second, we allowed for variation in survival for testing true positive or false negative from 32% and 25% (worst case)12 to 90% and 65% (best case),13,15 respectively. Third, we explored the impact of investment in laboratory infrastructure and instruments on the cost-effectiveness of surveillance, using base-case values of prevalence and cost for all of Africa. We assumed that district hospitals would have existing space allocated for clinical laboratory services, but allowed for variation in initial capital costs from $25,000 to $100,000. We assumed that facilities and instruments would have a useful life of 10 years and calculated annualized costs per person using interest rates set at 0%, 3%, 5%, and 10%. In addition, we allowed for variation in test volumes of between 1,000 and 10,000 blood cultures per annum. Costs of labor, reagents, consumables, and indirect costs were accounted for in cost per test estimates from Tanzania.
Results
Base case.
Compared with generic antimicrobials, the additional cost of laboratory monitoring for sepsis and evidence-based antimicrobial treatment was $25.35 per patient. Using clinical assessment with fixed sensitivity and specificity values as the entry point for the model, the additional cost per patient was identical across all subregions. Using all of Africa data, compared with generic antimicrobials, blood cultures and evidence-based antimicrobials saved 534 more lives per 100,000 patients (Table 4, Column 2). This corresponded to an ICER of $4,749 per life saved. The corresponding numbers for east Africa were 601 more lives saved per 100,000 patients (Table 4, Column 4), for north Africa were 367 (Table 4, Column 6), for southern Africa were 1,268 (Table 5, Column 2), and for west and central Africa were 1,134 (Table 5, Column 4). ICERs ranged from $2,000 (southern Africa) to $6,908 (north Africa) per life saved.
Table 4.
The base-case scenario for generic antimicrobials compared with the combination of blood culture monitoring and evidence-based antimicrobials (all of Africa, east Africa, and north Africa)
| Measure | All of Africa | East Africa | North Africa | |||
|---|---|---|---|---|---|---|
| Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | |
| (Column number) | (1) | (2) | (3) | (4) | (5) | (6) |
| Probability antimicrobials appropriate | 0.27 | 0.43 | 0.29 | 0.47 | 0.45 | 0.56 |
| Probability mortality if antimicrobials appropriate | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Probability mortality if antimicrobials inappropriate | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Probability patient lives | 0.94 | 0.95 | 0.94 | 0.95 | 0.95 | 0.95 |
| Expected cost per patient, $ | 6.84 | 32.19 | 6.84 | 32.19 | 6.84 | 32.19 |
| Deaths per 100,000 patients | 5,799 | 5,265 | 5,732 | 5,132 | 5,199 | 4,832 |
| Difference in survival | – | 534 | – | 601 | – | 367 |
| Difference in expected cost per patient, $ | – | 25.35 | – | 25.35 | – | 25.35 |
| ICER, $ | – | 4,749 | – | 4,221 | – | 6,908 |
ICER = incremental cost-effectiveness ratio.
Values in parentheses refer to columns as cited in text. For all scenarios, the prevalence of sepsis is 0.134. Sensitivity is set at 0.83 and specificity at 0.62. The cost of antimicrobials is $13.22 in the generic case and $26.46 in the evidence-based case. In the evidence-based case, there is an additional cost of $11.41 for a negative blood culture test and $71.96 for a positive blood culture test.
Numbers in parentheses refer to columns as cited in text.
Table 5.
The base-case scenario for generic antimicrobials compared with the combination of blood culture monitoring and evidence-based antimicrobials (southern Africa and west and central Africa)
| Measure | Southern Africa | West and central Africa | ||
|---|---|---|---|---|
| Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | |
| (Column number) | (1) | (2) | (3) | (4) |
| Probability antimicrobials appropriate | 0.24 | 0.62 | 0.22 | 0.56 |
| Probability mortality if antimicrobials appropriate | 0.20 | 0.20 | 0.20 | 0.20 |
| Probability mortality if antimicrobials inappropriate | 0.50 | 0.50 | 0.50 | 0.50 |
| Probability patient lives | 0.94 | 0.95 | 0.94 | 0.95 |
| Expected cost per patient, $ | 6.84 | 32.19 | 6.84 | 32.19 |
| Deaths per 100,000 patients | 5,899 | 4,631 | 5,966 | 4,832 |
| Difference in survival | – | 1,268 | – | 1,134 |
| Difference in expected cost per patient, $ | – | 25.35 | – | 25.35 |
| ICER, $ | – | 2,000 | – | 2,235 |
ICER = incremental cost-effectiveness ratio.
Values in parentheses refer to columns as cited in text. For all scenarios, the prevalence of sepsis is 0.134. Sensitivity of clinical assessment is set at 0.83 and specificity at 0.62. The cost of antimicrobials is $13.22 in the generic case and $26.46 in the evidence-based case. In the evidence-based case, there is an additional cost of $11.41 for a negative blood culture test and $71.96 for a positive blood culture test.
Numbers in parentheses refer to columns as cited in text.
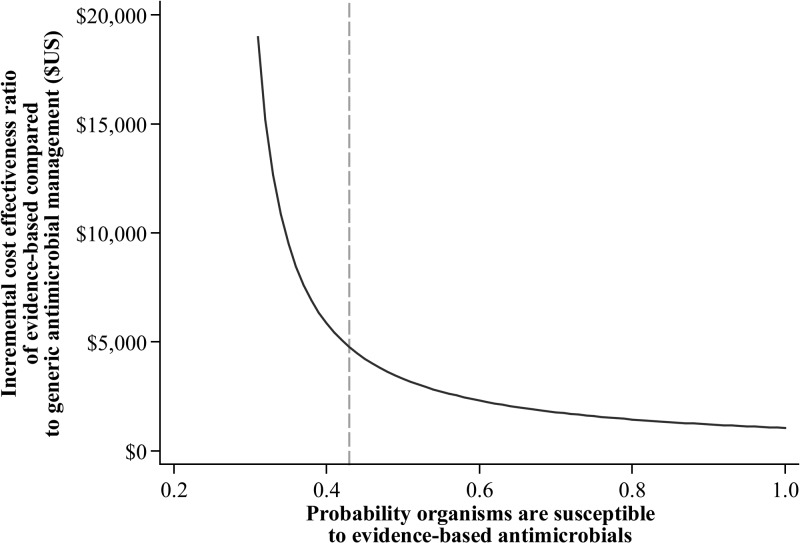
Figure 2 illustrates the ICER of evidence-based antimicrobial therapy, varying the susceptibility probabilities for evidence-based drugs between 0.00 and 1.00, compared with base-case generic therapy using all of Africa prevalence and susceptibility data. Above a probability of approximately 0.40 that an organism will be susceptible to evidence-based treatment, the ICER fell below $5,000 per life saved.
Figure 2.
Incremental cost-effectiveness ratio of evidence-based antimicrobial treatment at differing levels of susceptibility compared with base-case generic antimicrobial treatment using all of Africa prevalence and susceptibility data. The vertical dotted line indicates the probability of susceptibility to evidence-based antimicrobials at which the incremental cost-effectiveness ratio falls below $5,000 per life saved.
Table 6 includes the two hypothetical scenarios. In Table 6, Columns 1 and 2 show the relative cost-effectiveness of generic antimicrobials versus blood cultures and evidence-based antimicrobials using the prevalence values for the entire African continent but assuming that all Salmonella enterica and Streptococcus pneumoniae isolates were resistant to ampicillin. Under this scenario, blood cultures and evidence-based antimicrobials saved 934 more lives per 100,000 patients, corresponding to an ICER of $2,714 per life saved. Columns 3 and 4 show the relative cost-effectiveness of generic antimicrobials versus blood cultures and evidence-based antimicrobials using prevalence figures for north Africa assuming that the prevalence of Salmonella enterica and Brucella spp. is exchanged. Under this scenario, blood cultures and evidence-based antimicrobials saved 300 more lives per 100,000 patients, corresponding to an ICER of $8,443 per life saved.
Table 6.
The base-case scenario for generic antimicrobials compared with the combination of blood culture monitoring and evidence-based antimicrobials for two hypothetical scenarios
| Measure | Hypothetical 1 | Hypothetical 2 | ||
|---|---|---|---|---|
| Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | |
| (Column number) | (1) | (2) | (3) | (4) |
| Probability antimicrobials appropriate | 0.07 | 0.35 | 0.25 | 0.34 |
| Probability mortality if antimicrobials appropriate | 0.20 | 0.20 | 0.20 | 0.20 |
| Probability mortality if antimicrobials inappropriate | 0.50 | 0.50 | 0.50 | 0.50 |
| Probability patient lives | 0.94 | 0.94 | 0.94 | 0.94 |
| Expected cost per patient, $ | 6.84 | 32.19 | 6.84 | 32.19 |
| Deaths per 100,000 patients | 6,466 | 5,532 | 5,866 | 5,566 |
| Difference in survival | – | 934 | – | 300 |
| Difference in expected cost per patient, $ | – | 25.35 | – | 25.35 |
| ICER, $ | – | 2,714 | – | 8,443 |
ICER = incremental cost-effectiveness ratio.
Hypothetical 1 used the prevalence values for the entire African continent but was reconfigured such that all Salmonella enterica and Streptococcus pneumoniae isolates were resistant to ampicillin. Hypothetical 2 was based on prevalence figures for north Africa and was reconfigured such that the prevalence of Salmonella enterica and Brucella spp. is exchanged. Values in parentheses refer to columns as cited in text. For all scenarios, the prevalence of sepsis is 0.134. Sensitivity of clinical assessment is set at 0.83 and specificity at 0.62. The cost of antimicrobials is $13.22 in the generic case and $26.46 in the evidence-based case. In the evidence-based case, there is an additional cost of $11.41 for a negative blood culture test and $71.96 for a positive blood culture test.
Numbers in parentheses refer to columns as cited in text.
Sensitivity analysis.
When considering extreme susceptibility patterns of generic and evidence-based antimicrobial treatment, using the best-case susceptibility patterns for evidence-based antimicrobials and the worst-case susceptibility patterns for generic antimicrobials across all of Africa, evidence-based treatment saved 1,068 more lives per 100,000 patients (Table 7, Column 2). This corresponded to an ICER of $2,375 per life saved. Across the four regions, additional lives saved varied from 1,168 to 1,668 per 100,000 patients. ICERs ranged from $1,520 to $2,171 per life saved (Tables 7 and 8).
Table 7.
Susceptibility extremes for generic antimicrobials (worst case) compared with the combination of blood culture monitoring and evidence-based antimicrobials (best case) in all of Africa, east Africa, and north Africa
| Measure | All of Africa | East Africa | North Africa | |||
|---|---|---|---|---|---|---|
| Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | |
| (Column number) | (1) | (2) | (3) | (4) | (5) | (6) |
| Probability antimicrobials appropriate | 0.12 | 0.44 | 0.14 | 0.49 | 0.08 | 0.58 |
| Probability mortality if antimicrobials appropriate | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Probability mortality if antimicrobials inappropriate | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Probability patient lives | 0.94 | 0.95 | 0.94 | 0.95 | 0.94 | 0.95 |
| Expected cost per patient, $ | 6.84 | 32.19 | 6.84 | 32.19 | 6.84 | 32.19 |
| Deaths per 100,000 patients | 6,300 | 5,232 | 6,233 | 5,065 | 6,433 | 4,765 |
| Difference in survival | – | 1,068 | – | 1,168 | – | 1,668 |
| Difference in expected cost per patient, $ | – | 25.35 | – | 25.35 | – | 25.35 |
| ICER, $ | – | 2,375 | – | 2,171 | – | 1,520 |
ICER = incremental cost-effectiveness ratio.
Values in parentheses refer to columns as cited in text. For all scenarios, the prevalence of sepsis is 0.134. Sensitivity of clinical assessment is set at 0.83 and specificity at 0.62. The cost of antimicrobials is $13.22 in the generic case and $26.46 in the evidence-based case. In the evidence-based case, there is an additional cost of $US11.41 for a negative blood culture test and $71.96 for a positive blood culture test.
Numbers in parentheses refer to columns as cited in text.
Table 8.
Susceptibility extremes for generic antimicrobials (worst case) compared with the combination of blood culture monitoring and evidence-based antimicrobials (best case) (southern Africa and west and central Africa)
| Measure | Southern Africa | West and central Africa | ||
|---|---|---|---|---|
| Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | |
| (Column number) | (1) | (2) | (3) | (4) |
| Probability antimicrobials appropriate | 0.16 | 0.62 | 0.13 | 0.57 |
| Probability mortality if antimicrobials appropriate | 0.20 | 0.20 | 0.20 | 0.20 |
| Probability mortality if antimicrobials inappropriate | 0.50 | 0.50 | 0.50 | 0.50 |
| Probability patient lives | 0.94 | 0.95 | 0.94 | 0.95 |
| Expected cost per patient, $ | 6.84 | 32.19 | 6.84 | 32.19 |
| Deaths per 100,000 patients | 6,166 | 4,631 | 6,266 | 4,798 |
| Difference in survival | – | 1,535 | – | 1,468 |
| Difference in expected cost per patient, $ | – | 25.35 | – | 25.35 |
| ICER, $ | – | 1,652 | – | 1,727 |
ICER = incremental cost-effectiveness ratio.
Values in parentheses refer to columns as cited in text. For all scenarios, the prevalence of sepsis is 0.134. Sensitivity of clinical assessment is set at 0.83 and specificity at 0.62. The cost of antimicrobials is $13.22 in the generic case and $26.46 in the evidence-based case. In the evidence-based case, there is an additional cost of $11.41 for a negative blood culture test and $71.96 for a positive blood culture test.
Numbers in parentheses refer to columns as cited in text.
Table 9 shows the impact of different levels of capital investment on cost-effectiveness, at different volumes of blood cultures and interest rates. Allowing for capital investment of between $25,000 and $50,000 with interest rates of up to 10% per annum in facilities processing 1,000 blood cultures per annum, evidence-based treatment cost an expected $34.19–$39.59 per patient and yielded ICERs of between $5,124 and $6,134. For facilities processing 5,000 blood cultures per annum, the expected cost per patient of evidence-based treatment was $25.75–$26.83 with ICERs of $4,824–$5,026. Allowing for capital investment of $100,000 in facilities processing 5,000–10,000 blood cultures per annum and interest rates between 0% and 10%, the expected costs per patient of evidence-based treatment ranged from $26.35 to $28.31 and ICERs fell between $4,936 and $5,303.
Table 9.
Effect of different levels of capital investment on cost-effectiveness of blood culture monitoring and evidence-based antimicrobials
| All of Africa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial capital investment, $ | Number of blood cultures per annum | Interest rate, % | Generic antimicrobials | Blood culture monitoring + evidence-based antimicrobials | Difference in expected cost per patient, $ | ICER, $ | ||
| Expect cost per patient generic antimicrobials, $ | Cost of negative blood culture, $ | Cost of positive blood culture, $ | Expected cost per patient evidence based, $ | |||||
| 25,000 | 1,000 | 0 | 7.26 | 13.41 | 73.96 | 34.19 | 27.35 | 5,124 |
| 3 | 7.26 | 13.69 | 74.24 | 34.47 | 27.63 | 5,175 | ||
| 5 | 7.26 | 13.88 | 74.43 | 34.66 | 27.82 | 5,175 | ||
| 10 | 7.26 | 14.37 | 74.92 | 35.15 | 28.31 | 5,303 | ||
| 5,000 | 0 | 7.26 | 11.81 | 72.36 | 32.59 | 25.75 | 4,824 | |
| 3 | 7.26 | 11.87 | 72.42 | 32.65 | 25.81 | 4,834 | ||
| 5 | 7.26 | 11.90 | 72.45 | 32.69 | 25.85 | 4,842 | ||
| 10 | 7.26 | 12.00 | 72.55 | 32.79 | 25.95 | 4,860 | ||
| 50,000 | 1,000 | 0 | 7.26 | 16.41 | 76.96 | 37.19 | 30.35 | 5,686 |
| 3 | 7.26 | 17.10 | 77.65 | 37.88 | 31.04 | 5,815 | ||
| 5 | 7.26 | 17.58 | 78.13 | 38.36 | 31.52 | 5,904 | ||
| 10 | 7.26 | 18.81 | 79.36 | 39.59 | 32.75 | 6,135 | ||
| 5,000 | 0 | 7.26 | 12.41 | 72.96 | 33.19 | 26.35 | 4,936 | |
| 3 | 7.26 | 12.55 | 73.10 | 33.33 | 26.49 | 4,962 | ||
| 5 | 7.26 | 12.64 | 73.19 | 33.43 | 26.59 | 4,980 | ||
| 10 | 7.26 | 12.89 | 73.44 | 33.67 | 26.83 | 5,026 | ||
| 100,000 | 5,000 | 0 | 7.26 | 13.41 | 73.96 | 34.19 | 27.35 | 5,124 |
| 3 | 7.26 | 13.69 | 74.24 | 34.47 | 27.63 | 5,175 | ||
| 5 | 7.26 | 13.88 | 74.43 | 34.66 | 27.82 | 5,211 | ||
| 10 | 7.26 | 14.37 | 74.92 | 35.15 | 28.31 | 5,303 | ||
| 10,000 | 0 | 7.26 | 12.41 | 72.96 | 33.19 | 26.35 | 4,936 | |
| 3 | 7.26 | 12.55 | 73.10 | 33.33 | 26.49 | 4,962 | ||
| 5 | 7.26 | 12.64 | 73.19 | 33.43 | 26.59 | 4,980 | ||
| 10 | 7.26 | 12.89 | 73.44 | 33.67 | 26.83 | 5,026 | ||
ICER = incremental cost-effectiveness ratio.
All scenarios use all of Africa base-case values for both generic and evidence-based cases. Capital investment costs are added as annualized per patient costs to the base-case cost of blood culture monitoring and evidence-based antimicrobials, set at $71.96 per patient.
Discussion
Using a decision tree model applied to a hypothetical population of febrile patients presenting at the district hospital level in Africa, we compared costs and survival of generic antimicrobial management with management informed by local data on etiology and patterns of antimicrobial resistance. Our results showed that compared with generic antimicrobial management, blood culture surveillance-directed evidence-based antimicrobials saved 367–534 more lives per 100,000 patients at an additional cost of $25.35 per patient. Using all of Africa data, we found that evidence-based antimicrobial management could be considered cost-effective when the probability of susceptibility rose above approximately 0.40, costing no more than an additional $5,000 more per life saved (Figure 2). Furthermore, our results indicate that for facilities processing at least 5,000 blood cultures per year, evidence-based antimicrobial management would cost less than an additional $5,026 per patient given an initial investment of $25,000–$100,000.
Besides the development of laboratory services to support large vertical programs for HIV, tuberculosis, and malaria, clinical microbiology services have been neglected in many low-resource areas.4,5 Because of the structure of burden of disease estimates, bacterial sepsis after the neonatal period does not feature as a category in global burden of disease estimates.18,19 Nonetheless, bacteremia is commonly associated with severe febrile illness in inpatient studies in both Africa and Asia.1,2 Furthermore, the high case fatality ratio associated with sepsis, combined with the potential impact of timely and appropriate antimicrobial therapy,3 means that considerable survival gains can be achieved by carefully selecting empiric antimicrobial therapy. However, selection of locally appropriate antimicrobial regimens for suspected sepsis depends on local information about the epidemiology of bloodstream infections and patterns of antimicrobial resistance. Although conventional clinical microbiology services are not widely available in low-resource areas, we demonstrate that their use for surveillance for bloodstream infections is cost effective.
We focused on bloodstream infections because of their high case fatality ratio and the potential for better matching of antimicrobial therapy to patterns of antimicrobial resistance to improve patient survival.3 We studied only two antimicrobial regimens, ampicillin with gentamicin and ceftriaxone alone, because these are widely used and recommended for empiric sepsis management in low-resource areas.6,7 However, it is likely that closer tailoring of antimicrobial regimens to the local epidemiology of bloodstream infections would be possible if a wider range of antimicrobial agents and combinations was considered. This could be examined in future refinements of the model. In addition, pathogens that are either not detected or not readily detected by blood culture may be responsible for a large proportion of severe febrile illness in some settings.20,21 For example, rickettsial infections are very common in some areas of the tropics22 and would not be treated by either antimicrobial regimen evaluated in our study. Extending our model to include microbiologic evaluations beyond blood culture would allow for assessment of the cost-effectiveness of a wider group of diagnostic evaluations for pathogens causing severe disease in the tropics.
This study has a number of limitations. Our assumptions about sepsis prevalence were based on studies using a single blood culture, which lacks sensitivity. Therefore, it is likely that we underestimated the prevalence of bacteremia. We chose ampicillin plus gentamicin as our base-case antimicrobial regimen. Since ceftriaxone is replacing ampicillin and gentamicin for sepsis management and is more effective,23 we studied it as an alternative agent in our model. However, it is likely that empiric antimicrobial regimens other than those that we studied may be appropriate in other epidemiologic settings. For example, ceftriaxone may be a poor choice in areas where Enterobacteriaceae are often resistant to extended-spectrum cephalosporins. We did not account for externalities of antibacterial use, such as impacts of antimicrobial selection and use on the promotion of antimicrobial resistance.24 We also did not account for costs for patients who receive inadequate or inappropriate initial treatment and who subsequently seek additional care. As a result, our model likely underestimated costs for patients who receive inappropriate antimicrobial therapy. Incorporating these costs in the model would likely increase the cost-effectiveness of implementing blood culture surveillance and evidence-based antimicrobial treatment. In addition, we focus on the district hospital level since bloodstream infection surveillance may be feasible at this level of the health system in low-resource areas. We used data from Tanzania as an estimate of the average costs of operating a surveillance program. However, based on limited data available, there is likely to be heterogeneity in the costs of operating surveillance programs.
Our findings suggest that by informing empiric antimicrobial management of severe febrile illness, routine surveillance for bloodstream infections among patients admitted to district hospitals with febrile illness saves lives and is likely cost effective in low-resource settings. Although we ultimately need to compare surveillance to other relevant policy interventions, these results suggest that additional investment in clinical microbiology services in low-resource areas should be considered.
Disclaimer: This paper was presented in part at the 63rd American Society of Tropical Medicine and Hygiene annual meeting, New Orleans, LA, November 2–6, 2014, abstract 473.
Footnotes
Financial support: This research was supported by a Strategic Development Award of the Department of Preventive and Social Medicine, University of Otago. JAC is supported by the joint U.S. National Institutes of Health-National Science Foundation Ecology and Evolution of Infectious Disease program (R01 TW009237) and the UK Biotechnology and Biological Sciences Research Council (BBSRC) (BB/J010367/1), and by UK BBSRC Zoonoses in Emerging Livestock Systems awards BB/L017679, BB/L018926, and BB/L018845.
Authors' addresses: Erin C. Penno, Department of Preventive and Social Medicine, Centre for Health Systems, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand, E-mail: erin.penno@otago.ac.nz. Sarah J. Baird, Department of Global Health, Milken Institute School of Public Health, George Washington University, Washington, DC, E-mail: sbaird@gwu.edu. John A. Crump, Centre for International Health, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand, E-mail: john.crump@otago.ac.nz.
References
- 1.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis. 2012;12:480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 2.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244:379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 4.Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis. 2001;7:302–305. doi: 10.3201/eid0702.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Handbook: IMCI Integrated Management of Childhood Illness. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 7.World Health Organization . IMAI District Clinician Manual: Hospital Care for Adolescents and Children: Guidelines for the Management of Common Illnesses with Limited Resources. Vol. 1. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 8.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Cost Effectiveness and Strategic Planning (WHO-CHOICE). Threshold Values for Intervention Cost-Effectiveness by Region. 2015. http://www.who.int/choice/costs/CER_levels/en/ Available at. Accessed March 30, 2015.
- 10.Weber MW, Carlin JB, Gatchalian S, Lehmann D, Muhe L, Mulholland EK, WHO Young Infants Study Group Predictors of neonatal sepsis in developing countries. Pediatr Infect Dis J. 2003;22:711–717. doi: 10.1097/01.inf.0000078163.80807.88. [DOI] [PubMed] [Google Scholar]
- 11.Jamulitrat S, Meknavin U, Thongpiyapoom S. Factors affecting mortality outcome and risk of developing nosocomial bloodstream infection. Infect Control Hosp Epidemiol. 1994;15:163–170. doi: 10.1086/646884. [DOI] [PubMed] [Google Scholar]
- 12.Lueangarun S, Leelarasamee A. Impact of inappropriate empiric antimicrobial therapy on mortality of septic patients with bacteremia: a retrospective study. Interdiscip Perspect Infect Dis. 2012;2012:765205. doi: 10.1155/2012/765205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010;54:4851–4863. doi: 10.1128/AAC.00627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayner BL, Willcox PA. Community-acquired bacteraemia; a prospective survey of 239 cases. Q J Med. 1988;69:907–919. [PubMed] [Google Scholar]
- 15.Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, Mills A. Cost-effectiveness of malaria diagnostic methods in sub-Saharan Africa in an era of combination therapy. Bull World Health Organ. 2008;86:101–110. doi: 10.2471/BLT.07.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam T, Manzi F, Schellenberg JA, Mgalula L, de Savigny D, Evans DB. Does the integrated management of childhood illness cost more than routine care? Results from the United Republic of Tanzania. Bull World Health Organ. 2005;83:369–377. [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . AFRO Essential Medicines Price Indicator. Brazzaville, Republic of Congo: World Health Organization; 2007. WHO Regional Office for Africa (AFRO) [Google Scholar]
- 18.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Abdulhak AB, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, Leo DD, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 19.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J-P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayxay M, Castonguay-Vanier J, Chansamouth V, Dubot-Pérès A, Paris DH, Phetsouvanh R, Tangkhabuanbutra J, Douangdala P, Inthalath S, Souvannasing P, Slesak G, Tongyoo N, Chanthongthip A, Panyanouvong P, Sibounheuang B, Phommasone K, Dohnt M, Phonekeo D, Hongvanthong B, Xayadeth S, Ketmayoon P, Blacksell SD, Moore CE, Craig SB, Burns M-A, von Sonnenburg F, Corwin A, de Lamballerie X, González IJ, Christophel EM, Cawthorne A, Bell D, Newton PN. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013;1:e46–e54. doi: 10.1016/S2214-109X(13)70008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, Oliver LD, Massung RF, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Crump JA. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Nephrol Dial Transplant. 2011;53:e8–e15. doi: 10.1093/cid/cir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubach MP, Maro VP, Bartlett JA, Crump JA. Etiologies of illness among patients meeting Integrated Management of Adolescent and Adult Illness District Clinician Manual criteria for severe infections in northern Tanzania: implications for empiric antimicrobial therapy. Am J Trop Med Hyg. 2015;92:454–462. doi: 10.4269/ajtmh.14-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaier K, Frank U. Measuring the externality of antibacterial use from promoting antimicrobial resistance. Pharmacoeconomics. 2010;28:1123–1128. doi: 10.2165/11535640-000000000-00000. [DOI] [PubMed] [Google Scholar]