Abstract
Skp1 is a cytoplasmic and nuclear protein, best known as an adaptor of the SCF family of E3-ubiquitin ligases that label proteins for their degradation. Skp1 in Dictyostelium is posttranslationally modified on a specific hydroxyproline (Hyp) residue by a pentasaccharide, which consists of a Fucα1,2-Galβ-1,3-GlcNAcα core, decorated with two α-linked Gal residues. A glycopeptide derived form Skp1 was prepared to characterize the α-galactosyltransferase (AgtA) that mediates the addition of the α-Gal moieties, and to develop antibodies suitable for tracking the trisaccharide isoform of Skp1 in cells. A strategy was developed for the synthesis of the core trisaccharide-Hyp based on the use of 2-naphthylmethyl (Nap) ethers as permanent protecting groups to allow late stage installation of the Hyp moiety. Tuning of glycosyl donor and acceptor reactivities was critical for achieving high yields and anomeric selectivities of glycosylations. The trisaccharide-Hyp moiety was employed for the preparation of the glycopeptide using microwave-assisted solid phase peptide synthesis. Enzyme kinetic studies revealed that trisaccharide-Hyp and trisaccharide-peptide are poorly recognized by AgtA, indicating the importance of context provided by the native Skp1 protein for engagement with the active site. The trisaccharide-peptide was a potent immunogen capable of generating a rabbit antiserum that was highly selective toward the trisaccharide isoform of full-length Skp1.
Keywords: glycosylation, carbohydrate, glycopeptide, antibodies, posttranslational modification
Introduction
Skp1 is a small, highly conserved eukaryotic protein of 160–190 amino acids that is proposed to play multiple cellular functions. Skp1 is frequently complexed with the F-box domain of F-box proteins (FBP), and the resulting heterodimers are often incorporated into the SCF (Skp1, cullin-1, F-box protein, Roc1/Rbx1/Hrt1) family of E3-ubiquitin ligases that is expressed universally in the cytoplasmic and nuclear compartments of eukaryotes.[1] Considering the multitude of FBPs, the E3SCF-Ub ligases are expected to target hundreds of proteins for polyubiquitination and subsequent degradation by the 26S-proteasome.[2]
Glycosylation of Skp1 in the social amoeba Dictyostelium was established by metabolic incorporation of [3H]Fuc,[3] and confirmed by compositional analysis of the protein showing the presence of N-acetyl-glucosamine (GlcNAc), fucose (Fuc), and galactose (Gal).[4] Mass spectrometry based-sequencing studies[4b] have shown that Skp1 is modified by a pentasaccharide attached to a hydroxyproline (Hyp) residue at position 143. Based on exoglycosidase digestions, the core trisaccharide has the structure of the type 1 blood group H antigen and is modified by two additional α-linked Gal residues.
Assembly of the glycan of Skp1 requires the prior oxidation of Proline-143 by a cytoplasmic non-heme Fe(II)-dependent dioxygenase – the prolyl 4-hydroxylase PhyA, to give a Hyp residue. Modification of Hyp with a galactoside or arabinoside is common in plants and certain algae[5] but, due to the localization of the enzymes in the secretory compartment, these glycoproteins are secreted.[6] In Dictyostelium, the Skp1 Hyp143 is modified by αGlcNAc mediated by the enzyme N-acetylglucosaminyltransferase-1 (Gnt1). Addition of the second and third sugars of the Skp1 glycan is mediated by PgtA, which has dual β3GalT/α2FucT activity resulting in the formation of the blood group H type 1 antigen Fucα1,2Galβ1,3GlcNAcα-Hyp143. Two α-galactoside residues are added by the enzyme AgtA, which synthesizes the following pentasaccharide: Galα1,?Galα1,3Fucα1,2Galβ1,3GlcNAcα-Hyp143. The linkage of the terminal αGal residue is yet to be determined.
In Dictyostelium, PhyA has important roles in O2-dependent development. Genetically-induced changes in PhyA expression levels cause inverse changes in the level of O2-required for culmination, the developmental process in which the multicellular slug converts to a fruiting body.[7] Conversely, genetically-induced changes in Skp1 expression levels cause a corresponding change in the level of O2 required for culmination, in a way that depends on the presence of Pro143.[8] Glycosyltransferase mutants exhibit requirements for intermediate levels of O2, with disruption of AgtA causing a particularly disruptive effect,[9] implying a unique regulatory role for the trisaccharide form of Skp1 or additional functions of AgtA. Although AgtA can catalyze the addition of the first of the two αGal residues to αFuc and Fucα1,2Galβ-acceptors, evidence indicates that the acceptor trisaccharide must reside in the context of full-length Skp1 for efficient addition of the first αGal and is absolutely required for addition of the second αGal. This depends at least in part on second site recognition of Skp1 by the WD40-repeat domain in AgtA.[9b] Further studies are needed to investigate the role of the local peptide environment on conformation of the glycan that may also be an important determinant for glycan recognition. In vitro and in vivo studies stress the importance of the glycan in promoting interactions with F-box proteins,[10] which may represent the mechanism by which hydroxylation and glycosylation of Skp1 mediate or modulate O2-sensing during Dictyostelium development.
Studies on O2-dependent hydroxylation of Skp1 in cells, relative stability of Skp1 isoforms in cells, and competitive interactions of Skp1 isoforms with binding partners, have all been made possible by isoform-specific antibodies that differentiate unmodified, hydroxylated, and GlcNAcylated forms of Skp1 from each other and all other isoforms.[10–11] We reasoned that availability of an antibody specific for the trisaccharide form would be similarly exploitable for related studies and to investigate the contingency of Skp1 glycosylation in response to stress and during development. The failure of available anti-blood group (type 1) antibodies to react with Skp1 necessitates the generation of new Abs.
We report here the chemical synthesis of a glycopeptide derived from Skp1 that is modified by a blood group H type 1 trisaccharide, which was conjugated to KLH and employed for antibody production in rabbits. A synthetic strategy was developed in which a trisaccharide was synthesized that was modified by Hyp to give, after a number of chemical manipulations, a glycosylated amino acid that could be employed for glycopeptide synthesis. 2-Naphthylmethyl (Nap) ethers were employed as permanent protecting groups to allow late stage installation of the Hyp moiety. It was found that tuning glycosyl donor and acceptor reactivities was critical for achieving high yields and anomeric selectivities of the glycosylations. The suitability of the novel glycopeptide for probing AgtA acceptor substrate preference and to induce an isoform specific antibody is presented.
Results and Discussion
The target glycopeptide 1 was synthesized by first preparing trisaccharide 5, which was modified by an appropriately protected Hyp residue to give, after a number of protecting group manipulations, glycosylated amino acid 6, which was used in solid phase peptide synthesis (Figure 1). Late stage installation of the Hyp moiety was attractive because this amino acid can adopt cis/trans configurations complicating NMR analysis. It was anticipated that trisaccharide 5 could readily be prepared from monosaccharide building blocks 2, 3 and 4. The non-participating azido moiety of 5 would allow the installation of the Hyp moiety as an α-glycoside. Furthermore, Nap ethers were selected as permanent protecting groups because they can be readily removed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)[12] to give, after standard O-acetylation, acetyl esters that are desirable for glycopeptide synthesis. The more commonly employed benzyl ethers could not be employed because their removal by catalytic hydrogenation would result in reduction of the azido moiety. Also, possible late stage benzyl ether removal after installation of the Hyp residue did not offer an attractive approach because their removal is not compatible with the presence of an Fmoc protecting group.
Figure 1.
Strategy for the chemical synthesis of a glycopeptide derived from Skp1. L is appropriate leaving group.
Thus, a TMSOTf mediated glycosylation[13] of trichloroacetimidate 2a with acceptor 3 gave disaccharide 7a in a modest yield of 32%, surprisingly as a mixture of anomers (Table 1, entry 1). The low yield of coupling product was due to rearrangement of the trichloroacetimidate to give a trichloro-N-acetamide adduct. Furthermore, despite the presence of a participating acetyl ester at C-2 of the glycosyl donor, a mixture of anomers was obtained. The use of acetonitrile as a participating solvent[14] only marginally improved the yield and anomeric selectivity of the glycosylation (entry 2). It is known that the use of N-phenyl-trifluoroacetimidates[15] as glycosyl donors can suppress rearrangement, and indeed the use of glycosyl donor 2b improved the yield of the glycosylation, however, disaccharide 7a was still obtained as a mixture of anomers (entries 3 and 4). An N-iodosuccinimide (NIS) / trifluoromethanesulfonic acid (TfOH) mediated coupling[16] of 2c with 3 in a mixture of acetonitrile and dichloromethane at −30 °C gave the required disaccharide 7a in an acceptable yield of 71% mainly as the β-anomer (entries 5 and 6). The yield and anomeric selectivity could be further improved by employing glycosyl donors 2d or 2e, which have at C-2 a 2,5-difluorobenzoyl[17] or Lev[18] esters, respectively to give disaccharides 7b and 7c (entries 7–9). In each case, the use of acetonitrile as a cosolvent improved the β-anomeric selectivity of the glycosylation. Probably, acetonitrile stabilizes an acyloxonium ion intermediate thereby promoting 1,2-trans anomeric selectivity. Alternatively, it may promote the formation of an α-nitrilium ion which upon displacement by a sugar alcohol will give a β-glycoside. Disaccharides 7a–c were converted to glycosyl acceptor 8 by removal of the acetyl or dFBz protecting groups of 7a and 7b, respectively by using Zemplén conditions, whereas the Lev ester of 7c was cleaved using hydrazine acetate.
Table 1.
Optimizing galactosylation
Next, attention was focused on the fucosylation of glycosyl acceptor 8 using donor 4 to give trisaccharide 5 (Table 2). Various promoters and solvent systems were examined, and it was found that the most favorable anomeric selectivity was achieved when iodonium dicollidine triflate (IDCT)[19] was used as the promoter in a mixture of dioxane in toluene[20] (Table 2, entry 2). The best yield was, however, accomplished using NIS/TMSOTf as the activator in diethyl ether at −10 °C (Table 2, entry 6). The use of a corresponding trichloroacetimidate donor activated by TMSOTf in dichloromethane at −30 °C gave the trisaccharide 5 in a low yield of 11% (data not shown).
Table 2.
Optimization fucosylation
Although Nap ethers are commonly employed as temporary protecting groups,[12] their use for permanent alcohol protection is less well established and to the best of our knowledge the removal of several of these functionalities by oxidation with DDQ has not been reported. Hydrolysis of naphthylidene acetal of 5 using 10% trifluoroacetic acid (TFA) in DCM, subsequent acetylation in acetic anhydride and pyridine, followed by DDQ oxidation in a mixture of DCM and water gave the required compound in low yield. Byproducts that were identified included a derivative having a 4,6-O-naphthylidene acetal on the galactoside residue and compounds having a naphthoate ester at C-6 or C-4 of the galactoside moiety. The formation of these byproducts can be rationalized by a mechanism in which the removal of the first Nap ether of the galactoside moiety (C-4 or C-6) is followed by oxidation of the second Nap ether to give an intermediate carbocation that despite the presence of water can be trapped by the neighboring alcohol to give a naphthylidene acetal. The latter derivative can be further oxidized by DDQ to form the naphthoate ester.
These unexpected results led us to investigate an alternative approach for the deprotection of the Nap ethers. Hydrogenolysis using Pd/C could not be employed as the azido functionality, which would be required for the installation of an α-linked Hyp moiety, would also be reduced under these conditions. Lewis acids such as FeCl3 have found utility for the deprotection of benzyl ethers.[21] Hence, attention was turned to the use of FeCl3 for the removal of the Nap ethers (Scheme 1). Thus, trisaccharide 9 was subjected to anhydrous FeCl3 followed by acetylation with acetic anhydride and pyridine, which gratifyingly gave compound 10 (yield of 43%) in which the azide functionality had remained intact and all the Nap ethers cleaved together with the anomeric TDS protecting group. The anomeric acetyl ester of 10 was selectively cleaved with hydrazine acetate and the resulting lactol converted into trichloroacetimidate 11 by treatment with trichloroacetonitrile in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). A triflic acid promoted glycosylation of 11 with benzyl protected N-α-(9-fluorenylmethyloxycarbonyl)-l-trans-4-hydroxyproline gave compound 12 in a yield of 71% mainly as the α-anomer. Reduction of the azido moiety of 12 was accomplished using thioacetic acid[22] to provide 13 which was subjected to hydrogenolysis to give 6, which is appropriately protected for use in glycopeptide synthesis.
Scheme 1.
a) 10% TFA in DCM; b) Ac2O, Pyridine; c) FeCl3, DCM; d) Ac2O, pyridine; e) H2NNHOAc, DMF; f) CCl3CN, DBU, DCM; g) 9-fluorenylmethyloxycarbonyl-l-trans-4-hydroxyproline, TfOH, Et2O, 0 °C, 15 min; h) AcSH, pyridine; i) 10% Pd/C, DMF, H2
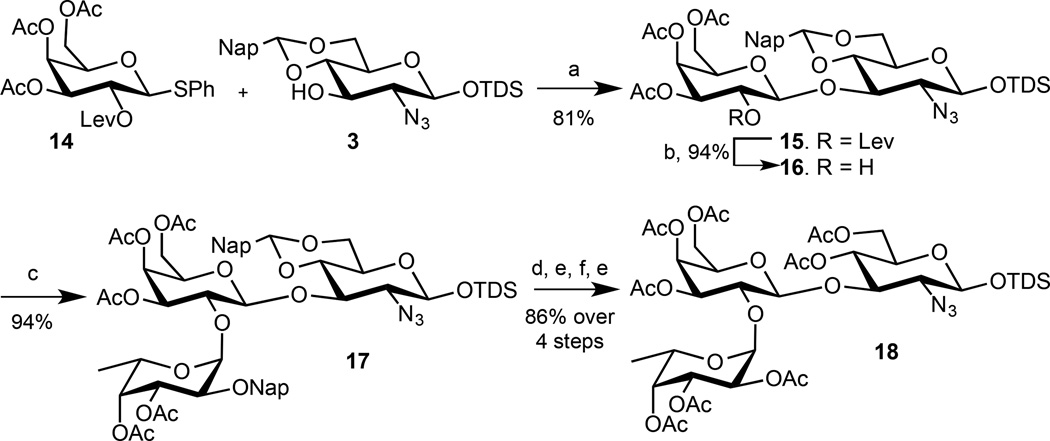
An alternative approach for the synthesis of 6 was explored to avoid the loss of expensive trisaccharide during the deprotection of the Nap ethers. It was envisaged that the use of galactosyl donor 14, which has permanent acetyl esters at C-3, 4 and 6 and a temporary Lev ester at C-2, would provide a trisacchararide that can be more readily converted into target trisaccharide 6 (Scheme 2). Thus, a glycosylation of 3 with 14 in the presence of NIS and TfOH in a mixture of DCM and acetonitrile gave disaccharide 15 in a yield of 81% as only the β-anomer. The Lev ester of 15 was removed using hydrazine acetate to give glycosyl acceptor 16 which was coupled with fucosyl donor 4 in diethyl ether at −10 °C using NIS/TMSOTf as the activator system, to give the trisaccharide 17 in a 94% yield as only the α-anomer.
Scheme 2.
a) NIS, TfOH, DCM:CH3CN, −30 °C, 30 min; b) H2NNH.OAc, EtOH:Toluene; c) 4, NIS, TMSOTf, Et2O, −10 °C, 20 min; d) 10% TFA in DCM; e) Ac2O, Pyridine; f) DDQ, DCM:H2O.
Subsequent treatment of 17 with 10% TFA in DCM to remove the naphthylidene acetal, acetic anhydride and pyridine to acetylate the resulting alcohols, DDQ to oxidatively remove the Nap ether, and finally acetic anhydride and pyridine for conversion of the resulting alcohol into acetyl ester, gave trisaccharide 18 in a much improved yield of 86% over four steps. A remarkable observation was that replacement of the ether protecting groups of galactosyl donor 2e with acetyl esters (14) did not only lead to a trisaccharide that could be more readily deprotected but also greatly facilitated the galactosylation and fucosylation reactions. Specifically, the lower reactivity of 14 led to an improvement in glycosylation yield and anomeric selectivity. Furthermore, fucosylation of the resulting acceptor (16), which has also a lower reactivity, also led to higher yielding and stereoselective glycosylation.
Glycopeptide 1 was synthesized by microwave-assisted solid phase glycopeptide synthesis[23] using Rink Amide AM LL resin (Scheme 3). The first eight amino acids were introduced using an HBTU-mediated HOBt ester activation protocol (→19). The glycosylated amino acid 6 was introduced manually using microwave-assisted solid phase peptide synthesis to give 20. The resin was returned to the automated peptide synthesizer to further elongate the glycopeptide. Cleavage from the resin was accomplished using 94% TFA, 2.5% H2O, 2.5% EDT, and 1% TIPS to afforded glycopeptide 22 which was treated with NaOMe buffered with guanidine.HCl to remove the acetyl esters,[24] to give after purification by C18 column chromatography, the target glycopeptide 1.
Scheme 3.
Assembly of Skp1 derived glycopeptide.
Substrate activity of the trisaccharide peptides toward AgtA
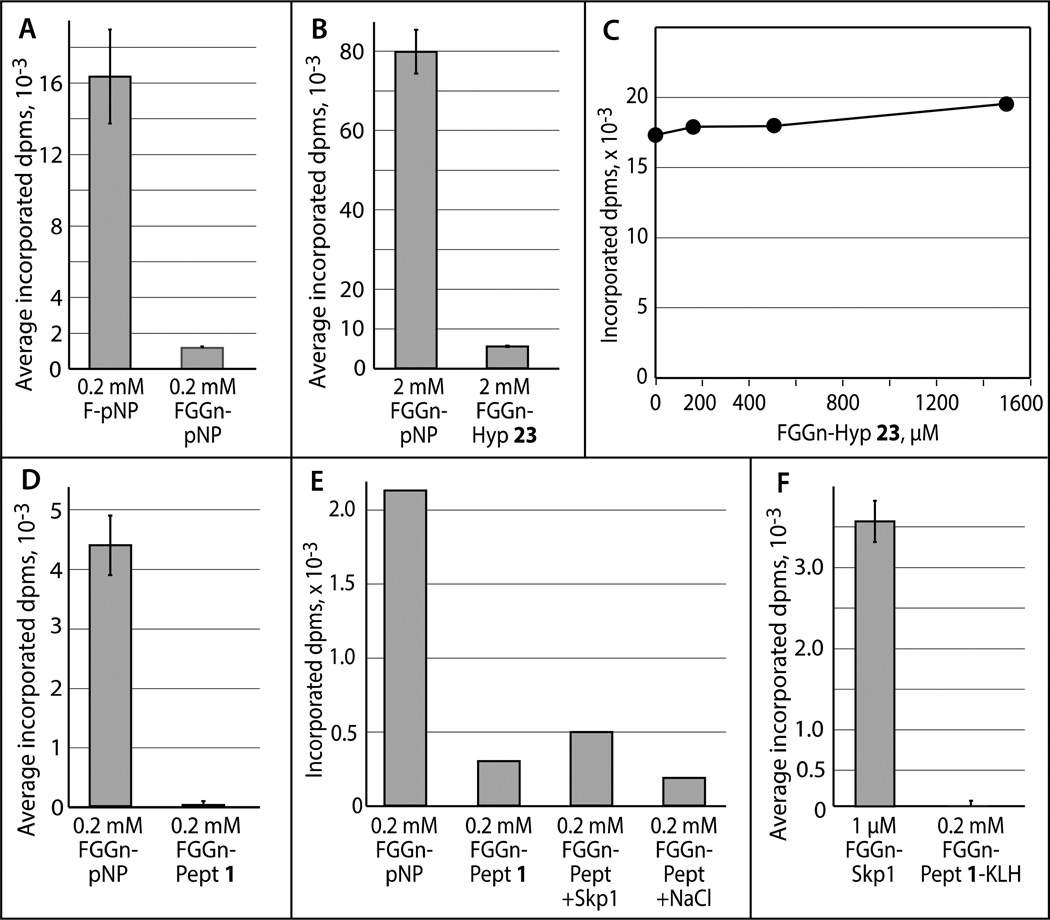
Previous studies showed that the order of preference of authentic and model acceptor substrates for AgtA is: FGGN-Skp1 > FG-pNP > F-pNP > FGGn-pNP, with little effect when the pNP aglycon is substituted with Bn or octyl.[9b] The substrate activities of FGGn-Hyp 23 and FGGn-peptide 1 were analyzed for ranking in this activity series, which might provide information regarding the importance of nearby amino acids for enzyme recognition and catalysis. Four methods of product detection were developed, to help avoid misinterpretation that could occur if substrate glycosylation interfered with detection. Under the conditions tested, activity toward FGGn-Hyp 23 was <10% that of FGGn-pNP (Figure 2B), and 1 exhibited even lower activity (Figures 2D–F). In addition, a 3-fold excess of 23 did not inhibit the reaction with F-pNP (Figure 2C). Further descriptions and analysis of the results are included in the supplement. The new acceptor activity hierarchy is: FGGN-Skp1 > FG-pNP > F-pNP > FGGn-pNP > FGGn-Hyp > FGGn-peptide. The inhibitory nature of the peptide and Hyp, relative to pNP, suggests that the local amino acids contribute to a conformation of the glycan that renders the 3-OH of Fuc less accessible to modification by AgtA, or otherwise renders the glycan unrecognizable to the AgtA active site.
Figure 2.
Acceptor activities of synthetic AgtA substrates. (A) Comparison of AgtA activity towards F-pNP and FGGn-pNP. Reactions were prepared with 10 µM UDP-[3H]Gal (2500 µCi/µmol) and His6AgtA, and incubated for 60 min. Incorporation was determined using method 1. (B) Activity towards FGGn-pNP and FGGn-Hyp 23 were determined in the presence of 5 µM UDP-[3H]Gal (10,000 µCi/µmol) and of 0.6× His6AgtA (relative to panel A) for 60 min. Incorporation was determined using method 2. (C) The effect of 23 on the 60-min reaction of 0.06× His6AgtA with 0.5 mM F-pNP in the presence of 5 µM UDP-[3H]Gal (5000 µCi/µmol), using method 2. (D) Activity towards FGGn-pNP and FGGn-peptide 1 was analyzed using 10 µM UDP-[3H]Gal (2500 µCi/µmol) and 1× His6AgtA for 180 min. Incorporation was determined using method 1. (E) Similar to D, except that 120-min reactions containing 10 µM UDP-[3H]Gal (5000 µCi/µmol) and 1.5× His6AgtA were analyzed using method 3. (F) Comparison of activities towards 1 coupled to KLH and FGGn-Skp1. Reactions were conducted using 5 µM UDP-[3H]Gal (10,000 µCi/µmol), the indicated concentrations of FGGn-peptide (0.66 mg/mL KLH-conjugate) or FGGn-Skp1, and 1× or 0.003× His6AgtA, respectively. Incorporation was determined using method 4. Error bars indicate ± SD.
Development of Skp1 specific antibodies
Glycoform-specific antibodies (Abs) are desirable for studying regulation of Skp1 glycosylation. To develop an Ab specific for the trisaccharide isoform of Skp1, FGGn-peptide 1 was conjugated to maleimide activated keyhole limpet hemocyanin (mcKLH) to give a conjugate carrying 641 glycan residues and used to immunize two rabbits. Ab specificity was examined by Western blotting using a panel of mutant cells uniquely expressing six of the seven isoforms of Skp1 generated by the Skp1 modification pathway. As shown in Figure 3A, pAb UOK104 exhibited a high degree of selectivity for trisaccharide (FGGn) modified Skp1, in the absence of affinity selection, and minor detection of pentasaccharide (GGFGGn) modified Skp1. pAb UOK105 exhibited similar, but weaker reactivity to all Skp1 isoforms (not shown).
Figure 3.
Specificity of anti-59/KLH UOK104 antibody. (A) Western blot analysis of a panel of Dictyostelium mutants that accumulate the indicated Skp1 glycoforms. Panels were probed with either pAb UOK104 or UOK77 (pan-specific for all Skp1 isoforms) at 1:1000 dilution. Note: An irregularity during placement of the gel on the blot membrane resulted in distortion of relative mobilities of the Skp1 isoforms. (B) The glycan specificity of UOK104 was tested against BSA conjugates of LNFP-I and LNFP-III, which were adsorbed in solutions containing the indicated pmol of glycan equivalents in 50 µL to wells of a 96-well ELISA plate. Coated wells were probed with UOK104 (1:500) or mAb ab3355 (1:10), with specificity for the blood group H type 1 antigen present in LNFP-I/BSA.
To test the basis for specific recognition of FGGn-Skp1, pAb UOK104 was tested for binding of a BSA-conjugate modified by lacto-N-fucopentaose-I (LNFP-I, Fucα1,2Galβ1,3GlcNAcβ1,3Galβ1,4Glc-) which is terminated by the same H-type trisaccharide as FGGn-Skp1 except the proximal sugar GlcNAc is in a β-linkage. As a negative control a BSA conjugate of lacto-N-fucopentaose-III (LNFP-III, Galβ1,3(Fucα1,3)GlcNAcβ1,3Galβ1,4Glc-) which has the same composition but different arrangement of the monosaccharides, was compared. Binding of UOK104 was not detected to either LNFP-I or LNFP-III (Figure 3B). As a positive control, LNFP-I was specifically recognized by ab3355, a mAb that recognizes Fucα1,2Galβ1,3GlcNAcβ1-, validating the assay.[25] Recognition of enzymatically assembled FGGn-Skp1 by an antibody raised against the synthetic glycopeptide supports the linkage assignments used to design the glycopeptide. Further studies are needed to determine whether the epitope(s) recognized by UOK104 is a peptide conformation imposed by the presence of the glycan, or consists jointly of both glycan and peptide determinants.
Conclusion
Reports dealing with the chemical synthesis of glycopeptides that are glycosylated at Hyp deal mainly with compounds having simple monosaccharide additions.[26] The preparation of glycopeptides having a complex glycan moiety linked to this amino acid has received even less attention, and to the best of our knowledge efforts have only been focused on the preparation of compounds derived from plants, which have very different oligosaccharide compositions compared to the glycopeptides described in this study. The properly protected trisaccharide-Hyp 6 was synthesized in excellent overall yield using a linear glycosylation strategy combined with the selection of appropriate protecting groups. The use of Nap ethers as a permanent protecting group made it possible to install a hydroxy-proline moiety at a late stage of synthesis. However, it was critical to avoid Nap ethers that were 1,2-cis to an alcohol due to unexpected byproduct formation. Furthermore, it was found that the application of galactosyl donor of low reactivity having acetyl esters at C-3, C-4 and C-6 and a temporary Lev ester at the C-2, gave markedly better yields and anomeric selectivities in glycosylations compared to the use of an ether protected and more reactive donor. Surprisingly, removal of the Lev ester of the resulting glycosylation product also provided a superior acceptor. The target glycopeptide was synthesized using automated microwave-assisted solid phase peptide synthesis using Rink Amide AM LL resin. The in vitro substrate dependence of AgtA revealed that galactose was transferred to trisaccharide-Hyp 23, however it was a poor substrate and the extended glycopeptide 1 was even less active. These results are consistent with folding of the acceptor hydroxyl of Fuc into a trisaccharide-dependent glycan conformation that is not recognized by AgtA.[27] The underlying hydroxyproline or glycopeptide may stabilize this unfavorable conformation leading to even lower activity levels towards these substrates.
The glycopeptide proved to be an excellent immunogen when conjugated to the carrier protein KLH, for inducing a highly glycoform-specific antiserum toward FGGn-Skp1. The Skp1 peptide appears to play an important role in recognition by pAb UOK104 as there was no recognition of LNFP I-BSA which contains the same Skp1 glycan attached to BSA. However, the possibility that UOK104 recognizes the glycan in a conformation induced by the Skp1 peptide cannot be excluded. pAb UOK104 is expected to be useful for studying the regulation of glycosylation in Dictyostelium, and the selective detection of FGGn-Skp1 in mixtures with other isoforms.
Experimental Section
General procedure for trichloroacetimidate glycosylations
Acceptor 3 and donor (2a or 2b) were dissolved in an appropriate solvent (DCM or a 1:1 mixture of DCM:CH3CN), and molecular sieves (4Å) were added. The mixture was stirred for 1 h at room temperature under an atmosphere of argon. The mixture was cooled to −30 °C and TMSOTf (0.2 eq) was added. The reaction mixture was left stirring at −30 °C for 15 min, after which it was diluted with DCM, filtered and the filtrate washed with a saturated solution of NaHCO3 and water, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc) to afford the target compound.
General procedure for thioglycoside glycosylations
Acceptor 3 and donor (2c, 2d, 2e, or 14) were dissolved in an appropriate solvent (DCM or a 1:1 mixture of DCM:CH3CN). Molecular sieves (4Å) were added and the mixture was stirred for 1 h at room temperature under an atmosphere of argon. NIS was added and the resulting solution was cooled to −30 °C, followed by the addition of TfOH. The reaction mixture was stirred in the dark at −30 °C for 15 min. The reaction mixture was diluted with DCM, filtered into a solution of sodium thiosulfate and stirred until the solution turned colorless. The organic layer was washed with a saturated solution of NaHCO3 and water, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc) to afford the disaccharides 7a–7c and 15.
General methods for automated microwave-assisted solid-phase peptide synthesis (MW-SPPS)
(Glyco)peptides were synthesized by established protocols on a CEM Liberty Automated Microwave Peptide Synthesizer equipped with a UV detector using N-α-Fmoc-protected amino acids and 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/1-hydroxybenzotriazole (HOBt) as the activating reagents. The compounds were prepared on a Rink Amide AM LL resin using the following amino acid building blocks: Fmoc-Ile-OH, Fmoc-Gln(Trt)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Hyp(tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Phe-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Pbf)-OH and Fmoc-Lys(Boc)-OH. Double couplings were employed for all amino acids. Deprotection of the N-α-Fmoc was achieved using 20% 4-methyl piperidine in DMF.
Dimethylthexylsilyl [3,4,6-tri-O-acetyl-β-D-galactopyranosyl]-(1→3)-4,6-O-(2-napthylidene)-2-deoxy-azido-β-D-glucopyranoside (16)
To a solution of compound 15 (1.91 g, 1.637 mmol) in EtOH (80 mL) and toluene (40 mL), hydrazine acetate (251 mg, 2.78 mmol) was added and the resulting reaction mixture was left stirring at room temperature for 1 h. The reaction mixture was concentrated under reduced pressure and the resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 2:1, v:v) to give 16 (1.64 g, 94%) as an amorphous white solid.15 1H NMR (CDCl3, 300 MHz): δ 0.00 (t, J = 3.9 Hz, 6H, CH3-Si-CH3), 0.71 (s, 12H, TDS-(CH3)2C-C(CH3)2), 1.46 (dd, J = 13.7, 6.8 Hz, 1H, TDS-CH), 1.59 (s, 3H, COCH3), 1.80–1.81 (m, 3H, COCH3), 1.90 (s, 3H, COCH3), 2.87 (s, 1H, OH), 3.22–3.29 (m, 2H, GlcN H-5, GlcN H-2), 3.56–3.76 (m, 6H, Gal H-5, GlcN H-4, GlcN H-3, Gal H-2, GlcN H-6a, Gal H-6a), 3.90 (dd, J = 11.1, 7.5 Hz, 1H, Gal H-6a), 4.14 (dd, J = 10.5, 5.0 Hz, 1H, GlcN H-6a), 4.39 (d, J = 7.8 Hz, 1H, Gal H-1), 4.46 (d, J = 7.6 Hz, 1H, GlcN H-1), 4.71 (dd, J = 10.3, 3.4 Hz, 1H, Gal H-3), 5.12 (d, J = 3.3 Hz, 1H, Gal H-4), 5.51 (s, 1H, Naphthylidene-H), 7.27–7.30 (m, 2H, 2x aromatic CH), 7.36 (dd, J = 8.5, 1.5 Hz, 1H, aromatic CH), 7.62–7.67 (m, 3H, 3x aromatic CH), 7.72 (s, 1H, aromatic CH). 13C NMR (75 MHz; CDCl3): δ 0.00, 1.03, 21.54, 21.65, 22.95, 23.08, 23.57, 23.77, 23.87, 27.97, 37.00, 64.37, 69.62, 70.11, 71.05, 71.71, 72.90, 74.39, 75.38, 82.80, 83.00, 100.80, 104.68, 107.03, 126.58, 128.53, 129.46, 129.73, 130.91, 131.44, 131.49, 135.97, 136.85, 137.31, 173.27, 173.32, 173.42. MALDI: [M+Na]+ C37H51N3NaO13Si, calcd 796.3089; obsvd 796.3134.
Dimethylthexylsilyl [3,4-di-O-acetyl-2-O-(2-methylnaphthyl)-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-D-galactopyranosyl]-(1→3)-4,6-O-(2-napthylidene)-2-deoxy-azido-β-D-glucopyranoside (17)
To a solution of donor 4 (2.24 g, 5.168 mmol) and acceptor 16 (2 g, 2.584 mmol) in anhydrous Et2O (52 mL), molecular sieves (4Å) were added and the resulting solution was left stirring for 1 h at room temperature under an atmosphere of argon. NIS (1.45 g, 6.46 mmol) was added, and the resulting solution was cooled to −10 °C, followed by the addition of a TMSOTf (46.8 µL). The reaction mixture was stirred in the dark at −10 °C for 20 min after which it was diluted with DCM and filtered into a solution of sodium thiosulfate and the solution was stirred until it turned colorless. The organic layer was extracted and washed with a saturated solution of NaHCO3 and water, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 2:1, v:v) to afford the trisaccharide 17 (2.79 g, 94%) as the α anomer. 1H NMR (CDCl3, 500 MHz): δ 0.14 (d, J = 13.88 Hz, 6H, CH3-Si-CH3), 0.70 (s, 12H, TDS-(CH3)2C-C(CH3)2), 0.94 (d, J = 6.4 Hz, 3H, Fuc CH3), 1.47 (t, J = 6.7 Hz, 1H, TDS-CH), 1.61 (dd, J = 11.3, 5.4 Hz, 6H, 2x COCH3), 1.74 (d, J = 8.5 Hz, 6H, 2x COCH3), 1.89 (s, 3H, COCH3), 3.23–3.28 (m, 3H, Gal H-5, GlcN H-2, GlcN H-5), 3.58–3.76 (m, 5H, GlcN H-3, GlcN H-6a, Fuc H-2, GlcN H-4, Gal H-6a), 3.80–3.86 (m, 2H, Gal H-6b, Gal H-2), 4.10 (dd, J = 10.5, 4.8 Hz, 1H, GlcN H-6b), 4.43–4.55 (m, 4H, Fuc H-5, CHHNap, CHHNap, GlcN H-1), 4.72 (d, J = 7.9 Hz, 1H, Gal H-1), 4.83 (dd, J = 9.7, 3.2 Hz, 1H, Gal H-3), 5.00 (d, J = 3.0 Hz, 1H, Gal H-4), 5.08 (d, J = 2.9 Hz, 1H, GlcN H-4), 5.17–5.21 (m, 2H, Fuc H-1, Fuc H-3), 5.46 (s, 1H, Naphthylidene-H), 7.16 (d, J = 8.3 Hz, 1H, aromatic CH), 7.24–7.31 (m, 4H, 4x aromatic CH), 7.40 (d, J = 8.5 Hz, 1H, aromatic CH), 7.49 (s, 1H, aromatic CH), 7.56–7.70 (m, 7H, 7x aromatic CH). MALDI: 13C NMR assigned from HSQC (125 MHz, CDCl3): δ 0.00, 18.59, 20.58, 22.24, 22.90 (x3), 36.18, 63.07, 67.72, 69.38, 70.38, 71.04 (x2), 72.04, 72.37, 74.03 (x2), 75.35, 76.02, 76.35, 79.67, 82.33, 98.92, 99.59, 102.91, 104.57, 126.15, 127.81, 128.47 (x4), 128.80 (x2), 130.13 (x2), 130.46 (x3). [M+Na]+ C58H73N3NaO19Si, calcd 1166.4505; obsvd 1166.6457.
Dimethylthexylsilyl [2,3,4-tri-O-acetyl-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-D-galactopyranosyl]-(1→3)-4,6-di-O-acetyl-2-deoxy-azido-β-D-glucopyranoside (18)
To a cooled solution of trisaccharide 17 (2.79 g, 2.44 mmol) in a mixture of DCM (100.8 mL) and water (10 mL), trifluoroacetic acid (11.2 mL) was added. The reaction mixture was stirred for 1 h after which it was neutralized with triethylamine, concentrated under reduced pressure and the residue azeotropically dried with toluene (3 × 15 mL). The resulting residue was dissolved in DCM (50 mL), pyridine (6 mL) and acetic anhydride (6 mL) and the resulting mixture was left stirring for 18 h. The reaction mixture was cooled (0 °C) and quenched with MeOH, concentrated in vacuo and azeotropically dried with toluene (4 × 20 mL) to give the product (~2.96 g), which was used without further purification. To a solution of the crude product (~2.96 g, 2.715 mmol) in a mixture of DCM (66 mL) and water (6.6 mL), 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) (1.2 g, 5.43 mmol) was added and the reaction mixture was left stirring vigorously for 80 min. The reaction mixture was concentrated in vacuo. The resulting residue was dissolved in DCM (50 mL), acetic anhydride (20 mL) and pyridine (20 mL) and the mixture left stirring for 24 h. The reaction mixture was cooled (0 °C) and quenched with MeOH, concentrated in vacuo and diluted with EtOAc, washed with a saturated aqueous solution of NaHCO3 and water. The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 1:1, v:v) to afford the product 18 (2.08 g, 86% over 4 steps). 1H NMR (CDCl3, 500 MHz): δ 0.00 (s, 6H, CH3-Si-CH3), 0.68 (s, 12H, TDS-(CH3)2C-C(CH3)2), 1.02 (d, J = 6.3 Hz, 3H, Fuc CH3), 1.47 (t, J = 6.5 Hz, 1H, TDS-CH), 1.75 (dd, J = 6.0, 0.9 Hz, 6H, 2x COCH3), 1.78 (s, 3H, COCH3), 1.84 (d, J = 0.8 Hz, 9H, 3x COCH3), 1.88 (d, J = 0.9 Hz, 3H, COCH3), 1.95 (d, J = 0.9 Hz, 3H, COCH3), 3.07 (dd, J = 9.5, 8.3 Hz, 1H, GlcN H-2), 3.40 (dd, J = 9.5, 4.3 Hz, 1H, GlcN H-5), 3.46 (t, J = 9.8 Hz, 1H, GlcN H-3), 3.59 (t, J = 8.7 Hz, 1H, Gal H-2), 3.63 (t, J = 6.8 Hz, 1H, Gal H-5), 3.81 (dd, J = 10.6, 8.0 Hz, 1H, Gal H-6a), 3.93–3.97 (m, 3H, GlcN H-6a, GlcN H-6b, Gal H-6b), 4.32 (dd, J = 15.8, 7.1 Hz, 2H, Fuc H-5, GlcN H-1), 4.60 (t, J = 9.7 Hz, 2H, Gal H-1, GlcN H-4), 4.79–4.82 (m, 2H, Fuc H2, Gal H-3), 5.07 (d, J = 3.3 Hz, 1H, Gal H-4), 5.12 (dt, J = 8.6, 3.9 Hz, 3H, Fuc H-3, Fuc H-4, Fuc H-1). 13C NMR assigned from HSQC (125 MHz, CDCl3): δ 0.33 (x2), 18.59, 21.25, 22.91, 23.57 (x8), 36.85, 63.75 (x2), 65.74, 67.74, 70.06, 70.73, 71.06, 71.39 (x2), 73.38, 74.05, 74.71, 75.04, 76.37, 79.03, 98.95, 100.28, 103.26.MALDI: [M+Na]+ C42H65N3NaO22Si, calcd 1014.3727; obsvd 1014.1285.
[2,3,4-tri-O-acetyl-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-d-galactopyranosyl]-(1→3)-4,6-di-O-acetyl-2-deoxy-azido-β-d-glucopyranosyl-2,2,2-trichloroacetimidate (11)
HF/pyridine (16.6 mL) was added drop wise to a cooled (0 °C) solution of the trisaccharide 18 (2.08 g, 2.1 mmol) in pyridine (40 mL) in an HDPE container. The reaction mixture was stirred at 0 °C for 30 min and then left stirring for an additional 3 h at room temperature. The reaction mixture was diluted with DCM, cooled (0 °C) and a solution of NaHCO3 was added and left stirring for 40 min. The organic layer was washed with a saturated aqueous solution of NaHCO3 and water. The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 1:2, v:v) to afford the hemiacetal (1.69 g, 95%) as an amorphous white solid. Trichloroacetonitrile (0.62 mL, 6.061 mmol) and DBU (44.1 µL, 0.242 mmol) were added sequentially to a solution of the hemiacetal (1.03 g, 1.21 mmol) in DCM (22 mL) and the reaction mixture was stirred under an atmosphere of Ar for 18 h. The reaction mixture was concentrated in vacuo and the resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 1:1, v:v) to afford donor 11 (860 mg, 72%) as an amorphous white solid. 1H NMR (CDCl3, 500 MHz): δ 1.17 (t, J = 6.9 Hz, 3H, Fuc CH3), 1.93 (s, 9H, 3x COCH3), 1.99 (d, J = 11.3 Hz, 6H, 2x COCH3), 2.04 (d, J = 4.4 Hz, 6H, 2x COCH3), 2.09 (d, J = 6.5 Hz, 3H, COCH3), 3.62 (dd, J = 10.4, 3.5 Hz, 1H, GlcN H-2), 3.80 (dd, J = 9.8, 7.7 Hz, 1H, Gal H-2), 3.86 (t, J = 6.6 Hz, 1H, Gal H-5), 3.98–4.19 (m, 6H, Gal H-6a, GlcN H-6a, GlcN H-6b, GlcN H-5, Gal H-6b, GlcN H-3), 4.47 (q, J = 6.5 Hz, 1H, Fuc H-5), 4.69 (d, J = 7.6 Hz, 1H, Gal H-1), 4.92–5.00 (m, 3H, Fuc H-2, Gal H-3, GlcN H-4), 5.24 (d, J = 3.4 Hz, 1H, Gal H-4), 5.27–5.29 (m, 2H, Fuc H-4, Fuc H-3), 5.34 (d, J = 3.8 Hz, 1H, Fuc H-1), 6.48 (d, J = 3.4 Hz, 1H, GlcN H-1), 8.80 (s, 1H, NH). 13C NMR assigned from HSQC (125 MHz, CDCl3): δ 15.39, 20.70 (x8), 61.21, 61.55, 61.88 (x2), 62.87, 64.87, 67.52 (x2), 67.85, 68.52, 70.51, 70.84, 71.17, 71.51, 73.50, 74.49, 94.41, 95.75, 100.39. MALDI: [M+Na]+ C36H47Cl3N4NaO22, calcd 1015.1645; obsvd 1015.1096.
N-α-(9-Fluorenylmethyloxycarbonyl)-l-trans-4-O-[[2,3,4-tri-O-acetyl-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-d-galactopyranosyl]-(1→3)-4,6-di-O-acetyl-2-deoxy-azido-α-d-glucopyranosyl]-proline benzyl ester (12)
To a solution of the donor 11 (1.08 g, 1.0846 mmol) and acceptor N-α-(9-fluorenylmethyloxycarbonyl)-L-trans-4-hydroxyproline (370 mg, 0.834 mmol) in Et2O (24 mL), molecular sieves (4Å) were added and the setup left to stir for 1 h at room temperature under an atmosphere of argon. The reaction flask was then cooled to 0 °C and TfOH (7.4 µL, 0.0834, as a 10:1 dilution in Et2O) was added and the reaction left to stir at 0 °C for 15 min. The reaction mixture was diluted with DCM, filtered and the filtrate washed with a saturated solution of NaHCO3 and water, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 1:1, v:v) to afford the product 12 (757 mg, 71%) as a separable 5:1 α-β mixture respectively.1H NMR (C6H6, 500 MHz): δ 1.34–1.37 (m, 3H, Fuc CH3), 1.42 (t, J = 4.6 Hz, 3H, COCH3), 1.56 (s, 3H, COCH3), 1.61 (d, J = 2.3 Hz, 3H, COCH3), 1.71–1.75 (m, 6H, 2x COCH3), 1.79–1.94 (m, 10H, 3x COCH3, HyPro-βHa), 2.04–2.07 (m, 1H, HyPro-βHb), 3.07–3.17 (m, 1H, Gal H-5), 3.22–3.26 (m, 1H, GlcN H-2), 3.51 (t, J = 5.8 Hz, 0.5H, HyPro-δHa), 3.57–3.61 (m, 1H, HyPro-δHb), 3.74–3.79 (m, 1H, Gal H-6a), 3.86–3.92 (m, 3H, Gal H-6a, HyPro-γH, HyPro-δHa, Fmoc-εH½), 3.94–3.98 (m, 1H, GlcN H-5), 4.07 (t, J = 6.9 Hz, 0.5H, Fmoc-εH½), 4.12 (td, J = 6.8, 3.2 Hz, 2H, Gal H-2, GlcN H-6a), 4.22–4.38 (m, 4H, GlcN H-6b, GlcN H-3, Fmoc-CH2), 4.51 (t, J = 3.5 Hz, 1H, HyPro-αH½, GlcN H-1½), 4.56 (d, J = 3.5 Hz, 0.5H, GlcN H-1½), 4.62–4.69 (m, 1.5H, Gal H-1, HyPro-αH½), 4.85–4.90 (m, 1.5H, Fuc H-5, Bn CHH½), 4.96–5.05 (m, 1.5H, Bn-CHH½), 5.07–5.12 (m, 1H, GlcN H-4), 5.20 (dt, J = 9.4, 4.4 Hz, 1H, Gal H-3), 5.28 (dd, J = 10.1, 3.3 Hz, 1H, Gal H-4), 5.43 (ddd, J = 10.6, 6.1, 4.2 Hz, 1H, Fuc H-2), 5.73 (d, J = 3.2 Hz, 1H, Fuc H-4), 5.87 (d, J = 3.84 Hz, 1H, Fuc H-1), 5.94 (ddd, J = 23.4, 11.0, 3.2 Hz, 1H, Fuc H-3), 6.93–7.05 (m, 3H, 3x aromatic CH), 7.15–7.28 (m, 6H, 6x aromatic CH), 7.42–7.60 (m, 4H, 4x aromatic CH). 13C NMR (125 MHz; C6D6): δ 15.58, 19.53, 19.87, 19.97, 20.14, 20.17, 20.31, 20.35, 20.39, 20.48, 35.91, 37.02, 47.48, 47.54, 51.80, 52.15, 58.00, 58.42, 60.44, 62.38, 62.83, 65.18, 65.23, 66.85, 66.91, 67.03, 67.62, 68.26, 68.45, 68.50, 69.01, 69.05, 69.33, 70.57, 71.68, 71.76, 71.87, 73.98, 74.02, 74.63, 75.08, 76.12, 78.13, 95.93, 97.29, 97.95, 100.79, 101.05, 120.07, 120.17, 120.19, 125.27, 125.39, 127.16, 127.23, 127.29, 127.34, 127.74, 128.33, 128.42, 128.50, 128.59, 128.62, 135.83, 136.01, 141.58, 141.61, 141.71, 144.27, 144.35, 144.42, 144.55, 154.25, 154.66, 169.10, 169.33, 169.71, 169.77, 169.81, 169.87, 170.03, 170.07, 170.25, 170.29, 170.61, 171.88, 171.98. MALDI: [M+Na]+ C61H70N4NaO26, calcd 1297.4176; obsvd 1297.4746.
N-α-(9-Fluorenylmethyloxycarbonyl)-l-trans-4-O-[[2,3,4-tri-O-acetyl-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-d-galactopyranosyl]-(1→3)-4,6-di-O-acetyl-2-deoxy-2-(N-acetamido)-α-d-glucopyranosyl]-proline benzyl ester (13)
Thiol acetic acid (10 mL) was added to a solution of compound 12 (470 mg, 0.3686 mmol) in pyridine (10 mL) and the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was diluted with EtOAc, washed with a saturated solution of NaHCO3 and a solution of CuSO4, dried (MgSO4), filtered and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexanes:EtOAc, 3:7, v:v) to afford 13 (360 mg, 75%). 1H NMR (CDCl3, 500 MHz) δ, 1.21–1.26 (m, 3H, Fuc CH3), 1.97–2.14 (m, 27H, 9x COCH3), 2.20–2.25 (m, 1H, HyPro-βHa), 2.57 (d, J = 0.6 Hz, 1H, HyPro-βHb), 3.65 (d, J = 10.7 Hz, 0.5H, HyPro-δHa½), 3.71–3.75 (m, 2.5H, Gal H-2, HyPro-δHb, HyPro-δHa½), 3.82 (t, J = 6.6 Hz, 1H, Gal H-5), 3.92 (q, J = 8.8 Hz, 2H, GlcNAc H-3, GlcNAc H-5), 3.99 (t, J = 8.9 Hz, 1.5H, Fmoc-εH½, Gal H-6a), 4.11 (d, J = 11.9 Hz, 1H, GlcNAc H-6a), 4.17–4.26 (m, 3.5H, Gal H-6b, GlcNAc H-6b, Fmoc CHH, Fmoc-εH½), 4.33–4.37 (m, 1.5H, GlcNAc H-2, HyPro-γH½), 4.43–4.52 (m, 3.5H, Gal H-1, HyPro-γH½, Fmoc CH½H, Fuc H-5, HyPro-αH½), 4.62 (d, J = 7.4 Hz, 1H, HyPro-αH½, Fmoc CH½H), 4.79 (d, J = 3.2 Hz, 1H, GlcNAc H-1), 4.86–4.87 (m, 1H, GlcNAc H-4), 4.94 (td, J = 9.8, 3.1 Hz, 2H, Gal H-3, Fuc H-2), 5.07 (d, J = 12.1 Hz, 0.5H, BnCH½H), 5.14 (dd, J = 21.6, 8.8 Hz, 1H, BnCHH), 5.24 (dd, J = 14.1, 10.5 Hz, 2.5H, BnCH½H, Fuc H-1, Gal H-4), 5.35 (s, 1H, Fuc H-4), 5.47 (dd, J = 10.8, 3.3 Hz, 1H, Fuc H-3), 7.31 (t, J = 6.9 Hz, 6H, 6x aromatic CH), 7.41–7.57 (m, 5H, 5x aromatic CH), 7.78 (d, J = 6.7 Hz, 2H, 2x aromatic CH). 13C NMR (125 MHz; C6D6): δ 14.05, 14.20, 15.36, 19.62, 19.92, 20.10, 20.20, 20.39, 20.49, 20.56, 22.95, 23.06, 23.10, 29.65, 29.95, 30.04, 32.17, 36.14, 36.22, 37.23, 47.30, 52.56, 55.57, 58.21, 59.92, 60.64, 60.89, 62.41, 62.60, 65.61, 66.98, 67.59, 67.80, 67.88, 67.91, 69.23, 69.95, 70.15, 72.46, 73.33, 73.82, 74.01, 77.30, 96.81, 97.62, 99.02, 100.85, 120.18, 125.05, 125.16, 125.19, 125.31, 125.49, 127.21, 127.32, 128.61, 135.69, 135.87, 141.48, 141.60, 143.77, 143.96, 144.60, 154.98, 168.97, 169.63, 169.85, 169.93, 170.00, 170.12, 170.22, 170.29, 170.54, 171.75. MALDI: [M+Na]+ C63H74N2NaO27, calcd 1313.4377; obsvd 1313.5216.
N-α-(9-Fluorenylmethyloxycarbonyl)-l-trans-4-O-[[2,3,4-tri-O-acetyl-β-l-fucopyranosyl]-(1→2)-[3,4,6-tri-O-acetyl-β-d-galactopyranosyl]-(1→3)-4,6-di-O-acetyl-2-deoxy-2-(N-acetamido)-α-d-glucopyranosyl]-proline (6)
10% Pd on activated carbon (30 mg) was added to a solution of 13 (151 mg, 0.117 mmol) in DMF (3.8 mL), and the mixture stirred for 20 min at room temperature. The argon was replaced with H2, and the reaction mixture was stirred for 2.5 h. The reaction mixture was filtered through celite, and the filtrate was concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (CHCl3:MeOH:AcOH, 99:2:0.2, v:v:v) to afford compound 6 (119 mg, 85%) as an amorphous white solid. 1H NMR (CDCl3, 500 MHz): δ 1.21 (s, 3H, Fuc CH3), 1.99–2.11 (m, 27H, 9x COCH3), 2.26–2.53 (m, 2H, HyPro-βHa, HyPro-βHb), 3.60 (d, J = 10.8 Hz, 0.5H, HyPro-δHa½), 3.72 (dt, J = 17.3, 8.2 Hz, 2.5H, HyPro-δHa½, HyPro-δHb, Gal H-2), 3.83 (d, J = 5.9 Hz, 1H, Gal H-5), 3.97 (dd, J = 19.6, 8.9 Hz, 3H, GlcNAc H-3, GlcNAc H-5, Gal H-6a), 4.11–4.61 (m, 11H, GlcNAc H-6a, GlcNAc H-6b, Gal H-6b, Fmoc-εH, Fmoc CHH, GlcNAc H-2, HyPro-γH, HyPro-αH, Fuc H-5, Gal H-1, Fmoc CHH), 4.80 (d, J = 3.3 Hz, 1H, GlcNAc H-1), 4.85–4.89 (m, 1H, GlcNAc H-4), 4.92–4.97 (m, 2H, Gal H-3, Fuc H-2), 5.25 (t, J = 4.0 Hz, 2H, Gal H-4, Fuc H-1), 5.34 (s, 1H, Fuc H-4), 5.45 (dd, J = 10.8, 3.0 Hz, 1H, Fuc H-3), 5.67 (d, J = 7.2 Hz, 1H, OH), 7.31 (t, J = 7.1 Hz, 2H, 2x aromatic CH), 7.37–7.41 (m, 2H, 2x aromatic CH), 7.56 (d, J = 6.2 Hz, 2H, 2x aromatic CH), 7.77 (d, J = 7.0 Hz, 2H, 2x aromatic CH). 13C NMR assigned from HSQC (125 MHz, CDCl3): δ 15.40, 20.72 (x8), 22.71, 35.65 (x2), 37.31 (x2), 47.27 (x2), 51.59, 51.92, 52.25, 57.56, 58.23, 60.22, 60.55 (x2), 62.54 (x2), 65.20, 66.86, 67.19, 67.86, 68.19 (x3), 68.85 (x2), 69.18, 69.85, 71.84, 72.84, 73.50, 73.83, 76.16, 76.82, 96.41, 97.73, 100.72, 120.31, 124.95, 127.28, 127.94. MALDI: [M+Na]+ C56H68N2NaO27, calcd 1223.3907; obsvd 1223.4296.
Glycopeptide (1)
The glycopeptide was synthesized by SPPS on a Rink Amide AM resin (147 mg, 0.05 mmol) as described above. After automated synthesis of the first 8 amino acids, the glycosylated amino acid 6 (90 mg, 0.075 mmol, 1.5) coupling was carried out manually on a CEM Discover SPS Microwave Synthesizer using HATU (30 mg, 0.075 mmol) and HOAt (10 mg, 0.075 mmol) as coupling reagents in the presence of DIPEA (35 µL, 0.2 mmol) in DMF (1 mL). The mixture was left at room temperature for 18 h, followed by microwave-irradiated coupling reaction, which was monitored by Kaiser test and was complete after 10 min. The resin was then returned to the synthesizer and peptide elongation was continued by automated synthesis as described above. The resin was rinsed with DCM (6 × 5 mL) and MeOH (6 × 5 mL) and dried under vacuum overnight. The resin was placed in a bubbler and treated with 10 mL of 94% TFA, 2.5% H2O, 2.5% EDT, 1% TIPS) for 2 h with occasional agitation. The resin was filtered off, and washed with DCM (2 × 8 mL). The filtrate was concentrated to 1/5 of its volume and the glycopeptide was precipitated out using ice-cold diethyl ether and recovered by centrifugation at 3,000 rpm for 15 min. The glycopeptide was dissolved in 50% aqueous acetonitrile and lyophilized. The obtained residue was dissolved in 5 mL of the stock solution (477 mg guanidine.HCl, 23 mg Na, 45 mL MeOH, 5 mL DCM) and left stirring at room temperature for 18 h. The reaction mixture was neutralized with AcOH and concentrated under reduced pressure. The glycopeptide 1 was purified by C18 reversed-phase HPLC and lyophilized. ESI: [C106H170KN25NaO45S]2+, calcd 2607.1027 [1304.055]2+; obsvd as doubly charged ion 1304.0514.
Conjugation of glycopeptide 1 to mcKLH
0.1 M sodium phosphate buffer pH 8.0 containing 0.15 M NaCl and 5 mM EDTA (200 µL) was added to a solution of Imject maleimide activated mcKLH (2.3 mg) in 0.1 M sodium phosphate buffer pH 7.2 containing 0.15 M NaCl (200 µL). This mixture (200 µL) was then added to glycopeptide 1 and the setup was left to incubate at room temperature for 18 h. Purification was performed via spin filtration through a 3 kDa filter and washed with 0.1 M sodium phosphate buffer pH 7.2 containing 0.15 M NaCl (500 µL), six times. The KLH conjugate was taken up in 0.1 M sodium phosphate buffer pH 7.2 containing 0.15 M NaCl (1 mL). This gave a conjugate with 641 residues per KLH molecule as determined by quantitative monosaccharide analysis by HPAEC/PAD and Bio-Rad protein concentration test.
Supplementary Material
Acknowledgements
This research was supported by NIH/NIGMS Grant Nos. R01GM084383 (West, Boons) and R01GM037539 (West).
Footnotes
Supporting information for this article is available. Provided are experimental procedures, copies of NMR spectra, HPLC traces of glycopeptides.
References
- 1.Willems AR, Schwab M, Tyers M. Biochim. Biophys. Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Skaar JR, Pagan JK, Pagano M. Nat. Rev. Mol. Cell Biol. 2013;14:369–381. doi: 10.1038/nrm3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Yanes B, Cicero JM, Brown RD, Jr, West CM. J. Biol. Chem. 1992;267:9595–9605. [PubMed] [Google Scholar]
- 4.a) Kozarov E, van der Wel H, Field M, Gritzali M, Brown RD, Jr, West CM. J. Biol. Chem. 1995;270:3022–3030. doi: 10.1074/jbc.270.7.3022. [DOI] [PubMed] [Google Scholar]; b) Teng-umnuay P, Morris HR, Dell A, Panico M, Paxton T, West CM. J. Biol. Chem. 1998;273:18242–18249. doi: 10.1074/jbc.273.29.18242. [DOI] [PubMed] [Google Scholar]
- 5.Ishiwata A, Kaeothip S, Takeda Y, Ito Y. Angew. Chem. Int. Ed. 2014;53:9812–9816. doi: 10.1002/anie.201404904. [DOI] [PubMed] [Google Scholar]
- 6.West CM, Wang ZA, van der Wel H. Biochim. Biophys. Acta. 2010;1800:160–171. doi: 10.1016/j.bbagen.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West CM, van der Wel H, Wang ZA. Development. 2007;134:3349–3358. doi: 10.1242/dev.000893. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZA, Singh D, van der Wel H, West CM. Dev. Biol. 2011;349:283–295. doi: 10.1016/j.ydbio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Zhang D, van der Wel H, Johnson JM, West CM. J. Biol. Chem. 2012;287:2006–2016. doi: 10.1074/jbc.M111.314021. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schafer CM, Sheikh MO, Zhang D, West CM. J. Biol. Chem. 2014;289:9076–9088. doi: 10.1074/jbc.M113.528679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Sheikh MO, Schafer CM, Powell JT, Rodgers KK, Mooers BH, West CM. Biochemistry. 2014;53:1657–1669. doi: 10.1021/bi401707y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sheikh MO, Xu Y, van der Wel H, Walden P, Hartson SD, West CM. Mol. Cell. Proteomics. 2015;14:66–80. doi: 10.1074/mcp.M114.044560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Wang ZA, Green RS, West CM. BMC Dev. Biol. 2012;12:31. doi: 10.1186/1471-213X-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Gaunt MJ, Yu JQ, Spencer JB. J. Org. Chem. 1998;63:4172–4173. [Google Scholar]; b) Xia J, Abbas SA, Locke RD, Piskorz CF, Alderfer JL, Matta KL. Tetrahedron Lett. 2000;41:169–173. [Google Scholar]
- 13.Schmidt RR, Kinzy W. Adv. Carbohydr. Chem. Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 14.a) Pougny JR, Sinay P. Tetrahedron Lett. 1976:4073–4076. [Google Scholar]; b) Ratcliffe AJ, Fraser-Reid B. J. Chem. Soc.-Perkin Trans. 1990;1:747–750. [Google Scholar]; c) Schmidt RR, Behrendt M, Toepfer A. Synlett. 1990:694–696. [Google Scholar]
- 15.a) Yu B, Tao HC. Tetrahedron Lett. 2001;42:2405–2407. [Google Scholar]; b) Cai S, Yu B. Org. Lett. 2003;5:3827–3830. doi: 10.1021/ol0353161. [DOI] [PubMed] [Google Scholar]
- 16.Veeneman GH, van Leeuwen SH, van Boom JH. Tetrahedron Lett. 1990;31:1331–1334. [Google Scholar]
- 17.Sjölin P, Kihlberg J. J. Org. Chem. 2001;66:2957–2965. doi: 10.1021/jo001584q. [DOI] [PubMed] [Google Scholar]
- 18.van Boom JH, Burgers PMJ. Tetrahedron Lett. 1976;17:4875–4878. [Google Scholar]
- 19.Veeneman GH, van Leeuwen SH, Zuurmond H, van Boom JH. J. Carbohydr. Chem. 1990;9:783–796. [Google Scholar]
- 20.Demchenko A, Stauch T, Boons GJ. Synlett. 1997:818–820. [Google Scholar]
- 21.Rodebaugh R, Debenham JS, Fraser-Reid B. Tetrahedron Lett. 1996;37:5477–5478. [Google Scholar]
- 22.Kale RR, McGannon CM, Fuller-Schaefer C, Hatch DM, Flagler MJ, Gamage SD, Weiss AA, Iyer SS. Angew. Chem. Int. Ed. 2008;47:1265–1268. doi: 10.1002/anie.200703680. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T, Hinou H, Kurogochi M, Shimizu H, Nishimura SI. Org. Lett. 2005;7:877–880. doi: 10.1021/ol0474352. [DOI] [PubMed] [Google Scholar]
- 24.Ellervik U, Magnusson G. Tetrahedron Lett. 1997;38:1627–1628. [Google Scholar]
- 25.Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S, Tattersall P, Agbandje-McKenna M, Cummings RD, Smith DF. J. Biol. Chem. 2012;287:44784–44799. doi: 10.1074/jbc.M112.425819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.a) Owens NW, Stetefeld J, Lattova E, Schweizer F. J. Am. Chem. Soc. 2010;132:5036–5042. doi: 10.1021/ja905724d. [DOI] [PubMed] [Google Scholar]; b) Corcilius L, Santhakumar G, Stone RS, Capicciotti CJ, Joseph S, Matthews JM, Ben RN, Payne RJ. Bioorg. Med. Chem. 2013;21:3569–3581. doi: 10.1016/j.bmc.2013.02.025. [DOI] [PubMed] [Google Scholar]; c) Kaeothip S, Ishiwata A, Ito Y. Org. Biomol. Chem. 2013;11:5892–5907. doi: 10.1039/c3ob41212a. [DOI] [PubMed] [Google Scholar]; d) Shinohara H, Matsubayashi Y. Plant Cell Physiol. 2013;54:369–374. doi: 10.1093/pcp/pcs174. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Xie N, Taylor CM. J. Org. Chem. 2014;79:7459–7467. doi: 10.1021/jo501191b. [DOI] [PubMed] [Google Scholar]
- 27.Karunaratne CV, Weldeghiorghis TK, West CM, Taylor CM. J. Am. Chem. Soc. 2014;136:15170–15175. doi: 10.1021/ja5033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.