Abstract
Objective
HIV-infected people are at increased risk of cancers of infectious origin. We estimated the burden of cancer attributable to infections among HIV-infected people in the United States in 2008.
Design
Incidence rates for cancer sites associated with infections were estimated from record linkage between HIV/AIDS registries and cancer registries.
Methods
Rates were applied to estimates of the population living with diagnosed HIV in the United States in 2008 to obtain the number of incident cancer cases. Site-specific attributable fractions (AF) and corresponding 95% confidence intervals (CI) were estimated from infection prevalence among cancer cases. Infection prevalence data were derived from literature review of case series.
Results
Of an estimated 6200 incident cancer cases (95%CI: 6000–6500), 2500 (95%CI: 2400–2700) were attributable to infection (AF=40%, 95%CI: 39–42). The most important infections were Kaposi sarcoma herpes virus, Epstein-Barr virus, and human papillomavirus, which together were responsible for 2200 new cancer cases (95%CI: 2100–2400), mainly Kaposi sarcoma, lymphomas, and ano-genital cancers. The AF in HIV-infected people was highest in the age group 20–29 years (69%, 95%CI: 65–72). Men who have sex with men were the HIV transmission group with the highest AF (48%, 95%CI: 46–50), due to the high incidence of both Kaposi sarcoma and anal cancer.
Conclusion
The very high fraction of cancer attributable to infection in HIV-infected people points to special opportunities to prevent these cancers, i.e., avoidance, detection, and early treatment of cancer-associated infections and universal early detection and uninterrupted treatment of HIV infection to avoid immunosuppression.
Keywords: Infection, cancer, attributable fraction, HIV, AIDS, United States
Introduction
People infected with human immunodeficiency virus (HIV) are at increased risk of developing cancers of infectious origin [1]. The prevalence of some oncogenic infectious agents is higher in HIV-infected people than in the general population (e.g., human papillomavirus [HPV], hepatitis C virus [HCV]), because many of these infectious agents share sexual and parenteral transmission routes with HIV [1]. Immunosuppression due to HIV also enhances the risk of persistent infection and neoplastic progression of several oncogenic viruses [2–4]. Non-infectious cofactors such as tobacco and alcohol consumption are also more frequent in HIV-infected people, and may contribute to excess cancer in this population [5–7]. Finally, since 1996, combined anti-retroviral treatment (cART) has reduced the incidence of some virus-associated malignancies, but increasing longevity provides more time for malignancies to develop [8–10].
We previously reported the burden of cancers attributable to infection in the general world population [11]. We now use the same methods to estimate this burden among HIV-infected people during the cART period. HIV-infected people in the United States were chosen on account of the wider range of information available compared to other countries in which only small cancer and HIV linkage studies have been possible [12, 13]. HIV has been classified as carcinogenic to humans by The International Agency for Research on Cancer (IARC), and is considered to cause cancer indirectly through immune suppression and the increased expression of the effects of oncogenic viruses [1]. Therefore, we do not attribute any cancers directly to HIV, but only to infections that may act in conjunction with HIV.
Methods
Choice of infectious agents and cancer sites
We consider infectious agents that have been classified as carcinogenic to humans by IARC [1], i.e., HPV, Epstein-Barr virus (EBV), hepatitis B virus (HBV), HCV, Helicobacter pylori (H. pylori) and Kaposi sarcoma-associated herpes virus (KSHV) (see Supplemental Digital Content 1, Appendix Table 1). Other carcinogenic infectious agents were not included because they are very rare in the United States, namely human T-lymphotrophic virus type 1, Opisthorchis viverrini, Clonorchis sinensis, and Schistosoma haematobium.
Cancer sites and histological types included (see Supplemental Digital Content 1, Appendix Table 1) are those for which an association with at least one infectious agent was reported in the same IARC volume [1]. Non-Hodgkin lymphoma (NHL) was subdivided by site and histology. NHL located in the central nervous system (CNS) was considered as a single cancer site regardless of histology. The remaining non-CNS NHLs were classified as diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma, and other NHL types collectively referred as “other non-CNS NHL”, which were not considered associated with infections.
Data sources
Cancer Incidence
The HIV/AIDS Cancer Match (HACM) Study (www.hivmatch.cancer.gov) is a population-based, registry-linkage study of HIV and cancer registries covering the period 1980–2010 in 14 U.S. states and metropolitan regions [9]. Seven HIV registries provided data on both people with acquired immune deficiency syndrome (AIDS) and people with HIV in the absence of AIDS (HIV-only) for the period 1996–2010, and 7 additional registries collected data only on people with AIDS [14]. Cases of HIV infection, with or without AIDS, are reportable to these registries through passive and active surveillance systems. Records from HIV-infected people were linked to cancer registry records using a probabilistic matching algorithm. Data from all registries active in any given year were merged, creating two separate cohorts (people with AIDS or HIV-only). In the sequel, the two cohorts are collectively referred to as HIV-infected people. Children (ages 0–14 years) were excluded, on account of the different cancer types involved compared to adults. Only aggregated counts of cases and person-years were retained for analysis. Institutional review boards at participating sites approved the HACM Study, as required.
Information on malignancies was obtained from cancer registries and coded according to the International Classification of Diseases for Oncology (third edition) [15]. Cancers were classified by site and histology using a modification of the Surveillance, Epidemiology, and End Results (SEER) Program’s “site recode with KS and mesothelioma” [15].
HIV-infected population
Estimates of the number of HIV-infected people in the United States in the year 2008 were obtained from the Centers for Disease Control and Prevention (CDC) using previously described methods [16].
Estimation of cancer incidence rates
Site-specific case counts and person-years at risk in the HACM Study were cross-tabulated by calendar year (1996–2010); age (from 15–19 years and then in 10-year age bands to ≥70 years); sex; race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latino, other/unknown); HIV transmission category (men who have sex with men (MSM), intravenous drug users (IDUs), MSM IDUs, heterosexual, other, unknown); AIDS or HIV-only; and, among people with AIDS, time since AIDS diagnosis (0–11 months, 12–35 months, 36–59 months, ≥60 months). For each cancer site associated with infection, Poisson regression models were used to estimate incidence rates using the above cross-classifying variables as covariates. A separate Poisson regression model was fitted to the total incidence of all cancer sites not associated with infection. All covariates were fitted as categorical variables except for calendar year, which was fitted as a continuous variable to give a log-linear trend with time. Bayesian regularization was used to avoid over-estimation of rate ratios when data were sparse [17]. To find the most parsimonious model, stepwise covariate selection was carried out, minimizing the Akaike Information Criterion (AIC). The stepwise selection allows the complexity of the model to adapt automatically to the quantity of available data, and the AIC chooses the model that gives the best predictions [18].
Model fit was evaluated by comparing observed and predicted case counts by calendar year. A poor fit was observed for KS, CNS NHL and non-CNS DLBCL due to the rapid decline in incidence of these cancers following the introduction of cART in 1996, which was not consistent with a linear time trend. Therefore, only data from the period 2002–2010 were used for KS and NHL.
Cases of non-CNS NHL of unknown histology were reclassified as one of DLBCL, Burkitt lymphoma, or “other specified non-CNS NHL” in proportion to the number of cases in each category. The proportions were estimated by fitting a multinomial regression model. Stepwise variable selection was again used to find the most parsimonious model for reclassification.
CDC estimates of the HIV-infected population in the year 2008 were cross-classified by the same risk factors as the HACM data (Table 1). Estimated incidence rates derived from the Poisson regression models fitted to the HACM data were then applied to the CDC population estimates to give the estimated number of incident cancers. Calculation of confidence intervals is described in the Appendix (see Supplemental Digital Content 1).
Table 1.
Estimated number of people living with diagnosed HIV in the United States in 2008
| Category | Female | % | Male | % | Total | % |
|---|---|---|---|---|---|---|
| Time since AIDS diagnosis (months) | ||||||
| No AIDS (HIV-only) | 98261 | 47.9 | 260654 | 42.8 | 358915 | 44.1 |
| 0 to 11 | 7767 | 3.8 | 21740 | 3.6 | 29507 | 3.6 |
| 12 to 35 | 15527 | 7.6 | 42660 | 7.0 | 58187 | 7.2 |
| 36 to 59 | 15453 | 7.5 | 43547 | 7.2 | 59000 | 7.3 |
| 60+ | 67953 | 33.2 | 239945 | 39.4 | 307898 | 37.8 |
| Total | 204961 | 100.0 | 608546 | 100.0 | 813507 | 100.0 |
| Risk group | ||||||
| MSM | 0.0 | 399992 | 65.7 | 399992 | 49.2 | |
| MSM & IDU | 0.0 | 48684 | 8.0 | 48684 | 6.0 | |
| IDU | 54929 | 26.8 | 88519 | 14.5 | 143448 | 17.6 |
| Heterosexual | 145285 | 70.9 | 65428 | 10.8 | 210713 | 25.9 |
| Other | 4747 | 2.3 | 5923 | 1.0 | 10670 | 1.3 |
| Total | 204961 | 100.0 | 608546 | 100.0 | 813507 | 100.0 |
| Race / ethnicity | ||||||
| Non-Hispanic White | 38474 | 18.8 | 241851 | 39.7 | 280325 | 34.5 |
| Non-Hispanic Black | 126245 | 61.6 | 228438 | 37.5 | 354683 | 43.6 |
| Hispanic / Latino | 33545 | 16.4 | 117750 | 19.3 | 151295 | 18.6 |
| Other / unknown | 6697 | 3.3 | 20507 | 3.4 | 27204 | 3.3 |
| Total | 204961 | 100.0 | 608546 | 100.0 | 813507 | 100.0 |
| Age group (years) | ||||||
| 15 – 19 | 3349 | 1.6 | 4220 | 0.7 | 7569 | 0.9 |
| 20 – 29 | 21670 | 10.6 | 50963 | 8.4 | 72633 | 8.9 |
| 30 – 39 | 51991 | 25.4 | 118079 | 19.4 | 170070 | 20.9 |
| 40 – 49 | 73213 | 35.7 | 239070 | 39.3 | 312283 | 38.4 |
| 50 – 59 | 41720 | 20.4 | 147687 | 24.3 | 189407 | 23.3 |
| 60 – 69 | 10505 | 5.1 | 40286 | 6.6 | 50791 | 6.2 |
| 70 + | 2513 | 1.2 | 8241 | 1.4 | 10754 | 1.3 |
| Total | 204961 | 100.0 | 608546 | 100.0 | 813507 | 100.0 |
Abbreviations: AIDS acquired immune deficiency syndrome; HIV human immunodeficiency virus; MSM men who have sex with men; IDU injecting drug users
Attributable Fraction
The attributable fraction (AF) for oncogenic infections is the proportion of new cancer cases that would have been prevented in a population if all infections had been avoided or treated before they cause cancer. Prevalence in cases is a valid AF estimate when the presence of infection in cancer using gold-standard methods is sufficient to infer that infection causes the cancer [11]. AF estimates in HIV-infected people for each infectious agent and its associated cancer site are given in the Supplemental Digital Content 1, Appendix Table 1. In most cancer sites except gastric cancer [19] we used the proportion of cancer cases positive for the infectious agent as the AF estimate. They were derived from case series conducted in the HIV population for lymphomas and liver cancers, or in the general population when robust data from HIV-infected people were not available or when the literature shows little or no difference in AF in HIV-infected people compared to the general population. Except for nasopharyngeal cancer, we only used findings from studies carried out in United States (when possible), North America, or Western countries. For EBV and DLBCL, we used an AF lower than estimates from the pre-cART era, reflecting improvements in immune function in the HIV-infected population (see Supplemental Digital Content 1, Appendix).
Comparison with general population
Estimates of the burden of cancer in the general population of the United States were calculated as previously described [11] in order to provide a comparison between the general population and the HIV-infected population.
Results
Table 2 shows the estimated numbers of incident cancer cases in HIV-infected people in the year 2008 in the United States for each infection-associated cancer site or histological type. Of the estimated 6231 new cancer cases (95%CI: 6041–6538), 2512 (95%CI: 2375–2709) were attributable to infection (AF=40%, 95%CI: 39–42). The most important infection-associated cancer sites were KS, lymphomas (especially NHL), and ano-genital cancers, comprising 85% of all cancer cases attributable to infection. Among ano-genital cancers, anal cancer was preponderant (Table 2) and 93% of anal cancer cases occurred in males. The three main infectious agents responsible for cancers in HIV-infected people were KSHV, which caused 789 cases, 95%CI: 703–882 (13% of all cancer); EBV, which caused 768 cases, 95%CI: 687–878 (12% of all cancer); and HPV, which caused 632 cases, 95%CI: 571–723 (10% of all cancer). Together, these three agents caused 2189 cases (95%CI: 2059–2389), i.e. 87% of all cancers attributable to infection and 35% of all cancers. HBV and HCV together caused 285 cases of liver cancer (95%CI: 234–341), which corresponds to an AF of 94% (95%CI: 90–97) of liver cancers for the two hepatitis viruses together. The contribution of HCV was nearly 4-fold larger than the one of HBV (Table 2).
Table 2.
Estimated number of incident cancer cases in HIV-infected people in the United States in 2008 by infectious agent and cancer site
| Infectious agent | Cancer site | AF (95% CI) | Number* of cancer cases (95% CI) | Number* of cancer cases attributable to infectious agent (95% CI) | ||
|---|---|---|---|---|---|---|
| Male | Female | Total | ||||
| KSHV | Kaposi sarcoma | 100% | 789 (703–882) | 764 (682–853) | 24 (15–37) | 789 (703–882) |
| EBV | Non-Hodgkin lymphoma | 1348 (1249–1497) | 422 (353–510) | 85 (63–113) | 508 (431–606) | |
| CNS-NHL | 96% (92–99) | 188 (152–236) | 142 (112–180) | 39 (25–59) | 181 (146–229) | |
| Non-CNS DLBCL | 27% (19–36) | 760 (677–860) | 170 (112–233) | 33 (18–49) | 203 (135–277) | |
| Non-CNS Burkitt | 45% (36–49) | 274 (225–345) | 110 (81–148) | 14 (7–23) | 124 (93–165) | |
| Other Non-CNS NHL | 126 (96–167) | 0 | 0 | 0 | ||
| Hodgkin lymphoma | 91% (87–96) | 280 (244–323) | 221 (187–258) | 35 (22–50) | 256 (217–296) | |
| Nasopharyngeal cancer | 85% (50–100) | 6 (2–14) | 3 (0–10) | 1 (0–6) | 5 (1–13) | |
| Total EBV related cancer sites | 1634 (1530–1791) | 646 (571–743) | 122 (98–155) | 768 (687–878) | ||
| HPV | Cervix uteri carcinoma | 100% | 86 (65–116) | 0 | 86 (65–116) | 86 (65–116) |
| Anal carcinoma | 91% (86–95) | 468 (407–536) | 395 (337–456) | 30 (18–44) | 425 (367–492) | |
| Vulva cancer | 80% (72–87) | 49 (32–75) | 0 | 39 (25–62) | 39 (25–62) | |
| Vaginal cancer | 70% (59–81) | 4 (0–9) | 0 | 2 (0–7) | 2 (0–7) | |
| Penile cancer | 46% (40–51) | 21 (13–38) | 9 (4–20) | 0 | 9 (4–20) | |
| Oropharyngeal cancer | 71% (67–75) | 99 (75–139) | 57 (41–81) | 13 (6–23) | 70 (51–100) | |
| Total HPV related cancer sites | 727 (664–830) | 461 (405–535) | 171 (142–211) | 632 (571–723) | ||
| HBV/HCV | Liver cancer due to HBV | 20% (15–26) | 303 (252–361) | 56 (36–80) | 6 (2–12) | 62 (41–87) |
| Liver cancer due to HCV | 73% (67–79) | 303 (252–361) | 201 (161–245) | 22 (11–35) | 223 (179–273) | |
| Total HBV and/or HCV related cancer sites | 303 (252–361) | 257 (210–311) | 28 (17–44) | 285 (234–341) | ||
| H. pylori | Non-cardia gastric cancer | 89% (79–95) | 43 (30–64) | 28 (18–43) | 11 (5–19) | 39 (25–59) |
| Total non-infection-related cancer sites | 2735 (2593–2896) | 0 | 0 | 0 | ||
| Total all cancer sites | 6231 (6041–6538) | 2155 (2031–2328) | 356 (316–415) | 2512 (2375–2709) | ||
Abbreviations: AF attributable fraction; KSHV Kaposi sarcoma herpes virus; EBV Epstein-Barr virus; HPV human papillomavirus; HBV hepatitis B virus; HCV hepatitis C virus; H. pylori Helicobacter pylori; CNS central nervous system; DLBCL diffuse large B-cell lymphoma; HIV human immunodeficiency virus
Numbers of cancer cases are estimations rounded to the nearest whole number
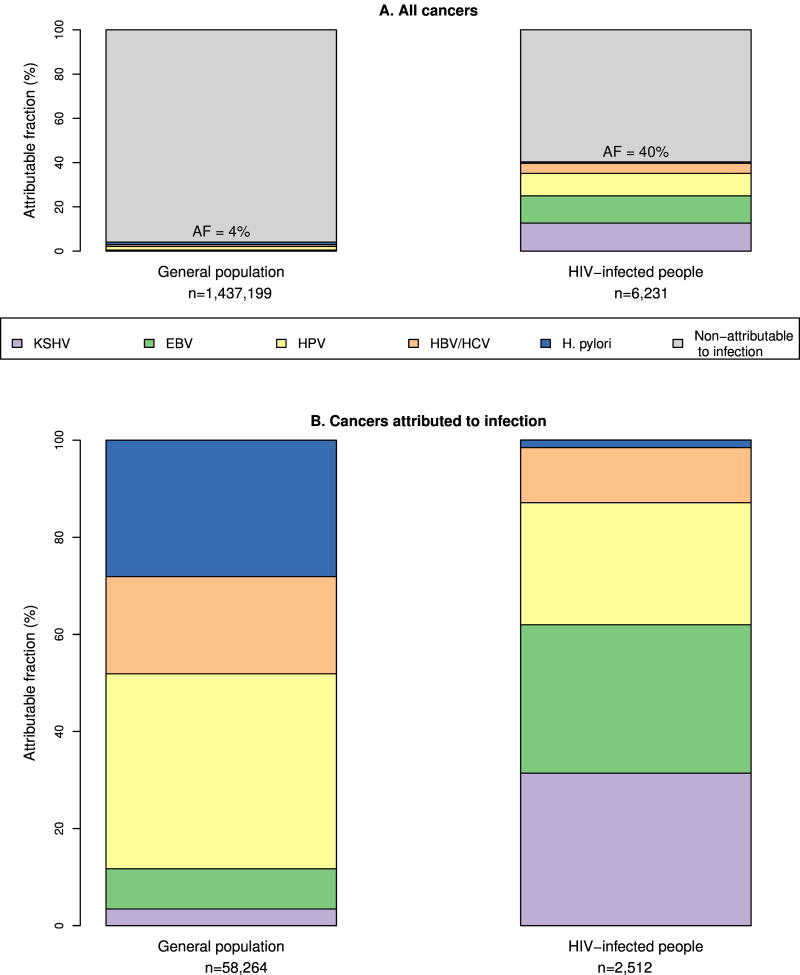
Figure 1 shows the burden of cancer attributable to infection in relation to the total cancer incidence (Figure 1A), and the breakdown of infection-attributable cancer by agent (Figure 1B) for both the general United States population and HIV-infected people (For further details see Appendix Table 3). In the general U.S. population, the proportion of cancers attributable to infection was 4% [11] compared to 40% in HIV-infected people (Figure 1A). In a sensitivity analysis adjusting the age and sex distribution of the general population to match that of the HIV-infected population, the proportion of infection-attributable cancers in the general population increased from 4% to 5%, showing that the much higher proportion of infection-attributable cancer in HIV-infected people is not due to demographic differences. Figure 1B shows infectious agents among cancers attributable to infection. This represents the same cases as the upper panel, but shows the distribution after removing cancers non-attributable to infection (shown in grey in Figure 1A). The spectrum of infectious agents causing cancer in the general population is quite different from the HIV-infected population. H. pylori causes a much smaller proportion of infection-attributable cancers in the HIV-infected population than in the general population. Conversely, EBV and KSHV cause a higher proportion of infection-attributable cancers. Liver cancers due to HBV and/or HCV account for a similar proportion of infection-attributable cancers in HIV-infected people (11%) and the general population (13%) (data not shown).
Figure 1. Cancer attributable to infection in the general population and HIV-infected people in the USA in 2008.
Abbreviations: AF attributable fraction; KSHV Kaposi sarcoma herpes virus; EBV Epstein-Barr virus; HPV human papillomavirus; HBV hepatitis B virus; HCV hepatitis C virus; H. pylori Helicobacter pylori
Table 3 shows the breakdown of infection-associated cancers and corresponding AFs by age, race/ethnicity, and a combination of sex and risk groups. The AF in HIV-infected people was highest in young people (AF = 69% in age group 20–29 years, 95%CI: 65–72), and decreased with age. Sixty-nine percent of the cases of infection-attributable cancers occurred in MSM and MSM-IDU, among whom the highest infection-attributable fractions are also found (AF=48%, 95%CI: 46–50 and 46%, 95%CI: 43–50, respectively), due to the high number of both KS (n=631 in MSM, n=60 in MSM-IDU) and anal cancer (n=313 in MSM, n=44 in MSM-IDU) in these groups compared to others.
Table 3.
Estimated number of incident cancer cases among HIV-infected people in the United States in 2008 by age, sex, risk group, race/ethnicity and fraction attributable to infection
| Category | N cancer cases (95% CI) | Incidence rate per 1000 person-years (95% CI) | N cases attributable to infection (95% CI) | Attributable Fraction (%) (95% CI) | |
|---|---|---|---|---|---|
| Age group (years) | |||||
| 15 – 19 | 17 (11–32) | 2.3 (2.0–3.5) | 9 (4–18) | 49 (39–63) | |
| 20 – 29 | 277 (243–323) | 3.8 (3.6–4.2) | 190 (162–228) | 69 (65–72) | |
| 30 – 39 | 846 (784–924) | 5 (4.8–5.2) | 499 (451–560) | 59 (57–61) | |
| 40 – 49 | 2061 (1963–2204) | 6.6 (6.4–6.9) | 964 (885–1061) | 47 (45–49) | |
| 50 – 59 | 1981 (1883–2111) | 10.5 (10.1–10.9) | 635 (585–705) | 32 (31–34) | |
| 60 – 69 | 860 (794–940) | 16.9 (16.1–18.0) | 183 (152–222) | 21 (20–24) | |
| 70 + | 189 (160–228) | 17.6 (16.2–20.0) | 32 (22–50) | 17 (14–23) | |
| Race / ethnicity | |||||
| Non-Hispanic White | 2370 (2268–2508) | 8.5 (8.2–8.8) | 970 (893–1060) | 41 (39–43) | |
| Non-Hispanic Black | 2622 (2507–2772) | 7.4 (7.2–7.7) | 958 (895–1053) | 37 (35–39) | |
| Hispanic / Latino | 1025 (960–1112) | 6.8 (6.6–7.1) | 493 (449–554) | 48 (46–51) | |
| Other / unknown | 214 (185–262) | 7.9 (7.3–8.9) | 91 (73–116) | 43 (39–47) | |
| Sex | Risk group | ||||
| Male | MSM | 3251 (3125–3436) | 8.1 (7.9–8.5) | 1550 (1442–1681) | 48 (46–50) |
| Male | MSM & IDU | 397 (355–448) | 8.1 (7.7–8.8) | 184 (156–222) | 46 (43–50) |
| Female | IDU | 380 (344–427) | 6.9 (6.6–7.4) | 115 (94–142) | 30 (28–33) |
| Male | IDU | 810 (750–882) | 9.2 (8.8–9.6) | 248 (213–289) | 31 (29–33) |
| Female | Heterosexual | 798 (738–875) | 5.5 (5.3–5.8) | 235 (202–277) | 29 (28–32) |
| Male | Heterosexual | 537 (493–597) | 8.2 (7.9–8.7) | 162 (136–195) | 30 (28–33) |
| Female | Other | 22 (14–35) | 4.7 (4.2–6.3) | 7 (3–14) | 30 (26–42) |
| Male | Other | 37 (26–55) | 6.2 (5.6–8.0) | 12 (6–24) | 34 (30–43) |
| Total | |||||
| All categories | 6231 (6041–6538) | 7.7 (7.5–8.0) | 2512 (2375–2709) | 40 (39–42) | |
Abbreviations: MSM men who have sex with men; IDU injecting drug users; HIV human immunodeficiency virus
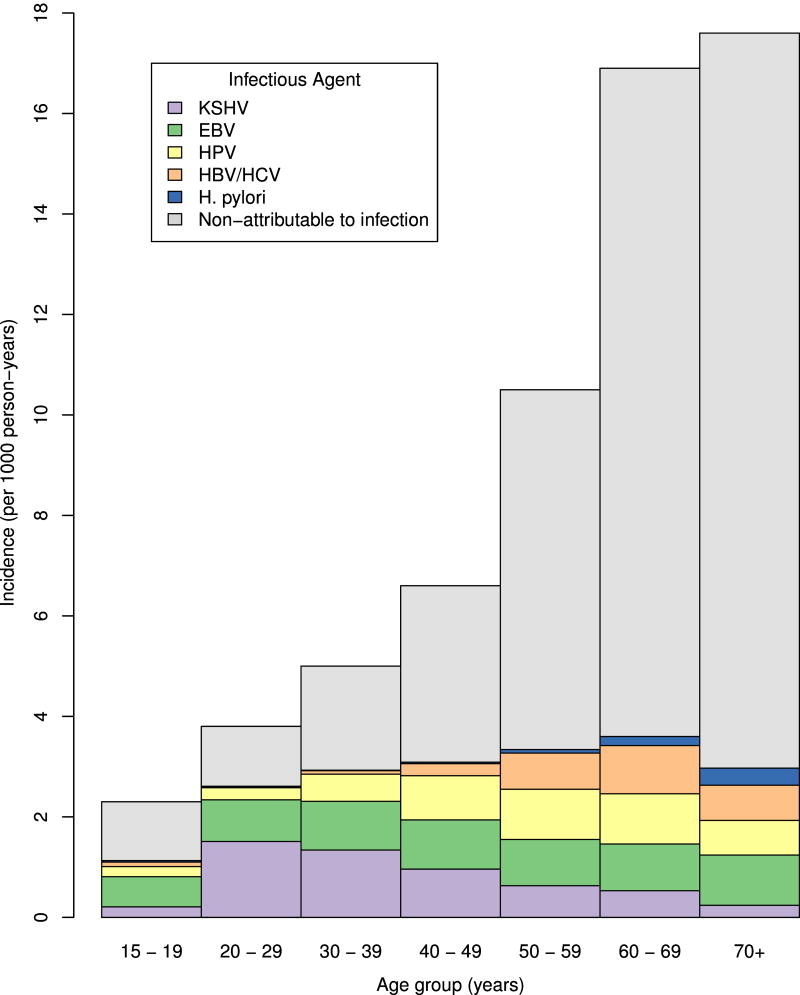
Figure 2 shows age-specific incidence rates of cancer attributable to individual infections or non-attributable to infection (for further details see Appendix Table 4). The incidence rate of cancer non-attributable to infection steeply increased with age, but the incidence of infection-attributable cancers increased very little after age 30 years. The composition of cancers due to different infectious agents, however, varied substantially by age group. Below age 30 years, nearly all infection-attributable cancers in HIV-infected people were due to KSHV or EBV. Incidence rates of cancers attributable to HPV increased after age 30, and those of cancers attributable to HBV/HCV and H. pylori increased after age 40.
Figure 2. Estimated cancer incidence among HIV-infected people in the USA in 2008 by age.
Abbreviations: KSHV Kaposi sarcoma herpes virus; EBV Epstein-Barr virus; HPV human papillomavirus; HBV hepatitis B virus; HCV hepatitis C virus; H. pylori Helicobacter pylori
Discussion
We estimate that 40% (95%CI: 39–42) of cancer cases occurring in HIV-infected people in the United States are attributable to infectious agents. This AF is ten times as high as in the general United States population (4%), and higher than the AF in the general population of any world region, including sub-Saharan Africa where 33% of cancers are attributable to infection [11].
The important infection-associated cancer sites in HIV-infected people are KS, NHL, and, especially in men, anal cancer. In contrast, in the general United States population they are non-cardia gastric, liver, and cervical cancer. Liver cancer accounts for a similar proportion of infection-attributable cancers in HIV-infected people and the general population. In the United States, KS is nearly exclusively detected in HIV-infected people, mainly in MSM patients with low CD4 cell count [20]. Our estimates show that even in the cART era, KS accounts for nearly a third of the burden of infection-attributable cancers in HIV-infected people. Its absolute and relative contribution is highest in the younger age groups. Approximately 14% of people with HIV in the United States are unaware of their HIV status [21], and this proportion is much higher in the youngest age groups, reaching 51% in the age group 15 to 24 [21]. This suggests that many cases of KS are occurring among people not previously aware of their HIV infection. Earlier detection and treatment of HIV infection is therefore the best available strategy to prevent KS.
Lymphomas due to EBV comprise around 30% of all infection-attributable cancers in HIV-infected people. The AF for EBV in lymphomas varies substantially by histological type and immune status (see Supplemental Digital Content 1, Appendix): it is larger for CNS lymphoma, immunoblastic DLBCL types, and in the severely immunosuppressed [14]. Nearly all adults are infected with EBV, and there is no preventive or therapeutic strategy against the infection. As with KS, early detection and uninterrupted treatment of HIV infection is currently the best way to prevent EBV-attributable NHLs. The beneficial effect of cART on the onset of centroblastic DLBCL, Burkitt lymphoma, or Hodgkin lymphoma is less well defined but certainly weaker than for CNS NHL and immunoblastic DLBCL [14, 22, 23].
Anal cancer is by far the most frequent HPV-attributable cancer in HIV-infected people in countries where MSM account for a large proportion of HIV-infected people [8]. As in the general population, nearly all anal cancers in HIV-infected people are caused by HPV infection, specifically HPV16 [24]. Screening has been advocated for some HIV-infected sub-populations, especially MSM, using cytology and high resolution anoscopy [25]. However, anal cancer screening is far less well validated and standardized than screening for cervical cancer. Better screening protocols are essential to improve outcomes and reduce rates of invasive cancer [26, 27].
The burden of HPV-attributable cervical, vulvar, and vaginal cancer in HIV-infected women in the United States is not negligible. Although screening can prevent most cervical cancer, HIV-infected women have substantially higher cervical cancer incidence than HIV-uninfected women [28] and additional efforts are needed to reduce barriers to screening and improve the management of HPV infections and cervical precancerous lesions in HIV-infected women [29]. Starting cART as soon as possible to avoid even mild degrees of immunosuppression will also help reduce the progression of HPV infection to high-grade anal or cervical lesions [28]. Finally, although a fraction of oropharyngeal cancer is caused by HPV, much of the excess in HIV-infected people is likely due to tobacco smoking [6], as shown by the similarly large excess observed for cancer of the lung and other head and neck sites in which HPV is not involved [14]. The key prevention strategy remains smoking cessation [6]. In the future, HPV vaccination of adolescents and young adults could prevent most HPV-associated cancers.
Nearly all liver cancers in HIV-infected people derive from chronic infection with HCV or HBV, in contrast to the general population among whom a large fraction of liver cancer is not of infectious origin [30]. HIV-infected people should therefore be tested for HBV and HCV, and should avoid habits that promote liver damage (alcohol drinking and tobacco smoking) [1]. The current program of HBV vaccination in children has the potential to prevent HBV-caused liver cancer in the future, but the contribution of HCV to liver cancer in United States HIV-infected people is nearly 4-fold larger than that of HBV. Fortunately, progress in the efficacy and tolerability of anti-viral drugs is making HCV treatment more compatible with cART [31]. The United States recommendation to screen all persons born during 1945–1965 for HCV infection and facilitate access to increasingly effective anti-HCV therapies is likely to favorably affect HIV-infected people, including those who are not aware of being infected by either virus [31].
Our study provides the first overall picture of the burden of cancer due to infections in HIV-infected people in the United States, using the fraction of cases that are attributable to infections rather than the combination of cancer sites that are more or less strongly associated with infections [32]. It is also the first to overcome the separation of cancer estimates for people with AIDS and people with HIV-only while being able to take into account the prevalence of the two groups. The combination of people with AIDS and people with HIV-only is consistent with the disappearance of a clear-cut separation between the two groups in the cART era.
Although our AF estimates were sometimes supported by limited information on cancer in the HIV-infected population, we expect them to be robust for KS, and cancers of the anus, cervix, and stomach, which are almost completely attributable to infections in any population. Little doubt exists also about the high AF for HBV and HCV in liver cancer in HIV-infected people [7]. Conversely, AFs for lymphomas are more challenging on account of the heterogeneity of the disease [33], the evolving influence of cART on the distribution of lymphoma sub-types, and the small number of recent NHL and Hodgkin lymphoma cases in which the presence of EBV has been evaluated (see Supplemental Digital Content 1, Appendix).
The HACM Study does not include data on cART use. However, some heuristic estimates of the impact of cART can be inferred from the trends in KS and NHL that jointly comprised 34% of all cancers in HIV-infected people and contributed 52% of cancers attributable to infection (Table 2). Appendix Figures 1 and 2 show the incidence rates of these cancers in the HACM Study, standardized by age and sex to the 2008 United States HIV population. Incidence of KS declined more than 8-fold between 1996 and 2008, while NHL declined nearly 5-fold in the same period, mainly due to decreases in non-CNS DLBCL and CNS NHL. The contribution of KS and NHL to the burden of infection-attributable cancer in the early cART era was therefore much larger than the 40% estimated for 2008.
Our estimates of the absolute numbers of cancer cases in HIV-infected people in the United States in 2008 are lower than those recently reported by Robbins et al. [32] for the year 2010. This difference partly reflects the growth of the United States HIV population over time. In addition, Robbins et al. [32] corrected their estimates for possible under-ascertainment of cancers in the HIV population due to imperfect sensitivity of the registry linkage and outmigration. We did not apply these corrections in our study since we expect the proportion of cancers attributable to infection to be largely unaffected. We also expect the proportions to be generalizable beyond the United States to other high-income countries in which medical standards and the prevalence of carcinogenic infectious agents are similar to the United States, but not to the rest of the world. Important differences affecting the cancer burden in low-income countries include lower life expectancy, a larger proportion of heterosexually acquired HIV infection, delayed and less universal access to cART, and lack of cervical cancer screening. In particular, KS and cervical cancer account for a higher proportion of cancer cases in sub-Saharan Africa than in the United States [12]. These sites should therefore be responsible for a substantially higher proportion of infection-attributable cancer in sub-Saharan Africa.
In conclusion, HIV-infected people in the United States show a proportion of cancer attributable to infection that is 10 times as high as in the general population. A few strategies for the prevention of infection-associated cancers have the potential to prevent an even larger fraction of disease in HIV-infected people, who have an increasingly similar life expectancy to the general population [34]. Universal early detection of HIV and uninterrupted cART remain the most powerful tools to prevent KS and lymphomas in HIV-infected people and may also substantially reduce the excess of cancer of the anus and cervix, provided that even moderate levels of immunodeficiency are avoided over the long latent phase of HPV infection.
Supplementary Material
Acknowledgments
The authors thank the staff at the following HIV/AIDS and cancer registries that provided data for the HIV/AIDS Cancer Match Study: California, Colorado, Connecticut, Washington D.C., Florida, Georgia, Illinois, Maryland, Massachusetts, Michigan, New Jersey, New York City, Seattle, and Texas.
This study was supported by a grant from the Fondation de France (Grant number: 00039621) and by the Intramural Research Program of the National Cancer Institute, NCI. Dr Catherine de Martel was partly supported by a grant from the Bill & Melinda Gates Foundation (OPP1053353).
Footnotes
Disclaimer: The findings and conclusions of the authors do not necessarily represent the views of the Centers for Disease Controls and Prevention.
Conflicts of Interest
The authors have no conflict of interest to declare.
CDM, MSS, SF, EPS, EAE and MP conceived and designed the study. HIH provided estimates of the HIV population; EAE and MSS provided cancer incidence data. MP and JV contributed to data analysis. CDM, SF, and MP wrote the manuscript. All authors contributed to the interpretation of data and approved the final manuscript.
References
- 1.IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100B : A Review of Human Carcinogens: Biological Agents. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 2.Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. Aids. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 3.Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan SG, Hirsch HH, Franceschi S, Steffen I, Amari EB, Mueller NJ, et al. Kaposi sarcoma herpes virus antibody response and viremia following highly active antiretroviral therapy in the Swiss HIV Cohort study. Aids. 2010;24:2245–2252. doi: 10.1097/QAD.0b013e32833b7830. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 6.Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. Aids. 2014;28:1499–508. doi: 10.1097/QAD.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 7.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer. 2010;103:416–422. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard EP, Pfeiffer RM, Engels EA. Mortality due to cancer among people with AIDS: a novel approach using registry-linkage data and population attributable risk methods. Aids. 2012;26:1311–1318. doi: 10.1097/QAD.0b013e328353f38e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 12.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, et al. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–990. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 13.Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. 2014;135:432–439. doi: 10.1002/ijc.28661. [DOI] [PubMed] [Google Scholar]
- 14.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin MD, et al. International classification of diseases for oncology (ICD-O) 3. Geneva (Switzerland): World Health Organization; 2000. [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed 2 December 2014];HIV Surveillance Report. 2012 24 http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2014. [Google Scholar]
- 17.Gelman A, Jaculin A, Pittau MG, Su YS. A weakly informative default prior distribution for logistic and other regression models. The Annals of Applied Statistics. 2009;2:1360–1383. [Google Scholar]
- 18.Stone M. An asymptotic equivalence of choice of model by cross-validation and Akaike’s criterion. J R Stat Soc Series B Stat Methodol. 1977;39:44–47. [Google Scholar]
- 19.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi S, Maso LD, Rickenbach M, Polesel J, Hirschel B, Cavassini M, et al. Kaposi sarcoma incidence in the Swiss HIV Cohort Study before and after highly active antiretroviral therapy. Br J Cancer. 2008;99:800–804. doi: 10.1038/sj.bjc.6604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data —United States and 6 dependent areas—2012. [Accessed 2 December 2014];HIV Surveillance Supplemental Report. 2014 19(3) http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2014. [Google Scholar]
- 22.Clifford GM, Rickenbach M, Lise M, Dal Maso L, Battegay M, Bohlius J, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113:5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 23.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 24.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 25.Palefsky JM. Anal cancer prevention in HIV-positive men and women. Curr Opin Oncol. 2009;21:433–438. doi: 10.1097/CCO.0b013e32832f511a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 27.Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schoni-Affolter F, et al. Risk factors for anal cancer in persons infected with HIV: a nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178:877–884. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 28.Abraham AG, D’Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62:405–413. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massad LS, Xie X, D’Souza G, Darragh TM, Minkoff H, Wright R, et al. Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol. 2015;212:606, e601–608. doi: 10.1016/j.ajog.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. doi: 10.1038/ajg.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afdhal NH, Zeuzem S, Schooley RT, Thomas DL, Ward JW, Litwin AH, et al. The new paradigm of hepatitis C therapy: integration of oral therapies into best practices. J Viral Hepat. 2013;20:745–760. doi: 10.1111/jvh.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-Infected people in the United States. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 34.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.