Summary
In vertebrates, mechano-electrical transduction of sound is accomplished by sensory hair cells. While mammalian hair cells are not replaced when lost, in fish they constantly renew and regenerate after injury. In vivo tracking and cell fate analyses of all dividing cells during lateral line hair cell regeneration revealed that support and hair cell progenitors localize to distinct tissue compartments. Importantly, we find that the balance between self-renewal and differentiation in these compartments is controlled by spatially restricted Notch signaling and its inhibition of Wnt-induced proliferation. The ability to simultaneously study and manipulate individual cell behaviors and multiple pathways in vivo, transforms the lateral line into a powerful paradigm to mechanistically dissect sensory organ regeneration. The striking similarities to other vertebrate stem cell compartments uniquely place zebrafish to help elucidate why mammals possess such low capacity to regenerate hair cells.
Introduction
Mammalian adult tissues differ dramatically in their respective regenerative capacities. While the sensory cells of the olfactory epithelium and taste buds regenerate readily, the sensory hair cells of the mature inner ear cannot (Cox et al., 2014). Because sensory hair cells are crucial for hearing, their loss in mammals due to noise exposure, ageing, chemotherapeutic drugs or antibiotics results in permanent loss (Furness, 2015). In contrast, the hair cells of the inner ear and lateral line (LL) system of non-mammalian vertebrates regenerate throughout the life of these animals (Rubel et al., 2013). The cellular and molecular basis of such striking difference between mammalian and non-mammalian vertebrates remains poorly understood. For instance, chicken and amphibian hair cells regenerate from dividing or transdifferentiating support cells (SC, Balak et al., 1990; Corwin and Cotanche, 1988; Jones and Corwin, 1996); while fish LL hair cells regenerate from mitotic SCs (Lush and Piotrowski, 2014b; Ma et al., 2008; Wibowo et al., 2011; Williams and Holder, 2000). Nevertheless, the location and regulation of the stem cells and progeny suspected to be involved in hair cell regeneration have yet to be fully characterized in any of the regenerating species. Likewise, our understanding of the molecular mechanisms controlling SC behavior is limited. Here we take advantage of the superficially located and experimentally accessible zebrafish sensory LL system to study the cell behaviors and signaling events that lead to newly formed hair cells.
The LL system of aquatic vertebrates serves to detect water motion. The sensory organs are called neuromasts (NMs) and are distributed along lines over the body of the animal (Metcalfe et al., 1985; Northcutt et al., 1994). Each NM consists of mechanosensory hair cells that are surrounded by SCs and a ring of peripheral mantle cells (MCs; Figures 1A-1D). LL hair cells are homologous to inner ear hair cells and mutations affecting LL hair cell function also cause deafness in humans (Nicolson, 2005; Whitfield, 2002). Previous studies of zebrafish LL regeneration described Notch-regulated proliferation patterns and localized quiescence in regenerating NMs; however, only differentiating divisions were described (Cruz et al., 2015; Ma et al., 2008; Wibowo et al., 2011). RNA-Seq analysis of regenerating NMs demonstrated that downregulation of Notch signaling is one of the earliest responses to hair cell death and therefore likely plays a crucial role in initiating regeneration (Jiang et al., 2014).
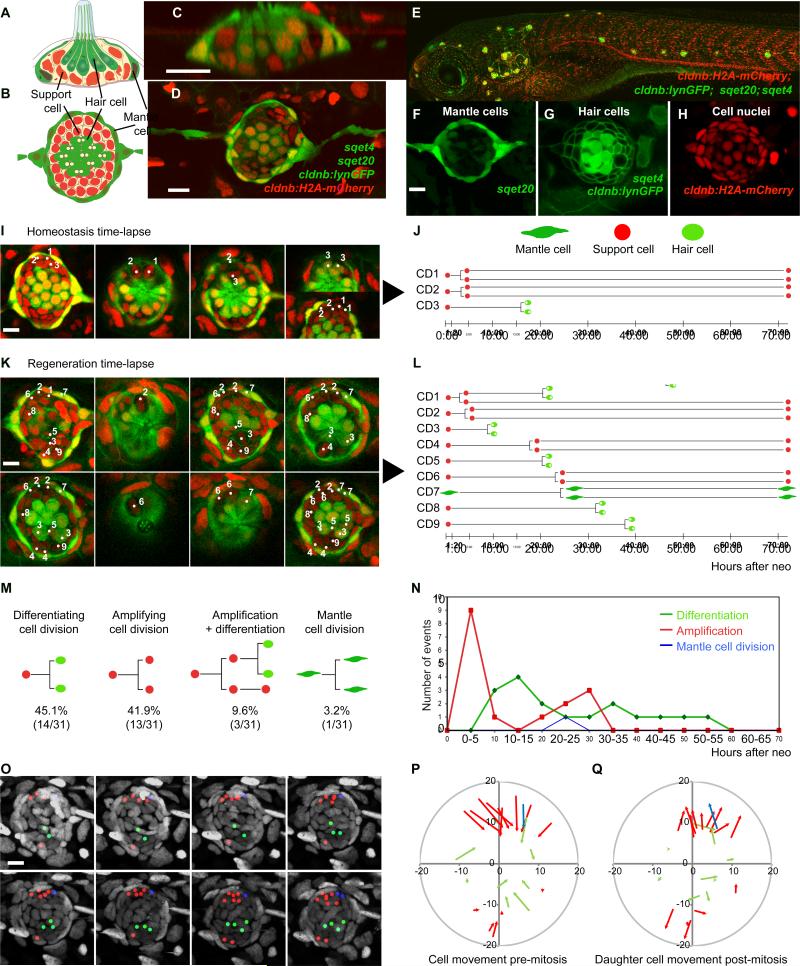
Figure 1. Support cells (SCs) are multipotent progenitors.
(A) Horizontal and (B) lateral views of a neuromast (NM).
(C-H) Quadruple transgenic larvae express the mantle cell (MC) marker sqet20
(F, green), the hair cell (HC) marker sqet4 (G, cytoplasmic green), the cell membrane marker cldnb:lynGFP (G) and the nuclear maker cldnb:H2A-mCherry (H).
(I) Still images of a time-lapse of a homeostatic NM (Movie S1). Split images show different focal planes. Numbers in NMs label the progenitors shown in (J). Time = hours : minutes.
(J) Lineage analysis of the mitotic events in (I) and Movie S1.
(K) Time-lapse of a regenerating NM (Movie S2B). CD1 is shown in Movie S2C.
(L) Lineage analysis in a regenerating NM (Figure 1K; Movie S2).
(M) SCs self-renew or differentiate into two hair cells: Quantification of lineages of three time-lapse movies of regenerating NMs from Figures S1F-S1H.
(N) Proliferation dynamics during regeneration. Amplifying divisions occur first (p<0.0001, Fisher's exact test).
(O) Proliferating cells and their progeny do not actively move in a regenerating NM. Lineages from Figure 1L are color-coded: red: amplifying cell divisions, green: differentiation, blue: MC divisions (Movie S3). mCherry nuclei are in grey.
(P) Vectors show directions and distances of cell displacement before mitosis (metaphase) for every cell division recorded during the first 24hrs in Figures S1F-S1H). Central HC progenitors are not displaced.
(Q) Vectors show cell displacements of one of the daughter SCs back to their original positions. Displacements for P and Q are quantified in Figure S1I.
Scale bars = 10μm.
In neonatal mice, downregulation of Notch signaling also induces SC proliferation, whereas in adults it leads to more hair cells via transdifferentiation (Mizutari et al., 2013). Similarly, canonical Wnt signaling activates proliferation of SCs and causes an increase in hair cells in neonatal mice, but has no effect in adult animals (Shi et al., 2013). In regenerating chicken and zebrafish sensory epithelia, Wnt signaling increases proliferation and a modest increase in hair cell numbers (Head et al., 2013; Jacques et al., 2014). However, the interactions between Notch and Wnt signaling and their effect on distinct SC fates have not been tested in regenerating species.
Because SCs look morphologically identical, we aimed to characterize NM cell populations by single cell lineage analyses. Manual tracking of every mantle and SC, combined with spatial analysis of proliferating cells provides a potent and unbiased approach to distinguish lineages. We find that peripheral MCs are a quiescent cell population that only re-enters the cell cycle after severe injury to the sensory organs. We also discovered that during homeostasis and regeneration SCs make lineage decisions according to their location in the NM. This phenomenon is reminiscent of stem cell behaviors in the intestine and hair follicle where stem cell fate is determined by the location of the cells within the niche (Ritsma et al., 2014; Rompolas et al., 2013). Our results show that SCs self-renew in the dorso-ventral (D-V) poles, differentiate in the center and are relatively quiescent in the antero-posterior (A-P) poles. Importantly, the balance between self-renewal and differentiation is controlled by spatially restricted Notch signaling and its inhibition of Wnt-induced proliferation.
Results
Time-lapse and fate analyses define dynamics of cell division and differentiation in homeostatic and regenerating NMs
Dying LL hair cells are replaced by surrounding SCs throughout life (Cruz et al 2015). To determine if NMs possess a distinct stem cell population, we performed time-lapse analyses of homeostatic and regenerating NMs and recorded the fate of each dividing cell. We generated fish expressing four transgenes (Figures 1C-1H). Et(krt4:EGFP)sqet20, (sqet20) labels MCs. Tg(cldnb:lynGFP) labels all LL cell membranes. Tg(atp2b1a-GFP), or sqet4 labels hair cells and their progenitors and Tg(cldnb:H2A-mCherry) labels all nuclei.
We tracked cell lineages in 70 hour time-lapse recordings of 5 days post fertilization (dpf) control NMs during homeostasis (n=4; Figures 1I-1J, S1B-S1E; Movie S1) and regeneration (n=3; Figures 1K, 1L, S1F-S1H, Movie S2). We determined the average number of cell types during regeneration in the first trunk NM (L1) at 0, 24, 48 and 72 hrs after hair cell loss (Figure S1A). In a homeostatic NM we observed 3 SC divisions (Movie S1; Figure 1I, 1J; Cell Divisions (CD) CD1-3) that we attribute to physiological hair cell turnover (Figure S1A, black, dotted line). The first two progenitors divided symmetrically and produced two SCs each (CD1, CD2), while the third SC (CD3) divided and the daughter cells differentiated into two GFP-positive hair cells (Figure 1J; Movie S1). The results of three other movies are described in Figures S1C-S1E.
To induce hair cell regeneration, we immersed larvae in the antibiotic neomycin. Complete regeneration occurs after 72hrs (Figure S1A, solid green line; (Ma et al., 2008). Time-lapse imaging revealed that hair cell death significantly increases the number of mitoses (Figures 1K, 1L and S1F-S1H; Movie S2B). Tracking all cell divisions and daughter cells in three regenerating NMs over 70hrs revealed that SCs are a mixture of self-renewing multipotent stem cells and progenitor cells that give rise to hair cells (Figures 1L-1M). 42% of the observed cells divided and produced two undifferentiated SCs (Amplifying cell divisions; Figures 1L, CD2, 4, 6, Figures S1F-S1H). This amplifying response led within 24hrs to a significant increase in SC numbers that slowly returned to control levels by 72hrs, while other SCs continued to differentiate into hair cells (Figures 1N and S1A, red lines). 45% of SCs produced two hair cells (Differentiating cell division; Figures 1L, CD3, 5, 8, 9, S1F-S1H). 10% of the dividing SCs first gave rise to two SCs with one of the daughter cells dividing a second time to give rise to two hair cells (Figures 1L-1M, CD1; S1G, CD10, CD11; Movie S2C). Therefore, SCs derived from symmetric, amplifying divisions have the potential to differentiate. MCs, on the other hand, rarely divided (Figures 1L, CD7; 1M; S1A, blue lines). These results show that SCs are the most likely source for stem cells, while MCs are unlikely to contribute to hair cell regeneration.
Our time-lapse recordings of regenerating NMs show that mitotic and quiescent cells maintain their relative positions and are not actively migrating during regeneration (Figures 1O and Movie S3). Neither amplifying, nor differentiating SCs were displaced by more than one cell diameter (5 pixels; Figure S1I). When we analyzed the direction of cell movements before division, we observed that cells are displaced toward the center (Figure 1P). The displacement is caused by the apical movement of dividing cells. After division, cells move back down to their original position (Figure 1Q).
Our time-lapse recordings show that all cell divisions are symmetric, with approximately half of the daughters undergoing self-renewal or amplification, and the other half differentiating into hair cells. Our analyses define 5 cell behaviors during both homeostasis and regeneration: 1) differentiating cell divisions 2) amplifying cell divisions 3) SCs that divide a second time and generate two HCs, 4) SC quiescence, and 5) MC quiescence.
Support cell lineages are restricted to different compartments
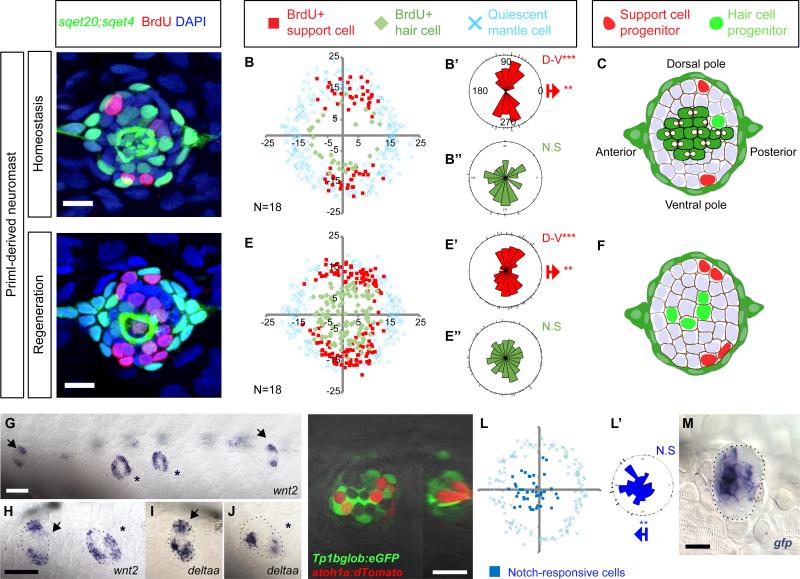
Given the limited cell movement, we tested whether SC behaviors are spatially confined within the NMs. Time-lapse analyses suggested that cells in the poles self-renew, whereas cells in the center differentiate into hair cells (Figures S1J-S1J”). To confirm this observation, we performed 24hrs BrdU incorporation experiments in sqet4;sqet20 homeostatic and regenerating larvae (Figures 2A and 2D). We plotted the location of BrdU+ GFP+ hair cells (Figures 2B and 2E, differentiating cell divisions, green diamonds), BrdU+;GFP- SCs (amplifying cell divisions; red squares), and quiescent sqet20+ MCs (blue crosses). In homeostatic NMs, amplifying cell divisions cluster in the D-V poles (Figure 2B’). Differentiating divisions occur randomly in a circle and are not clustered (Figure 2B”). Quiescent SCs are located in the center and A-P poles of the NMs (unlabeled, white areas in Figure 2B). Within 24hrs, one SC divides per pole to produce two SCs (amplifying division), while one central SC gives rise to two hair cells (differentiating division, Figure 2C).
Figure 2. Support cell amplification is restricted to polar compartments during homeostasis and regeneration.
(A, D) 24hr BrdU incorporation in primI-derived NMs during homeostasis and regeneration. Scale bar = 10μm.
(B, E) Amplifying cell divisions are clustered in the D/V compartments of NMs. BrdU plots show the positions of BrdU+ nuclei of 18 NMs superimposed on the same XY plane. Red squares indicate amplifying divisions; green diamonds indicate BrdU+ cells that differentiated into sqet4+ hair cells. Blue crosses indicate quiescent mantle cells. Axes are in pixels.
(B’, B”, E’, E”) Rose diagrams for the angular position of BrdU+ SCs (red), BrdU+ HC (green). Bipolar clustering (D-V) and directional bias to the posterior (red arrow) were statistically analyzed using the Binomial test (***p<0.001).
(C, F) Number and location of progenitors that divide within 24hrs.
(G-J) wnt2 and deltaa are expressed in poles. Arrowheads label primI-derived NMs. Asterisks label primII-derived NMs. Dashed lines outline NMs. Scale bar in (G) =100μm; in (H)=60μm.
(K-K’) EGFP driven by the Notch reporter Tg(Tp1bglob:eGFP) occurs in central cells beneath the red HCs labeled with Tg(atoh1a:dTomato). The Atoh1a reporter is mosaic, leaving some cells unlabeled. (K’) Orthogonal view of K. Scale bar = 10 μm
(L-L’) Superimposed EGFP+ Notch reporter cells of 15 NMs (blue squares) are biased toward the anterior side of the NM. (see rose diagram).
(M) In situ hybridization of egfp mRNA in Tg(Tp1bglob:eGFP). Scale bar = 10 μm.
See also Figure S2.
During regeneration, amplifying cell divisions increase but maintain their compartmentalized distribution in the D-V poles (Figures 2E and 2E’ and S2A). Differentiating divisions also increase but remain randomly distributed (Figures 2E-2E” and S2A). Also, differentiating divisions occur in previously quiescent, central SCs located immediately beneath the dying hair cells (Figure 2E). Interestingly, amplifying divisions occur almost exclusively adjacent to MCs, whereas differentiating divisions occur toward the center (Figures S2B-S2G).
To test if these localized cell behaviors correlate with hair cell orientation we studied neuromasts with a different developmental origin and epithelial planar cell polarity that is offset by 90° (primII-derived NMs; Figures 2A-2F and S2HS2M; López-Schier et al., 2004). We observed that amplifying SC divisions in primII-derived NMs are restricted to the A-P poles (Figures S2I-S2I’ and S2L-S2L’; A-P) mirroring the polar bias of amplifying cell divisions in primI-derived NMs.
We conclude from the consistent spatial restriction of cell lineages uncovered that attendant and similarly restricted molecular cues must exist to induce amplifying SC divisions in the poles and hair cell differentiating divisions closer to the center.
Notch and Wnt pathway members are expressed in complementary compartments during homeostasis
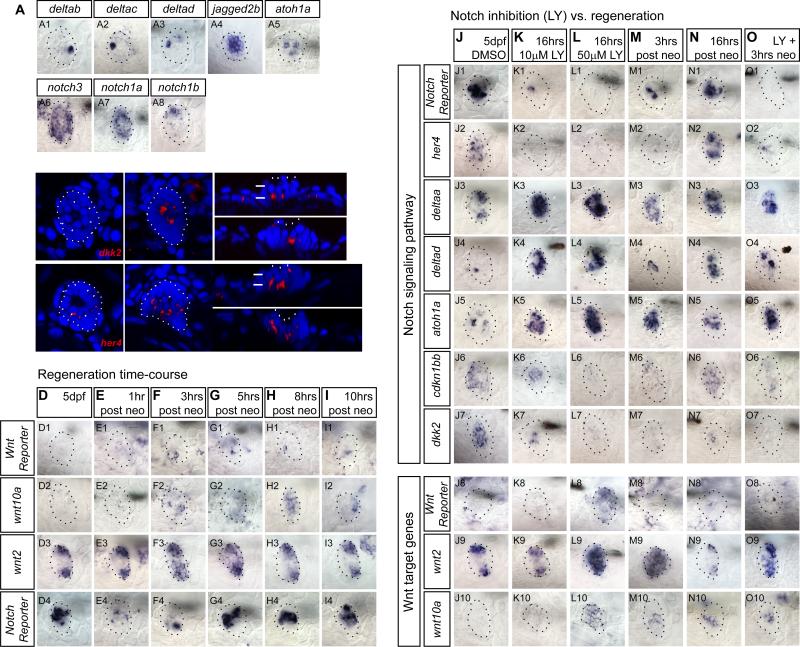
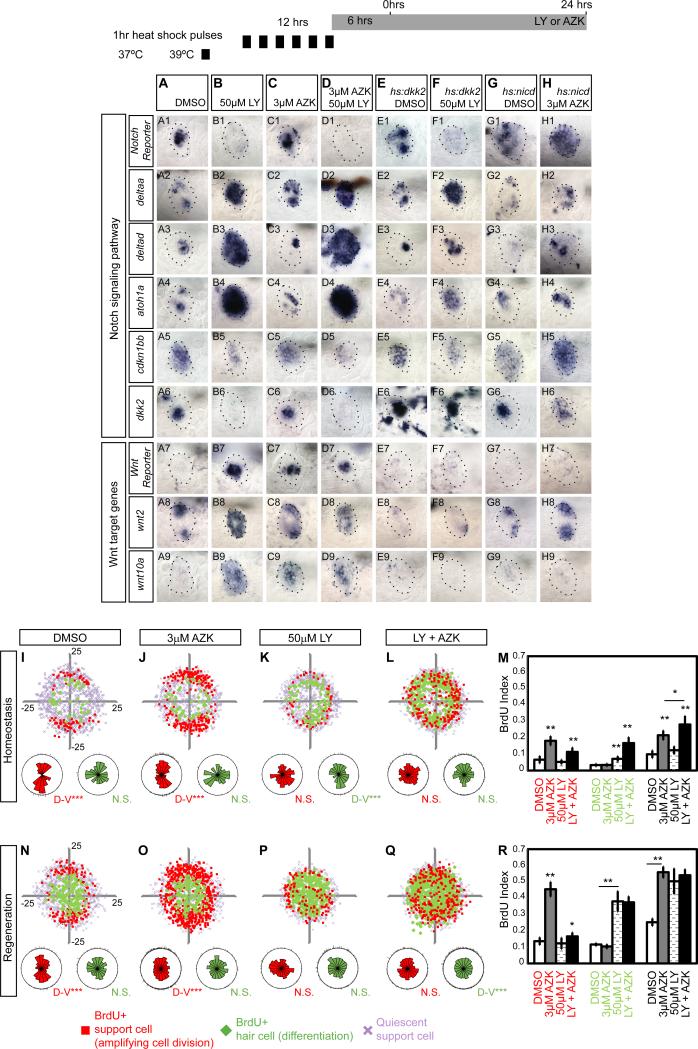
Notch and Wnt signaling control progenitor cell behavior and hair cell numbers in the LL (Head et al., 2013; Jacques et al., 2014; Ma et al., 2008; Wibowo et al., 2011). We discovered that members of these two signaling pathways are expressed in different NM compartments. wnt2 and the Notch ligand deltaa show clear restrictions to the D-V or A-P poles in primI- and primII-derived NMs, respectively (Figures 2G-2J). deltab, deltac and deltad and the hair cell precursor marker atoh1a are expressed in single cells resembling the localization of differentiating hair cells (Ma et al., 2008). On the other hand, the ligand jagged2b is expressed broadly in the NM center (Figures 3A1-3A5). Notch receptors also show heterogeneous expression patterns. notch3 is expressed in most SCs, with weaker expression in the D-V poles (Wibowo et al., 2011). notch1a is expressed robustly in all SCs, whereas notch1b is expressed only in a few cells (Figures 3A6-3A8). In contrast, the Wnt inhibitor dkk2 and the Notch target gene her4 are restricted to central SCs beneath hair cells (Figures 3B-3C’”).
Figure 3. Downregulation of Notch mimics expression changes during regeneration.
(A) Notch ligand and receptor expression in 5dpf neuromasts.
(B-C) dkk2 and her4 are expressed in SCs below HCs as shown by confocal imaging of the in situ hybridization signal. B and B’, C and C’ are different focal planes. B” and B’”, C” and C’” are orthogonal views. White arrows point at HCs.
(D-I) Time course of mRNA expression of the Wnt reporter Tg(6xTcf/LefBS-miniP:d2EGFP), the Wnt targets wnt10a and wnt2, and the Notch reporter Tg(Tp1bglob:EGFP) at different time points after neomycin treatment. Notch is downregulated first, followed by the activation of Wnt signaling.
(Row 2) wnt10a is inactive in control neuromasts but is activated 3-8hrs after hair cell death.
(Row 3) wnt2 is present in the poles of homeostatic neuromasts and is upregulated 3h after HC death.
(Row 4) mRNA of the Notch reporter shows that Notch downregulation occurs 1-3hrs after hair cell death.
(J-O) Notch inhibition with LY411575 mimics expression changes observed during the first 16hrs of regeneration. Larvae were pre-treated with LY or DMSO for 6hrs before starting the time-course (Figures 4A and 4F).
(J-K) Lower doses of LY (10μM) downregulate the Notch target her4 (K2) and the Notch reporter mRNA (K1), the cell cycle inhibitor cdkn1bb (K6), and the Wnt inhibitor dkk2 (K7). Also, 10μM LY activates the HC differentiation markers deltad (K4) and atoh1a (K5) and the polar marker delta (K3).
(L) After 50μM LY, dkk2 is absent (L7) and wnt2 and wnt10a are activated (L8-L10).
(M-N) deltaa (M3), deltad (M4), and atoh1a (M5) are upregulated after HC death. The Notch reporter (N1), her4 (N2), wnt2 (N9), wnt10a (N10) and cdkn1bb (N7) show that Notch downregulation is transient and is reactivated 16hrs after HC death.
(O) Notch downregulation during regeneration mimics changes in expressionduring regeneration. Larvae were pre-treated 6hrs in 50μM LY before neo.
The heterogeneous expression patterns of notch receptors and ligands suggest that different combinations may regulate progenitor proliferation or cell fate determination (Alunni et al., 2013; Okigawa et al., 2014). To determine in which cells Notch signaling is active, we analyzed the expression pattern of the Notch reporter Tg(Tp1bglob:eEGFP) crossed with Tg(atoh1a:dTomato) that labels hair cells. The Notch reporter expresses GFP in central SCs beneath the hair cells (Figures 2K-2K’), but is significantly biased toward the anterior poles of the NMs (Figures 2L-2L’, blue dots). Likewise, mRNA expression of the Notch reporter is shifted toward the anterior poles (Figure 2M). This bias in gene expression possibly corresponds to the slight bias of amplifying divisions towards the posterior poles in homeostatic and regenerating control NMs (Figures 2B’, 2E’; Cruz et al., 2015), suggesting that Notch signaling keeps SCs quiescent across the central region and in the anterior pole.
Even though wnt2 is strongly expressed in homeostatic NMs, we did not detect expression of the Wnt reporter Tg(6xTcf/LefBS-miniP:d2EGFP) or the Wnt target wnt10a (Lush and Piotrowski, 2014a) in the poles (Figures 3D1-3D3). However, wnt10a is transiently upregulated in central SCs during regeneration correlating with the downregulation of the Notch reporter (Figures 3E1-3F4). In contrast, the Wnt reporter is activated in only a few central cells during regeneration suggesting that the reporter requires high levels of Wnt signaling for activation (Figures 3F1-3I1; Head et al., 2013). The inhibition of wnt10a and the Wnt reporter during homeostasis is likely due to co-expression of the Wnt inhibitor dkk2 (Figures 3J7-3J10; Jiang et al., 2014; Wada et al., 2013b). The compartmentalized expression of Notch and Wnt pathway members and their expression changes during regeneration suggest that they might be involved in controlling proliferation and differentiation in distinct regions of homeostatic and regenerating NMs.
Inhibition of Notch signaling mimics expression changes observed during regeneration
To test if the Notch and Wnt pathways regulate each other we inhibited Notch signaling using the γ-secretase inhibitor LY411575, referred to as ‘LY’ (Mizutari et al., 2013). As Notch signaling exhibits dose-dependent effects we treated larvae with 10 and 50μM of LY (Chapouton et al., 2010; Ninov et al., 2012).
We first tested the effects on the transcription of Notch and Wnt pathway genes (Figures 3J-3L). Both doses of LY inhibit expression of the Notch reporter and the Notch target gene her4 and lead to the upregulation of deltad and atoh1a that are normally inhibited by Notch signaling (Figures 3K1-3K5 and 3L1-3L5; (Itoh and Chitnis, 2001). In homeostatic NMs, the cyclin-dependent kinase inhibitor cdkn1bb (p27) is expressed in a central region similar to where Notch signaling is active (Figure 3J6). In mammals, Cdkn1b keeps SCs quiescent (Chen et al., 2003). cdkn1bb is only downregulated by the high dose of LY (Figure 3L6). Likewise, the Wnt targets wnt2 and wnt10a are only upregulated after treatment with 50μM LY (Figures 3L8-3L10), correlating with loss of the Notch target and Wnt inhibitor dkk2 (Figure 3L7).
The expression changes induced by a 16hr exposure to 50μM LY closely mimic changes during the first few hours after hair cell death (Figures 3L-3M; (Jiang et al., 2014). 3hrs after neo exposure Notch pathway genes are downregulated and deltaa, deltad and atoh1a are upregulated (Figures 3M1-3M5). In contrast, wnt2 and wnt10a are transiently upregulated in the center of the NM, correlating with the downregulation of dkk2 (Figures 3M7-3M10). cdkn1bb is also transiently downregulated but its expression recovers by 16hrs post hair cell death (Figures 3M6 and 3N6). A 16hr treatment with 50μM LY also leads to gene expression changes that are not observed 3 or 16hrs after neo treatment, such as a complete loss of the Notch reporter and her4 and upregulation of Wnt target genes (Figures 3L). These differences are likely due to the sustained downregulation of Notch after LY treatment, whereas Notch is reactivated 5hrs after neo treatment (Figure 3G4).
We conclude from these data that Notch inhibits Wnt signaling in a dose dependent manner and that the expression changes observed during the first hours of regeneration can largely be attributed to the transient downregulation of Notch signaling. This interpretation is supported by the finding that the expression changes 3hrs after neo are very similar to the expression changes after 3hrs neo and LY (Figures 3M and 3O).
Notch inhibits proliferation and differentiation through different mechanisms
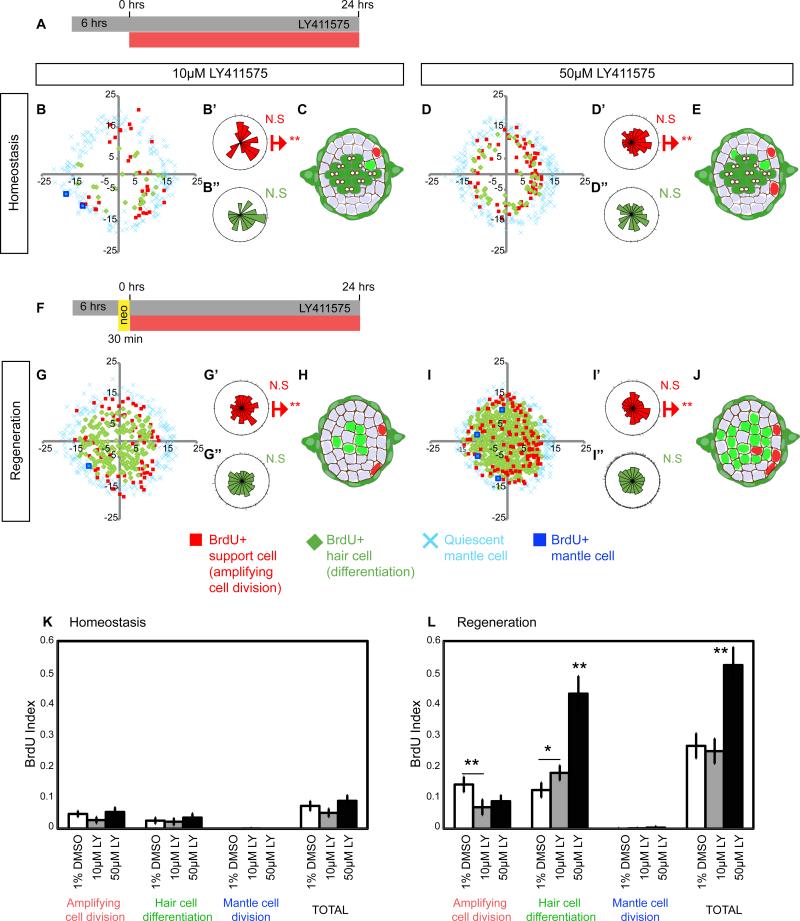
To quantify the effect of Notch downregulation on proliferation and differentiation, we performed 24hr BrdU experiments on LY-treated larvae (Figures 4A and 4F). Downregulation of Notch signaling with 10 and 50μM LY doses has no significant effect on the total proliferation rate during homeostasis (Figures 4B-4E and 4K, TOTAL; Ma et al., 2008). However, the polar distribution of amplifying divisions is disrupted (Figures 4B-4B” and 4D-4D”).
Figure 4. Notch inhibits proliferation and differentiation in a dose-dependent manner.
(A, F) sqet4;sqet20 larvae were treated for 30hrs with the γ-secretase inhibitor LY411575 (LY). After 6hrs in drug, BrdU was added for 24hrs. DMSO treated controls shown in Figures 2B and 2E.
(B-E) Notch inhibition disrupts the proliferative compartments. After 10μM LY, amplifying cell divisions are no longer clustered in the dorso-ventral poles.
(G-J) During regeneration, Notch inhibition enhances differentiating divisions in the central region of the NM and in the normally quiescent anterior and posterior compartments.
(K) Notch inhibition does not affect proliferation rates (BrdU index) during homeostasis.
(L) During regeneration 10μM LY does not affect total proliferation rates but induces differentiation at the expense of amplifying cell divisions. 50μM LY induces hyper-proliferation and an increase in differentiation.
Error bars show the 95% confidence interval (CI).
During regeneration the two doses of LY have different effects on total proliferation. 10μM LY does not increase the proliferation rate above the level of regenerating control NMs (Figures 4G and 4L), whereas 50μM increases total proliferation two-fold (Figures 4I and 4L). On the other hand, treatment with both doses of LY during regeneration causes loss of the D-V compartments and an increase in differentiation at the expense of amplifying divisions (Figures 4G-4J). Thus, upon Notch downregulation the majority of proliferating SCs differentiate into hair cells (Ma et al., 2008; Wibowo et al., 2011). These experiments show that Notch maintains neuromast size and the progenitor pool by inhibiting proliferation and differentiation during homeostasis. During regeneration, Notch is transiently downregulated, which triggers proliferation and differentiation (Figures 3, row 4). The finding that a low dose of the Notch inhibitor has no effect on total proliferation but promotes differentiation demonstrates that Notch signaling inhibits these two processes via independent mechanisms.
Notch signaling inhibits proliferation via inhibition of Wnt and by a Wnt-independent mechanism
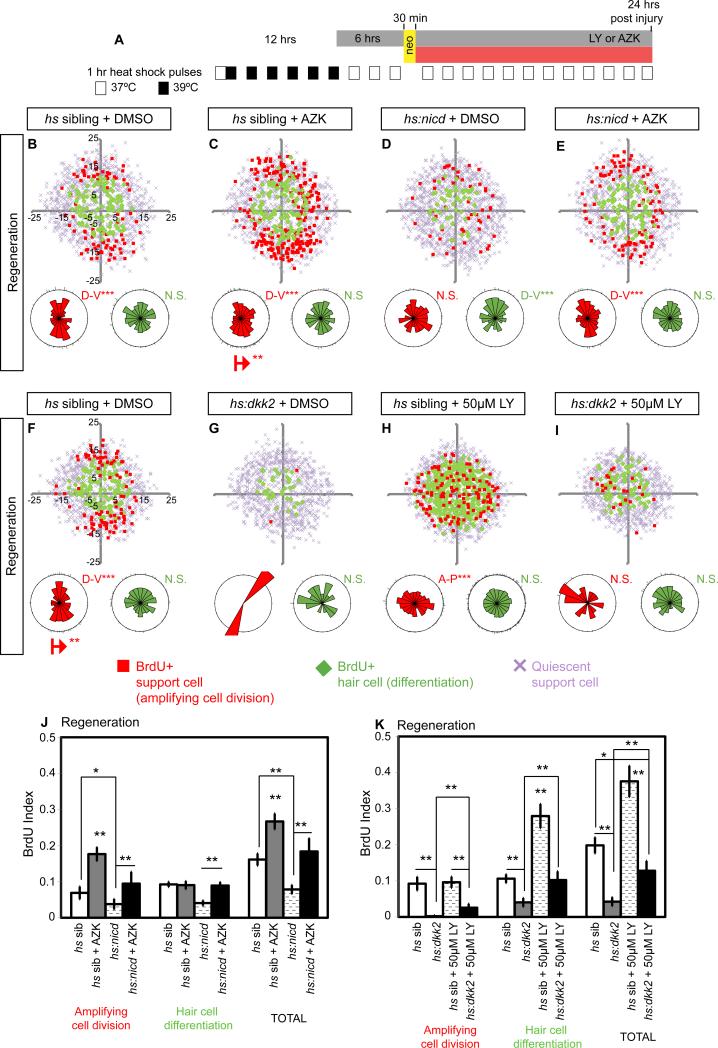
Wnt signaling induces proliferation in lateral line NMs (Head et al., 2013; Jacques et al., 2014). We confirmed this finding by treating NMs with the GSK3β inhibitor 1-Azakenpaullone (AZK) (Figures 5A and S3A). During homeostasis and regeneration AZK treatment significantly increases the BrdU index (Figures 5B-5C, 5J, S3B-S3C, S3J). We inhibited Wnt signaling with hs:dkk2 that blocks the binding of Wnt ligands to their Lrp co-receptor, and we observed a significant decrease in proliferation (Figures 5F-5G, 5K, S3F-S3G, S3K). Thus, Notch and Wnt signaling exert opposite effects on SC proliferation.
Figure 5. Notch signaling inhibits proliferation via Wnt inhibition and via a Wnt-independent mechanism during homeostasis and regeneration.
(A) Heat-shock protocol to experimentally induce hs:nicd or hs:dkk2 expression in regenerating NMs. Larva required 1hr-heat-shock pulses at least 12hrs before HC ablation in order to activate expression of the tagged reporter (c-Myc-tag or RFP respectively, not shown).
(B-E) BrdU plots for primI-derived regenerating NMs in sibling, Wnt activated (using the GSK3β inhibitor 1-Azakenpaullone, AZK) and Notch activated Tg(hsp70l:Gal4); Tg(UAS:myc-Notch1a-intra) transgenic larvae, referred to as (hs:nicd). All larvae carry the sqet4 transgene.
(B) In DMSO-treated, heat-shocked (hs) siblings amplifying cell divisions occur in the D-V poles and differentiating divisions in the center.
(C) AZK (3μM) increases SC proliferation in the D-V poles.
(D) Notch activation in hs:nicd larvae disrupts the proliferative compartments and reduces total proliferation.
(E) Activation of Wnt with AZK in hs:nicd larvae restores the clustering of SC amplification and proliferation rates.
(F-I) BrdU plots for primI-derived regenerating NMs in sibling or Wnt-inhibited Tg(hsp70l:Gal4); Tg(UAS:dkk2-RFP);sqet4, called hs:dkk2, larvae.
(G) Wnt inhibition in hs:dkk2 larvae depletes amplifying cell divisions and reduces differentiating divisions in the center.
(H) Notch inhibition disrupts polar compartments but maintains amplifying divisions and increases differentiating divisions in the center.
(I) Combined Notch and Wnt inhibition (hs:dkk2 + LY) depletes amplifying divisions in the poles but leaves differentiating divisions unaffected.
(J-K) BrdU indexes of amplifying, differentiating and total divisions after individual and combinatorial manipulations of the Wnt and Notch pathways. Error bar = 95% CI.
See also Figure S3.
The finding that Wnt pathway genes are upregulated after downregulation of Notch suggests that Notch inhibits Wnt signaling via dkk2 induction (Figures 3L7-3L10 and 6B6-6B9). To determine if the increase in proliferation after Notch inhibition during regeneration is due to upregulation of Wnt signaling, we performed epistasis experiments and treated hs:dkk2 larvae with 50μM LY (Figures 5H-5I and 5K). Indeed, the LY-induced increase in total proliferation is reduced to below normal levels, indicating that the majority of extra hair cells formed after LY treatment are likely due to an increase in Wnt signaling. This conclusion is supported by AZK-induced Wnt activation in myc-tagged hs:nicd larvae, in which the Notch pathway is constitutively active. hs:nicd induction causes a reduction in SC proliferation that is reverted by simultaneous activation of Wnt with AZK (Figures 5D-5E, 5J, S3D-S3E, S3J).
Figure 6. Wnt controls proliferation in the poles but does not affect hair cell differentiation.
(A) Notch pathway genes are active during homeostasis, whereas Wnt targets are absent, with the exception of wnt2 (A8).
(B) Notch inhibition causes activation of the Wnt reporter Tg(6xTcf/LefBS-miniP:d2EGFP) (B7) and Wnt targets wnt2 (B8) and wnt10a (B9).
(C) AZK-induced Wnt activation has no effect on HC differentiation markers, such as atoh1a (C4), Notch pathway genes (C1-2), or dkk2 (C6).
(D) LY and AZK combined phenocopy the effects of LY alone.
(E) hs:dkk2 does not affect the expression of Notch pathway genes
(F) LY-induced upregulation of Wnt target genes is reversed by hs:dkk2 induction.
(G) Increased Notch signaling in hs:nicd larvae enhances Notch reporter expression. Only 20% of neuromast cells express nicd (data not shown).
(H) hs:nicd inhibits the AZK-induced activation of wnt10a (H9) and the Wnt reporter (H7).
(I-Q) BrdU plots for primI-derived homeostatic and regenerating sqet4+ NMs. Larvae were treated with DMSO, AZK, 50μM LY or AZK+LY as in Figures 3A and 3F.
(I, N) In homeostatic and regenerating NMs amplifying cell divisions are clustered in the poles. During regeneration, centrally located SCs divide and differentiate.
(J, O) AZK enhances SC amplification in the polar compartments without affecting HC differentiation.
(K, P) LY enhances HC differentiation and disrupts the polar compartments.
(L, Q) Combined Wnt activation and Notch inhibition disrupts the polar compartments and randomizes amplification and differentiation.
(M, R) BrdU indexes of amplifying, differentiating and total divisions after single and combinatorial manipulations of the Wnt and Notch pathways. Error bar = 95% CI.
See also Figure S4.
Notch signaling also inhibits some degree of proliferation independently of Wnt signaling, as in LY treated hs:dkk2 larvae cell proliferation is not as severely reduced as in hs:dkk2 larvae (Figures 5I and 5K). Likewise, in the presence of NICD, AZK-induced proliferation is lower than if treated with AZK alone implying that Notch signaling also inhibits other proliferative signals (Figures 5E and 5J).
We also tested the effects of Notch and Wnt on the expression of the cell cycle inhibitor cdkn1bb. 50μM LY downregulates cdkn1bb (Figures 3L6, 6A5, 6B5, 6D5, and 6F5) and Notch activation by hs:nicd enhances cdkn1bb expression (Figures 6G5, 6H5). However, LY does not inhibit cdkn1bb completely and possibly other signals also control its expression. In contrast, AZK or hs:dkk2 do not affect cdkn1bb expression showing that cdkn1bb expression is not Wnt-dependent (Figures 6B5, 6C5, 6E5, 6F5 and 6H5). Together with the BrdU analyses, these data suggest that Notch signaling inhibits proliferation both in a Wnt-dependent fashion via the induction of dkk2 and independently of Wnt, possibly via the induction of cdkn1bb.
Wnt and Notch signaling control proliferation in overlapping and distinct compartments
AZK treatment specifically increases amplifying divisions in the poles and in other quiescent SCs without affecting differentiation in the center (Figures 6I, 6J, 6N, 6O, 6M and 6R). Hence, we hypothesized that central differentiating divisions are not affected by AZK, because Notch signaling is still present (Figure 6C). Indeed, Notch inhibition in AZK treated larvae induces central cell amplification (Figures 6K-6L and 6P-6Q). In contrast, loss of Wnt signaling significantly reduces amplifying divisions in the poles, periphery and center of the NMs (Figures 5F-5I, S3F-S3I). Importantly, Notch inhibition in hs:dkk2 NMs only induces differentiating divisions in the center (Figures 5I and S3I). Combined, these analyses support the notion that Notch inhibits proliferation and differentiation in the center of the NMs independently of Wnt signaling, putatively via activation of cdkn1bb (Figure 6C5).
Even though wnt2 is expressed in the poles (Figure 2G-2H), the Wnt reporter, when activated, is expressed only in central cells. We did not detect polar Wnt reporter expression during regeneration or after AZK treatment (Figures 3D1-3I4, and 6A7-6H7). Still, Wnt signaling has a clear activating effect on proliferation of the polar cells (Figure 6J). Therefore, we postulate that during regeneration, Notch downregulation in center cells activates Wnt signaling cell autonomously, which then non-cell autonomously upregulates proliferation in the periphery via an unknown mechanism. The role wnt2 plays in SC behavior is unknown.
Wnt does not affect hair cell differentiation during hair cell regeneration
Notch signaling inhibits hair cell differentiation via atoh1a inhibition (Itoh and Chitnis, 2001). However, Wnt is required for hair cell differentiation in the developing mouse inner ear, as downregulation of β-Catenin causes loss of Atoh1-positive cells (Jacques et al., 2014; Jacques et al., 2012; Shi et al., 2014; Shi et al., 2012). We therefore asked if Wnt signaling upregulates atoh1a expression in mature NMs. However, atoh1a is only upregulated after Notch downregulation (Figures 3K5-3L5, 6B4, 6D4, 6F4) and atoh1a is not affected by either AZK-induced Wnt activation or hs:dkk2 induction (Figures 6C4, 6E4 and 6H4). Also, differentiation is not enhanced in the poles after AZK treatment (Figures 6J and 6O). Only 72hr treatments with AZK modestly increase the number of hair cells, while most dividing cells remain SCs (Figures S4A-S4C; Head et al., 2013; Jacques et al., 2014). Therefore, AZK leads to a proportional increase of hair cell numbers because more SCs divide. Either Wnt signaling interacts with atoh1a only during NM development, or the disparate findings in mouse and zebrafish reflect species-specific differences. Future experiments are needed to identify the signal(s) that activate atoh1a and drive differentiation in mature NMs. Our results show that in NMs Wnt signaling activates SC amplification, but is not sufficient to induce hair cell differentiation, which is induced by Notch downregulation.
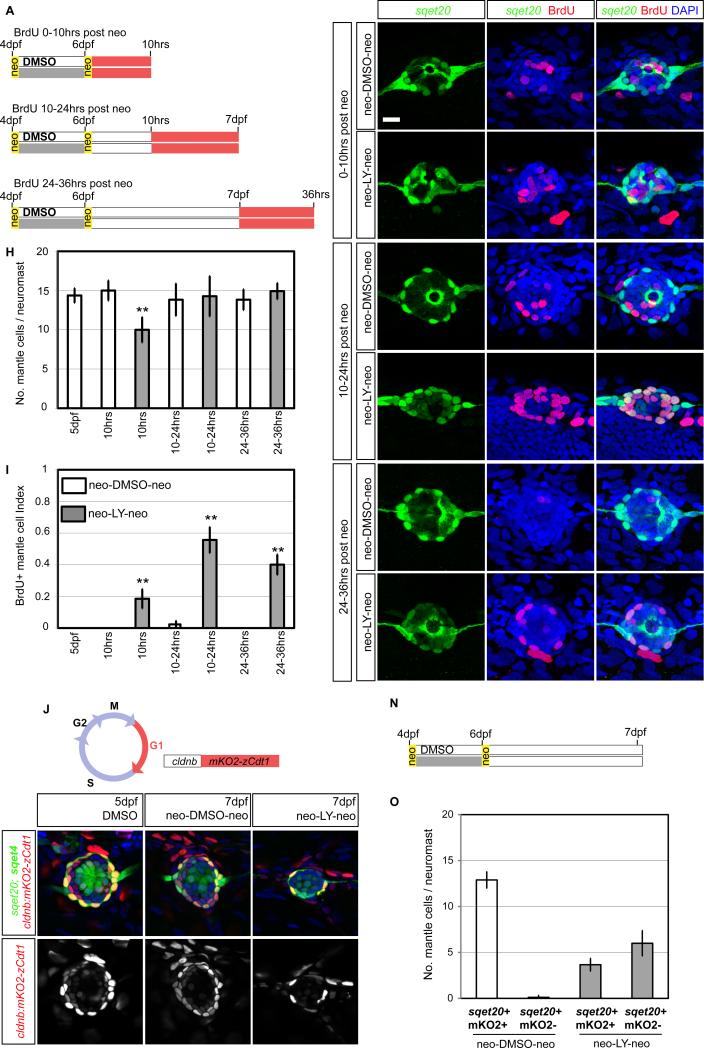
Mantle cells can re-enter the cell cycle
Mantle cells (MCs) do not respond to neo-induced hair cell death (Figures 4L and 7A-7B, 7D and 7F). However, MCs serve as stem cells for restoring NMs on regenerating tail tips (Dufourcq et al., 2006; Jones and Corwin, 1993). Hence, we tested if loss of SCs, in addition to hair cells would trigger MC proliferation. We depleted the SC pool by inhibiting Notch during regeneration to convert more SCs into hair cells, followed by a second dose of neomycin (neo) (Figures 7A, S5I). Indeed, 6hrs after the second neo treatment, NMs collapse and mainly consist of GFP+ MCs (Figures S5D-S5F’). NMs regain some of their shape by 10hrs but the number of MCs is reduced demonstrating that MCs were also killed (Figures 7H, S5D-D’). By 24hrs the MC number has recovered (Figure 7H, S5F-F’).
Figure 7. Mantle cells are quiescent stem cells.
(A) Protocol that transforms most SCs into hair cells, followed by neo treatment to test the MC response.
(B-G) BrdU incorporation in primI-derived sqet20 NMs at different time points. Scale bar = 10μm.
(H) sqet20+ MCs are reduced 10hrs after the second neo treatment but recover by 24hrs.
(I) MC BrdU index (No. of BrdU+, sqet20+ cells / total No. of sqet20+ cells). The proliferation of MCs significantly increases between 10-36hrs after the second neo treatment.
(J) In Tg(cldnb:mKO2-zCdt1) cldnb drives the Cdt1-tagged mKO2 fluorescent protein in NMs. The Cdt1 ubiquitination domain forces degradation of mKO2 once DNA replication begins.
(K) The quiescent state of MCs was analyzed 24hrs after the second neo treatment.
(L-L’) mKO2-zCdt1 expression is strong in MCs (O).
(M,M’) Treating embryos twice with neo does not affect the quiescent state of MCs.
(N-O) Depletion of SCs by LY in sqet20;sqet4 larvae causes some MCs to lose mKO2-zCdt1 expression suggesting that they re-entered the cell cycle. Error bar = 95% CI.
See also Figure S5.
BrdU incorporation between 0-10hrs after the second neo treatment shows an increase in the BrdU index of GFP+ MCs that further increases between 10-24hrs (Figures 7E and 7I). As the MC population recovers 24hrs after the second neo treatment, we also performed BrdU incorporation experiments between 24-36hrs. The BrdU index of MCs is still significantly increased, suggesting that MCs divide, possibly to restore the SC population (Figures 7G and 7I). The cell cycle re-entry of MCs also correlates with the disappearance of mKO2 fluorescence in Tg(cldnb:mKO2-zCdt1); sqet20 larvae (Figures 7J-7O). mKO2-zCdt1 labels quiescent cells in the G1 phase of the cell cycle (Sugiyama et al., 2009) and is highly expressed in MCs (Figures 7L-7O).
Because we experimentally transformed most SCs into hair cells using LY (Figures S5G-S5I), we wondered whether Notch downregulation was sufficient to activate MC proliferation. However, LY-treatment does not cause a significant change in MC proliferation (Figures 4K-4L). It remains to be demonstrated if MCs are stem cells and can differentiate into hair cells.
Discussion
In species that regenerate hair cells, SCs self-renew and give rise to new hair cells or they transdifferentiate into hair cells. In mammals, this ability is lost after birth leading to the hypothesis that mammalian SCs are highly differentiated (Burns and Corwin, 2014; Warchol, 2011). Yet, even in regenerating species SCs are not well characterized due to a dearth of molecular markers and the lack of distinct cytological characteristics. It is still unknown if SCs consist of different populations and which among them act as self-renewing stem cells (Groves, 2010; Ronaghi et al., 2012). To overcome these limitations we tracked cell behavior in vivo and in real time. Previous studies observed that SCs give rise to two hair cells without assessing amplifying divisions or MCs (López-Schier and Hudspeth, 2006; Ma et al., 2008; Wibowo et al., 2011). In adult zebrafish, anterior and posterior NM cells are label-retaining and SCs divide more often in the D/Vpoles (Cruz et al 2015). However, the identity and potency of the proliferating cells and the molecular underpinnings of their behavior could not be addressed.
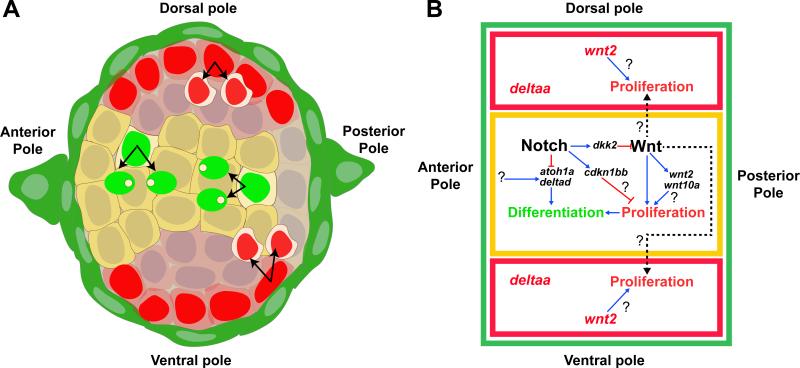
Self-renewal and differentiation occur in distinct compartments and are regulated by Notch/Wnt interactions
Our analyses of regenerating neuromasts revealed a striking spatial compartmentalization of cell behaviors and we identified at least three SC populations: 1) self-renewing cells located adjacent to peripheral MCs in the D-V poles; 2) cells located in the center and A-P poles that proliferate and differentiate and 3) quiescent peripheral MCs that only respond to severe injury.
We determined that the Notch and Wnt signaling pathways balance progenitor maintenance with hair cell differentiation during homeostasis and regeneration (Figures 8A-8B). The activation of Notch and Wnt pathways in the center of the NM, and deltaa and wnt2 expression in the poles, correlate with these different cell behaviors. Prior functional tests had determined that Notch signaling regulates hair cell differentiation via the downregulation of atoh1a (Itoh and Chitnis, 2001; Ma et al., 2008).
Figure 8. Model of the molecular control of cell behaviors during regeneration.
Notch signaling controls tissue homeostasis in the NM by restricting proliferation and differentiation through Wnt-dependent and -independent mechanisms. During regeneration Notch is transiently downregulated activating Wnt and proliferation in the center and D-V poles.
(A) SC amplification (red nuclei) occurs in the D-V compartments (red cytoplasm) next to peripheral, green MCs. Amplifying cells express wnt2 and deltaa. HC differentiation occurs in the central, Notch+ domain (yellow).
(B) Wnt/Notch signaling interactions. In the center (outlined in yellow), Notch inhibits differentiation by inhibiting atoh1a and delta ligands. Notch inhibits proliferation possibly via cdkn1bb and also by inhibiting Wnt signaling through the activation of dkk2. Wnt signaling activates proliferation of HC progenitors in the center and non-cell autonomously of SC progenitors in the poles. The mechanisms by which Wnt initiates proliferation in the poles and the roles of wnt10a and wnt2 have yet to be discovered. Red lines show inhibition, blue arrows indicate activation and dashed arrows show indirect, non-cell-autonomous activation of proliferation.
Importantly, downregulation of Notch signaling in center SCs also activates Wnt signaling (wnt10a, wnt2), as in murine inner ear development (Li et al., 2015). In addition, we show that the Wnt inhibitor dkk2 is a Notch target in center cells beneath hair cells and that its downregulation after loss of Notch is involved in the upregulation of Wnt signaling (Figures 3B and 6, rows 6 and 7). The regulation of dkk2 by Notch could be direct, as the human DKK2 enhancer possesses RBP-Jκ binding sites (Katoh and Katoh, 2007). Subsequently, Wnt signaling also activates proliferation non-cell autonomously in polar cells, as polar cells do not show signs of canonical Wnt pathway activation. The nature of this Wnt-induced signal to polar cells remains unknown. These data show that the activation of Wnt-induced proliferation and hair cell regeneration is controlled by the prior downregulation of Notch signaling. Our previous RNA-Seq analysis of regenerating SCs supports this conclusion. After hair cell death, Notch signaling is transiently downregulated before Wnt signaling is activated and we propose that loss of Notch signaling is triggering regeneration upstream of Wnt signaling (Jiang et al., 2014).
Expression of Notch pathway genes
Notch signaling cannot be equally active in all central cells, as it would prevent hair cell differentiation. To identify cells where the Notch-active cells, we performed in situ analyses with candidate ligands and receptors. The heterogeneous expression patterns of Notch receptors and ligands in NMs (Figures 3A) suggest that hair cell differentiation cannot simply be explained by lateral inhibition, where ligand expression predicts which cells differentiate (Eddison et al., 2000; Kageyama et al., 2008). For example, deltaa is strongly expressed in NM poles, where no hair cell differentiation occurs (Figures 2I-2J). Recent studies showed that different ligand/ receptor combinations regulate either progenitor proliferation or cell fate determination in the CNS and spinal cord (Alunni et al., 2013; Okigawa et al., 2014). As a result, the expression pattern of Notch pathway members is not sufficient to deduce in which cells Notch signaling is active (Perdigoto and Bardin, 2013; Petrovic et al., 2014).
We therefore relied on the expression of a Notch reporter that is active in the center and A-P poles. Because of the large number of ligands and receptors that are expressed in NMs and the lack of combinatorial mutants, a dissection of different combinations of ligand-receptor pairs, their effect on Notch signaling and LL cell behavior awaits to be performed.
Different dosages of Notch affect differentiation and proliferation
An effect of Notch dosage on cell quiescence, renewal, and cell differentiation occurs in the chick inner ear, mammary epithelial cells, fly intestine and pancreatic endocrine progenitors (Perdigoto and Bardin, 2013). Such dosage-dependency has been attributed to the fact that different ligands have different abilities to activate Notch signaling (Ninov et al., 2012; Petrovic et al., 2014). Interestingly, we also observed a correlation between Notch signaling strength and different cell behaviors in the NMs. A higher dose of the Notch inhibitor causes hair cell differentiation as well as induction of proliferation, whereas a lower dose only affects hair cell differentiation. These results suggest that different target genes regulate these two processes independently. We conclude that cell differentiation is inhibited by Notch cell-autonomously in center cells of the NM, whereas the inhibition of the majority of proliferation is mediated via the regulation of Wnt signaling.
Why is the Notch-regulated restriction of amplifying divisions to the poles important?
The significance of restricting amplifying divisions to the poles and keeping the anterior pole more quiescent than the posterior pole is not apparent. In other tissues, such as the fly midgut, stem cells are mosaically distributed throughout the epithelium (Ohlstein and Spradling, 2007). The observation that Notch signaling is linked to the establishment of mirror-symmetric planar cell polarity of the two daughter hair cells may provide an explanation (López-Schier and Hudspeth, 2006; Wibowo et al., 2011). A reduction in Notch leads to hair cell pairs that are polarized primarily in the same direction, rather than a 1:1 distribution of opposite polarities (Mirkovic et al., 2012; Wibowo et al., 2011). As such, identifying the signal(s) that activate the Notch pathway in the NM center, defining the mechanism underpinning the enrichment of Notch signaling in the anterior side of the NM and keeping it out of the poles (Figure 2K) will be quite informative. Likewise, we also have not yet identified the molecules that may be activating Wnt signaling in NMs.
Support cells throughout the NM are multipotent
NM support cells are cytologically undistinguishable, raising the question as to whether all SCs are stem cells, only a few stem cells are intermingled between progenitor cells, or if stem cells could be localized in discrete compartments such as the NM poles.
To regenerate an average of 14 hair cells in a 5 dpf NM, approximately 7 center SCs divide and produce two hair cells each, and 3-4 SCs per pole divide to maintain the progenitor pool (Figures 2F and S2A). We do not believe that these 3-4 cells constitute a special population of stem cells because a downregulation of Notch or ubiquitous activation of Wnt signaling abolishes the bias of amplifying divisions to the poles and posterior side of the NM. Thus, SCs throughout the NM are responsive to the Notch and Wnt signaling pathways and are capable to either amplify or give rise to hair cells. These findings are strikingly similar to hair follicle and intestinal stem cells, where the position within the niche determines the fate of the cells, as passive displacement exposes them to differentiation signals (Ritsma et al., 2014; Rompolas et al., 2013).
Mantle cells are quiescent but proliferate in response to severe injury
Transection of axolotl tails and zebrafish fins causes peripheral cells to proliferate and migrate onto the regenerating tail tips, where they form new sense organs (Jones and Corwin, 1993; Stone, 1937). MCs also give rise to postembryonic NMs suggesting that MCs are multipotent progenitors (Wada et al., 2013a). We were surprised that MCs do not react to neo-induced hair cell death. However, given that inner SC amplification and differentiation are balanced during regeneration, a MC response is not required (Figure 4L). Our finding that MCs re-enter the cell cycle after more severe damage to the NM suggests that MCs might represent a quiescent pool of progenitor cells that respond to signals triggered by severe injury. Quiescence is characteristic of a variety of stem cell populations in the liver, hair follicles, intestine and hematopoietic system (Li and Clevers, 2010; Tetteh et al., 2014). Alternatively, MCs could be specialized SCs that only proliferate to maintain the MC population. We will distinguish between these possibilities by generating transgenic lines that permit us to lineage-trace proliferating MCs. Interestingly, SC amplification almost exclusively occurs in cells that are in contact with MCs (Figures S2B-S2G), raising the possibility that MCs might constitute a niche for support stem cells.
Conclusion
We report a systematic in vivo analysis of progenitor cell lineages during homeostasis and regeneration. We also demonstrate how this approach can be used to investigate the function of signaling pathways during the poorly understood process of hair cell regeneration. Our combined methods have allowed to precisely identify different progenitor cell types that are restricted to particular tissue compartments, follow their behavior in real time and define a Notch-driven inhibition of Wnt-induced cell proliferation. These findings set the stage for a detailed characterization of signals that control progenitor cell maintenance versus differentiation in a vertebrate sensory organ, including the mammalian inner ear.
Experimental Procedures
Regeneration experiments, time-lapse imaging and image acquisition
To kill hair cells 5dpf larvae were treated for 30min with 300μM neo in 0.5 E2 (Fisher Bioreagents). Quadruple transgenic fish were obtained by crossing Tg(cldnb:lynGFP); Tg(cldnb:H2A-mCherry) and sqet20; sqet4. Before neo treatment, larvae were immobilized with tricaine (MS-222) up to 150 mg/L (100μL of 4g/L tricaine every 20min for 2 to 3hrs). Larvae were mounted in 1% low melting point agarose in 0.5× E2 with 100 mg/L tricaine on glass bottom dishes (Mat-Tek, USA). Images were acquired at 28°C on a Zeiss LSM780 confocal microscope using a 40× water objective and with appropriate Z-sampling for three-dimensional reconstruction and 4D-stacks. Except for Figure S2, only primI-derived pL1, pL2 or pL3 NMs were imaged. After neo treatment, single NMs were imaged every 6min for more than 70hrs. Three-dimensional rendering and image analysis of confocal z-stacks of single NMs were done using Imaris (Bitplane). Please, see Supplemental Experimental Procedures for fish lines used and specifics of the cell movement and spatial analyses.
Pharmacological inhibitors and BrdU incorporation
The γ-secretase inhibitor LY411575 (Selleckchem) and the GSK3β inhibitor 1-Azakenpaullone (Sigma-Aldrich) were diluted to their desired concentrations in 0.5× E2 media with a final concentration of 1% DMSO. Control larvae were treated with 1% DMSO. Bromodeoxyuridine (Sigma Aldrich) was diluted to 10mM in embryo medium containing 1%DMSO with or without the pharmacological inhibitors.
Heat-shock experiments
Tg(hsp70l:Gal4);Tg(sqet4) fish were crossed with either Tg(UAS:myc-Notch1a-intra) or Tg(UAS:dkk2-RFP)
GFP+ larvae were used for proliferation assays, and in situ hybridization was performed on GFP- siblings. To activate and sustain the expression driven by a heat shock-activated Gal4, 5 dpf larvae were heat shocked (HS) every other hour (1hr HS then 1hr at 28.5°C). Before drug treatments, larvae were heat shocked six times at 39°C (first HS at 37°C) in a water bath. After this initial activation, larvae were treated with neo or DMSO and transferred to E2 medium containing pharmacological inhibitors as described above. To maintain Gal4-activated expression, larvae were heat shocked every other hour for 24hrs in a 37°C incubator. Larvae were fixed in 4% paraformaldehyde at 4°C for 3 days. Activated Tg(UAS:dkk2-RFP) embryos were sorted after fixation by RFP fluorescence. Tg(UAS:myc-Notch1a-intra) fish were sorted after anti c-Myc antibody staining.
Immunohistochemistry
BrdU immunodetection was done according to Ma et al. (2008) with the following modifications: larvae were permeabilized for 15min using 20μg/ml proteinase-K and treated for 1hr in 2N hydrochloric acid. Antibodies used: monoclonal rat anti-BrdU (1:500; Accurate Chemical & Scientific Corp), rabbit anti-GFP (1:500; Invitrogen), monoclonal mouse anti c-myc (Santa Cruz). DAPI (Invitrogen) was used as counterstain. BrdU indexes were calculated as the number of BrdU+ cells over the total NM cell number. We compared samples by ANOVA and Tukey post-Hoc tests using the SAS 9.3 statistical software. The indexes were transformed using the formula arsin (sqrt(percentage/100)) to ensure the assumption of normality.
In situ hybridization
In situ hybridization was performed as described in (Kopinke et al., 2006) with modifications as in (Ma et al., 2008). See Supplemental Experimental Procedures for probe identities.
Supplementary Material
Acknowledgments
We are grateful to Drs. A. Sánchez Alvarado, L. Li, M. Lush, J. Kniss and M. Venero Galanternik for valuable comments on the manuscript, and Drs. V. Korzh, N. Lawson, T. Ishitani, H. Wada, K. Kawakami, B. Appel and P. Kulesa for strains and reagents. We are also thankful to R. Duncan and E. Young for preliminary experiments, members of the Piotrowski lab, and Dr. T. Xie for insightful discussions. We would also like to thank D. Fekete for suggesting the neo-LY-neo experiment. We thank the U. of Utah Zebrafish Core and the Aquatics Facility at the Stowers Institute for excellent fish care, J. Unruh and B. Rubinstein for support with the spatial analysis, J. Lange, S. McKinney and the Microscopy Core for imaging support and M. Miller for help with graphic design. This work was funded by an NIH (NIDCD) award RC1DC010631 to T.P, the Hearing Health Foundation, and by institutional support from the Stowers Institute for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
A.R.-C. and T.P. designed the study, analyzed and interpreted data, and wrote the paper.
A.R.-C., J.N.A., L.J. and A.K.-G. performed experiments.
H.L. performed the statistical analyses.
R.A. developed and performed the cell movement analyses.
References
- Alunni A, Krecsmarik M, Bosco A, Galant S, Pan L, Moens CB, Bally-Cuif L. Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development. 2013;140:3335–3347. doi: 10.1242/dev.095018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Corwin JT. Responses to cell loss become restricted as the supporting cells in mammalian vestibular organs grow thick junctional actin bands that develop high stability. J Neurosci. 2014;34:1998–2011. doi: 10.1523/JNEUROSCI.4355-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P, Skupien P, Hesl B, Coolen M, Moore JC, Madelaine R, Kremmer E, Faus-Kessler T, Blader P, Lawson ND, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche D.a. Regeneration of sensory hair cells after acoustic trauma. Science (New York, NY) 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen D-H, Chalasani K, Steigelman KA, Fang J, Rubel EW, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141:816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz IA, Kappedal R, Mackenzie SM, Hailey DW, Hoffman TL, Schilling TF, Raible DW. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol. 2015 doi: 10.1016/j.ydbio.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq P, Roussigné M, Blader P, Rosa F, Peyrieras N, Vriz S. Mechano-sensory organ regeneration in adults: the zebrafish lateral line as a model. Mol Cell Neurosci. 2006;33:180–187. doi: 10.1016/j.mcn.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN. Molecular basis of hair cell loss. Cell Tissue Res. 2015 doi: 10.1007/s00441-015-2113-z. [DOI] [PubMed] [Google Scholar]
- Groves AK. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 2010;235:434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JR, Gacioch L, Pennisi M, Meyers JR. Activation of canonical Wnt/β-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Dev Dyn. 2013;242:832–846. doi: 10.1002/dvdy.23973. [DOI] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev. 2001;102:263–266. doi: 10.1016/s0925-4773(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montgomery WH, Uribe PM, Yatteau A, Asuncion JD, Resendiz G, Matsui JI, Dabdoub A. The role of Wnt/β-catenin signaling in proliferation and regeneration of the developing basilar papilla and lateral line. Dev Neurobiol. 2014;74:438–456. doi: 10.1002/dneu.22134. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Puligilla C, Weichert RM, Ferrer-Vaquer A, Hadjantonakis A-K, Kelley MW, Dabdoub A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proc Natl Acad Sci U S A. 2014;111:E1383–1392. doi: 10.1073/pnas.1402898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13:1022–1034. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT antagonist, DKK2, is a Notch signaling target in intestinal stem cells: Augmentation of a negative regulation system for canonical WNT signaling pathway by the Notch-DKK2 signaling loop in primates. Int J Mol Med. 2007;19:197–201. [PubMed] [Google Scholar]
- Kopinke D, Sasine J, Swift J, Stephens WZ, Piotrowski T. Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev Dyn. 2006;235:2695–2709. doi: 10.1002/dvdy.20905. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science (New York, NY) 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, Li H. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A. 2015;112:166–171. doi: 10.1073/pnas.1415901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci U S A. 2006;103:18615–18620. doi: 10.1073/pnas.0608536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev Cell. 2004;7:401–412. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Lush ME, Piotrowski T. ErbB expressing Schwann cells control lateral line progenitor cells via non-cell-autonomous regulation of Wnt/β-catenin. eLife. 2014a;3:e01832. doi: 10.7554/eLife.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Piotrowski T. Sensory hair cell regeneration in the zebrafish lateral line. Dev Dyn. 2014b;243:1187–1202. doi: 10.1002/dvdy.24167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- Mirkovic I, Pylawka S, Hudspeth AJ. Rearrangements between differentiating hair cells coordinate planar polarity and the establishment of mirror symmetry in lateral-line neuromasts. Biology open. 2012;1:498–505. doi: 10.1242/bio.2012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge ASB. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T. The Genetics of Hearing and Balance in Zebrafish Teresa. Annu Rev Genet. 2005;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- Ninov N, Borius M, Stainier DYR. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development. 2012;139:1557–1567. doi: 10.1242/dev.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG, Catania KC, Criley BB. Development of lateral line organs in the axolotl. J Comp Neurol. 1994;340:480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science (New York, NY) 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Okigawa S, Mizoguchi T, Okano M, Tanaka H, Isoda M, Jiang Y-J, Suster M, Higashijima S-I, Kawakami K, Itoh M. Different combinations of Notch ligands and receptors regulate V2 interneuron progenitor proliferation and V2a/V2b cell fate determination. Dev Biol. 2014;391:196–206. doi: 10.1016/j.ydbio.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Perdigoto CN, Bardin AJ. Sending the right signal: Notch and stem cells. Biochim Biophys Acta. 2013;1830:2307–2322. doi: 10.1016/j.bbagen.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Petrovic J, Formosa-Jordan P, Luna-Escalante JC, Abelló G, Ibañes M, Neves J, Giraldez F. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development. 2014;141:2313–2324. doi: 10.1242/dev.108100. [DOI] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaghi M, Nasr M, Heller S. Concise review: Inner ear stem cells--an oxymoron, but why? Stem cells (Dayton, Ohio) 2012;30:69–74. doi: 10.1002/stem.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hear Res. 2013;297:42–51. doi: 10.1016/j.heares.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Edge ASB. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci U S A. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Jacques BE, Mulvaney JF, Dabdoub A, Edge ASB. β-Catenin is required for hair-cell differentiation in the cochlea. J Neurosci. 2014;34:6470–6479. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge ASB. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS. Further experimental studies of the development of lateral-line sense organs in amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115. [Google Scholar]
- Sugiyama M, Sakaue-Sawano A, Iimura T, Fukami K, Kitaguchi T, Kawakami K, Okamoto H, Higashijima S.-i., Miyawaki A. Illuminating cell-cycle progression in the developing zebrafish embryo. Proc Natl Acad Sci U S A. 2009;106:20812–20817. doi: 10.1073/pnas.0906464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Farin HF, Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2014;25:100–108. doi: 10.1016/j.tcb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Wada H, Dambly-Chaudiere C, Kawakami K, Ghysen A. Innervation is required for sense organ development in the lateral line system of adult zebrafish. Proc Natl Acad Sci U S A. 2013a;110:5659–5664. doi: 10.1073/pnas.1214004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Ghysen A, Asakawa K, Abe G, Ishitani T, Kawakami K. Wnt/Dkk negative feedback regulates sensory organ size in zebrafish. Curr Biol. 2013b;23:1559–1565. doi: 10.1016/j.cub.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species. Hear Res. 2011;273:72–79. doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Wibowo I, Pinto-Teixeira F, Satou C, Higashijima S.-i., López-Schier H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development. 2011;138:1143–1152. doi: 10.1242/dev.060566. [DOI] [PubMed] [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.