Abstract
Anxiety-related disorders are complex illnesses that underlying molecular mechanisms need to be understood. Mitochondria stand as an important link between energy metabolism, oxidative stress, and anxiety. The nuclear factor, erythroid-derived 2,-like 1(Nrf1) is a member of the cap “n” collar subfamily of basic region leucine zipper transcription factors and plays the major role in regulating the adaptive response to oxidants and electrophiles within the cell. Here, we injected small interfering RNA (siRNA) targeting Nrf1 in dorsal third ventricle of adult male albino Wistar rats and subsequently examined the effect of this silencing on anxiety-related behavior. We also evaluated apoptotic markers and mitochondrial biogenesis factors, along with electron transport chain activity in three brain regions: hippocampus, amygdala, and prefrontal cortex. Our data revealed that in the group that received Nrf1-siRNA, anxiety-related behavior did not show any significant changes compared to the control group. Caspase-3 did not increase in Nrf1-siRNA-injected rats even though Bax/Bcl2 ratio markedly elevated in Nrf1-knockdown rats in all three mentioned regions compared to control rats. Also, Nrf1 silencing of complex I and II–III did not alter, generally. In addition, Nrf1-knockdown affected mitochondrial biogenesis markers. The level of peroxisome proliferator-activated receptor gamma coactivator-1α and cytochrome-c increased, which indicates a possible role for mitochondrial biogenesis in anxiety.
Keywords: Anxiety, Nrf1, siRNA, Electron transport chain, PGC-1α
Introduction
Explained as sustained elevated apprehension in the absence of immediate threat, pathological anxiety is the most prevalent mental disorder around the world (Wu et al. 2008). Several molecular, neurological, and psychological mechanisms described underlying pathophysiology of anxiety; however, the actual pathogenesis is still poorly understood resulting in the lack of effective and consistent treatment (Wu et al. 2008). In a molecular point of view, a few intracellular stress axes, in brain neurons, are attributed to anxiety manifestations (Drake et al. 2007). Some are including oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and autophagy (Chevallier et al. 2011; Huang et al. 2013; Olsen et al. 2013). Consequently, neuronal dysfunction or neuronal death could occur as the result of sustained stress axis activity (Morales et al. 2010). Cumulating evidence from imaging and cellular studies suggest various brain regions affected by anxiety (Sun et al. 2013; Smith and Suckow 1985). As such, there has been shown an extensive structural plasticity in anxiety within the circuitry of the hippocampus, amygdala, and prefrontal cortex (Virok et al. 2011; Carlini et al. 2004; McHugh et al. 2004).
Mitochondria are the main sites of cellular energy production and, at the same time, these organelles stand for major intracellular sources of reactive oxygen species (ROS) (Huang et al. 2014). Interestingly, mitochondria are also primarily affected by ROS and are composed of rich antioxidative agents (Li et al. 2014). In this manner, mice models of anxiety-like behavior have been displayed mitochondrial dysfunction in different stages of mitochondrial activity (Kumar et al. 2012; Einat et al. 2005). Elevated expression levels of electron transport chain (ETC) complexes, impaired antioxidant defense system and disrupted mitochondrial transport processes are such pathologic changes, explained in anxiety (Einat et al. 2005). As a result, sustained and/or sever stress leads to mitochondrial dysfunction and actives mitochondrial-mediated apoptotic pathway. There are several proteins involved in apoptosis, such as Bax and B cell lymphoma 2 (Bcl2) which are pro- and anti-apoptotic molecules, respectively (Einat et al. 2005). Regarding the circuitry of anxiety within the broad range of brain regions, apoptosis has been documented in neurons of hippocampus, amygdala, and prefrontal cortex (Karimi et al. 2014; Khalifeh et al. 2015).
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcription coactivator that plays as the main stimulator of mitochondrial respiration and turnover, and also interacts with a broad range of transcription factors, including Nuclear respiratory factor-1 (NRF-1), NRF-2, and mitochondrial transcription factor A (TFAM) (Aquilano et al. 2010; Taherzadeh-Fard et al. 2011). Under oxidative stress conditions, PGC-1α up-regulates many ROS-detoxifying proteins, including glutathione peroxidase, catalase, and superoxide dismutase (SOD) which are found within the mitochondria (St-Pierre et al. 2006). Supportingly, overexpression of PGC-1α in neurodegenerative models restored neuronal capacity for oxidative phosphorylation and significantly abrogated mitochondrial apoptosis induced by oxidative insult (Strobel et al. 2014; St-Pierre et al. 2006).
Nuclear factor, erythroid-derived 2,-like 1(Nrf1) and nuclear factor, erythroid-derived 2,-like 2 (Nrf2) are members of the NF-E2 basic-leucine zipper family of proteins and interact with the antioxidant response element (ARE) that is present in the promoter regions of genes for phase 2 detoxifying enzymes (Biswas et al. 2013; Li et al. 2011). Nrf1 and Nrf2 are mostly known by their induction through an antioxidant response (Nguyen et al. 2009). However, Nrf1 and Nrf2 have a different physiological activities, for example, in development and inflammation, they are involved in transcription of proteasome subunits and induction of autophagosome formation (Stepkowski and Kruszewski 2011; Kwak et al. 2003). It is worth to be noted that recent studies indicated that Nrf2 and NRF-2 activities are converged on mitochondrial biogenesis in association with PGC-1α (Athale et al. 2012; Piantadosi et al. 2008).
Recently, we demonstrated that in vivo Nrf2 knockdown by means of specific short interfering RNA molecule (siRNA) developed significant anxiety-like behavior in rats (Khalifeh et al. 2015). This was associated with mitochondrial dysfunction and neuronal apoptosis in hippocampus, amygdala, and prefrontal cortex. We attempted here to silence Nrf1 by intra-dorsal third ventricle (D3V) siRNA injection, in order to assess the effect of this silencing on anxiety and also on the apoptotic markers in the mentioned brain regions. We also detected mitochondrial biogenesis markers, PGC-1α, NRF1, TFAM, and cytochrome-c, along with the activity of ETC enzymes to investigate the role of mitochondria on the anxiety-like behavior.
Materials and Methods
Reagents
Antibodies directed against caspase-3, Bax, Bcl2, cytochrome-c, and β-actin were purchased from Cell Signaling Technology (Beverly, USA). Anti-Nrf1, Nrf2 and PGC-1α antibodies were obtained from ABCAM (Cambridge, UK). NRF-1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody against mitochondrial TFAM was bought from BioVision (Palo Alto, CA, USA). Electrochemiluminescence (ECL) kit was provided from Amersham Bioscience (Piscataway, USA). Polyvinylidene fluoride (PVDF) membrane was from Millipore (Billerica, MA, USA). All other reagents were obtained from Sigma Aldrich (St. Louis, USA).
Animals and Experimental Design
Male albino rats of Wistar strain were purchased from Pasteur Institute (Tehran, Iran), weighting 200–250 g and kept under constant environmental conditions and fed on a normal laboratory diet. Three groups were designed for this study: control group 1 which received intra-D3V injection of 5 µl scrambled siRNA; control group 2 which received 5 µl RNase-free water in their D3V (note that scrambled siRNA has no homology to any known mammalian gene, and since the results of these two control groups had no significant difference, only scrambled one (control group 1) has been shown as control group); and third group which received intra-D3V injection of 5 µl Nrf1-siRNA (5 nmol siRNA/200 µl RNAase-free water). Each experimental group mentioned above was divided into two groups, which were decapitated 4 and 8 h after siRNA injection. Time of decapitating and doses of injected siRNA were chosen based on a preliminary experiment carried out before the main study (data not shown). Data obtained from this preliminary study indicated that the reduction in the protein level of Nrf1 and Nrf2 started from 2 h, and it was significant 3 h after siRNA injection.
Stereotaxic Surgery and siRNA Administration in Rat Brain
Rats were given an intraperitoneal injection of Ketamine Hydrochloride (50 mg/kg) and Xylezine (4 mg/kg) to produce sufficient depth of anesthesia for surgical procedures in stereotaxic apparatus. In the next step, guide cannula was placed in their D3V (anteroposterior: −0.5 mm relative to bregma, mediolateral: 0 mm, and dorsoventral: −3 mm from the skull surface) (Paxinos and Franklin 2001). The injector cannula was inserted into the guide cannula, and siRNA was delivered while the rat was free to move around a holding cage. To knockdown Nrf1 in the rats’ brain, Silencer Select pre-designed siRNA specific to Nrf1: 5′-CAACCUGCCUGUAGAAGAAtt-3′ (ID: s165931) was obtained from Ambion (Austin, TX, USA) and scrambled siRNA as control was obtained from Qiagen (Germany): 5′-UUCUCCGAACGUGUCACGUdTdT-3′.
Behavioral Testing by Elevated Plus Maze (EPM)
EPM test has been established based on creating a conflict between the rat’s exploratory drive and its innate fear of opened and exposed areas. As described in detail by Zarrindast et al. (2008), the apparatus consisted of a plus-shaped platform elevated 50 cm from the floor. Two open arms (50-cm long, 10-cm wide, 0.5-cm high borders) and two closed arms (50-cm long, 10-cm wide, 40-cm high walls). The maze was placed in an isolated room without any sort of guidance cues, away from any external noises or movement. The experimenter was as far away as possible from the maze and did not make noise or movement also avoiding from wearing perfumes. The animals were brought into the experiment room, at least 60 min before testing to recover from the transfer stress. Rats were allowed to acclimatize for 7 days before any handling. Animals were handled with experimenter 6 days before surgery and also day 2 and 3 after surgery in the test room to minimize acclimatizing stress. It should be note that rats did not see EPM apparatus before the test day. Rats were placed onto the central area (10 × 10 cm) of the maze. Then, rats were picked up and returned to their home cages. Percentage of time spent in open arms [%OAT: (time in open arm/time in “open + closed” arm) × 100] and percentage of open arm entries [%OAE: (number of open arm entries/number of “open + closed” arm entries) × 100] during the 5-min exposure were considered as an index of anxiety. The total number of enclosed arm entries was also considering as a measure of locomotor activity. Three hours after injection, all groups were examined for anxiety-related behavior by EPM.
Western Blotting
Total protein extracts from hippocampus, prefrontal cortex, and amygdala were prepared by homogenizing tissues in protein extraction buffer containing protease inhibitor cocktail and then centrifuged at 4000 × g at 4 °C (Niimura et al. 2006). The protein content of the supernatant was determined according to the Bradford’s method using bovine serum albumin as a reference standard (Bradford 1976). Next, 60 μg of total protein of each sample was subjected to sodium dodecyl sulfate–polyacrylamide (SDS)-PAGE separation. Electroblotted proteins onto PVDF membranes were incubated with the primary antibodies and the relevant secondary antibody. Following extensive washing, immunoreactivity was visualized using the enhanced chemiluminescence method. The bands were analyzed by densitometric quantification using ImageJ software and normalized to the appropriate loading controls.
Spectrophotometric Analysis of ETC Complexes Activities
Complex I (NADH ubiquinone reductase): Mitochondria were prepared from the brain, immediately after killing the animals by decapitation, according to Clark and Nicklas (1970) method. Decylubiquinone was used as the electron acceptor for determination of complex I activity according to Birch-Machin et al. method (1989). The assay medium (KH2PO4 35 mM, MgC12 5 mM, KCN 2 mM, pH 7.2) was supplemented with decylubiquinone 65 mM and NADH 10 mM. The reaction was started by the addition of sample and the oxidation rate of NADH was recorded at 340 nm, spectrophotometrically.
Complexes II–III (succinate ubiquinone reductase-ubiquinol-cytochrome-c reductase): Samples were added to medium buffer containing Tris-SO4 (50 mM), EDTA (100 mM), succinate (2 mM), and dodecylmaltoside (1 %). The reaction was initiated with the addition of 100 mM cytochrome-c and the absorbance was measured at 550 nm wavelengths at 30 °C (Veereshwarayya et al. 2006).
Complex IV (cytochrome-c oxidase): Mitochondrial samples were added to reaction buffer composed of 40 mM potassium phosphate (pH 7). Cytochrome-c oxidase activity was determined by measuring the oxidation of cytochrome-c, spectrophotometrically at 550 nm (37 °C) as described by Rustin et al. (1991).
Data Analysis
Each experiment was repeated at least three times. Mean ± SEM (standard error of mean) was used in order to express the data, which was processed by Graph Pad Prism® 5.0. Enzymatic and Western blot data were analyzed by one-way analysis of variance (ANOVA) with Tukey post hoc tests where appropriate. The behavioral data were analyzed by Student’s t test for between-group comparisons. A P value less than 0.05 (P < 0.05) was considered as statistically significant.
Results
Nrf1 mRNA Knockdown Significantly Reduced Its Protein Level in the Brain
It has been shown that in vivo siRNA injection results in specific knockdown of mammalian genes (Chen et al. 2006). We evaluated the level of Nrf1 by Western blotting, 4 and 8 h after Nrf1-siRNA injection. As shown in Fig. 1, protein level of Nrf1 reduced in consequence of Nrf1-siRNA injection. The most reduction of Nrf1 level was detected in prefrontal cortex, 8 h after siRNA injection (35.5 % compared to the control group). Furthermore, the level of Nrf1 protein decreased by 18.3 % and 27.4 % in hippocampus and amygdala, respectively, compared to the control group (8 h after Nrf1-siRNA injection). Although, the level of Nrf1 protein notably decreased 4 h after siRNA injection in all three brain regions in comparison with control rats (16.4, 23.3, and 27.4 % in hippocampus, prefrontal cortex, and amygdala, respectively), but these reductions were more 8 h after siRNA injection.
Fig. 1.
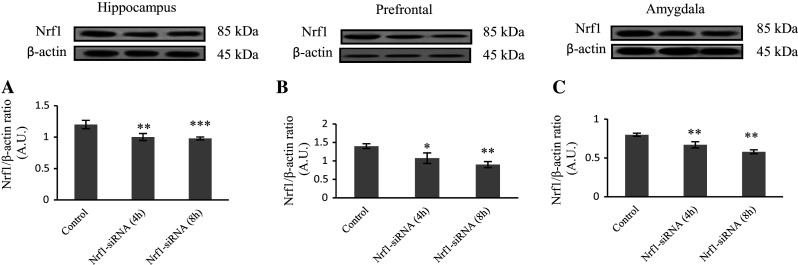
siRNA-mediated down regulation of Nrf1 in 3 areas of rat’s brain. Evaluation of protein level (Western blotting) after direct in vivo injection of scrambled siRNA (control) and Nrf1-siRNA in hippocampus (a), prefrontal (b), and amygdala (c). Sixty μg proteins were separated on SDS-PAGE, Western blotted, probed with anti-Nrf1 antibody, and reprobed with anti-β-actin antibody (one representative Western blot was shown). The densities of corresponding bands were measured and the ratio to β-actin bands was reported. Three rats were used for each 4 and 8 h groups, and experiment was repeated 3 times independently. *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
Nrf1 Silencing Did Not Affect Anxiety-Related Behavior
Knockdown of targeted genes is one of the best strategies in animals to increase our understanding about the molecular basis of anxiety-related behavior. One of the most commonly tests for measuring anxiety-related behavior is EPM. In the present study, anxiety-related behavior of Nrf1-knockdown rats was evaluated 3 h after siRNAs injection. Data relating to anxiety-related behavior are provided in Fig. 2. There was only a slight difference in OAE, OAT, and enclosed arm entries between Nrf1-siRNA-injected group and control rats which were not significant (Fig. 2a–c). Figure 2d–f shows time spend in open arm, closed arm, and in the central area of the EPM apparatus, respectively. There were also no significant differences in these parameters between Nrf1-injected rats in comparison with control group (Fig. 2d–f).
Fig. 2.
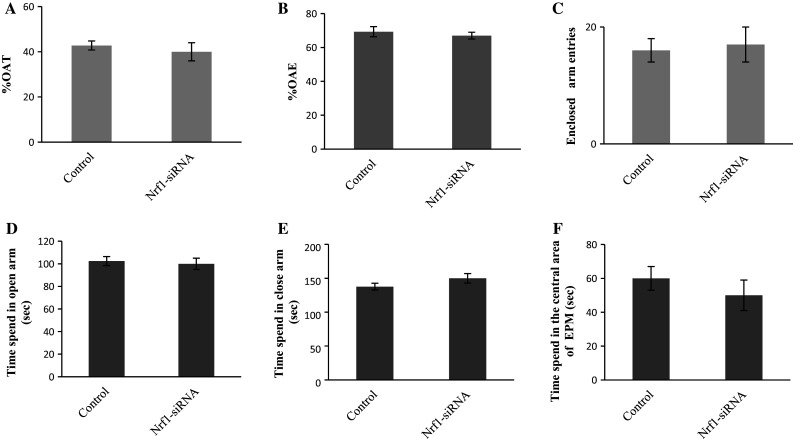
Effect of Nrf1-siRNA administration on rat anxiety-like behavior in the EPM. The animals received scrambled siRNA or Nrf1-siRNA into D3V. Mean ± SEM of a percentage of open arm time, b percentage of open arm entries, c enclosed arm enteries, d time spend in the open arm, e time spend in the close arm, and f time spend in the central area of EPM (n = 8 in each group)
Nrf1-siRNA Injection Did Not Change Apoptotic Factors Level Efficiently
Programmed cell death (Apoptosis) is mediated by some intracellular proteins including Bax, which increase mitochondrial membrane permeability and induce caspases cleavage (Elmore 2007). Activation of caspase-3 is the irreversible step in apoptosis (Desagher and Martinou 2000; Irony-Tur-Sinai et al. 2009). In this study, the ratio of Bax/Bcl2 level was evaluated 4 and 8 h after Nrf1-siRNA injection. As shown in Fig. 3, Bax/Bcl-2 ratio increased significantly 4 and 8 h after Nrf1-siRNA injection in all three regions (hippocampus, prefrontal cortex, and amygdala) in comparison with the control group (P < 0.001), while cleavage of caspase-3 did not change markedly in Nrf1-siRNA-injected group compared to the control group. In hippocampus, 8 h after Nrf1-siRNA injection, caspase-3 cleavage was quite a bit more, compared to the control group, but it was not statistically significant. Altogether, it seems that intraventricular injection of Nrf1-siRNA did not induce apoptosis in mentioned brain regions of rats.
Fig. 3.
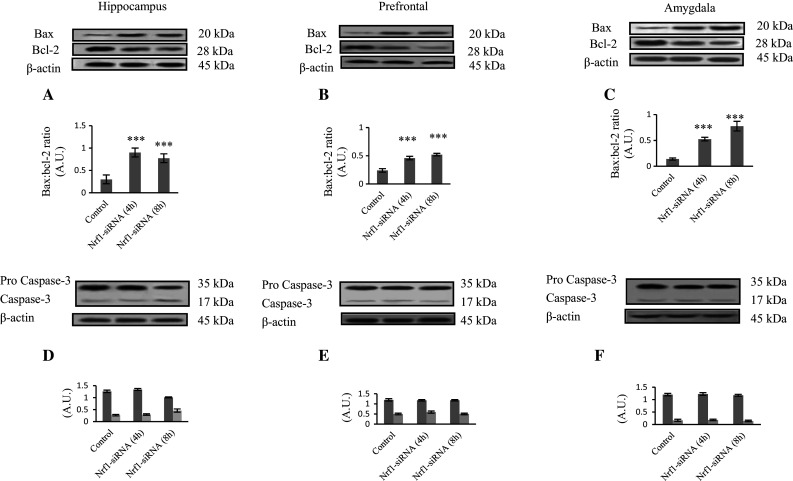
Bax/Bcl2 ratio and caspase-3 cleavage in hippocampus, amygdala, and prefrontal cortex after siRNA injection are shown by Western blotting. Sixty μg proteins were separated on SDS-PAGE, Western blotted, probed with anti-Bax, -Bcl2 and -caspase-3 antibodies, and reprobed with anti-β-actin antibody. The ratio to the β-actin bands was reported. Each point shows the mean ± SEM. ***P < 0.001 compared to the control group
Nrf1 Silencing Led to the Increase in Nrf2 Protein Level
Nrf2 and Nrf1 have some overlapping functions especially in regulating antioxidant system (Leung et al. 2003). Accordingly, we tested the possibility that if Nrf2 increase is compensatory consequence of Nrf1 knockdown. As shown in Fig. 4, our data revealed considerable increase in Nrf2 protein level especially in prefrontal cortex of about 1.3- and 1.8-fold, respectively, 4 and 8 h after Nrf1-siRNA injection. However, Nrf2 level showed slight increase in hippocampus and amygdala in comparison with the control group (P < 0.05).
Fig. 4.
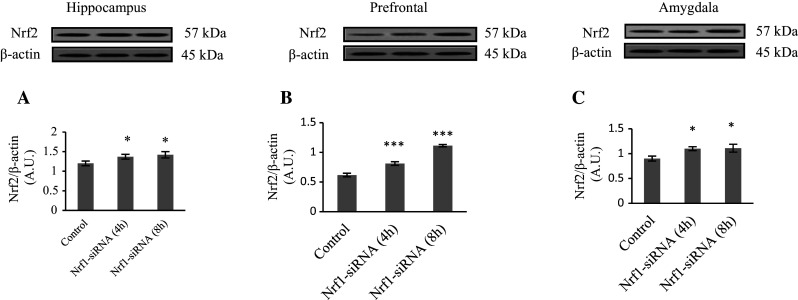
Western blot analysis of Nrf2/β-actin ratio in hippocampus, amygdala, and prefrontal cortex after Nrf1-siRNA injection. Method was as described in Fig. 1 legend. Each point shows the mean ± SEM. *P < 0.05 and ***P < 0.001 compared to the control group
Nrf1 Silencing Changed the Level of Proteins Involved in Mitochondrial Biogenesis
Mitochondrial biogenesis results from organized and precise activity of nuclear and mitochondrial genomes (Shao et al. 2010). In the present study, the level of some important factors involved in mitochondrial biogenesis has been evaluated after Nrf1-siRNA injection. As shown in Fig. 5, Nrf1-siRNA injection increased PGC-1α and cytochrome-c level compared to the control group (Fig. 5a–c, j–l). The most increase in PGC-1α level (2.3-fold compared to the control group) was in amygdala, 8 h after Nrf1-siRNA injection. Additionally, 8 h after Nrf1-siRNA injection, the level of PGC-1α increased 1.3- and two-fold in hippocampus and prefrontal cortex, respectively, relative to control rats. Moreover, 4 h after siRNAs injection, the level of PGC-1α increased as a result of Nrf1-knockdown (1.2-, 1.6-, and 1.7-fold in hippocampus, prefrontal cortex, and amygdala, respectively) compared to control rats.
Fig. 5.
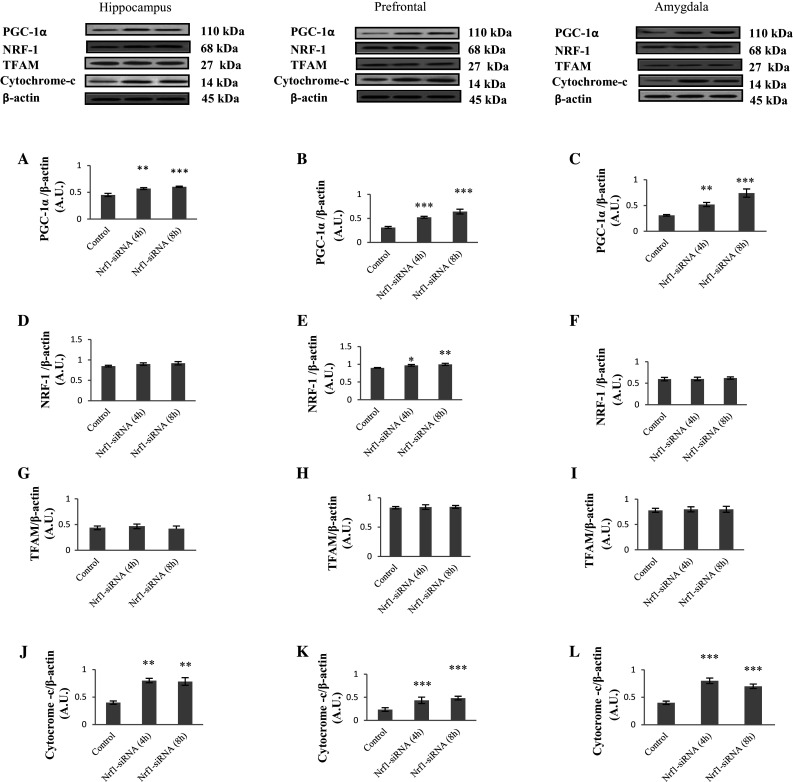
PGC-1α, cytochrome-c, NRF-1, and TFAM level in hippocampus, amygdala, and prefrontal after siRNA injection are shown by Western blotting. Method was as described in Fig. 1 legend. Each point shows the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group
The level of cytochrome-c increased in all three studied regions about two-fold, 4 h after Nrf1-siRNA injection. Also, 8 h after Nrf1-siRNA injection the level of cytochrome-c increased 1.8-, 1.9-, and 1.7-fold in hippocampus, prefrontal cortex and amygdala, respectively, compared to the control rats.
The pattern of changes for NRF-1 protein level is shown in Fig. 5d–f. The highest increase in NRF-1 protein level was 8 h after Nrf1-siRNA injection in prefrontal cortex (about 1.1-fold). Furthermore, TFAM level in siRNA-injected group did not show any marked changes compared to control rats in the studied regions (Fig. 5g–i).
Nrf1 Silencing Modulated Mitochondrial Respiratory Complexes’ Activity
Mitochondrial ETC is the most energy producer in the cell which consists of multi-subunit enzyme complexes (Wallace et al. 1998; Rhein et al. 2009). Tables 1, 2, and 3 illustrate the effect of Nrf1-siRNA injection on mitochondrial ETC’s enzymes activity. As shown in Table 1, complex I activity did not show any significant changes in all studied regions. Complexes II–III activity did not change significantly in prefrontal cortex and amygdala (Tables 1 and 2), but accumulated activity of complexes II–III in hippocampus increased to 32.13 and 33.76 nmol/min/mg protein, 4 and 8 h after Nrf1-siRNA knockdown, respectively. Otherwise, activity of complex IV in all three regions increased significantly. In the hippocampus, activity of complex IV reached to 8.97 and 16.61 nmol/min/mg protein, in consequence of Nrf1 knockdown, respectively, 4 and 8 h after Nrf1-siRNA injection as well as 16.61 and 35.05 nmol/min/mg protein in prefrontal cortex compared to control rats. Furthermore, in amygdala, activity of complex IV increased to 14.3 and 16.02 nmol/min/mg protein, 4 and 8 h, respectively, in Nrf1-siRNA-injected group compared to control rats (Tables 1, 2 and 3).
Table 1.
Nrf1 silencing effect on mitochondrial complex enzymes activity in hippocampus
| Group | Complex I (nmol/min/mg protein) | Complex II/III (nmol/min/mg protein) | Complex IV (nmol/min/mg protein) |
|---|---|---|---|
| Control | 57.35 ± 2.6 | 25.18 ± 2 | 4.13 ± 0.2 |
| Nrf1-siRNA, (4 h) | 58.05 ± 3.9 | 32.13 ± 1* | 8.97 ± 0.3** |
| Nrf1-siRNA, (8 h) | 58.32 ± 3.9 | 33.76 ± 2** | 16.61 ± 0.1*** |
n = 4
* P < 0.05; ** P < 0.01; *** P < 0.001
Table 2.
Nrf1 silencing effect on prefrontal cortex mitochondrial complex enzymes activity
| Group | Complex I (nmol/min/mg protein) | Complex II/III (nmol/min/mg protein) | Complex IV (nmol/min/mg protein) |
|---|---|---|---|
| Control | 79.18 ± 4.47 | 37.01 ± 3 | 10.02 ± 0.3 |
| Nrf1-siRNA, (4 h) | 80.57 ± 6.3 | 36.52 ± 2 | 16.61 ± 0.2*** |
| Nrf1-siRNA, (8 h) | 82.33 ± 3.9 | 45.69 ± 2 | 35.05 ± 0.2*** |
n = 4
* P < 0.05; ** P < 0.01; *** P < 0.001
Table 3.
Nrf1 silencing effect on amygdala mitochondrial complex enzymes activity
| Group | Complex I (nmol/min/mg protein) | Complex II/III (nmol/min/mg protein) | Complex IV (nmol/min/mg protein) |
|---|---|---|---|
| Control | 102.6 ± 6.2 | 36.09 ± 2 | 13.6 ± 0.2 |
| Nrf1-siRNA, (4 h) | 102.6 ± 5.2 | 35.95 ± 3 | 14.3 ± 0.1*** |
| Nrf1-siRNA, (8 h) | 107.11 ± 4.35 | 37.56 ± 1 | 16.02 ± 0.3*** |
n = 4
* P < 0.05; ** P < 0.01; *** P < 0.001
Discussion
Anxious state is characterized by intense, excessive, and persistent harm avoidance behavior such as irritability and difficulty in relaxing (Pauli et al. 1997; Zarrindast et al. 2012). In terms of epidemiology, one-eighth of the total population of the world suffers from anxiety with a 28 % of lifetime prevalence (Bouayed et al. 2009). Anxiety-like behavior is illustrated by EPM which is probably the most widely used model of animal anxiety state testing. Rats naturally prefer to stay in the enclosed arms of EPM. Therefore, drugs that elicit an increase in the time spent in the open arms are considered to be anxiolytic. Low amount of exploration in the closed arm of EPM reflects the anxiogenic effect of drug. This study indicated that intraventricular administration of siNrf1 did not develop anxiety in male Wistar rats by evaluating the previously explained circuity of anxiety in the brain. There were no significant differences in OAE, OAT, and enclosed arm entries between Nrf1-siRNA-injected group and control rats. Current data are summarized by elevated Nrf2 level following Nrf1 knockdown in hippocampus, amygdala, and prefrontal cortex. This was accompanied with increased PGC-1α, Bax/Bcl2 ratio, cytochrome -c protein levels, and the activity of mitochondrial complex IV. There was no significant alteration in intracellular level of caspase-3 and downstreams of PGC-1α. Notably, statistical significance of data from prefrontal cortex was more profound in some fields.
Recently, we implicated that Nrf2 silencing resulted in overt mitochondrial dysfunction and significant apoptosis leading to anxiety-like behavior by influencing anxiety neuronal circuitry (Khalifeh et al. 2015). Intriguingly, here we report that Nrf1 silencing neither affected anxious state in Wistar rats nor altered the apoptosis in the mentioned circuitry. It has been demonstrated that Nrf1 and Nrf2 have extensive convergence in transactivation of their downstreams with a little difference in activity. Totally, Nrf2 knockout has been shown to significantly blunt ARE-regulated genes compared to Nrf1; however, there may have been preferentiality in some genes to be activated by Nrf1. Our data suggest that compensatory upregulation of Nrf2 was followed by injection of intra-D3V siNrf1. In the searching for possible interaction between Nrf1 and Nrf2, Kwak et al. (2002) have reported that there are ARE-like sequences in the promoter of Nrf2, suggesting its activation by Nrf1. This seems paradoxical by our results; however, this is consistent with the activity of a p65 isoform of Nrf1, which has been shown to interact and suppress Nrf2-mediated activation of ARE-dependent reporter genes in cells (Wang et al. 2007). Moreover, due to basal expression of antioxidant genes by Nrf1 (Nguyen et al. 2003), its knockdown should increase intracellular oxidative state that otherwise robustly activated Nrf2. It should be noted that prefrontal cortex showed activation of Nrf2, more significantly compared to amygdala and hippocampus. This may highlight that prefrontal cortex receives dense projections from basolateral amygdala and hippocampus and is composed of widely distributed outputs to multiple levels of the anxiety circuit (Coplan and Lydiard 1998; Ghashghaei et al. 2007).
PGC-1α is characterized as the master regulator of mitochondrial biogenesis and ETC complexes along with a wide variety of biological responses, such as cell death and cellular adaptation under conditions of stress and mitochondrial dysfunction (Cui et al. 2006; Rasbach and Schnellmann 2007). In this regard, several aspects of mitochondrial dysfunction in the anxiety have been discussed by Filiou et al. (2011) and the contribution of oxidative stress with or without mitochondrial dysfunction has been widely reported. In addition to mitochondrial biogenesis, several genes that mediate the defense mechanism against oxidative stress, such as SOD, are downstream targets of PGC1-α (Chen et al. 2011). The data suggest that PGC-1α and cytochrome-c significantly upregulated following Nrf1 silencing, although downstream transcription factors, NRF-1 and TFAM, remained unchanged. To further explain the activation of PGC-1α by Nrf2, Adamovich et al. (2013) evidenced that PGC-1α is subjected to be protected by NAD(P)H dehydrogenase [quinone] 1 (NQO1), one of the Nrf2-dependent downstreams (Adamovich et al. 2013). NQO1 binds to PGC-1α, which is mediated by NADH, and prevents its proteasomal degradation (Adamovich et al. 2013). In another point of view, Nrf1 plays a significant role in the proper expression of proteasome subunits, indicating that Nrf1 silencing temporarily inhibited intracellular proteasomal activity (Digaleh et al. 2013; Filiou et al. 2011). Having NRF-1 and TFAM in the middle of (PGC-1α)-(NRF-1)-TFAM-(cytochrome-c) axis, further complicates the paradoxical upregulation of PGC-1α and cytochrome-c, without alteration in NRF-1 and TFAM levels. In a study by Herzig et al. (2000) it has been indicated that cytochrome-c promoter activity can be induced in a NRF-1 independent manner. This was accompanied by a rapid and transient phosphorylation of CREB and transactivation of cytochrome-c promoter by its functional CREB sites, before a delayed upregulation of NRF-1(Herzig et al. 2000). Interestingly, in our experimental setting, prefrontal cortex was the only site that showed a significant increase of NRF-1 level, both 4 and 8 h of siNrf1 post-injection. This may reflect the high intensity of stress in prefrontal cortex and/or its more sensitivity to Nrf1 depletion.
Mitochondrial pathway of apoptosis consist of pro- and anti-apoptotic factors that eventually ends in the activation of caspase-3, explained as the point of no return in apoptosis (Cheng et al. 1997). As a part of complex apoptosis pathway, Bcl2 inhibition along with Bax activation leads to increase in mitochondrial membrane permeability that let cytochrome-c translocation to the cytoplasm and formation of apoptosome (Cheng et al. 1997). Subsequently, procaspase-3 is cleaved by apoptosome and began an almost irreversible cascade to the end of apoptosis (Cheng et al. 1997; Rosse et al. 1998). The data indicated significant increase in Bax/Bcl2 ratio with the upregulation of cytochrome-c, although we did not observe significant procaspase-3 cleavage. In fact, one of the triggers of cytochrome-c translocation is elevated intracellular ROS and oxidative stress that seems to be prevented by simultaneous activation of antioxidant expression via compensatory upregulation of PGC-1α and Nrf2. Besides, ETC complexes are the main source of intracellular ROS and their inhibition in oxidative stress is a known tenet in neurodegenerative disorders (Parker et al. 1994). Mitochondrial complexes I and II have been recognized as the most participant in ROS production, whose activities were not altered in our study following Nrf1 knockdown (Garcia-Ruiz et al. 1997). This may be because of unaltered NRF-1 level and also increased NQO1 expression by Nrf2, which depleted NADH resources, considered as the main substrate of complex I. Conflicting increase in complex IV activity, supported by a study of Welchen and colleagues indicating that complex IV activity is independent of other ETC complexes and is also affected by cytochrome-c level (Welchen et al. 2012).
Overall, this study highlighted complex interaction of compensatory mechanisms in Nrf1 depletion and partly emphasized that anxiety pathophysiological manifestation is mostly beyond simplified Bcl2 or apoptosis point of view. Still, future experiments need to be devoted to study in detail how Nrf1, Nrf2, and PGC-1α interact each other and how mitochondrial dysfunction is connected to major antioxidant response transcription factors.
Acknowledgments
This work was supported financially by the Iran National Science Foundation (INSF, grant number 91003013).
Compliance with Ethical Standards
Conflict of interest
All authors have no conflict of interest.
Animal Rights and Informed Consent
All procedures involving the care of animals were performed according to the guidelines for animal experimentation issued by the National Institutes of Health Publication (No. 80-23, revised 1996) and the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences.
References
- Adamovich Y, Shlomai A, Tsvetkov P, Umansky KB, Reuven N, Estall JL, Spiegelman BM, Shaul Y (2013) The protein level of PGC-1alpha, a key metabolic regulator, is controlled by NADH-NQO1. Mol Cell Biol 33:2603–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR (2010) Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem 285:21590–21599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athale J, Ulrich A, MacGarvey NC, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA (2012) Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med 53:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin MA, Shepherd IM, Watmough NJ, Sherratt HS, Bartlett K, Darley-Usmar VM, Milligan DW, Welch RJ, Aynsley-Green A, Turnbull DM (1989) Fatal lactic acidosis in infancy with a defect of complex III of the respiratory chain. Pediatr Res 25:553–559 [DOI] [PubMed] [Google Scholar]
- Biswas M, Kwong EK, Park E, Nagra P, Chan JY (2013) Glycogen synthase kinase 3 regulates expression of nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) and inhibits pro-survival function of Nrf1. Exp Cell Res 319:1922–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouayed J, Rammal H, Soulimani R (2009) Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev 2:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR (2004) Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun 313:635–641 [DOI] [PubMed] [Google Scholar]
- Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW (2006) REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci 24:2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC (2011) Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278:1966–1968 [DOI] [PubMed] [Google Scholar]
- Chevallier N, Keller E, Maurice T (2011) Behavioural phenotyping of knockout mice for the sigma-1 (sigma(1)) chaperone protein revealed gender-related anxiety, depressive-like and memory alterations. J Psychopharmacol 25:960–975 [DOI] [PubMed] [Google Scholar]
- Clark JB, Nicklas WJ (1970) The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem 245:4724–4731 [PubMed] [Google Scholar]
- Coplan JD, Lydiard RB (1998) Brain circuits in panic disorder. Biol Psychiatry 44:1264–1276 [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D (2006) Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69 [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377 [DOI] [PubMed] [Google Scholar]
- Digaleh H, Kiaei M, Khodagholi F (2013) Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci 70:4681–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ (2007) Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond) 113:219–232 [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Manji HK (2005) Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res 165:172–180 [DOI] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiou MD, Zhang Y, Teplytska L, Reckow S, Gormanns P, Maccarrone G, Frank E, Kessler MS, Hambsch B, Nussbaumer M, Bunck M, Ludwig T, Yassouridis A, Holsboer F, Landgraf R, Turck CW (2011) Proteomics and metabolomics analysis of a trait anxiety mouse model reveals divergent mitochondrial pathways. Biol Psychiatry 70:1074–1082 [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem 272:11369–11377 [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig RP, Scacco S, Scarpulla RC (2000) Sequential serum-dependent activation of CREB and NRF-1 leads to enhanced mitochondrial respiration through the induction of cytochrome c. J Biol Chem 275:13134–13141 [DOI] [PubMed] [Google Scholar]
- Huang GB, Zhao T, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC (2013) Effects of chronic social defeat stress on behaviour, endoplasmic reticulum proteins and choline acetyltransferase in adolescent mice. Int J Neuropsychopharmacol 16:1635–1647 [DOI] [PubMed] [Google Scholar]
- Huang W, Yang X, Yao S, LwinOo T, He H, Wang A, Li C, He L (2014) Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol Biochem 82:76–84 [DOI] [PubMed] [Google Scholar]
- Irony-Tur-Sinai M, Lichtenstein M, Brenner T, Lorberboum-Galski H (2009) IL2-caspase3 chimeric protein controls lymphocyte reactivity by targeted apoptosis, leading to amelioration of experimental autoimmune encephalomyelitis. Int Immunopharmacol 9:1236–1243 [DOI] [PubMed] [Google Scholar]
- Karimi S, Jahanshahi M, Golalipour MJ (2014) The effect of MDMA-induced anxiety on neuronal apoptosis in adult male rats’ hippocampus. Folia Biol (Praha) 60:187–191 [DOI] [PubMed] [Google Scholar]
- Khalifeh S, Oryan S, Digaleh H, Shaerzadeh F, Khodagholi F, Maghsoudi N, Zarrindast MR (2015) Involvement of Nrf2 in development of anxiety-like behavior by linking Bcl2 to oxidative phosphorylation: estimation in rat hippocampus, amygdala, and prefrontal cortex. J Mol Neurosci 55:492–499 [DOI] [PubMed] [Google Scholar]
- Kumar A, Vashist A, Kumar P, Kalonia H, Mishra J (2012) Protective effect of HMG CoA reductase inhibitors against running wheel activity induced fatigue, anxiety like behavior, oxidative stress and mitochondrial dysfunction in mice. Pharmacol Rep 64:1326–1336 [DOI] [PubMed] [Google Scholar]
- Kwak MK, Itoh K, Yamamoto M, Kensler TW (2002) Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22:2883–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW (2003) Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23:8786–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY (2003) Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278:48021–48029 [DOI] [PubMed] [Google Scholar]
- Li M, Zhang X, Cui L, Yang R, Wang L, Liu L, Du W (2011) The neuroprotection of oxymatrine in cerebral ischemia/reperfusion is related to nuclear factor erythroid 2-related factor 2 (nrf2)-mediated antioxidant response: role of nrf2 and hemeoxygenase-1 expression. Biol Pharm Bull 34:595–601 [DOI] [PubMed] [Google Scholar]
- Li P, Zhao QL, Wu LH, Jawaid P, Jiao YF, Kadowaki M, Kondo T (2014) Isofraxidin, a potent reactive oxygen species (ROS) scavenger, protects human leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in p53-independent manner. Apoptosis 19:1043–1053 [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM (2004) Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci 118:63–78 [DOI] [PubMed] [Google Scholar]
- Morales P, Simola N, Bustamante D, Lisboa F, Fiedler J, Gebicke-Haerter PJ, Morelli M, Tasker RA, Herrera-Marschitz M (2010) Nicotinamide prevents the long-term effects of perinatal asphyxia on apoptosis, non-spatial working memory and anxiety in rats. Exp Brain Res 202:1–14 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura M, Takagi N, Takagi K, Mizutani R, Ishihara N, Matsumoto K, Funakoshi H, Nakamura T, Takeo S (2006) Prevention of apoptosis-inducing factor translocation is a possible mechanism for protective effects of hepatocyte growth factor against neuronal cell death in the hippocampus after transient forebrain ischemia. J Cereb Blood Flow Metab 26:1354–1365 [DOI] [PubMed] [Google Scholar]
- Olsen RH, Johnson LA, Zuloaga DG, Limoli CL, Raber J (2013) Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J Neurochem 2:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker WD Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK (1994) Electron transport chain defects in Alzheimer’s disease brain. Neurology 44:1090–1096 [DOI] [PubMed] [Google Scholar]
- Pauli P, Dengler W, Wiedemann G, Montoya P, Flor H, Birbaumer N, Buchkremer G (1997) Behavioral and neurophysiological evidence for altered processing of anxiety-related words in panic disorder. J Abnorm Psychol 106:213–220 [DOI] [PubMed] [Google Scholar]
- Paxinos GF, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. Academic, London [Google Scholar]
- Piantadosi CA, Carraway MS, Babiker A, Suliman HB (2008) Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG (2007) PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355:734–739 [DOI] [PubMed] [Google Scholar]
- Rhein V, Baysang G, Rao S, Meier F, Bonert A, Muller-Spahn F, Eckert A (2009) Amyloid-beta leads to impaired cellular respiration, energy production and mitochondrial electron chain complex activities in human neuroblastoma cells. Cell Mol Neurobiol 29:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C (1998) Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391:496–499 [DOI] [PubMed] [Google Scholar]
- Rustin P, Chretien D, Bourgeron T, Wucher A, Saudubray JM, Rotig A, Munnich A (1991) Assessment of the mitochondrial respiratory chain. Lancet 338:60 [DOI] [PubMed] [Google Scholar]
- Shao D, Liu Y, Liu X, Zhu L, Cui Y, Cui A, Qiao A, Kong X, Chen Q, Gupta N, Fang F, Chang Y (2010) PGC-1 beta-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR alpha. Mitochondrion 10:516–527 [DOI] [PubMed] [Google Scholar]
- Smith TM, Suckow RF (1985) Trazodone and m-chlorophenylpiperazine. Concentration in brain and receptor activity in regions in the brain associated with anxiety. Neuropharmacology 24:1067–1071 [DOI] [PubMed] [Google Scholar]
- Stepkowski TM, Kruszewski MK (2011) Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis. Free Radic Biol Med 50:1186–1195 [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408 [DOI] [PubMed] [Google Scholar]
- Strobel NA, Matsumoto A, Peake JM, Marsh SA, Peternelj TT, Briskey D, Fassett RG, Coombes JS, Wadley GD (2014) Altering the redox state of skeletal muscle by glutathione depletion increases the exercise-activation of PGC-1alpha. Physiol Rep 2:e12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Jia N, Guan L, Su Q, Wang D, Li H, Zhu Z (2013) Involvement of NR1, NR2A different expression in brain regions in anxiety-like behavior of prenatally stressed offspring. Behav Brain Res 257:1–7 [DOI] [PubMed] [Google Scholar]
- Taherzadeh-Fard E, Saft C, Akkad DA, Wieczorek S, Haghikia A, Chan A, Epplen JT, Arning L (2011) PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol Neurodegener 6:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW (2006) Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem 281:29468–29478 [DOI] [PubMed] [Google Scholar]
- Virok DP, Kis Z, Szegedi V, Juhasz G, Zvara A Jr, Muller G, Levay G, Harsing LG, Rajko R, Penke B, Janka Z, Janaky T, Puskas LG (2011) Functional changes in transcriptomes of the prefrontal cortex and hippocampus in a mouse model of anxiety. Pharmacol Rep 63:348–361 [DOI] [PubMed] [Google Scholar]
- Wallace DC, Brown MD, Melov S, Graham B, Lott M (1998) Mitochondrial biology, degenerative diseases and aging. BioFactors 7:187–190 [DOI] [PubMed] [Google Scholar]
- Wang W, Kwok AM, Chan JY (2007) The p65 isoform of Nrf1 is a dominant negative inhibitor of ARE-mediated transcription. J Biol Chem 282:24670–24678 [DOI] [PubMed] [Google Scholar]
- Welchen E, Hildebrandt TM, Lewejohann D, Gonzalez DH, Braun HP (2012) Lack of cytochrome c in Arabidopsis decreases stability of Complex IV and modifies redox metabolism without affecting Complexes I and III. Biochim Biophys Acta 1817:990–1001 [DOI] [PubMed] [Google Scholar]
- Wu LJ, Kim SS, Zhuo M (2008) Molecular targets of anxiety: from membrane to nucleus. Neurochem Res 33:1925–1932 [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A (2008) Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behav Pharmacol 19:716–723 [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khalifeh S, Rezayof A, Rostami P, Aghamohammadi Sereshki A, Zahmatkesh M (2012) Involvement of rat dopaminergic system of nucleus accumbens in nicotine-induced anxiogenic-like behaviors. Brain Res 1460:25–32 [DOI] [PubMed] [Google Scholar]


