Abstract
The role of host–microbe interactions in the pathobiology of oral mucositis is still unclear; therefore, this study aimed to unravel the effect of irradiation on behavioral characteristics of oral microbial species in the context of mucositis. Using various experimental in vitro setups, the effects of irradiation on growth and biofilm formation of two Candida spp., Streptococcus salivarius and Klebsiella oxytoca in different culture conditions were evaluated. Irradiation did not affect growth of planktonic cells, but reduced the number of K. oxytoca cells in newly formed biofilms cultured in static conditions. Biofilm formation of K. oxytoca and Candida glabrata was affected by irradiation and depended on the culturing conditions. In the presence of mucins, these effects were lost, indicating the protective nature of mucins. Furthermore, the Galleria melonella model was used to study effects on microbial virulence. Irradiated K. oxytoca microbes were more virulent in G. melonella larvae compared to the nonirradiated ones. Our data indicate that low-dose irradiation can have an impact on functional characteristics of microbial species. Screening for pathogens like K. oxytoca in the context of mucosits could be useful to allow early detection and immediate intervention.
Keywords: Irradiation, mucositis, oral microbiota, biofilm, virulence, Galleria melonella
Introduction
Alimentary mucositis is a severe side effect of both chemo- and radiotherapy and causes a range of symptoms throughout the gastrointestinal tract, including ulcers in the oral cavity, nausea, vomiting, diarrhea, and constipation. Accelerated fractionated schedules and concurrent chemotherapy have increased the incidence and severity of mucositis. Apart from being treatment limiting, the impacts on patient quality-of-life and the economic costs of supportive therapies are of significant concern.1–3 Limited therapeutic effects have been described for a variety of agents including radioprotectors, antibiotics, low-level laser therapy, and growth factors, but the evidence gathered to date does not support the use of any of these agents as a standard treatment. Therefore, there is an urgent need for original strategies to prevent and/or treat oral mucositis. This would not only reduce the suffering of the patients involved but would also dramatically reduce hospital and management costs associated with this condition. Although reports regarding the oral microbiota as a risk factor are scarce, prescription of alkaline mouthwashes is standard practice in the prevention of overgrowth by pathogens such as Candida albicans or Gram-negative bacilli.
Recently, we extensively reviewed the scientific literature on the link between oral mucositis and the endogenous microbiota.4 Overall, there is convincing data to conclude that irradiation changes the oral core microbiome over time5,6 with Gram-negative species becoming more abundant7–9 together with Candida spp.8,10–12 and Lactobacillus spp.9,10,12 The number and proportion of Lactobacillus spp. were found to be increased in swabs of tongue, buccal mucosa, vestibulum, supragingival plaque and subgingival region, and saliva after radiotherapy subjects, while data on Streptococcus spp. were inconclusive.9,10,12 C. albicans fungi seemed to be the most significant oral cavity pathogen in radiotherapy-induced mucositis patients, whereas Gram negatives such as Pseudomonas aeruginosa and Klebsiella pneumoniae were observed as main pathogens isolated from the blood of radiotherapy-treated patients.7
Radiation therapy uses high-energy irradiation to shrink tumors and kill cancer cells. X-rays, gamma rays, and charged particles are types of irradiation used for cancer treatment. In this study, we will mainly focus on the effect of gamma rays on microbial behavior. Gamma rays are released during isotopic negative beta decay that involves the conversion of a neutron into a proton, which releases an electron and an antineutrino.13 Gamma irradiation causes damage to nucleic acids and (phospho)lipids of the microbiota, whereas proteins, carbohydrates, and fats in food are not significantly affected by doses up to 10 kGy.14 It has further been shown that irradiation significantly changes the expression of heat shock proteins, the 50 S ribosomal protein, the transcriptional regulator (CtsR), and the enzyme formate acetyltransferase at doses >1 kGy.15,16 Surprisingly, effects of low-dose gamma irradiation on the viability, metabolic activity, and other functional properties of microbiota are hardly investigated. Only a few studies show that doses less than kilogray might indeed induce some effect. Recently, a study by Parikka et al.17 showed that two doses of 25 Gy with a one month’s interval were sufficient to reactivate a latent Mycobacterium marinum infection in adult zebrafish. In the reactivation group, a steep drop in survival after 16 days concomitant with high bacterial loads was seen and histological analysis of the moribund zebrafish revealed vast areas of free microbiota outside granulomas. Although in the zebra fish model the dampened immune suppression of the host is likely to be the trigger for reactivation, gamma irradiation is likely to have direct effects on Mycobacterium as in vitro studies with doses above 1 Gy have previously shown that the viability of M. tuberculosis is adversely affected.18
It is well known that gamma irradiation causes oxidative DNA damage and triggers oxidative stress responses, and compromised DNA repair mechanisms can lead to increased risk of carcinogenesis. It is currently unknown if or to what extent microbiota play a role in regulating radiosensitivity. A recent study by Maier et al.19 showed that the composition of the intestinal microbiome has an impact on the DNA repair response following irradiation. Mice with a conventional intestinal microbiota exhibited lower levels of acute chromosomal DNA lesions and γ-H2AX phosphorylation than mice with a restricted composition after total body irradiation (1 Gy). On the other hand, Crawford and Gordon20 showed that germ-free mice seem to be markedly resistant to lethal irradiation enteritis. Although limited, these studies indeed suggest a potential role for the microbiota in the host’s response to irradiation.
In this study, we wanted to determine whether radiation therapy might result in microbial dysfunction thereby providing a risk factor for developing or exacerbating oral mucositis. Therefore, the aim of this study was to unravel the effects of low-dose gamma irradiation on the functional behavior of a panel of oral species, namely C. albicans, Candida glabrata, Klebsiella oxytoca, and Streptococcus salivarius. C. albicans is found primarily in the intestines, colon, and the oral cavity and although a commensal, it can become pathogenic if a person’s immunity is lowered or if there is a dysbiosis.21 C. glabrata is considered a relatively harmless commensal of mucosal tissues, however, with the increased use of immunosuppressive agents, mucosal and systemic infections caused by C. glabrata have increased significantly.22 K. oxytoca is like other Klebsiella spp. an opportunistic pathogen and although it tends to colonize along the mucosal membranes, it can be found in all parts of the body. Infections often occur in patients with immunodeficient diseases and those being treated with antibiotics.23 S. salivarius is the principal commensal of the oral cavity in humans, the pioneer in colonizing dental plaque and infrequently pathogenic.24 To our knowledge, this type of study has not been performed so far, but would provide information on specific microbiota as risk factors for developing or progressive oral mucositis following radiation therapy.
Materials and methods
Cells, chemicals, and irradiation
C. albicans (ATCC 10231), C. glabrata (ATCC 2001), K. oxytoca (ATCC 49131), and S. salivarius (ATCC 7073) were obtained from the New Zealand Reference Culture Collection (ESR, Porirua, New Zealand) and grown on horse blood agar plates (Fort Richard Laboratories, Auckland, New Zealand). For growth studies, cells were cultured in Bacto Todd-Hewitt broth (Candida species) (Fort Richard Laboratories) supplemented with 0.5% yeast extract (THY), Bacto Brain Heart Infusion (BHI) broth (K. oxytoca and S. salivarius), Dulbecco’s modified Eagle medium (DMEM) (Life Technologies, Auckland, New Zealand) or DMEM supplemented with 5% (w/v) mucins from porcine stomach type III (Sigma-Aldrich). The live/dead BacLight bacterial viability staining kit was purchased from Life Technologies, and crystal violet, Fluka Calcofluor white and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) from Sigma-Aldrich. For the in vitro experiments, 60-cobalt irradiations were performed using an AECL Eldorado Model ‘G’ (Atomic Energy of Canada, Ltd., Commercial Products Division, Ottawa, Canada) at a dose rate of 0.5 Gy/min. For the experiments with Galleria mellonella, microbiota were irradiated with a single 6 MV photon beam generated using a linear accelerator SLi-18 (Elekta, Crawley, UK) at a dose rate of 430 cGy/min.
Determination of viability of test species
In order to assess the effect of different media (THY, BHI, DMEM, DMEM supplemented with mucins) on the viability of all test bacterial species with or without irradiation (10 Gy), different assays were performed. First, growth curves for each test species were prepared in presence of the different media with or without irradiation. The tested species were inoculated in 10 mL of culture broth and grown overnight. A 1:1000 dilution (optical density [OD] at 600 nm ∼0.03–0.05) was prepared in a 50-mL V-bottom polypropylene tube in presence of the different media and irradiation was performed prior to incubation at 37℃ and 200 r/min. Absorbance at 600 nm was measured spectrophotometrically every 30 min, using cuvettes with a path length of 1 cm, until cultures reached stationary phase (mQuant, Bio-Tek Instruments Inc., Winooski, Vermont, USA).
Second, to test the effect of irradiation upon biofilm formation, 200 µL of a 1:100 dilution of an overnight culture of all test species was transferred into two sterile 24-well microtiter plates (Fisher Scientific, Pittsburgh, PA) and 800 µL of the different media was added to the culture prior to irradiation. Plates were incubated for 24 h at 37℃ either statically in an atmosphere of 5% CO2 in air, or shaking at 200 r/min in an atmosphere of air. After 24 h, planktonic cells were gently removed and the remaining biofilm cells of one plate were stained with the live/dead BacLight bacterial viability kit (Life Technologies) according to manufacturer’s guidelines. Briefly, 3 µL of the dye mixture of SYTO9 and propidium iodide (PI) was added to the cells and incubated in the dark at room temperature. After 20 min, fluorescence was measured using a fluorimeter EnSpire 2300 Multilabel Reader (Perkin Elmer, Waltham, MA, USA) at Ex480/Em500 nm for SYTO9 and Ex490/Em635 nm for PI. Metabolic activity and viability of the biofilm cells in the second plate were evaluated using the MTT assay. In short, 1 mg/mL of MTT was added to each well after removal of the medium (containing the planktonic cells) and biofilm cells were incubated at 37℃. After 2 h, all media were discarded and formazan crystals were dissolved in 1 mL dimethyl sulfoxide (DMSO). Absorbance at 570 nm was measured spectrophotometrically (mQuant, Bio-Tek Instruments Inc., Winooski, Vermont, USA).
Determination of biofilm formation
Biofilm formation of all test species was measured in 24-well plates and in 50 mL V-bottom tubes. For the setup in plates, 200 µL of a 1:100 dilution of an overnight culture was transferred into 24-well plates to which 800 µL of medium was added. For the setup in tubes, 1 mL of a 1:100 dilution of an overnight culture of the test species was transferred into a sterile 50 mL V-bottom tube and 4 mL of the different test media was added. Subsequently, plates and tubes were subjected to irradiation or not (controls) and incubated statically for 24 h at 37℃ and 5% CO2 in air (plates) or in shaking conditions (plates: 200 r/min; tubes: 80 r/min). After 24 h, planktonic cells were gently removed. Biofilms grown on the bottom (plates) and sides of the wells (tubes: ring formation) were washed three times with tap water and stained with 0.2% crystal violet for 30 min. Excess of staining solution was removed by three washing steps with tap water. After overnight drying of the plates and the tubes, the crystal violet was dissolved by adding a mixture of methanol:acetic acid (1:4) and the OD 540 nm was measured spectrophotometrically (mQuant; Bio-Tek Instruments Inc., Winooski, Vermont, USA).
Calcofluor white staining assay
To test the influence of different media and irradiation on extracellular matrix components, β-linked polysaccharides such as cellulose and chitin, a calcofluor white staining was performed on biofilm cells. A total of 200 µL of the calcofluor white solution was added to the biofilms formed in 24-well plates (see above). Calcofluor staining of C. albicans and C. glabrata was enhanced by adding one drop of KOH to dissolve keratinized particles and to help emulsify solid, viscous material that may mask fungal elements. After 10 min incubation in the dark at room temperature, the dye was discarded and biofilms were thoroughly washed with filter-sterilized water to remove unbound dye. Fluorescence was measured using a fluorescence microplate reader with an excitation filter of 355 and an emission filter of 430 nm EnSpire 2300 Multilabel Reader (Perkin Elmer, Waltham, MA, USA).
G. mellonella larvae infection and survival assay
G. mellonella larvae (also known as the wax worm) were purchased from a local supplier (De Papegaai, Sint-Niklaas, Belgium), maintained at room temperature in the dark, and used within one week. Overnight bacterial cultures were divided in two equal parts after which one part was irradiated (10 Gy). Cultures were washed three times and resuspended in sterile PBS at the desired concentration. Larvae were each injected with 20 µl of inoculum into the lower left proleg using an insulin syringe. Each group of 9–10 larvae was incubated at 37℃ in 9-cm petri dishes without food for up to three days.
For tests with supernatant, supernatant of irradiated and nonirradiated overnight cultures of K. oxytoca and S. salivarius was collected 4 h postirradiation and cleared from remaining cells by centrifugation (10 min, 2200 g) and passed through a filter (0.2 µm). Larvae were injected with 20 µL of the supernatant or sterile broth as a control. For the experiments with dead bacteria, cells were fixed with ice-cold methanol for 10 min and washed with phosphate buffered saline (PBS) prior to injection.
Statistics
Statistical testing was conducted using Statistical Package for the Social Sciences statistics 22. Normality of the data was checked using the Shapiro–Wilk assay. When normality could be assumed, statistical differences between the growth conditions were determined using Student’s t-tests or analysis of variance. For nonparametric analyses, Mann–Whitney U or Kruskal–Wallis tests were used. Pairwise post hoc analysis was performed with Bonferroni correction. For the G. mellonella experiments, the survival between the different groups was compared using the log rank test. A 0.05 statistical significance level was used for all analyses.
Results
Effect of ionizing irradiation on growth of oral planktonic cells
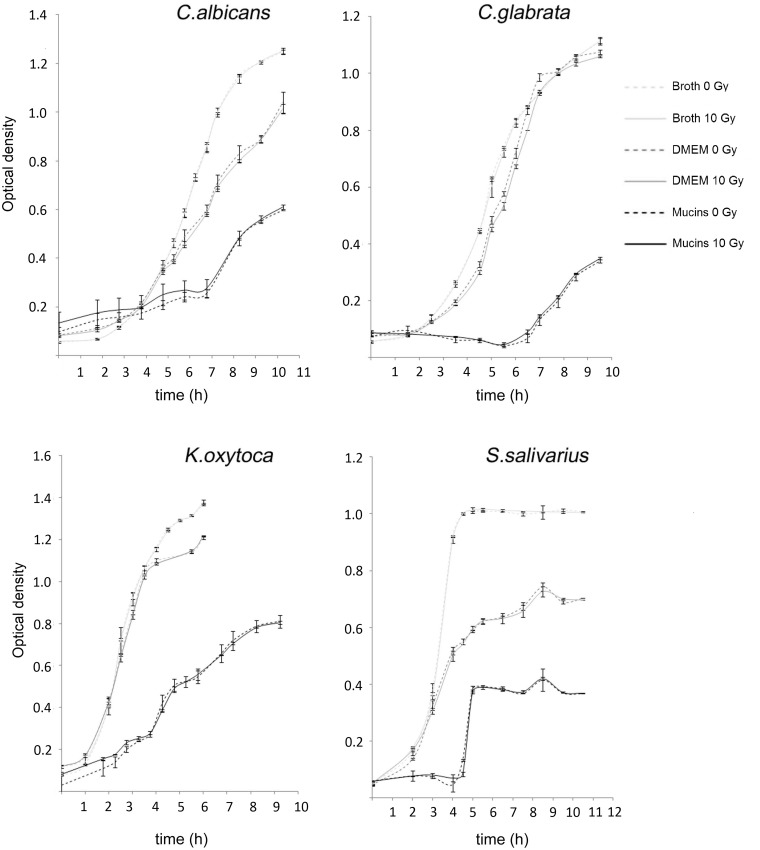
We first investigated the effect of ionizing irradiation (10 Gy) on the growth of different oral species (C. albicans, C. glabrata, S. salivarius, and K. oxytoca) under agitation. Growth curves were obtained by culturing planktonic cells in presence of rich culture broth (THY or BHI), glucose-rich DMEM and DMEM supplemented with mucins. As shown in Figure 1, a dose of 10 Gy did not affect subsequent outgrowth of test species. In contrast, comparative assessment of the data obtained with the different culture media (BHI or THY, DMEM, DMEM + mucins) showed a differential effect on growth of all test species depending on the culture medium. In general, the presence of mucins had a strong growth inhibitory effect on all test species.
Figure 1.
Growth curves of irradiated (10 Gy) and nonirradiated C. albicans, C. glabrata, K. oxytoca and S. salivarius planktonic cells cultured in different media (broth, DMEM, or DMEM + mucins). Vertical axis represents the OD of the cells measured at 600 nm using a spectrophotometer. Data of one experiment using two parallel cultures of each species are shown
Effect of ionizing irradiation on biofilm formation and viability of oral microbiota
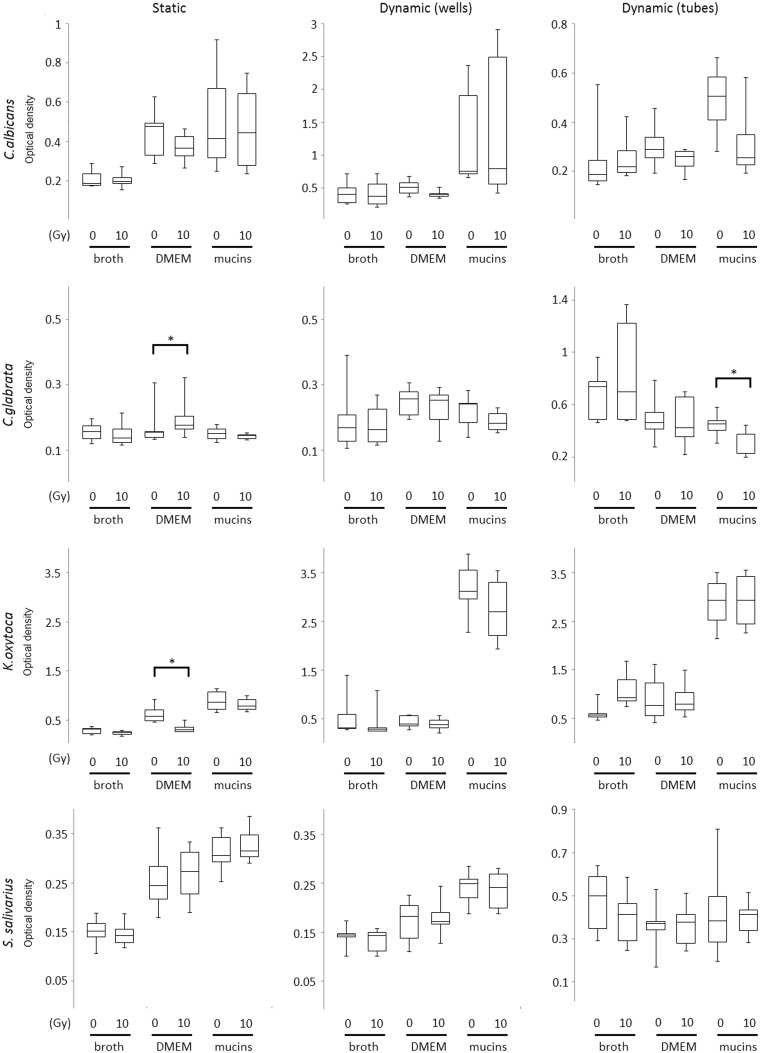
Biofilm formation was assessed after irradiation of the inocula. Biofilms were allowed to form in (1) static conditions, using 24-well plates and (2) dynamic conditions, using 24-well plates or 50 mL V-bottom tubes, in the presence of the different test media during a period of 24 h following irradiation of the inoculum. Crystal violet staining of the biofilms was performed as a measure of biofilm material, including both cells and extracellular matrix components. We observed that irradiation significantly reduced biofilm formation of K. oxytoca when cultured in static conditions and in the absence of mucins (P = 0.002) and of C. glabrata in dynamic conditions in the presence of mucins (P = 0.017) (Figure 2). However, thicker biofilms were observed for K. oxytoca cultured in dynamic (broth) conditions (not significant) and for C. glabrata in static conditions (P = 0.029) after irradiation (Figure 2). In addition, there was an overall positive effect of mucins on biofilm formation for most species, which was significant for K. oxytoca (static: P = 0.022; dynamic wells: P < 0.001; dynamic tubes: P < 0.001), C. albicans (dynamic wells: P = 0.012), and S. salivarius (dynamic wells: P < 0.001).
Figure 2.
Box and whiskers plot presentation of biofilms of irradiated (10 Gy) and nonirradiated C. albicans, C. glabrata, K. oxytoca, and S. salivarius cells formed during 24 h of culture in different media (broth, DMEM, or DMEM + mucins). Biofilms were generated both in static (24-well plates, first column) and dynamic conditions (24-well plates, second column; tubes, third column). Vertical axis represents the OD of dissolved crystal violet molecules measured at 540 nm using a spectrophotometer. Boxes and whiskers show median values and interquartile range of scores. Pooled data of at least four repetitions are shown. *P < 0.05
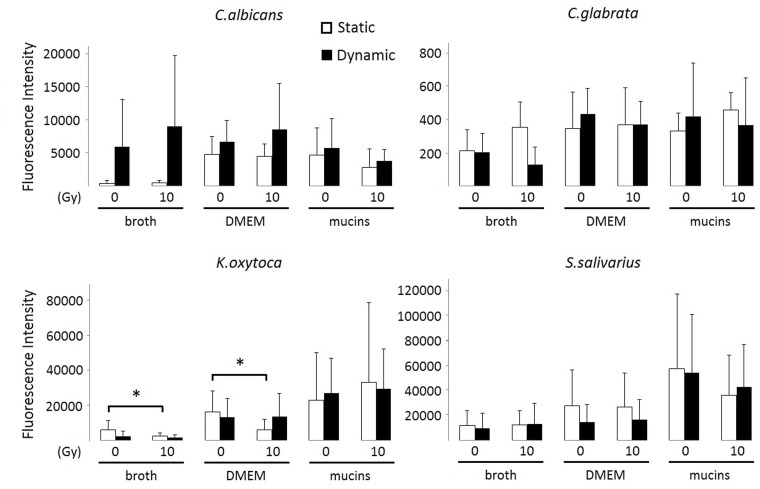
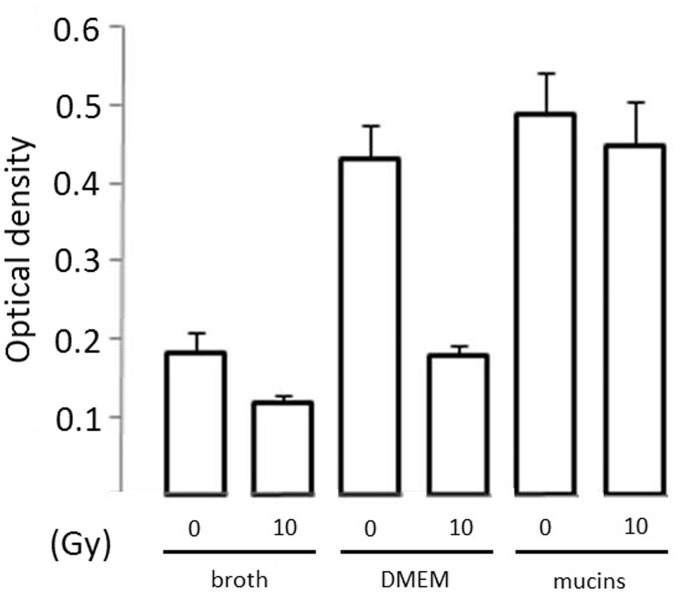
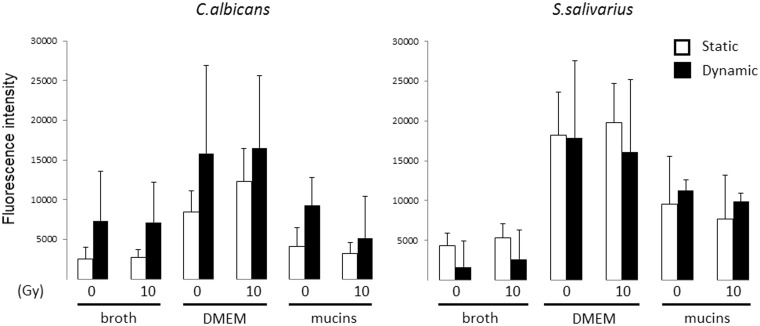
Next, we evaluated the number of cells in biofilms of all test species grown for 24 h after irradiation of the inoculum. To this end, we measured the fluorescence intensity of the biofilms after SYTO9 and PI staining. The SYTO9 stain generally labels all microbiota in a population, whereas PI penetrates only microbiota with damaged membranes, causing a reduction in the SYTO9 fluorescence when both dyes are present. We observed that irradiation significantly reduced the number of SYTO9-positive K. oxytoca cells, when the biofilms were grown in static conditions and in the absence of mucins (broth: P = 0.041; DMEM: P = 0.008) (Figure 3). The metabolic activity, measured as MTT reduction, of the K. oxytoca biofilms was also reduced in those specific conditions (Figure 4). Figure 3 further illustrates that Candida biofilm formation on polystyrene plates is poor, irrespective of the type of medium used, whereas K. oxytoca and S. salivarius form dense biofilms in all media as was also obvious upon visual inspection. There was a trend for the presence of mucins to enhance biofilm formation by K. oxytoca and S. salivarius, although this difference was not statistically significant. We were unable to detect PI values that significantly differed from the detection limit, which indicates that the majority of the biofilm cells were alive and not affected by irradiation.
Figure 3.
SYTO9-positive cells present in irradiated and nonirradiated biofilms after 24 h in different culture media. Biofilms were generated both in static and dynamic conditions (24-well plates with or without shaking). Vertical axis represents the SYTO9-positivity assessed by measurement of fluorescence intensity at Ex480/Em500 nm. Bars represent the means including the standard deviation. Pooled data of at least three repetitions are shown. *P < 0.05
Figure 4.
Metabolic activity of irradiated and nonirradiated biofilm cells of K. oxytoca after 24 h of culturing in different media (without agitation) as determined by measuring the OD at 490 nm of the dissolved formazan crystals (vertical axis). Bars represent the means including the standard deviation. Data of one representative experiment are shown (three replicates)
We further examined if irradiation had an impact on the production of extracellular matrix components of biofilm cells. The cellulose content of the biofilms grown for 24 h after irradiation of the inoculum was evaluated by a calcofluor white staining. In our setup, only C. albicans and S. salivarius produced an extracellular matrix that contained cellulose (Figure 5). Overall, no effect of irradiation on the cellulose content of the biofilm matrix could be observed.
Figure 5.
Calcofluor staining of biofilms of irradiated (10 Gy) and nonirradiated C. albicans and S. salivarius formed within 24 h in static (white bars) or dynamic conditions (black bars) in 24-well plates and in presence of the three test media. Vertical axis represents the calcofluor fluorescence intensity at Ex355/Em430 nm. Bars represent the means including the standard deviation. Pooled data of two repetitions are shown. *P < 0.05
Effect of ionizing irradiation on virulence of K. oxytoca and S. salivarius
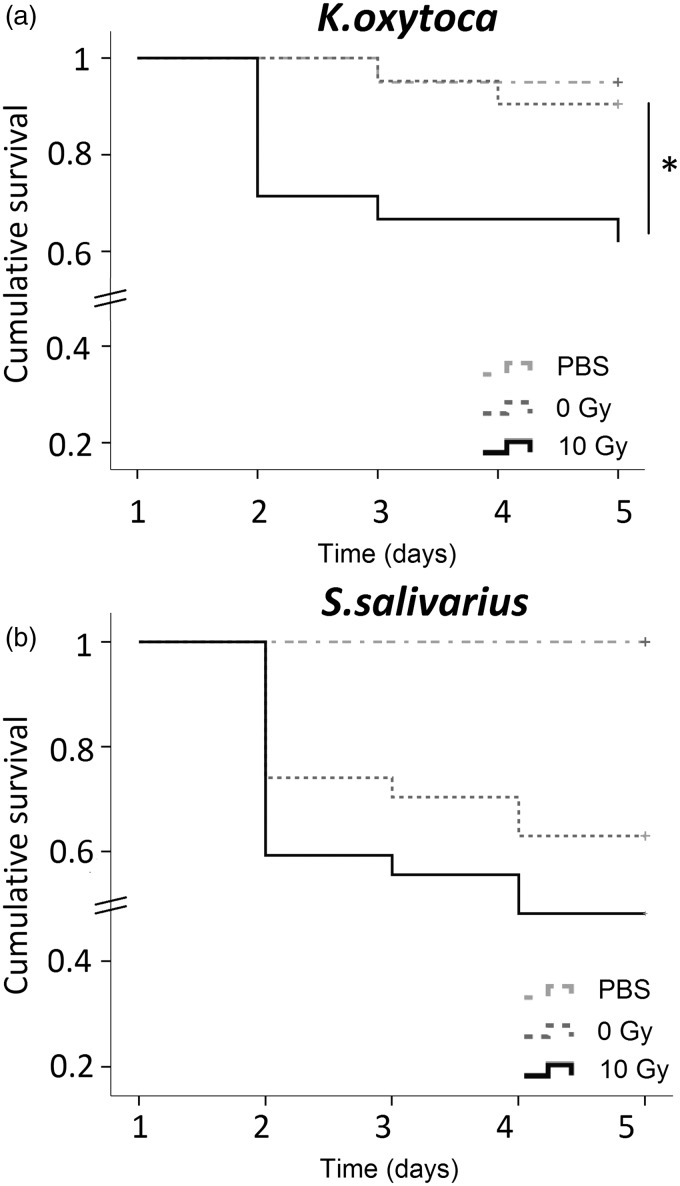
We further questioned if irradiation had an impact on the virulence of the pathogen K. oxytoca and the commensal S. salivarius. To test this, we used larvae of the wax moth G. mellonella as a model host for examining the virulence potential of the species of interest. For survival assays, wax moth larvae were infected and mortality was monitored every 24 h for four days. At a dose of 106 cfu/mL, irradiated K. oxytoca were significantly more virulent then nonirradiated cells (P = 0.027; log rank test, n = 20) (Figure 6(a)). At one day postinoculation, the survival probability of the wax moth larvae infected with irradiated cells was lower than with NIR. The time course of survival of the wax moth larvae depended on the pathogen load as injection of a dose of 107 or 108 cfu/mL resulted in a high mortality rate 24 h postinoculation, while a dose of 105 cfu/mL was too low to cause infection (data not shown). In contrast to K. oxytoca, the virulence of S. salivarius at a dose of 107 cfu/mL did not significantly change upon irradiation (Figure 6(b)). In order to find out if the increased virulence of K. oxytoca after irradiation was due to the secretion of particular factors, supernatants from nonirradiated and irradiated were collected and injected into the wax moth larvae. However, the supernatant of both groups had no effect on the overall survival of the wax larvae (data not shown). Also injection of dead cells had no influence on larval survival (data not shown), pointing to the need of living, metabolically active bacteria for virulence.
Figure 6.
Survival curves of Galleria melonella after inoculation with K. oxytoca (a) or S. salivarius (b), plotted using the Kaplan–Meyer method. For experiments with K. oxytoca, two independent tests were performed and pooled results are presented. Test groups were PBS controls (n = 20), nonirradiated (0 Gy; n = 21) and irradiated (10 Gy; n = 21). Vertical axis represents the cumulative survival probability of the wax larvae during four days postinjection. For experiments with S. salivarius, three independent tests were performed and pooled results are presented. Test groups were PBS controls (n = 27), nonirradiated (0 Gy; n = 27) and irradiated (10 Gy; n = 27). Vertical axis represents the cumulative survival probability of the wax larvae during four days postinjection. Differences in survival were calculated using the log rank test. *P < 0.05
Discussion
Overall, our data indicate that ionizing irradiation can induce behavioral changes in specific oral species (Table 1). Of all species tested, only K. oxytoca was consistently affected. Although we failed to detect an effect of irradiation on microbial growth, biofilm formation was clearly induced. We speculate that irradiation triggers a stress response that might be responsible for the induction of microbial adhesion. It is known that upon sensing a stress signal, free-living cells will initiate attachment to a surface and the formation of a biofilm that has a greater ability to withstand environmental challenges.25 Irradiation might have an effect on the different stages of biofilm formation i.e. initially when adhesion to a surface occurs through organelles including pili, flagella, and external microbial layers, or due to immobilization of microbiota,26–28 or through the secretion of quorum-sensing molecules such as 3-oxo-C12-homoserine lactone,29 or in mature biofilms through the increased production of extracellular matrix molecules like specific polysaccharides, proteins, and extracellular DNA (eDNA).30
Table 1.
Overview of effect of irradiation on different behavioral characteristics of oral species
| Static | Dynamic | ||
|---|---|---|---|
| Growth planktonic cells | C. albicans | NA | |
| C. glabrata | – | NA | |
| K. oxytoca | – | NA | |
| S. salivarius | – | NA | |
| Biofilm formation | C. albicans | NA | NA |
| C. glabrata | ↑ (pDMEM = 0.029) | ↓ (pDMEM + mucins = 0.017) | |
| K. oxytoca | ↓ (pDMEN = 0.002) | ↑ (broth: trend) | |
| S. salivarius | NA | NA | |
| Number of biofilm cells | C. albicans | NA | NA |
| C. alabrata | NA | NA | |
| K. oxytoca | ↓ (pbroth = 0.041; pDMEM = 0.008) | NA | |
| S. salivarius | NA | NA | |
| Cellulose content | C. albicans | NA | NA |
| C. alabrata | NA | NA | |
| K. oxytoca | NA | NA | |
| S. salivarius | NA | NA | |
| Virulence | K. oxytoca | ↑ (p = 0.027) | |
| S. salivarius | NA | ||
Note: -: not tested; NA: not affected.
Most importantly, we showed that irradiation significantly increased the virulence of K. oxytoca in vivo using the G. mellonella model system. This model allows the study of bacterial and fungal infections at 37℃ (in contrast to the Caenorhabditis elegans and Drosophilla melanogaster infection models) and the immune system of the larvae shares functional homology with the human innate immune system, possessing both humoral and cellular immunity.31,32 This observation is important and relevant in the context of oral mucositis. Indeed, K. pneumoniae is a prevalent isolate in blood and oral cavity of cancer patients undergoing (chemo)radiation therapy7,33 and pediatric patients undergoing high-dose chemotherapy or hematopoietic stem cell therapy.34,35 Both groups have a very high incidence of developing oral mucositis. Due to their high incidence rate, Klebsiella spp. are considered one of the most significant infectious pathogens, causing urinary tract infections, pneumonia, wound infections, septicemia, neonatal septicemia, and nosocomial infections.36,37 As K. pneumoniae is frequently identified in individuals wearing removable maxillary prosthesis and are assumed to play important roles in denture stomatitis, wearing a removable prosthesis might be an important risk factor for developing mucositis and/or infections during or after radiation therapy.38 It is therefore likely that the oral ulcerations are gateways for Klebsiella spp and other opportunistic pathogens to penetrate the underlying damaged mucosa. We are currently using the G. mellonella model to examine effects of radiation on different Candida spp. Results may explain the high incidence of local and/or systemic infections through fungal translocation associated with oral mucositis. Remarkably, the supernatant of irradiated bacteria did not mimic the toxic effects of living cells ruling out the interplay of a secretion factor. We speculate that irradiation induces a stress response in the microbial cells that changes the adhesive properties or surface hydrophobicity of the cells walls. Indeed, the microbial cell surface hydrophobicity is known to contribute to the adherence properties, which can be affected by stressors such as subinhibitory concentrations of antibiotics.39,40 This modified adhesive behavior could be linked to virulence since the number of cells attached to host cells is increased and therefore infection is more likely to occur.
We further observed that irradiated K. oxytoca cells formed less biofilm in static conditions where oxygen transfusion is limited. In dynamic, oxygen-rich conditions, an opposite trend could be observed. Interestingly, these changes were not seen when mucins were present in the culture medium. Mucins exhibit protective effects on the alimentary tract through reducing mechanical and chemical stress, preventing bacterial overgrowth and penetration of the mucosa. All oral microbiota are found in complex biofilms on and in the epithelial mucous layer (soft and hard tissues) or on the teeth (dental plaque), or as planktonic cells present in saliva.41 A number of recent studies5,6,42 has shown that after radiation therapy, the composition of the core oral microbiome is modulated over time. In this study, however, we show that also the functional behavior of oral microbiota can be affected by irradiation. We speculate that this change in functional behavior might have a dramatic effect on the epithelium, deprived from its protective mucous layer following radiation therapy due to reduced salivary production by the damaged salivary glands. As a consequence, the composition and texture of the mucous layer covering the oral epithelium are dramatically altered and protective proteins such as defensins, immunoglobulins, lysozyme, and trefoil proteins will be less abundant.43 Moreover, since mucins are important for growth of microbiota44 and for initiating host–microbiome interactions such as adhesion of microbiota,45 a mucous-deprived environment will leave the host more vulnerable to opportunistic species. Indeed, during progressive mucositis, desquamation of the epithelium and its overlaying mucin layer results in severe ulcerations often associated with local infections. Moreover, mucin composition and expression have been shown to be altered by chemotherapy and this is thought to be associated with mucositis.46–48 Therefore, our in vitro observations provide biological clues as to why patients with a damaged mucosa have an increased risk of developing infections.
Our data suggest that the overall effect of irradiation on biofilm formation by K. oxytoca is dependent on factors such as oxygen availability and the shear stress. In the oral cavity, mucin-poor, static, oxygen-deprived conditions are found in the gingival crevice, which is a reservoir of opportunistic pathogens. K. oxytoca has been frequently isolated in biofilms of HIV-positive patients with plaque-associated gingivitis or periodontitis, indicating their ability to colonize oxygen-deprived niches deprived from mucins in these patients49,50 and after bracket placement.51 Our data show that in oxygen-deprived conditions, a lower number of Klebsiella cells are found in newly formed biofilms, whereas in oxygen-rich conditions, a higher number could be observed. This suggests that radiation therapy might lead to a lower number of potentially harmful cells in the gingival crevices after radiation therapy, whereas the opposite could happen on the teeth or gum line, where oxygen is abundantly available. Therefore, patients with a history of gingivitis/periodontitis might be able to manage their condition (gingivitis/periodontitis) better after radiotherapy when maintaining good oral hygiene (as they are prone to develop denser K. oxytoca biofilms on the teeth surface). However, those patients might have an increased risk of developing infections related to C. glabrata as our data suggest that irradiation stimulates the biofilm formation of that species in static, oxygen-deprived conditions.
A potential relationship between radiation-induced mucositis and periodontitis has recently been suggested by Khaw et al.52 as both diseases are linked with chronic and systemic inflammation. This is clinically important, since it is estimated that up to 50% of the population is affected by periodontal disease as a result of the presence of multiresistant enterobacteria such as Klebsiella spp. in subgingival sites.53 Therefore, these patients may have a particular risk of developing oral mucositis complicated with systemic infections especially when they are immunocompromised and hospitalized. Various Candida species are frequently identified in oral mucositis patients, especially after high-dose chemotherapy and radiotherapy.11,33,54,55 In our study, no changes in biofilm formation of C. albicans were observed after irradiation. Therefore, the mechanism by which C. albicans is able to overgrow the affected mucosa during mucositis might not be associated with effects on growth or biofilm formation.
Overall, the risk of developing an infection after irradiation will ultimately also depend on other factors like the virulence of the microbiota, the shift in tempo-regional attachment, and colonization of the microbiota and the complexity of the composition of the biofilm. Recent data indicate that both chemo- and radiotherapy are associated with a decrease in microbial diversity,5,6,56 which is believed to be correlated with a poor health prognosis. Our data indicate that apart from the composition, radiation therapy can also change the functional behavior of the resident oral microbiome. Therefore, we hypothesize that local and systemic infections associated with mucositis are probably more likely to result from the cumulative effect of changes in composition, function, and behavior of the microbiome than solely from a compromised diversity. Therefore, functional changes in the oral microbiome following chemo- and radiation therapy should be studied more in depth.
Although this work is relevant in the context of identifying microbial risk factors for the development of oral mucositis, it is important to highlight its limitations. Oral biofilms are highly complex multispecies structures and it is the delicate interaction between all its members that will ultimately determine their behavior. Thus, further studies should address (1) if similar observations can be made with other mucositis-associated pathogens besides K. oxytoca and in multispecies biofilms containing K. oxytoca and (2) what the underlying mechanism of this increased virulence potential of K. oxytoca is. Based on our preliminary results, we hope to further clarify if treatments like chemo- and radiation therapy are likely to modify the functional behavior of the oral microbiome in a way that will eventually lead to dysbiosis, bacterial translocation, and the overgrowth of pathogens during the progression phase of mucositis.
ACKNOWLEDGEMENTS
TDR is a doctoral research fellow supported by the FWO-Flanders (G.0712.10N). TVdW is financially supported by FWO (1526012N) and GOA (BOF12/GOA/008). BVH’s research leading to these results has received funding from the Seventh Framework Programme (FP7/2011) under grant agreement n° 299169 (Mucositis Platform) and from the FWO (V.4.339.10.N.01). We also thank William Wilson for providing training for the use of the AECL Eldorado Model ‘G’.
Authors’ contributions
BVH, TDR, and KDb conducted all the experiments. BVH and TDR performed the data analysis and drafted the manuscript. TDR performed the statistical analysis. TB and BVH conducted the irradiation of the microbial cultures. SW trained BVH in the use of Galleria mellonella as a model system. TVdW supervised the work of TDR and SS of BVH, both TVdW and SS revised the manuscript.
Authors’ note
Barbara WA Vanhoecke and Tine RG De Ryck contributed equally to this work.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Sonis ST. New thoughts on the initiation of mucositis. Oral Dis 2010; 16: 597–600. [DOI] [PubMed] [Google Scholar]
- 2.Sonis ST. Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 2009; 45: 1015–20. [DOI] [PubMed] [Google Scholar]
- 3.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004; 4: 277–84. [DOI] [PubMed] [Google Scholar]
- 4.Vanhoecke B, De Ryck T, Stringer A, Van de Wiele T, Keefe D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis 2015; 21: 17–30. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Shao Z, Wang Q, Jiang Y, Ma R, Tang Z, Liu Z, Liang JP, Huang ZW. Exploring the dynamic core microbiome of plaque microbiota during head-and-neck radiotherapy using pyrosequencing. PLoS ONE 2013; 8: e56343–e56343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao Z-Y, Tang Z-S, Yan C, Jiang Y-T, Ma R, Liu Z, Huang ZW. Effects of intensity-modulated radiotherapy on human oral microflora. J Radiat Res 2011; 52: 834–9. [DOI] [PubMed] [Google Scholar]
- 7.Panghal M, Kaushal V, Kadayan S, Yadav JP. Incidence and risk factors for infection in oral cancer patients undergoing different treatments protocols. BMC Oral Health 2012; 12: 22–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonalika WG, Amsavardani Tayaar S, Bhat KG, Patil BR, Muddapur MV. Oral microbial carriage in oral squamous cell carcinoma patients at the time of diagnosis and during radiotherapy—a comparative study. Oral Oncol 2012; 48: 881–6. [DOI] [PubMed] [Google Scholar]
- 9.Tong HC, Gao XJ, Dong XZ. Non-mutans Streptococci in patients receiving radiotherapy in the head and neck area. Caries Res 2003; 37: 261–6. [DOI] [PubMed] [Google Scholar]
- 10.Almståhl A, Wikström M, Fagerberg-Mohlin B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis 2008; 14: 541–9. [DOI] [PubMed] [Google Scholar]
- 11.Belazi M, Velegraki A, Koussidou-Eremondi T, Andreadis D, Hini S, Arsenis G, Eliopoulou C, Destouni E, Antoniades D. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol 2004; 19: 347–51. [DOI] [PubMed] [Google Scholar]
- 12.Guobis Ž, Kareivienė V, Basevičienė N, Paipalienė P, Niedzelskienė I, Sabalys G, Kubilius R, Gervickas A. Microflora of the oral cavity in patients with xerostomia. Medicina (Kaunas) 2011; 47: 646–51. [PubMed] [Google Scholar]
- 13.Dickson JS. Radiation inactivation of microorganisms. In: Mollins R. (ed). Food irradiation: principles and applications, New York, NY: Willey-Interscience, 2001, pp. 23–35. [Google Scholar]
- 14.Farkas J. Irradiation as a method for decontaminating food. Int J Food Microbiol 1998; 44: 189–204. [DOI] [PubMed] [Google Scholar]
- 15.Caillet S, Millette M, Dussault D, Shareck F, Lacroix M. Effect of gamma radiation on heat shock protein expression of four foodborne pathogens. J Appl Microbiol 2008; 105: 1384–91. [DOI] [PubMed] [Google Scholar]
- 16.Trudeau K, Vu KD, Shareck F, Lacroix M. Capillary electrophoresis separation of protein composition of γ-irradiated food pathogens Listeria monocytogenes and Staphylococcus aureus. PLoS ONE 2012; 7: e32488–e32488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikka M, Hammarén MM, Harjula S-KE, Halfpenny NJA, Oksanen KE, Lahtinen MJ, Pajula ET, Livanainen A, Pesu M, Ramet M. Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog 2012; 8: e1002944–e1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zack MB, Stottmeier K, Berg G, Kazemi H. The effect of radiation on microbiologic characteristics of M Tuberculosis. Chest 1974; 66: 240–3. [DOI] [PubMed] [Google Scholar]
- 19.Maier I, Berry DM, Schiestl RH. Intestinal microbiota reduces genotoxic endpoints induced by high-energy protons. Radiat Res 2014; 181: 45–53. [DOI] [PubMed] [Google Scholar]
- 20.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A 2005; 102: 13254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A, Verma R, Murari A, Agrawal A. Oral candidiasis: an overview. J Oral Maxillofac Pathol 2014; 18: S81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis 2014; 33: 673–88. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez V, Bodey GP. Bacterial infections in immunosuppressed patients: diagnosis and management. Transplant Proc 1973; 5: 1249–54. [PubMed] [Google Scholar]
- 24.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res 1987; 95: 369–80. [DOI] [PubMed] [Google Scholar]
- 25.De la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol 2013; 16: 580–9. [DOI] [PubMed] [Google Scholar]
- 26.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284: 1318–22. [DOI] [PubMed] [Google Scholar]
- 27.O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 1998; 30: 295–304. [DOI] [PubMed] [Google Scholar]
- 28.Toole GO, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol 2000; 54: 49–79. [DOI] [PubMed] [Google Scholar]
- 29.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998; 280: 295–8. [DOI] [PubMed] [Google Scholar]
- 30.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science 2002; 295: 1487–1487. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann JA. Innate immunity of insects. Curr Opin Immunol 1995; 7: 4–10. [DOI] [PubMed] [Google Scholar]
- 32.Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2011; 2: 521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaetti-Jardim Júnior E, Ciesielski FIN, de Sousa FRN, Nwaokorie F, Schweitzer CM, Avila-Campos MJ. Occurence of yeast, pseudomonas and enteric bacteria in the oral cavity of patients undergoing head and neck radiotherapy. Brazilian J Microbiol 2011; 42: 1047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kersun LS, Propert KJ, Lautenbach E, Bunin N, Demichele A. Early bacteremia in pediatric hematopoietic stem cell transplant patients on oral antibiotic prophylaxis. Pediatr Blood Cancer 2005; 45: 162–9. [DOI] [PubMed] [Google Scholar]
- 35.Soares AF, de Aquino AR, de Carvalho CH, Nonaka CF, Almeida D, Pereira LP. Frequency of oral mucositis and microbiological analysis in children with acute lymphoblastic leukemia treated with 0.12% chlorhexidine gluconate. Braz Dent J 2011; 22: 312–6. [DOI] [PubMed] [Google Scholar]
- 36.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998; 11: 589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahly H, Podschun R. Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthropathic sequelae. Clin Diagn Lab Immunol 1997; 4: 393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira CA, Toledo BC, Santos CT, Pereira Costa ACB, Back-Brito GN, Kaminagakura E, Jorge AO. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn Microbiol Infect Dis 2013; 76: 419–24. [DOI] [PubMed] [Google Scholar]
- 39.Doyle R, Rosenberg M, Drake D. Hydrophobicity of oral bacteria. In: Doyle R, Rosenberg M. (eds). Microbial cell surface hydrophobicity, Washington, DC: American Society for Microbiology, 1990, pp. 387–419. [Google Scholar]
- 40.Wu Q, Wang Q, Taylor KG, Doyle RJ. Subinhibitory concentrations of antibiotics affect cell surface properties of Streptococcus sobrinus. J Bacteriol 1995; 177: 1399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol 2009; 28: 405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Ryck T, Duprez F, Bracke M, Van de Wiele T, Vanhoecke B. Dynamic shifts in the oral microbiome during radiotherapy. Clin Res Infect Dis 2015; 2: 1013–1013. [Google Scholar]
- 43.Amado F, Lobo MJC, Domingues P, Duarte JA, Vitorino R. Salivary peptidomics. Expert Rev Proteom 2010; 7: 709–21. [DOI] [PubMed] [Google Scholar]
- 44.Wei G-X, Campagna AN, Bobek LA. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J Antimicrob Chemother 2006; 57: 1100–9. [DOI] [PubMed] [Google Scholar]
- 45.Kesimer M, Kiliç N, Mehrotra R, Thornton DJ, Sheehan JK. Identification of salivary mucin MUC7 binding proteins from Streptococcus gordonii. BMC Microbiol 2009; 9: 163–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh ASJ, Al-Dasoogi N, Keefe DM. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 2009; 90: 489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Hamilton J, Keefe DM. Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood) 2009; 234: 430–41. [DOI] [PubMed] [Google Scholar]
- 48.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Laurence J, Keefe DM. Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol 2009; 64: 123–32. [DOI] [PubMed] [Google Scholar]
- 49.Gaetti-Jardim Júnior E, Nakano V, Wahasugui TC, Cabral FC, Gamba R, Avila-Campos MJ. Occurrence of yeasts, enterococci and other enteric bacteria in subgingival biofilm of HIV-positive patients with chronic gingivitis and necrotizing periodontitis. Braz J Microbiol 2008; 39: 257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zambon JJ, Reynolds HS, Genco RJ. Studies of the subgingival microflora in patient with acquired immunodeficiency syndrome. J Periodontol 1990; 61: 699–704. [DOI] [PubMed] [Google Scholar]
- 51.Naranjo AA, Triviño ML, Jaramillo A, Betancourth M, Botero JE. Changes in the subgingival microbiota and periodontal parameters before and 3 months after bracket placement. Am J Orthod Dentofacial Orthop 2006; 130: 275.e17–22. [DOI] [PubMed] [Google Scholar]
- 52.Khaw A, Logan R, Keefe D, Bartold M. Radiation-induced oral mucositis and periodontitis—proposal for an inter-relationship. Oral Dis 2014; 20: e7–18. [DOI] [PubMed] [Google Scholar]
- 53.Gonçalves MO, Coutinho-Filho WP, Pimenta FP, Pereira GA, Pereira JA, Mattos-Guaraldi AL, Hirata R., Jr Periodontal disease as reservoir for multi-resistant and hydrolytic enterobacterial species. Lett Appl Microbiol 2007; 44: 488–94. [DOI] [PubMed] [Google Scholar]
- 54.Kurnatowski P, Moqbil S, Kaczmarczyk D. Signs, symptoms and the prevalence of fungi detected from the oral cavity and pharynx of radiotherapy subjects with head and neck tumors, and their susceptibility to chemotherapeutics. Ann Parasitol 2014; 60: 207–13. [PubMed] [Google Scholar]
- 55.Laheij AMGA, de Soet JJ, von dem Borne PA, Kuijper EJ, Kraneveld EA, van Loveren C, Raber-Durlacher JE. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support Care Cancer 2012; 20: 3231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Vliet MJ, Tissing WJE, Dun CAJ, Meessen NEL, Kamps WA, de Bont ESJM, Harmsen HJ. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 2009; 49: 262–70. [DOI] [PubMed] [Google Scholar]