Abstract
Chronic nicotine administration in animals, and smoking in humans, causes up-regulation of α4β2* neuronal nicotinic receptors (nAChRs), which has been hypothesized to contribute to the addictive actions of nicotine. We used a rat model to test whether such up-regulatory effects differ in adolescents versus adults, and in males versus females. Following chronic treatment with nicotine or saline via subcutaneous osmotic minipumps, we measured α4β2 and α4β2α5 nAChRs in cerebral cortex using [3H]epibatidine to label assembled nAChRs, and selective antibodies to measure the individual subunits via immunoprecipitation. For the first time, we provide a detailed characterization of the response of both α4β2 and α4β2α5 nAChRs in female adolescent rat cerebral cortex. We found differences in nicotine-induced up-regulation between males and females in early adolescence that are absent in both late adolescence and adulthood. Males showed significant up-regulation at PN28 which was absent in age-matched females. These results demonstrate sex differences in the susceptibility of α4β2* nAChRs to the effects of chronic nicotine exposure in the cerebral cortex based on age.
Keywords: Chronic nicotine, adolescence, nicotinic receptor subtypes, immunoprecipitation, α5 subunit
1) Introduction
Adolescence is the most common period for initiation of recreational drug use (Spear et al., 2004). This use often begins with tobacco products, and evidence suggests that adolescents are differentially susceptible to the addictive and aversive effects of tobacco smoke (Levin et al., 2003; McQuown et al., 2007). Adolescent use of tobacco is associated with subsequent higher daily consumption and a lower probability of smoking cessation (Chen et al., 1998). It has also been demonstrated in animal studies that nicotine administration in early adolescence increased subsequent cocaine self-administration in rats (McQuown et al., 2007). Thus nicotine use during adolescence may have effects on brain function that are different and perhaps more prolonged than those that occur during adult tobacco use.
Increasing evidence indicates differences in smoking habits between males and females (Grunberg et al., 1991; Perkins 1999). When compared to males, females take fewer and shorter puffs from cigarettes, are less sensitive to some of the effects of nicotine, respond less to nicotine replacement therapy, and are more sensitive to smoking cues (Delfino et al., 2001). Sex differences in human response to nicotine could arise from multiple sources, including factors that directly affect tissue response to the drug (pharmacodynamics), factors that affect blood plasma levels of the drug (pharmacokinetics), factors that affect men and women’s socially defined roles and factors that affect sex differences in conditioned responses to the drug (Jensvold et al., 1996). In this study, we attempt to address the pharmacodynamics differences between males at females during early and late adolescence.
To explore the effects of chronic nicotine in male and female adolescents, we used a well-studied model employing osmotic mini-pumps to administer nicotine in rats. Periadolescence in rats is defined as the time between the earliest detection of diurnal gonadotropin cycling (approximately postnatal day 28 [PN28]) and reproductive maturity (approximately PN38–42) (Spear et al., 1983; Ojeda and Urbanski, 1994). Adolescence in the rat can be further sub-divided into early adolescence [starting at PN28] and late adolescence [around PN42]. Research regarding nicotine addiction during adolescence has shown that male adolescents rats are more likely to self administer nicotine than adult rats (Leslie et al., 2004; Vastola et al., 2002; Beluzzi et al., 2005). These findings have been reproduced in female rats as well (Levin et al, 2003). Previous studies have also shown that adolescent rats experience aversive effects from nicotine differently compared to adults. Specifically, adolescents display selective loss of striatal (and hippocampal) AMPA receptors, an effect not obtained with comparable treatment in the adult (Adriani et al., 2004), damage to striatal serotonergic terminals (Xu et al., 2001) and lasting changes in striatal cell signaling (Abreu-Villaca et al., 2003). Indeed, the striatum and its dopaminergic projections are still undergoing active remodeling and restructuring during adolescence (Goldman-Rakic 1987), factors which may render this region especially vulnerable to toxicant injury. Neurochemical, neuroanatomical and behavioral changes that occur during this period in rats are similar to those seen in human adolescents (Slotkin et al., 2004).
Chronic administration of nicotine increases the density of nAChRs in rat and mouse brains (Schwartz and Kellar, 1983; Marks et al., 1983). This up-regulation occurs in more than two-thirds of rodent brain regions (Marks et al., 1992). However, the extent of this up-regulation varies depending on the method of administration and brain region, with binding increased in the cerebral cortex by more than 60% (Kellar et al., 1989; Pauly et al., 1991; Marks et al., 1992; Perry et al., 2002; Nguyen et al., 2003). Similarly, studies of autopsied brains from human smokers and age-matched non-smokers (Benwell et al., 1988; Breese et al., 1997; Perry et al., 1999) found markedly higher densities of nAChR binding sites in the cerebral cortex of smokers compared to non-smokers. The cerebral cortex is of particular interest when studying nicotine addiction because of its probable role in cognition, memory, arousal, attention and anxiety. The cerebral cortex is known to be affected by nicotine (Levin, 1992). Indeed, acute nicotine improves performance on tasks regarding attention, cognition, learning and memory. When compared to adults, adolescents show increased novelty seeking behavior as well as elevated levels of impulsivity and restlessness, behaviors mediated by cortical activity (Laviola et al., 2003). Cortical development is not complete by adolescence and therefore may be disturbed by exposure to nicotine (Goriounova and Mansvelder 2012). In particular the medial prefrontal cortex (mPFC) which shows delayed development compared to other regions of the cortex is responsible for decision-making (Gogtay et al., 2004) and may play a role in the initiation of drug use in adolescents (Li et al., 1999).
Neuronal nAChRs are pentameric ligand-gated cation channels permeable to Na+, K+, and Ca2+. The channels are comprised of combinations of α and β subunits assembled from one of nine α (α2-α10) and three β (β2-β4) subunits. The majority of these receptors are heteromeric receptors comprised of two α and two β subunits, and a fifth subunit that is either an α or β subunit (Albuquerque et al., 2009; Millar et al., 2009). The major nAChR subtype in the CNS is the α4β2* nAChR, where the * indicates a variable fifth subunit (either α4, β2 or α5). These receptors account for >50% of all nAChR subtypes in the brain (Perry et al., 2002; Marks et al., 2010). Unlike other α subunits, the α5 subunit requires at least one other α subunit as well as a β2 and/or β4 subunit to form a functional nAChR, (Ramirez-Latorre et al., 1996) and does not participate in agonist binding (Fucile et al., 1997; Gerzanich et al., 1998). Previous studies suggest the importance of these nAChRs for nicotine self-administration: animals that don’t express α4 or β2 nAChR subunits fail to self-administer nicotine and the re-expression of the knocked out subunit in the ventral tegmental area can restore self-administration (Picciotto et al., 1998; Pons et al., 2008). The inclusion of the α5 subunit nAChRs alters channel conductance rates as well as desensitization rates and pharmacological properties (Alkondon et al., 1998; Tapia et al., 2007). As the majority of these nAChRs are presynaptic and modulate the release of neurotransmitters (Vizi and Lendvai 1999), projections from the prefrontal cortex are known to target dopaminergic and GABAergic neurons in the ventral tegmental area (Carr and Sesack, 2000), and cortical α5 subunit nAChRs drive development and morphology of cortical neurons (Bailey et al., 2012), we chose to look at nAChR regulation in the cerebral cortex. Using this design we are able to determine changes in the density of these nAChRs in a key region of the brain involved in the functional output of nicotine behaviors as mentioned above.
In the cerebral cortex of adult male rats, ~90% of the heteromeric nAChRs are the α4β2* subtype, and of these ~15% are the (α4β2)2α5 subtype (Mao et al., 2008). These nAChRs are highly sensitive to activation by nicotine (Brown et al., 2007) and are also highly Ca2+ permeable (Tapia et al., 2007). Interestingly, in the adult rat brain, α4β2* nAChRs that include the α5 subunit are resistant to the nicotine-induced up-regulation (Mao et al., 2008). Human genome-wide association studies have identified sequence variants in the region encoding the α5 subunit that increase the risk of nicotine dependence and lung cancer (Amos et al., 2008; Berrettini et al., 2008).
Previous work from our lab using autoradiography has indicated that adolescent and adult male rats differ significantly in the density of α4β2* nAChRs following chronic nicotine exposure (Doura et al., 2008), which suggests that these receptors may vary in their response to nicotine based on age or stage in development. In the present study we used subunit-selective antibodies in immunoprecipitation procedures to further elucidate differences in cortical α4β2* nAChR expression following chronic nicotine at different ages, comparing adults to early and late adolescence. In addition, we compared males and females at all three developmental stages. Our results indicate that both the age and the sex of the animal contribute to the net response of α4β2* nAChRs to chronic nicotine.
2 Materials and Methods
2.1 Radioligands and antibodies
[3H]Epibatidine ([3H]EB; 56.3 Ci/mmol and 62.2 Ci/mmol) was obtained from Perkin Elmer Life Sciences (Boston, MA, USA). Rabbit antisera directed at peptide sequences of the cytoplasmic domains of the nAChR α5 subunit were kind gifts from Robert P. Yasuda (Georgetown University, Washington, DC, USA). A monoclonal antibody (mAb 270) to the chick β2 subunit was made from hybridoma stocks (American Type Culture Collection, Manassas, VA, USA). This mAb was originally developed and characterized by Whiting and Lindstrom (1987). A monoclonal antibody selective for α4 subunits (mAb 299) (Whiting and Lindstrom 1988; Nakayama et al., 1995) was purchased from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA, USA). The antibodies used here are subunit-selective and their specificity for immunoprecipitation of radiolabeled nAChRs containing the corresponding subunits has been reported previously (Hernandez et al. 2004; Marritt et al. 2005; Perry et al. 2007). Additional studies to confirm the specificity of the alpha5 antibody have been previously published (Mao et.al 2008). Protein G Sepharose beads were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK). Normal rabbit serum (NRS) was purchased from Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA, USA). For simplicity, we use the term antibody to refer to unpurified antiserum, as well as to affinity purified antiserum and mAb.
2.2 Experimental Procedures
2.2.1 Animal treatment
Sprague–Dawley rats were obtained from Hilltop Lab Animals (Scottsdale, PA, USA) and delivered at specified ages based on the intended age of sacrifice. Osmotic minipumps (Alzet model 2002; Durect Corporation, Cupertino, CA, USA) were filled with sterile saline or with nicotine hydrogen tartrate (Sigma-Aldrich, St Louis, MO, USA) in saline. PN42 and PN84 animals were treated for 14 days. Implanting subcutaneous pumps can be technically challenging if it occurs prior to weaning. Therefore, in order to prevent issues with compromised nutrition or early weaning, PN28 animals were implanted at weaning (PN21) and treated for 7 days rather than 14. Preliminary studies from our lab (Supplemental Figure 1) indicated that the same level of nAChR up-regulation could be achieved in adult males with 7 or 14 days of treatment, which indicated that 7 days of treatment would be sufficient to induce up-regulation at PN28 if it were to occur. We administered nicotine for 14 days, whenever possible, as this is the standard of chronic nicotine exposure in the field when using this exposure method. The minipumps used deliver solution at a rate of 12 μL/day for a duration of 14 days (7 days for PN28). The nicotine tartrate concentration was calculated to provide a dose of 17 mg/kg/day (equivalent to 6 mg/kg/day nicotine free base = 37 μmol/kg/day). As the animals age and pregnancy progresses, the animals will gain weight, and the dose received at the time of sacrifice is less than the dose at implantation. However, the animals are still receiving relatively high doses of nicotine at sacrifice. Based on our measurements, the average weight gained by our female dams was 105g ± 9g at the time of sacrifice. Therefore, these animals were still receiving a nicotine concentration between 5.39 mg/kg/day and 5.74 mg/kg/day nicotine free base. The starting dose has been shown to yield blood levels of 0.56 μmol/L for nicotine and 3.5 μmol/L for cotinine in adult rats(Nguyen et al. 2004), which are comparable to levels achieved in humans who are moderate to heavy smokers. Male and female rats were anesthetized with isoflurane and the minipumps were inserted under sterile conditions into a subcutaneous pocket via a small incision made between the shoulders. While under anesthesia, animals were administered buprenorphine (0.1 mg/kg, s.c.) for post-operative pain. After recovery from anesthesia (10–30 min), animals were returned to individual cages. Fourteen days after minipump implantation (7 days for PN28 animals), animals were anesthetized with isoflurane and killed by decapitation. The brains were then rapidly removed and dissected, and cerebral cortex frozen on dry ice and stored at −80°C until use. Animal use and procedures were approved by the George Washington University Medical Center Institutional Animal Care and Use Committees, and performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Every effort was made to reduce animal suffering and to minimize the number of animals used.
2.2.2 Measurement of nAChR binding sites
Tissues were homogenized in 20 ml Tris buffer (50 mM Tris–HCl buffer, pH 7.4 at 4°C) using a Polytron Tissue Homogenizer (Brinkmann Instruments, Inc., Westbury, NY), at a moderate speed for 15–20 seconds in a large centrifuge tube. The samples were centrifuged twice at 30,000 G for 10 min in buffer and the membrane pellets were resuspended in fresh buffer. nAChR binding sites in the membrane homogenates from the tissues were then measured with 2 nM [3H]EB. Incubations were carried out in 200 μL Tris buffer for 24 h at 4°C. Bound nAChRs were separated from free ligand by vacuum filtration over Brandel GF/B glass-fiber filters (Gaithersburg, MD, USA) that were pre-wet with 0.5% polyethyleneimine; radioactivity on the filters was then measured in a liquid scintillation counter. Non-specific binding was determined in the presence of 300 μM nicotine, and specific binding was defined as the difference between total binding and non-specific binding. Animal numbers were 7–12 for PN84, 6–11 for PN42, and 6–8 for PN28.
2.2.3 Immunoprecipitation assays
Brain tissues from the same animals were used for both standard homogenate binding (above) and immunoprecipitation assays. Homogenates of brain tissues were prepared as above. The membranes were solubilized by incubating the homogenates in 2% Triton X-100 in 50 mM Tris buffer, with gentle rotation for 2 h at 4°C. This procedure results in ~90% solubilization of heteromeric nAChRs in the brain area measured. After centrifuging the mixture at 30,000 g for 10 min, aliquots of the clear supernatant cerebral cortex (equivalent to 10–15 mg tissue depending on the age of the animal) were added to sample tubes containing 2 nM [3H]EB. As all known heteromeric nAChRs display an affinity for [3H]EB between ~30 and 250 pM, this concentration is expected to occupy 86–98% of the nAChRs (Mao et al., 2008). One of the subunit-specific antibodies at an optimal concentration, which had been determined previously by titration in our lab to precipitate the maximum number of nAChRs possible from solution (1:30 [7μl in 200μl total] of mab299, 1:20 [10μl in 200μl total]of mab270 and 1:15 of α5 polyclonal), or 4μl of NRS, or Tris alone was then added. The final volume of the assay was 200 μL. The samples were then rotated gently overnight at 4°C. The next day, 50 μL of a 50% slurry of Protein G Sepharose beads was added and the rotation of the samples at 4°C was continued for another hour. The samples were then centrifuged at 8,000 g for 1 min and the supernatants were removed and filtered over Brandel GF/B glass-fiber filters that had been pre-wet with 0.5% polyethyleneimine. Pre-treating with PEI ensured the retention of solubilized nAChRs onto filters (Mao et al., 2008), which were counted by liquid scintillation and used as an internal control to confirm efficient immunoprecipitation and capture of labeled nAChRs. The immunoprecipated pellets were washed by resuspension in cold 1 mL 50 mM Tris–HCl buffer (pH 7.0), followed by centrifugation at 8,000 g for 1 min. The pellets were then dissolved in 200 μL of 0.1 N NaOH and the radioactivity was measured in a liquid scintillation counter. The counts precipitated in tubes containing NRS, which was used as control for non-specific precipitation of unpurified antiserum, or Protein G alone, which was used as a control for non-specific precipitation of mAbs, were subtracted. Immunoprecipitation assays were carried out to measure nAChR subtypes in the cerebral cortex from saline- and nicotine-treated rats. The calculated number of [3H]EB-labeled nAChRs immunoprecipitated by each antibody plus the remaining solubilized [3H]EB-labeled nAChRs in the supernatant were compared back to total radiolabeled nAChRs in the membrane binding assays. The former typically represented 75–80% of the latter measurements. This would be expected as some nAChRs would be lost due to repeated washing steps in the immunoprecipitation assay.
2.3 Statistics
The comparison between males and females under nicotine and saline treated conditions was made using a three-way ANOVA to define age × treatment × sex interactions based on the calculated density of nAChRs determined by immunoprecipitation (Table 1). We then performed two-way ANOVA in order to further define the age × treatment and sex × treatment interaction, followed by Tukey’s test for post-hoc analysis. Statistical significance was defined as p ≤ 0.05. These results are summarized in Table 2. Animal numbers were n=7 for nicotine and n=12 for saline treated PN84 males; n=8 for nicotine and n=7 for saline for PN84 females; n=6 for nicotine and n=11 for saline treated PN42 males; n=6 for both nicotine and saline treated PN42 females; and n=7 for nicotine and n=6 for saline treated PN28 males; n=6 for saline and n=8 for nicotine treated PN28 females.
Table 1. Three-way ANOVA of Immunoprecipitation Data (1.5 Column).
Three-way ANOVA of all imunoprecipitation data. The interactions of the independent variables ‘Sex’, Age’, ‘Treatment’ and ‘Subunit’ are examined and reported here. The F and p values for each analysis are reported, and statistical significance was determined as p<0.05 and designated by *.
| Three-Way ANOVA | ||
|---|---|---|
| Source | F | Significance |
| Sex | 18.726 | p<0.000* |
| Age | 12.648 | p<0.000* |
| Treatment | 88.235 | p<0.000* |
| Subunit | 418.399 | p<0.000* |
| Sex × Age | 6.630 | p<0.000* |
| Sex × Treatment | 0.772 | p<0.000* |
| Sex × Subunit | 3.375 | p<0.036* |
| Age × Treatment | 6.103 | p<0.003* |
| Age × Subunit | 2.901 | p<0.023* |
| Treatment × Subunit | 22.109 | p<0.000* |
| Sex × Age × Treatment | 11.662 | p< 0.000* |
| Sex × Age × Subunit | 1.405 | p= 0.233 |
| Sex × Treatment × Subunit | 1.174 | p= 0.311 |
| Age × Treatment × Subunit | 1.068 | p= 0.373 |
Dependent variable is amount of [3H]epibatidine-bound nicotinic acetylcholine receptor
Table 2. Two-way ANOVA of Immunoprecipitation Data (1.5 Column).
Two-way ANOVA of results shown in Figures 1 and 2. The effect of ‘treatment’, ‘sex’ and ‘treatment × sex’ interactions for each test (F and p values) are reported. Results were further analyzed by Tukey’s post-hoc test to identify differences between group means. Statistical results from post-hoc analysis are shown as asterisks in Figures 1 and 2.
| Two-Way ANOVA | ||
|---|---|---|
| Test | Effect | Significance |
| PN84 α4 Male vs. Female |
treatment sex treatment × sex |
F=21.81, p<0.0001* F=3.370, p=0.0763 F=0.0007295, p= 9.786 |
| PN84 β2 Male vs. Female |
treatment sex treatment × sex |
F=19.77, p=0.0001* F=1.879, p=0.1810 F=1.386, p=0.2486 |
| PN42 α4 Male vs. Female |
treatment sex treatment × sex |
F=36.94, p<0.0001* F=0.0006630, p=0.9797 F=22.52, p<0.0001* |
| PN42 β2 Male vs. Female |
treatment sex treatment × sex |
F=74.33, p<0.0001* F=1.395, p=0.2507 F=27.56, p<0.0001* |
| PN28 α4 Male vs. Female |
treatment sex treatment × sex |
F=5.573, p=0.0271 F=14.91, p=0.0008* F=8.079, p=0.0092* |
| PN28 β2 Male vs. Female |
treatment sex treatment × sex |
F=7.635, p=0.0111* F=23.65, p<0.0001* F=3.055, p<0.0938 |
Dependent variable is amount of [3H]epibatidine-bound nicotinic acetylcholine receptor
3 Results
3.1 Effect of Chronic Nicotine Treatment on nAChRs in Male Cortex
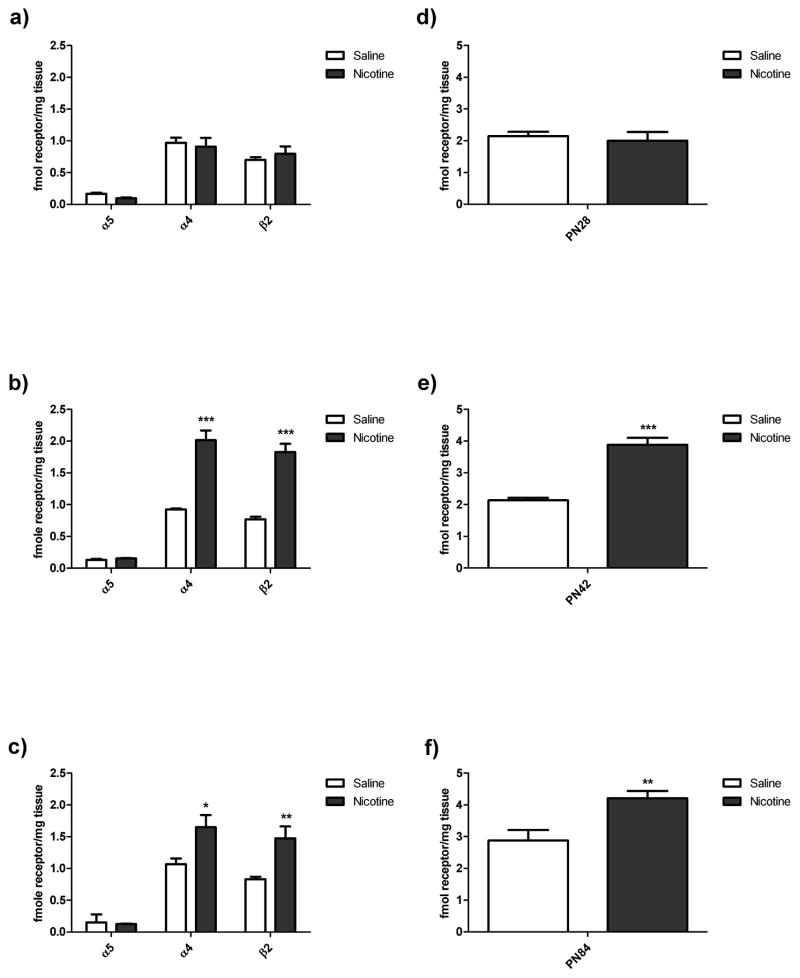
Immunoprecipitation experiments compared the effect of chronic nicotine on α4β2* nAChRs in the cerebral cortex of male rats at PN84 (adult), PN42 (late adolescence) and PN28 (early adolescence) (Figure 1). These data are shown as the effect of nicotine based on the subunit composition. Statistical significance was determined using a Tukey’s post-hoc following a 3-way ANOVA. We observed both α4- and β2-containing nAChRs significantly up-regulate in response to chronic nicotine exposure in adult males, while α5-containing nAChRs showed no response to chronic nicotine exposure in adult males (Figure 1c). These findings are in agreement with our previous report (Flores et al., 1992; Mao et al., 2008). Additional previous work from our lab suggested a reduced up-regulation in PN42 males in response to chronic nicotine (Doura et al., 2008). However, in our study we saw significant up-regulation in both α4- and β2-containing nAChRs (Figure 1b), which was not different from the up-regulation seen in males at PN84. Similarly, in cortex from early adolescent male cortex (PN28), we found significant nicotine-induced increases in nAChRs immunoprecipitated by both the α4 and β2 antibodies, and again no change in α5-containing nAChRs (Figure 1a).
Figure 1. Male Immunoprecipitation and Specific Binding of nAChRs (Double Column).
Immunoprecipitation of [3H]EB-labeled nAChRs using subunit specific antibodies in the cerebral cortex of male rats at PN28 (a), PN42 (b), and PN84 (c) following chronic treatment with either nicotine or saline control. Specific binding of [3H]EB in the cerebral cortex of male rats at (d) PN28, (e) PN42, and (f) PN84 after chronic treatment with either nicotine or saline control. N=6–12, statistical significance determined with ANOVA, *p<0.05, **p<0.01, ***p<0.001.
Confirming the immunoprecipitation data, conventional membrane binding studies with [3H]EB to label all α4β2* nAChRs expressed in the cerebral cortex of PN84 and PN42 and PN28 males indicated significant up-regulation in the number of assembled α4β2* nAChRs at all three ages following chronic nicotine treatment (Figure 1f, 1e, and 1d respectively).
3.2 Effect of Chronic Nicotine Treatment on nAChRs in Female Cortex
In parallel experiments, immunoprecipitation assays were also performed on the cerebral cortex of female rats at PN84, PN42 and PN28 to define the effects of chronic nicotine on α4β2* nAChR expression. As outlined above, statistical significance was determined using a two-way ANOVA to define age × treatment interactions. The calculated numbers of nAChRs under both nicotine and saline treated conditions were compared using a Tukey’s test for post-hoc analysis. Up-regulation of [3H]EB-labeled α4- and β2-containing nAChRs was seen in PN84 female rats following chronic nicotine exposure (Figure 2a). Similar to males, no nicotine-induced change was seen in α5-containing nAChRs in the adult females (Figure 2c). One of the notable differences in comparison to males was the highly significant nicotine-induced up-regulation of α4- and β2-containing nAChRs at 42 days postnatal (Figure 2b). Also, unlike the effect seen in male cortex, no significant change was seen in any of the three subunit-containing nAChRs labeled with [3H]EB at PN28 in female cortex (Figure 2a).
Figure 2. Female Immunoprecipitation and Specific Binding of nAChRs (Single Column).
Immunoprecipitation of [3H]EB-labeled nAChRs using subunit specific antibodies in the cerebral cortex of female rats at PN28 (a), PN42 (b), and PN84 (c) following chronic treatment with either nicotine or saline control. Specific binding of [3H]EB in the cerebral cortex of female rats at (d) PN28, (e) PN42, and (f) PN84 after chronic treatment with either nicotine or saline control. N=6–11, statistical significance determined with ANOVA,*p<0.05, **p<0.01, ***p<0.001.
As was the case in our assessment of the male cerebral cortex, the immunoprecipitation results in the female cortex were mirrored by binding experiments using [3H]EB to label all α4β2* nAChRs. Specifically, the binding results revealed significant up-regulation of [3H]EB-labeled nAChRs occurred following chronic nicotine exposure in adulthood and late adolescence (Figure 2f and e respectively), which is in agreement with the up-regulation of α4- and β2-containing nAChRs seen by immunoprecipitation (Figure 2c and b). In contrast, no significant change was seen at PN28 using either immunoprecipitation (Figure 2a) or receptor binding (Figure 2d) methodology.
3.3 Differences in Male and Female Adolescent Responses to Chronic Nicotine
There was an apparent pronounced difference in nicotine response between the sexes in early adolescence. This was evident by the large increase in α4- and β2-containing nAChRs in the PN28 males (Figure 1a) that was not observed in the female cortex (Figures 2a). Additionally, although we see a nicotine-induced up-regulation of nAChRs in both males and females at PN42, females at PN42 displayed a significantly greater increase in both α4- and β2- containing nAChRs in response to chronic nicotine than males at the same age as determined by two-way ANOVA. In concordance with previous literature, a significant up-regulation was seen in [3H]EB-labeled nAChRs in the adult male cortex (PN84) (Figure 1c), and this was also seen in female cortex at the same age (Figure 2c).
In order to statistically confirm these observed differences, a three-way ANOVA was used to compare the immunoprecipitation results in males and females for each subunit at each age following nicotine or saline treatment (Table 1). These data suggested that there was a significant Sex × Age × Treatment interaction. In order to further clarify the interactions between these variables, we performed a two-way ANOVA. There was a significant treatment effect but no significant interaction (Sex × Treatment) at PN84, indicating no effect of sex on nicotine treatment in adult rats. At PN42, there was a significant sex × treatment interaction where nicotine treatment affected the levels of α4- and β2-containing nAChRs in females to a greater extent than in males at the same age. At PN28, there was also a significant Sex × Treatment interaction in both the α4- and β2-containing nAChRs, indicative of sex differences in response to chronic nicotine exposure. Similarly, Tukey’s post-hoc analyses demonstrated there was a significant treatment effect on α4- and β2-containing nAChRs of males that was absent in females at PN28. Taken together, these data suggest that females at PN42, and males at PN28, may be more sensitive to the effects of chronic nicotine exposure on nAChR density in the cerebral cortex than age-matched animals of the opposite sex.
4 Discussion
Nicotine is one of the most socially acceptable drugs in our society, which makes it an early choice for adolescent experimentation with drugs of abuse. Epidemiological evidence suggests that earlier onset of drug experimentation is associated with an increased likelihood of the development of dependence (Schramm-Sapyta et al., 2009). This study provides a detailed analysis of the differences in how male and female rats respond to chronic nicotine exposure in early and late adolescence. Our data suggest that both age and sex play a significant role in the response of α4β2* nAChRs to chronic nicotine.
In adult male and female rat cerebral cortex, we saw a significant increase in α4- and β2- containing nAChRs, but no significant change in α5-containing nAChRs in either sex following nicotine administration. This confirms previous findings that indicated that α5-containing nAChRs are resistant to up-regulation in the presence of chronic nicotine administration (Mao et al., 2008). Work from other labs has suggested that there is a significant increase in α5- containing nAChRs in the caudal cortex of adult male rats (Moretti et al., 2010). However, these data were specific to the caudal cortex, rather than the cortex as a whole. Our current data reconfirm the previous work from the Kellar lab that showing that in the adult male cortex these nAChRs are resistant to up-regulation (Mao et al., 2008). In addition, our results demonstrate that these nAChRs are also resistant to up-regulation in female adult rats. This is the first characterization of the response, at a subunit-specific level, of adult female nAChRs to chronic nicotine administration in the cerebral cortex. From this we are able to conclude that there was no significant difference between the adult male and female α4β2* nAChR responses to nicotine.
Sex differences in human response to nicotine could arise from multiple sources, including factors that directly affect tissue response to the drug (pharmacodynamics), factors that affect blood plasma levels of the drug (pharmacokinetics), factors that affect men and women’s socially defined roles and factors that affect sex differences in conditioned responses to the drug (Jensvold et al., 1996). Evidence regarding sex differences in pharmacokinetics suggests that, in rats, males display 3- to 5- fold higher hepatic nicotine metabolism than female rats (Kato 1974; Kato 1979; El Defrawy El Masry and Mannering 1974). These pharmacokinetic differences may, however, differ depending on the species studied (Boyland and de Knock 1966; Booth and Boyland 1967; Stalhandske et al., 1969; Jenner et al., 1973; Jenner et al., 1973b). In humans, it has been demonstrated that female nonsmokers excrete more nicotine and less cotinine in their urine than female smokers following IV administration of nicotine hydrogen tartrate (Beckett et al., 1971). In this same study, they emphasize that studying sexual dimorphisms in nicotine metabolism in human chronic smokers may be complicated by the metabolic changes induced by smoking. Therefore, although there are reported species-dependent sex differences in the pharmacokinetic profiles of males and females, it is unlikely that these differences explain all of the epidemiological differences between male and female adolescents in response to nicotine administration. Therefore, we chose to further investigate the possible pharmacodynamics differences between male and female adolescent rats.
Previous work from our lab and others has shown age- and sex-related differences in nAChR expression following nicotine exposure (Doura et al., 2008). Our present work examined nicotine effects on the density and subunit composition of α4β2* nAChRs in the cerebral cortex in early and late adolescent male and female rats exposed to nicotine. Future studies in these ages and sexes are warranted to investigate possible differences in nAChR functionality in various brain regions following nicotine exposure.
Extensive work has shown that brain development is not complete by adolescence. In particular, growth in areas involved in cognition, like the medial prefrontal cortex (mPFC), continues throughout adolescence (Goriounova and Mansvelder, 2012). The development of cortical areas of the brain depends on age and experience and continues into adulthood (Sowell et al., 2003; Giedd, 2004). Because of these developmental differences and the complexity of the study design, we chose to look at the cortex as a whole to characterize α4β2 and α4β2α5 nAChRs in three ages and in both males and females. It will be interesting to investigate whether sex- and age-related differences in nAChR density and subunit composition in the cerebral cortex also exist in other brain regions.
Developmental differences in nAChRs have also been reported previously (Azam et al., 2007). This study showed differences between male rats at PN20 and adult rats in nAChR mRNA. In addition, they found a gradual decline in [3H]nicotine binding after birth. Slotkin and colleagues also reported higher [3H]cytisine binding in the cerebral cortex of PN45 rats compared to PN60 or PN75 rats (Trauth et al., 1999). This group also found that overall binding was higher in females than in males, and that overall binding decreased with age. Additionally, work from Kota and colleagues (Kota et al., 2009) has shown that male mice exposed to nicotine in early adolescence (PND 28–34) showed enhanced reward to nicotine as measured by conditioned place preference (CPP) when compared to middle adolescent (PN 35–48), late adolescent (PN49–58) and adult mice (PN 70+).
Nicotine is a psychoactive compound that may disturb the normal course of brain maturation (Richards et al., 2003). Prior work from our lab using autoradiography indicated that adolescent and adult male rats differ significantly in the density of α4β2* nAChRs following chronic nicotine exposure (Doura et al., 2008). This detailed study allowed regional and nAChR subtype specificity using subtype-specific ligands [125I]α-conotoxin MII, [125I]-5-iodo-3-(2(S)- azetidinylmethoxy)pyridine, and [125I]α-bungarotoxin. In that study, substantially reduced up-regulation of α4β2* nAChRs in the cerebral cortex of adolescents was observed compared to adults. Specifically, adolescents exhibited significant up-regulation in only 4 out of 10 cortical regions, compared to the up-regulation seen in all 10 of the same regions in adult males. These data suggest that nAChRs may vary in their response to nicotine based on age, and that adolescent males may have reduced receptor sensitivity to the effects of nicotine compared to adult males. The data presented in this study do not fully recapitulate the findings reported by Doura and colleagues (Doura et al., 2008), albeit the variations in these data may be explained by the differences in methodology employed by the two studies. In addition, our study examined the cortex as a whole using an immunological approach to define subtype specificity with particular emphasis on the response of α5-containing nAChRs to chronic nicotine at these ages. The present data showed significant up-regulation in the overall binding to α4β2* nAChRs in males during mid-adolescence (PN42) following nicotine treatment, however no differences were seen in the proportion of α5-containing nAChRs at this age. We did see a significant increase in α4- and β2- containing nAChRs at that age in male rats; however using a two-way ANOVA analysis we were able to demonstrate a significant difference in the response to chronic nicotine of both α4- and β2-containing nAChRs between male and female rats at PN42. Similarly, female α4β2* nAChRs showed pronounced up-regulation at PN42. We also observed that saline treated females express a significantly lower density of α4β2* nAChRs compared to saline treated males at every age (see Supplemental Table 1 for summary). These findings indicate that the sex difference in α4β2* nAChR densities in saline treated animals is consistent throughout all three developmental stages and therefore unlikely to account for the sexual dimorphism of nicotine response observed at PN28 and PN42.
Very little other work has investigated the receptor response to chronic nicotine in female rats. Trauth and colleagues used [3H]cytisine to measure the response of membranous α4β2* nAChRs in the midbrain, cerebral cortex and hippocampus to chronic nicotine in PN45 and, after cessation of treatment, in adult PN75 male and female rats (Trauth et al., 1999). They saw no significant difference between males and females in the overall binding of these receptors in adolescence with continuous treatment. Our data agree with what was observed in this paper with continuous treatment in both male and female rats in mid-adolescence. However, the study by Trauth et al., did not investigate the effect of nicotine on early adolescence in either males or females.
There has been virtually no work done on the effect of nicotine on nAChRs at PN28. This may be due to the technical difficulty of treating animals so soon after weaning. We were able to confirm, prior to starting these experiments that 7 days of treatment with nicotine was sufficient to induce nAChR up-regulation in adult male rats equivalent to that seen after the standard 14 day treatment (S1). Therefore, we were able to look at the effect of nicotine at the beginning of adolescence (PN28) in male and female rats. Unlike the results from PN84 and PN42 animals, we observed a clear sex difference to nicotine exposure in PN28 rats. Using a two-way ANOVA we found a significant interaction between sex and treatment in the α4- containing nAChRs of PN28 animals. Although no interaction was seen in the β2-containing nAChRs of PN28 rats, there was still a significant treatment and sex effect for this subtype. As with adult and late adolescent rats, there was no significant change in the density of α5- containing nAChRs following nicotine treatment in PN28 male or female rats. This suggests that, in early adolescence, males display a heightened sensitivity to the receptor effects of nicotine that is absent in females of the same age. These data are in agreement with the CPP observations made by Kota and colleagues (Kota et al., 2009), and may provide a pharmacodynamics explanation for the enhanced rewarding effect observed in males in early adolescence.
4.1 Conclusions
These data emphasize the necessity of looking at both male and female subjects when investigating the effects of nicotine during development. Further work in this area should focus on a more detailed mechanism of action for receptor regulation that involves possible hormonal influences. These data also suggest that nicotine self administration behavior may differ between male and female rats during adolescence, which may, in part, be due to the differential effect of nicotine on nAChRs at these ages. In humans, this may help clarify some of the mechanisms of addiction, and may help explain the behavioral differences observed.
Supplementary Material
Specific binding of [3H]EB in the cerebral cortex of male rats at PN84 following either 7 or 14 days of nicotine exposure or 14 days of saline exposure. One-way ANOVA was used to determine statistical significance. *p<0.05
Two-way ANOVA of immunoprecipitation data from saline-treated animals only. The effect of ‘age’, ‘sex’ and ‘age × sex’ interactions for each test (F and p values) are reported. Statistical significance was determined as p<0.05 and designated by *.
Dependent variable is amount of [3H]epibatidine-bound nicotinic acetylcholine receptor
Highlights.
Male rats at PN28, α4β2* nAChRs in the cortex up-regulate after chronic nicotine
No up-regulation of α4β2* nAChRs in female cortex at PN28 after chronic nicotine
Sex differences in nicotine-induced α4β2* nAChR were observed at PN42
No differences were observed in α5-containing nAChRs at any age or in either sex
Both age and sex play a role in α4β2* nAChR regulation in the cortex of rats
Acknowledgments
This work was supported by NIDA grant DA015767 (DCP and NHL). Special thanks to Dr. Kenneth J. Kellar for his advice and generous contribution. Thanks to Dr. Robert Yasuda from Georgetown University for his help with the α5 and β2 antibodies. Thanks also to Dan DeGuisto for his help with the handling and treatment of the animals.
Abbreviations
- EB
Epibatidine
- mPFC
medial prefrontal cortex
- NRS
normal rabbit serum
- PN
postnatal day
- s.c
sub-cutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bethany G. Hoegberg, Email: hoegberg@gwmail.gwu.edu.
Ermelinda Lomazzo, Email: lomazzo@uni-mainz.de.
Norman H. Lee, Email: nhlee@gwu.edu.
David C. Perry, Email: dcperry48@gmail.com.
References
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003;988:164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Albuquerque EX. α-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. doi: 10.1016/s0006-8993(98)00880-4. [DOI] [PubMed] [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CDC, Nyresa CA, Nashmi R, Mariella DB, Lambe EK. Nicotinic α5 subunits drive developmental changes in the activation and morphology of prefrontal cortex layer VI neurons. Biol Psychiatry. 2012;71:120–128. doi: 10.1016/j.biopsych.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett AH, Gorrod JW, Jenner P. The effect of smoking on nicotine metabolism in vivo in man. J Pharm Pharmac. 1971;23:62S–67S. doi: 10.1111/j.2042-7158.1971.tb08770.x. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. α5/α3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell MEM, Balfour DJK, Andersen JM. Evidence that tobacco smoking increases the density of (−)- [3H]nicotine binding sites in human brain regions. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic α5 subunit deletion locally reduces high affinity agonist activation without altering nicotinic receptor numbers. J Neurochem. 2007;103:204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- Booth J, Boyland E. Nicotine metabolism. Annu Rep Br Emp Cancer Camp. 1967;45:25. [Google Scholar]
- Boyland E, de Knock DH. Nicotine metabolism. Annu Rep Br Emp Cancer Camp. 1966;44:5–6. [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20(10):3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- Delfino RJ, Jammer LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine Tob Res. 2001;3:235–248. doi: 10.1080/14622200110050466. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Defrawy El Masry S, Mannering GJ. Sex-dependent differences in drug metabolism in the rat. Drug Metab Dispos. 1974;2:279–284. [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41(1):31–37. [PubMed] [Google Scholar]
- Fucile S, Barabino B, Palma E, Grassi F, Limatola C, Mileo AM, Alema S, Ballivet M, Eusebi F. α5 subunit forms functional α3β4α5 nAChRs in transfected human cells. Neuroreport. 1997;8:2433–2436. doi: 10.1097/00001756-199707280-00005. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 subunit alters desensitization, pharmacology Ca++ permeability and Ca++ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Front in Synaptic Neurosci. 2012;4(3):1–9. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Wewers ME. Gender differences in tobacco use. Health Psychol. 1991;10:143–153. [PubMed] [Google Scholar]
- Jenner P, Gerrod JW, Beckett AH. Factors affecting the in vivo metabolism of R-(+) and S-(−) nicotine by guniea-pig liver preparations. Xenobiotica. 1973;3:563–572. doi: 10.3109/00498257309151544. [DOI] [PubMed] [Google Scholar]
- Jenner P, Gerrod JW, Beckett AH. Species variation in the metabolism of R-(+) and S-(−) nicotine by α-C and N-oxidation in vitro. Xenobiotica. 1973b;3:573–580. doi: 10.3109/00498257309151545. [DOI] [PubMed] [Google Scholar]
- Jensvold MF, Hamilton JA, Halbreich U. Future research directions: methodological considerations for advancing gender sensitive pharmacology. In: Jensvold MF, et al., editors. Psychopharmacology and Women: Sex, Gender, and Hormone. Washington DC: American Psychiatric Press; 1996. pp. 415–430. [Google Scholar]
- Kato R. Sex-related differences in drug metabolism. Drug Metab Rev. 1974;3:1–32. doi: 10.3109/03602537408993737. [DOI] [PubMed] [Google Scholar]
- Kato R. Characteristics and differences in the hepatic mixed function oxidases of different species. Pharmacol Ther. 1979;6:41–98. doi: 10.1016/0163-7258(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Kellar KJ, Giblin BA, Lumpkin MD. Regulation of brain nicotinic cholinergic recognition sites by nicotine. Prog Brain Res. 1989;79:209–216. doi: 10.1016/s0079-6123(08)62480-2. [DOI] [PubMed] [Google Scholar]
- Kota D, Robinson SE, Damaj MI. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol. 2009;78:873–879. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzi JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann NY Acad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic systems and cognitive function. Psychopharmacology. 1992;108:417–431. doi: 10.1007/BF02247415. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self administration modeled in female rats. Psychopharm. 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans BI, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Laverty DS, Whiteaker P, Salmien O, Grady SR, McIntosh JM, Butt CM. John Daly’s compound epibatidine facilitates the identification of nicotinic receptor subtypes. J Mol Neurosci. 2010;40:96–104. doi: 10.1007/s12031-009-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotox and Terat. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Moretti M, Mugnaini M, Tessari M, Zoli M, Gaimarri A, Manfredi I, Pistillo F, Clementi F, Gotti C. A comparative study of the effects of the intravenous self-administration or subcutaneous minipump infusion of nicotine on the expression of brain neuronal nicotinic receptor subtypes. Mol Pharmacol. 2010;78:287–296. doi: 10.1124/mol.110.064071. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Shioda S, Okuda H, Nakashima T, Nakai Y. Immunocytochemical localization of nicotinic acetylcholine receptor in rat cerebral cortex. Mol Brain Res. 1995;32(2):321–328. doi: 10.1016/0169-328x(95)00092-7. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307(3):1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. Raven Press; New York: 1994. pp. 363–409. [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1999;64:295–299. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiographic studies. J Pharm Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeaux JP, Maskos U, Fratta W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain, regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cousins MM, Seidler FJ. Administration of nicotine to adolescent rats evokes regionally selective up-regulation of CNS alpha 7 nicotinic acetylcholine receptors. Brain Res. 2004;1030:159–163. doi: 10.1016/j.brainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent brain development and animal models. Ann NY Acad Sci. 2004;1021:23–26. doi: 10.1196/annals.1308.002. [DOI] [PubMed] [Google Scholar]
- Stalhandske T, Slanina P, Tjalve H, Hansson G, Schmiterlow CA. Metabolism in vitro of 14C-nicotine in livers of fetal, newborn and young mice. Acta Pharmacol Toxicol. 1969;27:363–380. doi: 10.1111/j.1600-0773.1969.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (α4)3(β2)2 stoichiometry greatly exceeded that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent up regulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Reviews. 1999;30(3):219–235. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8(9):3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specific binding of [3H]EB in the cerebral cortex of male rats at PN84 following either 7 or 14 days of nicotine exposure or 14 days of saline exposure. One-way ANOVA was used to determine statistical significance. *p<0.05
Two-way ANOVA of immunoprecipitation data from saline-treated animals only. The effect of ‘age’, ‘sex’ and ‘age × sex’ interactions for each test (F and p values) are reported. Statistical significance was determined as p<0.05 and designated by *.
Dependent variable is amount of [3H]epibatidine-bound nicotinic acetylcholine receptor