Abstract
Ultrasonography (USG) is a safe, easily available, and cost-effective modality, which has the additional advantage of being real time for imaging and image-guided interventions of the musculoskeletal system. Musculoskeletal interventions are gaining popularity in sports and rehabilitation for rapid healing of muscle and tendon injuries in professional athletes, healing of chronic tendinopathies, aspiration of joint effusions, periarticular bursae and ganglia, and perineural injections in acute and chronic pain syndromes. This article aims to provide an overview of the spectrum of musculoskeletal interventions that can be done under USG guidance both for diagnostic and therapeutic purposes.
Keywords: Interventions, pain, relief, sports, ultrasound
Introduction
The basis of image guided-intervention is the ability to reliably identify the region to be injected (target), confirm placement of the needle or other intervening device at the appropriate location, and subsequently confirm sampling of the appropriate location/lesion or delivery of the appropriate treatment.
Ultrasonography (USG) is ideally suited for musculoskeletal (MSK) interventions as it is convenient, inexpensive, safe, and allows for excellent visualization of MSK anatomy. An additional advantage of USG is the lack of ionizing radiation in comparison to other imaging modalities like fluoroscopy and computed tomography (CT). The time and special equipment required for magnetic resonance imaging (MRI)-guided interventions are also not needed.
This article aims to discuss the numerous ingenious ways in which USG is being used for therapeutic MSK interventions in current orthopedic and sports rehabilitation practice. A detailed review of the indications, technique, and results of individual MSK interventions is beyond the scope of this article. For purposes of this article, we will divide the use of USG in MSK into the areas of:
Muscle
Tendons and tendon sheaths
Ligaments
Bursae and ganglia
Joints
Bones
Nerves.
General Considerations
Partnering and practice building
Good clinical assessment, generating patient confidence and a coordinated diagnostic and rehabilitation plan are paramount to developing a successful practice. Good working relationships with orthopedic/sports medicine physicians are important before embarking on developing a therapeutic interventional practice.
Transducer selection
High-frequency (typically 7-12 MHz), linear array transducers provide a high spatial resolution for optimal visualization of superficial structures in the MSK system. Deep structures such as the hip joint require greater penetration and lower frequency curvilinear probes may be required. In general, the highest frequency transducer that allows visualization of the structure is used. Hockey stick transducers are particularly helpful for imaging superficial structures involving small or irregular parts which have small smooth surfaces for transducer placement, such as fingers, toes, and the ankle malleoli. Using larger volumes of USG jelly also helps in these areas.
Pre-procedure diagnostic assessment
A detailed pre-procedural USG examination of the area of interest (target), including color Doppler, is mandatory to identify and characterize the target and determine its relation to adjacent neurovascular structures before planning the needle trajectory. Initially, it is a good practice to mark the site for transducer placement and the expected site for needle entry with a marking pen. A written informed consent explaining the procedure, its risks, and response is mandatory prior to any procedure.
Sterility
All attempts to maintain stringent sterile conditions must be followed, especially during intra-articular injections, and in some instances, injection in the operating theater may also be considered. Sterility procedures involve cleaning the area thrice with 5% povidone-iodine antiseptic solution and subsequently cleaning the site and transducer with any generic 75% propanol containing or other disinfectant. The antiseptic skin solutions also provide a fluid interface for optimal visualization of the underlying structures. 5% povidone-iodine jelly may be used on the transducer during the procedure and provides a thicker interface. Sterile gloves are used and drapes may be applied to appropriately confine the area. Sterile probe covers can also be used if available.
Needle selection
This is based on the type of intervention being done. Larger needles (16-20 G) are generally required for aspiration of suspected thick material such as pus, ganglia, or organized hematoma. Smaller needles (22-24 G) suffice for most injections, but are inappropriate for aspirations unless the aspirate is thin. Needle visualization by USG can be improved by selecting a larger needle, and hence, in our practice, we commonly use a 22 G needle for most interventions. Furthermore, we find that the 22 G needle bends less than thinner needles allowing for a straight trajectory and accurate placement. Once experienced, thinner needles can be used to decrease patient discomfort.
Technical tips
Most MSK procedures are performed with a free-hand technique,[1] which allows direct, dynamic visualization of the needle and its tip throughout the procedure. The needle is best visualized when its long axis is parallel and in line with the long axis of the transducer face and in this plane, it is seen as a linear echogenic structure with reverberation artifact distally. In cases where the needle may be difficult to identify, holding the needle steady while moving the transducer helps in identifying the needle. Alternatively, the needle may be repositioned to run along the longitudinal axis of the transducer. Marking the skin site prior to cleaning and introducing the needle as close to the transducer as possible are good ways to ensure needle visualization [Figure 1A and B].
Figure 1 (A and B).
Needle visualization: (A) The needle is best visualized when its long axis is parallel and in line with the long axis of the transducer face and in this plane, the needle is seen as a linear echogenic structure with reverberation artifact distally (B) If the needle is not parallel to the long axis of the transducer as is often seen in deep-seated targets, it may be difficult to identify. Visualization can be improved either by holding the needle steady while moving the transducer to identify the needle or by repositioning the needle to run along the longitudinal axis of the transducer
Another less commonly used method is the safe technique method.[1] The area of interest is scanned by USG, its maximum dimensions are marked on the skin both in longitudinal and transverse planes, and a needle is inserted through the center of the cross made by joining these lines.
Injectables
A variety of agents are available for injection during USG-guided procedures as follows:
Local anesthetics
They are the most commonly used medications in MSK intervention and can be used for diagnostic purpose (diagnostic blocks) and pain relief, usually in combination with a steroid. Lignocaine hydrochloride 2% is commonly used, has a rapid onset within 1-2 min and a duration of action of up to 1 h.[2] Bupivacaine hydrochloride has a slower onset within 30 min, but a longer duration of action, lasting up to 8 h.[2] Newer local anesthetics like ropivacaine and levobupivacaine are also available for use in preservative-free forms. Although expensive, ropivacaine is associated with less motor blockade[3] compared to bupivacaine, making it ideal for perineural and postoperative analgesia. Ropivacaine is also associated with less cardiotoxicity,[4] and levobupivacaine with less cardiac and neurotoxicity,[5] as compared to bupivacaine.
The amount of local anesthetic injected varies depending on the type of procedure and the part being injected. Typically 1-2 ml of local anesthetic solution is used in combination with steroid[6] for a longer duration of action. Intra-articular local anesthetic injections have been reported to cause chondrolysis[7,8,9] and should be diluted (with normal saline), especially in larger joints where the injected volume could be larger. Ropivacaine and lidocaine are less chondrotoxic than bupivacaine.[10]
Most commonly available local anesthetics contain methylparaben as a preservative, which can be neurotoxic.[11] When performing perineural injections, preservative-free forms of these should be used.
Corticosteroids
These are potent anti-inflammatory and pain-modulating drugs that can be injected into articular, periarticular, intra- or peritendinous, and soft tissue structures, providing symptomatic relief in several MSK disorders like arthritis, bursitis, tenosynovitis, tendinopathies, stenosing tenosynovitis, entrapment neuropathies, and ganglion aspirations. Corticosteroids can be injected with the goals of short-term and medium-term pain relief, reduction of inflammation, and improved mobility. Steroid injections usually take 48-72 h to be effective and, hence, are combined with local anesthetics for a rapid onset of action. An important point is that steroid injections do not provide long-term pain relief and usually do not alter the course of underlying disease, hence should be used in conjunction with rehabilitative exercises.
Contraindications for the use of MSK injections include infection, known allergy or hypersensitivity to injectate, or known coagulopathies.[2]
Sterile synovitis (post-injection flare) is the most common local complication following an intra-articular steroid injection and has been attributed to the particulate nature of the injectate.[12] Patients should be counseled about this possibility and analgesics can be prescribed for pain relief. Other less common side effects of steroids are debatable and include effects on the articular cartilage, such as thinning and chondromalacia,[9] tendon ruptures after intratendinous[13,14,15,16] and, less commonly, peritendinous injections.[14,17,18,19] Local intradermal and subcutaneous injections can cause local skin atrophy, fat necrosis, and skin depigmentation.[20,21]
The specific choice of corticosteroid and the frequency of injection are guided in most instances by the clinician's experience with various therapeutic agents and steroid pharmacokinetics and there is limited systematic evidence available for the same.[2,22,23] Less-soluble, branched hydrocortisone esters like triamcinolone and methylprednisolone remain at the injection site longer, with a longer duration of action,[24] but are more likely to give rise to cutaneous adverse effects, as compared to unbranched esters.[2]
The most commonly used corticosteroids are triamcinolone acetonide and methylprednisolone acetate. Triamcinolone has a longer duration of action and is preferred for intra-articular injections.[2] Methylprednisolone is less soluble, shorter acting, and less prone to causing skin atrophy than triamcinolone,[24,25,26,27] and is therefore preferred for lesions near the skin surface.[28,29] Betamethasone has a high solubility, rapid onset of action, and less cutaneous side effects,[2] hence preferred for soft tissue injections.
The degree and duration of symptom relief following steroid injections varies among individuals and depends on the condition being treated. Repeat injections are advocated only if there is a significant relief following a single injection. There is no fixed consensus on the frequency and interval between repeat injections, but an interval of 3 months is preferred.[2]
Preservative-free triamcinolone and methylprednisolone without methyl paraben are available and should preferably be used during perineural injections. The most commonly used steroids in MSK interventions have been listed in the Table below[30] [Table 1].
Table 1.
Commonly used steroid preparations in musculoskeletal interventions

Autologous blood and platelet-rich plasma
Autologous blood injections contain fibroblast growth factors, which can restart a stalled healing process, modulate tissue healing, and have been used successfully in treatment of refractory medial and lateral epicondylitis of the elbow[31,32,33] and rotator cuff tears.[34,35,36] In autologous blood injections, a small amount of blood (2-3 cc) is taken from the patient's arm and injected back into the degenerative portion of the tendon under USG guidance. This provides the relatively avascular tendon tissue with healing growth factors that are otherwise difficult for the body to deliver because of the poor blood supply.
Platelet-rich plasma (PRP) injections have received significant media attention in MSK practice after many well-known professional athletes have used the same for rapid recovery from chronic tendinopathies and muscle and ligament injuries.[37] PRP is created from an autologous blood sample through a platelet separation and concentration process. A larger volume of blood (about 20-60 ml) is drawn from the patient, centrifuged to remove the red blood cells, and the concentrated platelets are harvested. Around 3-5 ml of PRP is locally injected into the injured tissue and modulates tissue healing by targeted release of growth factors like platelet-derived growth factor-BB, transforming growth factor-1,[38] vascular endothelial growth factor, endothelial growth factor, and insulin-like growth factor-1, which are chemotactic and mitogenic.
In the last few years, increasing interest in the concentration of individual growth factors has led to a variety of processes for producing PRP and PRP-like products.[38,39] The details of these are beyond the scope of this article and it is suffice it to say that the user should be aware of what they are injecting before they do so.
Autologous PRP minimizes the risk of transmissible diseases. The most common complaint is pain at the injection site from the ensuing local acute inflammatory response. PRP is contraindicated in thrombocytopenia or platelet dysfunction.
PRP has shown the greatest potential in treatment of chronic tendinopathies, commonly the common flexor/extensor origin at the elbow,[31,32,33,40] rotator cuff,[34,35,36] hamstring origin, patella (jumper's knee), and Achilles tendon.[41,42,43] Chronic epicondylitis has been reported to show 60% improvement in pain scores versus a 16% improvement observed in control patients.[40] Plantar fascitis has also shown good results in a study by Barrett et al., with 77.9% symptomatic improvement at 1 year.[44,45] PRP injections in chronic Achilles tendinopathy have shown variable results; some studies have reported faster recovery in athletes compared to controls.[41,42,43,46,47] PRP has shown beneficial effects in management of acute muscle injuries with faster recovery and is used among professional athletes during season.[48,49,50] The beneficial role of PRP in promoting bony proliferation and healing[51,52,53] articular cartilage has been reported in a few studies.
Recommendations for PRP use are constantly in evolution and currently no fixed guidelines exist for the same.[54] PRP is not a well-regulated substance and is used off label many times.
Prolotherapy
This is another promising treatment option where injection of an irritant solution (the proliferant) into a tendon or ligament incites a local inflammatory response[55] resulting in fibroblast proliferation and collagen synthesis, thereby encouraging healing and restoration of tensile strength. Fifteen percent hyperosmolar dextrose is a safe and inexpensive proliferant solution used in prolotherapy.[56]0.5 ml or less solution is injected into any one lesion/area; however, several lesions may be injected during a single treatment session. A point of note is that symptomatic improvement and imaging appearance are not necessarily correlated. Prolotherapy has been used to successfully treat various MSK syndromes,[56,57] including intervertebral disc disease,[58] mechanical low back pain,[58] plantar fasciitis,[59] refractory tendinopathies, especially lateral and medial epicondylitis,[60] Achilles tendinopathy,[57,61] and osteoarthritis-related pain.[62,63,64] Prolotherapy may be supplemented by agents such as PRP.
Viscosupplementation
Intra-articular injection of hyaluronic agent derivatives is increasingly popular for the alleviation of osteoarthritic symptoms.[65] Although the mechanism of action is not precisely understood, it aims to replace what is believed to be an important factor of joint lubrication. Common reactions to these injections include a significant flare, especially if injected extra-articularly. USG allows accurate delivery of these agents, minimizing the side effects.
Post-procedural instructions
After routine steroid injections, patients are advised to limit activity for 48-72 h and use local ice packs and analgesics for rare post-injection “flares,” which may be seen 2-4 h after the injection when the local anesthetic effect wears off and before the effect of the steroid sets in. Immediate resolution of symptoms following the injection is graded as a percentage of the pre-injection pain and confirms the appropriateness of the injection.
Following PRP or prolotherapy, rehabilitation programs vary greatly among practitioners. For tendon and muscle injections, following a 1-4 week rest period, there is graded return to activity, involving a good stretching program followed by an eccentric loading program. Post-procedure instructions also include avoiding use of nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief as they may inhibit the PRP-facilitated or dextrose-stimulated inflammatory reaction.
Interventions in Muscles
Muscle strains are a common source of pain and dysfunction, particularly with athletes. Muscles are rich in blood supply and generally heal with usual care; however, in an elite or impatient recreational athlete, faster return to activity may be desired. A systematic approach to muscle injuries is as follows.
Acute/subacute injuries
In acute/subacute grade II or III intramuscular injuries where there is no tendon avulsion, the role of PRP is being increasingly established for better pain relief, faster healing, and quicker return to play.[48,50,66] If there is a large associated hematoma, it is aspirated prior to the injection of 5-10 ml of PRP, depending on the size of the injury. Multiple injection sites may be considered for longer-length injuries.
In acute (traumatic) injuries, steroid/anesthetic injections can provide early pain relief and rehabilitation,[67] allowing objective assessment of the extent of injury. The downside of anti-inflammatory agents is delayed healing and scar formation, thereby risking re-injury.
Chronic injuries
Few patients with an old muscle injury present with a focal area of persistent pain, attributed to focal scar tissue. This may be identified on USG directly or by dynamic assessment during muscle contraction, and can be treated with guided trigger point injections. Trigger point injections have been proven useful to relieve myofascial pain.[68,69,70,71,72] USG guides accurate needle placement, improving the success rates and minimizing complications from inadvertent needle placement, especially in cervicothoracic injections where pneumothorax is a risk.
Interventions in Tendons and Tendon Sheaths
Tendon disorders can be a cause of pain and disability in recreational or professional athletes and in sedentary people. Tendon disorders include acute inflammatory tendonitis, chronic tendinopathies (calcific and non-calcific), and tenosynovitis (tendon sheath inflammation). Several ingenious non-surgical methods have been developed for management of these and USG plays an important role in guiding these therapies. Almost all tendinopathies are accessible to sonographic treatment. A general approach to tendinopathies is as follows:
Assess the tendon: A diagnostic sonographic assessment of the tendon is important to ensure that the tendon is not at risk of further damage. In large tendons like hamstrings, it is worthwhile to get a magnetic resonance imaging (MRI) done if one is not confident of tendon morphology sonographically. Finally, one must ensure that there is no ongoing infection, which could have disastrous consequences from a steroid or other injections
Acute/chronic tendinosis: mostly, these occur due to repetitive stress and/or abnormal biomechanics. Here, the injection is used to break this cycle and allow for appropriate rehabilitation measures enabling return to activity. USG-guided steroid injections can be given into the tendon sheath/adjacent bursa followed by rest and a graded physiotherapy program. Volumes of steroid and local anesthetic injected vary; usually, the approximate equivalent of 20-40 mg of triamcinolone acetonide is used.
Steroid injections show considerable symptomatic improvement, even after a single injection, and are the preferred treatment for de Quervain's tenosynovitis,[73,74] trigger finger,[74,75] and stenosing tenosynovitis of flexor hallucis longus[76] and peroneal tendons. Rotator cuff tendinosis (non-calcific)[77,78] also responds well to subdeltoid bursal injections. Peritendinous steroids are also effective in the management of medial[79] and lateral epicondylits[80] [Figures 2A-C and 3A and B].
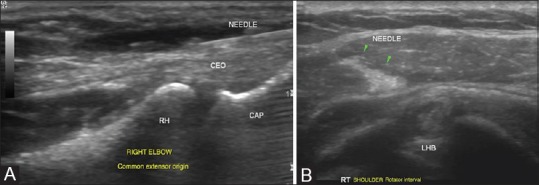
Figure 2 (A-C).
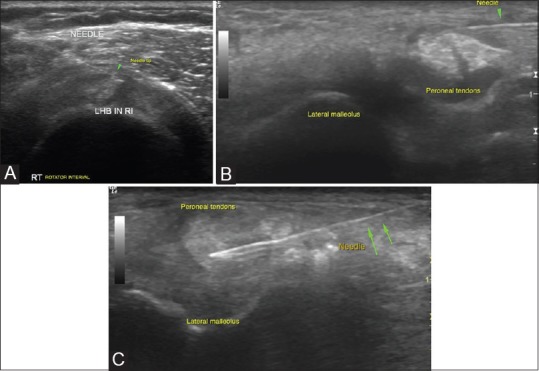
Tendon sheath injections: (A) Biceps tendon is identified in transverse in the bicipital groove and is traced till its intra-articular portion in the rotator interval, where targeted peritendinous steroid injections are given in cases of tendinosis. Rotator interval is also targeted for injections in adhesive capsulitis which is not controlled by medical therapy alone (B) Peritendinous steroid injections around the peroneal tendon are given as it traverses in the retromalleolar groove or at the site of maximum pain (C) Intratendinous injections with steroid or PRP injections have been attempted in some cases of tendinosis as in the above example of peroneal tendinosis
Figure 3 (A and B).
Peritendinous injections for release of trigger finger: (A) Trigger finger is caused by a tenosynovitis of the digital flexor tendons and presents with snapping fingers. Peritendinous steroid injections provide rapid symptomatic relief by reducing the inflammation. USG guidance, especially in smaller joints, aids and confirms an accurate delivery of the injectate into the area of interest (B) Peritendinous fluid distension confirms delivery of injectate which can be visualized real time with USG
Persistent/recalcitrant Tendinopathy
Autologous blood and PRP: Sometimes, especially in cases of tennis elbow or Achilles tendinopathy, despite multiple steroid and local anesthetic injections, symptoms persist. In such cases, the tendon is thought to be “stuck” in a state of “dysrepair” and PRP injections are thought to “kick-start” and accelerate the healing cascade.[81] In such situations, the injured tendon is gently or vigorously needled to re-create an injury and PRP injected into the tendon and adjacent structures acts as a catalyst in recovery. Generally, about 2-5 ml of PRP is injected into the tendon, depending on its size and an additional volume, if present, is injected into the tendon sheath. Some practitioners prefer using PRP primarily and not closely following a steroid[82,83] or local anesthetic[82,84] injection. In our practice, we only deliver PRP at least 4-6 weeks after the last steroid injection. Due to cost constraints, we often attempt a steroid and rehabilitation protocol before PRP. PRP has been consistently found useful in the treatment of various tendinopathies, including common extensor tendinosis.[31,32,33] There is no fixed consensus on the need and interval for repeat PRP injections for tendinopathies.[54] In our practice, we assess the patient 46 weeks after the first PRP injection to quantify symptomatic relief. In patients with significant (above 80%) symptomatic relief, usually a single PRP injection is administered and is followed by a graded rehabilitation program. In partially responsive cases, a second PRP injection can provide additional relief, especially when rehabilitative measures have not produced significant improvement. In less-responsive/unresponsive cases (below 20% improvement), we avoid further PRP injections
Prolotherapy: The general principles of prolotherapy (or reconstructive therapy) are similar to PRP,[55] except that usually an irritant, commonly 15% dextrose or normal saline, is injected to initiate an inflammatory response, produce re-injury, and stimulate a stalled healing response within the damaged tissue. There are various studies on the efficacy of prolotherapy in the management of chronic tendinopathies with good results in lateral epicondylitis,[60] but not enough data to develop an appropriate treatment regimen
Sclerotherapy: Neovascularity has been associated with painful tendinopathies and injection of sclerosing agents into these neovessels has been attempted. It is postulated that sclerosis of the neovessels in inflamed tendons and possibly the surrounding nerves is responsible for the pain relief associated with tendinopathies. Polidocanol is a widely used sclerosing agent and has been tried in the treatment of patellar and Achilles tendinopathies with promising results[85,86,87,88,89]
Fenestration or percutaneous tenotomy: chronic recalcitrant tendinosis, especially common extensor tendinopathy (tennis elbow), is ultimately treated with an open or arthroscopic selective tendon release. Since the pathologic tendon can be identified under USG and the patient is able to target the specific site of pain, an USG-guided tendon release using an 11-G scalpel or repeated fenestration of the tendon with a needle can also be performed.
The goal of this treatment is to convert a chronic, non-healing injury into an acute injury with increased healing potential. During tendon fenestration, the overlying area is anesthetized and the injured tendon is needled to create small fenestrations and induce bleeding, resulting in local release of platelets and growth factors to promote healing. Tenotomy procedures can be performed independently or combined with local PRP or dextrose injections. A study conducted by Mayo Clinic researchers found better results in over 70% patients treated with a combination of tenotomy and PRP injections, with 76% pain improvement.[90,91]
A similar technique has been described by McShane et al.[92] for USG-guided carpal tunnel release and has been described in the nerves section.
Newer advances: Matrix metalloproteinases (MMPs) have been implicated in chronic inflammation. Recent research shows that excess amounts of some MMPs have been found in patellar and rotator cuff tendinopathies and have been linked with the chronicity of these conditions.[93,94] Local injection of MMP inhibitors has been implicated to modulate tissue healing, hence providing an alternative treatment for chronic tendinopathies. Aprotinin (Trasylol), a broad-spectrum MMP inhibitor (normally used during cardiac surgery), was injected into the peritendinous space in patellar and Achilles tendinopathy and provided better pain control than corticosteroid or placebo injections.[95] The main side effect is a hypersensitivity reaction caused by repeated use; hence, recurrent use is strongly contraindicated for a year after exposure. We have never used these in our practice.
Calcific Tendonitis
This occurs secondary to the deposition of calcium hydroxyapatite crystals and usually affects the rotator cuff, the commonest site being the critical zone of the supraspinatus tendon. It is usually a self-limiting condition in which the pain is from leakage of calcium hydroxyapatite into the adjacent synovium. After a period of worsening pain, the condition usually improves with organization or resorption of the calcification;[96] however, some patients may develop chronic pain, disability, and functional impairment. Symptomatic improvement correlates well with resolution of the calcification[97,98,100,101] and treatment strategies are directed to promote removal of the deposited calcium. The clinical symptoms, imaging appearance, and physical consistency of the calcification differ significantly depending on the phase of disease (precalcific, calcific, and postcalcific stages).[102] USG plays an important role in the identification and characterization of the calcifications and subsequent conservative management strategies. Treatment approach in hyperacute and acutely symptomatic stages includes peritendinous/bursal steroid and local anesthetic injections to alleviate pain and explaining to the patient that the condition is self-limiting. In subacute and chronic cases, a barbotage can be attempted, which involves advancing an 18-20 G needle into the calcification and “pulsing” it with normal saline to dissolve the non-soluble calcium hydroxyapatite into soluble sodium hydroxyapatite and calcium chloride. This is usually followed by a bursal/adjacent steroid injection to limit the flare response. Barbotage is sometimes challenging when the calcification is mature and ossified, and radiographs are also helpful in determining the character of the calcification. We have attempted barbotage therapy for chronic calcific lateral epicondylitis in one case, with reduction in the size of the calcification on post-procedural radiographs and symptomatic improvement [Figure 4A-F].
Figure 4 (A-F).
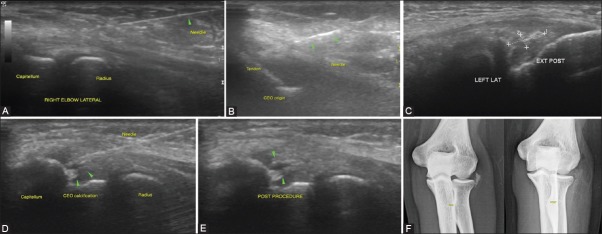
Spectrum of interventions in tennis elbow: (A) In acute tendinosis with minimal underlying tendon abnormality on USG, peritendinous steroids and local anesthetics are used for management, followed by appropriate physiotherapy (B) In cases of acute tendinosis with underlying tears at the common extensor origin, intratendinous steroids or PRP (especially in athletes) have also been attempted accompanied by a graded rehabilitation program (C-F) In chronic calcific tendonitis, a barbotage therapy which involves breakdown of the calcific deposit seen in (C) with continuous needling and irrigation with normal saline (D) till the deposit becomes smaller or more diffuse as seen in (E). (F) Comparison of pre- and post-procedure radiographs of the patient showing a reduction in the size and density of the calcific deposit following the procedure
Interventions in Ligaments
Histologically, ligaments are similar to tendons and undergo similar patterns of degeneration/injury. USG-guided PRP injections have been tried for rapid healing of ligamentous injuries[103] in sports medicine [Figure 5A and B].
Figure 5 (A and B).
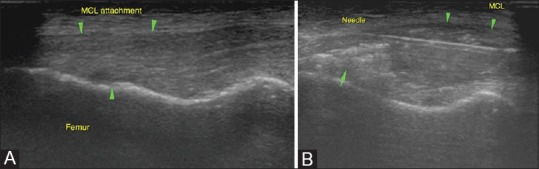
Acute MCL injuries: (A) Diffusely thickened and altered echotexture of the MCL near its femoral attachment in the left knee with an undersurface hypoechoic area suggesting a tear (single arrow) (B) Intraligamentous PRP injections in acute injuries have been beneficial in athletes, especially during the sporting season wherein rapid recovery and return to activity is desired. Targeted injection of PRP is given at the site of the injury
A relatively unique form of degeneration, seen more in ligaments than tendons, is mucoid degeneration with the formation of intraligamentous and periligamentous cysts/ganglia. These are discussed in greater detail in the next section [Figure 6A and B].
Figure 6 (A and B).
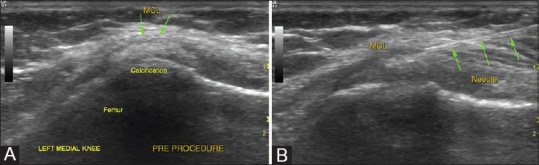
Painful Pelligrini-Stieda lesion: (A) USG examination shows a calcified focus within the fibers of the MCL referred to as Pelligrini-Stieda lesion (B) Local needling of the calcification followed by steroid injections can be done in occasional cases when these lesions are a cause of unresponsive pain
Interventions in Bursae and Ganglia
Bursae may be synovial-lined or adventitious, and can often be a cause of pain. Under USG guidance, these potential spaces can be identified and the needle position is confirmed, and during injection, distension of the potential space can be observed dynamically confirming an appropriate site of injection. Bursal injections are often performed for the subacromial bursa of the shoulder and the greater trochanteric bursa of the hip and almost always, they are injected with steroid and local anesthetic. In some instances, especially when the tendon has been fenestrated, PRP may also be injected. Subacromial bursal injections are routinely given in the management of tendon disorders, subacromial bursitis, and impingement syndromes[104] and have shown good results. Steroid and local anesthetic injections provide significant symptomatic relief in trochanteric bursitis.[105,106,107] In the knee joint, USG-guided aspirations/injections of the pes anserine bursa, semimembranosus medial collateral ligament bursa, and Baker's cysts have been done with relief of symptoms [Figure 7A-C].
Figure 7 (A-C).
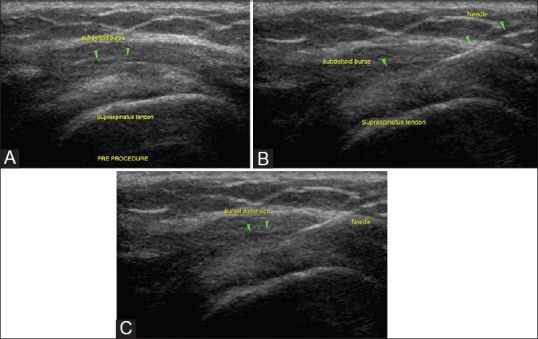
Subacromial/subdeltoid bursal injections: (A) Subacromial bursa is superficial and easily amenable to USG injections in the management of tendon disorders, subacromial bursitis, and impingement syndromes (B) Needle position within the bursa is confirmed prior to injection (C) Bursal distension during the injection is confirmatory of accurate delivery of injectate
Ganglia are outpouchings of synovium or may occur from degeneration of fibrocartilage in ligaments (scapholunate or anterior cruciate) or menisci and labra. They may cause pain from the underlying lesion itself (degeneration/tear) or secondary to the mass effect on adjacent structures. USG examination helps in identifying these ganglia, differentiating them from intra-articular joint fluid, and for targeted aspiration/injection. As these ganglia develop over time, their contents are often extremely viscus and gel like, making aspiration difficult. In these cases, repeated fenestration of the ganglia and injection of a small amount of steroid and local anesthetic into them can be attempted.[108] Ganglia, however, have a tendency to recur and so, we most often use these as the first attempt to alleviate the patients’ symptoms and make them aware that this is the most non-invasive approach to management. Adjunctive measures like hyaluronidase instillation prior to aspiration[109] and post-aspiration steroid injections[110] have been suggested to improve the success rates of ganglion aspirations.
In our practice, in addition to the easily accessible wrist ganglia, we commonly use USG guidance for the aspiration/fenestration and injection of ganglions associated with the cruciate ligaments[111,112] and meniscal cysts[113] with good symptomatic relief. Persistent pain from iliotibial band friction syndrome also responds to local injection therapy. We have treated many such cases [Figures 8-10A-C].
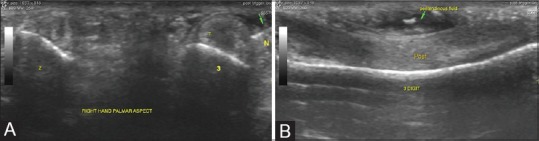
Figure 8(A-C).
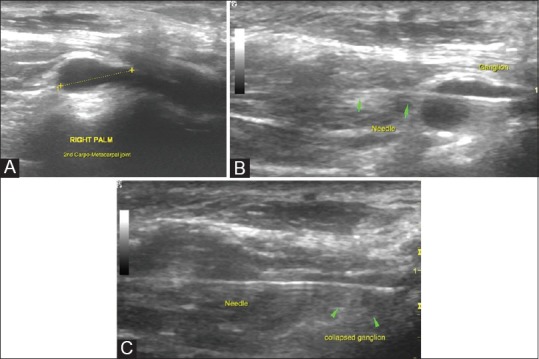
Aspiration of wrist ganglia: (A) USG examination revealed a ganglion on the dorsal aspect of the wrist overlying the scaphoid and trapezoid bones (scapho-trapezio-trapezoid ganglion) (B) A slightly thicker needle is used since the material within a ganglion is thicker and repeated fenestration of the ganglion may be required till reduction in size is confirmed (C)
Figure 10 (A-C).
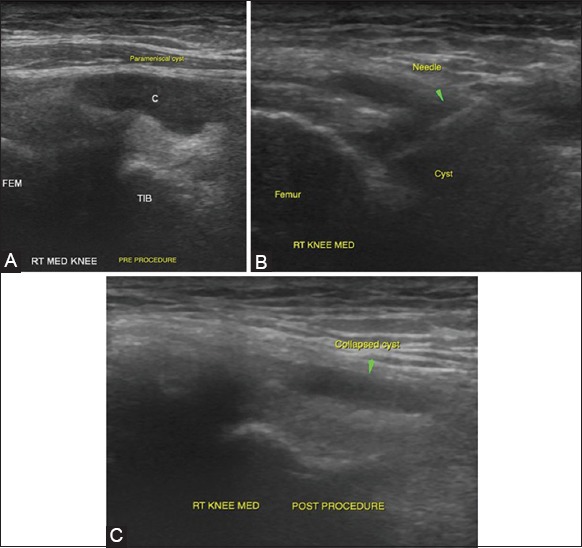
Parameniscal cyst aspiration: (A) Parameniscal cysts are relatively superficially located in the knee joint and can be visualized in the medial and lateral femoral gutters when scanning along the joint lines. The figure demonstrates a medial parameniscal cyst (B) The cyst is easily accessible with USG guidance. Needle position is confirmed by real-time visualization of the needle (C) Post-procedural scan shows near-complete collapse of the cyst
Figure 9(A-C).
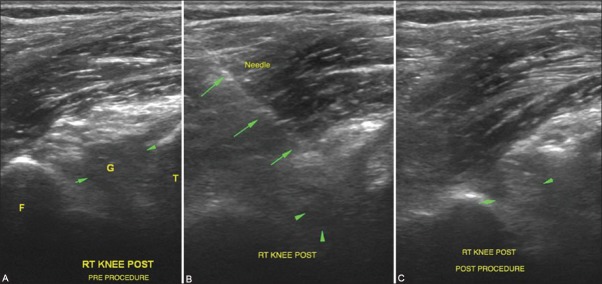
Aspiration of intra-articular ganglia related to the cruciates: (A) Deep-seated ganglia related to the cruciates in the knee are difficult to visualize. Anterior cruciate ligament ganglion is seen as a hypoechoic intra-articular area (G) when scanned from the posterior aspect of the knee joint (B) Needle position can be confirmed within the ganglion and aspiration/fenestration is attempted till reduction in size or alteration in echogenicity is visualized as in (C). This is then followed by a steroid injection
Interventions in Joints
The most common applications of USG interventions around the joints include diagnostic and therapeutic aspiration of intra-articular and periarticular fluid and collections, synovial biopsy, injection of local anesthetics to confirm a clinical diagnosis (diagnostic blocks), and injection of steroids and other drugs into joints and bursae for therapeutic purpose.
Joint aspirations
USG has been shown to be effective in guiding difficult joint aspirations throughout the body.[114,115] Comparison studies in cases of suspected joint effusions have reported a 97% success rate with USG-guided procedures and only a 32% success rate with blind procedures.[116]
Targeted approach with USG guidance is especially beneficial for deep-seated joints like the hip and shoulder and smaller peripheral joints like the acromioclavicular and small joints in the hands and feet.[117] Studies have shown that USG-guided aspiration had a higher accuracy and success rate in smaller peripheral joints[116] and in aspiration of hip joint effusions.[118] An additional advantage of USG being real time is in osteoarthritic joints where the needle can be manipulated under an osteophyte or osseous spur during the procedure itself [Figures 11 and 12A and B].
Figure 11.
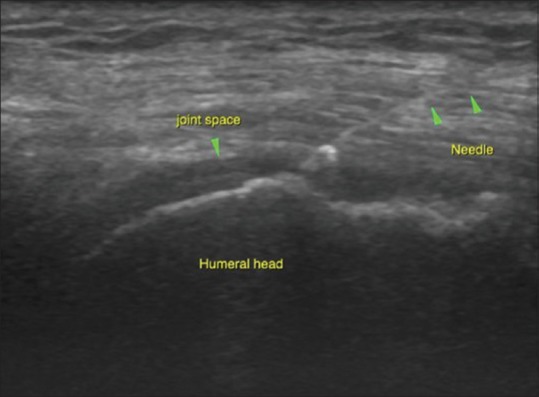
Targeted approach with USG guidance is especially beneficial for deep-seated joints like the hip and shoulder. The image shows accurate needle placement into the glenohumeral joint space for intra-articular injections
Figure 12 (A and B).
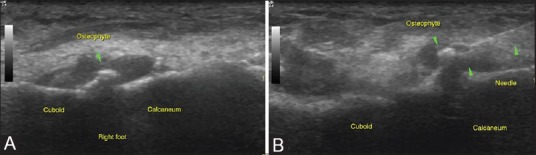
Targeted approach with USG guidance is especially beneficial for smaller peripheral joints like in the hands and feet: (A) USG images of the calcaneocuboid joint in the right foot demonstrate osteoarthritic changes with an overhanging cuboid osteophyte (B) An additional advantage of USG being real time is in osteoarthritic joints where the needle can be manipulated under an osteophyte or osseous spur during the procedure itself
Intra-articular and periarticular injections
USG guidance can be used for intra-articular injections of local anesthetic, corticosteroids, and drugs, both for diagnostic and therapeutic purposes.[119] Aspiration of the joint or bursa prior to injection avoids dilution of the injected medication. Free flow of the injectate and visualization of intra-articular or bursal fluid distension confirms optimal needle placement. The total volume of injectate depends on the size of the joint. Larger joints like the hip and shoulder can easily receive 10 ml, whereas the small joints of the hands and feet may take less than 1 ml. In all cases, injection should be terminated if the patient complains of excessive discomfort.
Diagnostic blocks are performed by injecting a small amount of anesthetic into a joint or bursa and then clinically assessing for improvement in symptoms after the procedure. Pain relief is graded on a verbal scale of 1-10 in comparison to the degree of pain before the injection. Symptomatic relief from intra-articular injection of local anesthetic confirms internal derangement as the source of pain[1] and, in many cases, is associated with improved outcome after surgical intervention.[120,121]
Combined intra-articular steroid and local anesthetic injections are used for short and medium relief of joint disorders and are commonly recommended for acute inflammatory monoarthritis, osteoarthritis,[122] frozen shoulders, and impingement syndromes. Common sites of injection for degenerative joint disease in sports include the knees,[122] ankles, shoulders, acromioclavicular joints, first metacarpophalangeal and metatarsophalangeal joints, as well as lumbar facet arthropathy, with better results seen among the smaller non-weight-bearing joints than the weight-bearing joints. Patients should be counseled that steroid injections alleviate pain and do not alter the course of the underlying pathology (except in inflammatory articular disorders). Steroids decrease inflammation and prevent capsular adhesion by fibrinolysis, when injected intra-articularly in frozen shoulder[68,123] and impingement syndromes. Steroids also help in postoperative intra-articular analgesia, especially for the knee and shoulder, making arthroscopy more acceptable as an outpatient procedure.[124]
Subtalar joint steroid injections[125,126] have been attempted for degenerative and inflammatory arthritis and impingement syndromes. Intermetatarsal ganglion cysts[125,126] can also be aspirated under USG guidance using either a plantar approach or a dorsal approach through the intermetatarsal space. The acromioclavicular joint space is also easily identified and accessible for USG-guided aspirations and steroid injections.
Viscosupplementation involves the injection of hyaluronic acid, or a derivative, directly into afflicted joints in the treatment of osteoarthritis.[65] Although the precise mechanism of action is not entirely understood, it aims to replace important factors of joint lubrication. Intra-articular hyaluronic acid injections should be considered in patients with significantly symptomatic osteoarthritis who have not responded adequately or developed an adverse reaction to standard nonpharmacologic and pharmacologic treatments.[127,128]
Intra-articular PRP injections have been used in postoperative cases of anterior cruciate ligament (ACL) repair,[129] in acute medial collateral ligament (MCL),[49] and meniscal injures in athletes to promote tissue healing and early recovery. Lab studies have demonstrated favorable results of PRP injections on articular cartilage,[130,131,132] with possible future prospects of PRP injections for osteoarthritis and greater trochanteric bursitis.
Spine injections
USG-guided facet joint injections have shown good accuracy rates in cadaveric studies performed for injection of the thoracic[133] and lumbar[34,125,134] vertebrae. During simulated facet injection, USG guidance consistently resulted in accurate needle placement within the facet joints, making this a feasible option for management of facet joint arthrosis. Sacroiliac joint can be visualized by USG with the patient in prone position, as a 4-5 mm hypoechoic cleft between the linear echogenic lines of the sacral ala and the iliac crest[135] at the level of the first sacral spinous process. Sacroiliac injections are given in the lower part of the joint approximately 1 cm above the lower end of the joint. The sacroiliac joint may be in close proximity to the sciatic nerve, which can be visualized under USG avoiding inadvertent nerve injuries.
Interventions in Bones
USG evaluation of the bone is limited due to beam attenuation; hence, USG-guided therapeutic interventions of the bone are relatively limited. Studies have suggested that PRP injections may enhance the rate of healing in non-united fractures of long bones, demonstrated by definitive radiographic evidence of healing; however, limited data is available on the same.[136]
In our practice, we have occasionally injected steroid and local anesthetics for painful osseous avulsion injuries of the distal fibular tip, coronoid process of ulna, medial and lateral epicondyles for symptomatic relief.
Interventions in Nerves
With high-resolution linear transducers (7-12 MHz), peripheral nerves can be identified on USG by a classic “salt and pepper appearance,” thereby guiding perineural injections. Deep-seated nerves are not as well identified; however, knowledge of the precise anatomic location of these nerves and sonographic identification of their close landmarks helps in accurate injection. USG guidance also confirms optimal spread of the injectate as presence of a hypoechoic halo surrounding the echogenic nerve. Accurate injections of local anesthetic minimize the volume of the injectate, thereby reducing the incidence of side effects, and improved the success rates.
Acute pain management: Perineural injections for acute pain management include post-operative and post-traumatic analgesia and mostly involve peripheral nerve blocks. Post-operative analgesia has gained popularity due to improved acceptance rates of arthroscopic surgeries on a day care basis. Commonly, axillary nerve blocks are used for upper extremity surgeries, interscalene[137] and suprascapular blocks[138] for shoulder surgeries, and femoral and sciatic nerve blocks for knee and foot surgeries.[139]
USG guidance can also be used for brachial plexus blocks, suprascapular nerve blocks in the suprascapular fossa, and cervical sympathetic block for pain management in the upper extremity. The femoral nerve is situated just lateral to the femoral vessels in the inguinal region and is superficial in location, making it easily accessible for perineural injections.[139] Sciatic nerve blocks[139] can be given in the subgluteal space, where the nerve is located deep to the gluteus maximus muscle, midway between the ischial tuberosity and greater trochanter. Distal sciatic blocks[140] at the level of the popliteal fossa can be done in case of foot and ankle surgeries. Being in an orthopedic setup, we give postoperative femoral nerve blocks on a daily basis in all patients of ACL grafting to help minimize postoperative pain. We have recently been using triple blocks,[141] wherein the iliac fascial plane is injected with local anesthetic and it aims at blocking the femoral, obturator nerves and also potentially the lumbar plexus, providing better pain relief [Figure 13A and B].
Figure 13 (A and B).
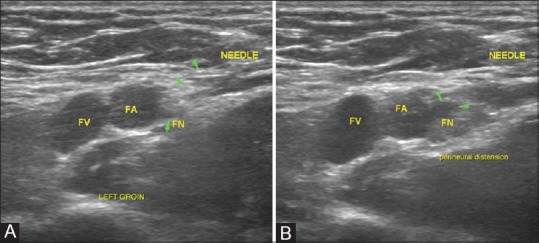
Femoral nerve blocks. (A) Femoral nerve is identified just lateral to the femoral vessels in the inguinal region by its classic “salt and pepper” appearance and being superficial, it is amenable to USG interventions (B) Optimal spread of the injectate is confirmed by presence of a hypoechoic halo surrounding the echogenic nerve (halo sign)
Chronic pain management: Perineural steroid injections for chronic pain management have been widely used in orthopedic practice, and USG guidance ensures accurate injection and improved success rates. Procedures include brachial and lumbar plexus blocks, peripheral nerve injections or local injections at the sites of trauma, entrapments, neuroma formation,[142] and epidural and spinal neural injections.[143]
Injections surrounding nerves, such as those for interdigital neuromas (Morton's neuroma), or the carpal tunnel area surrounding the interdigital nerve or the median nerve can only be achieved when the needle is inserted in the area surrounding the nerve rather than into the nerve itself. USG examination can easily identify a Morton's neuroma as a hypoechoic nodule in the second to third metatarsal web space and distinguish other causes of similar forefoot pain like metatarsophalangeal joint synovitis and intermetatarsal bursae. These neuromas show good response to injections of steroids and local anesthetics.[144] Post injection, all neuromas displayed increased echogenicity and/or the appearance of fluid surrounding it, confirming localization of the therapeutic mixture. USG-guided alcohol injections for Morton's neuroma have shown promising results[52,145] [Figure 14A and B].
Figure 14 (A and B).
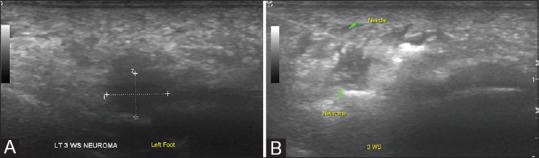
Morton's neuroma: (A) USG examination can easily identify a Morton's neuroma as a hypoechoic nodule in the second to third metatarsal web space (B) Post injection, all neuromas displayed increased echogenicity and/or appearance of fluid surrounding it, confirming localization of the therapeutic mixture
USG-guided perineural steroid injections are used for pain relief in patients with carpal tunnel syndrome. The median nerve in the carpal tunnel is superficial in location, and can be easily identified on USG and is amenable to USG-guided hydrodissection and percutaneous fenestration. The procedure involves mobilizing the nerve away from the deep surface of the flexor retinaculum by hydrodissection and then splitting the layers of the retinaculum to decompress the median nerve.[93]
Conclusion
Image guidance improves the accuracy of tissue sampling and delivery of treating agents. A large portion of the MSK system is easily visualized under USG guidance, and it is an easily available, safe, and easy-to-use modality, making it amenable for a huge variety of image-guided procedures of the MSK system. This article provides a brief overview of the potential USG has in managing MSK conditions, especially pertaining to internal derangements, and a basic overview of commencing and developing an USG MSK interventional practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Louis LJ. Musculoskeletal ultrasound intervention: Principles and advances. Radiol Clin North Am. 2008;46:515–33. doi: 10.1016/j.rcl.2008.02.003. vi. [DOI] [PubMed] [Google Scholar]
- 2.Stephens MB, Beutler AI, O’Connor FG. Musculoskeletal injections: A review of the evidence. Am Fam Physician. 2008;78:971–6. [PubMed] [Google Scholar]
- 3.Casati A, Vinciguerra F, Scarioni M, Cappelleri G, Aldegheri G, Manzoni P, et al. Lidocaine versus ropivacaine for continuous interscalene brachial plexus block after open shoulder surgery. Acta Anaesthesiol Scand. 2003;47:355–60. doi: 10.1034/j.1399-6576.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 4.Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989;69:563–9. [PubMed] [Google Scholar]
- 5.Glaser C, Marhofer P, Zimpfer G, Heinz MT, Sitzwohl C, Kapral S, et al. Levobupivacaine versus racemic bupivacaine for spinal anesthesia. Anesth Analg. 2002;94:194–8. doi: 10.1097/00000539-200201000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Wittich CM, Ficalora RD, Mason TG, Beckman TJ. Musculoskeletal injection. Mayo Clin Proc. 2009;84:831–7. doi: 10.4065/84.9.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liguori GA, Chimento GF, Borow L, Figgie M. Possible bupivacaine toxicity after intraarticular injection for postarthroscopic analgesia of the knee: Implications of the surgical procedure. Anesth Analg. 2002;94:1010–3. doi: 10.1097/00000539-200204000-00044. [DOI] [PubMed] [Google Scholar]
- 8.Dragoo JL, Korotkova T, Kanwar R, Wood B. The effect of local anesthetics administered via pain pump on chondrocyte viability. Am J Sports Med. 2008;36:1484–8. doi: 10.1177/0363546508318190. [DOI] [PubMed] [Google Scholar]
- 9.Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: A pilot study. Clin Orthop Relat Res. 2011;469:2941–7. doi: 10.1007/s11999-011-1834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb ST, Ghosh S. Intra-articular bupivacaine: Potentially chondrotoxic? Br J Anaesth. 2009;102:439–41. doi: 10.1093/bja/aep036. [DOI] [PubMed] [Google Scholar]
- 11.Gurun MS, Leinbach R, Moore L, Lee CS, Owen MD, Eisenach JC. Studies on the safety of glucose and paraben-containing neostigmine for intrathecal administration. Anesth Analg. 1997;85:317–23. doi: 10.1097/00000539-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 12.McCarty DJ, Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7:359–67. doi: 10.1002/art.1780070402. [DOI] [PubMed] [Google Scholar]
- 13.Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12:300–1. doi: 10.1136/emj.12.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford LT, DeBender J. Tendon rupture after local steroid injection. South Med J. 1979;72:827–30. doi: 10.1097/00007611-197907000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Jones JG. Achilles tendon rupture following steroid injection. J Bone Joint Surg Am. 1985;67:170. [PubMed] [Google Scholar]
- 16.Kleinman M, Gross AE. Achilles tendon rupture following steroid injection. Report of three cases. J Bone Joint Surg Am. 1983;65:1345–7. [PubMed] [Google Scholar]
- 17.Cigna E, Özkan Ö, Mardini S, Chiang PT, Yang CH, Chen HC. Late spontaneous rupture of the extensor pollicis longus tendon after corticosteroid injection for flexor tenosynovitis. Eur Rev Med Pharmacol Sci. 2013;17:845–8. [PubMed] [Google Scholar]
- 18.Bickel KD. Flexor pollicis longus tendon rupture after corticosteroid injection. J Hand Surg Am. 1996;21:152–3. doi: 10.1016/S0363-5023(96)80172-9. [DOI] [PubMed] [Google Scholar]
- 19.Unverferth LJ, Olix ML. The effect of local steroid injections on tendon. J Sports Med. 1973;1:31–7. doi: 10.1177/036354657300100404. [DOI] [PubMed] [Google Scholar]
- 20.Lemont H, Hetman J. Cutaneous foot depigmentation following an intra-articular steroid injection. J Am Podiatr Med Assoc. 1991;81:606–7. doi: 10.7547/87507315-81-11-606. [DOI] [PubMed] [Google Scholar]
- 21.Reddy PD, Zelicof SB, Ruotolo C, Holder J. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995:185–7. [PubMed] [Google Scholar]
- 22.Haslock I, MacFarlane D, Speed C. Intra-articular and soft tissue injections: A survey of current practice. Br J Rheumatol. 1995;34:449–52. doi: 10.1093/rheumatology/34.5.449. [DOI] [PubMed] [Google Scholar]
- 23.Centeno LM, Moore ME. Preferred intraarticular corticosteroids and associated practice: A survey of members of the American college of rheumatology. Arthritis Care Res. 1994;7:151–5. doi: 10.1002/art.1790070309. [DOI] [PubMed] [Google Scholar]
- 24.Beardwell A. Subcutaneous atrophy after local corticosteroid injection. Br Med J. 1967;3:600. doi: 10.1136/bmj.3.5565.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassidy JT, Bole GG. Cutaneous atrophy secondary to intra-articular corticosteroid administration. Ann Intern Med. 1966;65:1008–18. doi: 10.7326/0003-4819-65-5-1008. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs MB. Local subcutaneous atrophy after corticosteroid injection. Postgrad Med. 1986;80:159–60. doi: 10.1080/00325481.1986.11699543. [DOI] [PubMed] [Google Scholar]
- 27.Park SK, Choi YS, Kim HJ. Hypopigmentation and subcutaneous fat, muscle atrophy after local corticosteroid injection. Korean J Anesthesiol. 2013;65(Suppl 6):S59–61. doi: 10.4097/kjae.2013.65.6S.S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimbaud P, Meynadier J, Guilhou JJ, Meynadier J. Local dermatological complications secondary to corticosteroid injections. Nouv Presse Med. 1974;3:665–8. [PubMed] [Google Scholar]
- 29.Di Stefano V, Nixon JE. Skin and fat atrophy complications of local steroid injection. Pa Med. 1974;77:38. [PubMed] [Google Scholar]
- 30.Hameed F, Ihm J. Injectable medications for osteoarthritis. PM R. 2012;4(Suppl 5):S75–81. doi: 10.1016/j.pmrj.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Connell DA, Ali KE, Ahmad M, Lambert S, Corbett S, Curtis M. Ultrasound-guided autologous blood injection for tennis elbow. Skeletal Radiol. 2006;35:371–7. doi: 10.1007/s00256-006-0081-9. [DOI] [PubMed] [Google Scholar]
- 32.Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg Am. 2003;28:272–8. doi: 10.1053/jhsu.2003.50041. [DOI] [PubMed] [Google Scholar]
- 33.Longo UG, Franceschetti E, Rizzello G, Petrillo S, Denaro V. Elbow tendinopathy. Muscles Ligaments Tendons J. 2012;2:115–20. [PMC free article] [PubMed] [Google Scholar]
- 34.Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: A randomized controlled trial. Clin Rehabil. 2013;27:113–22. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 35.Muto T, Kokubu T, Mifune Y, Sakata R, Nagura I, Nishimoto H, et al. Platelet-rich plasma protects rotator cuff-derived cells from the deleterious effects of triamcinolone acetonide. J Orthop Res. 2013;31:976–82. doi: 10.1002/jor.22301. [DOI] [PubMed] [Google Scholar]
- 36.Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc. 2011;19:244–50. doi: 10.1097/JSA.0b013e318227b2dc. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz A. A Promising Treatment for Athletes, in Blood. New York Times Article; 2009. Epublished date. 2009 Feb 16; [Google Scholar]
- 38.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J Bone Joint Surg Br. 2009;91:987–96. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 39.Lubkowska A, Dolegowska B, Banfi G. Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents. 2012;26(Suppl 1):3S–22S. [PubMed] [Google Scholar]
- 40.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–8. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 41.Ferrero G, Fabbro E, Orlandi D, Martini C, Lacelli F, Serafini G, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. J Ultrasound. 2012;15:260–6. doi: 10.1016/j.jus.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monto RR. Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int. 2012;33:379–85. doi: 10.3113/FAI.2012.0379. [DOI] [PubMed] [Google Scholar]
- 43.Parafioriti A, Armiraglio E, Del Bianco S, Tibalt E, Oliva F, Berardi AC. Single injection of platelet-rich plasma in a rat Achilles tendon tear model. Muscles Ligaments Tendons J. 2011;1:41–7. [PMC free article] [PubMed] [Google Scholar]
- 44.Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014;35:313–8. doi: 10.1177/1071100713519778. [DOI] [PubMed] [Google Scholar]
- 45.Ragab EM, Othman AM. Platelets rich plasma for treatment of chronic plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:1065–70. doi: 10.1007/s00402-012-1505-8. [DOI] [PubMed] [Google Scholar]
- 46.Anitua E, Sanchez M, Nurden AT, Zalduendo M, de la Fuente M, Azofra J, et al. Reciprocal actions of platelet-secreted TGF-beta1 on the production of VEGF and HGF by human tendon cells. Plast Reconstr Surg. 2007;119:950–9. doi: 10.1097/01.prs.0000255543.43695.1d. [DOI] [PubMed] [Google Scholar]
- 47.Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthop Belg. 2013;79:10–5. [PubMed] [Google Scholar]
- 48.Hamid MS, Yusof A, Mohamed Ali MR. Platelet-rich plasma (PRP) for acute muscle injury: A systematic review. PloS One. 2014;9:e90538. doi: 10.1371/journal.pone.0090538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013;12:CD010071. doi: 10.1002/14651858.CD010071.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Bubnov R, Yevseenko V, Semeniv I. Ultrasound guided injections of platelets rich plasma for muscle injury in professional athletes. Comparative study. Med Ultrason. 2013;15:101–5. doi: 10.11152/mu.2013.2066.152.rb1vy2. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Zhai W. Histological observation of tendon-bone healing after anterior cruciate ligament reconstruction by platelet-rich plasma combined with deproteinized bone of calf. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:1323–9. [PubMed] [Google Scholar]
- 52.Chen L, Yang X, Huang G, Song D, Ye XS, Xu H, et al. Platelet-rich plasma promotes healing of osteoporotic fractures. Orthopedics. 2013;36:e687–94. doi: 10.3928/01477447-20130523-10. [DOI] [PubMed] [Google Scholar]
- 53.Camargo PM, Lekovic V, Weinlaender M, Vasilic N, Madzarevic M, Kenney EB. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37:300–6. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 54.Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, Dharamsi AS, et al. Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: A multicenter, retrospective review. PM R. 2013;5:169–75. doi: 10.1016/j.pmrj.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Banks AR. A Rationale for Prolotherapy. Journal of Orthopaedic Medicine. 1991;13:54–9. [Google Scholar]
- 56.Rabago D, Yelland M, Patterson J, Zgierska A. Prolotherapy for chronic musculoskeletal pain. Am Fam Physician. 2011;84:1208–10. [PubMed] [Google Scholar]
- 57.Distel LM, Best TM. Prolotherapy: A clinical review of its role in treating chronic musculoskeletal pain. PM R. 2011;3(Suppl 1):S78–81. doi: 10.1016/j.pmrj.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Dagenais S, Yelland MJ, Del Mar C, Schoene ML. Prolotherapy injections for chronic low-back pain. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004059.pub3. CD004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014;6:152–8. doi: 10.1016/j.pmrj.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: A pilot study. Clin J Sport Med. 2008;18:248–54. doi: 10.1097/JSM.0b013e318170fc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yelland MJ, Sweeting KR, Lyftogt JA, Ng SK, Scuffham PA, Evans KA. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: A randomised trial. Br J Sports Med. 2011;45:421–8. doi: 10.1136/bjsm.2009.057968. [DOI] [PubMed] [Google Scholar]
- 62.Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: A randomized controlled trial. Ann Fam Med. 2013;11:229–37. doi: 10.1370/afm.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabago D, Patterson JJ, Mundt M, Zgierska A, Fortney L, Grettie J, et al. Dextrose and morrhuate sodium injections (prolotherapy) for knee osteoarthritis: A prospective open-label trial. J Altern Complement Med. 2014;20:383–91. doi: 10.1089/acm.2013.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aldermann D. Prolotherapy for Musculoskeletal Pain. Practical Pain Management. 2007:10–5. [Google Scholar]
- 65.Tehranzadeh J, Booya F, Root J. Cartilage metabolism in osteoarthritis and the influence of viscosupplementation and steroid: A review. Acta Radiol. 2005;46:288–96. doi: 10.1080/02841850510016027. [DOI] [PubMed] [Google Scholar]
- 66.Hsu WK, Mishra A, Rodeo SR, Fu F, Terry MA, Randelli P, et al. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–48. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 67.Levine WN, Bergfeld JA, Tessendorf W, Moorman CT., 3rd Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28:297–300. doi: 10.1177/03635465000280030301. [DOI] [PubMed] [Google Scholar]
- 68.Baron EP, Cherian N, Tepper SJ. Role of greater occipital nerve blocks and trigger point injections for patients with dizziness and headache. Neurologist. 2011;17:312–7. doi: 10.1097/NRL.0b013e318234e966. [DOI] [PubMed] [Google Scholar]
- 69.Sarrafzadeh J, Ahmadi A, Yassin M. The effects of pressure release, phonophoresis of hydrocortisone, and ultrasound on upper trapezius latent myofascial trigger point. Arch Phys Med Rehabil. 2012;93:72–7. doi: 10.1016/j.apmr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Cotchett MP, Landorf KB, Munteanu SE. Effectiveness of dry needling and injections of myofascial trigger points associated with plantar heel pain: A systematic review. J Foot Ankle Res. 2010;3:8. doi: 10.1186/1757-1146-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venâncio Rde A, Alencar FG, Zamperini C. Different substances and dry-needling injections in patients with myofascial pain and headaches. Cranio. 2008;26:96–103. doi: 10.1179/crn.2008.014. [DOI] [PubMed] [Google Scholar]
- 72.Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourology and urodynamics. 2007;26:59–62. doi: 10.1002/nau.20393. [DOI] [PubMed] [Google Scholar]
- 73.Richie CA, 3rd, Briner WW., Jr Corticosteroid injection for treatment of de Quervain's tenosynovitis: A pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102–6. doi: 10.3122/jabfm.16.2.102. [DOI] [PubMed] [Google Scholar]
- 74.Wysocki RW, Biswas D, Bayne CO. Injection Therapy in the management of musculoskeletal injuries: Hand and wrist. Oper Tech Sports Med. 2012;20:132–41. [Google Scholar]
- 75.Dala-Ali BM, Nakhdjevani A, Lloyd MA, Schreuder FB. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263–8. doi: 10.4055/cios.2012.4.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehdizade A, Adler RS. Sonographically guided flexor hallucis longus tendon sheath injection. J Ultrasound Med. 2007;26:233–7. doi: 10.7863/jum.2007.26.2.233. [DOI] [PubMed] [Google Scholar]
- 77.Mellor SJ, Patel VR. Steroid injections are helpful in rotator cuff tendinopathy. BMJ. 2002;324:51. doi: 10.1136/bmj.324.7328.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: A meta-analysis. Br J Gen Pract. 2005;55:224–8. [PMC free article] [PubMed] [Google Scholar]
- 79.Stahl S, Kaufman T. The efficacy of an injection of steroids for medial epicondylitis. A prospective study of sixty elbows. J Bone Joint Surg Am. 1997;79:1648–52. doi: 10.2106/00004623-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Price R, Sinclair H, Heinrich I, Gibson T. Local injection treatment of tennis elbow--hydrocortisone, triamcinolone and lignocaine compared. Br J Rheumatol. 1991;30:39–44. doi: 10.1093/rheumatology/30.1.39. [DOI] [PubMed] [Google Scholar]
- 81.Lundquist W, Stanford R. Targeting systemic inflammation in patients with obesity-related pain: One practice's success with platelet-rich plasma therapy. J Fam Pract. 2013;62(Suppl CHPP):S10–5. [PubMed] [Google Scholar]
- 82.Carofino B, Chowaniec DM, McCarthy MB, Bradley JP, Delaronde S, Beitzel K, et al. Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: An in vitro study on human tendon cells. Arthroscopy. 2012;28:711–9. doi: 10.1016/j.arthro.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and tendon progenitor cell recruitment. J Orthop Res. 2006;24:173–82. doi: 10.1002/jor.20030. [DOI] [PubMed] [Google Scholar]
- 84.Scherb MB, Han SH, Courneya JP, Guyton GP, Schon LC. Effect of bupivacaine on cultured tenocytes. Orthopedics. 2009;32:26. doi: 10.3928/01477447-20090101-19. [DOI] [PubMed] [Google Scholar]
- 85.Hoksrud A, Torgalsen T, Harstad H, Haugen S, Andersen TE, Risberg MA, et al. Ultrasound-guided sclerosis of neovessels in patellar tendinopathy: A prospective study of 101 patients. Am J Sports Med. 2012;40:542–7. doi: 10.1177/0363546511433012. [DOI] [PubMed] [Google Scholar]
- 86.Hoksrud A, Bahr R. Ultrasound-guided sclerosing treatment in patients with patellar tendinopathy (jumper's knee).44-month follow-up. Am J Sports Med. 2011;39:2377–80. doi: 10.1177/0363546511417097. [DOI] [PubMed] [Google Scholar]
- 87.Alfredson H, Ohberg L. Sclerosing injections to areas of neo-vascularisation reduce pain in chronic Achilles tendinopathy: A double-blind randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. 2005;13:338–44. doi: 10.1007/s00167-004-0585-6. [DOI] [PubMed] [Google Scholar]
- 88.Ohberg L, Alfredson H. Sclerosing therapy in chronic Achilles tendon insertional pain-results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2003;11:339–43. doi: 10.1007/s00167-003-0402-7. [DOI] [PubMed] [Google Scholar]
- 89.Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: Pilot study of a new treatment. Br J Sports Med. 2002;36:173–7. doi: 10.1136/bjsm.36.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiavaras MM, Jacobson JA. Ultrasound-guided tendon fenestration. Semin Musculoskelet Radiol. 2013;17:85–90. doi: 10.1055/s-0033-1333942. [DOI] [PubMed] [Google Scholar]
- 91.Finnoff JT, Fowler SP, Lai JK, Santrach PJ, Willis EA, Sayeed YA, et al. Treatment of chronic tendinopathy with ultrasound-guided needle tenotomy and platelet-rich plasma injection. PM R. 2011;3:900–11. doi: 10.1016/j.pmrj.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 92.McShane JM, Slaff S, Gold JE, Nazarian LN. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: Preliminary report. J Ultrasound Med. 2012;31:1341–9. doi: 10.7863/jum.2012.31.9.1341. [DOI] [PubMed] [Google Scholar]
- 93.Castagna A, Cesari E, Garofalo R, Gigante A, Conti M, Markopoulos N, et al. Matrix metalloproteases and their inhibitors are altered in torn rotator cuff tendons, but also in the macroscopically and histologically intact portion of those tendons. Muscles Ligaments Tendons J. 2013;3:132–8. eCollection 2013. [PMC free article] [PubMed] [Google Scholar]
- 94.Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol (1985) 2013;115:884–91. doi: 10.1152/japplphysiol.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown R, Orchard J, Kinchington M, Hooper A, Nalder G. Aprotinin in the management of Achilles tendinopathy: A randomised controlled trial. Br J Sports Med. 2006;40:275–9. doi: 10.1136/bjsm.2005.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Speed CA, Hazleman BL. Calcific tendinitis of the shoulder. N Engl J Med. 1999;340:1582–4. doi: 10.1056/NEJM199905203402011. [DOI] [PubMed] [Google Scholar]
- 97.Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001:72–82. doi: 10.1097/00003086-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 98.Wang CJ, Ko JY, Chen HS. Treatment of calcifying tendinitis of the shoulder with shock wave therapy. Clin Orthop Relat Res. 2001:83–9. doi: 10.1097/00003086-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Wang CJ, Yang KD, Wang FS, Chen HH, Wang JW. Shock wave therapy for calcific tendinitis of the shoulder: A prospective clinical study with two-year follow-up. Am J Sports Med. 2003;31:425–30. doi: 10.1177/03635465030310031701. [DOI] [PubMed] [Google Scholar]
- 100.Loew M, Daecke W, Kusnierczak D, Rahmanzadeh M, Ewerbeck V. Shock-wave therapy is effective for chronic calcifying tendinitis of the shoulder. J Bone Joint Surg Br. 1999;81:863–7. doi: 10.1302/0301-620x.81b5.9374. [DOI] [PubMed] [Google Scholar]
- 101.del Cura JL, Torre I, Zabala R, Legórburu A. Sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: Short- and long-term results. AJR Am J Roentgenol. 2007;189:W128–34. doi: 10.2214/AJR.07.2254. [DOI] [PubMed] [Google Scholar]
- 102.Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management. J Am Acad Orthop Surg. 1997;5:183–91. doi: 10.5435/00124635-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 103.Yoshioka T, Kanamori A, Washio T, Aoto K, Uemura K, Sakane M, et al. The effects of plasma rich in growth factors (PRGF-Endoret) on healing of medial collateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2013;21:1763–9. doi: 10.1007/s00167-012-2002-x. [DOI] [PubMed] [Google Scholar]
- 104.Akgün K, Birtane M, Akarirmak U. Is local subacromial corticosteroid injection beneficial in subacromial impingement syndrome? Clin Rheumatol. 2004;23:496–500. doi: 10.1007/s10067-004-0930-7. [DOI] [PubMed] [Google Scholar]
- 105.Lustenberger DP, Ng VY, Best TM, Ellis TJ. Efficacy of treatment of trochanteric bursitis: A systematic review. Clin J Sport Med. 2011;21:447–53. doi: 10.1097/JSM.0b013e318221299c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farmer KW, Jones LC, Brownson KE, Khanuja HS, Hungerford MW. Trochanteric bursitis after total hip arthroplasty: Incidence and evaluation of response to treatment. J Arthroplasty. 2010;25:208–12. doi: 10.1016/j.arth.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 107.Brinks A, van Rijn RM, Bohnen AM, Slee GL, Verhaar JA, Koes BW, et al. Effect of corticosteroid injection for trochanter pain syndrome: Design of a randomised clinical trial in general practice. BMC Musculoskelet Disord. 2007;8:95. doi: 10.1186/1471-2474-8-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jose J, Silverman E, Kaplan L. Symptomatic ganglion cyst of the popliteus tendon treated with ultrasound-guided aspiration and steroid injection: A case report. Sports Health. 2011;3:393–5. doi: 10.1177/1941738111406223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paul AS, Sochart DH. Improving the results of ganglion aspiration by the use of hyaluronidase. J Hand Surg Br. 1997;22:219–21. doi: 10.1016/s0266-7681(97)80066-6. [DOI] [PubMed] [Google Scholar]
- 110.Breidahl WH, Adler RS. Ultrasound-guided injection of ganglia with coricosteroids. Skeletal Radiol. 1996;25:635–8. doi: 10.1007/s002560050150. [DOI] [PubMed] [Google Scholar]
- 111.Nam D, Macaulay A, Cross M, Shindle MK, Warren RF. Posterior cruciate ligament resection for ganglion cyst and associated ligament degeneration. Am J Orthop (Belle Mead NJ) 2011;40:E110–4. [PubMed] [Google Scholar]
- 112.Sloane J, Gulati V, Penna S, Pastides P, Baghla DP. Large intra-articular anterior cruciate ligament ganglion cyst, presenting with inability to flex the knee. Case Rep Med 2010. 2010 doi: 10.1155/2010/705919. 705919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Macmahon PJ, Brennan DD, Duke D, Forde S, Eustace SJ. Ultrasound-guided percutaneous drainage of meniscal cysts: Preliminary clinical experience. Clin Radiol. 2007;62:683–7. doi: 10.1016/j.crad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 114.van Holsbeeck M, Introcaso JH. Musculoskeletal ultrasonography. Radiol Clin North Am. 1992;30:907–25. [PubMed] [Google Scholar]
- 115.Chhem RK, Kaplan PA, Dussault RG. Ultrasonography of the musculoskeletal system. Radiol Clin North Am. 1994;32:275–89. [PubMed] [Google Scholar]
- 116.Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: A pilot study. J Rheumatol. 2002;29:2209–13. [PubMed] [Google Scholar]
- 117.Raza K, Lee CY, Pilling D, Heaton S, Situnayake RD, Carruthers DM, et al. Ultrasound guidance allows accurate needle placement and aspiration from small joints in patients with early inflammatory arthritis. Rheumatology (Oxford) 2003;42:976–9. doi: 10.1093/rheumatology/keg269. [DOI] [PubMed] [Google Scholar]
- 118.Smith J, Hurdle MF, Weingarten TN. Accuracy of sonographically guided intra-articular injections in the native adult hip. J Ultrasound Med. 2009;28:329–35. doi: 10.7863/jum.2009.28.3.329. [DOI] [PubMed] [Google Scholar]
- 119.Sibbitt WL, Jr, Peisajovich A, Michael AA, Park KS, Sibbitt RR, Band PA, et al. Does sonographic needle guidance affect the clinical outcome of intraarticular injections? J Rheumatol. 2009;36:1892–902. doi: 10.3899/jrheum.090013. [DOI] [PubMed] [Google Scholar]
- 120.Crawford RW, Gie GA, Ling RS, Murray DW. Diagnostic value of intra-articular anaesthetic in primary osteoarthritis of the hip. J Bone Joint Surg Br. 1998;80:279–81. doi: 10.1302/0301-620x.80b2.8299. [DOI] [PubMed] [Google Scholar]
- 121.Khoury NJ, el-Khoury GY, Saltzman CL, Brandser EA. Intraarticular foot and ankle injections to identify source of pain before arthrodesis. AJR Am J Roentgenol. 1996;167:669–73. doi: 10.2214/ajr.167.3.8751679. [DOI] [PubMed] [Google Scholar]
- 122.Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel-Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370–7. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 123.Oh JH, Oh CH, Choi JA, Kim SH, Kim JH, Yoon JP. Comparison of glenohumeral and subacromial steroid injection in primary frozen shoulder: A prospective, randomized short-term comparison study. J Shoulder Elbow Surg. 2011;20:1034–40. doi: 10.1016/j.jse.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 124.Wang JJ, Ho ST, Lee SC, Tang JJ, Liaw WJ. Intraarticular triamcinolone acetonide for pain control after arthroscopic knee surgery. Anesth Analg. 1998;87:1113–6. doi: 10.1097/00000539-199811000-00024. [DOI] [PubMed] [Google Scholar]
- 125.Yablon CM. Ultrasound-guided interventions of the foot and ankle. Semin Musculoskelet Radiol. 2013;17:60–8. doi: 10.1055/s-0033-1333916. [DOI] [PubMed] [Google Scholar]
- 126.Sofka CM, Adler RS. Ultrasound-guided interventions in the foot and ankle. Semin Musculoskelet Radiol. 2002;6:163–8. doi: 10.1055/s-2002-32362. [DOI] [PubMed] [Google Scholar]
- 127.Wen DY. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am Fam Physician. 2000;62:565–70. 72. [PubMed] [Google Scholar]
- 128.George E. Intra-articular hyaluronan treatment for osteoarthritis. Ann Rheum Dis. 1998;57:637–40. doi: 10.1136/ard.57.11.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mirzatolooei F, Alamdari MT, Khalkhali HR. The impact of platelet-rich plasma on the prevention of tunnel widening in anterior cruciate ligament reconstruction using quadrupled autologous hamstring tendon: A randomised clinical trial. (65-9).Bone Joint J. 2013:95–B. doi: 10.1302/0301-620X.95B1.30487. [DOI] [PubMed] [Google Scholar]
- 130.Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: A pilot study. Am J Phys Med Rehabil. 2010;89:961–9. doi: 10.1097/PHM.0b013e3181fc7edf. [DOI] [PubMed] [Google Scholar]
- 131.Saito M, Takahashi KA, Arai Y, Inoue A, Sakao K, Tonomura H, et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol. 2009;27:201–7. [PubMed] [Google Scholar]
- 132.Torrero JI, Aroles F, Ferrer D. Treatment of knee chondropathy with platelet rich plasma. Preliminary results at 6 months of follow-up with only one injection. J Biol Regul Homeost Agents. 2012;26(Suppl 1):71S–8S. [PubMed] [Google Scholar]
- 133.Stulc SM, Hurdle MF, Pingree MJ, Brault JS, Porter CA. Ultrasound-guided thoracic facet injections: Description of a technique. J Ultrasound Med. 2011;30:357–62. doi: 10.7863/jum.2011.30.3.357. [DOI] [PubMed] [Google Scholar]
- 134.Galiano K, Obwegeser AA, Bodner G, Freund M, Maurer H, Kamelger FS, et al. Ultrasound guidance for facet joint injections in the lumbar spine: A computed tomography-controlled feasibility study. Anesth Analg. 2005;101:579–83. doi: 10.1213/01.ANE.0000158609.64417.93. [DOI] [PubMed] [Google Scholar]
- 135.Harmon D, O’sullivan M. Ultrasound-guided sacroiliac joint injection technique. Pain Physician. 2008;11:543–7. [PubMed] [Google Scholar]
- 136.Zhu SJ, Choi BH, Jung JH, Lee SH, Huh JY, You TM, et al. A comparative histologic analysis of tissue-engineered bone using platelet-rich plasma and platelet-enriched fibrin glue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:175–9. doi: 10.1016/j.tripleo.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 137.Mariano ER, Afra R, Loland VJ, Sandhu NS, Bellars RH, Bishop ML, et al. Continuous interscalene brachial plexus block via an ultrasound-guided posterior approach: A randomized, triple-masked, placebo-controlled study. Anesth Analg. 2009;108:1688–94. doi: 10.1213/ane.0b013e318199dc86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heil JW, Ilfeld BM, Loland VJ, Mariano ER. Preliminary experience with a novel ultrasound-guided supraclavicular perineural catheter insertion technique for perioperative analgesia of the upper extremity. J Ultrasound Med. 2010;29:1481–5. doi: 10.7863/jum.2010.29.10.1481. [DOI] [PubMed] [Google Scholar]
- 139.Oberndorfer U, Marhofer P, Bösenberg A, Willschke H, Felfernig M, Weintraud M, et al. Ultrasonographic guidance for sciatic and femoral nerve blocks in children. Br J Anaesth. 2007;98:797–801. doi: 10.1093/bja/aem092. [DOI] [PubMed] [Google Scholar]
- 140.van Geffen GJ, Pirotte T, Gielen MJ, Scheffer G, Bruhn J. Ultrasound-guided proximal and distal sciatic nerve blocks in children. J Clin Anesth. 2010;22:241–5. doi: 10.1016/j.jclinane.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Christos SC, Chiampas G, Offman R, Rifenburg R. Ultrasound-guided three-in-one nerve block for femur fractures. West J Emerg Med. 2010;11:310–3. [PMC free article] [PubMed] [Google Scholar]
- 142.Bennett GL, Graham CE, Mauldin DM. Morton's interdigital neuroma: A comprehensive treatment protocol. Foot Ankle Int. 1995;16:760–3. doi: 10.1177/107110079501601204. [DOI] [PubMed] [Google Scholar]
- 143.Gofeld M, Bristow SJ, Chiu SC, McQueen CK, Bollag L. Ultrasound-guided lumbar transforaminal injections: Feasibility and validation study. Spine (Phila Pa 1976) 2012;37:808–12. doi: 10.1097/BRS.0b013e3182340096. [DOI] [PubMed] [Google Scholar]
- 144.Markovic M, Crichton K, Read JW, Lam P, Slater HK. Effectiveness of ultrasound-guided corticosteroid injection in the treatment of Morton's neuroma. Foot Ankle Int. 2008;29:483–7. doi: 10.3113/FAI-2008-0483. [DOI] [PubMed] [Google Scholar]
- 145.Musson RE, Sawhney JS, Lamb L, Wilkinson A, Obaid H. Ultrasound guided alcohol ablation of Morton's neuroma. Foot Ankle Int. 2012;33:196–201. doi: 10.3113/FAI.2012.0196. [DOI] [PubMed] [Google Scholar]


