Abstract
Pneumocystis jirovecii pneumonia (PCP) is one of the most common opportunistic infections in human immunodeficiency virus–infected adults. Colonization of Pneumocystis is highly prevalent among the general population and could be associated with the transmission and development of PCP in immunocompromised individuals. Although the microscopic demonstration of the organisms in respiratory specimens is still the golden standard of its diagnosis, polymerase chain reaction has been shown to have a high sensitivity, detecting Pneumocystis DNA in induced sputum or oropharyngeal wash. Serum β-D-glucan is useful as an adjunctive tool for the diagnosis of PCP. High-resolution computed tomography, which typically shows diffuse ground-glass opacities, is informative for the evaluation of immunocompromised patients with suspected PCP and normal chest radiography. Trimethoprim–sulfamethoxazole (TMP-SMX) is the first-line agent for the treatment of mild to severe PCP, although it is often complicated with various side effects. Since TMP-SMX is widely used for the prophylaxis, the putative drug resistance is an emerging concern.
Keywords: Pneumocystis pneumonia, human immunodeficiency virus, β-D-glucan, polymerase chain reaction, trimethoprim-sulfamethoxazole, prophylaxis
Introduction
Pneumocystis jirovecii pneumonia (PCP) is a potentially life-threatening fungal infection seen in immunocompromised individuals. In 1940s, Pneumocystis was recognized as a pathogen for pneumonia in malnourished or premature infants. Then, prior to 1980s, PCP was recognized as a rare but fatal infection primarily among patients with acute leukemia and other hematological malignancies.1 In 1980s, the worldwide epidemic of human immunodeficiency virus (HIV) dramatically increased the prevalence of PCP. Because of the progress in the antiretroviral therapy (ART) and the use of routine prophylaxis against PCP, the incidence of PCP in HIV- infected population was reduced in most of the industrialized countries. However, PCP still remains to be the most common opportunistic infection among patients with acquired immunodeficiency syndrome (AIDS) in many countries.2 This article will review the current understanding and future directions of the diagnosis, treatment, and prophylaxis of PCP in HIV-infected adults and adolescents. Because of the economic and health-care disparities among countries, some part of this review, especially epidemiology and diagnostic tools, may refer to developed countries but not be applicable to emerging ones.
Mycology
Pneumocystis was initially classified as a protozoa based on the histological characteristics of the two identified life-cycle forms, the small trophozoite and the larger cyst form, and the response of the infection to treatment with the antiprotozoa medication pentamidine.3 Based on DNA sequence analyses, Pneumocystis is now classified as a fungus, although it is difficult to culture in a standardized system. In 2012, the first fully assembled P. jirovecii genome from a single isolate was reported.4 The genome lacks virulence factors and most amino acid biosynthesis enzymes, suggesting that P. jirovecii is an obligate pathogen specialized in the colonization of human lungs and causes disease only in immunodeficient individuals.4 Although the whole-genome analysis of Pneumocystis has been completed, the life cycle and drug susceptibility of Pneumocystis were hindered by the difficulty in isolating it in pure culture.3,5 A recent comparative genomic study by Almeida et al suggested that primary homothallism is the system of reproduction of Pneumocystis species.6 In addition, Schildgen et al recently reported that P. jirovecii could be cultured in a permanent three-dimensional air–liquid interface culture system formed by CuFi-8 cells, a differentiated pseudostratified airway epithelial cell line.7 These advances in mycology are expected to provide a great advantage in understanding the characteristics of Pneumocystis.
Pneumocystis jirovecii lives almost exclusively in the pulmonary alveoli in human. Morphological studies have revealed three distinct stages: the trophozoite (trophic form), in which it often exists in clusters, the sporozoite (precystic form), and the cyst, which contains several intracystic bodies (spores).3 The trophic form, which adheres tightly to alveolar type I epithelial cell, is 1–4 µm in diameter and the mature cyst is 8–10 µm in diameter. During infection of the lung, the trophic forms predominate over the cyst forms by ~10:1.8,9
Colonization and Transmission
Based on serologic testing, most children acquire infection with P. jirovecii by age 4.10,11 In HIV-infected patients, the rates of colonization were reported to be as high as 69%.12 In addition, evidences showed frequent colonization of Pneumocystis also in non-HIV population.13,14
It is still controversial whether PCP occurs after reactivation of latent infection or colonization or is due to de novo acquisition. Earlier expert opinions suggested that PCP develops after reactivation of latent infection, but a growing body of evidence indicates that de novo exposure from individuals with PCP or those colonized with Pneumocystis may result in person-to-person transmission.15,16 In HIV-infected patients who experienced two or more episodes of PCP, genetically distinct isolates were associated with each episode, which suggests that the recurrent episodes of PCP were caused by reinfection rather than by reactivation of latent infection.17 In addition, in a large outbreak of PCP in renal transplant recipients, genotyping of the P. jirovecii isolates indicated airborne transmission from an index case at the outpatient clinic and ward.18,19 The results of these genetic epidemiological studies with genotyping support the person-to-person spread of Pneumocystis.5,19,20 Recently, Le Gal et al reported that P. jirovecii DNA was detected in the air samples collected at 5 m from the head of colonized patients, which suggested P. jirovecii exhalation from colonized patients and risk of nosocomial transmission.21 Pneumocystis-colonized individuals may not only pose a risk for developing PCP but also serve as a reservoir for disease transmission. To prevent transmission, placement of a patient developing PCP in the same room with an immunocompromised patient should be avoided.22 Furthermore, it may be reasonable to extend this measure to colonized patients.21
Host Response to Pneumocystis
A growing body of knowledge on the host response to Pneumocystis species has been accumulated based on the findings from rodent and human studies. The host immune response during PCP involves complex interactions between CD4+ T cells, CD8+ T cells, neutrophils, alveolar macrophages, and soluble mediators that facilitate the clearance of the infection.23,24 Alveolar macrophages serve as the primary host defense against P. jirovecii, playing an important role in the recognition, phagocytosis, and degradation of the organisms.25 In response to proliferation of Pneumocystis, the uptake of the organisms by macrophages occurs through multiple receptor systems, including the action of mannose receptors that interact with glycoprotein A or major surface glycoprotein (MSG) on the surface of Pneumocystis, and the interaction between (1→3)-β-D-glucan (β-D-glucan) of Pneumocystis and the macrophage surface receptors, dectin-1 and Toll-like receptor 2.3,23 IgG and other opsonic proteins in the alveolar spaces also participate in this uptake process. In addition, various proinflammatory cytokines and chemokines released from activated macrophages and epithelial cells are essential for the optimal elimination of the organisms.3,26,27 Despite their important roles in the host defense, alveolar macrophages in patients with PCP are defective in phagocytosis and fewer in number.28,29 Recently, Lei et al reported that, in a mouse model of PCP, myeloid-derived suppressor cells (MDSCs) accumulate in the lungs and impair the phagocytic activity of alveolar macrophages through activation of programmed death 1 protein pathway, which may be the key mechanism of the pathogenesis of PCP.30
CD4+ T lymphocytes, the count of which decreases in HIV infection, are essential to eradicate P. jirovecii infection and contribute to inflammatory lung damage. CD4+ T cells proliferate in response to Pneumocystis antigens and generate interferon (IFN)-γ, which induces further recruitment of macrophages. interleukin-8 (IL-8), which is released from epithelial cells and macrophages, strongly enhances the recruitment of neutrophils that not only contribute to the organism clearance but also mediate lung injury through the release of proteases and oxygen radicals. Severe PCP is characterized by neutrophilic lung inflammation that may result in diffuse alveolar damage, impaired gas exchange, and respiratory failure. The increased percentage of neutrophils in bronchoalveolar lavage (BAL) fluid has been shown to be predictive of 90-day mortality.31 The IL-8 level in BAL fluid, which is lower in PCP patients with HIV infection than in those without, also correlate closely with neutrophil accumulation in the alveolar space, impaired oxygenation, and mortality.32,33 These findings indicate that, once host immune response to Pneumocystis infection is excessive, pulmonary inflammation potently contributes to lung injury.5,20 The extent of the host immune response, especially neutrophilic lung inflammation, could be the determinant of the disease severity.
During PCP, pulmonary inflammation more potently contributes to lung injury than the direct effects of the organism. Mice with severe combined immunodeficiency (SCID) lacking functional T and B lymphocytes have spontaneous Pneumocystis infection by 3 weeks of age. In spite of the progressive infection, the SCID mice show normal oxygenation and lung function until the late stages of the disease.34 When the immune systems in these animals are reconstituted with the use of intact spleen cells, an intense T-cell–mediated inflammatory response ensues, resulting in substantially impaired gas exchange. Similarly, in HIV-infected patients, the initiation of ART during the course of PCP treatment is often associated with a paradoxical worsening of PCP with a relapse in their symptoms and deterioration in their respiratory status. This phenomenon, which is known as the immune reconstitution inflammatory syndrome, is a consequence of the recovery of immune function resulting from ART. These observations indicate that the development of lung injury requires cellular immune response besides Pneumocystis infection.3
Differences in Clinical Features of Pneumocystis Pneumonia Between Patients with HIV Infection and those without
PCP develops in patients with immunosuppression or immunomodulation due to the underlying disease or its treatment, such as anti-tumor necrosis factor (TNF) drugs for rheumatoid arthritis. Early in the AIDS epidemic, PCP occurred in 46% of the AIDS patients who did not receive chemoprophylaxis for PCP.5 Nowadays, AIDS-related PCP occurs primarily among individuals unaware that they have HIV infection and is an AIDS-defining illness.25 PCP usually develops in HIV-infected patients when the CD4+ lymphocyte count decreases to fewer than 200 cells/mm3 and particularly to fewer than 100 cells/mm3.35 Nevertheless, HIV-infected patients with CD4+ counts greater than 200 cells/mm3 account for 10%–15% of cases of PCP.20 Although decreasing CD4+ count is the strongest predictor of risk for opportunistic infection, increasing viral load (ie, HIV-RNA in plasma) is independently associated with increased risk.36
The clinical features of PCP are quite different between HIV-infected patients and those without HIV infection.37–39 In most case of PCP in HIV-infected patients, respiratory failure develops gradually and BAL fluid contains a large number of organisms, which facilitate precise diagnosis.37 The outcome of PCP is more favorable in HIV-infected patients than in those without HIV infection. The mortality rates of PCP range from 10% to 20% among HIV- infected patients, while it is 30%–60% among the non-HIV population.9,37,38 These differences in clinical features of PCP are thought to be due to the differences in the immune response of the host.
Presentation and Diagnosis
PCP may be difficult to diagnose owing to its nonspecific symptoms. Radiological presentation is also nonspecific or even normal in case of mild disease. Therefore, the single most important diagnostic tool for PCP is a high clinical suspicion. Because PCP is one of the most frequent opportunistic infection, physicians should consider it when an HIV-infected patient or a patient with oropharyngeal candidiasis or other signs suggestive of HIV infection complains of fever, shortness of breath, and/or cough. Microscopic demonstration of the organisms in a clinically relevant source such as specimens of induced sputum, BAL fluid, or lung tissue has been the golden standard for the diagnosis of PCP but a positive polymerase chain reaction (PCR) result in combination with an elevated level of serum β-D-glucan may be enough to start anti-PCP treatment.
Clinical presentation
In HIV-infected patients, PCP classically presents with low-grade fever, nonproductive or minimally productive cough, dyspnea, and malaise, which are not specific to PCP. Symptoms may be subtle at first but gradually progress and may be present for several weeks before diagnosis, and up to 7% of the patients can be asymptomatic.8 Physical examination is also nonspecific. The pulmonary auscultation is often normal, but, when abnormal, inspiratory crackles are the most common finding. Patients may have signs of respiratory compromise, including tachypnea, tachycardia, and cyanosis. Extrapulmonary manifestations of Pneumocystis infection are not common, but retinitis, thyroiditis, bone lesions, and pneumocystosis of brain, liver, spleen, and kidney have been described as rare manifestations.40–45 These findings tend to occur more frequently in patients who have been on prophylaxis with aerosolized pentamidine or who were extremely immunocompromised, generally from advanced AIDS.8
Microbiological diagnosis
Because Pneumocystis cannot readily be cultured in the laboratory, the microscopic demonstration of the organisms in respiratory specimens has been the golden standard for the diagnosis of PCP.2,5,25 Cysts can be stained with Grocott-Gomori methenamine-silver, toluidine blue O, or calcofluor white. Trophic forms, which predominate over the cyst forms during development of PCP, can be detected with Wright-Giemsa and Diff-Quik staining. The visualization of the organism may not be steady depending upon the skill and experience of the observer. Monoclonal antibodies for detecting Pneumocystis have a higher sensitivity and specificity in induced sputum samples than conventional tinctorial stains, but the difference is much less in BAL fluid.46,47 In HIV-infected patients, microscopic visualization of BAL specimens has a reported sensitivity of 98% or greater.48 Because bronchoscopy requires specialized personnel and equipment and carries an associated risk of complications, sputum induction with hypertonic saline, which has a diagnostic yield of 50%–90%, could be the initial procedure used to diagnose PCP.49 The sensitivity may be lower in patients receiving aerosolized pentamidine for prophylaxis.
Molecular diagnosis
The detection of Pneumocystis DNA in clinical specimens by using PCR assays is leading to important advances in the clinical diagnosis of PCP.50 Nested or conventional PCR, which uses PCR primers for the gene for Pneumocystis mitochondrial large-subunit ribosomal RNA, is a technically established method and is now widely used in clinical practice.51 PCR has 94%–100% sensitivity and 79%–96% specificity in the diagnosis of microscopically positive PCP.52–54 A meta-analysis by Lu et al showed a sensitivity of 99% and a specificity of 91% for PCP in AIDS population.55 Positive and negative likelihood ratios of PCR analysis in the diagnosis of PCP were 11.5 and 0.01, respectively.55 Although BAL fluid is the optimal specimen for PCR analysis, induced sputum has been shown to be acceptable, particularly in HIV-infected patients. Harris et al showed that the use of PCR technologies, when combined with less-invasive patient specimens such as induced sputum, represent more cost-effective options than any diagnostic procedure using BAL.56 It has been shown that Pneumocystis DNA can be detected by PCR in oropharyngeal washes and nasopharyngeal aspirates.8 In HIV-infected patients, PCR of oropharyngeal wash had a diagnostic sensitivity of up to 88% and a specificity of up to 90% for PCP, which is comparable to that of microscopic observation of BAL fluid and may exceed that of microscopic observation of induced sputum.57 In a recent report, de Leeuw et al showed that when targeting a small DNA fragment P. jirovecii, real-time PCR can be performed on formalin-fixed paraffin-embedded samples of BAL fluid with sensitivity up to 83.3%.58 This method will be useful in case the suspicion of PCP arises late after a patient starts to deteriorate rapidly.
Conventional PCR is known to often produce false- positive results. The imperfect specificity of conventional PCR for PCP likely relates to the highly sensitive nature of these assays and the fact that HIV-infected patients may have colonization of Pneumocystis.59 In case an HIV-infected patient colonized with Pneumocystis has bacterial pneumonia, PCR may show positive P. jirovecii DNA, leading to an incorrect diagnosis. In patients with positive PCR results in BAL fluid, sputum, or oropharyngeal wash but with negative smears, clinical management of the disease remains a challenge. Recently, Tasaka et al reported that, in patients with positive PCR, β-D-glucan is useful for differentiating between PCP and colonization.60 Considering the disease severity, positive results of PCR and a serum marker in immunocompromised patients with hypoxemia and typical radiological findings could be a good rationale for initiating treatment of PCP. As conventional PCR is highly sensitive, negative PCR results of BAL fluid may allow for the withdrawal of anti-Pneumocystis therapy in HIV-infected patients.
Quantitative real-time PCR assays have been reported to be more promising for the diagnosis of PCP than conventional PCR assays that lack specificity in distinguishing the disease from colonization.61 Multiple protocols that use various Pneumocystis gene targets have been developed. The main targeted genes include mitochondrial ribosomal large-subunit,62 heat shock protein gene (HSP70),61 dihydrofolate reductase gene (DHFR),63 dihydropteroate synthase gene (DHPS),54 and cell division cycle 2 gene (CDC2).64 Flori et al compared the sensitivity and specificity of standard staining, conventional PCR, and real-time PCR for MSG gene using 173 BAL fluid specimens from 150 patients (19 HIV-infected and 131 non-HIV patients).52 They described that the sensitivity and specificity of the techniques were 60% and 100% for staining, 100% and 87.0% for conventional PCR, and 100% and 84.9% for real-time PCR, respectively.52 The use of a concentration of 103 copies of DNA per capillary of BAL fluid as a cut-off increased the specificity to 98.6% without reducing the sensitivity of the real-time PCR.52 Matsumura et al described that the sensitivity and specificity for discriminating definite PCP from colonization were 100% and 80.0%, respectively, at a cut-off value of 1,300 copies/mL, while the values for discriminating probable PCP from colonization were 66.7% and 73.3%, respectively, at a cut-off value of 340 copies/mL.65 False negatives are still possible with PCR-based approaches if fewer copies are present than the lower limit of detection for a given assay. Although real-time PCR displayed high accuracy for discriminating colonization from PCP, the DNA sequences targeted for PCR and the cut-off values used in these assays remain to be standardized.
Despite increased sensitivity and specificity, these assays continue to have difficulty in discriminating between colonization and infection. New techniques that detect messenger RNA (mRNA) have been proposed as surrogate markers for organism viability.66 The rationale is that mRNA is less stable than DNA. Therefore, if the patient is not actively infected with viable organisms, the mRNA should be largely degraded and no longer detected. In contrast, DNA is far more stable and may still be present even if the organisms are dead. de Oliveira et al reported that in HIV-positive patients with suspected Pneumocystis pneumonia, reverse-transcriptase PCR targeting a heat shock protein of Pneumocystis mRNA (Phsb1) yielded a diagnostic sensitivity and specificity of 100% and 86%, respectively, in BAL specimens.66
The inability to culture Pneumocystis in a standardized culture system prevents routine susceptibility testing and detection of drug resistance. Because trimethoprim–sulfamethoxazole (TMP-SMX) is widely used not only for treatment but also for prophylaxis, the emergence of drug resistance is anticipated. PCR was recently used to detect mutations in the therapeutic target genes potentially responsible for drug resistance. In some microorganisms, sulfa drug resistance has resulted from specific point mutations in the DHPS gene. Similar mutations have been observed in P. jirovecii, and its association with prior sulfa prophylaxis failure was suggested.67,68 Prevalence of these mutations has been increasing, although there have been no data showing significant association between the DHPS gene mutations and TMP-SMX efficacy, that is, treatment failure.8,25,69
Serum markers for diagnosis
Because of invasiveness of BAL and imperfect specificity of PCR, the utility of serological diagnosis of PCP has been investigated. β-D-glucan is derived from the cell wall of several fungi, including Pneumocystis.70 Although the assay is not specific for Pneumocystis, the measurement of serum β-D-glucan level has been used as an adjunctive tool for the diagnosis of PCP.71–76 There remain, however, a couple of issues to be solved.71 First, at least four different methods of measurement are commercially available and not always compatible with each other.71 Fungitec G-Test MK™, a kinetic chromogenic assay using the serum of Tachy-pleus tridentatus as the lysate, and β-D-glucan Test Wako™, an endpoint chromogenic assay using the serum of T. tridentatus as the lysate, are widely used in Japan. When the same sample is assayed, the former method usually produces a higher value than the latter. In Western countries, Fungitell™, a kinetic chromogenic assay using the serum of Limulus polyphemus as the lysate, is widely used. Second, false-positive results due to factors such as the administration of immunoglobulin, bacteremia, histoplasmosis, hemodialysis, surgical gauze exposure, and certain antibiotics are known. Third, serum β-D-glucan levels are elevated in various fungal infection, and this test cannot distinguish among fungal etiologies (eg, PCP and Aspergillus species). Fourth, the cut-off value for the diagnosis of PCP still remains to be determined. In a retrospective case–control study of 295 patients with suspected PCP who had microscopy of BAL fluid for PCP and serum β-D-glucan assay with β-D-glucan Test Wako™, Tasaka et al described a cut-off value of 31.1 pg/mL with a sensitivity of 92% and a specificity of 86% for detecting PCP.73 On the other hand, Watanabe et al evaluated the diagnostic value of the assay in 111 patients with AIDS and described a cut-off value of 23.2 pg/mL with a sensitivity of 96.4% and a specificity of 87.8%.74 Because a meta-analysis revealed high sensitivity for PCP, the β-D-glucan assay could be useful at least for screening of the disease.71 It remains controversial whether or how serum β-D-glucan assay is utilized for the assessment of treatment response or prediction of the outcome of PCP.75
Krebs von den Lungen-6 (KL-6), a high–molecular weight mucin-like glycoprotein, is strongly expressed on type 2 alveolar pneumocytes and bronchiolar epithelial cells, and the serum KL-6 level is a sensitive indicator of various types of interstitial pneumonitis. Nakamura et al described that serum KL-6 levels were significantly higher in patients with AIDS-related PCP than in non-HIV patients with PCP.76 It was also shown that the detection rate of serum KL-6 in PCP patients with HIV infection was higher than that in those without HIV infection (88% vs. 66%).76 In a case–control study by Tasaka et al, elevated serum levels of KL-6 were observed in the patients with PCP, although the diagnostic significance of KL-6 was inferior to that of β-d-glucan.73 Since KL-6 is more a generalized marker for alveolar epithelial injury, its elevation in Pneumocystis is thought to be related to injury and regeneration of alveolar epithelium.
Lactate dehydrogenase (LDH) is a nonspecific biomarker and also goes up in bacterial pneumonia, histoplasmosis, and idiopathic interstitial pneumonia, as well as lymphoma. An elevated serum LDH level has been noted in patients with PCP; it is likely to be the result of the lung inflammation and injury rather than a specific marker for the disease.73,77 Whereas its diagnostic significance was inferior to that of β-D-glucan, serum LDH level showed a significant inverse correlation with the oxygenation index, indicating that serum LDH might reflect lung tissue injury.73
Although these biomarkers are promising, the diagnostic performance of the testing should be further evaluated to determine the predictive values in patients at risk for this infection. At present, the serum markers may be used as aids for the PCP diagnosis only in combination with the microbiological examination. Since this field has been intensely investigated, a standard for serological diagnosis of PCP will be established in the near future.
Radiological presentation
On chest radiograph, PCP typically presents with bilateral or diffuse ground-glass opacities (GGO). Chest radiograph can be normal, and other less common patterns have been reported, including lobar infiltrates, pulmonary nodules, and pneumatoceles and other cystic changes.78 PCP may also present with pneumothorax or bilateral pneumothoraces. Although relatively uncommon, pneumothorax presents a difficult problem, often requiring prolonged chest drainage. These less common patterns are more frequently observed in very severely immunocompromised individuals and those treated with aerosolized pentamidine.45
High-resolution computed tomography (HRCT) typically shows diffuse GGO with patchy distribution, reflecting accumulation of intra-alveolar fibrin, debris, and organisms (Fig. 1).79,80 Although the presence of GGO is nonspecific for PCP, their absence strongly argues against the diagnosis of PCP in HIV-infected patients, and no further diagnostic testing for PCP is generally warranted in these cases.81 In some patients with PCP, GGO is distributed in subpleural lung parenchyma, whereas peripheral sparing of GGO occurs in others.82,83 A study involving 32 patients with AIDS-related PCP showed a central distribution of GGO with relative peripheral sparing in 41% of patients, a mosaic pattern in 29%, and a diffuse distribution in 24%.83 A predilection for the upper lobes has also been described.78,82 With more advanced disease, septal lines with or without intralobular lines superimposed on GGO (crazy paving)83 and consolidation may develop.78,82
Figure 1.

High-resolution computed tomographic image of Pneumocystis jirovecii pneumonia in a 45-year-old male patient with AIDS, showing diffuse GGO with inhomogeneous distribution unrelated to secondary lobules and with spared peripheral lung parenchyma.
With widespread use of chemoprophylaxis, other HRCT manifestations of AIDS-related PCP are more commonly reported. Pulmonary cysts of varying shape, size, and wall thickness occur in as many as one-third of patients with PCP.78,82,83 Cysts are associated with an increased frequency of spontaneous pneumothorax, although spontaneous pneumothorax can occur in the absence of definable lung cysts (Fig. 2).84 Cysts may resolve after treatment and clearing of infection.78 It remains controversial whether cyst formation is a characteristic CT finding of PCP in AIDS patients.38,39,85
Figure 2.

High-resolution computed tomographic image of Pneumocystis jirovecii pneumonia, showing diffuse GGO with interlobular septal lines and cyst formation.
Granulomatous inflammation occurs in approximately 5% of patients (Fig. 3), usually early in the course of HIV infection while immunodeficiency is more limited, and can become evident at HRCT as a solitary nodule or mass mimicking lung carcinoma or as multiple nodules ranging from a few millimeters to more than 1 cm.78 However, small nodules and tree-in-bud opacities are uncommon in AIDS patients with PCP and usually indicate the presence of infectious bronchiolitis from other organisms.83 Patients recovering from PCP may have residual interstitial fibrosis.78,80,81 In addition, although rare, interstitial fibrosis can occur in AIDS patients with low-grade chronic PCP, a condition termed chronic PCP.86
Figure 3.
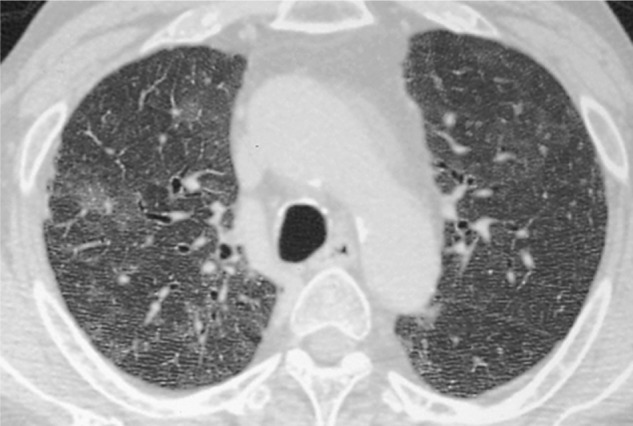
High-resolution computed tomographic image of Pneumocystis jirovecii pneumonia, showing small nodular lesions surrounded by diffuse GGO
Differential Diagnosis
AIDS patients can have a wide variety of pulmonary complications, such as bacterial pneumonia, pulmonary tuberculosis, invasive pulmonary aspergillosis, cytomegalovirus pneumonitis, lymphoma, and interstitial pneumonia. Because these pulmonary complications are developed with atypical presentation, a confident diagnosis requires a combination of clinical, radiological, and laboratory findings. HRCT is more sensitive than radiography for detecting parenchymal abnormalities in patients with AIDS and is superior to radiography in the differential diagnosis of the pulmonary complications.80
The HRCT findings of CMV pneumonitis are usually heterogeneous, including bilateral GGO and patchy consolidation, which resemble those seen in PCP.87 CMV is commonly detected in BAL fluid in AIDS patients. In most cases, it is an incidental finding, there being no associated pulmonary complication. In addition, the HRCT findings of AIDS-related lymphoma and nonspecific interstitial pneumonia, which are rarely developed in AIDS patients, may mimic those of PCP.80 In such cases, serum β-D-glucan assay and Pneumocystis PCR of induced sputum should be useful for the differential diagnosis.
Severity Assessment and Prognostication
Severity of PCP is not associated with radiological presentation or white blood cell count. Multivariate analysis identified factors associated with risk of death, including increasing patient age, prior receipt of PCP prophylaxis, poor oxygenation at hospitalization, elevated serum LDH levels, low hemoglobin and serum albumin levels, neutrophilia in BAL fluid, pneumothorax, presence of medical comorbidity, and the need for mechanical ventilation.88,89 The first episode of PCP causes more severe oxygenation impairment than the second or third episode of PCP, but mortality among patients with the first episode of PCP was lower than mortality among patients with subsequent episodes of PCP.88 Armstrong-James et al reported usefulness of a prognostic scoring tool (PST) to aid prediction of outcome from HIV-associated PCP.90 PST was calculated from repeat episode of PCP, patient’s age, hemoglobin and oxygen partial pressure on admission, and presence of medical comorbidity and pulmonary Kaposi sarcoma. Although it requires validation in patient cohorts from other health-care institutions, PST may facilitate rapid identification of patients who have severe HIV-associated PCP and are at high risk of in-hospital death.90 Although a trend toward increased mortality was observed in episodes of PCP containing mutant DHPS genotypes,89 it remains to be determined in a larger study whether DHPS mutation is associated with a worse outcome.8,25,69
Treatment
Anti-Pneumocystis agents
Because of the high efficacy and the availability of oral and parenteral forms, TMP-SMX is the first-line agent for the treatment of mild to severe PCP.8,25 Empiric treatment should be considered in severe patients who have risk factors and clinical manifestations for PCP including elevated serum β-D-glucan level. All severe cases should be treated intravenously in hospital. Oral administration of TMP-SMX is considered in mild cases or after initial improvement. For patients with AIDS, the duration of treatment is generally 3 weeks but longer if necessary. This therapy, however, is often complicated with adverse events, which include hepatotoxicity, nephrotoxicity, bone marrow depression, and skin rash, that sometimes become an obstacle to the completion of the treatment. The recommended daily dose is TMP 15–20 mg/kg plus SMX 75–100 mg/kg.8 Because this dose recommendation is not based on a randomized controlled trial, the optimal dose of TMP-SMX remains unclear. A retrospective investigation by Thomas et al revealed a good outcome with TMP 10 mg/kg/day plus SMX 50 mg/kg/day for PCP in HIV-infected patients.91 The efficacy of lower dose of TMP-SMX may be worth confirming in a randomized controlled trial.
Intravenous pentamidine isethionate is the most studied drug as an alternative to TMP-SMX. Although pentamidine is about as effective as TMP-SMX, the incidence of adverse events, such as nephrotoxicity and dysglycemia, during treatment with pentamidine is even higher compared to TMP-SMX. Atovaquone, which is less effective but better tolerated than TMP-SMX, is one of the oral treatment alternatives for mild and moderate PCP (alveolar-to-arterial oxygen difference of 45 mmHg or less).92 Oral atovaquone was shown to be as effective as intravenous pentamidine for the treatment of mild and moderate PCP and was associated with significantly fewer treatment-limiting adverse events.93 It remains to be determined whether atovaquone is effective for the treatment of severe PCP, partly because an intravenous formulation is not available. Clindamycin-primaquine is the salvage regimen of choice for those patients who fail standard therapy with TMP-SMX or pentamidine.94 Caspofungin, a lipopeptide antifungal agent, inhibits the synthesis of β-1,3-glucan, a major component of P. jirovecii cell wall. There have been several reports about severe PCP successfully treated with a combination of caspofungin and low-dose TMP-SMX.95
Treatment failure with an accepted regimen is uncommon. Although mutations in the DPHS and DHFR genes have been reported,67,68,89 such mutation is not consistently associated with clinical resistance and treatment failure.96 Therefore, changing antimicrobial agents other than for toxicity is not generally indicated. In general, those patients who need to be switched from TMP-SMX to pentamidine or other agents have worse survival rates than those who can tolerate 14–21 days of treatment. In patients with minor intolerance, such as minor rash and gastrointestinal intolerance, desensitization to TMP-SMX is preferred to the switching of agents for moderate to severe PCP.8,97
Adjunctive corticosteroid
In the guidelines, the addition of corticosteroids is recommended for HIV-infected patients with PCP.98 Adjunctive corticosteroid therapy is advocated for moderate to severe PCP patients with arterial oxygen pressure less than 70 mmHg while breathing on room air or an alveolar–arterial oxygen gradient greater than 35 mm Hg because it could attenuate lung injury by blunting the inflammatory response initiated by the degradation and clearance of the organisms.98 A systematic review showed a significant mortality-risk reduction with adjunctive corticosteroids in HIV-infected patients with PCP when substantial hypoxemia exists.99 It was also reported that, with corticosteroids, the mortality risk of severe PCP could be reduced by half, and significantly fewer patients require mechanical ventilation.100
Intensive care
Patients with the most critical forms of PCP often need intensive care. In France, PCP accounted for 35% of cases with respiratory failure among HIV-infected patients admitted to the ICU.101 In a recent cohort study by Barbier et al, however, the overall prevalence of PCP and other AIDS-defining diseases at ICU admission and of AIDS as the primary ICU diagnosis decreased steadily over time.102
When hospitalized in the ICU, at least two-thirds of PCP patients need mechanical ventilation, which generally is associated with a high in-hospital mortality.103 A prospective, case–control study by Confalonieri et al revealed that use of noninvasive positive pressure ventilation (NPPV) avoided intubation in 67% of patients, and avoidance of intubation was associated with a lower incidence of pneumothoraces and improved survival.104 NPPV might be considered as a first-line therapeutic choice for respiratory failure in AIDS patients with severe PCP.
Extracorporeal membrane oxygenation (ECMO) is becoming an accepted salvage therapy for respiratory and/or circulatory failure refractory to maximal medical treatment. Favorable outcomes in severe ARDS have been reported with ECMO use, which led to increased interest and use of ECMO for refractory respiratory failure. There have been several reported cases of respiratory failure due to PCP that were successfully managed with ECMO.105,106
Appropriate timing of ART initiation
Optimal timing of ART initiation for individuals presenting with AIDS-related opportunistic infections has not been defined. The AIDS Clinical Trials Group conducted a randomized trial comparing early ART – given within 14 days of starting antimicrobial treatment and deferred ART – given after completion of antimicrobial treatment.107 The early-ART arm had significantly fewer AIDS progression/deaths and no increase in adverse events, which supported the early initiation of ART in patients presenting with acute AIDS-related opportunistic infections.107
Prophylaxis
Despite intensive treatment, the mortality of PCP remains high, which is the rationale for chemoprophylaxis.
Primary prophylaxis
HIV-infected adults and adolescents, including pregnant women and those on ART, should receive chemoprophylaxis against PCP if they have CD4+ counts less than 200 cells/mm3 or a history of oropharyngeal candidiasis.108,109 Persons who have a CD4+ cell percentage less than 14% or a history of an AIDS-defining illness, but who do not otherwise qualify, should be considered for prophylaxis.108,109 Initiation of chemoprophylaxis at CD4+ counts between 200 and 250 cells/mm3 also should be considered when frequent monitoring of CD4+ counts, such as every 3 months, is impossible.108 Primary prophylaxis should be discontinued for HIV-infected patients who have responded to ART with an increase in CD4+ counts to or more for >3 months. In addition, data from a 12-cohort collaboration suggests that, in HIV-infected persons with CD4+ counts of 100–200 cells/mm3 and HIV-RNA levels less than 400 copies/mL, PCP incidence is low irrespective of PCP prophylaxis use, suggesting that it may be safe to stop prophylaxis earlier, though additional data are needed.110
TMP-SMX is the recommended prophylactic agent.109,111 One double-strength tablet daily is the preferred regimen, but one single-strength tablet daily also is effective and may be better tolerated than the double-strength tablet. One double-strength tablet three times weekly is also effective.112 For patients who cannot tolerate TMP-SMX even after desensitization, alternative prophylactic regimens include aerosolized pentamidine, atovaquone, and dapsone.111,113,114 In a large, open-label trial by Bozzette et al, the estimated 36-month cumulative risks of PCP in HIV-infected patients with CD4+ counts fewer than 200 cells/mm3 were 18%, 17%, and 21% in the TMP-SMX, dapsone, and aerosolized-pentamidine groups, respectively.111 Atovaquone is as effective as aerosolized pentamidine but substantially more expensive.114 It was reported that cytochrome b coenzyme Q–binding site mutations were observed in 33% of isolates from patients previously exposed to atovaquone, compared with 6% from those who were not. Cytochrome b mutations, which are associated with resistance to atovaquone, are more common in AIDS patients with atovaquone exposure.115
Secondary prophylaxis
Patients who have a history of PCP should be given chemoprophylaxis for life with TMP-SMX unless immune reconstitution occurs as a result of ART.116 For patients who are intolerant of TMP-SMX even after desensitization, the alternatives are atovaquone and aerosolized pentamidine. Secondary prophylaxis is recommended to be discontinued for adult patients whose CD4+ count has increased from <200 cells/mm3 to > 200 cells/mm3 for more than 3 months as a result of ART.98
Conclusion
At the present time, the microscopic demonstration of the organisms in BAL fluid or other respiratory specimens is still the golden standard of the diagnosis of PCP. However, recent advances in the molecular techniques are changing the situation. Nested or conventional PCR, which can detect Pneumocystis DNA in induced sputum or oropharyngeal wash, has a high negative predictive value. Although the targeted DNA sequences and the cut-off values remain to be standardized, quantitative real-time PCR is promising for discriminating PCP from colonization. In addition, PCR-based assays may be applied for the evaluation of drug resistance. The measurement of β-D-glucan and other serum markers is useful as an adjunctive tool for the diagnosis of PCP. HRCT is also informative for evaluation of immunocompromised patients with suspected PCP and normal chest radiography. These advances in the diagnostic tools have made (or even are making) the diagnosis of PCP less invasive and more accurate.
Footnotes
ACADEMIC EDITOR: Hussein D. Foda, Editor in Chief
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 2,396 words, excluding any confidential comments to the academic editor.
FUNDING: Author discloses no funding sources.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: ST. Wrote the first draft of the manuscript: ST. Developed the structure and arguments for the paper: ST. Made critical revisions: ST. The author reviewed and approved of the final manuscript.
REFERENCES
- 1.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Cattamanchi A, Davis JL, et al. International HIV-associated Opportunistic Pneumonias (IHOP) Study; Lung HIV Study. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8:294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gigliotti F, Limper AH, Pneumocystis Wright T. Cold Spring Harb Perspect Med. 2014;4:a019828. doi: 10.1101/cshperspect.a019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cissé OH, Pagni M, Hauser PM. De novo assembly of the Pneumocystis jirovecii genome from a single bronchoalveolar lavage fluid specimen from a patient. MBio. 2012;4:e428–12. doi: 10.1128/mBio.00428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck JM, Cushion MT. Pneumocystis workshop: 10th anniversary summary. Eukaryot Cell. 2009;8:446–60. doi: 10.1128/EC.00309-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida JM, Cissé OH, Fonseca Á, Pagni M, Hauser PM. Comparative genomics suggests primary homothallism of Pneumocystis species. MBio. 2015;6:e2250–14. doi: 10.1128/mBio.02250-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schildgen V, Mai S, Khalfaoui S, et al. Pneumocystis jirovecii can be productively cultured in differentiated CuFi-8 airway cells. MBio. 2014;5:e1186–14. doi: 10.1128/mBio.01186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmona EM, Limper AH. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther Adv Respir Dis. 2011;5:41–59. doi: 10.1177/1753465810380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderón EJ, Gutiérrez-Rivero S, Durand-Joly I, Dei-Cas E. Pneumocystis infection in humans: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:683–701. doi: 10.1586/eri.10.42. [DOI] [PubMed] [Google Scholar]
- 10.Pifer LL, Hughes WT, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978;61:35–41. [PubMed] [Google Scholar]
- 11.Peglow SL, Smulian AG, Linke MJ, et al. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990;161:296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Crothers K, Morris A, et al. Pneumocystis colonization in HIV-infected patients. J Eukaryot Microbiol. 2003;50(suppl):616–7. doi: 10.1111/j.1550-7408.2003.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Nevez G, Raccurt C, Jounieaux V, Dei-Cas E, Mazars E. Pneumocystosis versus pulmonary Pneumocystis carinii colonization in HIV-negative and HIV-positive patients. AIDS. 1999;13:535–6. doi: 10.1097/00002030-199903110-00020. [DOI] [PubMed] [Google Scholar]
- 14.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–53. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 15.Morris A, Beard CB, Huang L. Update on the epidemiology and transmission of Pneumocystis carinii. Microbes Infect. 2002;4:95–103. doi: 10.1016/s1286-4579(01)01514-3. [DOI] [PubMed] [Google Scholar]
- 16.Helweg-Larsen J, Lee CH, Jin S, et al. Clinical correlation of variations in the internal transcribed spacer regions of rRNA genes in Pneumocystis carinii f.sp. hominis. AIDS. 2001;15:451–9. doi: 10.1097/00002030-200103090-00003. [DOI] [PubMed] [Google Scholar]
- 17.Keely SP, Stringer JR. Sequences of Pneumocystis carinii f.sp. hominis strains associated with recurrent pneumonia vary at multiple loci. J Clin Microbiol. 1997;35:2745–7. doi: 10.1128/jcm.35.11.2745-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jirovecii pneumonia in renal transplant recipients: P. jirovecii is contagious to the susceptible host. Transplantation. 2009;88:380–5. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 19.Phipps LM, Chen SC, Kable K, et al. Nosocomial Pneumocystis jirovecii pneumonia: lessons from a cluster in kidney transplant recipients. Transplantation. 2011;92:1327–34. doi: 10.1097/TP.0b013e3182384b57. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Morris A, Limper AH, Beck JM. An official ATS workshop summary: recent advances and future directions in Pneumocystis pneumonia. Proc Am Thorac Soc. 2006;3:655–64. doi: 10.1513/pats.200602-015MS. [DOI] [PubMed] [Google Scholar]
- 21.Le Gal S, Pougnet L, Damiani C, et al. Pneumocystis jirovecii in the air surrounding patients with Pneumocystis pulmonary colonization. Diagn Microbiol Infect Dis. 2015;82:137–42. doi: 10.1016/j.diagmicrobio.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gigliotti F, Wright TW. Immunopathogenesis of Pneumosystis carinii pneumonia. Expert Rev Mol Med. 2005;7:1–16. doi: 10.1017/S1462399405010203. [DOI] [PubMed] [Google Scholar]
- 24.Kelly MN, Shellito JE. Current understanding of Pneumocystis immunology. Future Microbiol. 2010;5:43–65. doi: 10.2217/fmb.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catherinot E, Lanternier F, Bougnoux ME, Lecuit M, Couderc LJ, Lortholary O. Pneumocystis jirovecii pneumonia. Infect Dis Clin North Am. 2010;24:107–38. doi: 10.1016/j.idc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol. 2000;164:3755–63. doi: 10.4049/jimmunol.164.7.3755. [DOI] [PubMed] [Google Scholar]
- 27.Carmona EM, Vassallo R, Vuk-Pavlovic Z, Standing JE, Kottom TJ, Limper AH. Pneumocystis cell wall β-glucans induce dendritic cell costimulatory molecule expression and inflammatory activation through a Fas-Fas ligand mechanism. J Immunol. 2006;177:459–67. doi: 10.4049/jimmunol.177.1.459. [DOI] [PubMed] [Google Scholar]
- 28.Koziel H, Eichbaum Q, Kruskal BA, et al. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Invest. 1998;102:1332–44. doi: 10.1172/JCI560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleury J, Escudier E, Pocholle MJ, Carre C, Bernaudin JF. Cell population obtained by bronchoalveolar lavage in Pneumocystis carinii pneumonitis. Acta Cytol. 1985;29:721–6. [PubMed] [Google Scholar]
- 30.Lei GS, Zhang C, Lee CH. Myeloid-derived suppressor cells impair alveolar macrophages through PD-1 receptor ligation during Pneumocystis pneumonia. Infect Immun. 2015;83:572–82. doi: 10.1128/IAI.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azoulay E, Parrot A, Flahault A, et al. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am J Respir Crit Care Med. 1999;160:493–9. doi: 10.1164/ajrccm.160.2.9901019. [DOI] [PubMed] [Google Scholar]
- 32.Benfield TL, Vestbo J, Junge J, Nielsen TL, Jensen AB, Lundgren JD. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1995;151:1058–62. doi: 10.1164/ajrccm/151.4.1058. [DOI] [PubMed] [Google Scholar]
- 33.Tasaka S, Kobayashi S, Kamata H, et al. Cytokine profiles of bronchoalveolar lavage fluid in patients with Pneumocystis pneumonia. Microbiol Immunol. 2010;54:425–33. doi: 10.1111/j.1348-0421.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- 34.Roths JB, Marshall JD, Allen RD, Carlson GA, Sidman CL. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Natural history and pathobiology. Am J Pathol. 1990;136:1173–86. [PMC free article] [PubMed] [Google Scholar]
- 35.Phair J, Muñoz A, Detels R, Kaslow R, Rinaldo C, Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med. 1990;322:161–5. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan JE, Hanson DL, Jones JL, Dworkin MS, Adult and Adolescent Spectrum of HIV Disease Project Investigators Viral load as an independent risk factor for opportunistic infections in HIV-infected adults and adolescents. AIDS. 2001;15:1831–6. doi: 10.1097/00002030-200109280-00012. [DOI] [PubMed] [Google Scholar]
- 37.Limper AH, Offord KP, Smith TF, Martin WJ., II Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–9. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–71. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 39.Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of Pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med. 2010;49:273–81. doi: 10.2169/internalmedicine.49.2871. [DOI] [PubMed] [Google Scholar]
- 40.Panos GZ, Karydis I, Velakoulis SE, Falagas ME. Multi-skeletal Pneumocystis jirovecii (carinii) in an HIV-seropositive patient. Int J STD AIDS. 2007;18:134–7. doi: 10.1258/095646207779949583. [DOI] [PubMed] [Google Scholar]
- 41.Hagmann S, Merali S, Sitnitskaya Y, Fefferman N, Pollack H. Pneumocystis carinii infection presenting as an intra-abdominal cystic mass in a child with acquired immunodeficiency syndrome. Clin Infect Dis. 2001;33:1424–6. doi: 10.1086/322520. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett JA, Hulette C. Central nervous system pneumocystosis in a patient with AIDS. Clin Infect Dis. 1997;25:82–5. doi: 10.1086/514519. [DOI] [PubMed] [Google Scholar]
- 43.Guttler R, Singer PA, Axline SG, Greaves TS, McGill JJ. Pneumocystis carinii thyroiditis. Report of three cases and review of the literature. Arch Intern Med. 1993;153:393–6. doi: 10.1001/archinte.153.3.393. [DOI] [PubMed] [Google Scholar]
- 44.Dieterich DT, Lew EA, Bacon DJ, Pearlman KI, Scholes JV. Gastrointestinal pneumocystosis in HIV-infected patients on aerosolized pentamidine: report of five cases and literature review. Am J Gastroenterol. 1992;87:1763–70. [PubMed] [Google Scholar]
- 45.Edelstein H, McCabe RE. Atypical presentations of Pneumocystis carinii pneumonia in patients receiving inhaled pentamidine prophylaxis. Chest. 1990;98:1366–9. doi: 10.1378/chest.98.6.1366. [DOI] [PubMed] [Google Scholar]
- 46.Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988;318:589–93. doi: 10.1056/NEJM198803103181001. [DOI] [PubMed] [Google Scholar]
- 47.Shelhamer JH, Gill VJ, Quinn TC, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med. 1996;124:585–99. doi: 10.7326/0003-4819-124-6-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 48.Huang L, Hecht FM, Stansell JD, et al. Suspected Pneumocystis carinii pneumonia with a negative induced sputum examination: is early bronchoscopy useful? Am J Respir Crit Care Med. 1995;151:1866–71. doi: 10.1164/ajrccm.151.6.7767533. [DOI] [PubMed] [Google Scholar]
- 49.Armbruster C, Pokieser L, Hassl A. Diagnosis of Pneumocystis carinii pneumonia by bronchoalveolar lavage in AIDS patients: comparison of Diff-Quik, fungif-luor stain, direct immunofluorescence test and polymerase chain reaction. Acta Cytol. 1995;39:1089–93. [PubMed] [Google Scholar]
- 50.Durand-Joly I, Chabé M, Soula F, Delhaes L, Camus D, Dei-Cas E. Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol Med Microbiol. 2005;45:405–10. doi: 10.1016/j.femsim.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Wakefield AE, Guiver L, Miller RF, Hopkin JM. DNA amplification on induced sputum samples for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1991;337:1378–9. doi: 10.1016/0140-6736(91)93062-e. [DOI] [PubMed] [Google Scholar]
- 52.Flori P, Bellete B, Durand F, et al. Comparison between real-time PCR, conventional PCR and different staining techniques for diagnosing Pneumocystis jirovecii pneumonia from bronchoalveolar lavage specimens. J Med Microbiol. 2004;53:603–7. doi: 10.1099/jmm.0.45528-0. [DOI] [PubMed] [Google Scholar]
- 53.Caliendo AM, Hewitt PL, Allega JM, Keen A, Ruoff KL, Ferraro MJ. Performance of a PCR assay for detection of Pneumocystis carinii from respiratory specimens. J Clin Microbiol. 1998;36:979–82. doi: 10.1128/jcm.36.4.979-982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez-Martínez MJ, Miró JM, Valls ME, et al. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jirovecii in clinical specimens. Diagn Microbiol Infect Dis. 2006;56:153–60. doi: 10.1016/j.diagmicrobio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y, Ling G, Qiang C, et al. PCR diagnosis of Pneumocystis pneumonia: a bivariate meta-analysis. J Clin Microbiol. 2011;49:4361–3. doi: 10.1128/JCM.06066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris JR, Marston BJ, Sangrujee N, DuPlessis D, Park B. Cost-effectiveness analysis of diagnostic options for Pneumocystis pneumonia (PCP) PLoS One. 2011;6:e23158. doi: 10.1371/journal.pone.0023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larsen HH, Huang L, Kovacs JA, et al. A prospective, blinded study of quantitative touch-down polymerase chain reaction using oral-wash samples for diagnosis of Pneumocystis pneumonia in HIV-infected patients. J Infect Dis. 2004;189:1679–83. doi: 10.1086/383322. [DOI] [PubMed] [Google Scholar]
- 58.de Leeuwa BH, Voskuil WS, Maraha B, van der Zee A, Westenend PJ, Kusters JG. Evaluation of different real time PCRs for the detection of Pneumocystis jirovecii DNA in formalin-fixed paraffin-embedded bronchoalveolar lavage samples. Exp Mol Pathol. 2015;98:390–2. doi: 10.1016/j.yexmp.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Davis JL, Welsh DA, Beard CB, et al. Pneumocystis colonisation is common among hospitalised HIV-infected patients with non-Pneumocystis pneumonia. Thorax. 2008;63:329–34. doi: 10.1136/thx.2007.088104. [DOI] [PubMed] [Google Scholar]
- 60.Tasaka S, Kobayashi S, Yagi K, et al. Serum (1→3) β-D-glucan assay for discrimination between Pneumocystis jirovecii pneumonia and colonization. J Infect Chemother. 2014;20:678–81. doi: 10.1016/j.jiac.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Huggett JF, Taylor MS, Kocjan G, et al. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jirovecii DNA in bronchoalveolar lavage fluid of HIV-infected patients. Thorax. 2008;63:154–9. doi: 10.1136/thx.2007.081687. [DOI] [PubMed] [Google Scholar]
- 62.Montesinos I, Brancart F, Schepers K, Jacobs F, Denis O, Delforge ML. Comparison of 2 real-time PCR assays for diagnosis of Pneumocystis jirovecii pneumonia in human immunodeficiency virus (HIV) and non-HIV immunocompromised patients. Diagn Microbiol Infect Dis. 2015;82:143–7. doi: 10.1016/j.diagmicrobio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Bandt D, Monecke S. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jiroveci. Transpl Infect Dis. 2007;9:196–202. doi: 10.1111/j.1399-3062.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 64.Arcenas RC, Uhl JR, Buckwalter SP, et al. A real-time polymerase chain reaction assay for detection of Pneumocystis from bronchoalveolar lavage fluid. Diagn Microbiol Infect Dis. 2006;54:169–75. doi: 10.1016/j.diagmicrobio.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Matsumura Y, Ito Y, Iinuma Y, et al. Quantitative real-time PCR and the (1→3)-β-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect. 2012;18:591–7. doi: 10.1111/j.1469-0691.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 66.de Oliveira A, Unnasch TR, Crothers K, et al. Performance of a molecular viability assay for the diagnosis of Pneumocystis pneumonia in HIV-infected patients. Diagn Microbiol Infect Dis. 2007;57:169–76. doi: 10.1016/j.diagmicrobio.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Nahimana A, Rabodonirina M, Bille J, Francioli P, Hauser PM. Mutations of Pneumocystis jirovecii dihydrofolate reductase associated with failure of prophylaxis. Antimicrob Agents Chemother. 2004;48:4301–5. doi: 10.1128/AAC.48.11.4301-4305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang L, Crothers K, Atzori C, et al. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg Infect Dis. 2004;10:1721–8. doi: 10.3201/eid1010.030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alvarez-Martínez MJ, Moreno A, Miró JM, et al. Pneumocystis jirovecii pneumonia in Spanish HIV-infected patients in the combined antiretroviral therapy era: prevalence of dihydropteroate synthase mutations and prognostic factors of mortality. Diagn Microbiol Infect Dis. 2008;62:34–43. doi: 10.1016/j.diagmicrobio.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 70.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1→3)-β-D-glucan assay for the diagnosis of invasive fungal infections – a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 71.Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for Pneumocystis jirovecii pneumonia, invasive candidiasis, and invasive asper-gillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marty FM, Koo S, Bryar J, Baden LR. (1→3) beta-D-glucan assay positivity in patients with Pneumocystis (carinii) jirovecii pneumonia. Ann Intern Med. 2007;147:70–2. doi: 10.7326/0003-4819-147-1-200707030-00018. [DOI] [PubMed] [Google Scholar]
- 73.Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest. 2007;131:1173–80. doi: 10.1378/chest.06-1467. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe T, Yasuoka A, Tanuma J, et al. Serum (1→3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis. 2009;49:1128–31. doi: 10.1086/605579. [DOI] [PubMed] [Google Scholar]
- 75.Koga M, Koibuchi T, Kikuchi T, et al. Kinetics of serum β-D-glucan after Pneumo-cystis pneumonia treatment in patients with AIDS. Intern Med. 2011;50:1397–401. doi: 10.2169/internalmedicine.50.5296. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura H, Tateyama M, Tasato D, et al. Clinical utility of serum β-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med. 2009;48:195–202. doi: 10.2169/internalmedicine.48.1680. [DOI] [PubMed] [Google Scholar]
- 77.Quist J, Hill AR. Serum lactate dehydrogenase (LDH) in Pneumocystis carinii pneumonia, tuberculosis, and bacterial pneumonia. Chest. 1995;108:415–8. doi: 10.1378/chest.108.2.415. [DOI] [PubMed] [Google Scholar]
- 78.Boiselle PM, Crans CA, Jr, Kaplan MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172:1301–9. doi: 10.2214/ajr.172.5.10227507. [DOI] [PubMed] [Google Scholar]
- 79.Kanne JP, Yandow DR, Meyer CA. Pneumocystis jirovecii pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol. 2012;198:W555–61. doi: 10.2214/AJR.11.7329. [DOI] [PubMed] [Google Scholar]
- 80.Marchiori E, Muller NL, Soares Souza A, Jr, Escuissato DL, Gasparetto EL, Franquet T. Pulmonary disease in patients with AIDS: high-resolution CT and pathologic findings. AJR Am J Roentgenol. 2005;184:757–64. doi: 10.2214/ajr.184.3.01840757. [DOI] [PubMed] [Google Scholar]
- 81.Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967–75. doi: 10.2214/ajr.169.4.9308446. [DOI] [PubMed] [Google Scholar]
- 82.Kuhlman JE, Kavuru M, Fishman EK, Siegelman SS. Pneumocystis carinii pneumonia: spectrum of parenchymal CT findings. Radiology. 1990;175:711–4. doi: 10.1148/radiology.175.3.2343118. [DOI] [PubMed] [Google Scholar]
- 83.Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV infection: clinical manifestations, laboratory findings, and radiological features. J Infect Chemother. 2007;13:1–7. doi: 10.1007/s10156-006-0484-5. [DOI] [PubMed] [Google Scholar]
- 84.Chow C, Templeton PA, White CS. Lung cysts associated with Pneumocystis carinii pneumonia: radiographic characteristics, natural history, and complications. AJR Am J Roentgenol. 1993;161:527–31. doi: 10.2214/ajr.161.3.8352098. [DOI] [PubMed] [Google Scholar]
- 85.Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii pneumonia in immunocompromised patients with and without AIDS. Lung. 2010;188:159–63. doi: 10.1007/s00408-009-9214-y. [DOI] [PubMed] [Google Scholar]
- 86.Wassermann K, Pothoff G, Kirn E, Fätkenheuer G, Krueger GR. Chronic Pneumocystis carinii pneumonia in AIDS. Chest. 1993;104:667–72. doi: 10.1378/chest.104.3.667. [DOI] [PubMed] [Google Scholar]
- 87.McGuinness G, Scholes JV, Garay SM, Leitman BS, McCauley DI, Naidich DP. Cytomegalovirus pneumonitis: spectrum of parenchymal CT findings with pathologic correlation in 21 AIDS patients. Radiology. 1994;192:451–9. doi: 10.1148/radiology.192.2.8029414. [DOI] [PubMed] [Google Scholar]
- 88.Walzer PD, Evans HE, Copas AJ, Edwards SG, Grant AD, Miller RF. Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis. 2008;46:625–33. doi: 10.1086/526778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crothers K, Beard CB, Turner J, et al. Severity and outcome of HIV-associated Pneumocystis pneumonia containing Pneumocystis jirovecii dihydropteroate synthase gene mutations. AIDS. 2005;19:801–5. doi: 10.1097/01.aids.0000168974.67090.70. [DOI] [PubMed] [Google Scholar]
- 90.Armstrong-James D, Copas AJ, Walzer PD, Edwards SG, Miller RF. A prognostic scoring tool for identification of patients at high and low risk of death from HIV-associated Pneumocystis jirovecii pneumonia. Int J STD AIDS. 2011;22:628–34. doi: 10.1258/ijsa.2011.011040. [DOI] [PubMed] [Google Scholar]
- 91.Thomas M, Rupali P, Woodhouse A, Ellis-Pegler R. Good outcome with trimethoprim 10 mg/kg/day-sulfamethoxazole 50 mg/kg/day for Pneumocystis jirovecii pneumonia in HIV-infected patients. Scand J Infect Dis. 2009;41:862–8. doi: 10.3109/00365540903214256. [DOI] [PubMed] [Google Scholar]
- 92.Hughes W, Leoung G, Kramer F, et al. Comparison of atovaquone (566C80) with trimethoprim-sulfamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med. 1993;328:1521–7. doi: 10.1056/NEJM199305273282103. [DOI] [PubMed] [Google Scholar]
- 93.Dohn MN, Weinberg WG, Torres RA, et al. Oral atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS. Atovaquone Study Group. Ann Intern Med. 1994;121:174–80. doi: 10.7326/0003-4819-121-3-199408010-00003. [DOI] [PubMed] [Google Scholar]
- 94.Helweg-Larsen J, Benfield T, Atzori C, Miller RF. Clinical efficacy of first- and second-line treatments for HIV-associated Pneumocystis jirovecii pneumonia: a tri-centre cohort study. J Antimicrob Chemother. 2009;64:1282–90. doi: 10.1093/jac/dkp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tu GW, Ju MJ, Xu M, et al. Combination of caspofungin and low-dose trimethoprim/sulfamethoxazole for the treatment of severe Pneumocystis jirovecii pneumonia in renal transplant recipients. Nephrology. 2013;18:736–42. doi: 10.1111/nep.12133. [DOI] [PubMed] [Google Scholar]
- 96.Navin TR, Beard CB, Huang L, et al. Effect of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of P carinii pneumonia in patients with HIV-1: a prospective study. Lancet. 2001;358:545–9. doi: 10.1016/S0140-6736(01)05705-1. [DOI] [PubMed] [Google Scholar]
- 97.Fishman JA. Treatment of infection due to Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1309–14. doi: 10.1128/aac.42.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2009. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. [PubMed]
- 99.Briel M, Boscacci R, Furrer H, Bucher HC. Adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in patients with HIV infection: a meta-analysis of randomised controlled trials. BMC Infect Dis. 2005;(5):101. doi: 10.1186/1471-2334-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in patients with HIV infection. Cochrane Database Syst Rev. 2015;(4):CD006150. doi: 10.1002/14651858.CD006150.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbier F, Coquet I, Legriel S, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. 2009;35:1678–86. doi: 10.1007/s00134-009-1559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barbier F, Roux A, Canet E, et al. Temporal trends in critical events complicating HIV infection: 1999–2010 multicentre cohort study in France. Intensive Care Med. 2014;40:1906–15. doi: 10.1007/s00134-014-3481-7. [DOI] [PubMed] [Google Scholar]
- 103.Forrest DM, Zala C, Djurdjev O, et al. Determinants of short- and long-term outcome in patients with respiratory failure caused by AIDS-related Pneumocystis carinii pneumonia. Arch Intern Med. 1999;159:741–7. doi: 10.1001/archinte.159.7.741. [DOI] [PubMed] [Google Scholar]
- 104.Confalonieri M, Calderini E, Terraciano S, et al. Noninvasive ventilation for treating acute respiratory failure in AIDS patients with Pneumocystis carinii pneumonia. Intensive Care Med. 2002;28:1233–8. doi: 10.1007/s00134-002-1395-2. [DOI] [PubMed] [Google Scholar]
- 105.De Rosa FG, Fanelli V, Corcione S, et al. Extra corporeal membrane oxygenation (ECMO) in three HIV-positive patients with acute respiratory distress syndrome. BMC Anesthesiol. 2014;(14):37. doi: 10.1186/1471-2253-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cawcutt K, Gallo De, Moraes A, Lee SJ, Park JG, Schears GJ, Nemergut ME. The use of ECMO in HIV/AIDS with Pneumocystis jirovecii pneumonia: a case report and review of the literature. ASAIO J. 2014;60:606–8. doi: 10.1097/MAT.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 107.Zolopa AR, Andersen J, Komarow L, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaplan JE, Hanson DL, Navin TR, Jones JL. Risk factors for primary Pneumocystis carinii pneumonia in human immunodeficiency virus-infected adolescents and adults in the United States: reassessment of indications for chemoprophy-laxis. J Infect Dis. 1998;178:1126–32. doi: 10.1086/515658. [DOI] [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention Guidelines for prophylaxis against Pneumocystis carinii pneumonia for persons infected with human immunodeficiency virus. MMWR Morb Mortal Wkly Rep. 1989;38(suppl 5):1–9. [PubMed] [Google Scholar]
- 110.Opportunistic Infections Project Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) Mocroft A, Reiss P, et al. Is it safe to discontinue primary Pneumocystis jirovecii pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL? Clin Infect Dis. 2010;51:611–9. doi: 10.1086/655761. [DOI] [PubMed] [Google Scholar]
- 111.Bozzette SA, Finkelstein DM, Spector SA, et al. A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. N Engl J Med. 1995;332:693–9. doi: 10.1056/NEJM199503163321101. [DOI] [PubMed] [Google Scholar]
- 112.Huang L, Beard CB, Creasman J, et al. Sulfa or sulfone prophylaxis and geographic region predict mutations in the Pneumocystis carinii dihydropteroate syn-thase gene. J Infect Dis. 2000;182:1192–8. doi: 10.1086/315824. [DOI] [PubMed] [Google Scholar]
- 113.Schneider MM, Hoepelman AI, Eeftinck Schattenkerk., JK A controlled trial of aerosolized pentamidine or trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. N Engl J Med. 1992;327:1836–41. doi: 10.1056/NEJM199212243272603. [DOI] [PubMed] [Google Scholar]
- 114.Chan C, Montaner J, Lefebvre EA, et al. Atovaquone suspension compared with aerosolized pentamidine for prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected subjects intolerant of trimethoprim or sulfonamides. J Infect Dis. 1999;180:369–76. doi: 10.1086/314893. [DOI] [PubMed] [Google Scholar]
- 115.Kazanjian P, Armstrong W, Hossler PA, et al. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J Infect Dis. 2001;183:819–22. doi: 10.1086/318835. [DOI] [PubMed] [Google Scholar]
- 116.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons – 2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002;137:435–78. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]


