Abstract
Background: AdADOSE was a 12-week, international, observational study conducted in the Middle East and Russia where patients received nifedipine gastrointestinal therapeutic system (GITS) at a daily dose of 30, 60, or 90 mg as part of an antihypertensive combination therapy. This subgroup analysis of the AdADOSE study assesses the efficacy and tolerability of nifedipine GITS combination therapy when used specifically at the 60-mg strength. Methods: Patients with hypertension who received a daily nifedipine GITS dose of 60 mg, either at constant dose (n = 686) or up-titrated from 30 mg (n = 392), were analyzed. Target blood pressure (BP) was <140/90 mmHg (or <130/80 mmHg for those at high/very high cardiovascular risk). Results: Following nifedipine GITS combination therapy, target BP was achieved by 33.7% patients in the 60 mg group (previously untreated, 42.5%; previously treated, 32.0%) and 32.4% patients in the 30–60 mg group (previously untreated, 45.2%; previously treated, 30.7%). Mean systolic BP/diastolic BP changes were −40.3/−20.7 mmHg and −35.6/−18.5 mmHg, respectively, and were similar regardless of previous antihypertensive treatment or the number of concomitant diseases. Incidences of drug-related adverse events (AEs) were low (3.2%, 60 mg; 2.0%, 30–60 mg group), few patients discontinued because of AEs (0.6% and 1.0%, respectively), and there were no serious AEs. Conclusion: Combination therapy with nifedipine GITS 60 mg in a real-life observational setting was effective and well tolerated in hypertensive patients, with low rates of treatment-related AEs.
Keywords: AdADOSE, cardiovascular, hypertension, nifedipine GITS, observational study
Introduction
Most patients with elevated blood pressure (BP) require a combination of 2 or more antihypertensive drugs in order to achieve their BP target (1). Nifedipine is a dihydropyridine calcium channel blocker (CCB) with demonstrated efficacy in combination with other antihypertensive drugs, including β-blockers, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers (2,3). Nifedipine gastrointestinal therapeutic system (GITS) is an extended-release dosage formulation that provides sustained blood concentrations of nifedipine over 24 hours and which is established in clinical trials to achieve smooth and continuous BP control in hypertensive patients (4–7). Guidelines recommend such CCB combinations as first-line treatment in hypertension (1,8,9).
A large, international, observational study (AdADOSE) investigated the efficacy and safety of nifedipine GITS (30, 60, or 90 mg, once daily) combination therapy for 12 weeks in routine clinical practice (10). Patients were enrolled in AdADOSE from regions associated with particularly high cardiovascular risk, including the Middle East, Pakistan, and Russia (11,12). Despite a high level of pretreatment (85% of patients), usually with 1 or 2 antihypertensive therapies, nearly one-half of the patients enrolled in AdADOSE had systolic BP (SBP) of 160–179 mmHg and one-third had SBP ≥180 mmHg at the start of the study period. During the study, nifedipine GITS in initial combination therapy or as add-on therapy provided highly effective BP-lowering efficacy in the overall patient population, as well as in patients stratified according to baseline antihypertensive medication and the number of concomitant diseases (10). Treatment increased the proportion of patients achieving BP treatment goals current at the time of study (1) from 31% to 65% in patients without additional comorbidities, and from 7% to 23% in those with at least 3 concomitant diseases. Nifedipine GITS combination therapy was also well tolerated, with a very low incidence of drug-related adverse events (AEs; 2.6%) (10).
The main AdADOSE study included all patients who received nifedipine GITS at doses of 30, 60, or 90 mg once daily in combination with other antihypertensive agents (10). The decision to prescribe 30 or 60 mg nifedipine GITS tablets, and to up-titrate or down-titrate during the study, was made on an individual patient basis by the treating physician. The proportion of patients with grade 2 or grade 3 hypertension was higher in the 60 mg dose group compared with the overall AdADOSE population.
Previous publications have identified that increased doses of antihypertensive medication, including CCBs, may be associated with reduced tolerability (13,14). In the present study, post-hoc subanalyses were performed specifically on patients who received the 60 mg dose of nifedipine GITS, either as a constant daily dose of 60 mg or adjusted from 30 to 60 mg during the study period, with the aim to investigate whether the potential for greater efficacy at this higher dose was accompanied by change in adverse event profile.
Methods
Study design and patients
Detailed methods for the AdADOSE study have been published previously (10). Briefly, AdADOSE was a 12-week, prospective, international, multicenter, observational phase IV study that monitored the efficacy and safety of antihypertensive therapy with once-daily nifedipine GITS in combination with other antihypertensive agent(s) (ClinicalTrials.gov registration number NCT01118286). Selection of other antihypertensive medications (and doses) was at the discretion of the treating physician, and reflected daily practice. Patients were enrolled from 318 clinical practices in 10 countries (Bahrain, Egypt, Jordan, Lebanon, Oman, Pakistan, Qatar, Russia, Saudi Arabia, and the United Arab Emirates) between January 2010 and September 2011.
Eligible patients were male or female, aged ≥18 years, with primary hypertension (i.e. BP >140/90 mmHg or >130/80 mmHg in patients at high or very high cardiovascular risk) (1). Patients could be either previously untreated or insufficiently controlled on current antihypertensive regimens not containing CCBs. Baseline BP measurements in the AdADOSE study were taken prior to the first dose of study medication (nifedipine GITS), but while the patient was on concomitant background medication in those patients previously treated.
Observational parameters
The primary aim of the AdADOSE study was to assess the proportion of patients reaching the target BP of <140/90 mmHg (or <130/80 mmHg for those at high or very high cardiovascular risk), in accordance with European Society of Hypertension/European Society of Cardiology (ESH/ESC) guidelines current at the time of study (1). SBP, diastolic BP (DBP), pulse pressure, and heart rate were measured at the initial study visit and at up to 3 follow-up clinic visits during the 12-week observation period. Target BP was defined as a composite of the proportion of patients not at high/very high cardiovascular risk achieving their BP target of <140/90 mmHg and the proportion of patients at high/very high cardiovascular risk achieving their BP target of <130/80 mmHg.
All AEs that occurred throughout the study period were recorded on standardized case report forms at study visits. No information is available on whether investigators actively questioned patients on AEs at study visits or whether only those AEs that were spontaneously reported by patients were recorded; this may have been handled differently by individual investigators. However, since the case report forms contained a note requiring documentation of AEs by investigators at each visit and since the patients were made aware of their participation in a clinical trial by signing the informed consent form prior to study entry, an elevated awareness of AEs and AE reporting can be assumed for both investigators and patients. Efficacy and tolerability were also evaluated in the physician’s final assessment of therapy (with potential responses using the scales: very good, good, sufficient, and insufficient) and in the physician’s satisfaction with therapeutic effect (either very satisfied, satisfied, unsatisfied, or very unsatisfied).
Subgroup analyses
The total efficacy population, reported previously (10), included all patients who received once-daily nifedipine GITS 30, 60, or 90 mg in combination with other antihypertensive agent(s) with at least 1 follow-up BP measurement. The present subanalyses report data from the efficacy population for 2 subgroups of patients – those who received a constant daily dose of nifedipine GITS 60 mg (“the 60-mg group”), and those who had their daily dose adjusted from 30 to 60 mg nifedipine GITS during the study period (“the 30–60 mg group”).
Descriptive summary statistics for categorical and quantitative (continuous) data are reported. Percentage values were calculated as the proportion of each category including the category of missing values. Analyses were also stratified according to pretreatment status, initial BP, and concomitant diseases. Safety analyses are also reported for the 2 selected subgroups of patients.
Results
Patients at baseline
In the main AdADOSE study, 4477 patients were included in the safety analysis and 3430 patients in the efficacy analysis (10). Of the total efficacy population, 686 (20.0%) patients received a constant daily dose of nifedipine GITS 60 mg and 392 (11.4%) patients received nifedipine GITS 30 mg up-titrated to 60 mg. In the 60-mg and 30–60-mg groups, respectively, patients were enrolled from Saudi Arabia (n = 221 and n = 113), Pakistan (n = 137 and n = 52), Lebanon (n = 98 and n = 24), Egypt (n = 84 and n = 53), Russia (n = 60 and n = 74), Qatar (n = 32 and n = 29), Jordan (n = 27 and n = 3), the United Arab Emirates (n = 25 and n = 31), Bahrain (n = 2 and n = 8), and Oman (n = 0 and n = 5).
Baseline characteristics for the 2 patient subgroups are presented in Tables 1 and 2, alongside the total AdADOSE study population. Most patients were male (58–63%), mean age was 53 years, mean body mass index was 30 kg/m2, and the most prevalent race was Asian (36–40%). Mean baseline SBP/DBP was 171.2/101.6 mmHg (60 mg group) and 167.9/100.1 mmHg (30–60 mg group), and most patients in both groups (87%) had previously received antihypertensive medication(s). Specific concomitant diseases/conditions were reported in 91% of patients in each of the 2 subgroups. The most common specific concomitant diseases/conditions in the 60 mg and 30–60 mg groups, respectively, were dyslipidemia (38.3% and 51.8%), obesity (27.4% and 29.1%), and fatty liver (27.4% and 29.1%).
Table 1. Baseline characteristics in patients who received nifedipine GITS 60 mg (constant daily dose or up-titrated from 30 mg) combination therapy.
| Characteristic | Total AdADOSE Population (n = 3430) | Nifedipine GITS 60 mg (n = 686) | Nifedipine GITS 30–60 mg (n = 392) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 1993 (58.1) | 430 (62.7) | 227 (57.9) |
| Female | 1387 (40.4) | 244 (35.6) | 157 (40.1) |
| Missing | 50 (1.5) | 12 (1.7) | 8 (2.0) |
| Mean age, years ± SD (range) | (n = 3322) 53.4 ± 10.4 (19.0–89.0) | (n = 659) 53.2 ± 10.1 (23.0–89.0) | (n = 385) 53.5 ± 11.1 (22.0–80.0) |
| Age, n (%) | |||
| <65 years | 2836 (82.7) | 571 (83.2) | 327 (83.4) |
| ≥65 years | 486 (14.2) | 88 (12.8) | 58 (14.8) |
| Missing | 108 (3.1) | 27 (3.9) | 7 (1.8) |
| Race, n (%) | |||
| Asian | 1212 (35.3) | 276 (40.2) | 139 (35.5) |
| White | 966 (28.2) | 206 (30.0) | 139 (35.5) |
| Black | 96 (2.8) | 21 (3.1) | 8 (2.0) |
| Other | 970 (28.3) | 135 (19.7) | 74 (18.9) |
| Missing | 186 (5.4) | 48 (7.0) | 32 (8.2) |
| Mean BMI, kg/m2 ± SD (range) | (n = 3214) 29.9 ± 5.2 (15.1–57.4) | (n = 642) 29.8 ± 5.0 (15.1–52.1) | (n = 372) 30.1 ± 5.3 (17.4–54.2) |
| Mean BP, mmHg ± SD (range) | |||
| SBP | (n = 3413) 166.4 ± 16.7 (100.0–260.0) | (n = 685) 171.2 ± 17.1 (140.0–250.0) | (n = 390) 167.9 ± 17.0 (125.0–230.0) |
| DBP | (n = 3418) 99.7 ± 9.9 (55.0–170.0) | (n = 686) 101.6 ± 10.4 (60.0–150.0) | (n = 389) 100.1 ± 9.4 (70.0–140.0) |
| Stages of hypertension, n (%)a | |||
| Normal | 8 (0.2) | 0 | 0 |
| High normal | 16 (0.5) | 0 | 2 (0.5) |
| Grade 1 (mild) | 407 (11.9) | 52 (7.6) | 42 (10.7) |
| Grade 2 (moderate) | 1620 (47.2) | 312 (45.5) | 199 (50.8) |
| Grade 3 (severe) | 1142 (33.3) | 285 (41.5) | 131 (33.4) |
| Isolated systolic hypertension | 218 (6.4) | 36 (5.2) | 15 (3.8) |
| Missing | 19 (0.6%) | 1 (0.1) | 3 (0.8) |
| Duration of hypertension, n (%) | |||
| Newly diagnosed | 479 (14.0) | 83 (12.1) | 44 (11.2) |
| Previously diagnosed | 2947 (85.9) | 602 (87.8) | 346 (88.3) |
| Missing | 4 (0.1) | 1 (0.1) | 2 (0.5) |
| <1 year | 434 (14.7) | 72 (12.0) | 58 (16.8) |
| 1–5 years | 1210 (41.1) | 264 (43.9) | 135 (39.0) |
| 6–10 years | 692 (23.5) | 152 (25.2) | 80 (23.1) |
| >10 years | 554 (18.8) | 99 (16.4) | 67 (19.4) |
| Missing | 57 (1.9) | 15 (2.5) | 6 (1.7) |
| Previous antihypertensive therapy, n (%) | |||
| 0 | 421 (12.3) | 73 (10.6) | 42 (10.7) |
| 1 | 1314 (38.3) | 249 (36.3) | 123 (31.4) |
| 2 | 1067 (31.1) | 216 (31.5) | 143 (36.5) |
| ≥3 | 521 (15.2) | 131 (19.1) | 76 (19.4) |
| Missing | 107 (3.1) | 17 (2.5) | 8 (2.0) |
| Concomitant antihypertensive therapy at initial visit, n (%) | (n = 3430) | (n = 686) | (n = 392) |
| 0 | 152 (4.4) | 39 (5.7) | 19 (4.8) |
| 1 | 1792 (52.2) | 344 (50.1) | 162 (41.3) |
| 2 | 105 (30.7) | 214 (31.2) | 134 (34.2) |
| ≥3 | 433 (13.0) | 89 (13.0) | 77 (19.8) |
| Missing | 0 | 0 | 0 |
| Previous classes of antihypertensive therapy, n (%)b | (n = 2922) | (n = 600) | (n = 342) |
| ACE inhibitors | 1210 (41.4) | 236 (39.3) | 145 (42.4) |
| ARBs | 833 (28.5) | 200 (33.3) | 118 (34.5) |
| CCBsc | 348 (11.9) | 108 (18.0) | 32 (9.4) |
| β-blockers | 1389 (47.5) | 271 (45.2) | 156 (45.6) |
| Thiazide diuretics | 940 (32.2) | 211 (35.2) | 142 (41.5) |
| Other | 97 (3.3) | 29 (4.9) | 18 (5.3) |
| Not codable | 294 (10.1) | 53 (8.8) | 39 (11.4) |
| Missing | 20 (0.7) | 4 (0.7) | 0 |
| Concomitant classes of antihypertensive therapy at initial visit, n (%)b | (n = 3430) | (n = 686) | (n = 392) |
| None | 152 (4.4) | 39 (5.7) | 19 (4.8) |
| ACE inhibitors | 1104 (32.2) | 197 (28.7) | 126 (32.1) |
| ARBs | 938 (27.3) | 212 (30.9) | 140 (35.7) |
| CCBsc | 275 (8.0) | 52 (7.6) | 30 (7.7) |
| β-blockers | 1496 (43.6) | 295 (43.0) | 167 (42.6) |
| Thiazide diuretics | 1062 (31.0) | 222 (32.4) | 168 (42.9) |
| Other | 92 (2.7) | 29 (4.4) | 15 (3.8) |
| Not codable | 304 (8.9) | 53 (7.7) | 25 (6.4) |
| Missing | 0 | 0 | 0 |
| Smoking history, n (%) | |||
| Current smoker | 725 (21.1) | 182 (26.5) | 74 (18.9) |
| Never | 1940 (56.6) | 338 (49.3) | 234 (59.7) |
| Past smoker | 606 (17.7) | 123 (17.9) | 62 (15.8) |
| Missing | 159 (4.6) | 43 (6.3) | 22 (5.6) |
Data for all variables were not available for all patients. ACE inhibitors, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BMI, body mass index; BP, blood pressure; CCBs, calcium channel blockers; DBP, diastolic blood pressure; GITS, gastrointestinal therapeutic system; SBP, systolic blood pressure; SD, standard deviation.
aDefinitions according to ESH/ESC 2007 guidelines.
bMultiple responses possible.
cOther than nifedipine GITS.
Table 2. Specific concomitant diseases at baseline in patients who received nifedipine GITS 60 mg (constant daily dose or up-titrated from 30 mg) combination therapy.
| Total AdADOSE population (n = 3430) | Nifedipine GITS 60 mg (n = 686) | Nifedipine GITS 30–60 mg (n = 392) | |
|---|---|---|---|
| Patients with specific concomitant diseases, n (%) | 3109 (90.6) | 625 (91.1) | 356 (90.8) |
| Dyslipidemia | 1417 (41.3) | 263 (38.3) | 203 (51.8) |
| Obesity | 917 (26.7) | 188 (27.4) | 114 (29.1) |
| Fatty liver | 917 (26.7) | 188 (27.4) | 114 (29.1) |
| Stable angina pectoris | 445 (13.0) | 74 (10.8) | 49 (12.5) |
| Diabetic neuropathy | 396 (11.5) | 89 (13.0) | 63 (16.1) |
| Microalbuminuria | 275 (8.0) | 73 (10.6) | 60 (15.3) |
| Diabetic retinopathy | 271 (7.9) | 53 (7.7) | 52 (13.3) |
| Myocardial infarction | 230 (6.7) | 42 (6.1) | 28 (7.1) |
| Renal insufficiency | 167 (4.9) | 42 (6.1) | 31 (7.9) |
| Peripheral vascular disease | 158 (4.6) | 35 (5.1) | 14 (3.6) |
| Congestive heart failure | 141 (4.1) | 35 (5.1) | 14 (3.6) |
| Coronary revascularization | 137 (4.0) | 35 (5.1) | 14 (3.6) |
| Transient ischemic attack | 115 (3.4) | 24 (3.5) | 16 (4.1) |
| Cerebrovascular accident | 97 (2.8) | 16 (2.3) | 9 (2.3) |
| Number of concomitant diseases, n (%)a | |||
| 1 | 622 (18.1) | 121 (17.6) | 61 (15.6) |
| 2 | 863 (25.2) | 193 (28.1) | 75 (19.1) |
| 3 | 685 (20.0) | 122 (17.8) | 83 (21.2) |
| 4 | 442 (12.9) | 92 (13.4) | 50 (12.8) |
| 5 | 264 (7.7) | 53 (7.7) | 35 (8.9) |
| 6 | 149 (4.3) | 27 (3.9) | 32 (8.2) |
| 7 | 82 (2.4) | 15 (2.2) | 15 (3.8) |
| 8 | 34 (1.0) | 8 (1.2) | 7 (1.8) |
| 9 | 12 (0.3) | 2 (0.3) | 2 (0.5) |
| 10 | 5 (0.1) | 1 (0.1) | 0 |
| >10 | 8 (0.2) | 1 (0.1) | 1 (0.3) |
GIT, gastrointestinal therapeutic system.
aCoded according to Medical Dictionary for Regulatory Activities System Organ Class.
In general, the 60 mg and 30–60 mg nifedipine GITS groups had similar baseline characteristics. However, the 60 mg group had a higher proportion of patients with grade 3 (severe) hypertension than the 30–60-mg group (41.5% vs 33.4%), as well as a greater proportion of patients with previous treatment with CCBs (18.0% vs 9.4%) (Table 1). Notably, the incidence of grade 2 or grade 3 hypertensions at baseline was higher in the 60 mg and 30–60 mg nifedipine GITS groups compared with the total AdADOSE population.
Concomitant treatments
At the initial visit, most patients in the nifedipine GITS 60 mg and 30–60 mg groups were taking 1 (50.1% and 41.3%) or 2 (31.2% and 34.2%) concomitant antihypertensive drugs, with lower proportions taking 3 (10.2% and 17.3%), 4 (2.3% and 2.0%), and 5 (0.4% and 0.3%) concomitant antihypertensive drugs. The most frequently used concomitant antihypertensive drugs at initial and final visits, respectively, were β-blockers (60 mg group, 43.0% and 44.6%; 30–60 mg group, 42.6% and 42.1%) and thiazide diuretics (60-mg group, 32.4% and 34.0%; 30–60-mg group, 42.9% and 42.6%). The most frequent non-antihypertensive concomitant medications in the 60-mg and 30–60-mg groups, respectively, were acetylsalicylic acid (29.4% and 34.2%), atorvastatin calcium (12.8% and 17.1%), and metformin (14.7% and 18.4%).
Efficacy
By the end of the 12-week observational study period, the target BP goal (i.e., BP > 140/90 mmHg or >130/80 mmHg in patients at high/very high cardiovascular risk) was reached by 33.7% (231/686) of patients in the 60-mg nifedipine GITS group and by 32.4% of patients (127/392) in the 30–60-mg group (Figure 1). By comparison, the target BP goal was reached by 36.3% of the total population receiving all nifedipine GITS doses in the AdADOSE study.
Figure 1.
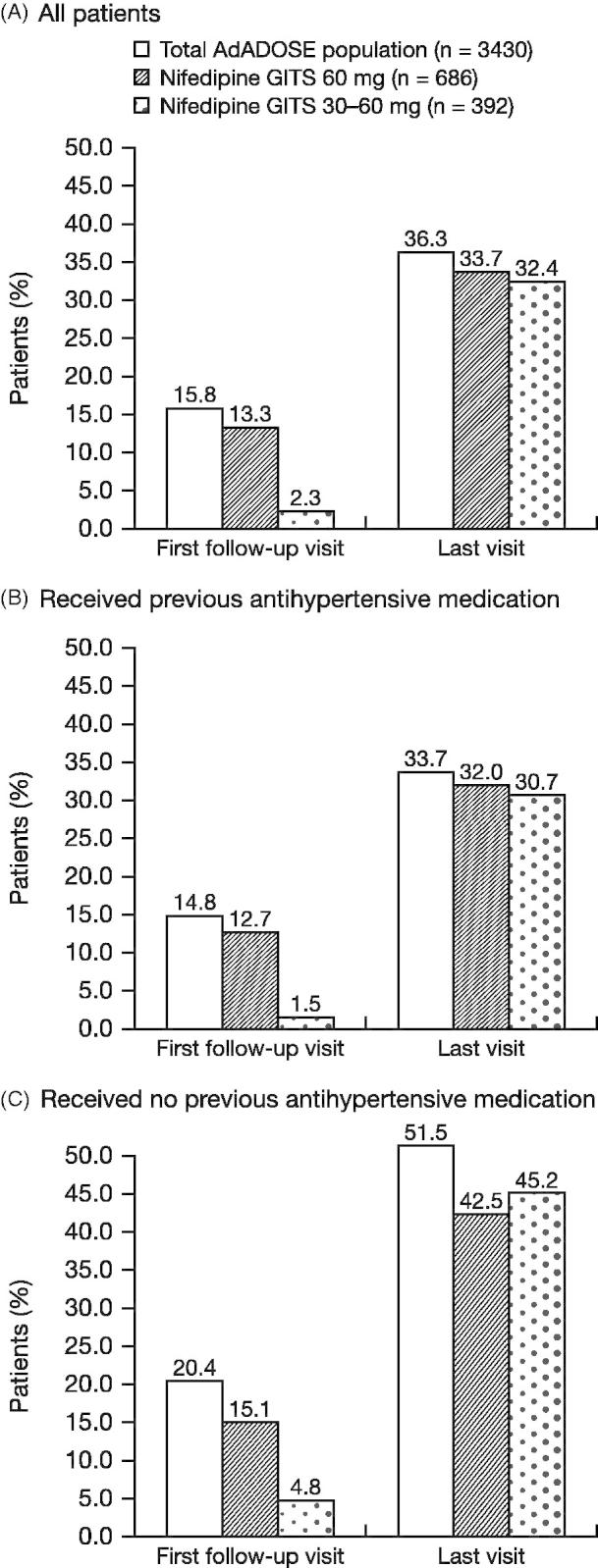
Proportion of patients who reached target BPa following treatment with nifedipine GITS 60mg (constant daily dose or up-titrated from 30mg) combination therapy. aBP < 140/90mmHg (or BP < 130/80mmHg in those at high or very high cardiovascular risk). BP, blood pressure; GITS, gastrointestinal therapeutic system.
In patients not at high/very high cardiovascular risk, the target BP of <140/90 mmHg was reached by 67.2% (170/253) of patients in the 60 mg group and by 70.8% (92/130) of patients in the 30–60 mg group. In patients who were at high/very high risk, the lower target BP of <130/80 mmHg was reached by 14.1% (61/433) of patients in the 60 mg group and 13.4% (35/262) of patients in the 30–60 mg group. The BP target was achieved in higher proportions of patients who were previously untreated (42.5%, 60 mg group; 45.2%, 30–60 mg group) compared with those who were previously treated with antihypertensive medications (32.0%; 30.7%) (Figure 1).
Combination therapy with nifedipine GITS provided a mean absolute reduction in SBP/DBP of −40.3/−20.7 mmHg (60 mg group) and −35.6/−18.5 mmHg (30–60 mg group). BP reductions were similar irrespective of the number of concomitant diseases present, ranging from 37.4 to 44.0/19.0 to 23.1 mmHg (60-mg group) and 34.1 to 37.4/16.4 to 21.0 mmHg (30–60-mg group). In previously untreated patients, mean absolute reductions were −45.4/−23.8 mmHg (60-mg group) and −36.6/−20.3 mmHg (30–60-mg group). In previously treated patients, mean absolute reductions were −39.4/−20.2 mmHg (60-mg group) and −36.0/−18.5 mmHg (30–60-mg group).
When patients were stratified according to initial BP values, mean BP reductions were greater for higher baseline SBP and DBP. In the 60-mg group, the change in SBP ranged from −18.0 mmHg (initial SBP 140-149 mmHg) to −56.6 mmHg (initial SBP ≥180 mmHg) and the change in DBP ranged from −2.0 mmHg (initial DBP <75 mmHg) to −31.2 mmHg (initial DBP ≥110 mmHg). In the 30–60-mg group, the change in SBP ranged from −5.1 mmHg (initial SBP 130–139 mmHg) to −52.9 mmHg (initial SBP ≥180 mmHg) and the change in DBP ranged from −2.7 mmHg (initial DBP 80–84 mmHg) to −31.4 mmHg (initial DBP ≥110 mmHg).
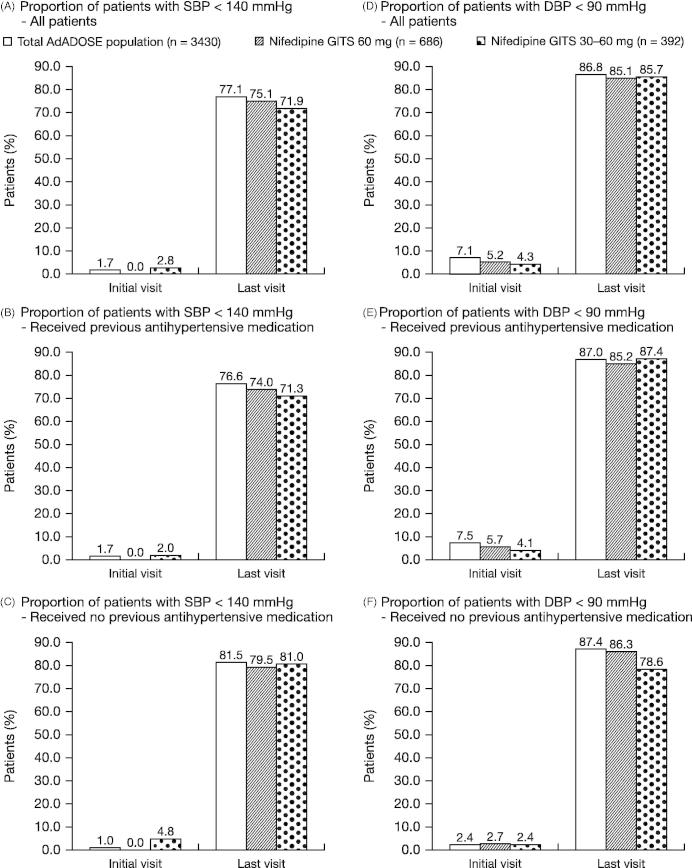
The proportion of patients with SBP <140 mmHg increased from the initial visit to the last visit: 60-mg group, from 0% to 75.1%; 30–60-mg group, from 2.8% to 71.9% (Figure 2). The proportion of patients with DBP <90 mmHg also increased from the initial visit to the last visit: 60-mg group, from 5.2% to 85.1%; 30–60-mg group, 4.3% to 85.7%. The increased rates of SBP <140 mmHg and DBP <90 mmHg from initial to final visit occurred in both previously treated and untreated patients.
Figure 2.
Proportion of patients with SBP <140 mmHg or DBP <90 mmHg following treatment with nifedipine GITS 60 mg (constant daily dose or up-titrated from 30 mg) combination therapy.
Between the initial and last visit, mean pulse pressure (± standard deviation [SD]) decreased from 69.6 ± 14.9 mmHg to 50.0 ± 9.3 mmHg (60 mg group) and from 67.8 ± 15.9 mmHg to 50.6 ± 9.8 mmHg (30–60 mg group). Mean heart rate (± SD) decreased from 79.3 ± 10.9 beats per minute (bpm) to 75.9 ± 8.2 bpm (60-mg group) and from 77.0 ± 10.7 bpm to 74.7 ± 7.6 bpm (30–60 mg group). Changes in these parameters were similar for patients regardless of the use of previous antihypertensive medication (data not shown).
Tolerability
The incidence of AEs was low in the 60 mg group (39 AEs in 24/686 [3.5%] patients) and in the 30–60 mg group (14 AEs in 11/392 [2.8%] patients). The most common AEs were peripheral edema (9 [1.3%] patients, 60-mg group; 4 [1.0%] patients, 30–60 mg group,) and headache (6 [0.9%]; 3 [0.8%] patients, respectively) (Table 3).
Table 3. Incidence of AEs and drug-related AEs (>1 patient in either treatment group) in patients during treatment with nifedipine GITS 60 mg (constant daily dose or up-titrated from 30 mg) combination therapy.
| Nifedipine GITS 60 mg (n = 686) |
Nifedipine GITS 30–60 mg (n = 392) |
|||
|---|---|---|---|---|
| AEs | Drug-related AEs | AEs | Drug-related AEs | |
| All patients (%) | 24 (3.5) | 22 (3.2) | 11 (2.8) | 8 (2.0) |
| AE, n (%) | ||||
| Peripheral edema | 9 (1.3) | 9 (1.3) | 4 (1.0) | 3 (0.8) |
| Headache | 6 (0.9) | 6 (0.9) | 3 (0.8) | 3 (0.8) |
| Edema | 3 (0.4) | 2 (0.3) | 0 | 0 |
| Flushing | 3 (0.4) | 3 (0.4) | 1 (0.3) | 1 (0.3) |
| Dizziness | 3 (0.4) | 3 (0.4) | 0 | 0 |
| BP inadequately controlled | 0 | 0 | 2 (0.5) | 0 |
| Tachycardia | 2 (0.3) | 2 (0.3) | 0 | 0 |
AEs experienced by only 1 patient in the 60-mg group were: allergic dermatitis, anxiety, constipation, dry mouth, fatigue, hypokalemia, hypotension, increased heart rate, loss of consciousness, orthopnea, palpitations, postural dizziness, and somnolence; and in the 30–60-mg group were: dyspnea, flushing, hypotension, nausea, and palpitations. Drug-related AEs experienced by only 1 patient in the 60-mg group were: allergic dermatitis, anxiety, constipation, hypotension, increased heart rate, orthopnea, and somnolence; and in the 30–60-mg group were: dyspnea, flushing, hypotension, nausea, and palpitations.
AEs, adverse events; BP, blood pressure; GITS, gastrointestinal therapeutic system.
Low incidences of AEs were considered by the treating physician to be study drug related: 32 events in 22/686 (3.2%) patients in the 60 mg group and 11 events in 8/392 (2.0%) patients in the 30–60 mg group. These included peripheral edema (9 [1.3%] patients, 60 mg group; 3 [0.8%] patients, 30–60 mg group) and headache (6 [0.9%]; 3 [0.8%] patients). No serious AEs or serious drug-related AEs were reported during the study period. Few AEs resulted in permanent discontinuation of study drug in the 60 mg group (5 AEs in 4 [0.6%] patients) or in the 30–60 mg group (4 AEs in 4 [1.0%] patients). In the 60 mg group, these events were peripheral edema (n = 3 [0.4%]), constipation (n = 1 [0.2%]), and orthopnea (n = 1 [0.2%]). In the 30–60 mg group, these were inadequately controlled BP (n = 2 [0.5%]), peripheral edema (n = 1 [0.3%]), and hypotension (n = 1 [0.3%]).
The incidence of all AEs, as well as drug-related AEs, in patients who received exclusively the 60-mg nifedipine GITS dose was compared in an explanatory way with the incidence in patients who received exclusively the 30 mg dose in the AdADOSE study (Table 4). There were slightly higher rates of AEs and drug-related AEs at the higher dose, but statistical analysis showed that these differences were not significant (p = 0.370 for all AEs [odds ratio with 95% CI: 1.24 [0.76–2.01]] and p = 0.208 for drug-related AEs [odds ratio with 95% CI: 1.40 [0.84–2.33]]). There was also no statistically significant difference in the rate of AEs resulting in permanent discontinuation of study drug between the 30 and 60 mg dose groups (p = 0.623, odds ratio with 95% CI: 0.67 [0.23–1.99]); for this comparison, the AE rate was actually higher in the 30-mg group (0.87% vs 0.58%, p = 0.623), without statistical significance.
Table 4. Incidence of AEs and drug-related AEs (>1 patient in either treatment group) in patients during treatment with nifedipine GITS 60 mg versus 30 mg (constant daily doses) combination therapy.
| Nifedipine GITS 60 mg (n = 686) | Nifedipine GITS 30 mg (n = 2073) | p Value* | Odds ratio [95% CI] | |||
|---|---|---|---|---|---|---|
| AE, n (%) | 24 (3.50) | 59 (2.85) | 0.370 | 1.24 [0.76–2.01] | ||
| Drug-related adverse events, n (%) | 22 (3.21) | 48 (2.32) | 0.208 | 1.40 [0.84–2.33] | ||
| Serious adverse events, n (%) | 0 (0.00) | 0 (0.00) | – | – | ||
| AEs resulting in permanent discontinuation of study drug, n (%) | 4 (0.58) | 18 (0.87) | 0.623 | 0.67 [0.23–1.99] |
*Fisher’s exact test.
Physician satisfaction with nifedipine GITS combination treatment
In the 60 mg and 30–60 mg groups, respectively, high proportions of treating physicians assessed the efficacy of nifedipine GITS combination treatment as “very good” (66.9% and 57.1%) and “good” (27.4% and 32.9%); low proportions assessed efficacy as “sufficient” (2.6% and 6.9%) and “insufficient” (1.9% and 2.8%).
The tolerability of nifedipine GITS combination treatment in the 60 mg group and 30–60 mg groups, respectively, was assessed as “very good” (64.7% and 54.3%), “good” (29.4% and 37.8%), “sufficient” (2.0% and 5.9%), and “insufficient” (0.9% and 0.8%).
Overall, treating physicians were “very satisfied” (62.4% and 54.8%), “satisfied” (31.3% and 40.1%), “unsatisfied” (2.9% and 2.6%), and “very unsatisfied” (0 and 0.3%) with the therapeutic effect of nifedipine GITS combination treatment in the 60 and 30–60 mg groups, respectively (missing data not reported).
Discussion
In this subgroup analysis of the 12-week, international, observational AdADOSE study, nifedipine GITS 60 mg, at a constant daily dose or up-titrated from 30 mg, was highly effective and well-tolerated in combination with other antihypertensive(s). The efficacy and tolerability of nifedipine GITS in patients taking the 60 mg dose were generally consistent with the total study population, which included nifedipine GITS doses of 30, 60, or 90 mg daily (10).
Observational studies can provide important information on the efficacy and safety of hypertensive treatments in real-life clinical practice. In such studies, the choice of antihypertensive agent (and dose) is likely to be based on the treating physician’s clinical experience and preferences. In the present analysis, 87% of patients prescribed nifedipine GITS 60 mg had received prior antihypertensive treatment and 91% had at least 1 concomitant disease. However, despite a high level of pretreatment, mean SBP/DBP at the start of the study was 171.2/101.6 mmHg (60 mg group) and 167.9/100.1 mmHg (30–60 mg group), and more than 84% of patients in each group had moderate-to-severe hypertension. The proportion of patients already at their target BP (based on ESH/ESC guidelines at the time of the study [1]) was only 2.3% (30–60 mg group) and 13.3% (60 mg group). This highlights the poor BP control (and, therefore, uncontrolled cardiovascular risk) present in the patients enrolled in this study.
Data at the start of the study also revealed that patients who received a constant daily dose of nifedipine GITS 60 mg had more severe hypertension compared with the total population (10); this was evidenced by a higher mean SBP (171.2 mmHg vs 166.4 mmHg) and a higher proportion of patients with grade 3 (severe) hypertension (41.5% vs 33.3%). Patients in the 60 mg group also had a higher rate of prior CCB use than the total population (18.0% vs 11.9%). In addition, both groups who received nifedipine GITS 60 mg had a higher rate of prior treatment with at least 3 antihypertensive medications compared with the total study population (19.1%, 60 mg group; 19.4%, 30–60 mg group; 15.2%, total population).
Mean SBP/DBP changes were greater in the 60-mg group (−40.3/−20.7 mmHg) than in the 30–60 mg group (−35.6/−18.5 mmHg) and the total population (−36.1/−18.8 mmHg). In all patient groups, the changes were similar irrespective of previous treatment or the number of concomitant diseases.
During the 12-week study period, nifedipine GITS in initial combination therapy or as add-on therapy improved the achievement of BP goals. In the 30–60 mg group, an additional 30.1% of patients reached target BP (from 2.3% to 32.4%) after 12 weeks of nifedipine GITS treatment. In the 60 mg group, an additional 20.4% of patients reached target BP (from 13.3% to 33.7%). Data were similar in those who were previously treated with antihypertensive therapy. For comparison, in the main study population of AdADOSE, an additional 20.5% of all patients reached target BP (from 15.8% to 36.3%) after 12 weeks of nifedipine GITS treatment (10).
It should be noted that the target blood pressure values of <140/90 mmHg for patients not at high risk and <130/80 mmHg in high-risk patients in the ESC/ESH guidelines of 2007, which were considered when the protocol for the AdADOSE study was initiated, were revised in the 2013 ESC/ESH guidelines (15). These newer ESC/ESH guidelines recommend a BP target of <140/90 mmHg for most patients, irrespective whether they are at high risk or not. In this context, the achievement of the higher target BP in the current subpopulation of the AdADOSE study was even better. The proportion of patients with SBP <140 mmHg increased from the initial visit to the last visit in the 60 mg group (0–75.1%) and in the 30–60 mg group (2.8–71.9%), with data being consistent with the total population (1.7–77.1%). Similarly, the proportion of patients with DBP <90 mmHg also increased from the initial visit to the last visit in the 60 mg group (5.2–85.1%) and 30–60 mg group (4.3%–85.7%); again, data were consistent with the total population (7.1–86.8%). The increased rates of SBP <140 mmHg and DBP <90 mmHg from initial to final visit occurred in both previously treated and untreated patients.
Findings from the present observational study are consistent with the BP reductions and improved BP control seen in previous observational studies of nifedipine GITS. In a large observational study conducted in the Far East, the Middle East, Pakistan, and Mexico, nifedipine GITS 30 mg or 60 mg (monotherapy or add-on therapy) provided a mean BP reduction of −28/−14 mmHg (16). The extent of BP reduction was associated with a number of factors, including hypertension grade, age, presence of at least 5 additional cardiovascular risk factors, and prior treatment. Target BP (<140/90 mmHg; 130/80 mmHg in patients with diabetes) was achieved in 29.5% of patients overall, and was slightly higher in those receiving monotherapy (33.4%) versus combination therapy (25.3%), suggesting that patients prescribed combination therapy had more severe hypertension. Smaller observational studies in China have also demonstrated the BP-lowering efficacy of nifedipine GITS in real-life clinical practice (17,18).
In the present subgroup analysis, the incidence of drug-related AEs was low (2.0%–3.2%). Furthermore, there were no serious AEs and few patients discontinued due to AEs (0.6%–1.0%). The AE profile in the subgroups (e.g., low rates of edema, headache, and hypotension) was consistent with that in the total AdADOSE population (10) and with the known safety profile of nifedipine GITS. Slightly higher AE rates, not considered as clinically relevant, were observed when patients treated exclusively with the 60-mg dose were compared with patients treated with the 30-mg dose. Reasons for this small difference in AE rates between the dose groups might be that co-medications, in particular angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors, decrease the rate of edema caused by CCBs (19–21). On the other hand, since AdADOSE was not a randomized trial and included a low number of AEs, the lack of difference in AE rates between patients receiving the 60-mg dose and the 30-mg nifedipine GITS dose might also, at least partly, be due to the fact that investigators did not allocate patients at higher risk for edema to the 60-mg nifedipine GITS dose. Whatever the explanation, the low overall AE rate and the lack of a relevant increase in AE rates at the 60 mg dose underlines the very good tolerability of nifedipine GITS at both 30 and 60 mg doses under real-life conditions when used as part of antihypertensive combination therapy.
Data for physician satisfaction with nifedipine GITS combination treatment mirrored data for the total population (10). In patients taking the 60 mg dose, physicians rated the overall efficacy of nifedipine GITS as “very good”/“good” in 90–94% of patients, the tolerability as “very good”/“good” in 92–94% of patients, and their satisfaction with therapeutic efficacy as “very good”/“good” for 94–95% of patients.
Limitations of the AdADOSE study are its observational design with lack of control group, and the absence of information that underlies the selection of nifedipine GITS dose and the types of concomitant medication chosen by study investigators. However, observational studies can provide important information on efficacy and tolerability of antihypertensive agents in the real-life clinical setting that is likely to inform daily practice. In that light, the present subgroup analysis provides useful data on the efficacy and tolerability of the 60 mg dose of nifedipine GITS. Another limitation of the AdADOSE study, as discussed previously (10), is that international guidelines on treatment targets have since been updated for patients at high cardiovascular risk, although the broad conclusions of the study remain applicable for informing practice (8,9).
Conclusion
Combination therapy with nifedipine GITS 60 mg once daily in a real-life observational setting was highly effective in hypertensive patients, with low rates of treatment-related AEs. This subanalysis from AdADOSE supports the value of GITS 60 mg in the treatment of patients with hypertension including those with additional cardiovascular risk factors and comorbidities.
Acknowledgements
Bayer Pharma provided support in the design and conduct of the study, and in the collection, management, and analysis of the data. The roles of the authors who are employed by Bayer Pharma are itemized in the section above. Caroline Schneider and Klaus Hechenbichler at the Dr. Schauerte Contract Research Organization provided project management and statistical support. Bill Wolvey from PAREXEL provided medical writing support funded by Bayer Pharma.
Declaration of interest
A.K.M., E.U., W.H., and Z.D. have declared that they have no competing interests. T.P. is an employee of Bayer HealthCare, Germany.
References
- Mancia G, DeBacker G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- Brennan F, Flanagan M, Blake S, Cannon P. Nifedipine in the treatment of hypertension. Eur J Clin Pharmacol. 1983;25:713–15. doi: 10.1007/BF00542507. [DOI] [PubMed] [Google Scholar]
- Gradman AH, Basile JN, Carter BL, et al. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:90–8. doi: 10.1016/j.jash.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson PA, Lubsen J, Kirwan BA, et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364:849–57. doi: 10.1016/S0140-6736(04)16980-8. [DOI] [PubMed] [Google Scholar]
- Hasebe N, Kikuchi K. Controlled-release nifedipine and candesartan low-dose combination therapy in patients with essential hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J Hypertens. 2005;23:445–53. doi: 10.1097/00004872-200502000-00028. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356:366–72. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- Saito I, Saruta T. Controlled release nifedipine and valsartan combination therapy in patients with essential hypertension: the Adalat CR and Valsartan cost-effectiveness combination (ADVANCE-combi) study. Hypertens Res. 2006;29:789–96. doi: 10.1291/hypres.29.789. [DOI] [PubMed] [Google Scholar]
- Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–58. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence: Clinical Management of Primary Hypertension in Adults. NICE Guidelines [CG127]. UK National Institute for Health and Clinical Excellence (NICE) Clinical Guideline. Available from: http://publications.nice.org.uk/hypertension-cg127 [last accessed 27 April 2015]
- Motaweih AK, Usova E, Hussain W, et al. Effectiveness of combination therapy with nifedipine GITS: a prospective, 12-week observational study (AdADOSE). BMC Cardiovasc Disord 2015;15:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- European Society of Cardiology: Euro Heart Survey. Cardiovascular diseases in Europe in 2006. Available from: http://www.escardio.org/static_file/Escardio/EORP/about/EHS-CVD-report-2006.pdf [last accessed 27 April 2015]
- Haller H. Effective management of hypertension with dihydropyridine calcium channel blocker-based combination therapy in patients at high cardiovascular risk. Int J Clin Pract. 2008;62:781–90. doi: 10.1111/j.1742-1241.2008.01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutel JM. The use of combination drug therapy in the treatment of hypertension. Prog Cardiovasc Nurs. 2002;17:81–8. doi: 10.1111/j.0889-7204.2002.01308.x. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- Ueng KC, Ningling S, El MA, et al. Efficacy and tolerability of long-acting nifedipine GITS/OROS monotherapy or combination therapy in hypertensive patients: results of a 12-week international, prospective, multicentre, observational study. Clin Drug Investig. 2011;31:631–42. doi: 10.2165/11588970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Huo Y, Zhang J, He Q, et al. Efficacy and safety of nifedipine GITS in Chinese patients with hypertension--a post-marketing surveillance study. Blood Press Suppl. 2007;1:18–23. doi: 10.1080/08038020601154575. [DOI] [PubMed] [Google Scholar]
- Runlin G, Junren Z, Guozhang L, et al. Efficacy and safety of nifedipine GITS in Asians with hypertension: results of a post-marketing surveillance study in China. Clin Drug Investig. 2007;27:565–72. doi: 10.2165/00044011-200727080-00005. [DOI] [PubMed] [Google Scholar]
- Chrysant SG, Sugimoto DH, Lefkowitz M, et al. The effects of high-dose amlodipine/benazepril combination therapies on blood pressure reduction in patients not adequately controlled with amlodipine monotherapy. Blood Press Suppl. 2007;1:10–17. doi: 10.1080/08038020701189828. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Oparil S, Feng Z. Comparison of efficacy and side effects of combination therapy of angiotensin-converting enzyme inhibitor (benazepril) with calcium antagonist (either nifedipine or amlodipine) versus high-dose calcium antagonist monotherapy for systemic hypertension. Am J Cardiol. 2000;86:1182–7. doi: 10.1016/s0002-9149(00)01199-1. [DOI] [PubMed] [Google Scholar]
- Kjeldsen SE, Sica D, Haller H, et al. DISTINCT Investigators Nifedipine plus candesartan combination increases blood pressure control regardless of race and improves the side effect profile: DISTINCT randomized trial results. J Hypertens. 2014;32:2488–98. doi: 10.1097/HJH.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]