Abstract
As a type of secreted membrane vesicle, exosomes are an emerging mode of cell-to-cell communication. Yet as exosome samples are commonly contaminated with other extracellular vesicles, the biological roles of exosomes in regulating immunity and promoting oncogenesis remain controversial. Wondering whether existing methods could distort our view of exosome biology, we compared two direct methods for imaging extracellular vesicles and quantified the impact of different production and storage conditions on the quality of exosome samples. Scanning electron microscope (SEM) was compared to transmission electron microscope (TEM) as alternatives to examine the morphology of exosomes. Using SEM, we were able to distinguish exosomes from other contaminating extracellular vesicles based on the size distribution. More importantly, freezing of samples prior to SEM imaging made it more difficult to distinguish exosomes from extracellular vesicles secreted during cell death. In addition to morphology, the quality of RNA contained within the exosomes was characterized under different storage conditions, where freezing of samples also degraded RNA. Finally, we developed a new flow cytometry approach to assay transmembrane proteins on exosomes. While high-copy-number proteins could be readily detected, detecting low-copy-number proteins was improved using a lipophilic tracer that clustered exosomes. To illustrate this, we observed that exosomes derived from SKBR3 cells, a cell model for human HER2+ breast cancer, contained both HER1 and HER2 but at different levels of abundance. Collectively, these new methods will help to ensure a consistent framework to identify specific roles that exosomes play in regulating cell-to-cell communication.
Introduction
Coordinated action among a diverse collection of individual cell types within a tissue requires robust modes for intercellular communication. Cells receive and transmit information through a variety of mechanisms, some of which are well-characterized while others remain hazy. Conventional study of these mechanisms focuses on direct modes of communication that require cell-to-cell contact or indirect modes that rely on the release of soluble biochemical cues. These soluble cues are relayed through diffusive and convective transport mechanism and signal via receptors. An emerging and controversial mode of communication that blends aspects from both categories are exosomes1–3.
Exosomes are nanoscale vesicles derived from the luminal membranes of multivesicular bodies (MVBs) and are released to the extracellular milieu when MVBs fuse with the cell membrane4,5. Exosomes are reported to convey coding RNA6, non-coding RNA7,8, oncoproteins9–11, and antigen presentation molecules12, or even DNA13 between cells. By carrying a complex payload of proteins and RNAs, exosomes may manipulate recipient cells and other organs over a long distance6,7,10,14–21. For instance, tumor-derived exosomes have been reported to prepare distant sites for metastatic colonization10,22,23 or serve as disease biomarkers7,31-34. Alternatively, exosomes may play a local role in shaping the development and homeostasis of normal tissues35–38 or in oncogenesis; including melanoma10,24,25, ovarian24,26, colorectal27, breast8,20,28, and prostate cancer29,30. The spatial organization of tissues depends on convective and diffusive transport mechanisms to relay intercellular information. Thus, the specific role that exosomes play may depend highly on size, given the inverse relationship between diffusivity in extracellular matrices and molecular size39.
However, one of the challenges with identifying the biological role of exosomes in transmitting information between cells is that they are one of a number of extracellular nano- and micro-scaled vesicles that are constitutively produced by cells and vary in size, molecular composition, and biological function1,40. While the extracellular vesicles field has grown significantly in recent years, the controversy associated with the exosome literature is fueled by inconsist ent nomenclature and isolation methods that result in impure preparations41,42. Wondering whether existing met hods could distort our view of exosome biology, we focused on three important aspects related to obtaining and assessing the quality of exosome samples. First, we compared electron microscopy methods for assessing population-level exosome morphology, as a way to distinguish between exosomes and other confounding extracellular vesicles based on their size distributions. Second, we compared conditions for producing and storing exosomes in vitro in relation to the quality of exosome samples. Third, we developed methods to improve the sensitivity of flow cytometry to assay of protein expression on exosomes.
Experimental
Materials and reagents
B16F0 cells, a murine model of metastatic melanoma, were acquired from American Type Culture Collection (ATCC, Manassas, VA). A HER2+ human breast cancer cell line, SKBR3, was kindly provided by Dr. Jia Luo (University of Kentucky, Lexington, KY). Dulbecco's Modification of Eagle's Medium (DMEM) was from Cellgro (Manassas, VA). Improved Modified Eagle Medium Zn2+ option (IMEM, no phenol red) was from Invitrogen (Grand Island, NY). Dulbecco's Phosphate-Buffered Saline (DPBS, 0.1 μm sterile filtered) and heat inactivated fetal bovine serum (FBS) was from Hyclone, Inc (Logan, Utah).
TEM (transmission electron microscopy) copper grids (200 mesh and coated by formvar carbon film), glutaraldehyde solution, paraformaldehyde (16% paraformaldehyde aqueous solution) and 4% uranyl acetate solution were from Electron Microscopy Sciences (Hatfield, PA). DNA-free™ DNA removal kit, mirVana™ miRNA isolation kit, and SuperScript III First-Strand Synthesis System were from Invitrogen. RNA 6000 pico kit, reagents and ladders for Agilent 2100 Bioanalyzer was from Agilent Technologies (Santa Clara, CA). DEPC water was made by mixing distilled water with 0.1 % v/v diethylpyrocarbonate for at least 2 hours at 37 °C and then the water was autoclaved for 30 min.
Allophycocy anin (APC) conjugated mouse anti-human Her1/ErbB1 monoclonal antibody (SC-120), phycoerythrin (PE) conjugated mouse anti-human HER2/Neu/ErbB2 mAb (SC-23864) and PE conjugated mouse isotype control mAbs (SC-2866) were purchased from Santa Cruz Biotechnology (Dallas, TN). Human IgG and mouse IgG were from Jackson ImmunoResearch Laboratories (West Grove, PA). APC or PE conjugated mouse anti human/mouse IL-12 receptor beta 2 (IL12RB2) mAbs (FAB1959) was from R&D systems (Minneapolis, MN). Quantum Simply Cellular microspheres conjugated to anti-mouse IgG were purchased from Bangs Laboratories (Fishers, IN). Polystyrene beads with respective diameters 2.19, 0.84 and 0.054 μm and labeled with Nile red were from Spherotech, Inc. (Lake Forest, IL). PBSAz for cells was prepared by mixing 2% FBS and 0.02% sodium azide in DPBS. PBSAz for exosomes was prepared by mixing 0.5% BSA and filtered twice using sterile 0.22 μm filters to exclude nanoparticles. Vybrant® DiI lipophilic tracer (DiI, Cat#: V22888) was from Invitrogen (Grand Island, NY).
Tissue culture
B16F0 was maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C, 5% CO2, passaged at 1:5 every 2 days or until 100% confluence of cells as described previously43. SKBR3 cell culture was maintained in 37°C, 5% CO2, correspondingly in supplemented medium as described previously20,44. Briefly, SKBR3 was maintained in IMEM supplemented with 10% FBS and penicillin/streptomycin that was replaced of every 2 days, and passaged at 1:3 every 4-6 days when cells reached 80% confluence. To detach adherent cells, B16F0 or SKBR3, trypsin-EDTA was applied for 7-10 min at 37°C, 5% CO2 after one wash by DPBS. After trypsin treatment, FBS was added to neutralize and resuspend cells. Cells were washed once in medium before further culture or any treatment.
Exosome preparation and isolation
Exosomes were isolated as described previously45. In brief, cell lines were cultured until 70% confluence, washed once by DPBS, and incubated with fresh serum-free medium for 30min at 37°C and 5% CO2. After 30 minutes, the medium was replaced either by serum-free medium (SFM) or exosome-free serum containing medium (EFM) and returned to the incubator for the indicated exosome production period. The cell-conditioned media were collected at different time points and exosomes were isolated by differential centrifugation as follows: 300 ×g for 10 minutes to remove cells, 2,600 ×g for 10 minutes to remove residual cells and debris, 10,000 ×g for 60 minutes to remove microvesicles, and 100,000 × g for 2 hours to collect nano-scaled vesicles in pellets. The resulting pellet was resuspended, washed once in DMEM, and repelleted at 100,000 ×g for 2 hours. Differential centrifugation was conducted using a Beckman Coulter X-14R centrifuge and a Beckman Coulter XL90 ultracentrifuge with proper rotors, open-top (Cat#: 355631) or capped (Cat#: 355618, Cat#: 355655) thickwall polycarbonate tubes (Beckman Coulter). Once isolated, nano-scaled vesicles were resuspended in DPBS and kept on ice.
Exosome cryopreservation
Exosomes were preserved in two different ways: directly frozen at −80°C and cryopreserved in liquid nitrogen (−196°C) as is typically done with mammalian cells. The cryopreservation protocol involved mixing isolated exosomes with equal volume of medium containing 2 × DM SO (10% or 20%), aliquoting 1 ml into each cryopreservation tube, wrapping tubes in heat isolated materials and storing in a −80°C freezer overnight (about 16 hr). Samples were then put in liquid nitrogen for preservation. To remove from cryopreservation, exosomes were thawed on ice for 30min according to45, or 1-2 min at 37°C according to recovering cell lines, washed once in 20ml PBS, and ultracentrifuged to pellet. The supernatant was discarded and exosome pellet was resuspended in 200μL-1mL PBS for SEM inspection.
Induction of apoptotic and necrotic cell death in B16F0 cells
Extracellular vesicles constitutively produced by cells were compared to vesicles released during cell death. To induce apoptosis, B16F0 cells were cultured in serum-free medium containing 10μM 7-ethyl-10-hydroxycamptothecin (Cayman Chemical Company, USA) for 24 hours, as reported previously46. After treatment, B16F0 cells were 70-80% confluence, with visible detached and dead cells in the culture. Extracellular vesicles (i.e., apoptotic vesicles) were subsequently collected from the B16F0 cell conditioned medium. To induce necrotic cell death, B16F0 cells exposed to high shear conditions by vigorous vortex and extracellular vesicles (i.e., necrotic bodies) were subsequently collected from conditioned media. Apoptotic vesicles and necrotic bodies were isolated by different ial (ultra)-centrifugation that was identical with the exosome isolation. Biological experiments were performed independently at least three times. Each biological sample was diluted 4-6 fold, fixed and mounted on substrates, and imaged by electron microscopy (EM). At least 3 EM images were acquired for each sample.
Scanning electron microscopy (SEM)
Pellets containing extracellular vesicles isolated from healthy cells (i.e., exosomes) and from apoptotic and necrotic cells were vortexed and resuspended in 0.2-1 ml DPBS. Exosomes and nano-scaled apoptotic vesicles or necrotic bodies (microscaled parts of apoptotic vesicles or necrotic bodies were removed prior to ultracentrifugation) were fixed in a 2% EMS-quality paraformaldehyde aqueous solution. The samples were then diluted in distilled (dl) water in serial dilutions, added in 1-5 μl vesicle mixtures to cleaned silicon chips, which were sonicated in acetone, ethanol and distilled water for 5 min in each solvent, flushed by water and blown dry, and immobilized after drying vesicles under a ventilation hood. Samples on silicon chips were mounted on a SEM stage by carbon paste. To make surface conductive, a coating of 2-5 nm gold-palladium alloy was applied by sputtering (SPI-Module Sputtering, Argon as gas for plasma) before imaging by scanning electron microscopy Hitachi S-4700 or a JEOL JSM-7600F SEM. SEM was performed under low beam energies (5.0-10.0 kV). For best vesicle morphology under SEM, fresh isolated exosomes were fixed and immobilized on silicon right after isolation, and imaged within 7 days. Analysis of exosome sizes were done using the SEM images via ImageJ and the density distribution of exosome diameters were obtained using R/Bioconductor.
Transmission electron microscopy (TEM)
Exosomes were produced by incubating cells in serum free medium for 24 hrs. Freshly isolated exosomes from mouse melanoma B16F0 cells were resuspended in cold DPBS containing 2% para-formaldehyde. Exosome samples were prepared for TEM inspection as described previously45. Briefly, exosomes were mounted on copper grids, fixed by 1% glutaraldehyde in cold DPBS for 5 min to stabilize the immunoreaction, washed in sterile distilled water, contrasted by uranyl-oxalate solution at pH 7 for 5 min, and embedded by methyl cellulose-UA for 10 min on ice. Excess cellulose was removed and samples were dried for permanent preservation. A JEOL 1010 TEM was used to image exosome samples at a voltage of 80kV.
Statistical analysis
Extracellular vesicle sizes are reported in terms of diameters (mean ± standard deviation). Comparisons among multiple groups were performed with one-way or two-way ANOVA methods depending on the factors using SPSS statistics 20. The statistical difference in means between two different groups were comp ared using a two-sided Student's t-test with unequal variance, where a P value < 0.05 was considered as significant.
Exosomal RNA (esRNA)
To characterize exosomal RNA, total RNA were isolated from cells, fresh exosome or frozen exosome pellets using a mirVana™ miRNA isolation kit (Life Technologies) according to the manufacturer's protocol. DNase digestion was then performed to remove possible contaminating DNA (DNA-free™ DNA removal kit). After isolation, cellular RNA and exosomal RNA was st ored at −80°C until characterized using a NanoDrop 2000 Spectrophotometer (Thermo Scientific) and on-chip-electrophoresis via Agilent 2100 Bioanalyzer and RNA 6000 pico kit. Strict RNA handling guidelines were followed during assays with RNA.
B16F0 cellular RNA and esRNA were reversely transcribed with the SuperScript III system. B16F0 genomic DNA was isolated using Genomic DNA Purification Kit (Thermo Scientific). The cDNAs, together with genomic DNA about 10% of RNA input, were used to amplify the full-length coding sequences (ORFs) or part of introns of the indicated genes by semi-quantitative PCR, in which the amplified DNA products were monitored from certain cycles after the desired fragments showed up, and comp ared before the amplification was saturated. The primer sequences are listed in supplementary Table S1.
Western blot analysis
Western blot analysis was used to confirm that exosome samples contained prot eins commonly associated with exosomes. For immunoblotting, rabbit anti- CD9, CD63, CD81, Hsp70 antibodies were from System Biosciences (Mount ain View, CA), mouse anti- β-actin and β-tubulin were from Sant a Cruz Biotechnology, Inc. (Dallas, Texas). B16F0 cells and exosomes were lysed with ice-cold radio immunoprecipitation assay buffer (RIPA, 150 mM sodium chloride, 1.0% Triton X-100, 0.5% sodium deoxy cholate, 0.1% SDS, 50 mM Tris, pH 8.0) supplemented with prot ease and phosphatase inhibitors. The protein concentration was determined using Pierce BCA Protein Assay Kit (Life Technologies), and 20μg of each sample was resolved for SDS-poly acrylamide gel electrophoresis. Proteins were transferred to Bio Trace PVDF membrane (PALL Life Sciences, Pensacola, FL) and detected using Pierce ECL Western Blotting Substrate (Life Technologies).
Flow cytometry
Isolated exosomes and cells were resuspended in PBSAz, blocked by 1/100 (v/v) human IgG in PBSAz for 15 min on ice, and stained using appropriate antibodies for 30 min on ice in the dark. After staining, exosomes were washed with PBSAz and ultracentrifuged by 150,000 ×g for 1 hour at 4°C. The exosome pellet was resuspended in PBSAz and a final volumetric percent of 2% paraformaldehyde was added to preserve samples at 4°C for flow cytometry for up to 2 weeks.
Flow cytometry was performed as described previously using a FACSAria or a LSRFortessa flow cytometer and analysis software (BD Biosciences). Briefly, cellular copy numbers of HER1 and HER2 were estimated using Quantum Simply Cellular calibration beads. The fluorescent intensity for each parameter was reported as a pulse area using 18-bit resolution. Unstained cells and exosomes were used as negative flow cytometry controls and singly stained cells and exosomes were used to establish fluorescent compensation parameters. When exosomes were analyzed by flow cytometry, events were detected when the side scatter area was above a threshold that was established using running buffer. Following acquiring at least 20,000 events, flow cytometry data was exported as FCS3.0 files and analyzed using R/Bioconductor, as described previously48.
Results and discussion
SEM provides a quick alternative to TEM for characterizing the morphology and distribution of exosomes
Exosomes are one of a number of different micro- and nanoscaled vesicles released by cells that can be distinguished based upon their morphology and size distribution. Electron microscopy (EM) is necessary to characterize their morphology since particles smaller than 300 nm are invisible in optical methods49. Though transmission EM (TEM) is considered a standard tool for characterizing the morphology of exosomes, scanning EM (SEM) is an alternative approach that has recently emerged50. In general, TEM and SEM both require ultracentrifugation to isolate exosomes, include sample processing steps prior to EM, and use electron beams to detect the nanostructures with high resolution. However, the number of sample processing steps are different between the two methods. The first aim of this study was to compare these two EM methods for characterizing the morphology of exosomes, as to distinguish them from other extracellular vesicles. To illustrate these methods, exosomes were isolated by differential centrifugation from two cell lines – B16F0, a mouse melanoma cell line, and SKBR3, a human breast cancer cell line – and were processed immediately for electron microscopy to image fresh vesicles.
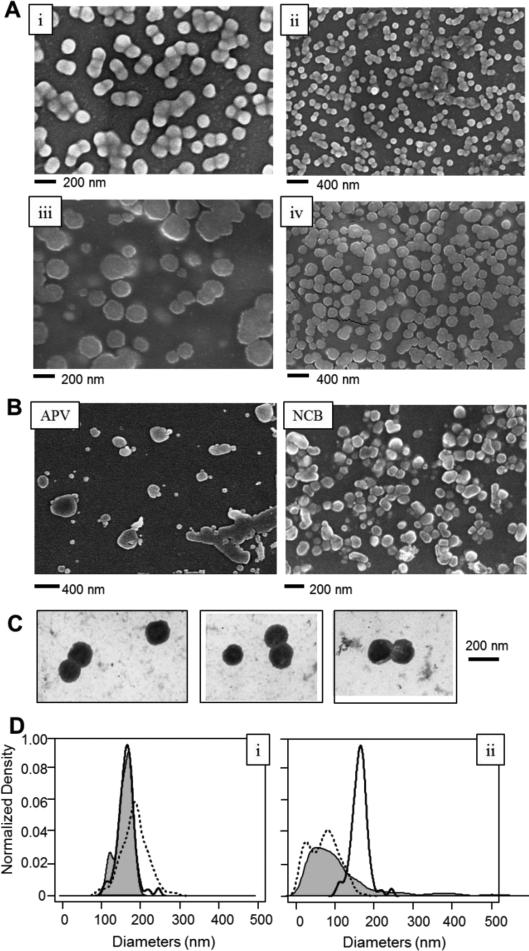
Under SEM, extracellular vesicles isolated from normal cells exhibited a round morphology and uniform, unimodal distribution in size that was consistent with exosomes (Figure 1A, subpanels i and ii for B16F0 exosomes, subpanels iii and iv for SKBR3 exosomes). In contrast to exosomes, apoptotic vesicles and necrotic bodies derived from B16F0 cells displayed irregular shapes and a heterogeneous size distribution, as observed by SEM (Figure 1B, apoptotic vesicles, APV (left); necrotic bodies, NCB (right)). TEM was also used to characterize the morphology of B16F0 exosomes, where a central depression was observed, which is a characteristic for exosomes under TEM (Figure 1C). The size distributions of the different extracellular vesicles and exosomes imaged using different EM methods were compared (Figure 1D and Table 1). Using both EM methods, we also noted that dispersing samples containing extracellular vesicles as a monolayer on the silicon substrate improved the image quality significantly. In summary, we found that B16F0 exosome diameters were not significantly different between the two EM methods (P value > 0.05). This is interesting as it suggests that the two EM methods provide the same measurement of exosome sizes regardless of the morphological differences observed under the two EMs, such as the “cup-shape” observed by TEM. In contrast, APC and NCB from B16F0 cells did not exhibit unimodal distributions as observed with exosomes. In addition, the size distributions of APC and NCB were significantly different from exosomes (P < 0.001 ). B16F0 exosomes were also slightly smaller than the SKBR3 exosomes (162 nm vs. 183 nm, respectively; P < 0.001).
Figure 1.
Different extracellular vesicles exhibited different morphologies and size distributions, as imagpd by SEM and TEM. (A) Exosomes (EXO) isolated from B16F0 mouse melanoma cells (subpanels i and ii) and SKBR3 human breast cancer cells (subpanels iii and iv) were imagpd by SEM. (B) Extracellular vesicles isolated from apoptotic B16F0 cells that were treated with 7-ethyl-10-hydroxycamptothecin (left panel, apoptotic vesicles APV). Necrotic bodies (NCB) were isolated from B16F0 cells following exposure to high shear conditions (right panel). APV and NCB were imagpd by SEM. (C) Exosomes from B16F0 cells were imagsd by TEM (scale bar = 200 nm). (D) The size distributions of EXO, APV, and NCB observed by electron microscopy, (left panel) B16F0 EXO observed by SEM (black solid line; n = 113) or TEM (gray shaded; n = 14), and SKBR3 EXO under SEM (dotted line; n = 237). (right panel) B16F0 vesicles observed by SEM, including EXO (black solid line; same data as in left panel), APV (gray shaded) and NCB (dotted line), All imagps were representative of a least three biological replicates. See Table 1 for comparisons and statistics.
Table 1.
Size comparisons and statistics of exosomes, apoptotic vesicles and necrotic bodies that were isolated and observed by electron microscopy.
| Vesicle Isolated | EM Methods | Diameter ± SD | N | **P values for statistical significance |
|---|---|---|---|---|
| B16F0 Exosomes (EXO) | SEM | 162 ± 23 nm | 113 | N.S., B16F0 EXO imaged by SEM vs. TEM; P<0.001, B16F0 EXO vs. APV or NCB in SEM. |
| B16F0 Exosomes | TEM | 158 ± 19 nm | 14 | |
| SKBR3 Exosomes | SEM | 183 ± 34 nm | 237 | P<0.001, SKBR3 EXO vs. B16F0 EXO, in SEM |
| B16F0 Apoptotic vesicles (APV) | SEM | 131 ± 148 nm * | 602 | P<0.001, B16F0 APV vs. B16F0 EXO or NCB, in SEM. |
| B16F0 Necrotic bodies (NCB) | SEM | 70 ± 38 nm * | 222 | P<0.001, B16F0 NCB vs. B16F0 EXO or APV, in SEM. |
Size distributions did not exhibit unimodal distributions. Mean diameters were calculated from projected areas of all particles that exhibit round or irregular shapes.
P values were accessed using one-way ANOVA or Student's t-test.
SD is standard deviation. N is sample number of observed vesicles in EM. N.S. is not significant.
Similar diameters have been observed by EM imaging for cancer exosomes from B16F10 (closely related to B16F0) mouse melanoma cells25, from human plasma of melanoma patients10 and from SKBR3 human breast cancer cells20. While the average sizes of the extracellular vesicles that we have observed are slightly higher than previous reports of exosomes50,51, their uniform size distribution rules out apoptotic and necrotic bodies, which were heterogeneously distributed and irregularly shaped (Figure 1 A-D). Discrepancies could be attributed to the cellular source of exosomes or to differences in sample isolation and SEM preparation. For instance, it has been reported that exosomes derived from the human HEK cell line 293T shrunk in size by 50% within 8 days, from 116 to 63 nm in diameter, when stored in PBS at 4 °C50.
Morphological differences were apparent when identical samples of exosomes were imaged with these two different EM techniques, where TEM images show a characteristic central depression in the exosomes and SEM images show exosomes as round spheroids. We observed that the SEM images for exosomes derived from two different cell lines were consistently spheroidal, as similarly observed with exosomes from human saliva51. While early work suggests that exosomes exhibit a characteristic central depression, this trait is an artifact attributed to TEM sample preparation, such as embedding the exosomes in polymeric cellulose, rather a physiological features of exosomes41,52. In comparing these two EM methods, TEM sample preparation includes steps for gradient dehydration, contrasting staining by heavy metals and embedding the sample in polymeric cellulose, which are omitted in preparing SEM samples. We also note that all the images shown in Figure 1 were obtained using fresh samples. Differences in methods for storing extracellular vesicles can also introduce artifacts, as we will discuss a later section.
Collectively, the imaging results suggested that SEM and TEM provide similar information regarding the size distribution but a slightly different morphological view of exosomes and other extracellular vesicles. Currently, EM methods are the standard practice for direct imaging the size and morphology of exosomes. However, there are some trade-offs to consider when comparing the TEM and SEM. For instance, preparing TEM samples requires more steps compared with SEM sample preparation. The SEM images of exosomes, apoptotic vesicles, necrotic bodies, shown in Figure 1, exhibited intact membrane structures and the morphologies were distinctly different for each type of nanoscale vesicles. This finding suggested that SEM is a valid alternative to TEM for direct imaging of extracellular vesicles, with advantages based on the improved sample process methods.
The morphology and size of extracellular vesicles, including exosomes, are critical in defining their physiological roles, distribution, and concentrations in organs53,54,55. Observing nanoscale vesicles under physiological conditions in situ using an electron beam is a challenge, as a high vacuum, 10−5 to 10−8 Torr, and anhydrous atmosphere are required. However, reducing the number of sample fixing and processing steps streamlined the pursuit of the native morphologies of exosomes. To illustrate this point, we also found that B16F0 and SKBR3 exosomes frequently appeared as clusters of multiple exosomes (2-10 particles, approximately) with membranes connected tightly or even merged in SEM images, suggesting that the natural morphology of B16F0 and SKBR3 exosomes are as multi-exosome clusters instead of isolated exosomes (Figure 1A). In contrast, the clustering phenomena were not observed under SEM with other nanoscale vesicles, such as apoptotic vesicles or necrotic bodies (Figure 1B). This suggests that clustering is not due to sample processing, such as forming clusters due to the capillary effects of water drops during evaporation. This observation may be due to minimal processing of the samples afforded by SEM imaging. However, clustering of native exosomes has implications for other indirect methods for characterizing exosomes, like dynamic light scattering or nanoparticle tracking analysis, that assume that the particles are isolated vesicles49,56,57. The results also imply that filtering samples through a 0. 2 micron filter prior to ultracentrifugation, which is common practice, would actually enrich for vesicles associated with cell death. In addition, TEM imaging using nanoparticle-conjugated antibodies can be used to identify whether exosomes contain membrane proteins45, The use of SEM for immuno-gold labeling studies of cell samples remains in development58,59. Alternative methods also exist for quantifying membrane protein expression on exosomes, as we discuss in a later section. Next, we used SEM to evaluate the effects of other process parameters associated with producing, isolating, and storing exosomes from cell-conditioned media.
A survey of exosome production methods and their impact on exosomal RNA quality
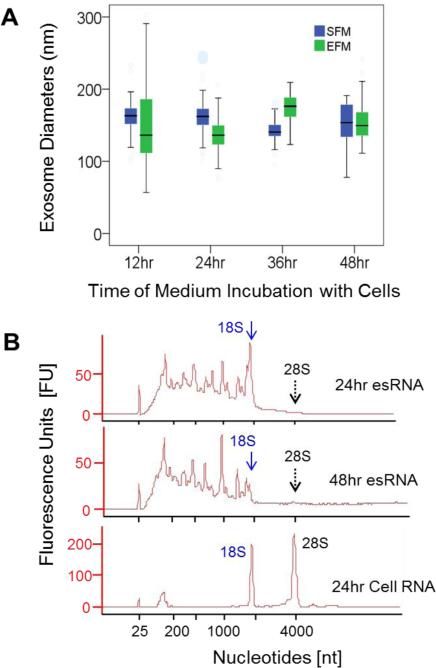
Fetal bovine serum (FBS) is a common supplement to eukaryotic cell culture media; yet, animal serum can contain exosomes that can contaminate cell-derived exosome populations. In this study, we evaluated the impact of two media options for limiting FBS-derived exosome contamination on in vitro production of cell-derived exosomes. The two media options were serum-free medium (SFM) and medium supplemented with serum that had been cleared of exosomes by ultracentrifugation, that is exosome-free serum-containing medium (EFM). Media were conditioned with B16F0 cells for different lengths of time. In both SFM and EFM, B16F0-derived exosomes could be isolated under all conditions. The size distributions of exosomes isolated from the B16F0-conditioned SFM and EFM at each time point were determined from SEM images (Figure 2A), The size distributions of B16F0 exosomes purified from SFM or EFM, at the indicated incubation times (12-48 hrs) with the B16F0 parental cells, were assessed for statistical differences by two-way and one-way ANOVA analysis. These size distributions were found to be not significantly different with respect to the incubation medium types and with respect to the incubation times. A shift in particle size distribution towards smaller sizes would suggest that the culture conditions decreased cell viability, as observed by the tail in the distribution of vesicles obtained after conditioning for 48 hours in serum free media.
Figure 2.
Exosome quality was evaluated in terms of vesicle morphology and exosomal RNA as a function of production condtions. (A) B16F0 exosomes were produced using serum free medium (SFM) or exosome-free serum medium (EFM) for 12, 24, 36 and 48 hours and characterized using SEM (n = 51, 73, 76 and 53 for SFM exosomes, from 12-48 hrs,; n = 68, 213, 91, 210 for EFM exosomes, from 12-48 hrs, respectively). In the box plots, the top and bottom of the boxes indicate the 75% and 25% of the distribution in sizes, black bands on the boxes indicate the median sizes, top and bottom bars indicated the maximum and minimum of the sizes. The exosome sizes were assessed using two-way and one-way ANOVA and found to be not significantly different. Multiple SEM pictures and biological samples, n ≥ 3, were used to determine the exosome sizes. (B) Electrophoresis spectrums of exosomal and cellular RNA derived from B16F0 cells using Agilent Bioanalyzer. The positions of 18S and 28S peaks on the RNA spectrums were indicated in diagrams by arrows and labels. esRNAis for exosomal RNAs. Representative figures of RNA analy ses were shown, n > 3.
In addition, exosomal RNA (esRNA) was also characterized by on-chip-electrophoresis using an Agilent Bioanalyzer (Figure 2B), which can quickly assess the quality of RNAs purified from exosomes. As RNA become degraded, longer RNAs become fragmented such that the distribution shifts entirely towards the low end (< 200 nt in length). Following a single wash of exosome pellets in DMEM alone, exosomal RNAs were isolated from exosome pellets obtained from B16F0-conditioned serum free medium after 24 (top panel) and 48 (middle panel) hours incubation with cells. For comp arison, RNA was isolated from whole B16F0 cell lysates and analyzed similarly (bottom panel). The results are representative of at least three replicates.
Overall, we found that fresh exosomes contained high quality RNA. Analysis of esRNA did not contain the characteristic peaks associated with ribosomal RNA, as observed previously7 (see reduced 18S and 28S peaks labeled in Figure 2B). The presence of the 28S peak, in particular, may indicate the presence of contaminating cell debris. The distribution in esRNA was primarily uniform but was limited to below 1800 nucleotides in length. This distribution in RNAs is not surprising, as we used the mirVana miRNA isolation kit that uses a column to enrich small RNAs. Finally, longer production times were also associated with a shift in distribution in esRNA to smaller length RNA, as illustrated by the difference between the 24 hr and 48 hr esRNA samples. While this shift in esRNA was slight, degradation of RNA shifts the distribution towards lengths below 200 nucleotides7. Collectively, we observed no difference in exosome quality between SFM and EFM and that prolonged production conditions slightly degraded the quality of esRNA.
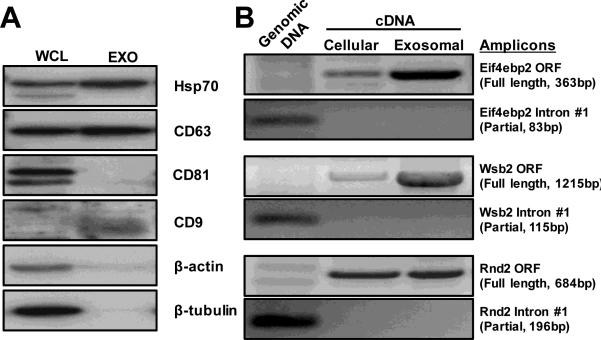
We also biochemically characterized samples to support the claim that these samples contain exosomes with high quality RNA. Specifically, Western blot analysis of samples indicated the presence of common markers of exosomes, such as Hsp70, CD63, and CD9 (Figure 3A), while CD81, β-actin and β-tubulin were absent. Whole cell lysates were used as a positive control; yet, the absence of appropriate loading controls renders the analysis qualitative. In our hands, we found that exosomal RNA contained mRNA transcripts of protein open reading frames with no contaminating genomic DNA (Figure 3B). This is in contrast to reports that suggest that exosomes contain mRNA fragments63, which may be an artifact of storage conditions that we will discuss next
Figure 3.
Biochemical characterization of exosomes from B16F0 cells. (A). Immunoblotting analysis of common exosome markers, where 20μg of total protein was loaded in each lane (WCL, whole cell lysate; Exo, exosome lysate). (B). Amplification of the full-length protein ORF s and partial introns by semi-quantitative RT-PCR suggested that a group of functional mRNAs are enriched in exosomes. 100ng of RNAs were reverse-transcribed into cDNA and subject to PCR amplification as indicated. 10ng of genomic DNA (10%) was also used as quality control (left lane).
Preservation and stability of exosomes and RNAs at low temperatures
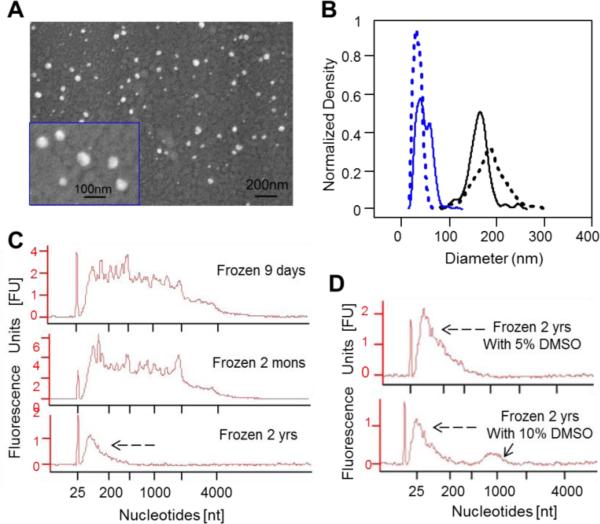
To understand the role that exosomes play in intercellular communication, cellular assays need to be performed that identify how exosomes influence cellular function in a dose-dependent fashion and independent from direct cell-to-cell interaction. To demonstrate these influences in a reproducible manner, exosomes should be stored under conditions that preserve their biological activity once isolated from cell-conditioned media. Conventional methods suggest that exosomes can be preserved by freezing at −80°C12. Alternatively, dimethyl sulfoxide (DM SO) is used as a cryoprotectant when cells are preserved in liquid nitrogen. To determine the impact of these two different freezing options on exosome quality, we froze B16F0 and SKBR3 exosomes at −80°C in serum free medium and in serum free medium supplemented with 10% DM SO. Following thawing on ice, the exosomes were imaged using SEM (see Figure 4A for B16F0 frozen exosomes, and Supplemental Figure S1B for SKBR3 frozen exosomes). Images show that cryopreservation in serum free medium alone resulted in smaller (10-100 nm in diameter) and more heterogeneous shapes to the frozen exosome samples, in comparison with the fresh exosomes (Figure 4B). Specifically, SEM images indicated that the diameters of the frozen exosomes were 44 ± 15 nm for B16F0 (n = 508) and 34 ± 8 nm for SKBR3 (n = 354). These size distributions were significantly different from each other as well as from fresh exosomes (see Figure 1D and Table 1), which was assessed using one-way ANOVA (P value < 0.001). Our analyses suggested that direct freezing affects the stability of the exosome membranes and degrades the samples.
Figure 4.
Freezing of exosomes decreased their size and degraded exosomal RNA. (A) SEM pictures of B16F0 exosomes subjected to a freeze-and-thaw cycle. (B) The size distributions of frozen exosomes processed from B16F0 (blue solid line, diameter 44 ± 15 nm, n = 508) and SKBR3 (blue dotted line, diameter 34 ± 8 nm, n = 354) cells, in comparison with the fresh exosomes from B16F0 (black solid line, diameter 162 ± 23 nm, n = 113) and SKBR3 (black dotted line, diameter 183 ± 34 nm, n = 237) cells. The data for fresh exosomes are also shown in Figure 1D-i and Table 1. The sizes of the four exosome samples are statically different as assessed by one-way ANOVA (P value < 0.001). (C and D) Bioanalyzer results for exosomal RNAs isolated from exosomes frozen at −80 °C to −196 °C in media alone (C) or with 5-10% DM SO as cryoprotectant (D). Exosomes were stored frozen for 9 days (C top panel), 2 months (C middle panel), or 2 years (C bottom panel and D). The shift in nucleotide size towards small, degraded RNA is indicated by black dashed arrows. A hump around 1000 nt was observed in samples frozen for 2 years in 10% DM SO (solid arrow in D bottom panel). Representative figures were shown, n < 3 as for SEM of the biological samples; n<3 for RNA analysis and isolation.
In contrast, the morphology of exosomes cryopreserved using DM SO was similar to SEM images of fresh exosomes (compare panels C and D in Figure S1, with Figure 1A). Though sizes and shapes of a certain percentage of exosomes were preserved, DM SO was unable to preserve the morphology of all vesicles in the sample. Collectively, the results suggested that DM SO could be used as a cryoprotectant to help maintain the morphology of these vesicles for long-term storage. However, cryostorage also negatively impacted the morphology of exosomes, as the exosomes became smaller and more het erogeneous in size. This implies that cryostorage makes it more difficult to assess the quality of the sample by distinguishing between exosomes and vesicles derived from dead cells. Without confirming the quality of the exosome sample, the biological implications of downstream assays using these samples would be unclear.
Besides morphology, preserving the biological activity of RNAs in exosomes is also crucial for studying exosome biology in a reproducible manner. Using an Agilent Bioanalyzer, we characterized exosomal RNAs that were isolated from exosomes frozen for various periods of time (Figure 4C) or cryopreserved with DM SO (Figure 4D). In Figure 4C, RNAs isolated from exosomes frozen for 9 days and 2 months, still contained a variety of RNA molecules spanning from 18S to smaller length nucleotides, featuring the characteristics of RNAs isolated from fresh exosomes (compare with Figure 2B, top and middle). However, freezing those exosomes for 2 years resulted loss of the 18S and obvious shifting in RNA distributions towards smaller nucleotides (Figure 4C, bottom), as indicated by a dashed black arrow. Collectively, the results suggest that exosomal RNA was preserved in frozen exosomes for a couple of months, but was degraded with longer times. This degraded RNA signature can also be observed in previous exosome studies (for example6, 64,65). To check whether DM SO could also preseve exosomal RNAs, exosomal RNAs purified from frozen exosomes stored for 2 years with 5-10 % DM SO were analyzed by Bioanalyzer (see Figure 4D). In these samples, RNA appeared to be degraded, as shown by the enrichment of smaller RNAs (see the dashed black arrows in Figure 4D). There was also an odd peak at around 1000 nucleotides of the RNA spectrum (see the solid arrow in Figure 4D bottom), which is possibly an artifact attributed to DM SO. The results here indicated that DM SO was unable to preserve exosomal RNAs (Figure 4D), though the size and morphology of vesicles could be protected by DM SO during low temperature storage and the thawing process (C and D in Figure S1). Collectively, the results suggest that sample quality should be established using fresh exosomes and that prolonged storage in the freezer degrades biological activity.
Flow cytometry as an unbiased tool to characterize exosomal membrane protein expression
A number of different techniques have been proposed to isolate exosomes from biological fluids. To bypass the timeconsuming ultra-centrifugation step, exosomes have been isolated using “exosome markers”, that are proteins contained in the external lipid bilayer. Exosomes can be purified using these exosome markers and capture beads. Exosomes bound to these capture beads can then be analyzed using a conventional flow cytometer60. Collectively, flow cytometric analysis of exosomes is high-throughput with the capacity for quantitative protein characterization60. Recently, a high-end dedicated flow cytometer with higher sensitivity forward scatter detection and fluorescent amplification has been developed to separate stained exosomes from background contaminants61. However, it is unclear whether these isolation and detection methods are biased. A potential source of bias may be exosome heterogeneity, as translating cellular transmembrane protein copy number to nanometer-sized exosomes suggests that the protein copy numbers on exosomes may be in the single digits. Since many institutions do not have dedicated instruments for studying extracellular vesicles, we modified a conventional flow cytometer to use SSC instead of FSC to detect flow cytometric events and characterized the heterogeneity of transmembrane protein abundance among an exosome population and assessed the sensitivity of this approach.
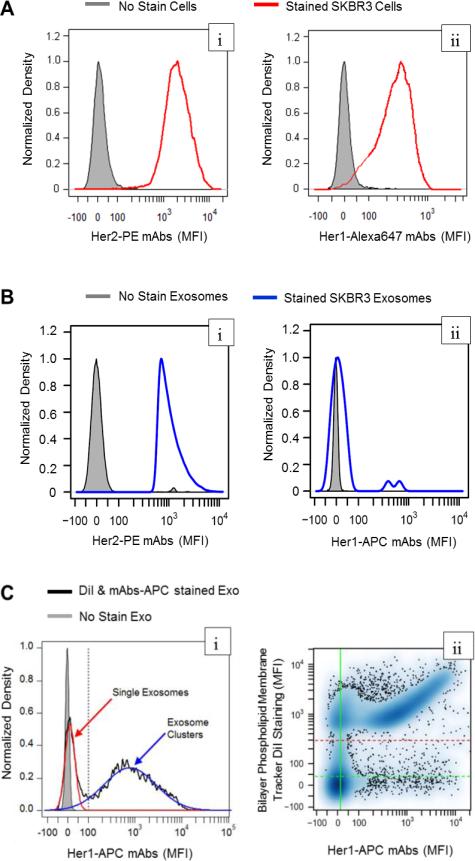
SKBR3 cells and exosomes isolated from conditioned media were stained using fluorophore conjugated antibodies against two members of the epidermal growth factor family of receptors: HER1 and HER2 (Figure 5). As the SKBR3 cell line is considered a cell model for HER2+ breast cancer, the cells exhibited positive staining for both HER1 and HER2 (Figure 5A). Using antibody calibration beads (see Supplemental Figure S2), the median cellular copy numbers of HER1 and HER2 on SKBR3 cells were estimated to be 1.41×105 copies of HER1 and 1.43×106 copies of HER2 per cell. As exp ected, SKBR3 cells contained ten times higher copy numbers of HER2 than HER1 on the surface. Next, we assayed HER1 and HER2 abundance in SKBR3 exosomes by flow cytometry (see Figure 5B). The SKBR3 exosomes were clearly positive for HER2 staining, while HER1 staining was not significantly different from unstained exosomes. Cells and exosomes stained using isotype controls also displayed no difference from unstained cells or exosomes (data not shown).
Figure 5.
The abundance of the membrane proteins, HER1 and HER2, were quantified on SKBR3 exosomes and the parental cells by flow cytometry. SKBR3 cells (A) and SKBR3 exosomes (B) were assayed for HER2 (left panels) and HER1 (right panels) abundance using fluorophore-conjugated antibodies and flow cytometry. Unstained exosomes and SKBR3 cells were used as negative controls (gray shaded). (C) Flow cytometric analysis of SKBR3 exosomes double-stained with APC-conjugated HER1 mAbs and DiI, a lipophilic fluorescent dye. (left panel: (i)) The density distribution of fluorescence associated with HER1 staining in DiI-positive events (black line) was bimodal and was deconvoluted into two normal distributions associated with single (red curve) and DiI-clustered exosomes (blue curve). The upper limit for APC-MFI of single exosomes is indicated by the gray vertical line. (right panel (ii)) A scatterplot of MFIs associated with HER1 staining versus DiI staining of SKBR3 exosome samples. The threshold to detect DiI-MFI is indicated by the red dashed horizontal line. The vertical green line indicates a data-driven threshold where 95 percent of unstained exosomes (gray shaded in left panel) exhibited a lower MFI associated with APC fluorescence.
Conventionally, cellular events are recognized in flow cytometry by their forward and side scatter properties. The forward scatter area is proportional to the cross-sectional area of an object as it flows by the laser and side scatter is related to the complexity of the object to scatter light. To identify whether we could distinguish exosomes from background debris, we used flow cytometry to quantify the forward scatter properties of fluorescent nanoparticles that bracketed the expected size range of exosomes (Supplemental Figure S3). In comparison to the fluorescent nanoparticles, we found that exosomes exhibited forward scatter areas between the forward scatter area of 54 nm PE beads and 840 nm PE beads and similar to background debris. These results suggest that non-exosome events may confound the interpretation of HER1 staining of SKBR3 exosomes. In addition, flow cytometry using fluorophoreconjugated ant ibodies may not be sufficiently sensitive to detect the low copy number of HER1 on exosomes. Using the cellular copy numbers of HER1 on SKBR3 cells, the average HER1 copy number on each exosome is 47 and ranges from 30-70, assuming that a SKBR3 cell can be modelled as a sphere with a diameter of 10 μm52 and that SKBR3 exosomes have an average diameter of 183 ± 34 nm (Figure 1B).
Similar to the detection of fluorescent nanoparticles, fluorescence can be used to distinguish a flow cytometric event associated with true particle different from background debris. Therefore, we used a lipophilic dialkylcarbocyanine dye, DiI, to label exosomes for flow cytometric detection. To determine whether DiI improved exosome detection by the flow cytometry, we stained B16F0 exosomes using a 2×2 factorial experimental design with APC-conjugated monoclonal antibody against IL12RB2 and DiI as the two factors (see Supplemental Figure S4). We found that the no stain and the mAb-stained only groups were very similar in their forward scatter area. Interestingly, we also found that DiI-stained samples increased the forward scatter areas above the background, suggesting that DiI may be clustering the exosomes. To demonstrate how the lipophilic tracer DiI can be used to enhance detection of proteins on exosomes using flow cytometry, we revised the flow cytometry assay to detect HER1 proteins on SKBR3 exosomes. As single exosomes, SKBR3 exosomes were negative for HER1 staining (see Figure 5B) in our result, to increase the HER1 signal, we stained SKBR3 exosomes using an APC-conjugated HER1 mAb and then clustered them into larger particles using DiI (Figure 5C). The DiI positive events exhibited a bimodal distribution, where single exosomes exhibited a distribution similar to the unstained control (mean MFI = 11.4 a.u. versus mean MFI = 0 a.u. for unstained) and exosome clust ers exhibited a broad distribution (mean MFI = 778 a.u.). On a flow cytometric event basis, the single exosomes seem to be a large fraction of the population. However considering that events exhibiting a MFI great er than 100 a.u. are comprised of multiple exosomes, the number of single exosomes was estimated to be less than 1% of the total exosome population.
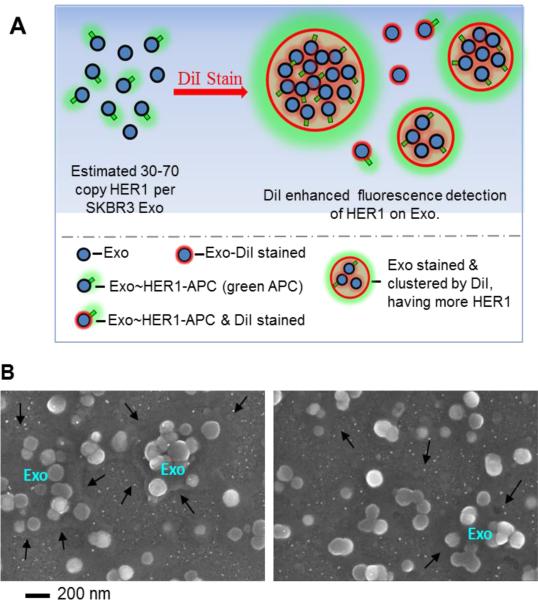
Collectively, the flow cytometry results suggested two points. First, the sensitivity of the flow cytometry assay limited our ability to assess exosome biomarkers to those proteins that exhibited high copy numbers. As an alternative to using antibodies that are directly conjugated to fluorophores, a primary antibody directed against the protein of interest plus a fluorophore-conjugated secondary antibody that binds to multiple epitopes on the primary antibody may improve the ability to detect lower abundant proteins, as used in62. While a polyclonal secondary antibody may improve the detection signal, the mean fluorescent intensity cannot be used in this context to quantify exosomal copy number, as described here. Second, a lipophilic dialkylcarbocyanine tracer (i.e. DiI, in this work) can be used to enhance the detection of low copy number proteins through unbiased clustering of exosomes and creating a lipophilic phase that favour the clustering of exosomes. This observation is schematically illustrated in (Figure 6A) and also supported by SEM imaging of exosomes stained by DiI (Figure 6B). Moreover, the addition of the lipophilic tracer enabled discriminating exosome-related flow cytometric events from background debris. This unbiased clustering increased the detection of the fluorescent signal associated with antibody binding. Collectively, these results illustrate how conventional flow cytometric analysis can be improved to leverage the high-throughput and quantitative potential of the method to characterize protein expression on exosomes with minimal potential bias.
Figure 6.
Exosomes were clustered using the lipophilic tracer DiI. Exosome clusters had more HER1 copies and bigger particle sizes, which enhanced HER1 detection by conventional flow cytometry using anti HER1-APC mAbs. (A) A schematic diagram illustrating that DiI was used to cluster nanoscaled exosomes into microscaled clusters. (B) A representative SEM image of DiI clustered exosomes, where “Exo” indicates the clusters of exosomes and black arrows indicates the edges of microscaled clusters induced by DiI.
Conclusions
In summary, we found that subtle differences in producing and storing exosomes can distort experimental observations of the purity of exosome samples, the size of exosomes, and the quality of exosomal RNA. In comparing two methods for direct imaging using electron microscopy, scanning electron microscope (SEM) provided a less time-consuming alternative to transmission electron microscope (TEM) to image the native morphology of exosomes and to assess sample purity. Using SEM, we were able to assess the impact of two different cell culture media on exosome production and of storage conditions on exosome quality. Within the first 24-36 hours, using serumfree media during exosome production did not appreciably alter exosome quality. The isolated exosomes also contained intact RNA transcripts. If the isolated exosomes need to be stored for a prolonged period, DM SO can be used to cryopreserve the morphology of exosomes but the sample quality is best characterized using fresh samples. In terms of biological activity, exosomal RNAs were preserved by freezing for short periods of time but became degraded with prolonged cryostorage, which may alter function. We also show that flow cytometry can be used to detect the presence of membrane-bound proteins on exosomes without specific bias toward any potential subpopulation in exosomes. Protein epitopes at high copy numbers could be readily detected while the lipophilic tracer, DiI, was used to cluster exosomes and enhance the detection of lower copy number proteins. The flow cytometry results also demonstrate that exosomes isolated from SKBR3 cells, a model of human HER2+ breast cancer, contain HER1 and HER2, two members of the epidermal growth factor family of receptors. Exosomes are approximately 100-times smaller in diameter than the mammalian cells and, therefore, contain less HER1 or HER2 copies in comparison with the parental cells. In addition, we found that the relative densities of HER1 and HER2 on the membrane surfaces were similar in SKBR3 exosomes as they were in the parental SKBR3 cells. In closing, we hope that, by identifying key process parameters, these improved methods will help to ensure a consistent framework in identifying the role that exosomes play in regulating cell-to-cell communication.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Science Foundation (NSF CAREER 1053490), the National Cancer Institute (NCI R15CA123123), and the National Institutes of Health (NIH P30GM103488, P30RR032138, RR020866, OD016165). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, the NIH, or the NSF. The authors thank K. M. Brundage, for assistance with flow cytometry, A. C. MacLeod, J. S. Hardinger, M. L. Redigolo, and K. S. Brown for assistance with SEM imaging, the WVU Tissue Processing and Analysis Core (TPAC) for assist ance with TEM imaging, and W. Szeszel-Fedorowicz for assistance with Bioanalyzer and NanoDrop assays, V. A. Cuppett and C. N. Byrne-Hoffman for assistance with tissue culture, R. Kanj for manuscript comments.
Footnotes
Electronic Supplementary Information (ESI) available: The supporting information includes the following additional figures. Figure S1: SEM images of frozen exosomes from mouse melanoma cells B16F0 and human breast cancer cells SKBR3. Figure S2: Calibration of HER1 and HER2 expression on SKBR3 cells by flow cytometry. Figure S3: Quantification of the forward scatter properties of fluorescent nanoparticles that bracketed the expected size range of exosomes. Figure S4: Flow cytometric analysis of B16F0 exosomes stained using APC conjugated IL12RB2 mAb and the lipophilic dye DiI. Table S1: Primers for semi-quantitative PCR.
Notes and references
- 1.Théry C, Ostrowski M, Segura E. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Andaloussi SEL, Mäger I, Breakefield XO, Wood MJ. Nat. Rev. Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 3.Robbins PD, Morelli AE. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding C, Heuser J, Stahl P. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. J. Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 7.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Carter BS, Krichevsky AM, Breakefield XO. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor DD, Gercel-Taylor C. Gynecol. Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 10.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia B. a, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peinado H, Lavotshkin S, Lyden D. Semin. Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, Gauldie J, Bramson J, Wan Y. J. Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- 13.Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakefield XO, Skog J. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronisz A, Wang Y, Nowicki MO, Peruzzi P, Ansari KI, Ogawa D, Balaj L, De Rienzo G, Mineo M, Nakano I, Ostrowski MC, Hochberg F, Weissleder R, Lawler SE, Chiocca EA, Godlewski J. Cancer Res. 2014;74:738–750. doi: 10.1158/0008-5472.CAN-13-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z, Linnane S. J. Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 17.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 18.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 19.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni YM, Klinke DJ, II, Wu Y, Byrne-Hoffman C. Biotechnol. Bioeng. 2014;111:1853–1863. doi: 10.1002/bit.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 22.Somasundaram R, Herlyn M. Nat. Med. 2012;18:853–854. doi: 10.1038/nm.2775. [DOI] [PubMed] [Google Scholar]
- 23.Hood JL, San RS, Wickline SA. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 24.Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 25.Hood JL, Pan H, Lanza GM, Wickline SA. Lab. Investig. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor DD, Gerçel-Taylor C. Br. J. Cancer. 2005;92:305–311. doi: 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X. Clin. Cancer Res. 2005;11:7554–7563. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 28.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Br. J. Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z, Clayton A. J. Transl. Med. 2009;7:4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Semin. Cancer Biol. 2014:1–11. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Semin. Cancer Biol. 2012;22:342–349. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Keller S, Ridinger J, Rupp A-K, Janssen JWG, Altevogt P. J. Transl. Med. 2011;9:86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton A, Mason MD. Curr. Oncol. 2009;16:46–49. doi: 10.3747/co.v16i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Nat. Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 36.Xu D, Tahara H. Adv. Drug Deliv. Rev. 2013;65:368–75. doi: 10.1016/j.addr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P, Schiapparelli L, Cline HT. Curr. Opin. Neurobiol. 2013;23:997–1004. doi: 10.1016/j.conb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming AB, Saltzman WM. J. Control. Release. 2001;70:29–36. doi: 10.1016/s0168-3659(00)00318-7. [DOI] [PubMed] [Google Scholar]
- 40.Cocucci E, Racchetti G, Meldolesi J. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Raposo G, Stoorvogel W. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathivanan S, Ji H, Simpson RJ. J. Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni YM, Chambers E, McGray AJR, Ware JS, Bramson JL, Klinke DJ., II Integr. Biol. 2012;4:925–936. doi: 10.1039/c2ib20053h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni YM, Klinke DJ., II Proteome Sci. 2012;10:11. doi: 10.1186/1477-5956-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Théry C, Amigorena S, Raposo G, Clayton A. Curr. Protoc. Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3, Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 46.Hu W, Zhang C, Fang Y, Lou C. Toxicol Vitr. 2011;25:513–520. doi: 10.1016/j.tiv.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Théry C, Boussac M, Véron P, Raposo G, Garin J, Amigorena S. J. Immunol. 2001 doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 48.Klinke DJ, II, Brundage KM. Cytometry. A. 2009;75:699–706. doi: 10.1002/cyto.a.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Pol E, Hoekstra a G., Sturk A, Otto C, van Leeuwen TG, Nieuwland R. J. Thromb. Haemost. 2010;8:2596–2607. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- 50.Sokolova V, Ludwig A-K, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Colloids Surf. B. Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Sharma S, Rasool HI, Palanisamy V, Mathisen C, Schmidt M, Wong DT, Gimzewski JK. ACS Nano. 2010;4:1921–1926. doi: 10.1021/nn901824n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Cell. Mol. Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Fan TM, Borst LB, Cheng J. ACS Nano. 2012;6:3954–3966. doi: 10.1021/nn300149c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy ST, van der Vlies AJ, Simeoni E, O'Neil CP, Swartz MA, Hubbell JA. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 55.Irvine DJ, Swartz MA, Szeto GL. Nat. Mater. 2013;12:978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG. J. Thromb. Haemost. 2012;10:919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 57.Maas SLN, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, Schiffelers RM, Wauben MHM, Broekman MLD, Nolte-'t Hoen ENM. J. Control. Release. 2015;200:87–96. doi: 10.1016/j.jconrel.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erlandsen SL, Bemrick WJ, Schupp DE, Shields JM, Jarroll EL, Sauch JF, Pawley JB. J. Histochem. Cytochem. 1990;38:625–632. doi: 10.1177/38.5.2332623. [DOI] [PubMed] [Google Scholar]
- 59.Speirs V, Eich-Bender S, Youngson CR, Cutz E. J. Histochem. Cytochem. 1993;41:1303–1310. doi: 10.1177/41.9.8394853. [DOI] [PubMed] [Google Scholar]
- 60.Nolte-'t Hoen EN, van der Vlist EJ, Aalberts M, Mertens HC, Bosch BJ, Bartelink W, Mastrobattista E, van Gaal EVB, Stoorvogel W, Arkesteijn GJ, Wauben MH. Nanomedicine. 2012;8:712–720. doi: 10.1016/j.nano.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Vlist EJ, Nolte-'t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Nat. Protoc. 2012;7:1311–1326. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 62.Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. Curr. Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batagov AO, Kurochkin IV. Biol. Direct. 2013;8:12. doi: 10.1186/1745-6150-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ekstrom K, Valadi H, Sjostrand M, Malmhall C, Bossios A, Eldh M, Lotvall J. J. Extracell Vesicles. 2012;1:18389. doi: 10.3402/jev.v1i0.18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Proc Natl Acad Sci. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.