Abstract
Colorectal cancer (CRC) is the third leading cause of death in both men and women in North America. Despite chemotherapeutic efforts, CRC is associated with a high degree of morbidity and mortality. Thus, to develop effective treatment strategies for CRC, one needs knowledge of the pathogenesis of cancer development and cancer resistance. It is suggested that colonic tumors or cell lines harbor truncated adenomatous polyposis coli (APC) without DNA repair inhibitory (DRI)-domain. It is also thought that the product of the APC gene can modulate base excision repair (BER) pathway through an interaction with Pol-β and flap endonuclease 1 (Fen-1) to mediate CRC cell apoptosis. The proposed therapy with temozolomide (TMZ) exploits this particular pathway; however, a high percentage of colorectal tumors continue to develop resistance to chemotherapy due to mismatch repair (MMR)-deficiency. In the present communication, we have comprehensively reviewed a critical issue that has not been addressed previously: a novel mechanism by which APC-induced blockage of single nucleotide (SN)- and long-patch (LP)-BER play role in DNA-alkylation damage-induced colorectal carcinogenesis.
Keywords: Adenomatous polyposis coli, base excision repair, DNA damage, colorectal carcinogenesis
Graphical Abstract
Introduction
Colorectal cancer (CRC) is the third most common cause of cancer-related death in both men and women in the Western hemisphere. In 2015, the American Cancer Society estimated that 132,700 new CRCs (93,000 colon and 39,610 rectal cancers) would be diagnosed and that 49,700 deaths would occur with this disease in the United States [1]. Prognosis and therapy depends on the stage of the tumor at the time of diagnosis. Therapeutic interventions include surgery, chemotherapy and/or radiation. Of the three modalities, surgery appears to be the most effective treatment for early stages that have adequate surgical margins with no invasive characteristics. Chemotherapy and/or radiation are often an adjunctive therapy for stages II and III of CRC patients; however, such therapy is less effective due to high rates (nearly 30–50%) of recurrence and/or drug resistance [2–4]. To understand why CRC is associated with high recurrence and drug resistance rates, one needs a better understanding of CRC pathogenesis.
CRC develops through a series of histologically distinct stages from “adenoma to carcinoma” [5] and follows a temporal order in which genetic mutations and epigenetic events occurring in different genes generate multiple pathways for CRC progression [6–9]. One of the earliest events during this multistep process of tumorigenesis in CRC stems arises from mutations in the APC gene [10–13]. In mice that carry the targeted conditional mutation of Apc, the development of CRC from the loss of APC function has been observed [14]. Additionally, Apc knockout demonstrates a DNA damage signature and genomic instability in the liver that is further enhanced with loss of p53 [15]. However, the role of wild-type APC in response to carcinogen-induced DNA damage in colorectal carcinogenesis is not clear.
The focus of this review article is to highlight the important role of wild-type and mutant APC in the repair of abasic DNA by the BER pathway, and the potential link between BER and colorectal carcinogenesis. We will focus primarily on the following critical aims that have not been previously described before: (i) Understand the link between APC and BER, (ii) Determine the factors that may affect the cellular consequences (transformation vs. apoptosis) of the APC block of BER, (iii) Understand the mechanisms by which APC/BER interaction influences CRC carcinogenesis and (iv) Determine whether the APC/BER pathway interaction may be an alternative chemotherapeutic target.
APC gene and mutation in CRC
The APC gene product is a 310-kDa-homodimeric protein, which is localized in both the cytoplasm and nucleus [16–21]. It is expressed constitutively within the normal colonic epithelium. At the cellular level, wild-type APC is critical for cytoskeletal integrity [17, 22], cellular adhesion [23, 24], cell migration [21, 25], and Wingless/Wnt signaling [26–28]. In addition, it can act to suppress tumor growth through several different mechanisms. Wild-type APC binds to EB1 and DLG (a tumor suppressor protein) to regulate microtubule polymerization [29–32] and cell cycle progression from Go/G1 to S phase [33], respectively. APC may also act as a negative regulator of β-catenin signaling in the transformation of colonic epithelial cells [34, 35]. The β-catenin/Tcf4 complex regulates the proto-oncogene and cell cycle regulator c-myc [36], the G1/S-regulating cyclin D1 [37], the gene encoding the matrix-degrading metalloproteinase, matrilysin [38], the AP-1 transcription factors c-jun and fra-1, and the urokinase-type plasminogen activator receptor gene, uPAR [39]. The β-catenin/Tcf4 complex-mediated expression of c-myc regulates the expression of Cdk4 and links the APC mutation to β-catenin stabilization, c-myc upregulation and abrogation of Cdk4-mediated G1/S transition [40, 41]. Furthermore, c-myc deletion has been shown to rescue Apc deficiency in the small intestine of mice [42, 43].
In colorectal tumorigenesis, mutations of the APC, Ki-ras, deleted in colorectal cancer (DCC), and p53 genes play important roles at different stages of colorectal cancer development [26, 27]. About 60 to 80% of sporadic colorectal cancers and adenomas have mutations in the APC gene [8, 10, 12]. Mutation of the APC gene is an early event in familial adenomatous polyposis (FAP), a syndrome in which there is an inherited predisposition to colon cancer [10, 11]. An association has also been shown between FAP and germline mutations in the mutation cluster region (MCR) of APC [44–47]. A truncation mutation involving the MCR is thought to be necessary for the loss of β-catenin binding and nuclear localization signals in APC that promotes tumor progression [10, 26, 48–50]. A recent study has shown that animals with homozygous truncating mutations at codon 1638 of APC do not develop tumors and survive through adulthood [51]. Patients with mutations outside of the MCR region may exhibit a milder phenotype in CRC development [52–54]. Thus, it is now well established that mutations in APC may be necessary for the early onset of polyposis.
Base excision repair (BER) in CRC
The damage to genomic DNA base-pairs can occur by both exogenous and endogenous mutagenic agents. It has been estimated that approximately 106 abasic sites are generated per mammalian cell per day [55]. Abasic sites are unstable and degrade spontaneously into DNA-strand breaks by β-elimination and are also highly mutagenic due to creation of non-template DNA and RNA synthesis. Despite the large number of abasic sites generated by each cell per day, the number of mutations is extremely low. This is because most of the damaged DNA bases can be efficiently repaired by several DNA repair systems [56]. However, deficiencies in the DNA repair pathways results in unrepaired damaged DNA bases, which are thought to be a major source of intermediate precursors for carcinogenesis and tumorigenesis [57].
In humans, deficiency in DNA repair has been linked to a number of genetic diseases characterized by radiation sensitivity and cancer-prone syndromes [58]. It is proposed that about 15% of hereditary nonpolyposis colon cancers (HNPCC) have defects in one or more proteins in the DNA mismatch repair (MMR) pathway. A significant concordance between the in vitro replication errors of Pol-β and in vivo point mutations of the APC gene has been suggested as a leading cause of CRC [59]. Most DNA repair mechanisms involve the participation of enzymes and other proteins that recognize structural alterations in DNA [60–63].
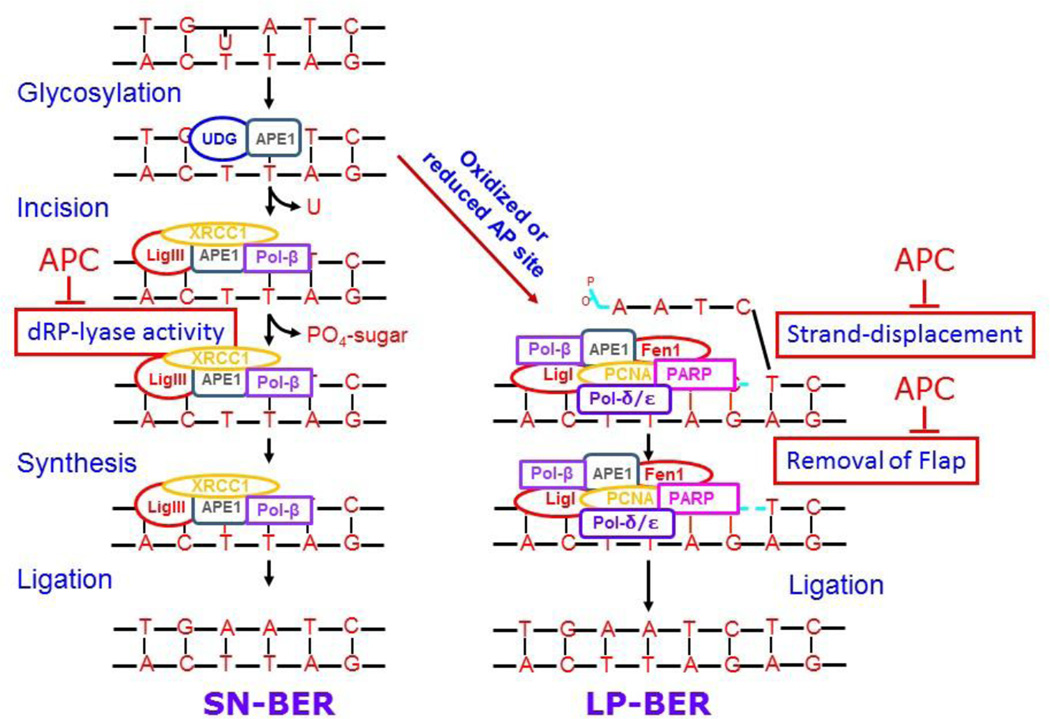
In mammalian cells, BER can proceed through at least two pathways designated as the “single nucleotide (SN)-BER” and “multi-nucleotide or long-patch (LP)-BER” pathways (Fig. 1). These two pathways are differentiated by the repair patch size, as well as, by the contribution of different proteins to the pathway [64–66]. In both pathways, repair is first initiated by the recognition and removal of the modified base by a DNA glycosylase. The two types of DNA glycosylases – monofunctional and bifunctional – generate an abasic site (AP). Monofunctional DNA glycosylases cleave only the glycosidic bond between N and C1. This cleavage protects the abasic site until apurinic/apyrimidinic (AP) endonuclease 1 (APE-1) cleaves the DNA backbone at the 5’-end of the AP-site. The bifunctional DNA glycosylases have an additional AP-lyase activity between the sugar and the base to establish an abasic-site [67]. At the latter DNA cleavage site, APE-1 generates a 3′-OH and 5’-deoxyribose phosphate (5′-dRP) ends [68–70]. Second, the remaining 5′-dRP residue is cleaved by a 5’-deoxyribose phosphate lyase (dRP-lyase) activity of Pol-β to yield a 5’-phosphorylated gapped-DNA strand. Third, Pol-β incorporates the correct base at the site of the damaged base with its polymerizing activity. Lastly, the DNA ligase-I or III seals the nick [71]. This multi-step repair process becomes more complicated once the AP-site becomes oxidized or reduced and cannot undergo the dRP-lyase activity of Pol-β . Under these circumstances, the repair of DNA is accomplished through LP-BER. The Pol-β-dependent strand-displacement synthesis generates a longer repair patch and a 5’-overhang of a single-stranded DNA-flap with a modified sugar at its 5’-end. The 5’-overhang DNA-flap is cleaved by flap endonuclease 1 (Fen-1), and finally the nick is sealed by DNA ligase I or III [56, 65, 72–76].
Figure 1. Model of BER pathway.
DNA repair of abasic sites diverge after the generation of the 3’-hydroxyl group. The SN- or LP-BER pathways and their known protein components are summarized. The interaction of APC with BER pathway is also depicted.
The regulation of the SN- and LP-BER pathways becomes more complex when accessory proteins interact directly with one or more of the BER proteins and alter their activity. For example, Werner’s syndrome protein [77, 78], poly(ADP-ribose)polymerase-1 [79, 80], proliferating cell nuclear antigen [64, 81–83], p53 [84], replication protein A [85], X-ray cross-complementing group 1 (XRCC1) [86], and arginine methyltransferase 6 (PRMT-6) [66] function as accessory proteins in the BER pathway. Our studies suggest that APC is another accessory protein that interacts with Pol-β and Fen-1 and blocks SN- and LP-BER activities [68, 87–89]. Since Arg83 of Pol-β is one of the amino acids involved in the interaction with APC, it would be interesting to determine whether the methylation of Pol-β at this residue affects APC-binding and its function.
APC/BER interaction and CRC carcinogenesis
The APC block of BER in response to exposure to DNA-alkylation damage can result in the transformation of colonic epithelial cells. This has also been shown in cigarette smoke carcinogen-induced neoplastic transformation of normal breast epithelial cells both in vitro and in vivo models [90–92]. The role of APC in BER is based upon the finding of PCNA-interacting protein (PIP)-like box Qxx(h)xx(aa) in APC (amino acids Gln1256, ILe1259 and Tyr1262) [68]. Using site-directed mutagenesis, the APC amino acid residues ILe1259 and Tyr1262 are important for the interaction and functional activity of Pol-β; this interacting domain of APC is known as the DNA repair inhibitory (DRI)-domain [87]. The DRI-domain of APC is located in the N-terminal region and is spared by mutations of MCR-associated truncation, which compromises the function of APC and contributes to chromosomal instability [93, 94] (Fig. 2). In a series of reconstituted or cell-based in vitro experiments, APC blocks Pol-β-directed LP-BER and SN-BER by blocking the dRP-lyase and strand-displacement activities of Pol-β [54, 68, 87, 89].
Figure 2. Structures of APC and Pol-β.
Panel A, the 2843 amino acid sequence displays an armadillo domain near the N-terminus. There are two β-catenin binding domains. The first 15-amino acid repeat can bind β-catenin, but its functional significance is still obscure, while the 20-amino acid repeat can bind β-catenin with a high affinity upon phosphorylation. The DRI-domain (showing the Pol-β interacting amino acids ILe1259 and Tyr1264), just upstream of MCR, is involved in the regulation of the BER pathway. Asef, APC-stimulated guanine nucleotide exchange factor; DLG, Drosophila discs large; EB1, end-binding protein 1, KAP3A, kinesin superfamily-associated protein 3A; NES, nuclear export signal; NLS, nuclear localization signal; PP2-B56α, protein phosphates 2A B56α subunit. Panel B, depicts the crystal structure of Pol-β that shows the APC interacting amino acids Thr79, Lys81 and Arg83.
Although, APC is present in the purified BER complex assembled onto the basic DNA substrate [95], it is not clear whether APC directly interacts with the abasic DNA, is recruited by or recruits BER proteins. In recent studies, a direct interaction of APC with DNA has been shown [96, 97]. The DNA binding activity of APC has been implicated in the blockade of DNA replication, S phase progression, and drug-induced apoptosis [98, 99]. In mouse embryonic fibroblast cells, MMS-induced levels of APC block LP-BER and increase apoptosis [88]. Our recent findings [100–103] indicate that the wild-type APC can induce sensitivity of DNA-alkylating drugs to CRC cells by blocking the BER pathway (Fig. 3). On the other hand, mutations in the APC gene can cause resistance of DNA-alkylating drugs to CRC cells by removing its interference with the BER pathway. This view has been supported by subsequent studies showing that mutations in the APC gene influences 5-fluorouracil (5-FU) resistance to CRCs [99, 104].
Figure 3.
A hypothetical model for the role of APC blockade of BER in carcinogenesis and chemotherapy.
APC/BER in determining transformation versus apoptosis
The precise relationship between the extent of DNA damage, duration of DNA damage, DNA repair capacity of the cell, and the role of APC in colorectal carcinogenesis is not well-established. The current understanding suggests that when a threshold limit of DNA damage is incurred, the cell tries to repair the damage; however, a few cells may escape the repair, acquire additional cell survival characteristics, and become tumorigenic. When the extent of DNA damage sustained is higher than the repair capacity, the cell becomes apoptotic [105–107]. Hence, the risk for the development of cancer is based on the inter-individual variation in carcinogen metabolism, amount of carcinogen-DNA adducts and DNA repair capacity of the cell [108, 109].
Molecules that regulate cellular proliferation and transformation include APC, claudin-1, and azoxymethane (AOM) amongst many others. Previous studies show that APC regulates cellular proliferation and transformation induced by the activation of RAS and β-catenin signaling pathways and/or cooperated by other genes, such as Dnah3, Ahnak, Stk17b, and Rbm9 [110, 111]. Claudin-1 overexpression enhances intestinal epithelial cell susceptibility to APC-mediated colon tumorigenesis [112]. The carcinogen AOM causes transformation of normal colonic epithelial cells in culture and animal models in a dose- and duration-dependent manner [113]. AOM-induced transformation and survival or apoptosis of rat colonic epithelial cells is measured by the level and repair of 6O-methyl guanine adducts in DNA [114, 115]. In AOM-induced mouse colon tumors, the development of colon cancer is thought to be associated with loss of wild-type APC protein and by mutations in the Apc gene that alters its expression [116, 117]. Later, mutations in Apc gene were determined in AOM-induced colorectal carcinogenesis in rats [117]. However, the precise relationship between the extents of DNA damage, the duration of DNA damage, the DNA repair capacity of the cell, and the role of APC in colorectal carcinogenesis is not well-understood. Therefore, it is imperative to define the mechanism(s) that may explain whether the cells harboring wild-type APC that sustain DNA damage survive, enter the carcinogenesis cascade, or become apoptotic.
APC/BER interaction as a chemotherapeutic target
DNA-alkylating agents are commonly used in the treatment of brain tumors, ovarian cancer, malignant melanomas, and various hematological tumors [118–120]. These agents have either one or two reactive groups that interact covalently with nucleophilic centers in DNA. Such reactive sites are present in all four bases, and are attacked with different affinities and specificities. The most reactive sites are the ring nitrogen atoms (N7 of guanine (N7mG) and N3 of adenine (N3mA)) and under normal circumstances they are repaired by the BER pathway [121, 122]. Alkylation also occurs at the nucleophilic oxygen sites, such as the O6 position of guanine (O6mG), which is repaired by O6-guanine DNA methyltransferase (MGMT) [123–125] or mismatch repair (MMR) pathways [126–128]. N7mG and N3mA are most frequently methylated adducts comprising 80–85% and 8–18% of total DNA methylated adducts, respectively. N3mA adducts are readily hydrolyzed, while N7mG adducts are stable for longer times with an in vitro half-life of 40–80 hours [129]. The O6meG are approximately 8% of the total methylated DNA adducts, and they are more stable and persist longer in DNA in the absence of MGMT [123]. The inappropriate pairing of O6MeG lesions during DNA replication causes pre-mutagenic and pre-toxic GC to AT transitions [130]. On the other hand, although N7MeG and N3MeA lesions do not result in mismatch, they do have indirect genetic consequences through secondary lesions of abasic sites [131–134]. Unrepaired abasic sites hinder DNA replication and give rise to base pair substitutions including GC to AT transitions and AT to TA and GC to TA transversions [131, 134–136]. Thus, N7MeG and N3MeA lesions, while less mutagenic than O6MeG lesions, are effective in stimulating the recombinational change that leads to genetic duplications. Since N7MeG and N3MeA lesions are repaired by BER, it is clear that the BER pathway is critical for determining DNA alkylation-induced carcinogenesis. Any defect in the BER pathway that causes an accumulation of these lesions can also produce cytotoxicity, a fact that has been exploited as a chemotherapeutic target for cancer cell death [118–122, 137–139].
Regardless the attempts to improve patient outcomes by incorporating new active systemic agents into clinical practice, there has been little improvement in the metastatic CRC patient cure rate. In spite of the best practice of 5-FU with or without additional therapy to eradicate micrometastatic disease after “curative” surgery for early stage CRC or in those with oligometastatic disease, most cancers relapse within the first few years following treatment completion. This indicates a relatively rapid repopulation of neoplastic progeny, i.e., CRC stem cells [140, 141]. Treatment with higher or prolonged doses of multiple drug combinations, such as 5-FU and oxaliplatin, compromises the patient’s quality of life and leads to serious side-effects [142, 143]. Despite decades of use, a thorough understanding of the mechanisms of action of 5-FU and interventions to overcome resistance are still relatively limited. Thus, there is critical need for a rational design of mechanism-based systemic therapy with well-characterized, specific targets [144, 145]. The efficacy of many chemotherapeutic drugs, such as 5-FU and oxaliplatin can be improved by blocking specific DNA repair pathways. Since multiple pathways are involved in the repair of 5-FU and oxaliplatin-induced DNA lesions, multiple inhibitors will be required to achieve the desired goal. Therefore, we propose that targeting one specific pathway, such as Pol-β and using the therapeutic drug, such as TMZ, may prove to be a good future strategy for significantly improved outcome for patients with colorectal cancer.
Numerous Pol-β inhibitors, with limited success that have been reported in recent years, are summarized in Table 1 [100–102, 146–164]. Despite the number of available drugs, more potent and selective inhibitors of DNA Pol-β are still needed. A new and emerging concept is to sensitize cancer cells to DNA-damaging agents by inhibiting various proteins in the DNA repair pathways [138]. Small chemical compounds have been identified by molecular docking or NMR studies to target the BER pathway by inhibiting AP endonuclease 1 (APE1) and Pol-β activities [153, 165]. For Pol-β, the most active compound identified by NMR chemical shift mapping is pamoic acid [153]. However, this compound, which inhibits dRP-lyase activity, blocks only SN-BER of Pol-β, and achievement of this block requires administration of a high concentration of the reagent. Since abasic DNA damage can also be repaired by LP-BER, there is a need for agents that can block both Pol-β-directed SN- and LP-BER pathways.
Table 1.
Summary of the Pol-β inhibitors.
| Inhibitors | Activity | Reference | |
|---|---|---|---|
| 1 | Thymidine 5’-monophosphate analogs | Irreversible inhibitor of the lyase activity |
146 |
| 2 | Dideoxythymidine triphosphate (ddTTP) | Polymerase activity | 147 |
| 3 | Nigranoic acid | Polymerase activity | 148 |
| 4 | Suramin | Polymerase activity | 149 |
| 5 | Fatty acids | Competing with both the substrate- and template-primer binding |
150 |
| 6 | Koetjapic acid | Competing with both the substrate- and template-primer binding |
151 |
| 7 | D-mandelonitrile-beta-D-glucoside | Competing with both the substrate- and template-primer binding |
152 |
| 8 | Pamoic acid | Binds with 8 kDa domain | 153, 164 |
| 9 | Fomitellic acids | Polymerase activity | 154 |
| 10 | Lithocholic acid | Competitive inhibition with substrate and non-competitive inhibition with template-primer |
155 |
| 11 | 3,4,5-Tri-O-galloylquinic acid | Competing with template-primer binding |
156 |
| 12 | Sulfated glycoglycerolipids | Competitive inhibition with template-primer and non- competitive inhibition with the substrate |
157 |
| 13 | Biscoumarin and sterols | Inhibitor of the lyase activity | 158, 159 |
| 14 | Sesquiterpenoids and triterpenoids | Inhibitor of the lyase activity | 160, 161 |
| 15 | Rhodamine-based small molecules | Inhibits polymerization and TdT activity |
162, 163 |
| 16 | NSC124854 | Inhibitor of the lyase and strand- displacement activities |
100 |
| 17 | NSC666715 | Inhibitor of the lyase and strand- displacement activities |
101, 102 |
Taking the advantage that APC interacts with Pol-β and Fen1, blocks both SN- and LP-BER pathways [54, 68, 87–90, 92], and interacts with amino acid residues Thr79, Lys81 and Arg83 of Pol-β [89], specific potent small molecule inhibitors (SMIs) targeting APC have been identified by molecular docking. The SMIs can enhance the efficacy of the current chemotherapeutic drug TMZ to reduce the growth of colon cancer cells both in vitro and in vivo [100–102]. Based upon these studies, we have suggested the role of APC in cancer cells and a novel site for the development of therapeutic drugs. This idea has been further supported by other laboratories [99, 104, 166–168].
Conclusion
Overall this review establishes, for the first time, the involvement of APC in DNA damage-induced carcinogenesis and how the DNA damage strategy can be used for APC-mediated apoptosis of colon cancer cells. The small molecular inhibitors mimic the interaction of APC with Pol-β and block Pol-β-directed BER and increase the sensitivity of TMZ in both MMR-proficient and MMR-deficient colorectal tumors. This is an important asset for the SMI because it can bypass the colorectal cancer resistance due to MMR-deficiency. Further studies are needed to understand how the interaction of APC or small molecular weight inhibitors may improve the use of TMZ and other drugs to modulate the antitumor activity of the targeted cell without affecting the normal cell. These studies will help to advance the field of colorectal carcinogenesis.
Acknowledgment
Authors are very thankful to Nirupama Gupta, MD, for the critical reading of the manuscript.
Grant support
The studies summarized in this work were supported by NCI-NIH grants CA-097031 and CA-100247 and Flight Attendant Medical Research Institute (Miami, FL) to S.N.
Abbreviations
- AOM
azoxymethane
- AP
apurinic/apyrimidinic
- APC
adenomatous polyposis coli
- APE1
apurinic/apyrimidinic endonuclease
- BER
base excision repair
- DRI
DNA repair-inhibitory
- dRP
2’-deoxyribose 5’-phosphate
- LP
long-patch
- MCR
mutation cluster region
- Pol-β
DNA polymerase β
- SN
single nucleotide
- TMZ
temozolomide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Aghili M, Izadi S, Madani H, Mortazavi H. Clinical and pathological evaluation of patients with early and late recurrence of colorectal cancer. Asia Pac. J. Clin. Oncol. 2010;6:35–41. doi: 10.1111/j.1743-7563.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell MJ, Campbell ME, Goldberg RM, Grothey A, Seitz JF, Benedetti JK, Andre T, Haller DG, Sargent DJ. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J. Clin. Oncol. 2008;26:2336–2341. doi: 10.1200/JCO.2007.15.8261. [DOI] [PubMed] [Google Scholar]
- 4.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36:2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Fearon ER. Molecular genetics of colorectal cancer. Ann. Rev. Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 8.Fearnhead NS, Wilding JL, Bodmer WF. Genetics of colorectal cancer: hereditary aspects and overview of colorectal tumorigenesis. Br. Med. Bull. 2002;64:27–43. doi: 10.1093/bmb/64.1.27. [DOI] [PubMed] [Google Scholar]
- 9.Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Path. Res. Int. 2012;2012:509348. doi: 10.1155/2012/509348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum. Mol. Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 11.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 12.Tsao J, Shibata D. Further evidence that one of the earliest alterations in colorectal carcinogenesis involves APC. Am. J. Path. 1994;145:531–534. [PMC free article] [PubMed] [Google Scholar]
- 13.Ichii S, Horii A, Nakatsuru S, Furuyama J, Utsunomiya J, Nakamura Y. Inactivation of both APC alleles in an early stage of colon adenomas in a patient with familial adenomatous polyposis (FAP) Hum. Mol. Genet. 1992;1:387–390. doi: 10.1093/hmg/1.6.387. [DOI] [PubMed] [Google Scholar]
- 14.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, Kanamaru R, Kanegae Y, Saito I, Nakamura Y, Shiba K, Noda T. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 15.Meniel V, Megges M, Young MA, Cole A, Sansom OJ, Clarke AR. Apc and p53 interaction in DNA damage and genomic instability in hepatocytes. Oncogene. 2014 doi: 10.1038/onc.2014.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat. Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 17.Munemitsu S, Souza B, Muller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- 18.Neufeld KL, White RL. Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc. Nat. Acad. Sci. USA. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neufeld KL, Nix DA, Bogerd H, Kang Y, Beckerle MC, Cullen BR, White RL. Adenomatous polyposis coli protein contains two nuclear export signals and shuttles between the nucleus and cytoplasm. Proc. Nat. Acad. Sci. USA. 2000;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumour suppressor has a nuclear export function. Nature. 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
- 21.Wong MH, Hermiston ML, Syder AJ, Gordon JI. Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc. Nat. Acad. Sci. USA. 1996;93:9588–9593. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994;54:3672–3675. [PubMed] [Google Scholar]
- 23.Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J. Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bienz M, Hamada F. Adenomatous polyposis coli proteins and cell adhesion. Curr. Opin. Cell Biol. 2004;16:528–535. doi: 10.1016/j.ceb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Harris ES, Nelson WJ. Adenomatous polyposis coli regulates endothelial cell migration independent of roles in beta-catenin signaling and cell-cell adhesion. Mol. Biol. Cell. 2010;21:2611–2623. doi: 10.1091/mbc.E10-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum. Mol. Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 27.Narayan S, Roy D. Role of APC and DNA mismatch repair genes in the development of colorectal cancers. Mol. Cancer. 2003;2:41. doi: 10.1186/1476-4598-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J. Clin. Oncol. 2000;18:1967–1979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 29.Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- 30.Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- 31.Kanaba T, Maesaki R, Mori T, Ito Y, Hakoshima T, Mishima M. Microtubule-binding sites of the CH domain of EB1 and its autoinhibition revealed by NMR. Biochim. Biophys. Acta. 2013;1834:499–507. doi: 10.1016/j.bbapap.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Stypula-Cyrus Y, Mutyal NN, Dela Cruz M, Kunte DP, Radosevich AJ, Wali R, Roy HK, Backman V. End-binding protein 1 (EB1) up-regulation is an early event in colorectal carcinogenesis. FEBS Lett. 2014;588:829–835. doi: 10.1016/j.febslet.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19:365–372. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
- 34.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 35.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 36.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 37.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Nat. Acad. Sci. USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, Matrisian LM. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 39.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Nat. Acad. Sci. USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myant K, Sansom O. Efficient Wnt mediated intestinal hyperproliferation requires the cyclin D2-CDK4/6 complex. Cell Division. 2011;6:3. doi: 10.1186/1747-1028-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, O’Connell BC, Mateyak MK, Tam W, Kohlhuber F, Dang CV, Sedivy JM, Eick D, Vogelstein B, Kinzler KW. Identification of CDK4 as a target of c-MYC. Proc. Nat. Acad. Sci. USA. 2000;97:2229–2234. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 43.Athineos D, Sansom OJ. Myc heterozygosity attenuates the phenotypes of APC deficiency in the small intestine. Oncogene. 2010;29:2585–2590. doi: 10.1038/onc.2010.5. [DOI] [PubMed] [Google Scholar]
- 44.Ficari F, Cama A, Valanzano R, Curia MC, Palmirotta R, Aceto G, Esposito DL, Crognale S, Lombardi A, Messerini L, Mariani-Costantini R, Tonelli F, Battista P. APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis. Br. J. Cancer. 2000;82:348–353. doi: 10.1054/bjoc.1999.0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagase H, Miyoshi Y, Horii A, Aoki T, Ogawa M, Utsunomiya J, Baba S, Sasazuki T, Nakamura Y. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res. 1992;52:4055–4057. [PubMed] [Google Scholar]
- 46.Polakis P. Wnt signaling and cancer. Genes & development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 47.Wu JS, Paul P, McGannon EA, Church JM. APC genotype, polyp number, and surgical options in familial adenomatous polyposis. Ann. Surg. 1998;227:57–62. doi: 10.1097/00000658-199801000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW, Jones DA. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y. The role of the adenomatous polyposis coli (APC) gene in human cancers. Adv. Cancer Res. 1993;62:65–87. doi: 10.1016/s0065-230x(08)60315-2. [DOI] [PubMed] [Google Scholar]
- 51.Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, Kucherlapati R, Khan PM, Fodde R. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plawski A, Banasiewicz T, Borun P, Kubaszewski L, Krokowicz P, Skrzypczak-Zielinska M, Lubinski J. Familial adenomatous polyposis of the colon. Hered. Cancer Clin. Pract. 2013;11:15. doi: 10.1186/1897-4287-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwong LN, Dove WF. APC and its modifiers in colon cancer. Adv. Exp. Med. Biol. 2009;656:85–106. doi: 10.1007/978-1-4419-1145-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaiswal AS, Narayan S. A novel function of adenomatous polyposis coli (APC) in regulating DNA repair. Cancer Lett. 2008;271:272–280. doi: 10.1016/j.canlet.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holmquist GP. Endogenous lesions, S-phase-independent spontaneous mutations, and evolutionary strategies for base excision repair. Mutation research. 1998;400:59–68. doi: 10.1016/s0027-5107(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 56.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 57.Krokan HE, Standal R, Slupphaug G. DNA glycosylases in the base excision repair of DNA. Biochem. J. 1997;325(Pt 1):1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Modrich P. Mismatch repair, genetic stability, and cancer. Science. 1994;266:1959–1960. doi: 10.1126/science.7801122. [DOI] [PubMed] [Google Scholar]
- 59.Muniappan BP, Thilly WG. The DNA polymerase beta replication error spectrum in the adenomatous polyposis coli gene contains human colon tumor mutational hotspots. Cancer Res. 2002;62:3271–3275. [PubMed] [Google Scholar]
- 60.Sancar A, Sancar GB. DNA repair enzymes. Ann. Rev. Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- 61.Sarasin AR, Hanawalt PC. Carcinogens enhance survival of UV-irradiated simian virus 40 in treated monkey kidney cells: induction of a recovery pathway? Proc. Nat. Acad. Sci. USA. 1978;75:346–350. doi: 10.1073/pnas.75.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantha AK, Sarkar B, Tell G. A short review on the implications of base excision repair pathway for neurons: relevance to neurodegenerative diseases. Mitochondrion. 2014;16:38–49. doi: 10.1016/j.mito.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Dianov GL, Hubscher U. Mammalian base excision repair: the forgotten archangel. Nucleic Acids Res. 2013;41:3483–3490. doi: 10.1093/nar/gkt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biade S, Sobol RW, Wilson SH, Matsumoto Y. Impairment of proliferating cell nuclear antigen-dependent apurinic/apyrimidinic site repair on linear DNA. J. Biol. Chem. 1998;273:898–902. doi: 10.1074/jbc.273.2.898. [DOI] [PubMed] [Google Scholar]
- 65.Klungland A, Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El-Andaloussi N, Valovka T, Toueille M, Steinacher R, Focke F, Gehrig P, Covic M, Hassa PO, Schar P, Hubscher U, Hottiger MO. Arginine methylation regulates DNA polymerase beta. Mol. Cell. 2006;22:51–62. doi: 10.1016/j.molcel.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Huffman JL, Sundheim O, Tainer JA. DNA base damage recognition and removal: new twists and grooves. Mut. Res. 2005;577:55–76. doi: 10.1016/j.mrfmmm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Narayan S, Jaiswal AS, Balusu R. Tumor suppressor APC blocks DNA polymerase beta-dependent strand displacement synthesis during long patch but not short patch base excision repair and increases sensitivity to methylmethane sulfonate. J. Biol. Chem. 2005;280:6942–6949. doi: 10.1074/jbc.M409200200. [DOI] [PubMed] [Google Scholar]
- 69.Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc. Nat. Acad. Sci. USA. 1991;88:11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kane CM, Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J. Biol. Chem. 1981;256:3405–3414. [PubMed] [Google Scholar]
- 71.Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- 72.Podlutsky AJ, Dianova II, Podust VN, Bohr VA, Dianov GL. Human DNA polymerase beta initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J. 2001;20:1477–1482. doi: 10.1093/emboj/20.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bambara RA, Murante RS, Henricksen LA. Enzymes and reactions at the eukaryotic DNA replication fork. The Journal of biological chemistry. 1997;272:4647–4650. doi: 10.1074/jbc.272.8.4647. [DOI] [PubMed] [Google Scholar]
- 74.Lieber MR. The FEN-1 family of structure-specific nucleases in eukaryotic DNA replication, recombination and repair. BioEssays. 1997;19:233–240. doi: 10.1002/bies.950190309. [DOI] [PubMed] [Google Scholar]
- 75.Memisoglu A, Samson L. Base excision repair in yeast and mammals. Mut. Res. 2000;451:39–51. doi: 10.1016/s0027-5107(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 76.Norbury CJ, Hickson ID. Cellular responses to DNA damage. Ann. Rev. Pharmacol. and toxicology. 2001;41:367–401. doi: 10.1146/annurev.pharmtox.41.1.367. [DOI] [PubMed] [Google Scholar]
- 77.Harrigan JA, Opresko PL, von Kobbe C, Kedar PS, Prasad R, Wilson SH, Bohr VA. The Werner syndrome protein stimulates DNA polymerase beta strand displacement synthesis via its helicase activity. J. Biol. Chem. 2003;278:22686–22695. doi: 10.1074/jbc.M213103200. [DOI] [PubMed] [Google Scholar]
- 78.Harrigan JA, Wilson DM, 3rd, Prasad R, Opresko PL, Beck G, May A, Wilson SH, Bohr VA. The Werner syndrome protein operates in base excision repair and cooperates with DNA polymerase beta. Nucleic Acids Res. 2006;34:745–754. doi: 10.1093/nar/gkj475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- 80.Prasad R, Lavrik OI, Kim SJ, Kedar P, Yang XP, Vande Berg BJ, Wilson SH. DNA polymerase beta -mediated long patch base excision repair. Poly(ADP-ribose)polymerase-1 stimulates strand displacement DNA synthesis. J. Biol. Chem. 2001;276:32411–32414. doi: 10.1074/jbc.C100292200. [DOI] [PubMed] [Google Scholar]
- 81.Kedar PS, Kim SJ, Robertson A, Hou E, Prasad R, Horton JK, Wilson SH. Direct interaction between mammalian DNA polymerase beta and proliferating cell nuclear antigen. J. Biol. Chem. 2002;277:31115–31123. doi: 10.1074/jbc.M201497200. [DOI] [PubMed] [Google Scholar]
- 82.Fortini P, Pascucci B, Parlanti E, Sobol RW, Wilson SH, Dogliotti E. Different DNA polymerases are involved in the short- and long-patch base excision repair in mammalian cells. Biochemistry. 1998;37:3575–3580. doi: 10.1021/bi972999h. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto Y, Kim K, Bogenhagen DF. Proliferating cell nuclear antigen-dependent abasic site repair in Xenopus laevis oocytes: an alternative pathway of base excision DNA repair. Mol. Cell. Biol. 1994;14:6187–6197. doi: 10.1128/mcb.14.9.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou J, Ahn J, Wilson SH, Prives C. A role for p53 in base excision repair. EMBO J. 2001;20:914–923. doi: 10.1093/emboj/20.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeMott MS, Zigman S, Bambara RA. Replication protein A stimulates long patch DNA base excision repair. J. Biol. Chem. 1998;273:27492–27498. doi: 10.1074/jbc.273.42.27492. [DOI] [PubMed] [Google Scholar]
- 86.Thompson LH, West MG. XRCC1 keeps DNA from getting stranded. Mut. Res. 2000;459:1–18. doi: 10.1016/s0921-8777(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 87.Jaiswal AS, Balusu R, Armas ML, Kundu CN, Narayan S. Mechanism of adenomatous polyposis coli (APC)-mediated blockage of long-patch base excision repair. Biochemistry. 2006;45:15903–15914. doi: 10.1021/bi0607958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kundu CN, Balusu R, Jaiswal AS, Narayan S. Adenomatous polyposis coli-mediated hypersensitivity of mouse embryonic fibroblast cell lines to methylmethane sulfonate treatment: implication of base excision repair pathways. Carcinogenesis. 2007;28:2089–2095. doi: 10.1093/carcin/bgm125. [DOI] [PubMed] [Google Scholar]
- 89.Balusu R, Jaiswal AS, Armas ML, Kundu CN, Bloom LB, Narayan S. Structure/function analysis of the interaction of adenomatous polyposis coli with DNA polymerase beta and its implications for base excision repair. Biochemistry. 2007;46:13961–13974. doi: 10.1021/bi701632e. [DOI] [PubMed] [Google Scholar]
- 90.Kundu CN, Balusu R, Jaiswal AS, Gairola CG, Narayan S. Cigarette smoke condensate-induced level of adenomatous polyposis coli blocks long-patch base excision repair in breast epithelial cells. Oncogene. 2007;26:1428–1438. doi: 10.1038/sj.onc.1209925. [DOI] [PubMed] [Google Scholar]
- 91.Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene. 2004;23:5880–5889. doi: 10.1038/sj.onc.1207792. [DOI] [PubMed] [Google Scholar]
- 92.Jaiswal AS, Panda H, Pampo CA, Siemann DW, Gairola CG, Hromas R, Narayan S. Adenomatous polyposis coli-mediated accumulation of abasic DNA lesions lead to cigarette smoke condensate-induced neoplastic transformation of normal breast epithelial cells. Neoplasia. 2013;15:454–460. doi: 10.1593/neo.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH, Clevers H. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat. Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 94.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 95.Jaiswal AS, Narayan S. Assembly of the base excision repair complex on abasic DNA and role of adenomatous polyposis coli on its functional activity. Biochemistry. 2011;50:1901–1909. doi: 10.1021/bi102000q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deka J, Herter P, Sprenger-Haussels M, Koosch S, Franz D, Muller KM, Kuhnen C, Hoffmann I, Muller O. The APC protein binds to A/T rich DNA sequences. Oncogene. 1999;18:5654–5661. doi: 10.1038/sj.onc.1202944. [DOI] [PubMed] [Google Scholar]
- 97.Qian J, Sarnaik AA, Bonney TM, Keirsey J, Combs KA, Steigerwald K, Acharya S, Behbehani GK, Barton MC, Lowy AM, Groden J. The APC tumor suppressor inhibits DNA replication by directly binding to DNA via its carboxyl terminus. Gastroenterology. 2008;135:152–162. doi: 10.1053/j.gastro.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perchiniak EM, Groden J. Mechanisms Regulating Microtubule Binding, DNA Replication, and Apoptosis are Controlled by the Intestinal Tumor Suppressor APC. Curr. Colorectal Cancer Rep. 2011;7:145–151. doi: 10.1007/s11888-011-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brocardo MG, Borowiec JA, Henderson BR. Adenomatous polyposis coli protein regulates the cellular response to DNA replication stress. Int. J. Biochem. Cell Biol. 2011;43:1354–1364. doi: 10.1016/j.biocel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 100.Jaiswal AS, Banerjee S, Aneja R, Sarkar FH, Ostrov DA, Narayan S. DNA polymerase beta as a novel target for chemotherapeutic intervention of colorectal cancer. PloS One. 2011;6:e16691. doi: 10.1371/journal.pone.0016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jaiswal AS, Banerjee S, Panda H, Bulkin CD, Izumi T, Sarkar FH, Ostrov DA, Narayan S. A novel inhibitor of DNA polymerase beta enhances the ability of temozolomide to impair the growth of colon cancer cells. Mol. Cancer Res. 2009;7:1973–1983. doi: 10.1158/1541-7786.MCR-09-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jaiswal AS, Panda H, Law BK, Sharma J, Jani J, Hromas R, Narayan S. NSC666715 and Its Analogs Inhibit Strand-Displacement Activity of DNA Polymerase beta and Potentiate Temozolomide-Induced DNA Damage, Senescence and Apoptosis in Colorectal Cancer Cells. PloS one. 2015;10:e0123808. doi: 10.1371/journal.pone.0123808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Panda H, Jaiswal AS, Corsino PE, Armas ML, Law BK, Narayan S. Amino acid Asp181 of 5’-flap endonuclease 1 is a useful target for chemotherapeutic development. Biochemistry. 2009;48:9952–9958. doi: 10.1021/bi9010754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martino-Echarri E, Henderson BR, Brocardo MG. Targeting the DNA replication checkpoint by pharmacologic inhibition of Chk1 kinase: a strategy to sensitize APC mutant colon cancer cells to 5-fluorouracil chemotherapy. Oncotarget. 2014;5:9889–9900. doi: 10.18632/oncotarget.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quiros S, Roos WP, Kaina B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell cycle. 2010;9:168–178. doi: 10.4161/cc.9.1.10363. [DOI] [PubMed] [Google Scholar]
- 106.Guerard M, Baum M, Bitsch A, Eisenbrand G, Elhajouji A, Epe B, Habermeyer M, Kaina B, Martus HJ, Pfuhler S, Schmitz C, Sutter A, Thomas AD, Ziemann C, Froetschl R. Assessment of mechanisms driving non-linear dose-response relationships in genotoxicity testing. Mutation research. Rev. Mut. Res. 2015;763:181–201. doi: 10.1016/j.mrrev.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Becker K, Thomas AD, Kaina B. Does increase in DNA repair allow “tolerance-to-insult” in chemical carcinogenesis? Skin tumor experiments with MGMT-overexpressing mice. Environ. Mol. Mutagen. 2014;55:145–150. doi: 10.1002/em.21834. [DOI] [PubMed] [Google Scholar]
- 108.Vahakangas K, Autrup H, Harris CC. Interindividual variation in carcinogen metabolism, DNA damage and DNA repair. IARC scientific publications. 1984:85–98. [PubMed] [Google Scholar]
- 109.Harris CC, Trump BF, Grafstrom R, Autrup H. Differences in metabolism of chemical carcinogens in cultured human epithelial tissues and cells. J. Cell. Biochem. 1982;18:285–294. doi: 10.1002/jcb.1982.240180304. [DOI] [PubMed] [Google Scholar]
- 110.Park KS, Jeon SH, Kim SE, Bahk YY, Holmen SL, Williams BO, Chung KC, Surh YJ, Choi KY. APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J. Cell Sci. 2006;119:819–827. doi: 10.1242/jcs.02779. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka M, Jin G, Yamazaki Y, Takahara T, Takuwa M, Nakamura T. Identification of candidate cooperative genes of the Apc mutation in transformation of the colon epithelial cell by retroviral insertional mutagenesis. Cancer Sci. 2008;99:979–985. doi: 10.1111/j.1349-7006.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis. Mol. Cancer. 2014;13:167. doi: 10.1186/1476-4598-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moyer MP, Aust JB. Human colon cells: culture and in vitro transformation. Science. 1984;224:1445–1447. doi: 10.1126/science.6328655. [DOI] [PubMed] [Google Scholar]
- 114.Nyskohus LS, Watson AJ, Margison GP, Le Leu RK, Kim SW, Lockett TJ, Head RJ, Young GP, Hu Y. Repair and removal of azoxymethane-induced O6-methylguanine in rat colon by O6-methylguanine DNA methyltransferase and apoptosis. Mut. Res. 2013;758:80–86. doi: 10.1016/j.mrgentox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Bugni JM, Meira LB, Samson LD. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28:734–741. doi: 10.1038/onc.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maltzman T, Whittington J, Driggers L, Stephens J, Ahnen D. AOM-induced mouse colon tumors do not express full-length APC protein. Carcinogenesis. 1997;18:2435–2439. doi: 10.1093/carcin/18.12.2435. [DOI] [PubMed] [Google Scholar]
- 117.De Filippo C, Caderni G, Bazzicalupo M, Briani C, Giannini A, Fazi M, Dolara P. Mutations of the Apc gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. Br. J. Cancer. 1998;77:2148–2151. doi: 10.1038/bjc.1998.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCormick JE, McElhinney RS. Nitrosoureas from chemist to physician: classification and recent approaches to drug design. Eur. J. Cancer. 1990;26:207–221. doi: 10.1016/0277-5379(90)90214-e. [DOI] [PubMed] [Google Scholar]
- 119.Chaney SG, Sancar A. DNA repair: enzymatic mechanisms and relevance to drug response. J. Nat. Cancer Inst. 1996;88:1346–1360. doi: 10.1093/jnci/88.19.1346. [DOI] [PubMed] [Google Scholar]
- 120.Fishel ML, He Y, Smith ML, Kelley MR. Manipulation of base excision repair to sensitize ovarian cancer cells to alkylating agent temozolomide. Clin. Cancer Res. 2007;13:260–267. doi: 10.1158/1078-0432.CCR-06-1920. [DOI] [PubMed] [Google Scholar]
- 121.Denny BJ, Wheelhouse RT, Stevens MF, Tsang LL, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33:9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- 122.Mutter N, Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Exp. Rev. Anticancer Ther. 2006;6:1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 123.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair. 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 124.Margison GP, Santibanez Koref MF, Povey AC. Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis. 2002;17:483–487. doi: 10.1093/mutage/17.6.483. [DOI] [PubMed] [Google Scholar]
- 125.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 126.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Ann. Rev. Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 127.Umar A, Kunkel TA. DNA-replication fidelity, mismatch repair and genome instability in cancer cells. Eur. J. Biochem. 1996;238:297–307. doi: 10.1111/j.1432-1033.1996.0297z.x. [DOI] [PubMed] [Google Scholar]
- 128.Jiricny J. Mediating mismatch repair. Nat. Genet. 2000;24:6–8. doi: 10.1038/71698. [DOI] [PubMed] [Google Scholar]
- 129.Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mut. Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 130.Shrivastav N, Li D, Essigmann JM. Chemical biology of mutagenesis and DNA repair: cellular responses to DNA alkylation. Carcinogenesis. 2010;31:59–70. doi: 10.1093/carcin/bgp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Ann. Rev. Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 132.Laval J, Boiteux S, O’Connor TR. Physiological properties and repair of apurinic/apyrimidinic sites and imidazole ring-opened guanines in DNA. Mut. Res. 1990;233:73–79. doi: 10.1016/0027-5107(90)90152-t. [DOI] [PubMed] [Google Scholar]
- 133.Vogel EW, Nivard MJ. International Commission for Protection Against Environmental Mutagens and Carcinogens. The subtlety of alkylating agents in reactions with biological macromolecules. Mut. Res. 1994;305:13–32. doi: 10.1016/0027-5107(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 134.Glaab WE, Tindall KR, Skopek TR. Specificity of mutations induced by methyl methanesulfonate in mismatch repair-deficient human cancer cell lines. Mut. Res. 1999;427:67–78. doi: 10.1016/s0027-5107(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 135.Strauss BS. The 'A rule' of mutagen specificity: a consequence of DNA polymerase bypass of non-instructional lesions? BioEssays. 1991;13:79–84. doi: 10.1002/bies.950130206. [DOI] [PubMed] [Google Scholar]
- 136.Goodman MF, Cai H, Bloom LB, Eritja R. Nucleotide insertion and primer extension at abasic template sites in different sequence contexts. Ann. New York Acad. Sci. 1994;726:132–142. doi: 10.1111/j.1749-6632.1994.tb52804.x. discussion 142-133. [DOI] [PubMed] [Google Scholar]
- 137.Liu L, Nakatsuru Y, Gerson SL. Base excision repair as a therapeutic target in colon cancer. Clin. Cancer Res. 2002;8:2985–2991. [PubMed] [Google Scholar]
- 138.Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat. Rev. 2005;31:603–617. doi: 10.1016/j.ctrv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Wilson SH, Beard WA, Shock DD, Batra VK, Cavanaugh NA, Prasad R, Hou EW, Liu Y, Asagoshi K, Horton JK, Stefanick DF, Kedar PS, Carrozza MJ, Masaoka A, Heacock ML. Base excision repair and design of small molecule inhibitors of human DNA polymerase beta. Cell. Mol. Life Sci. 2010;67:3633–3647. doi: 10.1007/s00018-010-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kosmider S, Lipton L. Adjuvant therapies for colorectal cancer. World J. Gastroenterol. 2007;13:3799–3805. doi: 10.3748/wjg.v13.i28.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Desch CE, Benson AB, 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJO. American Society of Clinical, Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 142.Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin. Oncol. 2002;29:11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- 143.Sanoff HK, Carpenter WR, Freburger J, Li L, Chen K, Zullig LL, Goldberg RM, Schymura MJ, Schrag D. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer. 2012;118:4309–4320. doi: 10.1002/cncr.27422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 145.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 146.Arian D, Hedayati M, Zhou H, Bilis Z, Chen K, DeWeese TL, Greenberg MM. Irreversible inhibition of DNA polymerase beta by small-molecule mimics of a DNA lesion. J. Am. Chem. Soc. 2014;136:3176–3183. doi: 10.1021/ja411733s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Izuta S, Saneyoshi M, Sakurai T, Suzuki M, Kojima K, Yoshida S. The 5'-triphosphates of 3'-azido-3'-deoxythymidine and 2', 3'-dideoxynucleosides inhibit DNA polymerase gamma by different mechanisms. Biochem. Biophys. Res. Commun. 1991;179:776–783. doi: 10.1016/0006-291x(91)91884-f. [DOI] [PubMed] [Google Scholar]
- 148.Sun HD, Qiu SX, Lin LZ, Wang ZY, Lin ZW, Pengsuparp T, Pezzuto JM, Fong HH, Cordell GA, Farnsworth NR. Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J. Nat. Prod. 1996;59:525–527. doi: 10.1021/np960149h. [DOI] [PubMed] [Google Scholar]
- 149.Ono K, Nakane H, Fukushima M. Differential inhibition of various deoxyribonucleic and ribonucleic acid polymerases by suramin. Eur. J. Biochem. 1988;172:349–353. doi: 10.1111/j.1432-1033.1988.tb13893.x. [DOI] [PubMed] [Google Scholar]
- 150.Mizushina Y, Yoshida S, Matsukage A, Sakaguchi K. The inhibitory action of fatty acids on DNA polymerase beta. Biochim. Biophys. Acta. 1997;1336:509–521. doi: 10.1016/s0304-4165(97)00067-6. [DOI] [PubMed] [Google Scholar]
- 151.Sun DA, Starck SR, Locke EP, Hecht SM. DNA polymerase beta inhibitors from Sandoricum koetjape. J. Nat. Prod. 1999;62:1110–1113. doi: 10.1021/np990104r. [DOI] [PubMed] [Google Scholar]
- 152.Mizushina Y, Takahashi N, Ogawa A, Tsurugaya K, Koshino H, Takemura M, Yoshida S, Matsukage A, Sugawara F, Sakaguchi K. The cyanogenic glucoside, prunasin (D-mandelonitrile-beta-D-glucoside), is a novel inhibitor of DNA polymerase beta. J. Biochem. 1999;126:430–436. doi: 10.1093/oxfordjournals.jbchem.a022468. [DOI] [PubMed] [Google Scholar]
- 153.Hu HY, Horton JK, Gryk MR, Prasad R, Naron JM, Sun DA, Hecht SM, Wilson SH, Mullen GP. Identification of small molecule synthetic inhibitors of DNA polymerase beta by NMR chemical shift mapping. J. Biol. Chem. 2004;279:39736–39744. doi: 10.1074/jbc.M402842200. [DOI] [PubMed] [Google Scholar]
- 154.Tanaka N, Kitamura A, Mizushina Y, Sugawara F, Sakaguchi K. Fomitellic acids, triterpenoid inhibitors of eukaryotic DNA polymerases from a basidiomycete, Fomitella fraxinea. J. Nat. Prod. 1998;61:193–197. doi: 10.1021/np970127a. [DOI] [PubMed] [Google Scholar]
- 155.Ogawa A, Murate T, Suzuki M, Nimura Y, Yoshida S. Lithocholic acid, a putative tumor promoter, inhibits mammalian DNA polymerase beta. Jpn. J. Cancer Res. 1998;89:1154–1159. doi: 10.1111/j.1349-7006.1998.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parker WB, Nishizawa M, Fisher MH, Ye N, Lee KH, Cheng YC. Characterization of a novel inhibitor of human DNA polymerases: 3,4,5-tri-O-galloylquinic acid. Biochem. Pharmacol. 1989;38:3759–3765. doi: 10.1016/0006-2952(89)90582-0. [DOI] [PubMed] [Google Scholar]
- 157.Ogawa A, Murate T, Izuta S, Takemura M, Furuta K, Kobayashi J, Kamikawa T, Nimura Y, Yoshida S. Sulfated glycoglycerolipid from archaebacterium inhibits eukaryotic DNA polymerase alpha, beta and retroviral reverse transcriptase and affects methyl methanesulfonate cytotoxicity. Int. J. Cancer. 1998;76:512–518. doi: 10.1002/(sici)1097-0215(19980518)76:4<512::aid-ijc12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 158.Li SS, Gao Z, Feng X, Hecht SM. Biscoumarin derivatives from Edgeworthia gardneri that inhibit the lyase activity of DNA polymerase beta. J. Nat. Prod. 2004;67:1608–1610. doi: 10.1021/np040127s. [DOI] [PubMed] [Google Scholar]
- 159.Li SS, Gao Z, Feng X, Jones SH, Hecht SM. Plant sterols as selective DNA polymerase beta lyase inhibitors and potentiators of bleomycin cytotoxicity. Bioorg. Med. Chem. 2004;12:4253–4258. doi: 10.1016/j.bmc.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 160.Cao S, Gao Z, Thomas SJ, Hecht SM, Lazo JS, Kingston DG. Marine sesquiterpenoids that inhibit the lyase activity of DNA polymerase beta. J. Nat. Prod. 2004;67:1716–1718. doi: 10.1021/np049849+. [DOI] [PubMed] [Google Scholar]
- 161.Chaturvedula VS, Zhou BN, Gao Z, Thomas SJ, Hecht SM, Kingston DG. New lupane triterpenoids from Solidago canadensis that inhibit the lyase activity of DNA polymerase beta. Bioorg. Med. Chem. 2004;12:6271–6275. doi: 10.1016/j.bmc.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 162.Strittmatter T, Brockmann A, Pott M, Hantusch A, Brunner T, Marx A. Expanding the scope of human DNA polymerase lambda and beta inhibitors. ACS Chem. Biol. 2014;9:282–290. doi: 10.1021/cb4007562. [DOI] [PubMed] [Google Scholar]
- 163.Strittmatter T, Bareth B, Immel TA, Huhn T, Mayer TU, Marx A. Small Molecule Inhibitors of Human DNA Polymerase lambda. ACS Chem. Biol. 2011;6:314–319. doi: 10.1021/cb100382m. [DOI] [PubMed] [Google Scholar]
- 164.Hazan C, Boudsocq F, Gervais V, Saurel O, Ciais M, Cazaux C, Czaplicki J, Milon A. Structural insights on the pamoic acid and the 8 kDa domain of DNA polymerase beta complex: towards the design of higher-affinity inhibitors. BMC Struct. Biol. 2008;8:22. doi: 10.1186/1472-6807-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Madhusudan S, Smart F, Shrimpton P, Parsons JL, Gardiner L, Houlbrook S, Talbot DC, Hammonds T, Freemont PA, Sternberg MJ, Dianov GL, Hickson ID. Isolation of a small molecule inhibitor of DNA base excision repair. Nucleic Acids Res. 2005;33:4711–4724. doi: 10.1093/nar/gki781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lesko AC, Goss KH, Prosperi JR. Exploiting APC function as a novel cancer therapy. Curr. Drug Targets. 2014;15:90–102. doi: 10.2174/1389450114666131108155418. [DOI] [PubMed] [Google Scholar]
- 167.Brocardo M, Henderson BR. APC shuttling to the membrane, nucleus and beyond. Trends Cell Biol. 2008;18:587–596. doi: 10.1016/j.tcb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 168.Das D, Preet R, Mohapatra P, Satapathy SR, Siddharth S, Tamir T, Jain V, Bharatam PV, Wyatt MD, Kundu CN. 5-Fluorouracil mediated anti-cancer activity in colon cancer cells is through the induction of Adenomatous Polyposis Coli: Implication of the long-patch base excision repair pathway. DNA Repair. 2014;24:15–25. doi: 10.1016/j.dnarep.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]