Abstract
The central trade-off between reproduction and longevity dominates most species’ life history. However, no mortality cost of reproduction is apparent in eusocial species, particularly social insects in the order Hymenoptera: one or a few individuals (typically referred to as queens) in a group specialize on reproduction and are generally longer-lived than all other group members (typically referred to as workers), despite having the same genome. However, it is unclear whether this survival advantage is due to social facilitation by the group or an intrinsic, individual property. Furthermore, it is unknown whether the correlation between reproduction and longevity is due to a direct mechanistic link or an indirect consequence of the social role of the reproductives. To begin addressing these questions, we performed a comparison of queen and worker longevity in the ant Cardiocondyla obscurior under social isolation conditions. Survival of single queens and workers was compared under laboratory condition, monitoring and controlling for brood production. Our results indicate that there is no intrinsic survival advantage of queens relative to workers unless individuals are becoming reproductively active. This interactive effect of caste and reproduction on life expectancy outside of the normal social context suggests that the positive correlation between reproduction and longevity in social insect queens is due to a direct link that can activate intrinsic survival mechanisms to ensure queen longevity.
Keywords: Ageing, Social Evolution, Fertility, Longevity, Caste, Formicidae
Background
Across and within most species, a negative correlation between reproductive effort and survival exists, which has led to the “cost or reproduction” paradigm (Williams, 1966; Harshman & Zera, 2007). A pronounced exception is presented by eusocial species, in which some individuals specialize on reproduction and have exceptionally long lifespans. The reproductive castes attain lifespans that are much longer than those of the non-reproductive castes and also are extremely long-lived compared to closely-related non-social species (Carey, 2001). The link between longevity and high reproductive rates in the queen caste compared to the non-reproductive female worker caste is particularly pronounced in ants (Keller & Genoud, 1997). Ultimately, the evolution of queen longevity in social insects can be explained by low extrinsic mortality rates for queens in mature colonies and a delayed reproductive schedule (Williams, 1966), as well as the queen’s role as the colony’s universal stem cell in the context of the disposable soma theory (Kirkwood 1977). In contrast, the proximate causes of the longevity of social insect queens are not sufficiently understood, despite some data on candidate mechanisms, such as oxidative stress resistance (Corona et al., 2007).
Experimental evidence from species in which workers can become reproductively active suggests that this reversal of the life-history trade-off between reproduction and longevity depends on individuals attaining reproductive status (Hartmann & Heinze, 2003). Egg laying changes their internal physiology and their social environment: reproductives receive more care from their nestmates and rarely leave the nest, which reduces extrinsic mortality. Thus, evolutionary theory predicts selection for long-life in reproductives of social insects (Keller & Genoud, 1997), but the proximate causes for the combination of long life span and high fertility in ant queens and other social insect reproductive castes are unclear. The lack of the common fertility-longevity trade-off could be due to external, social facilitation by worker care (Rueppell et al., 2004) or intrinsic processes that up-regulate survival and reproduction (Corona et al., 2007). Profound effects of social environment may act through internal physiological processes (Amdam et al., 2005, 2009), which complicates the distinction between social facilitation and physiological causes as proximate causes for the longevity of social insects reproductives.
The facultatively polygynous ant Cardiocondyla obscurior (Wheeler) is an invasive species, facilitated by frequent brother-sister mating, and a rapid life cycle (Heinze et al., 2006). Mating appears to prolong the life span of queens independently of their actual reproductive rate (Schrempf et al., 2005, 2008, 2015). In addition, fertility and longevity tend to be positively correlated in fertile queens of C. obscurior and a closely related species (Heinze & Schrempf, 2012; Heinze et al., 2013). Regardless of the specific findings, no study in a natural colony context can definitely determine whether the observed longevity is due to a change in worker behavior towards reproductives or intrinsic changes of the reproductives themselves. To distinguish these two alternative explanations and additionally test the general hypothesis that the longevity-fecundity trade-off is not an intrinsic property of all organisms, we performed a survival comparison of workers and queens of C. obscurior in social isolation. Cardiocondyla obscurior is ideally suited for this study because queens readily mate in the nest under laboratory conditions, workers are sterile, and the species is very adaptable to laboratory conditions and reasonably short-lived (Schrempf et al., 2005; Heinze et al., 2006).
Material and methods
All individuals were derived from 8 large, highly polygynous C. obscurior laboratory colonies that had been maintained in the laboratory for at least 10 generations and were derived from a single polygynous Brazilian colony by splitting queens, workers and some brood into new nest boxes. Hence, all individuals included in this study have the same genetic background. Forty-eight experimental pairs of newly emerged queens and workers were set up. Each pair was comprised of one queen and one worker with the same emergence date from the same colony. Each individual ant was housed in a separate petri dish that contained a plaster base, a small artificial nest and two metal food dishes for feeding with honey and small cockroach pieces. Food was provided ad libitum twice per week. For the first two weeks, all queens were individually paired with one random, wingless male to allow for mating to occur. Each corresponding worker was also paired with one random, wingless male during the initial two weeks of the experiment to control for any potential social interaction effect other than mating. Workers of C. obscurior lack reproductive organs completely and do not mate.
For the remainder of the experiment, workers and queens were kept solitarily, except for the presence of brood produced. All queens had the opportunity to mate but only queens that were observed egg-laying were classified as reproductive. One queen produced only a single egg and dissection of its spermatheca showed that it was not inseminated. Consequently, this queen was classified as non-reproductive. Workers that were paired with a reproducive queen received the same number of eggs to control for a possible brood effect, but all brood was removed before adults could emerge. Mortality and egg production was surveyed three to five days per week by visual inspection until all but the longest-lived queen had died. This longest-lived queen was coded as censored data, as were two other queens that accidentally drowned in the honey dish.
Survival was compared overall between workers and queens by Cox-regression analysis (SPSS V21, IBM), accounting for reproduction/brood as a second, independent factor. Specific queen-worker pairs were compared in follow-up Wilcoxon Signed Ranks tests. We categorized individuals as reproductive queens (n=6), workers that were paired with reproductive queens and thus received brood from their original colony (n=6), non-reproductive queens (n=42), and workers without brood (n=42).
Results
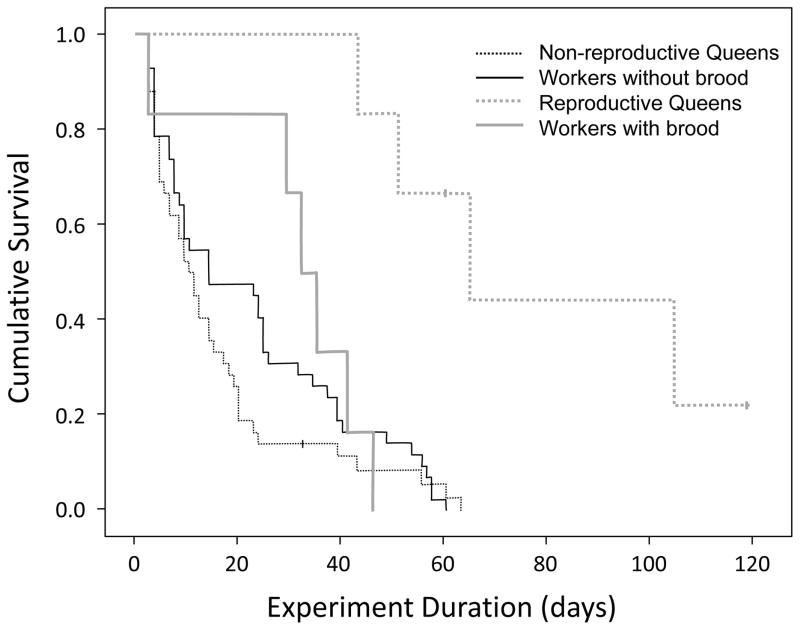
Observed lifespans ranged for workers from three to 63 days with a median of 24.5 and for queens from three to 120 days with a median of 13 (original data available in Appendix S1: “Dataset of survival and reproduction”). One queen was still alive when we terminated the experiment. Six of the 48 queens activated their ovaries and started consistent egg production, successfully raising worker pupae in three cases. The workers that were paired with those queens received corresponding numbers of eggs, except for one worker that had died before the paired queen had produced brood. Overall, queen and worker survival did not differ significantly (worker versus queen hazard ratio (HR) = 0.77, P = 0.235), while reproduction/brood significantly increased survival relative to non-reproductive queens/workers without brood (HR = 0.02, P = 0.002; Fig. 1). However, a significant interaction between the caste and reproduction factors (P = 0.01) required separate queen-worker comparisons for reproductive and non-reproductive dyads.
Figure 1.
The survival of queens and workers of the ant Cardiocondyla obscurior indicating a reversal of the general fecundity-lifespan trade-off. Individuals were kept in isolation under laboratory conditions to compare the intrinsic rates of aging between the castes. A small fraction of queens initiated reproduction, and corresponding workers received equivalent amounts of eggs to control for potential brood effects. Overall survival was not significantly different (not shown) but reproductively active queens were longer-lived than their worker complements, while survival of non-reproductive queens was not significantly different from that of the corresponding workers.
Queens that were reproductively active lived significantly longer than their paired workers with brood (Z6 = 2.2, p = 0.028), but there was no significant difference in survival between paired non-reproductive queens and workers without brood (Z21 = 1.9, p = 0.059). In fact, the worker outlived the queen in the majority of these pairings (Fig. 2). Accordingly, reproductive activation significantly increased survival in queens (HR = 0.14, p = 0.001), but addition of brood did not significantly affect worker survival (HR = 0.84, p = 0.702). All of these results were qualitatively identical when the analyses were limited to individuals that lived at least for two weeks and consequently died only after the removal of the males.
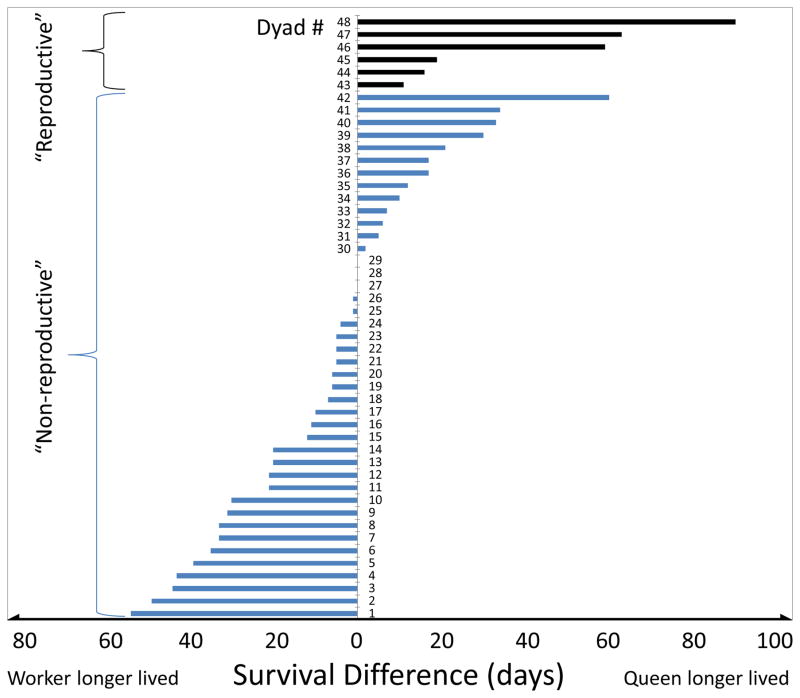
Figure 2.
Survival differences in the specific queen-worker dyads that were initially paired based on emergence date and colony source. Subsequently, the worker in a pair received eggs equivalent to what the corresponding queen produced (dyads # 43–48) or remained brood-less when the queen did not produce any eggs (dyads #1–42) to control for potential brood effects. While all of the reproductive queens were longer-lived than the corresponding workers (upper, black bars), only 13 of 42 non-reproductive queens were longer-lived than the corresponding workers (lower, blue bars), which illustrates the significant interaction effect of caste and reproductive status on survival (Fisher’s exact test: p = 0.002).
Discussion
Our comparison of queen and worker survival in social isolation under laboratory conditions revealed that reproductive C. obscurior queens have a survival advantage compared to workers that is independent of social colony environment. This result supports the hypothesis that the longevity-fecundity trade-off is not an intrinsic property of all organisms. The ants’ reversal of the reproduction-longevity trade-off is particularly remarkable because the long-lived, reproductive queen phenotype is based on the same genome as the short-lived, non-reproductive worker phenotype. Specifically, the workers and queens in this study were derived from the same genetic background. Thus, the evolutionary reverse relation between fertility and longevity is based on phenotypic plasticity within species, as well as genetic differentiation between social and solitary species.
Additionally, the study provides the first direct demonstration that the remarkable reversal of the reproduction-longevity trade-off in the reproductively specialized queen caste in ants and other eusocial Hymenoptera (Keller & Genoud, 1997) can be achieved without food transfers, grooming, or other forms of preferential treatment by workers. However, queens (Schrempf et al., 2005) and workers (unpublished data: A. Schrempf & J. Giehr) in colonies kept under similar laboratory conditions live generally much longer than their socially isolated counterparts in this study. Thus, social facilitation of longevity occurs in both castes of social insects (Rueppell et al., 2004), but our study demonstrates that individual, intrinsic properties of social insect reproductives contribute significantly to their high life expectancy.
In contrast to the reproductively active queens, the non-reproductive queens exhibited a comparable or even lower life expectancy than workers. These distinct results suggest that the activation of intrinsic mechanisms significantly contribute to the relative longevity of the queens. Genomic studies in C. obscurior are suggesting that these may be related to genes that affect aging in solitary species (von Wyschetski et al., 2015). Presumably, similar inducible mechanisms are also present in queen and worker castes of social insect species in which both, queens and workers can become reproductively active. The relative longevity of reproductively active workers of two ant species (Tsuji et al., 1996; Hartmann & Heinze, 2003) and honey bees (Dixon et al., 2014) in the colony context favor this hypothesis, although reproductive workers do not always attain lifespans comparable to queens (Dixon et al., 2014). A future test of the aging effects of worker reproductive activation outside the social environment is needed. However, this test cannot be performed in C. obscurior because its workers have lost all reproductive capacity, making a direct comparison between reproductive queens and workers in this species impossible.
Unmated C. obscurior queens in a normal colony context experience a shortened life expectancy (Schrempf et al., 2005) and in some ant species unmated young queens shift to a worker phenotype (Nehring et al., 2012). Accordingly, the short lifespans of socially isolated, non-reproductive queens may be due to the induction of worker-specific mechanisms that lead to a short life or the failure of inducing queen-specific mechanisms that lead to an extended life. The addition of brood to the workers that were paired with reproductive queens was not designed to mimic worker reproduction but controlled for potential brood effects. Brood can facilitate survival of adult ants either by food secretions (Hölldobler & Wilson, 1990) or serving as direct food source (Rueppell & Kirkman, 2005). In our social isolation paradigm with food ad libitum, added brood did not significantly increase worker life expectancy. Thus, the higher life expectancy of queens that produced brood than queens that did not may be explained better by reproductive activation than by brood presence.
Previous comparisons of lifespans of unmated, sham-mated, and mated C. obscurior queens under natural colony conditions suggested that insemination by males, rather than reproductive activity increases the lifespan of queens (Schrempf et al., 2005). In other cases, reproduction is important (Hartmann & Heinze, 2003) and perhaps both factors, insemination and reproductive activation, influence longevity to a different extent, depending on social environment and experimental circumstances. We could eliminate social environment here but the design of our study does not allow a distinction between the effect of insemination and reproduction. Male insemination could be the ultimate factor, simultaneously activating egg production in the ovary and mechanisms that lead to a lifespan extension in these queens compared to workers or non-reproductive queens. In any case, the distinction between reproductive and non-reproductive queens seems more prudent here because we do not have spermatheca dissection data from most individuals but regularly observed the queens’ egg production.
Our study demonstrates for the first time that reproduction is intrinsically linked to longevity in C. obscurior outside a social context. This result has profound consequences for understanding individual longevity in a social context and highlights the necessity of accounting for reproductive status in any mechanistic studies of aging in social insects. Social insect evolution is dominated by kin selection, which makes resource transfers an important consideration for the evolution of aging (Lee 2003). However, our study shows that these resources transfers are not necessary to generate an aging difference between queens and workers. Instead, our study suggests intrinsic mechanisms that have evolved to be phenotypically plastic based on caste development and in response to reproduction.
Comparative results from honey bees suggest that the intrinsic, positive link between reproduction and longevity may be due to a reconfiguration of endocrine regulation in social species, such as the negative link between juvenile hormone and egg yolk proteins (Amdam & Omholt, 2003; Corona et al., 2007). Such egg yolk proteins play a key role in maturing oocytes but also convey longevity benefits (Seehuus et al., 2006; Corona et al., 2007). Similar co-option of reproductive physiology to defend against molecular damage and prolong life of reproductive individuals may have independently evolved in ants (Morandin et al., 2014). The evolution of exceptional longevity in reproductive compared to non-reproductive individuals can be explained by the disposable soma theory (Kirkwood 1977) when the colony is regarded as the unit of selection (=superorganism concept). However, at an individual and proximate level our study suggests that the almost ubiquitous trade-off between reproduction and longevity may have a general, evolved regulatory basis that guides the optimization of resource allocation and determines aging rates (Tatar et al., 2003) and that this regulatory architecture has been altered by social evolution (von Wyschetski et al., 2015).
Supplementary Material
Acknowledgments
Funding
This research was supported by a Guest Researcher Fellowship of the Regensburger Universitätsstiftung, and the National Institute of Aging (grant # R21AG046837).
We thank S. Cremer for providing us initially with the laboratory colonies and L. Kalb for help during the initial set-up of the experimental groups. The suggestions of 3 anonymous reviewers improved an earlier version of this manuscript.
Footnotes
Authors’ contributions
OR, AS, and JH designed the experiment, FK and AS performed the experiment and collected the data, OR analyzed and interpreted the data, initially drafting the manuscript, and OR, AS and JH, contributed equally to the final version of the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Data accessibility
The dataset supporting this article has been uploaded as part of the Supplementary Material (“Dataset of survival and reproduction”).
References
- Amdam GV, Aase ALTO, Seehuus SC, Fondrk MK, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Experimental Gerontology. 2005;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J Theor Biol. 2003;223(4):451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Rueppell O, Fondrk MK, Page RE, Nelson CM. The nurse’s load: Early-life exposure to brood-rearing affects behavior and lifespan in honey bees (Apis mellifera) Experimental Gerontology. 2009;44:467–471. doi: 10.1016/j.exger.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR. Demographic mechanisms for the evolution of long life in social insects. Exp Gerontol. 2001;36:713–722. doi: 10.1016/s0531-5565(00)00237-0. [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci U S A. 2007;104(17):7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L, Kuster R, Rueppell O. Reproduction, social behavior, and aging trajectories in honeybee workers. Age. 2014;36(1):89–101. doi: 10.1007/s11357-013-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends Ecol Evol. 2007;22(2):80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Heinze J. Lay eggs, live longer: Division of labor and life span in a clonal ant species. Evolution. 2003;57(10):2424–2429. doi: 10.1111/j.0014-3820.2003.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Heinze J, Cremer S, Eckl N, Schrempf A. Stealthy invaders: the biology of Cardiocondyla tramp ants. Insectes Sociaux. 2012;53(1):1–7. [Google Scholar]
- Heinze J, Frohschammer S, Bernadou A. Queen life-span and total reproductive success are positively associated in the ant Cardiocondyla cf. kagutsuchi. Behav Ecol Sociobiol. 2013;67(10):1555–1562. [Google Scholar]
- Heinze J, Schrempf A. Terminal investment: individual reproduction of ant queens increases with age. PLoS One. 2012;7(4):e35201. doi: 10.1371/journal.pone.0035201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Cambridge, Mass: The Belknap Press of Harvard University Press; 1990. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Kirkwood TB. Evolution of ageing. Nature. 1977;270(5635):301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Lee R. Rethinking the evolutionary theory of aging: Transfers, not births, shape social species. Proceedings of the National Academy of Sciences USA. 2003;100(16):9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandin C, Havukainen H, Kulmuni J, Dhaygude K, Trontti K, Helanterä H. Not only for egg yolk - Functional and evolutionary insights from expression, selection, and structural analyses of Formica ant vitellogenins. Molecular Biology and Evolution. 2014;31(8):2181–2193. doi: 10.1093/molbev/msu171. [DOI] [PubMed] [Google Scholar]
- Nehring V, Boomsma JJ, d’Ettorre P. Wingless virgin queens assume helper roles in Acromyrmex leaf-cutting ants. Current Biology. 2012;17(11):R671–R673. doi: 10.1016/j.cub.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Jr, Carey JR. From genes to society: Social insects as models for research on aging. Sci Aging Knowl Environ. 2004;5:pe5. doi: 10.1126/sageke.2004.5.pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Kirkman RW. Extraordinary starvation resistance in Temnothorax rugatulus (Hymenoptera, Formicidae) colonies: Demography and adaptive behavior. Insectes Sociaux. 2005;52(3):282–290. doi: 10.1007/s00040-005-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf A, Heinze J. Mating with stressed males increases the fitness of ant queens. PLoS One. 2008;3(7):e2592. doi: 10.1371/journal.pone.0002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf A, Heinze J, Cremer S. Sexual cooperation: Mating increases longevity in ant queens. Current Biology. 2005;15(3):267–270. doi: 10.1016/j.cub.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Schrempf A, von Wyschetzki K, Klein A, Schrader L, Oettler J, Heinze J. Mating with an allopatric male triggers immune response and decreases longevity of ant queens. Mol Ecol. 2015;24(14):3618–3627. doi: 10.1111/mec.13267. [DOI] [PubMed] [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proceedings of the National Academy of Sciences USA. 2006;103(4):962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1350. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Nakata K, Heinze J. Lifespan and reproduction in a queenless ant. Naturwissenschaften. 1996;83(12):577–578. [Google Scholar]
- von Wyschetski K, Rueppell O, Oettler J, Heinze J. Transcriptomic signatures mirror the lack of the fecundity / longevity trade-off in ant queens. Molecular Biology and Evolution. 2015 doi: 10.1093/molbev/msv186. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat. 1966:687–690. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.