Abstract
Globally, enterotoxigenic Escherichia coli (ETEC) is a leading cause of childhood and travelers' diarrhea, for which an effective vaccine is needed. Prevalent intestinal colonization factors (CFs) such as CFA/I fimbriae and heat-labile enterotoxin (LT) are important virulence factors and protective antigens. We tested the hypothesis that donor strand-complemented CfaE (dscCfaE), a stabilized form of the CFA/I fimbrial tip adhesin, is a protective antigen, using a lethal neonatal mouse ETEC challenge model and passive dam vaccination. For CFA/I-ETEC strain H10407, which has been extensively studied in volunteers, an inoculum of 2 × 107 bacteria resulted in 50% lethal doses (LD50) in neonatal DBA/2 mice. Vaccination of female DBA/2 mice with CFA/I fimbriae or dscCfaE, each given with a genetically attenuated LT adjuvant (LTK63) by intranasal or orogastric delivery, induced high antigen-specific serum IgG and fecal IgA titers and detectable milk IgA responses. Neonates born to and suckled by dams antenatally vaccinated with each of these four regimens showed 78 to 93% survival after a 20× LD50 challenge with H10407, compared to 100% mortality in pups from dams vaccinated with sham vaccine or LTK63 only. Crossover experiments showed that high pup survival rates after ETEC challenge were associated with suckling but not birthing from vaccinated dams, suggesting that vaccine-specific milk antibodies are protective. In corroboration, preincubation of the ETEC inoculum with antiadhesin and antifimbrial bovine colostral antibodies conferred a dose-dependent increase in pup survival after challenge. These findings indicate that the dscCfaE fimbrial tip adhesin serves as a protective passive vaccine antigen in this small animal model and merits further evaluation.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of bacterial diarrhea in countries where resources are limited. It accounts for an estimated 121,000 deaths annually, a third of which occur in children under 5 years of age (1, 2), and is the leading cause of travelers' diarrhea (3). ETEC diarrhea and resultant dehydration are due to the elaboration of fimbrial colonization factors (CFs) that facilitate small intestinal adherence and production of a heat-stable (ST) and/or heat-labile (LT) enterotoxin that promotes the loss of electrolytes and water, though the details of pathogenesis are arguably more complex (4). Among the many ETEC CFs that have been implicated in human disease, CFA/I fimbria is one of the most common and is archetypal of eight genetically related class 5 ETEC fimbriae (5, 6). CFA/I fimbriae are composed of a polymeric tract of the major subunit CfaB, arranged in a helical stalk, and the tip-localized minor subunit CfaE with adhesive properties (7, 8). Accumulated evidence indicates that CFA/I fimbriae and LT can function as protective antigens (9–14).
Due to the appreciable morbidity and mortality attributable to ETEC diarrhea, development of a vaccine has been pursued, though none are as yet licensed for use (15, 16). LT and LT-like vaccines have been shown to confer protection but are hampered by limited coverage and durability (12–14). New whole-cell live and inactivated vaccines containing both fimbriae and LT-based components are currently in clinical evaluation (17–19). Elucidation of the structure and function of CFA/I fimbriae suggests an alternative approach that targets the tip-localized adhesin to more specifically elicit antiadhesive immunity to abrogate the initial step of ETEC colonization. Toward this end, a highly stable form of CfaE has been engineered using in cis donor strand complementation (referred to here as dscCfaE) that assumes native, Ig-like domain structure and retains its adhesive function (7, 20).
Physiologically true animal models of disease for human-specific ETEC are lacking, although several imperfect models have been used to study pathogenesis and evaluate vaccines and therapeutics (21–29). A lethal neonatal mouse model has been used to study the pathogenesis of bovine ETEC and more recently to assess virulence features and test vaccines of human ETEC (30–37). In the present study, we developed a lethal neonatal mouse challenge with ETEC strain H10407, a CFA/I-ETEC challenge strain that has been extensively used in volunteer challenges (38) and then applied it to test the hypothesis that the dscCfaE fimbrial tip adhesin serves as a protective antigen. We showed that dscCfaE antibodies confer protection against lethal challenge when provided through passive transfer in maternal milk or exogenously by administration of hyperimmune antiadhesin bovine colostrum.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
ETEC strain H10407 (serotype O78:H11; CFA/I; LTSThSTp) was originally isolated from a Bangladeshi patient with severe diarrhea (39) and has been used extensively in volunteer challenge studies (10, 38). ETEC strain 258909-3 strain (serotype O128:H?; CFA/I; LTSTh), another severe diarrhea isolate from Bangladesh (40, 41) has previously been established as pathogenic in the neonatal mouse challenge model (31, 33). 258909-3M is a derivative of 258909-3 that has been cured of the plasmid that encodes CFA/I, LT, and STh (40). These strains were kindly provided by Ann Mari Svennerholm (Göteborg University, Göteborg, Sweden). For routine bacterial propagation, bacteria were grown in Luria-Bertani medium. For pup challenge, hemagglutination, and Caco-2 cell adherence assays, cultures were grown on CFA agar (42).
Preparation of CFA/I fimbriae, dscCfaE, and LTK63.
CFA/I fimbriae were purified from ETEC strain WS1933D (serotype O71:H-; CFA/I; STh), with a serotype distinct from the ETEC challenge strains used here. Purified CFA/I (lot 1096) was produced at the Walter Reed Army Institute of Research Pilot Bioproduction Facility (Silver Spring, MD) under current good manufacturing practice (cGMP) conditions. WS1933D was grown in a fermentor containing Dulbecco modified Eagle medium and Ham F-12 medium at 37°C to late logarithmic phase, and the cell pellet was harvested by continuous flow centrifugation and resuspended in phosphate-buffered saline (PBS). The suspension was treated at 62°C for 20 min and then centrifuged. The resulting supernatant was subjected to hollow fiber tangential flow microfiltration (0.22-μm filter and 500,000 molecular-weight-cutoff ultrafilters), and the filter retentate was precipitated with ammonium sulfate to a final saturation of 25%. The precipitate was diafiltered into PBS and filtered (0.22-μm-pore-size membrane) for sterilization.
Cloning and characterization of the recombinant dscCfaE fimbrial tip adhesin followed previously described procedures (20). In brief, a pET-24(a)+T7 expression plasmid was engineered to express CfaE with a C-terminal extension consisting of a short hairpin linker, followed by the N-terminal 19 amino acid residues of CfaB (CFA/I major subunit) and a hexahistidine affinity tag. Induced cultures of the expression clone were disrupted by microfluidization, and dscCfaE was purified in a two-step process of nickel affinity and cation-exchange chromatography as described previously (43).
The mucosal adjuvant, LTK63 (Ser63 to Lys), a nontoxic derivative of LT, was constructed via site-directed mutagenesis in the genetic background of the LT (LTh) produced by the H10407 strain and purified as previously described (44). LTK63 has been previously been shown to have undetectable ADP-ribosylation activity (45).
The final preparations of CFA/I, dscCfaE and LTK63 used here were shown to be in excess of 95% purity. Endotoxin removal was not required for dscCfaE preparations, whereas purified CFA/I was treated with Triton X-114 to lower the endotoxin content. The residual endotoxin contents in both antigens (CFA/I and dscCfaE) were ≤1 endotoxin unit (EU)/μg of purified protein. Contaminating lipopolysaccharide present in the LTK63 samples was removed by using Detoxi-Gel (Pierce), resulting in final preparations that contained ≤20 EU/μg of purified protein, as determined by the LAL assay (Lonza).
Immunodetection of CFA/I, dscCfaE, and LTK63 in purified fimbrial, subunit, and adjuvant preparations.
Purified CFA/I, dscCfaE, and LTK63 were separated by SDS-PAGE and stained with Coomassie blue or transferred to nitrocellulose membranes. The latter were subject to immunoblot analyses, using polyclonal anti-CFA/I, anti-CfaE, or anti-LTK63 specific antisera in the primary reaction and horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (Sigma) as the secondary detection reagent, followed by development with a SuperSignal detection kit (Pierce).
Preparation of hyperimmune anti-CFA/I and anti-CfaE bovine colostral IgG.
The two bovine colostral IgG preparations were produced by ImmuCell Corp. (Portland, ME). Pregnant cows were immunized during the dry period by the intramuscular route with a series of three doses at 3-week intervals of dscCfaE (500 μg/dose) or CFA/I (250 μg/dose), each mixed with an oil-based adjuvant. After parturition, bovine colostrum was collected and the IgG-rich whey fraction was obtained as the by-product of a standard cheese making process. The whey was further processed by pasteurization, diatomaceous earth clarification, diafiltration, heat treatment, and freeze-drying. Final preparations were milled to a semifine off-white powder. Each preparation was characterized for total protein (Kjeldahl method) and IgG content (SDS-PAGE and densitometry). Skim milk was used as a placebo preparation in the passive protection studies.
The two bovine colostral IgG preparations were tested for potency by measurement of antibody levels to the homologous antigen by enzyme-linked immunosorbent assay (ELISA) in a 96-well microtiter format. Cross-reactivity of each product to either dscCfaE or CFA/I was also measured by ELISA. Functional (antiadhesive) antibody activity was measured in a hemagglutination inhibition (HAI) assay in which the milk antibodies were tested for inhibition of CFA/I-ETEC (H10407) induced mannose-resistant hemagglutination (MRHA) of human type A erythrocytes. Activity was expressed as the lowest IgG concentration (μg/ml) that completely inhibited MRHA.
Vaccination regimens.
Mice were supplied by the Isogenic Mouse Breeding Facility of the Department of Immunology, Biomedical Sciences Institute (ICB), University of São Paulo (USP), and all procedures of this study followed the ethical principles for animal experimentation adopted by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee on Animal Experiments of the Institute of Biomedical Sciences (protocol 106:36v2), University of São Paulo. Groups of 10 female DBA/2 mice ranging from 6 to 8 weeks old were subjected to light anesthesia (75 mg/kg ketamine [10%] plus 10 mg/kg xylazine [2%]) for inoculation via the intranasal (i.n.) route at a volume of 10 μl administered dropwise to the external nares of each mouse using a 2- to 20-μl Pipetteman (Rainin Instrument). Vaccination by the orogastric (o.g.) route was performed with a stainless-steel round tip gavage cannula with a total volume of 500 μl. Sham-vaccinated mice had with the same volumes of PBS administered. CFA/I or dscCfaE were administered at doses of 25 μg for i.n. vaccine regimens, or 125 μg for o.g. vaccination, combined or not with LTK63 adjuvant (10 μg for both administration routes). The vaccination regimen consisted of three doses given at 2-week intervals. Bleeds from the retro-orbital plexus were performed 1 day before each inoculation and 2 weeks after the last dose. One week before the last dose, male DBA/2 mice were introduced into the cages of the vaccinated females for a maximum period of 1 week. Two weeks after the last dose, sera and fecal samples were collected. Fecal samples were collected for one night and per mouse group subjected to the same immunization procedure. Fecal pellets were freeze-dried and stored at −20°C. Fifteen pellets (∼0.6 g) were homogenized in PBS and centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants were collected to detect LTK63-, CFA/I-, or CfaE-specific IgA by ELISA. Milk samples were surgically removed from the stomachs of 6- to 7-day-old suckling mice. The stomach contents were removed, homogenized with 2 volumes of PBS per sample weight, and centrifuged at 10,000 × g for 10 min. The supernatants were collected and kept frozen at −20°C until measurement of the LTK63-, CFA/I-, or CfaE-specific IgA.
ELISA.
LTK63-specific serum IgG and mucosal IgA titers were determined by GM1-ELISA (44), using purified LTK63 as GM1-binding molecule, whereas anti-CFA/I and CfaE antibodies levels were evaluated by conventional ELISA, using plates coated with CFA/I fimbriae or dscCfaE (32, 33). Serum anti-LTK63 and anti-CFA/I and anti-CfaE IgG (total IgG and IgG1/IgG2a subclasses) titers were measured with a horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (Sigma) diluted to 1:3,000. Titers of LTK63-, CFA/I-, or CfaE-specific IgA antibodies in fecal and milk extract were determined using horseradish peroxidase-conjugated goat anti-mouse IgA (Sigma). The assays were conducted using duplicate samples and repeated at least three times. The results are expressed as titers and are defined as the highest sample dilution with an A492 of ≥0.2 compared to preimmune sera or fecal or milk extracts.
Inhibition of ETEC adherence to Caco-2 cells and HAI.
Polarized Caco-2 cell adherence assays were performed as described previously (32) with minor modifications. Briefly, Caco-2 cells were maintained in Dulbecco modified Eagle high-glucose medium (DMEM high glucose) supplemented with gentamicin (100 μg/ml), kanamycin (50 μg/ml), l-glutamine (2 mM), 100 μM minimum essential medium nonessential amino acid solution (Invitrogen), and 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. Cells were seeded in 24-well plates (Costar), loaded with tissue culture-treated glass coverslips (Fisher Scientific), incubated for 14 days (±1 day) to confluence and to reach for differentiation phenotype, washed with PBS, and covered with 750 μl of the supplemented DMEM high glucose prior to the assay. Bacterial strains were grown on CFA agar overnight at 37°C and suspended to 109 bacteria/ml in supplemented DMEM high glucose with 1% (wt/vol) d-mannose. The suspension was incubated with serum pools collected from vaccinated mice (at a normalized concentration corresponding to a final reciprocal titer of 400 with the tested antigen) and then added to the tissue culture wells at a final concentration of 107 bacteria/ml. The plates were incubated for 3 h and then washed, fixed, stained, and mounted as described previously (6) and were then observed microscopically. Incubation with the different polyclonal sera did not reduce viability of the bacterial cells. The number of adherent bacteria in 100 randomly selected cells was determined and is represented both as the percentage of the average number of cells with at least one adherent bacterium and as the number of adherent bacteria per cell with at least one adherent bacteria. For each serum sample, a minimum of three experiments was performed in duplicate to determine the adherence indices, and the results were expressed as the means ± the standard deviations (SD). HAI was performed at room temperature for 30 min in humidified chambers with aliquots (20 μl) of a 3% suspension of human group A erythrocytes that had been washed in PBS and added to bacteria previously incubated with the diluted serum samples. Twofold serial dilutions of the tested serum samples were incubated with 107 cells of the ETEC strain H10407 and incubated at room temperature for 1 h before admixing with the red blood cells. The results were evaluated by visual inspection. The maximal dilutions inhibiting the hemagglutination reaction were recorded. d-Mannose was added at a concentration of 1% (wt/vol) to the dilution buffer (PBS) to avoid binding mediated by type 1 fimbriae. The test was repeated independently at least three times.
Neonatal mouse lethal ETEC challenge model.
The neonatal mouse ETEC challenge model was described by Duchet-Suchaux et al. (36, 46) and was subsequently adapted to test the protective passive immunity conferred by CFA/I-based vaccines (31–33). In keeping with a prior report, neonatal CBA mice were found to be the most susceptible of several inbred mouse strains to intragastric CFA/I-ETEC challenge in screening experiments, but high rates of cannibalism after CBA pup handling confounded outcomes (unpublished observations). Hence, the DBA/2 mouse strain was selected for use here based on moderate susceptibility to CFA/I-ETEC challenge and more docile maternal behavior with a lower associated incidence of cannibalism.
One- or two-day-old DBA/2 mouse pups were challenged by direct intragastric inoculation with different bacterial loads using disposable syringes with ultrathin needles for insulin administration (needle size, 12.7 by 0.33 mm; BD). Inoculation of the bacterial strain was facilitated by the presence of previously ingested milk that had accumulated in the stomachs of newborn mice. The 50% lethal dose (LD50) was determined after inoculating neonatal mice with different bacterial loads ranging from 105 to 109 CFU. For challenge experiments with vaccinated dams, a bacterial load of 4 × 108 CFU, corresponding to approximately 20× LD50 for ETEC strain H10407, was routinely used. Survival was monitored for a period of 8 days. Only pups dying >24 h after ETEC challenge were considered in calculating survival because deaths associated with manipulation occur up to 24 h after handling. Deaths associated with ETEC challenge were recorded from days 1 to 8 postchallenge. Litters from 8 to 10 dams receiving each vaccination regimen were used in the challenge experiments. Because the number of pups per litter varied from 4 to 6, the final numbers of challenged newborns ranged from 11 to 32 for each tested condition. Sixteen pups were challenged with ETEC H10407 (20× LD50) and, after 48 to 72 h, were euthanized to collect systemic blood used in blood culture and PCR assays. Blood samples (100 μl) were poured on MacConkey agar plates and incubated at 37°C overnight. The presence of ETEC was screened by PCR amplification of the genes cfaB (with the primers CFA5Fw [5′-GAGGAAAGATCTATGGAGAAAAATATTACTGTAACAG] and CFA3Rv [5′-CGACGTCTCTAGAACTGGATCCCAAAGTCATTACAA]) and eltB (with the primers ELTBFw [5′ TCT ATG TAG ATC TAT GGC TCC TCA GTC TAT TAC AGA 3′] and ELTB2Rv [5′-TTT TAA TTC TAG ATT AGT TTT CCA TAC TGA TTG CCG C-3′]).
Passive protection conferred by bovine colostral IgG preparations.
Bacterial cells cultivated on CFA plates for 18 h at 37°C were gently suspended in pyrogen-free physiological saline to a final concentration of 109 CFU/ml. Bacterial cell aliquots containing 2 × 107 CFU, corresponding to approximately 20× LD50 for ETEC strains 258909-3 (31–33), were mixed with bovine colostral IgG preparations. The mixtures were incubated at room temperature for 1 h and then inoculated (in a volume of 70 μl) intragastrically into neonatal mice born to nonimmunized dams. As a negative control, we used the 258909-3M strain (mETEC). Newborns that died between 24 h and 8 days after the lethal challenge were considered in the determination of mortality rates.
Statistical analyses.
The results were analyzed with GraphPad Prism 5 software. The statistical significance for the Kaplan-Meier survival curve was calculated with a Mantel-Cox test. The significance of neonatal passive protective efficacy was determined in dam vaccination, crossover, and bovine colostral IgG preincubation experiments by applying a two-tailed Fisher exact test comparing survival rates between each treatment group and the corresponding control group. P values of <0.05 were considered statistically significant. Comparisons between immunized groups were by one-way analysis of variance (ANOVA) with Bonferroni's multiple-comparison test, and significance was assessed at P values of <0.05, <0.01, or <0.001 throughout the study.
RESULTS
Identity and purity of vaccine components.
Examination of the protein vaccine antigens and adjuvant used in the present study was carried out by SDS-PAGE and Western blot analyses, confirming their identity and purity (see Fig. S1 in the supplemental material).
Induction of systemic and secreted antibody responses in vaccinated mice.
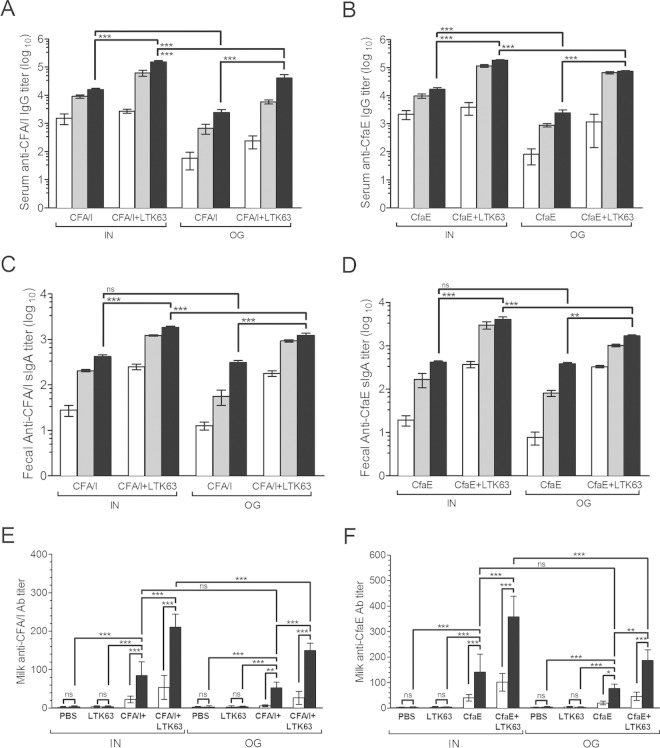
Female DBA/2 mice immunized via one of two mucosal (i.e., orogastric [o.g.] or intranasal [i.n.]) routes with CFA/I fimbriae or dscCfaE developed high vaccine-specific antibody responses that were detected in both serum (IgG) and feces (IgA) starting after a single vaccine dose (Fig. 1A to D). Further increases in antibody titers were detected following a second and third dose. Coadministration of LTK63 with each vaccine augmented anti-CFA/I and anti-CfaE serum IgG and fecal IgA titers. Mice vaccinated via the i.n. route mounted higher vaccine-specific anti-CFA/I and anti-CfaE serum responses compared to mice immunized via the o.g. route, while these differences were only detected in the vaccine-specific fecal IgA responses when the mice received LTK63 as adjuvant (Fig. 1A to D). Antibodies elicited in mice vaccinated with dscCfaE cross-reacted with CFA/I fimbriae and vice versa (see Fig. S2A to D in the supplemental material), explainable by the composition of CFA/I fimbriae. LTK63-specific antibody responses in serum (IgG) and feces (IgA) were also detected in mice receiving LTK63, and higher titers were achieved after i.n. vaccination (see Fig. S3A and B in the supplemental material).
FIG 1.
CFA/I- and CfaE-specific antibody responses elicited in female DBA/2 mice exposed to the vaccine regimen. Serum IgG (A and B) and fecal IgA (C and D) responses were measured in samples collected from mouse groups vaccinated via the i.n. or o.g. route with three doses of CFA/I fimbriae (A and C) or dscCfaE (B and D) admixed or not with LTK63. (A to D) Blood and fecal samples were collected 2 weeks after the first (□), second (), and third (■) vaccinations. (E and F) Gastric samples of milk were collected from suckling pups born from dams vaccinated via the i.n. or o.g. route with CFA/I fimbriae (E) or dscCfaE (F) and pooled before testing (IgG, □; IgA, ■). Experiments to determine the CFA/I- and CfaE-specific antibody responses were independently performed between three and six times. Serum samples were processed and tested individually. Values represent means of endpoint titers ± the SD of three independent measurements. Statistically significant differences are indicated with brackets (one-way ANOVA with Bonferroni's test). P values are indicated by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The serum IgG subclass response pattern detected in vaccinated mice indicated a predominant IgG1 response with IgG1/IgG2a ratios ranging from 2.2 (mice vaccinated via the o.g. route with CFA/I+LTK63) to 39.8 (mice vaccinated via the i.n. route with CFA/I) (see Fig. S2E and F in the supplemental material). Notably, mice immunized via the i.n. route showed a tendency to develop a more type 2-biased immune response pattern, with higher antigen-specific IgG1 titers, than mice immunized via the o.g. route.
Vaccine-specific IgA antibodies were detected in milk of vaccinated dams, as determined from gastric milk samples collected from suckling mice, while in milk samples of sham-immunized dams or dams immunized with LTK63 alone, only trace amounts of vaccine-specific antibodies were measured (Fig. 1E and F). In contrast, low or no significant amounts of vaccine-specific IgG were detected in the milk samples of vaccinated dams. Similar results were also found after measurement of LTK63-specific milk IgA responses (see Fig. S3C in the supplemental material).
Anticolonization effects of antibodies generated in mice vaccinated with CFA/I or dscCfaE.
An effective ETEC vaccine should elicit antibodies that can block CFA/I-mediated adhesion of bacteria to host cell receptors. To evaluate the adhesion-neutralization effect of antibodies raised in mice vaccinated with CFA/I or dscCfaE, serum samples collected from vaccinated mice were tested in a hemagglutination inhibition assay (HAI) using the ETEC strain H10407 and human red blood cells. Similar tests were conducted with Caco-2 cells using bacteria that were preincubated with immune serum samples. As indicated in Table 1, sera from mice immunized via the i.n. route with CFA/I or dscCfaE plus LTK63 adjuvant showed higher HAI titers than sera from mice immunized via the o.g. route with the same vaccine formulations. Indeed, the ratios between the HAI titer and corresponding vaccine-specific IgG titer were approximately 2- to 3-fold higher among mice vaccinated via i.n. delivery than among mice vaccinated via the o.g. route. When a fixed dilution of immune sera was tested for the inhibition of binding to Caco-2 cells, the anti-CFA/I and anti-CfaE sera showed adherence inhibition effects, and no significant differences were measured between serum samples collected from mice immunized via different administration routes (Table 1 and see Fig. S4 in the supplemental material).
TABLE 1.
Antiadhesive effects (HAI and Caco-2 binding inhibition activity) of serum samples from mice after different vaccination regimens
| Test seruma | Titerb |
Mean ± SD |
||
|---|---|---|---|---|
| IgG ELISA | HAI | Caco-2 cell binding (%)c | No. of bacteria/celld | |
| Non-immune serum | — | — | 95.3 ± 2.7 | 24.0 ± 5 |
| α-CFA/I+LTK63 (IN) | 153,374 | 16,384 (0.10) | 28.3 ± 4.7 | 5.0 ± 2.7 |
| α-CfaE+LTK63 (IN) | 183,584 | 32,768 (0.17) | 13.7 ± 2.3 | 3.5 ± 1.7 |
| α-CFA/I+LTK63 (OG) | 40,402 | 2,048 (0.05) | 35.7 ± 5.3 | 5.0 ± 2.3 |
| α-CfaE+LTK63 (OG) | 73,470 | 4,096 (0.05) | 27.0 ± 2 | 4.2 ± 2.7 |
| α-LTK63 (IN) | — | — | 93.3 ± 2.7 | 25.0 ± 2.7 |
| α-LTK63 (OG) | — | — | 92.7 ± 4.3 | 22.7 ± 4.3 |
Pooled serum samples were tested independently at least three times, and each experiment was performed in duplicate.
The antigen-specific (CFA/I or dscCfaE) IgG titers of serum pools were determined by ELISA using the corresponding homologous antigen. Hemagglutination inhibition (HAI) titers are expressed as the reciprocal of the highest serum dilution that inhibited mannose-resistant hemagglutination (MRHA). Numbers in parentheses represent the HAI titer/total IgG titer ratio for each tested vaccine formulation. —, below limit of detection.
Values are expressed as percentages of Caco-2 cells with at least one adherent bacterial cell. Tests were performed using H10407 incubated with serum pools diluted to a final concentration corresponding to a reciprocal titer of approximately 400.
Values are expressed as mean numbers of bacteria bound per Caco-2 cell.
Dam immunization confers passive protection to neonates.
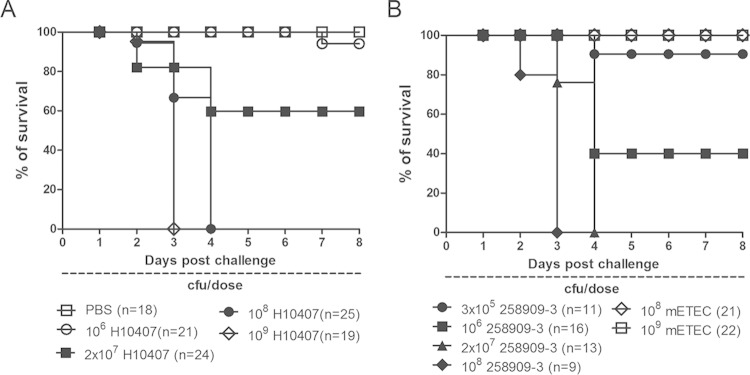
Prerequisite to passive protection studies, the LD50s of CFA/I-ETEC strains H10407 and 258909-3 were defined. By interpolation from pup survival rates in groups that received intragastric challenge with each strain over a several log range of doses, the LD50 for H10407 was found to be approximately 2 × 107 CFU, and that for 258909-3 was approximately 1 × 106 CFU (Fig. 2), indicating similar virulence levels in this model. By comparison, 100% of pups survived challenge with the virulence plasmid-cured derivative of 258909-3 (258909-3M; CFA/I−, enterotoxin negative) at doses of 108 to 109 CFU, which was 10 to 100 times higher than the lowest inoculum of 258909-3 that resulted in 100% mortality (Fig. 2B). The absence of bacteremia in newborns challenged with 20× LD50 of H10407 was ascertained by blood culture and PCR analyses for ETEC genes, using blood of pups sacrificed 48 to 72 h after the challenge. Here, in the passive vaccination-challenge studies, an inoculum corresponding to 20× LD50 for H10407 (4 × 108 CFU) or 258909-3 (2 × 107 CFU) was used in pup challenges.
FIG 2.
Determination of the survival curves of newborn DBA/2 mice after challenge with ETEC strains H10407 and 258909-3. Different numbers of viable cells of H10407 (A), 258909-3 (B), and 258909-3M (mETEC) (B) were inoculated into the milk filled stomachs of neonatal mice up to 48 h after birth. The y axis values correspond to the percentage of surviving animals over an observation period of 7 days. Deaths recorded within the first 24 h after the challenge were not included in the results.
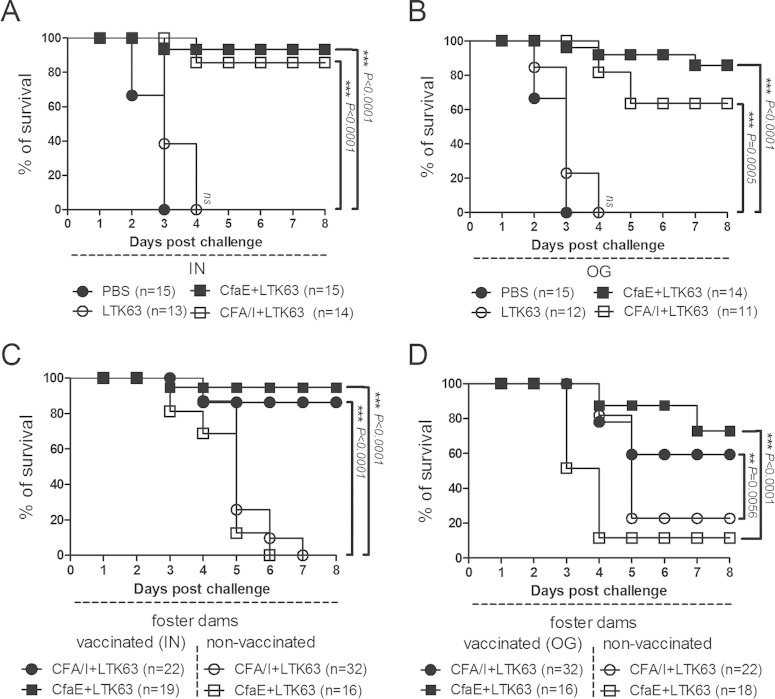
Neonatal mice born from dams that were vaccinated i.n. with adjuvanted CFA/I fimbriae or dscCfaE showed more than 85% survival after 20× LD50 challenge with H10407, while neonates born from dams vaccinated by the o.g. route with CFA/I or dscCfaE admixed with LTK63 showed survivals of 63 and 85%, respectively (Fig. 3A and B). In contrast, no significant protection was observed in neonates born from dams immunized with just LTK63 when challenged with H10407 (Fig. 3A and B). The attribution of significant neonatal survival advantage to maternal mucosal vaccination with dscCfaE or CFA/I prompted further study to define the contribution of transplacental versus lacteal antibodies.
FIG 3.
Mucosal immunization of female DBA/2 mice confers passive protection to newborns to lethal challenge with the CFA/I-ETEC strain H10407. (A and B) Survival curves of newborn mice challenged with 4 × 108 CFU (20× LD50) of H10407 and born from dams vaccinated via the i.n. (A) or o.g. (B) route. Dams were mated during the vaccination period and allowed to feed their offspring during the observation period. (C and D) Neonatal mice born from nonvaccinated dams were transferred to foster dams vaccinated with different immunization regimens, and neonatal mice born to vaccinated dams were transferred to nonvaccinated dams, as indicated in the figure. Pup survival was monitored over a total period of 8 days. Mice that died in the first 24 h after challenge were not included in the results. The numbers of animals challenged in each vaccination group are indicated (in parentheses) at the bottom of the panels. P values were calculated by two-tailed Fisher exact test comparing survival rate to the referent PBS-treated control group (PBS) (A and B) or between groups fostered by vaccinated (closed symbols) or nonvaccinated (open symbols) dams treated with the same vaccine formulation (C and D). P values are indicated for each comparison by asterisks (*, P < 0.05; **, P < 0.001; ***, P < 0.001). In addition, a Mantel-Cox test was applied to the same groups (not indicated in the figures), taking into account the complete curves leading to a P value of <0.0001.
In crossover experiments, litters born from nonvaccinated dams experienced survival rates ranging from 62 to 96% when fed by foster dams vaccinated with CFA/I plus LTK63 (o.g.) or dscCfaE plus LTK63 (i.n.), respectively (Fig. 3C and D). In contrast, irrespective of maternal vaccination regimen, litters born to vaccinated dams but suckled by nonvaccinated dams showed survival rates of ≤20% after 20× LD50 challenge with H10407 (Fig. 3C and D). These differences were statistically significant and indicate that the protective immunity acquired by neonatal mice involves the passive lacteal route. In agreement, relatively low anti-CFA/I, anti-CfaE, or anti-LTK63 IgG titers were detected in sera of the offspring of vaccinated dams (see Fig. S5 in the supplemental material). In addition, gastric milk samples from pups fed by vaccinated dams had elevated levels of CFA/I and CfaE-specific IgA antibodies (Fig. 1E and F). Similarly, anti-LTK63 milk IgA levels were elevated in all groups receiving LTK63 (see Fig. S3C in the supplemental material).
Characterization of anti-CFA/I and anti-CfaE bovine colostral IgG preparations and passive protection studies.
Bovine hyperimmune anti-CfaE and anti-CFA/I colostral antibody preparations were produced, which were rich in IgG (37 and 43% IgG by weight, respectively) and exhibited high antigen-specific antibody titers (see Fig. S6). When compared in the HAI assay, the lowest concentration of the anti-CfaE (12 μg/ml) and anti-CFA/I (12 μg/ml) bovine colostral IgG (bIgG) preparations that completely inhibited human erythrocyte MRHA was identical.
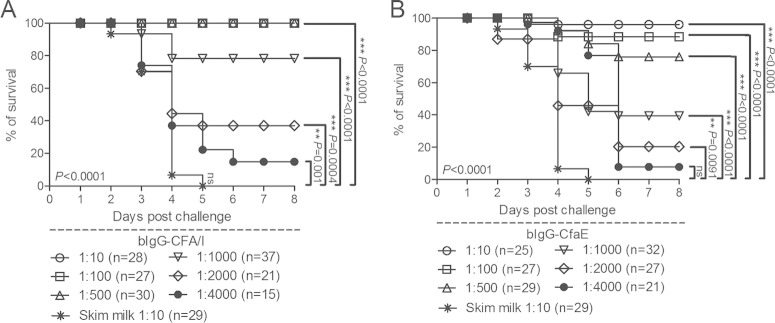
Different dilutions of both the anti-CFA/I and the anti-CfaE bIgG preparations (ranging from 5 to 0.001 mg/dose) were mixed with a 20× LD50 of CFA/I-ETEC strain 258909-3 bacteria before intragastric inoculation into neonatal mice born to nonvaccinated dams. Each preparation was associated with a dose-dependent improvement in neonatal survival after ETEC challenge, conferring ≥76% survival at amounts at or above 0.005 mg (corresponding to 1:1,000 dilution) for the anti-CFA/I preparation, and at or above 0.010 mg (corresponding to 1:500 dilution) for the anti-CfaE preparation (Fig. 4).
FIG 4.
Anti-CFA/I and anti-CfaE bovine colostral IgG preparations protected newborn mice against a lethal challenge with the CFA/I-ETEC strain 258909-3. Groups of DBA/2 mice were inoculated with 2 × 107 CFU (20× LD50 for 258909-3) previously incubated with different amounts of the anti-CFA/I (A) or the anti-CfaE (B) bIgG preparation. The survival curves were established over a period of 1 week. Deaths recorded in the first 24 h after the challenges were not considered. The number of pups used to test each bIgG preparation ranged from 21 to 37. P values were calculated by two-tailed Fisher exact test in which the last values of the survival curves were compared to those corresponding to the reference group (skim milk) and are indicated by asterisks (*, P < 0.05; **, P < 0.001; ***, P < 0.001). In addition, a Mantel-Cox test was applied to the same groups (not indicated in the figures), taking into account the complete curves leading to a P value of <0.0001.
DISCUSSION
As greater attention is focused on the development of human vaccines against ETEC, it has exposed the need for improved animal models for vetting new vaccine candidates. This is particularly the case for vaccines directed against human CFs, since these factors are species specific and are not evaluable in native animal ETEC disease models (47, 48). Here, the lethal neonatal mouse challenge model was used to test a novel, prototype ETEC fimbrial tip adhesin vaccine. We first expanded the model by establishing the conditions for lethal challenge with a second CFA/I-ETEC strain, H10407, and then demonstrated the efficacy of dam vaccination with CFA/I fimbriae or its tip adhesin dscCfaE in preventing suckling pup mortality following H10407 challenge. Findings from crossover experiments implicated passive lacteal immunity in protection. In concordance with these findings, a dose-dependent improvement in pup survival was observed following preincubation of hyperimmune antifimbrial (CFA/I) or antiadhesin (dscCfaE) bovine colostral IgG with the inoculum of a second previously established CFA/I-ETEC challenge strain before challenge of pups from nonimmunized dams.
To adequately test the hypothesis that the CFA/I fimbrial tip adhesin functions as a protective antigen in the neonatal mouse model, it was important to first define regimens for dam vaccination that elicited solid vaccine-specific antibody responses in both the systemic and mucosal compartments. When administered to adult female mice by i.n. or o.g. vaccination, CFA/I and dscCfaE stimulated significant vaccine-specific serum IgG and fecal IgA responses, which were potentiated by coadministration of the LTK63 adjuvant. In the adjuvanted groups, fimbria- and adhesin-specific serum and fecal antibody responses were significantly different between the two routes of administration. The functionality of serum antibodies elicited by CFA/I and dscCfaE vaccination (each with LTK63) was apparent from their inhibitory effects in an HAI assay and on H10407 binding to Caco-2 cells in tissue culture. Notably, administration of the vaccine formulations by the i.n. route consistently resulted in higher antigen-specific IgG and IgA responses compared to animals immunized via the o.g. route. The superiority of immune responses after i.n. administration of 5-fold lower doses of adhesin or fimbriae may be explained in part by more efficient vaccine uptake in the nasopharynx and the avoidance of exposure to acid hydrolysis and enzymatic degradation on gastrointestinal transit after o.g. administration.
Based on the robustness of induced immune responses, four different dam vaccination regimens, CFA/I+LTK63 by i.n. and o.g. vaccination and dscCfaE+LTK63 vaccination by the same two routes of delivery, were carried forward into subsequent dam vaccination-pup challenge experiments. Indeed, pups produced and suckled by dams vaccinated antenatally with each of these four regimens experienced a significant survival advantage over pups from sham-immunized dams after H10407 challenge. Concurrently, we also showed that vaccine-specific IgA titers were detectable in ingested milk samples after dam vaccination with CFA/I+LTK63 and dscCfaE+LTK63. In crossover experiments, pups born to nonimmunized dams and suckled by dams immunized with each of these four regimens experienced higher survival rates after H10407 challenge than did pups born to dams receiving the corresponding vaccination regimen and suckled by nonimmunized dams. This fits with our understanding that newborn mice primarily acquire maternal antibodies in the postnatal period by absorption from colostrum and milk, while prenatal transplacental transfer of antibodies plays a minor role (49). Lastly, preincubation of CFA/I-ETEC strain 258909-3 with anti-CFA/I and anti-CfaE bovine colostral IgG over a range of concentrations prior to use in intragastric challenge of pups resulted in a dose-dependent increase in survival compared to controls in which the inoculum was incubated with skim milk. Its use here provided a bridge back to previous neonatal mouse challenge studies where this strain, distinct from H10407 in its O:H serotype, had been used to test CfaB-based ETEC vaccines (31–33).
The weight of evidence presented here supports the hypothesis that dscCfaE functions as a protective antigen in the neonatal mouse challenge model. Establishment of challenge conditions and the LD50 for H10407 enabled the incorporation of this CFA/I-ETEC strain into the dam vaccination-pup challenge experiments, in harmonization with volunteer studies in which H10407 has been widely used (38). Demonstration of the protective efficacy of antifimbrial antibodies in the neonatal mouse model is concordant with a prior neonatal mouse study (32) and, more importantly, with a volunteer study in which hyperimmune anti-CFA/I bovine milk IgG conferred passive protection against challenge with H10407 (10), lending credibility to the innovative finding that dscCfaE vaccination also conferred passive protection in the neonatal mouse challenge model. It is important to note that dam vaccination with LTK63 alone by either i.n. or o.g. routes did not confer any survival benefit to suckling pups, indicating that protection in this model was specifically attributable to antifimbrial and antiadhesin antibodies. The absence of protection attributable to LTK63 as shown here contrasts with vaccine field trial findings showing that LT and the related cholera toxin B-subunit (CTB) were associated with short-term, partial protection against ETEC (12–14). Possible explanations for this incongruity include the use here of an overwhelming challenge dose (20× LD50) and the fact that H10407 expresses two ST toxins (STh and STp) in addition to LT.
It is worth considering some limitations of the studies presented herein. First, more specific, corroborative evidence for the role of CFA/I fimbriae in disease and death of neonatal mice might have been obtained by comparative challenge with an isogenic CFA/I− mutant of H10407, an experiment precluded by our lack of such a strain. However, the nonlethality of a plasmid-cured, CFA/I− and enterotoxin-negative derivative of wild-type strain 258909-3 suggests that intestinal adherence and/or enterotoxicity contributes to virulence in this model. Second, a relatively high inoculum was required to achieve lethality following neonatal DBA/2 mouse challenge with these human ETEC strains. The LD50 of both 258909-3 and H10407 (106 to 107 CFU) is several logs higher than that for lethal neonatal mice challenge models with bovine and porcine ETEC (30, 37, 46, 50), and other inbred mouse strains such as CBA may offer higher pup susceptibility at lower doses of H10407 (36). We found, however, that maternal aggression and high rates of pup cannibalism limited the utility of the CBA mouse strain in the evaluation of passive protective neonatal immunity (unpublished observations). Lastly, the cause of pup death after CFA/I-ETEC challenge was not explicitly investigated, other than ruling out bacteremia as a contributory factor. Although pathophysiological studies indicated that death following outbred murine pup challenge with porcine ETEC strains is attributable to diarrhea and hemodynamic instability (30), similar evidence in the context of our model with DBA/2 mice and CFA/I-ETEC challenge must be gathered to reinforce its relevance to human disease.
In prior studies of an infant mouse colonization model with outbred ICR mice, H10407 more efficiently colonized the intestine than H10407P, a derivative strain missing the large, CFA/I-encoding virulence plasmid, suggesting that CFA/I plays a role in mouse intestinal colonization (35). It has also been shown that dam vaccination with CfaB, the major subunit of CFA/I fimbriae, using DNA vaccine priming and boosting with CfaB-expressing live vaccines, improved the survival of suckling mice (31, 33). These findings, together with those presented here, imply that CFA/I-mediated adherence to the intestinal mucosa is a necessary step in the disease process leading to death and is impeded by antifimbrial and antiadhesin antibodies.
The relevance of the findings presented herein to human ETEC diarrhea and prevention, particularly as it relates to the protective role of CfaE, remains to be established. There is convincing evidence indicating that CFA/I fimbriae can serve as a protective antigen (9–11), and further proof-of-principle is awaited from clinical studies of CFA/I-containing live and inactivated whole-cell ETEC vaccines currently in development (17–19). In vitro studies indicate that CfaE plays an essential role in binding of CFA/I-ETEC to intestinal cells (6, 51). The findings reported here further suggest that dscCfaE, a highly stable, conformationally intact variant of the CFA/I fimbrial tip adhesin, can elicit antibodies that confer passive protection in the neonatal mouse challenge model. A forthcoming passive oral immunoprophylaxis study in volunteers with hyperimmune antiadhesin (CfaE) bovine colostral antibodies will clarify the clinical relevance of our findings in this small animal model.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grant 10/52167-8) to L.C.S.F. and grants from the U.S. Office of Naval Research (A0706) to S.J.S. and Peer Reviewed Medical Research Program (PR0033239) to S.J.S.
We acknowledge the helpful technical assistance of E. Gimenes, L. C. da Silva, and M. R. de Jesus, as well as F. Cassels and S. Poole, for the production of CFA/I fimbriae and dscCfaE, respectively.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. S.J.S. is a military service member, and this work was prepared as part of his official duties. Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00858-15.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Steffen R, Hill DR, DuPont HL. 2015. Traveler's diarrhea: a clinical review. JAMA 313:71–80. doi: 10.1001/jama.2014.17006. [DOI] [PubMed] [Google Scholar]
- 4.Fleckenstein JM, Hardwidge PR, Munson GP, Rasko DA, Sommerfelt H, Steinsland H. 2010. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect 12:89–98. doi: 10.1016/j.micinf.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaastra W, Sommerfelt H, van Dijk L, Kusters JG, Svennerholm AM, Grewal HM. 2002. Antigenic variation within the subunit protein of members of the colonization factor antigen I group of fimbrial proteins in human enterotoxigenic Escherichia coli. Int J Med Microbiol 292:43–50. doi: 10.1078/1438-4221-00189. [DOI] [PubMed] [Google Scholar]
- 6.Anantha RP, McVeigh AL, Lee LH, Agnew MK, Cassels FJ, Scott DA, Whittam TS, Savarino SJ. 2004. Evolutionary and functional relationships of CFA/I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect Immun 72:7190–7201. doi: 10.1128/IAI.72.12.7190-7201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YF, Poole S, Rasulova F, McVeigh AL, Savarino SJ, Xia D. 2007. A receptor-binding site as revealed by the crystal structure of CfaE, the colonization factor antigen I fimbrial adhesin of enterotoxigenic Escherichia coli. J Biol Chem 282:23970–23980. doi: 10.1074/jbc.M700921200. [DOI] [PubMed] [Google Scholar]
- 8.Li YF, Poole S, Nishio K, Jang K, Rasulova F, McVeigh A, Savarino SJ, Xia D, Bullitt E. 2009. Structure of CFA/I fimbriae from enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A 106:10793–10798. doi: 10.1073/pnas.0812843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DJ Jr, Evans DG, Opekun AR, Graham DY. 1988. Immunoprotective oral whole-cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiol Immunol 1:9–18. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DJ, Tacket CO, Delehanty A, Maneval DR, Nataro J, Crabb JH. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J Infect Dis 177:662–667. doi: 10.1086/514227. [DOI] [PubMed] [Google Scholar]
- 11.Rao MR, Wierzba TF, Savarino SJ, Abu-Elyazeed R, El-Ghoreb N, Hall ER, Naficy A, Abdel-Messih I, Frenck RW Jr, Svennerholm AM, Clemens JD. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J Infect Dis 191:562–570. doi: 10.1086/427662. [DOI] [PubMed] [Google Scholar]
- 12.Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, Huda N, Khan MU, Kay BA, Khan MR, Ansaruzzaman M, Yunus M, Rao MR, Svennerholm AM, Holmgren J. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis 158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 13.Peltola H, Siitonen A, Kyronseppa H, Simula I, Mattila L, Oksanen P, Kataja MJ, Cadoz M. 1991. Prevention of travellers' diarrhoea by oral B-subunit/whole-cell cholera vaccine. Lancet 338:1285–1289. doi: 10.1016/0140-6736(91)92590-X. [DOI] [PubMed] [Google Scholar]
- 14.Behrens RH, Cramer JP, Jelinek T, Shaw H, von Sonnenburg F, Wilbraham D, Weinke T, Bell DJ, Asturias E, Pauwells HL, Maxwell R, Paredes-Paredes M, Glenn GM, Dewasthaly S, Stablein DM, Jiang ZD, DuPont HL. 2014. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers' diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 14:197–204. doi: 10.1016/S1473-3099(13)70297-4. [DOI] [PubMed] [Google Scholar]
- 15.Walker RI. 2015. An assessment of enterotoxigenic Escherichia coli and shigella vaccine candidates for infants and children. Vaccine 33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. 2013. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Syst Rev 7:CD009029. doi: 10.1002/14651858.CD009029.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. 2012. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 19:1921–1931. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren A, Leach S, Tobias J, Carlin N, Gustafsson B, Jertborn M, Bourgeois L, Walker R, Holmgren J, Svennerholm AM. 2013. Clinical trial to evaluate safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli prototype vaccine containing CFA/I overexpressing bacteria and recombinantly produced LTB/CTB hybrid protein. Vaccine 31:1163–1170. doi: 10.1016/j.vaccine.2012.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, Holmgren J, Petzold M, Walker R, Svennerholm AM. 2014. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine 32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 20.Poole ST, McVeigh AL, Anantha RP, Lee LH, Akay YM, Pontzer EA, Scott DA, Bullitt E, Savarino SJ. 2007. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol Microbiol 63:1372–1384. doi: 10.1111/j.1365-2958.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 21.Wadstrom T. 1984. Streptococcus faecium M 74 in control of diarrhoea induced by a human enterotoxigenic Escherichia coli strain in an infant rabbit model. Zentralbl Bakteriol Mikrobiol Hyg A 257:357–363. [PubMed] [Google Scholar]
- 22.Svennerholm AM, Wenneras C, Holmgren J, McConnell MM, Rowe B. 1990. Roles of different coli surface antigens of colonization factor antigen II in colonization by and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect Immun 58:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spira WM, Sack RB, Froehlich JL. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun 32:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mynott TL, Chandler DS, Luke RK. 1991. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect Immun 59:3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Vidal Y, Ahren C, Svennerholm AM. 1987. Colonization, diarrhoea and protective immunogenicity of a CFA-deficient, enterotoxin-producing Escherichia coli mutant in a non-ligated intestine experimental model. Acta Pathol Microbiol Immunol Scand B 95:123–130. [DOI] [PubMed] [Google Scholar]
- 26.Klipstein FA, Engert RF, Clements JD. 1981. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect Immun 34:637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans DG, Silver RP, Evans DJ Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun 12:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrd W, Mog SR, Cassels FJ. 2003. Pathogenicity and immune response measured in mice following intranasal challenge with enterotoxigenic Escherichia coli strains H10407 and B7A. Infect Immun 71:13–21. doi: 10.1128/IAI.71.1.13-21.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen KP, Randolph MM, Fleckenstein JM. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun 74:869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newsome PM, Burgess MN, Burgess MR, Coney KA, Goddard ME, Morris JA. 1987. A model of acute infectious neonatal diarrhoea. J Med Microbiol 23:19–28. doi: 10.1099/00222615-23-1-19. [DOI] [PubMed] [Google Scholar]
- 31.Luiz WB, Cavalcante RC, Paccez JD, Souza RD, Sbrogio-Almeida ME, Ferreira RC, Ferreira LC. 2008. Boosting systemic and secreted antibody responses in mice orally immunized with recombinant Bacillus subtilis strains following parenteral priming with a DNA vaccine encoding the enterotoxigenic Escherichia coli (ETEC) CFA/I fimbriae B subunit. Vaccine 26:3998–4005. doi: 10.1016/j.vaccine.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 32.Lasaro MO, Luiz WB, Sbrogio-Almeida ME, Nishimura LS, Guth BE, Ferreira LC. 2004. Combined vaccine regimen based on parenteral priming with a DNA vaccine and administration of an oral booster consisting of a recombinant Salmonella enterica serovar Typhimurium vaccine strain for immunization against infection with human-derived enterotoxigenic Escherichia coli strains. Infect Immun 72:6480–6491. doi: 10.1128/IAI.72.11.6480-6491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasaro MO, Luiz WB, Sbrogio-Almeida ME, Ferreira LC. 2005. Prime-boost vaccine regimen confers protective immunity to human-derived enterotoxigenic Escherichia coli. Vaccine 23:2430–2438. doi: 10.1016/j.vaccine.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Guevara CP, Luiz WB, Sierra A, Cruz C, Qadri F, Kaushik RS, Ferreira LC, Gomez-Duarte OG. 2013. Enterotoxigenic Escherichia coli CS21 pilus contributes to adhesion to intestinal cells and to pathogenesis under in vivo conditions. Microbiology 159:1725–1735. doi: 10.1099/mic.0.065532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldhar J, Zilberberg A, Ofek I. 1986. Infant mouse model of adherence and colonization of intestinal tissues by enterotoxigenic strains of Escherichia coli isolated from humans. Infect Immun 52:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duchet-Suchaux M, Le Maitre C, Bertin A. 1990. Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J Med Microbiol 31:185–190. doi: 10.1099/00222615-31-3-185. [DOI] [PubMed] [Google Scholar]
- 37.Bertin A. 1983. Virulence factors of enterotoxigenic Escherichia coli studied in the infant mouse model. Ann Rech Vet 14:169–182. [PubMed] [Google Scholar]
- 38.Porter CK, Riddle MS, Tribble DR, Louis Bougeois A, McKenzie R, Isidean SD, Sebeny P, Savarino SJ. 2011. A systematic review of experimental infections with enterotoxigenic Escherichia coli (ETEC). Vaccine 29:5869–5885. doi: 10.1016/j.vaccine.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Evans DG, Graham DY, Evans DJ Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers: response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87:934–940. [PubMed] [Google Scholar]
- 40.Gothefors L, Ahren C, Stoll B. 1985. Presence of colonization factor antigens in fresh isolates of fecal Escherichia coli: a prospective study. J Infect Dis 152:1128–1132. doi: 10.1093/infdis/152.6.1128. [DOI] [PubMed] [Google Scholar]
- 41.Ahren C, Svennerholm AM. 1985. Experimental enterotoxin-induced Escherichia coli diarrhea and protection induced by previous infection with bacteria of the same adhesin or enterotoxin type. Infect Immun 50:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DG, Evans DJ Jr, Tjoa WS, DuPont HL. 1978. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun 19:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YF, Poole S, Rasulova F, Esser L, Savarino SJ, Xia D. 2006. Crystallization and preliminary X-ray diffraction analysis of CfaE, the adhesive subunit of the CFA/I fimbriae from human enterotoxigenic Escherichia coli. Acta Crystallogr Sect F Struct Biol Crystallogr Commun 62:121–124. doi: 10.1107/S1744309105043198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues JF, Mathias-Santos C, Sbrogio-Almeida ME, Amorim JH, Cabrera-Crespo J, Balan A, Ferreira LC. 2011. Functional diversity of heat-labile toxins (LT) produced by enterotoxigenic Escherichia coli: differential enzymatic and immunological activities of LT1 (hLT) AND LT4 (pLT). J Biol Chem 286:5222–5233. doi: 10.1074/jbc.M110.173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magagnoli C, Manetti R, Fontana MR, Giannelli V, Giuliani MM, Rappuoli R, Pizza M. 1996. Mutations in the A subunit affect yield, stability, and protease sensitivity of nontoxic derivatives of heat-labile enterotoxin. Infect Immun 64:5434–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duchet-Suchaux M. 1988. Protective antigens against enterotoxigenic Escherichia coli O101:K99,F41 in the infant mouse diarrhea model. Infect Immun 56:1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuPont HL, Formal SB, Hornick RB, Snyder MJ, Libonati JP, Sheahan DG, LaBrec EH, Kalas JP. 1971. Pathogenesis of Escherichia coli diarrhea. N Engl J Med 285:1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- 48.Blanco J, Blanco M, Garabal JI, Gonzalez EA. 1991. Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals. Microbiologia 7:57–73. [PubMed] [Google Scholar]
- 49.Guyer RL, Koshland ME, Knopf PM. 1976. Immunoglobulin binding by mouse intestinal epithelial cell receptors. J Immunol 117:587–593. [PubMed] [Google Scholar]
- 50.Bertin A. 1985. F41 antigen as a virulence factor in the infant mouse model of Escherichia coli diarrhoea. J Gen Microbiol 131:3037–3045. [DOI] [PubMed] [Google Scholar]
- 51.Baker KK, Levine MM, Morison J, Phillips A, Barry EM. 2009. CfaE tip mutations in ETEC CFA/I fimbriae define critical human intestinal binding sites. Cell Microbiol 11:742–754. doi: 10.1111/j.1462-5822.2009.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.