Abstract
Background
With the diverse origin of neointimal cells, previous studies have documented differences of neointimal cell-lineage composition across models, but the animal-to-animal difference has not attracted much attention though the cellular heterogeneity may impact neointimal growth and its response to therapeutic interventions.
Methods
The R26R+;Myh11-CreER+ and R26R+;Scl-CreER+ mice were utilized to attach LacZ tags to the pre-existing smooth muscle cells (SMCs) and endothelial cells (ECs), respectively. Neointimal lesions were created via complete ligation of the common carotid artery (CCA) and transluminal injury to the femoral artery (FA).
Results
LacZ-tagged SMCs were physically relocated from media to neointima and changed to a de-differentiated phenotype in both CCA and FA lesions. The content of SMCs in the neointimal tissue, however, varied widely among specimens, ranging from 5–70% and 0–85%, with an average at low levels of 27% and 29% in CCA (n=15) and FA (n=15) lesions, respectively. Bone marrow cells, while able to home to the injured arteries, did not differentiate fully into SMCs after either type of injury. Pre-existing ECs were located in the sub-endothelial region and produced mesenchymal marker α-actin, indicating endothelial-mesenchymal-transition (EndoMT), however, EC-derived cells represented only 7% and 3% of the total neointimal cell pool of CCA (n=7) and FA (n=7) lesions, respectively. ECs located on the luminal surface exhibited little evidence for EndoMT.
Conclusion
Neointimal hyperplasia proceeds with a wide range of variation in its cellular composition between individual lesions. Relative to ECs, SMCs are major contributors to the lesion-to-lesion heterogeneity in neointimal cell-lineage composition.
Neointimal hyperplasia is the primary pathology that leads to early failure of vascular procedures aimed at treating occlusive arterial diseases. Animal models, particularly those created with transgenic mice, have been utilized widely in studies to obtain mechanistic insights into the hyperplastic response of injured vessels. Of these models, common carotid artery ligations, transluminal injury to femoral arteries, and interpositional vein grafting have been used widely for these studies. Using these models, studies have identified numerous potential therapeutic molecular targets, but treatments developing from preclinical studies have, so far, failed to prove to be translational in clinical practice. While an easy explanation for the failure is that “animals are not humans”, the potential for true translational application may require a better understanding of the cellular events occurring in animal models.
In humans, work in early 1970s suggested that neointimal hyperplasia is an adaptive response of the vessel wall to injuries, with smooth muscle cells (SMCs) being the key player in this process 1–3. Later experimental studies, however, suggested that the source of neointimal cells is not limited to SMCs. Over 40% of neointimal cells were of bone marrow origin 4, 5. Other sources, including both local vessel wall 6, 7 and remote perivascular tissues 8, have also been documented as donating progenies to the neointimal cell population. Such a diverse origin of neointimal cells has sparked a hot debate on the contribution of SMCs to neointimal cell population 7, 9, 10. Although genes in SMCs may be manipulated to alter the progression of neointimal thickening 11, uncertainty has remained about whether SMCs make up the majority of neointimal cells. A study tracing the lineage fate of these cells demonstrated that over 80% of neointimal cells originated from the pre-existing SMCs 12, however, juvenile mice (<6 weeks of age) were chosen in this study, and given the significant disparity of the in vivo SMC biology between young and adult or aged animals 13, the contribution of SMCs to the neointimal cell repopulation remains to be evaluated with models that are more relevant to patients who often develop this pathology after adult age. More recently, endothelial cells (ECs) were identified as sources for neointimal cell population 14, 15. In a model of vein bypass grafting, EC-derived cells donated more than 50% of total neointimal cell population via a process termed endothelial-mesenchymal transition (EndoMT) 15. The discovery of non-SMC origin of neointimal cells has challenged the long-standing paradigm that assumes SMCs to be the predominant source for neointimal cell population.
The current study sought to tackle this fundamental issue with a focus on the fate of SMCs and ECs. Using inducible Cre-loxP systems driven by a SMC- or EC- specific promoter, we mapped the fate of these cell groups in neointimal lesions induced by different types of arterial injury. The results show that medially derived SMCs lose their markers of differentiation and assume a more dedifferentiated state on relocating to neointimal lesions. To our knowledge, it is the first direct evidence that reaffirms the concept of “SMC dedifferentiation” suggested by Regan et al 16. In addition, we show that, in adult animals, SMCs donate to the total neointimal cell pool with a wide range of animal-to-animal variation in models that have been used widely in the past in both mechanistic and pre-clinical studies. EndoMT, while serving as a major mechanism for neointimal cell repopulation in selected vascular settings 15, appears to occur infrequently in neointimal lesions induced by complete cessation of blood flow or intraluminal injury. Our findings suggest that experimental neointimal lesions are highly heterogeneous pathologically, which raises a question as to the pathologic property, and thus the response to therapeutic intervention in humans.
METHODS
Animal models
This study conforms to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Institutional Animal Care and Use Committee of the University of Florida. The Gt(ROSA)26Sor (R26R), CAG-EGFP, and C57BL/6-Ly5.1 mice were purchased from Jackson Laboratory. The Myh11-CreER and Scl-CreER strains were kindly provided by Drs. Offermanns and Gothert, respectively. All animals were on C57BL/6 background. To avoid the potential issue of sexually dimorphic response to vascular injury, only male mice were used in this study, and the responses in female mice were not studied. The Cre lines were bred with R26R, and offspring littermates were screened for R26R+;Myh11-CreER+ and R26R+;Scl-CreER+ animals with PCR-based genotyping. The R26R+;Myh11-CreER+ mice received 10 consecutive doses of tamoxifen (2.5 mg/mouse×day, I.P.), while the R26R+;Scl-CreER+ mice were dosed with five daily injections (2.5 mg per mouse×day, I.P.) followed by additional five doses administered every other day. These protocols were selected from our pilot experiments, where various dosing schedules were executed to achieve maximal efficiency of the Cre mediated activation of the reporter gene. Neointimal lesions were induced in the right common carotid artery (CCA) and right femoral artery (FA) in the same animal via complete ligation and a metal wire injury, respectively. For the carotid ligation, an 8-0 ligature was placed immediately proximal to the bifurcation. Mechanical injury to femoral artery was produced via a one-minute distention by a 0.015″ guidewire inserted through the profunda femoris artery 4. Animals received tamoxifen induction at 11 weeks of age, and operative procedures to create vascular injuries were performed on the day following the completion of tamoxifen induction. Surgical samples were perfusion-fixed with 4% paraformaldehyde in PBS and collected at various time points as specified in Table 1. At the time of sample collection, uninjured CCAs and FAs from the contralateral side were also harvested to examine the LacZ-labeling of SMCs and ECs.
Table 1.
Experimental design
| Target | BMT | Pre-op TM | Surgery | Post-op TM | Evaluation |
|---|---|---|---|---|---|
| SMCs (n=15) | No | −d10 | d0 | No | d38 |
| BMCs (n=5–7) | −d38 | No | d0 | d14, d28, or d49 | d24, d38, or d59 |
| ECs (n=6) | No | −d15 | d0 | No | d38 |
Note: BMT, bone marrow transplantation; TM, tamoxifen; BMCs, bone marrow-derived cells; d, days pre or post –operatively; and “−”, preoperatively.
Experimental Design
The R26R;Myh11-CreER system, in conjunction with a timely and controlled tamoxifen induction, selectively labels cells carrying the active Myh11 promoter with LacZ tags. This system allows tracking of SMCs and detecting those with acquired ability to express Myh11 in neointimal lesions. As detailed in table 1, the SMC group received tamoxifen only pre-operatively. Under this scenario, any LacZ-tagged cells in neointimal lesions would be of SMC origin. The bone marrow transplant experiment (e.g. the bone marrow-derived cell (BMC) group) was performed to examine the SMC lineage fate of BMCs in neointimal lesions. Chimeras were created with R26R+;Myh11-CreER+ bone marrow, and tamoxifen induction was completed prior to histologic evaluation at time points of d24, d38, or d59. Selective labeling of mature ECs was completed with the R26R;Scl-CreER system. Two Scl-CreER mouse lines with different specificities of EC labeling were created, and the strain utilized in this study was the one with minimal off-target Cre production in hematopoietic cells 17.
Cell tracing
X-gal staining (MIR 2600, Mirus, Madison, WI) was performed on frozen sections (5 μm in thickness, one section per sample) that were collected at a site 500 μm proximal to the ligature or site of wire insertion. Sections were counterstained with nuclear fast red. Unmanipulated CCAs and FAs collected from R26R+;Myh11-CreER+ (n=6) and R26R+;Scl-CreER+ (n=5) animals were assayed to estimate the efficiency of LacZ labeling in SMCs and ECs, respectively. To quantify LacZ-tagged SMCs, x-gal positive area was measured using Axiovision as we have detailed previously 18. The percent of the x-gal positive area in the medial layer of unmanipulated arteries was considered as the baseline for the efficiency of LacZ labeling of SMCs. Because of the fusion of the neointima with the media, especially in the FA lesions, determination of the border between these layers was technically challenging. Therefore, the neointima and media were viewed as one layer during morphometric analysis. The x-gal positive area was normalized to the total medial and neointimal (M+I) area of the corresponding section, and normalized data were used for subsequent analyses. The phenotype of the LacZ-tagged SMCs was characterized with immuno-fluorescent assays for SM α-actin and myosin heavy chain (SMMHC).
To quantify LacZ-labeled ECs, the length of the x-gal positive surface on cross sections of unmanipulated arteries was measured and indexed to the circumference of the corresponding section. The percent of the x-gal positive surface was considered as the baseline efficiency of LacZ labeling in ECs. All relocated LacZ-tagged (e.g. EC-derived) cells in the sub-EC neointimal/medial region were taken as events of EndoMT and counted on the x-gal staining sections. The percent of EC-derived cells was calculated to estimate the contribution of ECs to the total neointimal cell pool. The phenotype of the luminal ECs and the EC-derived cells (e.g. those located in the sub-EC region) was further characterized with immuno-fluorescent assays against LacZ tags, the EC lineage marker CD31, and mesenchymal makers of α-actin and collagen 1.
SMC and bone marrow cell culture
Primary aortic SMCs were explanted from tamoxifen-treated R26R+;Myh11-CreER+ mice using the method we reported previously 19. Cells were expanded in DMEM plus 10% FBS, and the SMC lineage was confirmed with flow cytometry against α-actin (F3777, Sigma, St. Louis, MO). To examine the durability of LacZ tags during de-differentiation and proliferation, SMCs were treated with 10 ng/ml of PDGF-BB (SRP3138, Sigma, St. Louis, MO) for 24 h 16 and assayed with x-gal staining. SMCs separated from non-treated R26R+;Myh11-CreER+ mice were included as negative controls. Acquisition of the LacZ tags by bone marrow cells was evaluated via PDGF-promoted Myh11 activation 5. Whole bone marrow (1×106 cells/mL) collected from non-treated R26R+;Myh11-CreER+ mice were incubated in RPMI 1640 plus 10% FBS for 3 h. After three gentle washes with serum-free RPMI 1640 to remove non-adherent cells, the remaining cells were treated with 50 ng/ml PDGF-BB for 72 h, followed by induction with the active form of tamoxifen (4-hydroxytamoxifen, H-7904, Sigma, St. Louis, MO) at a concentration of zero or 0.5 μM for 48 h 20. Activity of the Myh11 promoter in these cells was then examined with x-gal staining assays. All assays were done in triplicates.
Immuno-fluorescent assays
Immuno-fluorescent assays were performed on frozen sections (5μm) with specimens omitting primary antibodies included as negative controls. Primary antibodies applied to the assays were cy3 conjugated anti α-actin (c6198, Sigma, St. Louis, MO), chicken anti β-galactosidase (ab9361, abcam, Cambridge, MA), rabbit anti CD31 (ab28364, abcam, Cambridge, MA), rat anti CD31 (NB100-1642, Novus, Littleton, CO), rabbit anti SMMHC (ab53219, abcam, Cambridge, MA), goat anti vimentin (sc-7557, Santa Cruz, Dallas, Texas), and rabbit anti collagen 1 (NBP1-30054, Novus, Littleton, CO). Secondary antibodies were Alexa 488 goat anti-chicken, Alexa 546 goat anti-rabbit, and Alex 546 goat anti-rat, all purchased from Life Technologies (Grand Island, NY). Antigen retrieval was required for CD31 and β-galactosidase staining and achieved by incubating sections with citrate acid (H-3300, Vector Labs, Burlingame, CA) in a pressure cooker for 10 min. All assays were evaluated with confocal microscopy to determine co-localization of the examined markers.
Bone marrow transplantation
The R26R+;Myh11-CreER+ or EGFP+ marrow cells (on a Ly5.2 background) were harvested from femurs by flushing with buffer-containing PBS plus 2%FBS. Cell clumps were broken by gentle passing through a 27G needle and debris was removed with a 100 μm cell strainer. After lyses of red blood cells with ammonium chloride buffer (0.8% in 0.01 M Tris-HCl), the remaining cells were re-suspended in flush buffer and evaluated for cell concentration with a hemocytometer. Six-week-old C57BL/6-Ly5.1 male recipients were lethally irradiated with two doses of 500 Rads separated by a period of 4 h and then received 2×106 donor cells for bone marrow transplantation (BMT) via retro-orbital injection. Four weeks after BMT, the presence of ly5.2 positive cells in peripheral blood was evaluated with flow cytometry analysis. All chimeras demonstrated engraftment efficiency greater than 90%.
Statistical analyses
All data are expressed as mean ± SEM. Comparisons were done using an unpaired t-test and linear regression as appropriate. P< 0.05 was considered significant.
RESULTS
Medial SMCs migrate to the neointimal layer and become de-differentiated in neointimal lesions
In the tamoxifen-treated R26R+;Myh11-CreER+ mice, LacZ tags (appearing in blue) were attached exclusively to SMCs in the media (Fig S1, A–C), with a labeling efficiency of 92% and 94% for un-manipulated CCAs and FAs, respectively (Fig S1, D). Aortic outgrowths were nearly homogeneously α-actin positive and had LacZ tags (Fig. S1, E–G), indicating that these cells were SMCs. During neointimal hyperplasia, LacZ-tagged cells accumulated in the neointimal layer of both CCA (Fig 1, A–B) and FA (Fig 1, C–D) lesions on d38. Colocalization analyses revealed that some SMC-derived neointimal cells (Fig 1, E–H, green) lost their contractile protein α-actin (Fig 1, E and G, red). Analyses of the neighboring sections uncovered more severe loss of SM myosin heavy chain (SMMHC, Fig 1, F and H, red) than α-actin in SMC-derived neointimal cells in both the CCA and FA lesions. In contrast, SMCs in the media, particularly those in CCA lesions where the tunica media was well-preserved, displayed robust α-actin and SMMHC staining (Fig 1, E and F). Such a pattern was observed consistently in all 15 CCA and FA lesions. Evidently, SMCs physically relocate (e.g. migration) from the tunica media to the developing neointima and de-differentiate with loss of mature SMC markers.
Fig 1.
SMCs from the tunica media repopulate neointimal lesions via a process of physical relocation and de-differentiation. A–D: x-gal staining of CCA (A–B) and FA (C–D) lesions. B and D, magnified view of the boxed area in A and C; Blue, LacZ-tagged SMCs; Purple, nuclear counterstain. Arrows point to internal elastic lamina (IEL). The residual media of FA lesions was torn apart during frozen sectioning due to the weak mechanical strength of this layer. Note the accumulation of LacZ-tagged SMCs in the neointimal layer of both CCA and FA lesions. E–H: immunofluorescent images representative of 15 CCA and FA lesions. Green, β-gal (LacZ gene product); Red, α-actin or SMMHC. White dash lines delineate IEL. Note the loss of α-actin (E and G) and SMMHC (F and H, arrows) in the majority of the SMC-derived neointimal cells (arrows). Scale bars, 50 μm.
SMCs populate the neointima with significant animal to animal variation
The content of LacZ-tagged SMCs in the neointimal layer varied widely, from a majority (Fig 2, column A) to nearly complete absence (Fig 2, column C) in both CCA and FA lesions. In some extreme cases, although the neointimal lesions were highly cellular and the underlying SMCs were well-preserved as evidenced by robust x-gal staining in the media, LacZ-tagged cells were hardly detected in the neointimal layer (Fig 2, column C). Such a striking phenomenon was noted in both CCA and FA lesions, suggesting that SMCs do not always donate progenies to the neointimal cell population. The paucity of LacZ-tagged cells seems not to be a result of random loss of the LacZ tags since SMCs remained homogenously on LacZ tags even after several generations of continuous passaging or PDGF stimulation (Fig S1, F–G). Measurements of LacZ-tagged cells in individual CCA and FA lesions are plotted in Fig 2D (top panel); those having more LacZ-tagged cells in CCA lesions displayed more LacZ-tagged cells in their FA lesions as well, and such a correlation was statistically significant (Fig 2, D), indicating an individual dependency of the contribution of SMCs to the neointimal cell population. Overall, the content of LacZ-tagged cells was surprisingly low and averaged 27% and 29% in CCA and FA lesions, respectively, P>0.05 (Fig 2, D).
Fig 2.
SMCs from the tunica media repopulate neointimal lesions with a degree varying widely between animals. A–C: x-gal staining of CCA (top row) and FA (bottom row) lesions (d38). Blue, LacZ-tagged SMCs; Purple, nuclear counterstain. Red dash lines delineate IEL. Note the remarkable variation in the number of LacZ-tagged SMCs in both CCA and FA lesions. Scale bars, 100 μm. D: accumulation of LacZ-tagged SMCs in FA and CCA lesions of individual animals. Regression analysis detected a significant individual dependency (top panel) though the averaged content of LacZ-tagged SMCs was not significantly different between CCA and FA lesions (lower panel).
Bone marrow-derived cells cannot fully differentiate into SMC in the developing neointima
In order to capture the activation of Myh11 promoter in bone marrow-derived cells during neointimal hyperplasia, tamoxifen induction was performed at various time points (Table 1) during neointimal formation. The injured CCAs and FAs produced a robust neointima hyperplasia by d38, with a combined neointimal/medial area measured as 37,293 +/− 10,983 and 32,926 +/− 6,221 μm2 for the CCA and FA lesions, respectively, which were not significantly different from the amount of neointimal formation in non-irradiated mice (34,748 +/− 5,596 and 41,630 +/− 7,107 μm2 in CCA (P=0.86) and FA (P=0.60) lesions, respectively); however, microscopic examination did not detect any LacZ-tagged cells in CCA or FA lesions at all indicated time points (Fig 3, A). To confirm the recruitment of the bone marrow cells to neointimal lesions created with our experimental protocol, we generated EGFP bone marrow chimeras and evaluated the recruitment of EGFP cells in FA lesions (n=3). The FA lesions displayed abundant bone marrow-derived cells on postoperative d38 (Fig 3, B). We further validated the negative finding with directed marrow cell differentiation experiments. The R26R+;Myh11-CreER+ bone marrow cells were stimulated with PDGF, a growth factor known to induce Myh11 expression in bone marrow progenitor cells 5. After induction with the active form of tamoxifen, a subset of marrow cells were labeled successfully by LacZ tags (Fig 3, C), while the untreated bone marrow cells were completely LacZ-negative (Fig 3, D). These results suggest that the absence of the Myh11-positive cells in the CCA and FA lesions is the result of the inability of bone marrow cells to acquire the Myh11 promoter activity rather than a labeling artifact or failed homing of bone marrow cells to the neointimal lesions.
Fig 3.
Bone marrow cells home to neointimal lesions but cannot acquire Myh11 promoter activity. A: x-gal staining of the CCA and FA neointimal lesions created in R26R+;Mhy11-CreER+ bone marrow chimeras. Samples were assayed at time points specified on the left of the panel. Purple, nuclear counterstain. Note the absence of β-gal positive cells in both the CCA and FA lesions at various time points. B: fluorescent imaging of an FA lesion (d38) created in an EGFP bone marrow chimera. Green, bone marrow derived EGFP positive cells; Blue, nuclear counterstain; L, lumen; M, media. C–D: x-gal staining of the R26R+;Myh11-CreER+ bone marrow cells treated with (C) and without (D) PDGF. Note that LacZ tags (blue) are attached to a subset of bone marrow cells. Scale bars, 50 μm.
Mature ECs undergo EndoMT infrequently to donate progenies to neointimal cell population
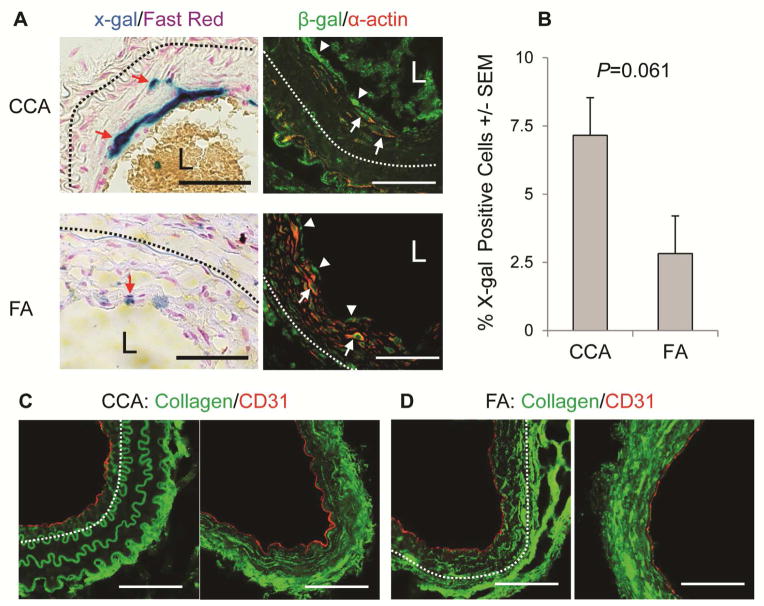
Neointimal lesions were created in CCAs and FAs of tamoxifen-treated R26R+;Scl-CreER+ mice. In un-manipulated arteries, LacZ tags were attached exclusively to cells lining the luminal surface (Fig S2, A). Confocal Z-stack scanning showed a precise co-localization of the LacZ tags with the EC lineage marker CD31 (Fig S2, B), indicating that the LacZ labeling is restricted to mature ECs. The labeling efficiency was calculated as 62% and 82% for CCAs and FAs, respectively (Fig S2, C; P=NS). In the CCA and FA lesions, a few LacZ-tagged cells were detected in the sub-endothelial region (Fig 4, column A). In addition to the compartmental relocation, these cells also acquired the ability to produce α-actin (Fig 4, column B), the definitive mesenchymal marker usually not expressed in ECs 14, 21, indicating the occurrence of EndoMT in neointimal lesions. This cellular event, however, occurred at a very low frequency and represented only 7% and 3% of the total neointimal cell pool of CCA and FA lesions, respectively (Fig 4, B, P=0.061).
Fig 4.
ECs repopulate the neointima with a very low frequency. A: x-gal (left panels) and immunofluorescent (right panels) staining images representing CCA (n=6) and FA (n=6) lesions on d38. Dash lines, IEL. L, lumen; Blue, LacZ tagged ECs; Purple, nuclear counterstain; Green, β-gal; Red, a-actin. Red arrows point to EC-derived neointimal cells, white arrows denote cells stained positive for both β-gal and α-actin, and arrow heads point to LacZ-tagged luminal ECs. B: Fraction of EC-derived cells in the sub-endothelial region of CCA (7±1%, n=6) and FA (3±1%, n=6) lesions. P=0.061, by unpaired t-test.; Scale bars, 50 μm.
ECs may also acquire a mesenchymal phenotype without completely losing their lineage markers 22. We examined the “in situ EndoMT” in both CCA and FA lesions. Several markers have been utilized to determine EndoMT in injured or diseased organs. Of them, α-actin and collagen 1 are accepted widely as a definitive marker for this process 14, 15, 23. Therefore, we examined both markers in luminal ECs. As shown in the right column of Fig 4 A, the LacZ-tagged ECs did not produce α-actin on luminal surface of both CCA and FA lesions. Collagen 1 was detected primarily in the adventitia and deep neointima of both CCA (Fig 4, C) and FA lesions (Fig 4, D). Cells lining the luminal surface maintained their ability to produce CD31, and we were unable to detect collagen 1 in these cells (Fig 4, C–D).
DISCUSSION
Using a strategy of cell tracing, the current study has provided in vivo evidence suggesting that neointimal lesions pathologically are highly heterogeneous among individual lesions. We showed that SMCs relocate from the tunica media to neointimal lesions, donating progenies to the neointimal cell population. During neointimal hyperplasia, SMCs become de-differentiated and lose the contractile proteins α-actin and SMMHC when populating the neointimal cell response. These cells, however, do not always comprise the major group in the neointimal cell population, which underscores the importance of individual differences and the importance of individualized therapy that might need to be targeted to control neointimal hyperplasia. EndoMT, though serving as a major mechanism for neointimal cell repopulation in some vascular settings, occurred infrequently in the models examined in the current study.
The Myh11-CreER mouse line was chosen in this study for two reasons: 1) Myh11 is the most definitive marker identified to date for SMCs; and 2) the Cre activity is inducible, which enables attachment of LacZ tags to SMCs from the media in a timely, controlled fashion. Other systems, such as the SM22-LacZ 5 and Myh11-LacZ 16, were utilized in previous studies to map the fate of SMCs. Because of the mosaic and episodic expression of the reporter cassettes, a binary Cre-loxP system was developed later and achieved a more homogenous labeling of SMCs 7, 24, 25, however, both SM22 and Myh11 promoters are expressed transiently in non-SMCs during embryonic development 24. At the adult stage, the SM22 promoter remains active in myeloid cells 26. To avoid off-target Cre activity, several inducible Cre lines have been created, and the Myh11-CreER strain 27 has gained popularity in studies to fate-map SMCs during pathogenesis of vascular diseases 9, 11, 12. Consistent with these previous reports, we showed that about 95% of the SMCs in the tunica media in CCAs and FAs were labeled successfully with LacZ tags. Once attached, these tags become permanent, as evidenced by the results obtained from continuous SMC passaging and PDGF-induced dedifferentiation (Fig S1).
Recent work has raised a fundamental question as to the role of SMCs during neointimal hyperplasia 9, 10. Early evidence supporting SMCs as a source for the neointimal cell response comes from observations that SMCs underwent active proliferation during early neointimal hyperplasia, and cells expressing active SM22 promoter prior to vascular injury accumulated in neointimal lesions 1, 16. This concept has now gained a solid foundation from two recent studies 11, 12. Consistent with these reports, our results showed that SMCs served as a source of progenies to the neointimal cell pool in lesions induced by different types of vascular injury. The incidence of this event appears, however, to be age-dependent. In juvenile mice, Herring et al observed that ~80% of neointimal cells were of SMC origin 12, while in adult mice, we found cells of SMC origin represented less than 30% of the total neointimal cell pool. Another widely accepted concept is that SMCs undergo “phenotypic switching” during neointimal hyperplasia 28. Using an SM22 promoter assay, Regan et al demonstrated loss of SM22 expression in SMCs derived from the tunica media of the vessel wall 16. In the current study, we provided in vivo evidence showing that the expression of α-actin and SMMHC proteins are also severely inhibited or completely lost in neointimal SMCs, which reaffirms the concept of “SMC de-differentiation”. Additionally, there was an uncertainty on the lineage identity of the cells collected from aortic outgrowth 7, 9, 10. In this study, we provided direct evidence demonstrating that cells explanted from aortic media are truly SMCs.
A surprising finding in our study was the wide-range of mouse-to-mouse variation in the contribution of SMCs to the neointimal cell population in both CCA and FA lesions. Previous studies suggested that the number of living SMCs is the major determinant for the fraction of SMCs in the total neointimal cell pool 4. While this theory is applicable to FA lesions where the structure of the tunica media is usually severely injured during creation of the model we employed, it is difficult to reconcile the variation among CCA lesions where the media layer is well preserved and contains LacZ-tagged SMCs (Fig 2). Another explanation would be a random loss of the LacZ tags in the de-differentiated SMCs. Using total RNA extracted from whole tissue block, Cuttler et al detected a decrease in LacZ expression in neointimal lesions 29. Because of the small fraction of SMCs in the total neointimal cell pool, it is not surprising that the mean LacZ expression is low in neointimal lesions compared to normal arteries where the major cell group is LacZ-expressing SMCs. Our results show that SMCs remained homogeneously on LacZ tags after continuous passaging or PDGF-induced de-differentiation, supporting the concept that the variation seen in our experiments is not the result from random loss of LacZ tags. Complete carotid artery ligation and mechanical injury to the femoral artery are popular models utilized in both mechanistic studies and preclinical evaluations 30. Given the extremely high disparity of the dominant cell group between individual neointimal lesions forming in the same vascular setting, a critical but still unanswered question is whether neointimal cells of different origin would respond to molecular interventions and/or pharmaceutical therapies in a similar fashion. The answer to this question will guide the future development of therapeutic strategies to control neointimal growth.
Previous studies have led to confusion about the potential of bone marrow cells to differentiate into SMCs. Some investigators demonstrated that bone marrow cells were transient visitors and unable to acquire the ability to express SM α-actin in neointimal lesions 31–33, while other investigators provided evidence that supports the opposite conclusion 34, 35. These studies used an EGFP reporter driven by various promoters, and the labeling efficiency of the EGFP reporter varies among different strains and cell lineages 36. In the current study, we evaluated the SMC-lineage potential of bone marrow cells with a LacZ reporter driven by the Myh11 promoter. Examination at various time points could not detect any LacZ-tagged cells in either the CCA or the FA lesions. With our observations, it seems unlikely that there was a refractory response of the bone marrow cells to the labeling system, because LacZ tags were activated successfully in bone marrow cells after PDGF-stimulation that is known to induce Myh11 promoter activity in marrow progenitor cells 5. Our results, therefore, support the concept that bone marrow cells cannot acquire an SMC lineage fate in neointimal lesions.
Emerging evidence suggests that ECs can differentiate into SMC-like mesenchymal cells via EndoMT. These cells migrate to the sub-endothelial region and participate in the pathogenesis of cardiovascular diseases, such as pulmonary and cardiac fibrosis 21, 37. We evaluated the importance of EndoMT as a mechanism for repopulating neointimal lesions. In the neointima forming in injured CCAs and FAs, we could only detect a small number of EC-derived cells in the sub-endothelial region. This observation is consistent with the report from Chen et al who utilized a Cre line different from ours and showed that EC-derived cells represented only 5% of the total neointimal cell population 14. Interestingly, in neointimal responses forming in bypass vein grafts, Cooley et al observed that over 50% of the neointimal cells were of EC origin 15. In contrast, Chen et al reported that EC-derived cells constituted only 6.7% of the neointimal cells in a similar vein bypass model 14. All these studies, including ours, have utilized different lineage-tracing systems to investigate ECs as a source for neointimal cell population in different settings of vascular injuries. It appears that both the lineage-tracking system and the vascular setting are important factors for the inconsistency between these studies.
In addition to relocation to pathologic sites, another feature of EndoMT is the alteration of their molecular signature, including loss of the markers of EC lineage CD31 and VE-cadherin and the gain of the mesenchymal markers α-actin, collagen 1, and vimentin 14, 21. We evaluated these markers in ECs lining the luminal surface of developing neointimal lesions and could not detected any convincing evidence for “in situ EndoMT” in both CCA and FA lesions. This finding appears inconsistent with the report from Chen et al who observed EndoMT in 46% of luminal ECs in the same model and at the same time point as we chose in the current study 14. Major differences between the study of Chen et al 14 and our studies was the age (6 vs. 11 weeks) of animals used in these experiments; indeed an age-dependent phenotypic expression has been documented recently for vascular remodeling in maturing mice 13.
A few limitations exist in our study. LacZ-labeling of the mature ECs was achieved with the Cre-mediated recombination driven by the Scl promoter. Although the Cre activity of the Scl-CreER mouse line used in our study is highly restricted to mature ECs with little leakage to the hematopoietic cells, the recombination driven by this mouse line is incomplete in ECs lining the luminal surface of CCAs and FAs. Given the heterogeneity of the EC population 38, our system might have labeled selectively a subset of ECs, and, therefore, the fate map obtained with this mouse line may not be extrapolated to the entire EC population. In addition, the expression of SMMHC is a marker of differentiation gained by SMCs at the late stage of lineage maturation39. In the BMT experiment, the SMC lineage potential of bone marrow cells was judged by the ability to acquire the Myh11 promoter activity, which could not exclude the possibility that some of these cells may be able to differentiate into immature SMCs in neointimal lesions. Also, we only studied male mice, and therefore, we cannot comment on whether a similar response occurs in female mice.
In summary, SMCs are important players that physically relocate/migrate from the tunica media to the neointima, contributing to neointimal hyperplasia. The consistency of this cellular event varies widely between models and between individual animals, causing considerable disparity of the neointimal cell composition between different models and among lesions forming in the same animal model. Although ECs are important contributors to the hyperplastic neointimal thickening, the importance of EndoMT in this process appears limited to certain vascular settings. The extremely high pathologic heterogeneity of neointimal lesions seen in experimental models calls for future studies to characterize the cellular composition of human neointimal lesions.
Supplementary Material
Figure S1. The R26R+;Myh11-CreER+ system facilitates specific and nearly homogenous labeling of mSMCs with durable LacZ tags. (A and B) X-gal staining images representing un-manipulated CCAs (A) and FAs (B). Blue, LacZ-tagged SMCs; purple, nuclear counterstain; FV, femoral vein. (C) Immunofluorescence confocal evaluation for LacZ labeling specificity in aortas. Orthogonal views at the plane indicated by green and red lines are placed on top and right side of the main panel. (D) LacZ-labeling efficiency in mSMCs of unmanipulated CCAs and FAs. (E) Flow cytometry analysis of primary aortic outgrowths with α-actin specific antibody or isotype control (ISO Contl). (F and G) X-gal staining of PDGF treated primary SMCs (p10) isolated from R26R+;Myh11-CreER+ aortas with (F) or without (G) tamoxifen induction. Scale bars, 100 μm.
Figure S2. The R26R+;Scl-CreER+ system facilitates specific labeling of mature ECs with LacZ tags. (A) X-gal staining images representing un-manipulated R26R+;Scl-CreER+ CCAs and FAs. Blue, LacZ tagged ECs; purple, nuclear counterstain. (B) EC LacZ-labeling efficiency, calculated as percent of LacZ positive surface in circumference of the cross sections. Scale bars, 100 μm.
Acknowledgments
Supported by NIH1R01HL105764
We thank Drs. Stefan Offermanns (University of Haidelberg) and Mary C.M. Weiser-Evans (University of Colorado) for their generosity of providing us the Myh11-CreER breeding pairs. We also thank Dr. Joachim R. Göthert (University Hospital Essen) for his kindness to share the Scl-CreER line with us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Imai H, Lee KJ, Lee SK, Lee KT, O’Neal RM, Thomas WA. Ultrastructural features of aortic cells in mitosis in control and cholesterol-fed swine. Lab Invest. 1970;23:401–415. [PubMed] [Google Scholar]
- 2.Lee KT, Thomas WA, Florentin RA, Reiner JM, Lee WM. Evidence for a polyclonal origin and proliferative heterogeneity of atherosclerotic lesions induced by dietary cholesterol in young swine. Ann N Y Acad Sci. 1976;275:336–347. doi: 10.1111/j.1749-6632.1976.tb43366.x. [DOI] [PubMed] [Google Scholar]
- 3.Clowes AW, Clowes MM, Reidy MA. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986;54:295–303. [PubMed] [Google Scholar]
- 4.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13–e20. doi: 10.1161/01.res.0000037090.34760.ee. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, O’Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–2693. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 7.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Z, Wang A, Wang D, Li S. Smooth muscle cells: to be or not to be? Response to Nguyen et Al. Circ Res. 2013;112:23–26. doi: 10.1161/CIRCRESAHA.112.281055. [DOI] [PubMed] [Google Scholar]
- 11.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, et al. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300–1308. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21. doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, et al. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124:755–767. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, et al. FGF Regulates TGF-beta Signaling and Endothelial-to-Mesenchymal Transition via Control of let-7 miRNA Expression. Cell Rep. 2012;2:1684–1696. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooley BC, Nevado J, Mellad J, Yang D, St HC, Negro A, et al. TGF-beta Signaling Mediates Endothelial-to-Mesenchymal Transition (EndMT) During Vein Graft Remodeling. Sci Transl Med. 2014;6:227ra34. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA. Established neointimal hyperplasia in vein grafts expands via TGF-{beta} mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009;297:200–207. doi: 10.1152/ajpheart.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu C, Yu P, Tao M, Gupta T, Moldawer LL, Berceli SA, et al. Monocyte chemoattractant protein-1/CCR2 axis promotes vein graft neointimal hyperplasia through its signaling in graft-extrinsic cell populations. Arterioscler Thromb Vasc Biol. 2012;32:2418–2426. doi: 10.1161/ATVBAHA.112.255786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jokela T, Vainio S. Conditional tamoxifen Cre induced mutagenesis in the embryonic kidney in organ culture. Genesis. 2007;45:757–761. doi: 10.1002/dvg.20352. [DOI] [PubMed] [Google Scholar]
- 21.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 22.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 24.Regan CP, Manabe I, Owens GK. Development of a smooth muscle-targeted cre recombinase mouse reveals novel insights regarding smooth muscle myosin heavy chain promoter regulation. Circ Res. 2000;87:363–369. doi: 10.1161/01.res.87.5.363. [DOI] [PubMed] [Google Scholar]
- 25.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Z, Li C, Frieler RA, Gerasimova AS, Lee SJ, Wu J, et al. Smooth muscle protein 22 alpha-Cre is expressed in myeloid cells in mice. Biochem Biophys Res Commun. 2012;422:639–642. doi: 10.1016/j.bbrc.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 28.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 29.Cuttler AS, LeClair RJ, Stohn JP, Wang Q, Sorenson CM, Liaw L, et al. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis. 2011;49:673–680. doi: 10.1002/dvg.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sata M, Fukuda D, Tanaka K, Kaneda Y, Yashiro H, Shirakawa I. The role of circulating precursors in vascular repair and lesion formation. J Cell Mol Med. 2005;9:557–568. doi: 10.1111/j.1582-4934.2005.tb00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation. 2007;116:2053–2061. doi: 10.1161/CIRCULATIONAHA.107.722355. [DOI] [PubMed] [Google Scholar]
- 32.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 33.Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, et al. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. [DOI] [PubMed] [Google Scholar]
- 34.Kumar AH, Metharom P, Schmeckpeper J, Weiss S, Martin K, Caplice NM. Bone marrow-derived CX3CR1 progenitors contribute to neointimal smooth muscle cells via fractalkine CX3CR1 interaction. FASEB J. 2010;24:81–92. doi: 10.1096/fj.09-132225. [DOI] [PubMed] [Google Scholar]
- 35.Metharom P, Kumar AH, Weiss S, Caplice NM. A specific subset of mouse bone marrow cells has smooth muscle cell differentiation capacity-brief report. Arterioscler Thromb Vasc Biol. 2010;30:533–535. doi: 10.1161/ATVBAHA.109.200097. [DOI] [PubMed] [Google Scholar]
- 36.Giel-Moloney M, Krause DS, Chen G, Van Etten RA, Leiter AB. Ubiquitous and uniform in vivo fluorescence in ROSA26-EGFP BAC transgenic mice. Genesis. 2007;45:83–89. doi: 10.1002/dvg.20269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 38.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 39.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The R26R+;Myh11-CreER+ system facilitates specific and nearly homogenous labeling of mSMCs with durable LacZ tags. (A and B) X-gal staining images representing un-manipulated CCAs (A) and FAs (B). Blue, LacZ-tagged SMCs; purple, nuclear counterstain; FV, femoral vein. (C) Immunofluorescence confocal evaluation for LacZ labeling specificity in aortas. Orthogonal views at the plane indicated by green and red lines are placed on top and right side of the main panel. (D) LacZ-labeling efficiency in mSMCs of unmanipulated CCAs and FAs. (E) Flow cytometry analysis of primary aortic outgrowths with α-actin specific antibody or isotype control (ISO Contl). (F and G) X-gal staining of PDGF treated primary SMCs (p10) isolated from R26R+;Myh11-CreER+ aortas with (F) or without (G) tamoxifen induction. Scale bars, 100 μm.
Figure S2. The R26R+;Scl-CreER+ system facilitates specific labeling of mature ECs with LacZ tags. (A) X-gal staining images representing un-manipulated R26R+;Scl-CreER+ CCAs and FAs. Blue, LacZ tagged ECs; purple, nuclear counterstain. (B) EC LacZ-labeling efficiency, calculated as percent of LacZ positive surface in circumference of the cross sections. Scale bars, 100 μm.