Abstract
Lipid oxidation-induced quality problems can be minimized with the use of natural antioxidants. Antioxidant potential of tomato puree (10 %; T-1), tomato pulp (12.5 %; T-2), lyophilized tomato peel (6 %; T-3), and pink guava pulp (10 %; T-4) was evaluated in raw pork emulsion during refrigerated storage for 9 days under aerobic packaging. The lycopene and β-carotene content varied in pork emulsion as T-3 > T-1 > T-2 > T-4 and decreased (P < 0.05) during storage. The surface redness (a* value) increased (P < 0.05) with the incorporation of tomato products and pink guava pulp. Furthermore, metmyoglobin formation and lipid oxidation were lower (P < 0.05) in tomato- and guava-treated emulsions than in control. Overall, incorporation of tomato products and pink guava pulp improved the visual colour and odour scores of raw pork emulsion. These results indicated that tomato products and guava pulp can be utilized as sources of natural antioxidants in raw pork products to minimize lipid oxidation, off-odour development, and surface discolouration.
Keywords: Raw pork emulsion, Tomato products, Pink guava, Antioxidant, Lipid oxidation
Introduction
Lipid oxidation is a major factor abbreviating the shelf-life of meat and meat products. It accelerates meat discolouration and results in off-odour development, which compromise consumer acceptance (Descalzo and Sancho 2008). Furthermore, lipid oxidation also leads to loss of nutritive value of muscle foods (Valenzuela and Nieto 1996). The quality deterioration due to lipid oxidation can be prevented by the use of antioxidants. Synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tertiary butylhydroquinone (TBHQ) have been widely used in muscle foods (Valencia et al. 2007). In the last two decades, interest in using natural antioxidants in food has increased dramatically due to the reported carcinogenic effects of synthetic antioxidants (Georgantelis et al. 2007; Fellenberg and Speisky 2006) and the consensus that foods rich in natural antioxidants can mitigate the pathology of chronic diseases (Bernardini et al. 2011). Lycopene is an acyclic red carotenoid without provitamin A activity and is a potent quencher of singlet oxygen and scavenger of peroxyl radicals (DiMasico et al. 1989). The antioxidant activity of lycopene is twice as of β-carotene and ten times greater than α-tocopherol (Shi and Maguer 2000). Lycopene is also responsible for the red colour of fruits such as tomatoes, pink guava, watermelon, and pink grape fruit.
Tomato, a solanaceous vegetable, is a rich source of lycopene, β-carotene, ascorbic acid, vitamin E, and phenolic compounds (Eyiler and Oztan 2011). Red-fleshed guava contains several antioxidants such as phytofluene, β-carotene, β-cryptoxanthin, γ-carotene, lycopene, ascorbic acid, rubixanthin, cryptoflavin, lutein, and neochrome (Mercadante et al. 1999). Previous studies reported the use of tomato products and lycopene as antioxidants in meat products, such as tomato powder in frankfurters (Eyiler and Oztan 2011), dried tomato peel in beef hamburgers (García et al. 2009; Selgas et al. 2009), dried tomato peel in dry fermented sausages (Calvo et al. 2008), tomato paste in frankfurters (Deda et al. 2007), sun-dried tomatoes, tomato paste and crystalline lycopene in minced meat (Osterlie and Lerfall 2005), and tomato paste in beef patties (Candogan 2002). However, scientific information on the use of pink guava pulp as an antioxidant in meat products is very limited.
Retailing of raw pork meat emulsion is a practical strategy to meet the increasing consumer demand for meat products. However, processed pork products contain high levels of fat, and therefore are highly susceptible to oxidation. Osterlie and Lerfall (2005) reported that the addition of lycopene-rich natural ingredients in minced meat can improve colour and increase the fiber content in the diet, leading to various health benefits. Therefore, the present study was undertaken to evaluate the efficacy of different tomato products and pink guava pulp, as sources of natural antioxidants, in raw pork emulsion during refrigerated storage under aerobic packaging.
Materials and methods
Preparation of tomato products and pink guava pulp
Fresh, ripened tomatoes (Lycopersicon esculentum var. esculentum) purchased from local market were washed before the preparation of different products such as puree, pulp, and peel powder.
Tomato puree (TPE)
The tomatoes were scalded in hot water (80 °C) for 2 min, which facilitated the manual removal of peel and seeds. The tomato pulp was heated at 80 °C for 30 min with regular stirring. The resultant mass of TPE was cooled, filled into polypropylene bottles, and frozen at −18 °C.
Lyophilized tomato peel (LTP)
The manually removed tomato peel (after scalding) was freeze-dried in a lyophilizer (MSW-137, Mac India, Mumbai, India) at −40 °C for 18 h. The freeze-dried peel was pulverized in a grinder (Inalsa Technologies, New Delhi, India), and the fine powder was packaged in moisture impermeable high-density polyethylene (HDPE) bags. Packaged LTP was stored in a deep freezer (−18 °C) until used.
Tomato pulp (TPP)
The pulp, free off seeds and peel, was ground in a food mixer (Inalsa Technologies, New Delhi, India) to a paste and packed in low-density polyethylene (LDPE) bags. Packaged TPP was stored at −18 °C in a freezer until used.
Pink guava pulp (PGP)
Pink guava (Psidium guajava) fruits were purchased from local farm. The skin and seeds were separated, and the pulp was packed in LDPE bags and stored at −18 °C until used.
Preparation of pork emulsion
Animal Ethical Committee of Guru Angad Dev Veterinary and Animal Sciences University (GADVASU) has approved the experimental protocols for this study. The castrated Large White Yorkshire male pigs (8–10 months age and 90–100 kg weight), were selected from Instructional Livestock Farm, GADVASU reared under similar conditions of management and feeding. The selected animals were humanely harvested in the experimental abattoir of GADVASU. The carcasses were dressed, and chilled at 4 °C for 18 h, and then manually deboned. The deboned meat, free from excessive connective tissue and fat, was packaged in LDPE bags and stored at −18 °C in a freezer.
Frozen deboned lean pork was cut into cubes (2.54 cm × 2.54 cm × 2.54 cm) and double minced through 6 mm, and then 4 mm plate using a meat mincer (KL-32, Kalsi, Ludhiana, India). Similarly, back fat trimmings were also minced separately.
The levels of tomato products and pink guava pulp were selected on the basis of our previous investigations on optimization of level of incorporation (Joseph et al. 2010a, b, 2012). The formulation of five different treatments includes control (78 % pork and 10 % water), T-1 (78 % pork and 10 % TPE), T-2 (75.5 % pork and 12.5 % TPP), T-3 (78 % pork, 6 % LTP and 4 % water), and T-4 (78 % pork and 10 % PGP) and in addition 10 % back fat and 2 % common salt is added in all the treatments. All the ingredients as per the formulation were first hand-mixed for 5 min and subsequently for 60 s in a kneader-cum-blender (Inalsa Technologies, New Delhi, India) to prepare the emulsion. The prepared emulsions were packaged in LDPE bags, thermally sealed, and stored for 9 days at 4 ± 1 °C in darkness. The samples were drawn on 1, 3, 5, 7, and 9 days for analyses of physico-chemical properties and sensory attributes.
Meat pH and titratable acidity
Ten gram of emulsion was homogenized with 50 ml distilled water, and the pH of the suspension was recorded using digital pH meter (SAB 5000, LABINDIA, New Delhi, India). The titratable acidity was measured as described by Shelef and Jay (1970). Ten gram of meat emulsion was blended with 200 ml distilled water, and the volume was made up to 250 ml. The slurry was filtered through Whatman filter paper No. 2. Twenty-five milliliter of filtrate was collected, and 75 ml distilled water and three drops of 1 % phenolphthalein indicator were added. The resulting solution was titrated against 0.1 N NaOH till the endpoint (pink colour) was achieved. The titratable acidity was calculated as given below and expressed as percent lactic acid.
 |
Estimation of β-carotene content
Beta-carotene content in meat emulsion was measured using AOAC (1995) protocol with modifications. One gram meat emulsion sample was triturated with 20 ml of acetone in the presence of anhydrous sodium sulfate. The mixture was transferred to a centrifuge tube and was held at 4 °C for 15 min with occasional shaking and centrifuged at 3,500 g for 10 min (MP400R, Eltek, India Limited, New Delhi, India). The supernatant was decanted in separate tube, and the sample was re-extracted again with 20 ml of acetone. Both the supernatants were combined and filtered using Whatman filter paper No. 42. The absorbance of the extract at 449 nm was determined using a UV–VIS spectrophotometer (SL-159, Elico India Limited, Hyderabad, India). The concentration of β-carotene (mg/kg) was determined by an external standard method substituting respective absorbance in the linear regression formula (y = 0.087x; where y = absorbance, x = β-carotene concentration)
Estimation of lycopene content
The lycopene content was estimated following spectrophotometric method according to Fish et al. (2002) with minor modifications. Representative portions of the emulsion (2 g samples) were taken in amber-coloured vials, and 5 ml of 0.05 % (w/v) BHT in acetone, 5 ml of 95 % ethanol and 10 ml of hexane were added. The vials were kept in ice and stirred for 45 min. Then, 3 ml of deionized water was added to each vial, and the samples were again shaken for 10 min in ice. Then, it was incubated at 25 °C for 15 min to allow the separation of aqueous and oil phases. The absorbance of the hexane (upper layer) was recorded at 503 nm versus a blank of hexane solvent using a UV–VIS spectrophotometer (SL-159, Elico India Limited, Hyderabad, India). The lycopene content was estimated using the formula indicated below, where A503 is the absorbance at 503 nm and 31.2 is the constant.
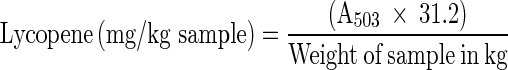 |
Instrumental colour evaluation
At the conclusion of storage periods, emulsions were removed from the packages, and the samples were spread in Petri dishes of 10 cm diameter and 1.2 cm thickness. CIE L* (lightness), a* (redness), and b* (yellowness) values, and hue angle were measured at three random locations on each Petri dish using a HunterLab MiniScan XE Plus colourimeter (HunterLab Associates, Reston, VA, USA) with illuminant D65, 2.54-cm diameter aperture, and 2° standard observer (AMSA 1991).
Visual colour and odour evaluation
The visual colour and odour scores were determined using a 5-point descriptive score card. The panel consisted of seven semi-trained experienced members selected among post graduate students and faculty members of the department. The panelists were briefed on the visual colour and odour of the pork samples. They were also detailed on colour and odour score card. On each storage day, the panelists were provided with fresh pork as reference samples. The test samples were presented to the panelists after assigning the suitable codes. The samples were evaluated for colour attributes on a numeric scale (1 = grayish pink, 2 = light reddish pink, 3 = moderately pink, 4 = dark reddish pink, and 5 = purplish red). In addition, panelists also evaluated odour on a numeric scale (1 = very unpleasant, 2 = moderately unpleasant, 3 = moderately pleasant, 4 = pleasant, and 5 = very pleasant).
Percent metmyoglobin formation
The percent metmyoglobin in pork emulsions was measured using the method of Trout (1989). Three gram of meat emulsion samples were homogenized with 30 ml of ice-cold 0.04 M phosphate buffer (pH 6.8) solution and centrifuged at 7,200 g for 5 min at 4 °C (MP400R, Eltek, India Limited, New Delhi, India). The supernatant was collected and filtered through Whatman filter paper No. 42. The absorbance was measured at 525, 572 and 700 nm using a UV–VIS spectrophotometer (SL-159, Elico India Limited, Hyderabad, India). Metmyoglobin percent was calculated using the formula of Krzywicki 1979.
 |
Thiobarbituric acid reactive substances (TBARS)
TBARS was measured using the extraction method described by Witte et al. 1970 with minor modifications. Ten grams samples were triturated with 25 ml of ice-cooled 20 % trichloroacetic acid (TCA) in 2 M orthophosphoric acid solution for 2 min. The contents were then quantitatively transferred to a beaker by rinsing with 25 ml of chilled distilled water, and filtered through Whatman filter paper No. 1. Three milliliter of filtrate were mixed with 3 ml of 0.005 M thiobarbituric acid (TBA) in a test tube and incubated in darkness for 16 h at 25 °C. A solution containing 3 ml of 10 % TCA and 3 ml of 0.005 M TBA reagent was used as a blank. The absorbance was measured at 532 nm using UV–VIS spectrophotometer (SL-159, Elico India Limited, Hyderabad, India), and TBARS value was calculated as mg malonaldehyde per kg of sample.
Peroxide value (PV)
PV was measured as described by Koniecko 1979 with modifications. Five gram of meat sample was blended for 2 min with 30 ml chloroform in the presence of anhydrous sodium sulfate. The mixture was filtered through Whatman filter paper No. 1. To a 25 ml aliquot of the filtrate, 30 ml glacial acetic acid and 2 ml saturated potassium iodide solution were added. The mixture was allowed to stand for 2 min with occasional shaking, and 100 ml of distilled water and 2 ml of fresh 1 % starch solution were added. This mixture was titrated against 0.1 N sodium thiosulfate till the endpoint (non-aqueous layer turning colourless) was reached. The peroxide value (meq/kg) was calculated as per the formula.
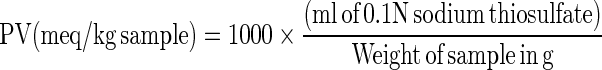 |
Free fatty acids (FFA) content
FFA content was estimated using method described by Koniecko 1979. Five grams of the meat emulsion were blended for 2 min with 30 ml of chloroform in the presence of anhydrous sodium sulfate. The homogenate was filtered through Whatman filter paper No. 1, and the filtrate was titrated against 0.1 N alcoholic potassium hydroxide using 0.2 % phenolphthalein indicator till the endpoint (permanent pink colour). FFA content was calculated as indicated below.
 |
Statistical analysis
Data were analyzed statistically using SPSS-16.0 (SPSS Inc. Chicago IL, USA) software package. The duplicate samples were drawn for each parameter, and the same sets of experiments were repeated three times. Data were analyzed with two-way ANOVA on the basis of 5 treatments × 5 storage days. Differences among means were detected at 5 % level (P < 0.05) using Duncan’s Multiple Range Test.
Results and discussion
Meat pH and titratable acidity
Results of pH and titratable acidity (Table 1) indicated that the pH of the pork emulsion varied (P < 0.05) among the treatments and with storage days. Control exhibited greater (P < 0.05) pH than other treatments. Overall, the pH decreased (P < 0.05) in all the treatments during storage. However, an increase (P < 0.05) in pH was observed in T-2 on day 9. In contrast, the pH of control decreased (P < 0.05) up to day 5 and increased thereafter. The pH was lower (P < 0.05) for T-3 than for other antioxidant treatments, and this may be attributed to the low pH (pH 4.51 ± 0.07) of the lyophilized tomato peel powder. The variation in pH amongst antioxidant treatments could be due to the inherent differences in the pH of tomato products and PGP. The pH of additives was 5.02 ± 0.09 for TPE and TPP, and was 4.96 ± 0.12 for PGP. The titratable acidity increased (P < 0.05) during storage and corresponded to the changes in pH. Furthermore, control samples demonstrated lower (P < 0.05) titratable acidity than the tomato products and pulp guava -treated emulsions throughout the storage.
Table 1.
Effect of different tomato products and pink guava pulp on pH and titratable acidity of raw pork emulsion during refrigerated (4 ± 1 °C) storage
| Treatments | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| pH1 | |||||
| Control | 5.94 ± 0.01cx | 5.87 ± 0.01cwx | 5.81 ± 0.02cw | 5.89 ± 0.01cwx | 5.88 ± 0.01cwx |
| T-1 | 5.80 ± 0.01bx | 5.78 ± 0.01bx | 5.76 ± 0.01bwx | 5.75 ± 0.01bw | 5.73 ± 0.01bw |
| T-2 | 5.82 ± 0.01bx | 5.81 ± 0.01bx | 5.78 ± 0.01bw | 5.78 ± 0.01bw | 5.82 ± 0.01bcx |
| T-3 | 5.54 ± 0.02ay | 5.49 ± 0.02axy | 5.46 ± 0.03ax | 5.41 ± 0.02aw | 5.38 ± 0.02aw |
| T-4 | 5.79 ± 0.01bx | 5.77 ± 0.01bx | 5.74 ± 0.02bwx | 5.73 ± 0.01bw | 5.72 ± 0.01bw |
| Titratable acidity1 | |||||
| Control | 0.032 ± 0.035aw | 0.035 ± 0.002awx | 0.043 ± 0.001ay | 0.039 ± 0.002axy | 0.039 ± 0.002axy |
| T-1 | 0.041 ± 0.002bw | 0.049 ± 0.002cx | 0.049 ± 0.001bxy | 0.049 ± 0.001bxy | 0.053 ± 0.001cy |
| T-2 | 0.040 ± 0.001bw | 0.040 ± 0.001abw | 0.043 ± 0.002awx | 0.050 ± 0.001bxy | 0.047 ± 0.002by |
| T-3 | 0.049 ± 0.001cw | 0.052 ± 0.002cw | 0.058 ± 0.001cx | 0.064 ± 0.001cy | 0.072 ± 0.001dz |
| T-4 | 0.040 ± 0.001bw | 0.043 ± 0.002bcw | 0.049 ± 0.001bx | 0.052 ± 0.001bxy | 0.055 ± 0.002cz |
T-1 = 10 % tomato puree; T-2 = 12.5 % tomato pulp; T-3 = 6 % lyophilized tomato peel; T-4 = 10 % pink guava pulp
Mean ± Standard Error for the same trait in a column (a–d) and in a row (w–z) without a common letter differ (P < 0.05)
1(n = 6)
Beta-carotene and lycopene contents
Beta-carotene and lycopene were absent in control samples. The levels of β-carotene and lycopene (Fig. 1) were greatest in T-3 followed by T-1, T-2, and T-4 in the order. The highest lycopene content observed in T-3 can be attributed to the fact that tomato peel contains 5 times more lycopene than pulp (Sharma and Le Mauger 2006). The relatively high content of active compounds (β-carotene and lycopene) in T-1 might be due to the consequence of the heat treatment during TPE preparation, which leads to breakdown of the cell wall of tomato matrix and thus enhances the release and bioavailability of lycopene and β-carotene (Shi and Le Maguer 2000; Dewanto et al. 2002). The availability of lycopene also depends on the moisture content in the tomato and guava products (Rao and Agrawal 1999). The moisture content varied from 71.84 ± 1.52 for TPP, 63.49 ± 1.76 for TPE, 3.81 ± 0.46 for LTP, and to 65.38 ± 0.89 for PGP. The lower level of β-carotene in T-4 could be attributed to its low concentration in guava fruits (Padula and Rodryuez Amaya 1986). The level of β-carotene in emulsion decreased (P < 0.05) during the storage period irrespective of the treatment, and the decline was dependent on the type of fruit product added to the emulsion. The loss of β-carotene was pronounced in T-3 during storage and might be due to the greater initial content of β-carotene in T-3 than the other antioxidant treatments. Lycopene content also decreased (P < 0.05) during storage, and the decrease was estimated as 5.82, 6.14, 14.01 and 16.43 % in T-1, T-2, T-3 and T-4, respectively. The stability of lycopene varies with the storage conditions such as light, temperature, water activity, oxygen as well as matrix characteristics (Goula and Adamopoulos 2005). The main causes of lycopene degradation are isomerization and oxidation.
Fig. 1.

Changes in β-carotene and lycopene content of raw pork emulsion incorporated with different tomato products and pink guava pulp during refrigerated (4 ± 1 °C) storage (n = 6) T-1 = 10 % tomato puree; T-2 = 12.5 % tomato pulp; T-3 = 6 % lyophilized tomato peel; T-4 = 10 % pink guava pulp a–d Mean ± Standard Error between treatments on the same storage day without a common letter differ (P < 0.05) w–x Mean ± Standard Error between storage days for the same treatment without a common letter differ (P < 0.05)
Instrumental colour evaluation
L* value (lightness) varied (P < 0.05) among treatments and storage days (Table 2). L* value was lower (P < 0.05) in tomato- and guava-treated pork emulsions than in control throughout the storage, and was lowest for T-3 except on day 9. These results indicated that the emulsions containing tomato and guava products were darker than the controls. Calvo et al. (2008) also reported a decrease in L* value in tomato peel incorporated beef and beef products. Similar observations were recorded in raw beef hamburgers incorporated with dried tomato peel (García et al. 2009), in pork patties incorporated with tomato powder (Kim et al. 2009), and in beef patties containing commercial lycopene preparation (Escalante et al. 2003).
Table 2.
Effect of different tomato products and pink guava pulp on instrumental colour and percent metmyoglobin of raw pork emulsion during refrigerated (4 ± 1 °C) storage
| Treatments | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| L* value 1 | |||||
| Control | 52.3 ± 0.19dwx | 53.9 ± 0.70dx | 50.9 ± 0.58dw | 52.5 ± 0.12ewx | 51.8 ± 0.36cw |
| T-1 | 45.3 ± 0.64cw | 47.1 ± 0.42cx | 46.1 ± 0.26cwx | 45.9 ± 0.42cwx | 45.9 ± 0.51bwx |
| T-2 | 42.1 ± 0.23bw | 44.7 ± 0.48bx | 44.7 ± 0.38bx | 43.2 ± 0.39bwx | 42.7 ± 0.48aw |
| T-3 | 38.0 ± 0.35aw | 39.6 ± 0.18ax | 38.7 ± 0.34awx | 39.3 ± 0.38ax | 41.6 ± 0.46ay |
| T-4 | 45.5 ± 0.31cw | 46.5 ± 0.41cwx | 45.7 ± 0.51bcw | 47.8 ± 0.49dx | 46.5 ± 0.80bwx |
| a* value 1 | |||||
| Control | 4.4 ± 0.03az | 4.1 ± 0.14ayz | 3.8 ± 0.23axy | 3.2 ± 0.24ax | 1.5 ± 0.07aw |
| T-1 | 8.6 ± 0.19cw | 8.5 ± 0.21cw | 8.4 ± 0.25cw | 7.9 ± 0.18cw | 7.4 ± 0.44cw |
| T-2 | 15.1 ± 0.19dx | 14.6 ± 0.35dx | 14.4 ± 0.47dwx | 13.3 ± 0.13dw | 13.9 ± 0.34dwx |
| T-3 | 16.7 ± 0.27ex | 15.0 ± 0.21dw | 14.7 ± 0.17dw | 14.6 ± 0.11ew | 14.5 ± 0.20dw |
| T-4 | 7.8 ± 0.36by | 6.5 ± 0.05bx | 6.5 ± 0.09bx | 6.1 ± 0.29bx | 4.6 ± 0.31bw |
| b* value 1 | |||||
| Control | 10.5 ± 0.09axy | 10.8 ± 0.18by | 10.0 ± 0.27bx | 9.4 ± 0.17aw | 9.3 ± 0.15aw |
| T-1 | 12.5 ± 0.16bx | 11.5 ± 0.17cwx | 11.5 ± 0.14cwx | 11.2 ± 0.15bw | 11.0 ± 0.31bw |
| T-2 | 14.2 ± 0.10cx | 13.2 ± 0.20dw | 13.5 ± 0.20dw | 13.4 ± 0.21cw | 12.7 ± 0.13cw |
| T-3 | 15.5 ± 0.21dy | 14.7 ± 0.21exy | 14.1 ± 0.18dx | 14.0 ± 0.17dx | 13.4 ± 0.19dw |
| T-4 | 10.3 ± 0.12ay | 9.8 ± 0.11axy | 9.3 ± 0.13ax | 8.9 ± 0.17awx | 8.5 ± 0.29aw |
| Hue angle1 | |||||
| Control | 67.4 ± 0.18cw | 69.1 ± 0.47cw | 69.4 ± 0.40cwx | 71.5 ± 0.54cx | 80.9 ± 0.25dy |
| T-1 | 53.1 ± 0.40bw | 53.4 ± 0.38bw | 53.8 ± 0.23bw | 54.9 ± 0.19bx | 56.0 ± 0.35by |
| T-2 | 43.3 ± 0.27aw | 42.1 ± 0.29aw | 43.1 ± 0.23aw | 45.2 ± 0.30ax | 42.4 ± 0.92aw |
| T-3 | 42.8 ± 0.37aw | 44.3 ± 0.45ax | 43.9 ± 0.46awx | 43.8 ± 0.28aw | 44.2 ± 0.43ax |
| T-4 | 52.7 ± 0.18bw | 56.4 ± 0.24bx | 55.0 ± 0.55bwx | 55.6 ± 0.30bx | 61.4 ± 0.43cy |
| Percent metmyoglobin2 | |||||
| Control | 54.3 ± 0.98bw | 67.3 ± 0.89cx | 70.5 ± 1.32cx | 78.7 ± 0.85cy | 91.5 ± 1.67dz |
| T-1 | 45.9 ± 1.76aw | 49.0 ± 2.98awx | 55.5 ± 1.57abx | 61.8 ± 1.23 ay | 69.9 ± 0.87bz |
| T-2 | 45.5 ± 1.09aw | 50.8 ± 0.54ax | 59.4 ± 1.97by | 73.7 ± 1.98bz | 78.40 ± 1.29cz |
| T-3 | 46.5 ± 2.16aw | 58.2 ± 1.44bx | 60.2 ± 1.32bxy | 63.9 ± 2.37ay | 68.2 ± 0.71bz |
| T-4 | 45.6 ± 0.87aw | 51.6 ± 1.85ax | 52.0 ± 1.05ax | 57.4 ± 0.81ay | 64.0 ± 0.89az |
T-1 = 10 % tomato puree; T-2 = 12.5 % tomato pulp; T-3 = 6 % lyophilized tomato peel; T-4 = 10 % pink guava pulp
Mean ± Standard Error for the same trait in a column (a–d) and in a row (w–z) without a common letter differ (P < 0.05)
1(n = 12)
2(n = 6)
Meat colour is the most important characteristic influencing the purchase decisions because consumers often consider surface redness as an indicator of wholesomeness. Surface redness (a* value) was greater (P < 0.05) in tomato-and guava-treated raw pork emulsions than the control (Table 2). Among the tomato products and guava pulp treatments, redness was greatest in T-3, followed by T-2 and T-1, and was lowest in T-4. This can be correlated with the lycopene and β-carotene content in the raw pork emulsion. Selgas et al. 2009 also observed an increase in a* value (P < 0.05) in hamburger with dried tomato powder. Furthermore, Escalante et al. 2003 reported greater (P < 0.05) a* values in lycopene-treated beef patties than in red pepper-treated ones. The a* values decreased during storage in all the treatments, and these observations are in agreement with previous reports in meat products incorporated with tomato products (Kim et al. 2009; Escalante et al. 2003; Candogan 2002). This might be due to decrease of lycopene content during storage. Hence, this can be interpreted as a* value depend on the concentration of lycopene in the meat emulsion.
The b* value (yellowness) also varied (P < 0.05) among the treatments (Table 2). The lowest b* value was recorded for T-4 and the highest for T-3, which suggested that LTP exerted more effect on yellowness than PGP. Similar to a* values, the b* values also decreased during storage irrespective of the treatment. In support, García et al. (2009) and Selgas et al. (2009) reported same trend for a* and b* values in hamburgers containing dried tomato powder.
Hue angle, which correlates with visual assessment of meat discolouration (Giroux et al. 2001), was greater (P < 0.05) in control than in the antioxidant treatments(Table 2). This could be attributed to the greater a* values in antioxidant treatments, which confirmed the colour-improving effect of tomato products and pink guava pulp in pork emulsion. Furthermore, this observation concurred with the results of visual colour evaluation. Among the antioxidant treatments, hue angle was greater (P < 0.05) in T-1 and T-4 than in T-2 and T-3. Overall, hue angle increased during the storage in all the treatments.
Percent metmyoglobin formation
Formation of brown metmyoglobin results in discolouration of raw meat and meat products. In the present study, metmyoglobin formation was greater (P < 0.05) in the controls than in tomato products and guava pulp -treated emulsions (Table 2). Nonetheless, percent metmyoglobin increased (P < 0.05) in control as well as tomato products and guava pulp -treated pork emulsions throughout the storage. However, the rate of increase was greater (P < 0.05) in control than in the antioxidant treatments. The observed lower metmyoglobin formation in the antioxidant-treated samples than controls could be attributed to the decrease in lipid oxidation, which is a major factor promoting myoglobin oxidation (Eyiler and Oztan 2011). Among the antioxidant-treated emulsions, percent metmyoglobin was greater (P < 0.05) in T-3 on day 3 and in T-2 on days 7 and 9 than the others. Furthermore, on day 9 % metmyoglobin was lowest in T-4. On the other hand, T-1 and T-3 exhibited comparable metmyoglobin formation during the storage, except on day 3.
Lipid oxidation
The PV was lower (P < 0.05) in T-1 and T-4 than control throughout the storage, whereas T-2 and T-3 exhibited PV comparable to control on days 1, 5, and 7 (Table 3). Nonetheless, PV in antioxidant-treated emulsions was numerically lower than in control. PV increased (P < 0.05) during the storage in all the treatments. Among antioxidant treatments, PV was greater (P < 0.05) for T-2 than for other treatments. The greater PV of T-2 may be explained on the basis of its greater pH than other antioxidant treatments. In partial agreement, Osterlie and Lerfall 2005 argued that low pH could minimize hydrolytic activity of microorganisms and reduce oxidative rancidity in meat products.
Table 3.
Effect of different tomato products and pink guava pulp on the lipid oxidation and odour scores of raw pork emulsion during refrigerated (4 ± 1 °C) storage
| Treatments | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| Peroxide value (meq/kg)1 | |||||
| Control | 5.1 ± 0.08cw | 5.4 ± 0.17bw | 6.1 ± 0.34bx | 7.1 ± 0.25cy | 7.5 ± 0.12cy |
| T-1 | 4.4 ± 0.10abw | 4.7 ± 0.12aw | 5.3 ± 0.13ax | 5.7 ± 0.12ax | 6.4 ± 0.27ay |
| T-2 | 4.7 ± 0.16bcw | 5.0 ± 0.17abw | 5.7 ± 0.17abx | 6.9 ± 0.22cy | 7.1 ± 0.12bcy |
| T-3 | 4.7 ± 0.13bcw | 4.8 ± 0.16aw | 5.5 ± 0.19abx | 6.7 ± 0.22bcy | 6.9 ± 0.07aby |
| T-4 | 4.2 ± 0.17aw | 4.6 ± 0.17aw | 5.3 ± 0.29ax | 6.1 ± 0.16aby | 6.5 ± 0.19ay |
| Thio barbituric acid residual substances (mg MDA/kg)1 | |||||
| Control | 0.89 ± 0.02dw | 1.1 ± 0.06dwx | 1.4 ± 0.03dx | 1.9 ± 0.06cy | 2.4 ± 0.03dz |
| T-1 | 0.68 ± 0.02bw | 0.88 ± 0.04cx | 0.98 ± 0.02bx | 1.1 ± 0.02ay | 1.6 ± 0.01bz |
| T-2 | 0.75 ± 0.01cw | 0.82 ± 0.01bwx | 1.1 ± 0.01cx | 1.3 ± 0.09by | 1.9 ± 0.06cz |
| T-3 | 0.58 ± 0.03aw | 0.69 ± 0.03aw | 0.85 ± 0.05bx | 1.3 ± 0.02by | 1.5 ± 0.04abz |
| T-4 | 0.67 ± 0.02bw | 0.83 ± 0.01bx | 0.95 ± 0.03abxy | 1.1 ± 0.05ay | 1.5 ± 0.04az |
| Free fatty acids (%)1 | |||||
| Control | 0.088 ± 0.003bw | 0.093 ± 0.002bwx | 0.103 ± 0.008bx | 0.115 ± 0.002by | 0.122 ± 0.002bz |
| T-1 | 0.080 ± 0.002abw | 0.086 ± 0.003ax | 0.088 ± 0.003ax | 0.096 ± 0.003aby | 0.105 ± 0.002az |
| T-2 | 0.085 ± 0.003abw | 0.090 ± 0.003abwx | 0.095 ± 0.003abx | 0.101 ± 0.002aby | 0.110 ± 0.002az |
| T-3 | 0.083 ± 0.004abw | 0.084 ± 0.003aw | 0.088 ± 0.002ax | 0.097 ± 0.003aby | 0.105 ± 0.002az |
| T-4 | 0.077 ± 0.002aw | 0.085 ± 0.002ax | 0.086 ± 0.004ax | 0.092 ± 0.005axy | 0.106 ± 0.001ay |
T-1 = 10 % tomato puree; T-2 = 12.5 % tomato pulp; T-3 = 6 % lyophilized tomato peel; T-4 = 10 % pink guava pulp
Mean ± Standard Error for the same trait in a column (a–d) and in a row (w–z) without a common letter differ (P < 0.05)
1(n = 6)
TBARS values were greater (P < 0.05) in control than in tomato- and guava-treated pork emulsions and increased (P < 0.05) during storage for all the treatments (Table 3). In control, TBARS was greater than the threshold limit of 2 mg MDA/kg (Eyiler and Oztan 2011; Greene and Cumuze 1982) on day 9 of storage, whereas it was lower than the threshold limit in all tomato products and guava pulp-treated products throughout the storage. TBARS values varied (P < 0.05) among the treatments and were lowest in T-3. On the last day of storage, TBARS was greater (P < 0.05) in T-2 than other antioxidant treatments. The TBARS values in T-1, T-2, and T-3 can be correlated to their lycopene and β-carotene contents (Fig. 1), and these results are in agreement with Osterlie and Lerfall (2005) and Djuric and Powell (2001). Martinez-Valverde et al. 2002 also reported that the antioxidant activity of tomato extracts was correlated to the levels of lycopene, ferulic acid, and caffeic acid, and was dependent on the tomato cultivar. On the other hand, T-4 demonstrated lower (P < 0.05) TBARS than most other antioxidant treatments on days 7 and 9. The lower TBARS in T-4, albeit its low lycopene content, may be attributed to the high content (200–300 mg/100 g) of vitamin C in fresh pink guava pulp (Holland et al. 1991), which exerts synergistic antioxidant effect with lycopene and other phytochemicals (Jacob et al. 2008; Escalante et al. 2003). The aforementioned results indicated improved oxidative stability of raw pork emulsion containing tomato products and pink guava pulp.
Similar to TBARS and PV, FFA also increased during storage in all the treatments (Table 3). FFA values were comparable in all the antioxidant treatments, but was greater (P < 0.05) in controls than the tomato products and guava pulp -treated products on day 9 of storage. This may be due to possible low lipolysis and lipolytic enzyme activity in antioxidant-treated products, leading to low production of free fatty acids (Aguirrezabal et al. 2000).
Visual colour and odour evaluation
Visual colour scores were greater (P < 0.05) for antioxidant-treated pork emulsions than control (Table 4). The colour scores decreased (P < 0.05) throughout the storage period in control and tomato products and guava pulp -treated raw pork emulsion. The results of visual colour scores demonstrate that LTP contributes more purplish-red appearance than PGP as well as other tomato products. García et al. 2009 observed an orange-red colouration of hamburgers with the addition of dried tomato powder.
Table 4.
Effect of different tomato products and pink guava pulp on the sensory quality of raw pork emulsion during refrigerated (4 ± 1 °C) storage
| Treatments | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|---|
| Visual colour scores1 | |||||
| Control | 2.7 ± 0.11ay | 2.3 ± 0.11axy | 2.5 ± 0.18ay | 2.1 ± 0.08ax | 1.7 ± 0.45aw |
| T-1 | 3.7 ± 0.11cy | 3.3 ± 0.11bx | 3.3 ± 0.17bx | 3.3 ± 0.11bx | 2.7 ± 0.11bw |
| T-2 | 3.8 ± 0.17cx | 3.8 ± 0.11cx | 3.8 ± 0.11cx | 3.6 ± 0.15cx | 3.1 ± 0.15bcw |
| T-3 | 4.2 ± 0.11dx | 4.1 ± 0.08dwx | 4.0 ± 0.00cwx | 4.0 ± 0.13dwx | 3.8 ± 0.11cw |
| T-4 | 3.3 ± 0.11bx | 3.3 ± 0.11bx | 3.1 ± 0.15bx | 3.0 ± 0.13bx | 2.3 ± 0.11abw |
| Odour scores1 | |||||
| Control | 4.3 ± 0.11aby | 3.5 ± 0.22abx | 3.2 ± 0.11abx | 2.3 ± 0.11aw | 2.0 ± 0.13aw |
| T-1 | 4.3 ± 0.11bz | 4.0 ± 0.13cy | 3.3 ± 0.11bcx | 3.2 ± 0.11bwx | 2.8 ± 0.11bw |
| T-2 | 4.3 ± 0.11bz | 4.0 ± 0.00cy | 3.5 ± 0.00cx | 3.3 ± 0.11bx | 3.0 ± 0.00bw |
| T-3 | 4.0 ± 0.00az | 3.3 ± 0.11ay | 3.0 ± 0.00axy | 2.8 ± 0.11ax | 2.3 ± 0.11aw |
| T-4 | 4.3 ± 0.11abz | 3.8 ± 0.11bcy | 3.3 ± 0.11bcx | 3.2 ± 0.11bwx | 3.0 ± 0.00bw |
T-1 = 10 % tomato puree; T-2 = 12.5 % tomato pulp; T-3 = 6 % lyophilized tomato peel; T-4 = 10 % pink guava pulp
Mean ± Standard Error for the same trait in a column (a–d) and in a row (w–z) without a common letter differ (P < 0.05)
1(n = 21)
On day 1 of storage, the odour scores for control were comparable (P < 0.05) to those of the antioxidant treatments. However, among the antioxidant treatments, the odour scores were lower (P < 0.05) for T-3 than T-1 and T-2 on day 1 of storage. The lower odour scores for T-3 could be possibly due to its higher acidity. Furthermore, the odour scores were lower (P < 0.05) for T-3 than all the treatments throughout storage. Odour scores decreased throughout storage (P < 0.05). On day 9, the sensory panelists recorded greater (P < 0.05) odour scores for T-1, T-2 and T-4 than T-3 and control. The observed odour scores were in agreement with the indicators for lipid oxidation (TBARS, PV, and FFA) in raw pork emulsion.
Conclusions
Tomato products (tomato puree, tomato pulp, and lyophilized tomato peel) and pink guava pulp are rich sources of natural antioxidants, such as β-carotene and lycopene, which can prevent lipid oxidation in complex food systems. The results of the present study demonstrated that the incorporation of tomato products and pink guava pulp improves colour and minimizes lipid oxidation in raw pork emulsion. Meat industry may utilize these natural ingredients as sources of antioxidants to minimize oxidation-induced quality deterioration in raw meat products.
References
- Aguirrezabal MM, Mateo MC, Dominguez MC, Zumalacarregui JM. The effect of paprika, garlic and salt on rancidity in dry sausages. Meat Sci. 2000;54:77–81. doi: 10.1016/S0309-1740(99)00074-1. [DOI] [PubMed] [Google Scholar]
- AMSA . Guidelines for meat colour evaluation. Chicago: American Meat Science Association; 1991. [Google Scholar]
- AOAC . Official methods of analysis of AOAC International. 16. Washington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Bernardini RD, Harnedy P, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and byproducts. Food Chem. 2011;124:1296–1307. doi: 10.1016/j.foodchem.2010.07.004. [DOI] [Google Scholar]
- Calvo MM, Garcia ML, Selgas MD. Dry fermented sausages enriched with lycopene from tomato peel. Meat Sci. 2008;80:167–172. doi: 10.1016/j.meatsci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Candogan K. The effect of tomato paste on some quality characteristics of beef patties during refrigerated storage. Eur Food Res Technol. 2002;215:305–309. doi: 10.1007/s00217-002-0567-1. [DOI] [Google Scholar]
- Deda MS, Bloukas JG, Fista GA. Effect of tomato paste and nitrite level on processing and quality characteristics of frankfurters. Meat Sci. 2007;76:501–508. doi: 10.1016/j.meatsci.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Descalzo AM, Sancho AM. A review of natural antioxidants and their effects on oxidative status, odour and quality of fresh beef produced in Argentina. Meat Sci. 2008;79:423–426. doi: 10.1016/j.meatsci.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- DiMasico P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Powell LC. Antioxidant capacity of lycopene containing foods. Int J Food Sci Nutri. 2001;52:142–149. doi: 10.1080/713671775. [DOI] [PubMed] [Google Scholar]
- Escalante SA, Torrescano G, Djenene D, Beltran JA, Roncales P. Combined effect of modified atmosphere packing and addition of lycopene rich tomato pulp, oregano and ascorbic acid and their mixtures on the stability of beef patties. Food Sci Technol Int. 2003;9:72–74. [Google Scholar]
- Eyiler E, Oztan A. Production of frankfurters with tomato powder as a natural additive. LWT-Food Sci Technol. 2011;44:307–311. doi: 10.1016/j.lwt.2010.07.004. [DOI] [Google Scholar]
- Fellenberg MA, Speisky H. Antioxidants: their effects on broiler oxidative stress and its meat oxidative stability. Poult Sci J. 2006;62:53–69. [Google Scholar]
- Fish WW, Perkins-Veazie P, Collins JK. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J Food Comp Anal. 2002;15:309–317. doi: 10.1006/jfca.2002.1069. [DOI] [Google Scholar]
- García ML, Calvo MM, Selgas MD. Beef hamburgers enriched in lycopene using dry tomato peel as an ingredient. Meat Sci. 2009;83:45–49. doi: 10.1016/j.meatsci.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Georgantelis D, Blekas G, Katikou P, Ambrosiadis I, Fletouris DM. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007;75:256–264. doi: 10.1016/j.meatsci.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Giroux M, Ouattara B, Refsah R, Smoragiewicz W, Saucier L, Lacroix M. Combined effect of ascorbic acid and gamma irradiation on microbial and sensorial characteristics of beef patties during refrigerated storage. J Agric Food Chem. 2001;49:919–925. doi: 10.1021/jf000544k. [DOI] [PubMed] [Google Scholar]
- Goula AM, Adamopoulos KG. Stability of lycopene during spray drying of tomato pulp. LWT-Food Sci Technol. 2005;38:479–487. doi: 10.1016/j.lwt.2004.07.020. [DOI] [Google Scholar]
- Greene BE, Cumuze TH. Relationship between TBA numbers and inexperienced panelists assessments of oxidized flavour in cooked beef. J Food Sci. 1982;47:52–58. doi: 10.1111/j.1365-2621.1982.tb11025.x. [DOI] [Google Scholar]
- Holland B, Welch AA, Unwin ID, Buzz DH, Paul AA, Southgate AT. McCance and Widdowson’s the composition of foods. 5. UK: The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food; 1991. [Google Scholar]
- Jacob K, Periago MJ, Bohm V, Berruezo GR. Influence of lycopene and vitamin-C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2008;99:137–146. doi: 10.1017/S0007114507791894. [DOI] [PubMed] [Google Scholar]
- Joseph S, Chatli MK, Biswas AK, Sahoo J (2010a) Oxidative stability of lycopene rich pork emulsion incorporated with different variants of tomatoes and pink guava. National Symposium on ‘Strategies for sustainable meat production and processing for nutritional security and employment generation’, Indian Veterinary Research Institute, Izatnagar, India November 19–20, Abstract No. 5.10, 143–144
- Joseph S, Chatli MK, Biswas AK, Sahoo J (2010b) Efficacy of lyophilized tomato peel as an antioxidant in pork emulsion during aerobic refrigerated storage. National Symposium on ‘Strategies for sustainable meat production and processing for nutritional security and employment generation’, Indian Veterinary Research Institute, Izatnagar, India, November 19–20, Abstract No. 5.17, 147–148
- Joseph S, Chatli MK, Biswas AK, Sahoo J (2012) Efficacy of pink guava pulp as an antioxidant in raw pork emulsion. J Food Sci Technol. doi:10.1007/s13197-012-0668-1 [DOI] [PMC free article] [PubMed]
- Kim LS, Jin SK, Hur IC, Choi SY, Jung HJ, Lee JK, Kang SH, Woo GM, Kang SN. Effect of tomato powder on meat patties as nitrite alternatives. Korean J Food Sci Anim Res. 2009;29:382–390. doi: 10.5851/kosfa.2009.29.3.382. [DOI] [Google Scholar]
- Koniecko R. Handbook for meat chemists. Wayne: Avery Publishing Group, Inc; 1979. pp. 53–55. [Google Scholar]
- Krzywicki K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of meat. Meat Sci. 1979;3:1–10. doi: 10.1016/0309-1740(79)90019-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Valverde I, Periago MJ, Provan G, Chesson A. Phenolic compounds lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum) J Food Sci. 2002;56:1489–1492. [Google Scholar]
- Mercadante AZ, Steck A, Pfander H. Carotenoids from guava (Psidium guajava L): isolation and structure elucidation. J Agric Food Chem. 1999;47:145–151. doi: 10.1021/jf980405r. [DOI] [PubMed] [Google Scholar]
- Osterlie M, Lerfall J. Lycopene from tomato products added minced meat: effect on storage quality and colour. Food Res Int. 2005;38:925–929. doi: 10.1016/j.foodres.2004.12.003. [DOI] [Google Scholar]
- Padula M, Rodryuez Amaya DB. Carotenoids and assessment of vitamin A of Brasilian Guava (Psidium guajava L) Food Chem. 1986;20:11–19. doi: 10.1016/0308-8146(86)90163-9. [DOI] [Google Scholar]
- Rao AV, Agrawal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases; a review. Nutr Res. 1999;19:305–323. doi: 10.1016/S0271-5317(98)00193-6. [DOI] [Google Scholar]
- Selgas MD, Garcia ML, Calvo MM. Effects of irradiation and storage on the physico-chemical and sensory properties of hamburgers enriched with lycopene. Int J Food Sci Technol. 2009;44:1983–1989. doi: 10.1111/j.1365-2621.2009.02017.x. [DOI] [Google Scholar]
- Sharma SK, Le Mauger M. Lycopene in tomato and tomato pulp fractions. Ital J Food Sci. 2006;8:107–113. [Google Scholar]
- Shelef LA, Jay JM. Use of a titrimetric method to assess the bacterial spoilage of fresh beef. Appl Microbiol. 1970;19:902–905. doi: 10.1128/am.19.6.902-905.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Maguer ML. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40:1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- Trout GR. Variations in myoglobin denaturation and colour of cooked beef, pork and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate and cooking temperature. J Food Sci. 1989;54:536–540. doi: 10.1111/j.1365-2621.1989.tb04644.x. [DOI] [Google Scholar]
- Valencia I, Ansonera D, Astiasaran I. Development of dry fermented sausages rich in docosahexaenoic acid with oil from the microalgae Schizochytrium sp: influence on nutritional properties, sensorial quality and oxidation stability. Food Chem. 2007;104:1087–1096. doi: 10.1016/j.foodchem.2007.01.021. [DOI] [Google Scholar]
- Valenzuela A, Nieto S. Synthetic and natural antioxidants: food quality protectors. Grasay Aceites. 1996;47:186–196. doi: 10.3989/gya.1996.v47.i3.859. [DOI] [Google Scholar]
- Witte VC, Krause GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid values of pork beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]


