Abstract
Chronic stress alters the hypothalamic-pituitary-adrenal (HPA) axis and enhances visceral and somatosensory pain perception. It is unresolved whether chronic stress has distinct effects on visceral and somatosensory pain regulatory pathways. Previous studies reported that stress-induced visceral hyperalgesia is associated with reciprocal alterations of endovanilloid and endocannabinoid pain pathways in DRG neurons innervating the pelvic viscera. In this study, we compared somatosensory and visceral hyperalgesia with respect to differential responses of peripheral pain regulatory pathways in a rat model of chronic, intermittent stress. We found that chronic stress induced reciprocal changes in the endocannabinoid 2-AG (increased) and endocannabinoid degradation enzymes COX-2 and FAAH (decreased), associated with down-regulation of CB1 and up-regulation of TRPV1 receptors in L6-S2 DRG but not L4-L5 DRG neurons. In contrast, sodium channels Nav1.7 and Nav1.8 were up-regulated in L4-L5 but not L6-S2 DRGs in stressed rats, which was reproduced in control L4-L5 DRGs treated with corticosterone in vitro. The reciprocal changes of CB1, TRPV1 and sodium channels were cell-specific and observed in the sub-population of nociceptive neurons. Behavioral assessment showed that visceral hyperalgesia persisted, whereas somatosensory hyperalgesia and enhanced expression of Nav1.7 and Nav1.8 sodium channels in L4-L5 DRGs normalized 3 days after completion of the stress phase. These data indicate that chronic stress induces visceral and somatosensory hyperalgesia that involves differential changes in endovanilloid and endocannabinoid pathways, and sodium channels in DRGs innervating the pelvic viscera and lower extremities. These results suggest that chronic stress-induced visceral and lower extremity somatosensory hyperalgesia can be treated selectively at different levels of the spinal cord.
Keywords: Chronic stress, pain, visceral hyperalgesia, somatosensory hyperalgesia, endocannabinoid, CB1, TRPV1, sodium channel
Introduction
Exposure to internal or external sources of stress including chronic psychological stress activates the hypothalamic–pituitary–adrenal (HPA) axis which leads to profound, region-specific alterations in dendrite and spine morphology, neurogenesis, receptor expression and neuronal function in the central nervous system (Joels et al., 2007). A number of studies support the association of stress with lowered pain sensation thresholds and enhanced pain perception that parallels the altered brain circuit activity (Lightman, 2008). Both visceral and somatic hyperalgesia have been reported in human patients under chronic stressful conditions (Larauche et al., 2012;Hannibal & Bishop, 2014). Several pathophysiological mechanisms have been implicated in the development and modulation of this stress-enhanced pain perception (Johnson & Greenwood-Van, 2014). The corticotrophin-releasing factor (CRF) signaling pathway has been widely studied, including but not limited to the exploration of its region-specific expression in the central nervous system and its functional correlation in stress-related activation of the HPA axis and modulation of pain pathways (Chappell et al., 1986;Tran et al., 2014). Chronic stress also alters the brain derived neurotropic factor (BDNF) pathway and glucocorticoid receptor (GR) expression in specific brain regions in humans and animal models related to depression and post-traumatic stress disorder (Gray et al., 2013;Suri & Vaidya, 2013).
In the peripheral nervous system, there is emerging evidence that animal models of neuropathic pain demonstrate region- and cell-specific changes in primary sensory pain regulatory pathways. For example, femoral artery occlusion increases expression of acid-sensing ion channels in dorsal root ganglia (DRG) neurons innervating skeletal muscle (Liu et al., 2010), and selective spinal nerve (L5 DRG) ligation causes changes in expression of sensory organ-specific microRNAs in rat DRGs resulting in mechanical hypersensitivity (Aldrich et al., 2009). These changes were observed in both myelinated and unmyelinated primary afferent neurons. The diabetic rat demonstrates increased expression in the endovanilloid TRPV1 receptor that correlates with enhanced cell stress in large-soma L4-L5 DRG neurons contributing to somatosensory hyperalgesia (Hong & Wiley, 2005;Hong et al., 2008). These observations suggest that different models of neuropathic pain can have distinct effects on primary sensory nerve function, particularly L4-L5 DRG neurons that contribute to the sciatic nerves in rodents (Swett et al., 1991).
Functional visceral pain and neuropathic pain disorders have been associated with activation of the ATP ion-gated channels, voltage-gated sodium and calcium channels, as well as up-regulation of protease-activated receptors (PAR2), TRPV1 receptor, serotonin, cannabinoids and cholecystokinin (de Carvalho Rocha et al., 2014;Wood, 2004). Voltage-gated sodium channels (Nav) are arguably the most thoroughly studied ion channels (Bagal et al., 2014;Catterall, 1992). Tetrodotoxin (TTX)-sensitive Nav1.7 is broadly expressed in DRG neurons, while TTX-resistant Nav1.8 and Nav1.9 are largely restricted to the subset of small- and medium-soma, nociceptive neurons (Akopian et al., 1999). The role of Nav1.7, Nav1.8, and Nav1.9 in pain signaling has been well established in pharmacological, molecular and genetic studies with preclinical species (Cummins et al., 2007;Liu & Wood, 2011). Missense mutations in Nav1.7 are causally linked to inherited erythromelalgia (Yang et al., 2004). Neonatal maternal deprivation stress enhanced expression of TTX-resistant Nav1.8 sodium channels in DRGs innervating the colon in adult rats (Hu et al., 2013). We reported previously that chronic stress induced visceral hyperalgesia that was associated with reciprocal changes in anti-nociceptive endocannabinoid CB1 receptor (down-regulated) and pro-nociceptive endovanilloid TRPV1 receptor (up-regulated) in primary sensory L6-S2 DRG neurons innervating pelvic organs, including the distal colon (Hong et al., 2009).
The goal of the current study was two-fold: 1. Investigate whether chronic stress-associated changes observed in endocannabinoid levels, CB1 and TRPV1 receptor levels are expressed in a region-specific manner limited to the L6-S2 DRGs innervating pelvic visceral including the distal colon or, alternatively, expressed more broadly and include the L4-L5 DRG neurons that contribute to the somatosensory innervation in the lower extremities; and 2. Is the earlier recovery in somatosensory hyperalgesia compared to visceral hyperalgesia after chronic, intermittent stress associated with reversible changes in sodium channel levels. Our results demonstrate that chronic, intermittent stress produces differential, region- and cell-specific changes in pain regulatory pathways in primary sensory DRG neurons innervating the pelvic viscera compared to the somatosensory innervation of the lower extremities, and suggest that distinct regulatory pathways are involved in chronic stress-induced visceral and somatosensory hyperalgesia that demonstrate differential, time-dependent recovery.
Materials and methods
Animals and experimental design
Male Sprague-Dawley rats (weighting 160-180 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in the animal facility that was maintained at 22°C with an automatic 12 h light/dark cycle. The animals received a standard laboratory diet and tap water ad libitum. All experiments were approved by the University of Michigan Committee on Use and Care of Animals according to National Institutes of Health guidelines. The experimenter was blinded to animal treatment during behavioral experiments.
Animals were hosted in the animal facility for 5-7 days after their arrival before starting the experiments. Then the animals, weighing 200-220 g, were randomly grouped and were subjected to 10-day water avoidance (WA) stress or repeated corticosterone administration. Behavioral assessment were conducted 24-hour/72-hour (day 11, day 14 respectively) after completing the 10-day stress procedure. A separate group of stressed rats was assessed for visceral pain sensation 6 weeks after completing the 10-day WA stress. Biochemical and molecular characterization of pain regulatory pathways were performed using separate groups of animals without behavioral measurements.
Water avoidance stress
Repeated exposure of male rats to water avoidance (WA) stress was conducted as described previously (Hong et al., 2009;Hong et al., 2011). The rats were placed on a glass platform in the middle of a tank filled with water (25 °C) to 1 cm below the height of the platform. The animals were maintained on the tank for 1 h in the morning daily for 10 consecutive days. The control (sham-stress) rats were placed similarly for 1 h daily for 10 days in a tank without water. In a separate study, several groups of healthy control rats were received the optimal dose of CORT (3 mg/kg; Sigma-Aldrich, St. Louis, MO) in 5% ethanol and 95% saline via subcutaneous (s.c.) injection in the morning, daily, for 10 days to reproduce the serum corticosterone (CORT) level observed in the stressed rats as described previously (Hong et al., 2011).
Measurement of serum corticosterone
Serum corticosterone levels were determined as described previously (Hong et al., 2009). Briefly, blood was collected from the rat after 1 hour WA stress on day 10 of the stress procedure or corticosterone injection. Total corticosterone in serum samples was analyzed by the Correlate-EIA corticosterone enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) according to the manufacturer’s instructions. All samples were run in triplicates with standard corticosterone controls and kit calibrators in each analysis. Absorbance at 405 nm was measured with a reference wavelength of 680 nm.
Visceral motor response (VMR) to colorectal distention (CRD)
Routine procedures were conducted as previously described (Hong et al., 2011). Briefly, rats were deeply anaesthetized with subcutaneous injection of a mixture of ketamine (60 mg/kg) and xylazine (5 mg/kg). An incision was made in the skin of the lower abdomen, and two perfluoroalkoxy-coated, 32-gauge stainless steel wires were inserted into the external oblique pelvic muscles superior to the inguinal ligament. Animals were injected with 500 μL of 0.9% saline and artificial tears eye ointment was placed onto their eyes. The animals were then allowed to recover for 3-5 days prior to the VMR measurement.
Measurement of the VMR to CRD was conducted in separate groups of rats on day 11 (WA group), day 14 (WA-Rec group) and 6-week after the completion of 10-day WA stress procedure. The VMR was quantified by measuring electromyographic activity in the external oblique musculature in the awake animals. CRD was conducted to constant pressures of 10, 20, 40, and 60 mmHg by a custom-made distension control device. The responses were considered stable if there was less than 20% variability between 2 consecutive trials of CRD at 60 mm Hg. The increase in the area under curve (AUC), which is the sum of all recorded data points multiplied by the sample interval (in seconds) after baseline subtraction, was presented as the overall response during the course of the CRD test.
Thermal Hyperalgesia (Hargreaves test)
Thermal analgesia was measured using an IITC analgesia machine (Model 336TG IITC, Woodland Hills, CA) as described previously (Sullivan et al., 2007). To quantify thermal sensitivity, stressed rats (WA and WA-Rec groups) and the matched controls were placed on a temperature controlled glass plate (32 °C) inside a plexiglas compartment and allowed to acclimate for at least 1 h. A light source was placed under the hindpaw and the time it took for a hindpaw withdrawal from the light source was measured in seconds. The light source reached a maximum of 75 °C. A cutoff time of 15 seconds was applied to protect the rats from injury. The latency to foot withdrawal (escape) served as the behavioral measure of thermal nociception. Each foot was tested 5 times with 3 min between stimulations of either foot to avoid peripheral sensitization effects. As compared with the baseline (control) latency, a significant decrease in the latency of foot withdrawal in response to the thermal stimulus was interpreted as indicating the presence of thermal hyperalgesia.
Mechanical Allodynia (von Frey test)
Mechanical allodynia was determined by a significant decrease in mechanical threshold of the stressed rat compared to that of the control rat. To quantify mechanical sensitivity of the foot, brisk foot withdrawal in response to a normally innocuous mechanical stimulus was measured as described previously (Cheng et al., 2009). Control and stressed (WA and WA-Rec groups) animals were placed in Plexiglas cage with mesh flooring and allowed to acclimate for 1 h. Response to the mechanical stimulus was measured with von Frey filaments (von Frey hairs; Stoelting, Wood Dale, IL). Testing started with a 6 g filament and increased until a response was elicited. Brisk withdrawal of the hindpaw indicated a positive response. Once there was a response, we used the up-down method (Cheng et al., 2009). Six positive and negative data points were collected and a 50% paw withdrawal threshold was calculated. A significant decrease in the pressure necessary to elicit a brisk foot withdrawal in response to this mechanical stimulus was interpreted as mechanical allodynia.
Mass Spectrometry (LC-APCI/MS-MS)
DRG samples used for mass spectrometry analysis were prepared according to the method described (Hong et al., 2009). Briefly, L4-L5 and L6-S2 DRGs from control and WA stressed rats (n = 5 each group) that had not undergone surgery or colorectal distention were dissected on day 11 of the WA stress procedure, combined and homogenized in 2.0 ml acetonitrile containing 114 pmol of deuterated Arachidonic Acid. The supernatants were concentrated under a constant stream of nitrogen and subjected to LC-APCI/MS analysis. Analysis of 2-AG (2-Arachidonoylglycerol) was performed using a ThermoFinnigan Surveyor HPLC system in conjunction with a triple quadrupole mass spectrometer (Thermo Finnigan TSQ Quantum Ultra AM, West Palm Beach, FL). For quantitative analysis, the peak area of 2-AG ions from the test samples was compared and normalized. The standard curve was obtained by injecting known amounts of 2-AG ranging between 1 pM and 100 μM and plotting peak area versus molar concentration.
Administration of corticosterone in vitro
For in vitro studies, DRGs from control rats were isolated and treated with corticosterone as previously described (Hong et al., 2011). Briefly, L4-L5 and L6-S2 DRGs from control rats were isolated, chopped and incubated in Minimal Essential Medium (Gibco) supplemented with 3% fetal bovine serum and 50 ng/ml nerve growth factor in 95% air + 5% CO2 at 37°C. Corticosterone (10 μM) was added to the incubation wells and incubated for 24 hours. The DRGs were then collected for Western blot analysis.
Western blot analysis
DRG neurons from T5-T6, L4-L5 and L6-S2 regions in rats that had not undergone surgery or VMR measurement were taken on day 11 of the WA stress procedure. Protein were extracted and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE blotting and probed with specific antibodies for CB1 (Cayman Chemical, Ann Arbor, MI), TRPV1 (Santa Cruz Biotechnology, Dallas, TX), COX-2 (Cell Signaling Tech, Boston, MA), FAAH (Cayman Chemical), Nav1.7 (Cell Signaling Tech), Nav1.8 (Sigma-Aldrich), and β-actin (Sigma-Aldrich) at 4°C, overnight, and subsequently with secondary antibodies (GE Healthcare Bio-Sciences, Pittsburgh, PA) for 1 h. The X-ray films were developed using SuperSignal West Dura Chemiluminescent Substrate Kit (Thermo Fisher Scientific, Rockford, IL).
Immunohistochemistry
L4-L5 and L6-S2 DRG neurons from rats that had not undergone surgery or colorectal distention were removed on day 11 (WA group) or day 14 (WA-Rec group) of the WA stress procedure for immunofluorescence staining. Primary antibodies used for overnight incubation were anti-TRPV1 (1:500; Santa Cruz Biotechnology), anti-CB1 (1:300; Cayman Chemical), anti-sodium channel NaV1.7 (1:500; Cell Signaling Tech), and anti-sodium channel NaV1.8 (1:500; Sigma-Aldrich). Other primary antibodies used in some experiments were the antibodies of peripherin, a marker of small C-fibers (1:250; Life Technologies, Grand Island, NY) and NF200, a marker of large A-β fibers (1:500; Sigma-Aldrich). Secondary antibodies Alexa Fluor 488 (1:400) and Alexa Fluor 594 (1:500) from Molecular Probes (Life Technologies, Grand Island, NY) were used for the 2 h incubation. Sections were mounted with an anti-fade fluorescence mounting medium for microscope viewing.
Imaging analysis
Immunofluorescence stained DRG sections were viewed under a Zeiss Axioplan fluorescence microscope, and digitized images were acquired covering the entire DRG in one field under low magnification. Images from 4 to 5 different DRG sections from each rat were pooled for counting in each group. In each rat, 1000–1500 total DRG neurons were counted. Neurons were judged to be positive if they had mean brightness values greater than the corresponding control value stained with the secondary antibody alone.
Statistical analysis
To examine the VMR in response to CRD pressures, the electromyographic amplitudes, represented by calculating the AUC, were normalized as percentage of baseline response for the highest pressure (60 mm Hg) for each rat and then averaged for each group of rats. The effects of stress and/or pharmacologic treatment on the VMR to CRD was analyzed using a repeated-measures two-way ANOVA followed by Bonferroni post-test comparisons. The differences in the thermal and mechanical sensitivity between control and WA or WA-Rec groups were compared by using 2-tail Student t test. Unpaired Student t test was used to examine the data for the protein expression obtained from Western blot and immunohistochemistry. Results were expressed as means ± standard error. P < 0.05 was considered statistically significant.
Results
Chronic WA Stress induced region-specific changes of TRPV1 and CB1 receptor expression levels in rat DRGs
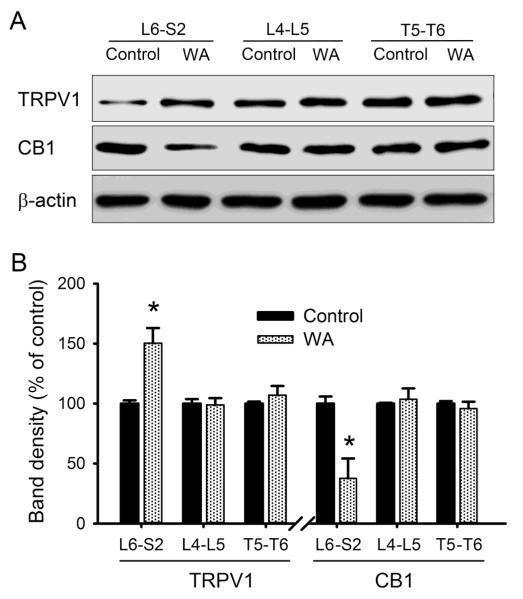
After 10 days of intermittent WA stress, the serum CORT level was 330.2 ± 28.2 ng/ml in stressed rats, which is significant higher than that in control rats (102.9 ± 19.5 ng/ml; P < 0.05). To determine the expression of TRPV1 and CB1 in other DRG regions, T5-T6, L4-L5 and L6-S2 DRGs were isolated from control and WA stressed rats for immunoblotting. As shown in Fig. 1A, chronic WA stress was not associated with significant changes in either CB1 or TRPV1 levels in L4-L5 DRG neurons that contribute to the somatosensory innervation of the lower extremities via the sciatic nerve or T5-T6 DRGs that innervate the sensory innervation of the lower thorax and upper abdomen. In contrast, WA stress caused significant increase in TRPV1 protein level (50.3±12.6%, P < 0.05) and significant decrease in CB1 protein level (62.3±16.5%, P < 0.05) in L6-S2 DRGs that innervate the pelvic viscera (Fig. 1B), which is consistent with our previous report (Hong et al., 2009).
Fig. 1.
Western blot analysis revealed region-specific alterations of TRPV1 and CB1 receptors in DRGs in WA stressed rats compared with controls. (A) Typical blotting bands demonstrating the expression levels of TRPV1 and CB1 in T5-T6, L4-L5 and L6-S2 DRG regions. (B) Graph depicting the percentage changes in the receptor expression in DRGs from control and stressed animals (n = 5). Data are expressed as mean ± standard error. *, P < 0.05.
Chronic WA stress was associated with preferential increase of TRPV1 receptor expression in the subpopulation of nociceptive neurons in L6-S2 DRGs
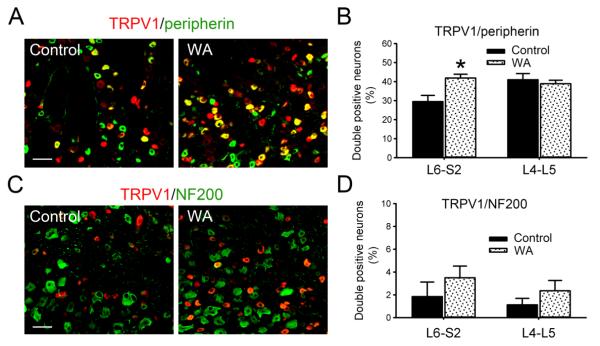
As shown in Fig. 2A, the TRPV1 immunoreactivity signal was increased in L6-S2 DRGs from WA stress rats compared with controls. In L6-S2 DRGs, the number of double-positive neurons for TRPV1 and small-sized, nociceptive C-fiber neuronal marker, peripherin, was 29.5±3.1% in controls rats and increased significantly to 42.0±2.0% in WA stressed rats (P < 0.05). In L4-L5 DRGs, the number of double-positive neurons for TRPV1 and peripherin was 41.4±3.3% and 38.9±1.8% in control and WA stressed rats, respectively, and no significant difference was observed (P = 0.6; Fig. 2B). The expression level of TRPV1 in large soma, myelinated Aβ-fiber neuronal subtypes (NF200 immunoreactivity-positive) was low as shown in Fig. 2C. The difference in the number of double-positive neurons for TRPV1 and NF200 between control and WA stressed rats was not significant in either L6-S2 DRGs or L4-L5 DRGs (Fig. 2D).
Fig. 2.
Colocalization of TRPV1 in neuronal subpopulations in L6-S2 DRGs. (A) Double immunofluorescence staining of TRPV1 (red) and small-sized nociceptive C-fiber marker, peripherin (green), in L6-S2 DRGs from control and WA stressed rats. (B) Statistical data of the double immuoreactivity-positive neurons for TRPV1 and peripherin in L4-L5 and L6-S2 DRGs (n = 4). (C) Double immunofluorescence staining of TRPV1 (red) and large-sized Aβ-fiber marker, NF200 (green), in L6-S2 DRGs from control and WA stressed rats. (D) Statistical data of the double immunoreactivity-positive neurons for TRPV1 and NF200 in L4-L5 and L6-S2 DRGs (n = 4). Scale bar, 60 μm. Data are expressed as mean ± standard error. *, P < 0.05.
Chronic WA stress induced decrease in CB1 expression in both small unmyelinated C-fiber and large myelinated Aβ-fiber neurons in L6-S2 but not L4-L5 DRGs
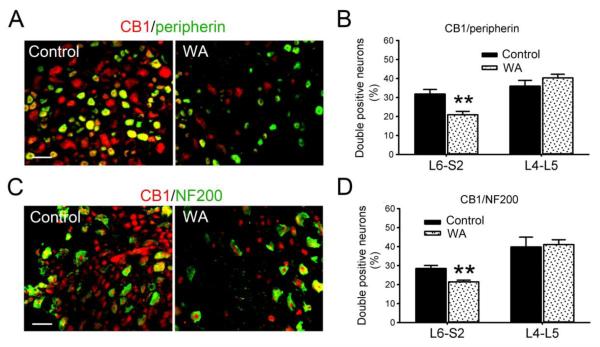
The CB1 immunoreactivity was greatly reduced in L6-S2 DRGs from WA stressed rats compared with controls (Fig. 3A, C). The number of double-positive neurons for CB1 and peripherin was significantly decreased in WA stressed rats (20.9±1.7%) compared with the controls (31.8±2.3%) in L6-S2 DRGs (P < 0.01). This number was not changed significantly in L4-L5 DRGs in control (35.9±3.0%) and WA stressed (40.3±1.9%) rats (P = 0.5; Fig. 3B). Similarly, the number of double-positive neurons for CB1 and NF200 decreased 25.0% in L6-S2 DRG neurons in WA stressed rats compared with controls (P < 0.01), whereas this number did not change significantly in L4-L5 DRGs in control and WA stressed rat groups as shown in Fig. 3D.
Fig. 3.
Decreased expression of CB1 in both small-sized and large-sized L6-S2 DRG neurons in WA stressed rats. (A) Double immunofluorescence staining of CB1 (red) and peripherin (green) in L6-S2 DRG neurons in control and WA stressed rats. (B) Statistical data of the double immuoreactivity-positive neurons for CB1 and peripherin in L4-L5 and L6-S2 DRGs (n = 4). (C) Double immunofluorescence staining of CB1 (red) and NF200 (green) in L6-S2 DRG neurons in control and WA stressed rats. (D) Statistical data of the double immunoreactivity-positive neurons for CB1 and NF200 in L4-L5 and L6-S2 DRGs (n = 4). Scale bar, 60 μm. Data are expressed as mean ± standard error. **, P < 0.01.
Chronic WA stress induced region-specific changes of endocannabinoid 2-AG content in DRGs
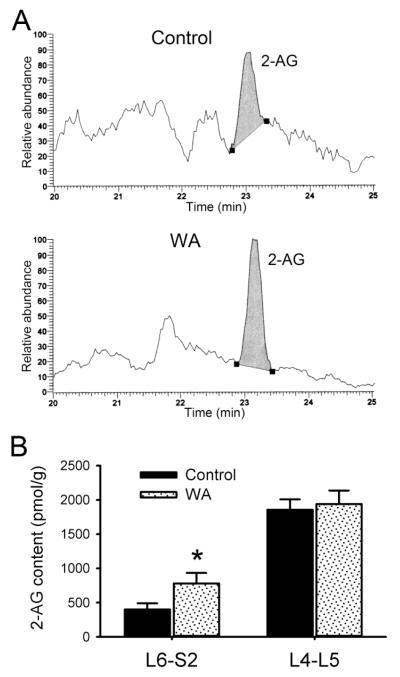
Representative chromatographs depicting the content of 2-AG in DRG tissue extracts are shown in Fig. 4A. The value from L6-S2 DRGs of control rats was 400.2±90.7 pmol/g tissue weight for 2-AG, compared to 781.0±153.4 pmol/g tissue weight in WA stressed rats, corresponding to 95% higher 2-AG content compared to controls (Fig. 4B). This difference was significant (P < 0.05), which is consistent with the enhanced content of endocannabinoid anandmide in L6-S2 DRGs of stressed rats that we reported previously (Hong et al., 2009). The 2-AG content was higher in L4-L5 DRGs compared to L6-S2 DRGs. However, no significant difference was observed between control (1857.5 ± 152.3 pmol/g) and WA stressed (1941.6 ± 198.8 pmol/g) rats (Fig. 4B).
Fig. 4.
WA stressed induced differential alterations of the endocannabinoid 2-AG content in L4-L5 and L6-S2 DRGs. (A) Representative chromatograph from L6-S2 DRG extracts depicting the relative abundance of endocannabinoid 2-AG in control and WA stressed rats. (B) The bar graph illustrated the increase in 2-AG content in L6-S2 but not L4-L5 DRGs in rats following chronic WA stress compared to controls (n = 5). Data are expressed as mean ± standard error. *, P < 0.05.
Chronic WA stress and corticosterone administration in situ in healthy rats induced region-specific decreases of endocannabinoid degradation enzymes
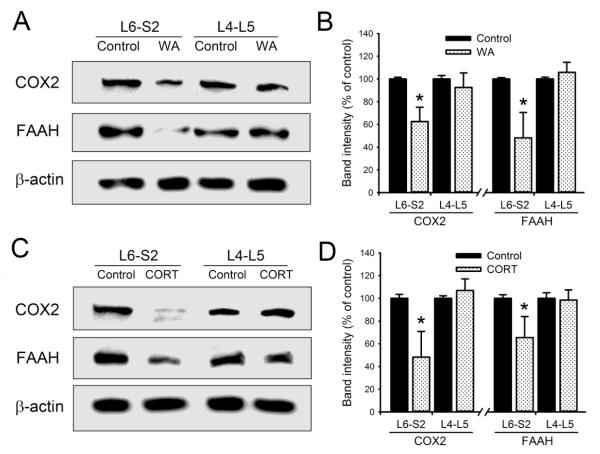
We examined the protein levels of endocannabinoid degradation enzymes in L4-L5 and L6-S2 DRGs from both chronic WA stressed and corticosterone-injected rats. Repeated injection of corticosterone in healthy control rats increased serum corticosterone level to 393.6 ± 50.8 ng/ml on day 10, which is significantly higher compared with controls and similar to the level observed in WA stress rats. As shown in Fig. 5A, the protein levels of both cyclooxygenase-2 (COX2) and fatty acid amide hydrolase (FAAH) decreased significantly in L6-S2 DRGs from WA stressed rats compared with controls. The levels of COX2 and FAAH decreased 37.6±12.6% and 51.8±22.4% in L6-S2 DRGs from WA stressed rats compared with controls, respectively (P < 0.05; Fig. 5B). The changes of COX2 and FAAH expression levels were not significantly in L4-L5 DRGs between control and WA stressed rats. Similarly, repeated corticosterone injection in control rats reproduced the changes in COX2 and FAAH in L6-S2 DRGs that were observed in the WA stress rats (Fig. 5C). The levels of COX2 and FAAH decreased 51.7±20.6% and 35.1±18.7% in L6-S2 DRGs from corticosterone-injected rats compared to the vehicle-treated controls, respectively (P < 0.05). No significant changes of COX2 and FAAH expression levels were observed in L4-L5 DRGs from control and corticosterone-injected rats (Fig. 5D).
Fig. 5.
Differential changes in endocannabinoid degradation enzymes in L4-L5 and L6-S2 DRGs. (A) Western blot analysis showed preferentially decreased expression levels of COX2 and FAAH protein in L6-S2 but not L4-L5 DRGs in WA stressed rats compared with controls. (B) Statistical analysis of the percentage change of COX2 and FAAH proteins in DRGs in control and WA stressed rats (n = 4). (C) Repeated corticosterone treatment of healthy control rats induced down-regulation of COX2 and FAAH in L6-S2 DRGs but not L4-L5 DRGs compared to untreated controls. (D) Bar graph depicting the percentage change of COX2 and FAAH proteins in L6-S2 and L4-L5 DRGs in the rats treated with corticosterone (n = 4). Data are expressed as mean ± standard error. *, P < 0.05.
Chronic WA stress resulted in increased visceral hyperalgesia that persisted longer than 3 days
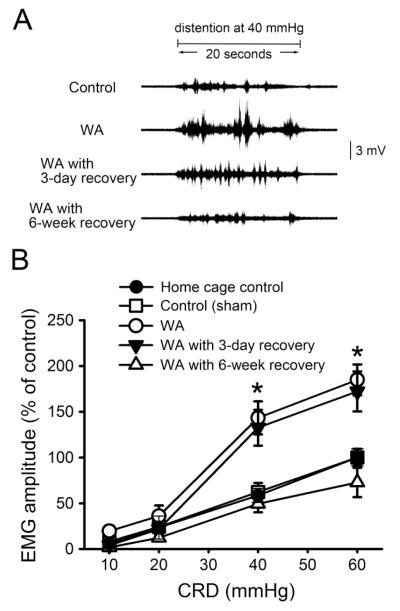
Visceral nociception was assessed by measuring VMR to graded levels of CRD in control, WA stressed rats at 24 h, 3 days, and 6 weeks after completing the WA stress phase. As shown in Fig. 6A, the frequencies and amplitude of abdominal muscle contraction signals in response to colorectal distention were larger at 40 mmHg distention pressure in WA stressed rats and in WA stressed rats with 3-day recovery compared with the controls, while they returned to the control level in WA stressed rats 6 weeks after completing the stress phase. In response to CRD, the changes of electromyographic (EMG) amplitude, expressed as AUC percentage, were significantly higher in the WA stressed rats compared with the levels of control (sham) and home cage control (without any treatment) rats (Fig. 6B). No difference in EMG amplitude was observed between control (sham) and home cage control groups at the distention pressures from 10 to 60 mmHg. The EMG amplitudes increased to 143.2±18.1% and 184.9±16.8% in WA stressed rats compared to controls at 40 and 60 mmHg, respectively (P < 0.05). The EMG amplitudes in WA stressed rats at 3 days after completion of the stress phase were 132.6±19.6% and 172.2±21.5% at 40 and 60 mmHg, respectively, which is significantly increased compared to controls (P < 0.05). Six weeks after completing the WA stress phase, the EMG amplitudes in WA stressed rats were 49.5±9.2% and 72.8±16.0% at pressures of 40 and 60 mmHg respectively, were not significantly different when compared to controls (62.4±9.8% and 100±9.5%, respectively).
Fig. 6.
Effect of chronic WA stress on visceral motor response (VMR) in response to colorectal distention (CRD) at different time duration after WA stress. (A) Representative EMG recordings depicting the VMR to CRD at the distention pressure of 40 mmHg in control rats, WA stress rats, and WA stress rats with recovery for 3 days and 6 weeks, respectively, after completing the stress procedure. (B) Electromyographic amplitude expressed as area under curve (AUC) of the raw electromyographic response was significantly increased following chronic WA stress on day 11 (WA) and day 14 (WA with 3-day recovery), and returned to baseline level 6 weeks after stress (WA with 6-week recovery). Data are expressed as mean ± standard error, n = 6-8 in each group. *, P < 0.05 between the following groups: control and WA, control and WA with 3-day recovery, WA or WA with 3-day recovery and WA with 6-week recovery.
Somatosensory hyperalgesia returned to control levels within 3 days after completion of the WA stress phase
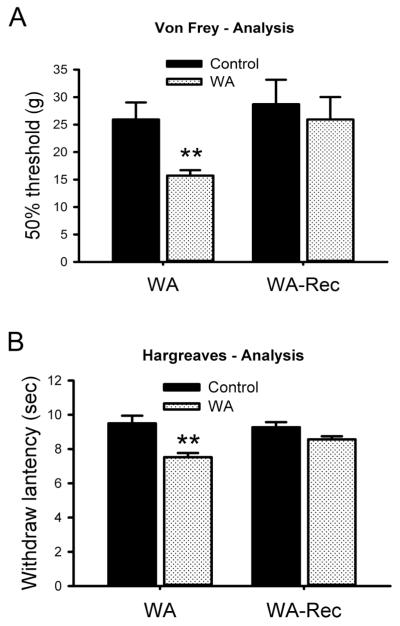
Somatosensory mechanical allodynia was determined by measuring the hind paw withdrawal threshold in response to application of a von Frey probe (Fig. 7A). WA stressed rats showed a significant decrease in the pressure required to elicit paw withdrawal as compared with the response of controls at the completion of the WA stress phase. The 50% threshold of withdrawal pressure was 25.9±3.1 g in controls and 15.7±2.8 g in WA stressed rats (P < 0.01). The paw pressure withdrawal threshold returned to control levels in WA stressed rats 3 days after completing the chronic stress phase. Somatosensory thermal hyperalgesia was determined by measuring the withdrawal latency to a radiant heat stimulus applied to the hind foot. Fig. 7B illustrates a significant decrease in the withdrawal latency to thermal stimulation in hind paws of WA stressed rats. The withdrawal latency was 9.5±0.5 sec and 7.5±0.2 sec in control and stressed rats, respectively (P < 0.01). No significant difference in withdrawal latency was observed in WA stressed rats after 3-day recovery compared to the controls (P = 0.2).
Fig. 7.
Line graphs show acute development of somatosensory pain in WA stressed rats compared with controls. (A) Comparison the differences in pain-related behaviors in response to mechanical (Von Frey test) stimuli in the hind paws of the control, WA and WA with 3-day recovery (WA-Rec) rat groups. (B) Comparison the differences in pain-related behaviors in response to thermal (Hargreaves test) stimuli in the hind paws of the control, WA and WA with 3-day recovery (WA-Rec) rat groups. Data are expressed as mean ± standard error, n = 6 in each group. **, P < 0.01.
Chronic WA stress increased expression of sodium channels in L4-L5 DRGs that returned to control levels 3 days after completion of the chronic stress phase
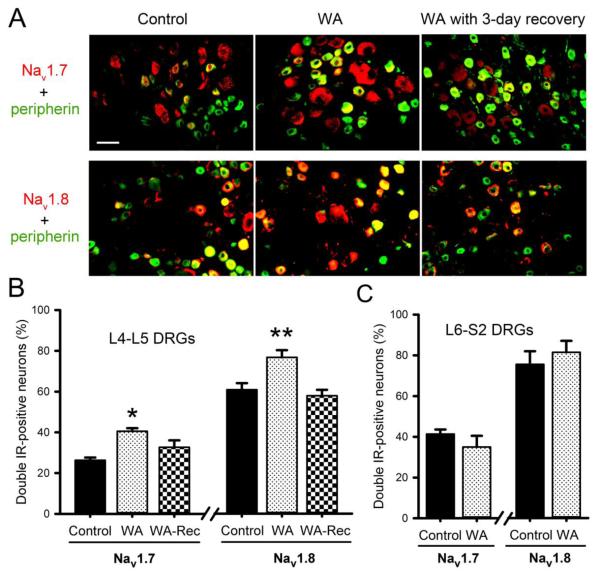
In painful diabetic neuropathy animal model, enhanced somatosensory pain perception was associated with increased expression and function of both tetrodotoxin (TTX)-sensitive Nav1.7 and TTX-resistant Nav1.8 sodium channels (Hong et al., 2004). Here we examined the expression of Nav1.7 and Nav1.8 in L4-L5 DRGs of control and WA stressed rats by immunofluorescence staining. As shown in Fig. 8A, both Nav1.7 and Nav1.8 were increased in L4-L5 DRGs in WA stressed rats compared to controls, which returned to their corresponding control levels 3 days after completion of the WA stress phase. The number of double-positive neurons for Nav1.7 and peripherin was significantly increased in L4-L5 DRGs from WA stressed rats (41.0±2.1%) compared with controls (27.5±1.9%; P < 0.05). After 3-day recovery, this number returned to 32.7±3.4% in WA-Rec group, which is significantly different compared to the WA group (P < 0.05). Similarly, the percentage of double-positive neurons for Nav1.8 and peripherin was significantly increased in L4-L5 DRGs from WA stressed rats (75.1±5.7%) compared to controls (60.3±5.8%; P < 0.01), which returned to control levels 3 days after completion of the chronic stress phase. (Fig. 8B). In contrast, the expression levels of Nav1.7 and Nav1.8 channels were not significantly changed in L6-S2 DRG neurons in stressed rats compared to the controls (Fig. 8C).
Fig. 8.
Double immunofluorescence staining of DRG neurons with C-type fiber marker peripherin (green) and sodium channel (red) antibodies. The neurons of double immunoreactivity positive are shown in yellow. (A) Increased expression of TTX-S Nav1.7 and TTX-R Nav1.8 sodium channels in peripherin-positive neurons in L4-L5 DRGs from WA stressed rats (middle panel) compared with the control (left panel). After 3-day recovery following the stress procedure (right panel), the enhanced expression of Nav1.7 returned to control level. Scale bar, 50 μm. (B) Percentage changes of the number of double-positive neurons for sodium channels and C-fiber marker peripherin in L4-L5 DRG neurons in WA stressed rats compared to the controls. (C) The number of double-positive neurons for Nav1.7 / Nav1.8 and peripherin in L6-S2 DRG neurons in control and stressed rats. Data are expressed as mean ± standard error, n = 4 in each group. *, P < 0.05; **, P < 0.01.
Corticosterone treatment increased expression of sodium channels in L4-L5 DRGs in vitro
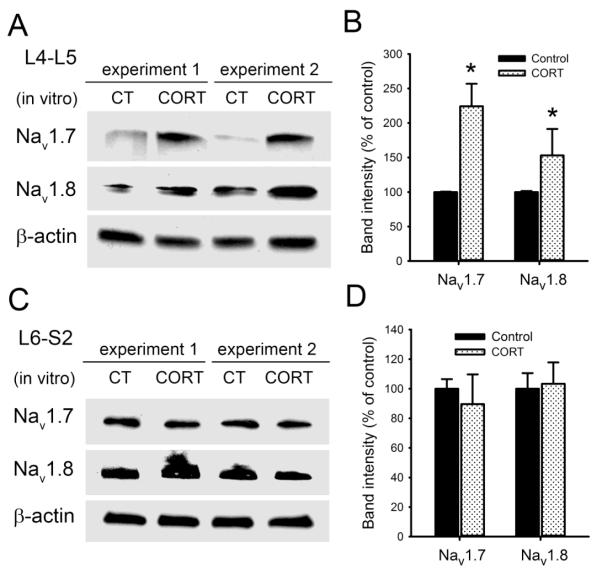
As shown in Fig. 9A&B, both Nav1.7 and Nav1.8 sodium channels were significantly increased in isolated L4-L5 DRGs from healthy control rats after corticosterone treatment in vitro. Nav1.7 increased 124±32.6% in corticosterone-treated L4-L5 DRGs compared to the non-treated, while Nav1.8 increased 53±38.5% after corticosterone treatment (P < 0.05). In contrast, no significant changes in Nav1.7 and Nav1.8 protein levels were observed in L6-S2 DRGs treated with corticosterone in vitro compared with the untreated controls (Fig. 9C&D). These results support the interpretation that up-regulation of sodium channels in L4-L5 DRGs in chronic stress is directly linked to the stress axis via elevation in CORT levels.
Fig. 9.
Corticosterone treatment induced up-regulation of Nav1.7 and Nav1.8 sodium channels in L4-L5 DRGs in vitro. (A) Western blot analysis showed significant increases in the expression levels of Nav1.7 and Nav1.8 proteins after corticosterone (CORT) treatment in vitro in isolated L4-L5 DRGs from control rats. Two replicates from different animal groups were shown. (B) Statistical analysis of the percentage change of Nav1.7 and Nav1.8 proteins in L4-L5 DRGs after corticosterone treatment. (C) Western blot analysis of Nav1.7 and Nav1.8 protein expression after corticosterone treatment in vitro in isolated L6-S2 DRGs from control rats. (D) No significant changes in Nav1.7 and Nav1.8 protein levels were observed in L6-S2 DRGs treated with corticosterone in vitro. Data are expressed as mean ± standard error, n = 4 in each group. *, P < 0.05.
Discussion
The results presented in this study indicate that chronic, intermittent water avoidance (WA) stress produces preferential, region- and cell-specific changes in primary sensory neuron pain regulatory pathways innervating the pelvic viscera, including the distal colon compared to the somatosensory innervation of the lower extremities. Specifically, the WA stress rat model demonstrated: 1. Reciprocal changes in CB1 (decreased levels) and TRPV1 (increased levels) receptors were observed L6-S2 DRG neurons but not L4-L5 DRG neurons; 2. Corticosterone treatment of healthy control rats in situ reproduced the region-specific, reciprocal changes in CB1 and TRPV1 receptor levels; 3. Elevated levels of the endocannabinoid 2-AG and decreased levels of the endocannabinoid degradation enzymes COX2 and FAAH were observed in L6-S2 DRGs but not L4-L5 DRGs; 4. The reciprocal changes in expression of CB1 and TRPV1 receptors in L6-S2 DRG neurons were cell-specific and observed in the sub-population of nociceptive neurons, e.g. C-type (peripherin-positive) fibers with small soma; 5. While the visceral hyperalgesia required up to one month to recover, somatosensory hyperalgesia recovered within 3 days after completion of the chronic stress phase; 6. The L4-L5 DRGs demonstrated reversible up-regulation in Nav1.7 and Nav1.8 that reverted to control levels 3 days after completion of the chronic WA stress phase which correlated with reversible somatosensory hyperalgesia; and 7. Corticosterone treatment in vitro increased the expression levels of Nav1.7 and Nav1.8 sodium channels in L4-L5 DRGs.
The endocannabinoid system is involved in a variety physiologically important processes including regulation of appetite, immune system, mood, memory and pain sensation (Carrier et al., 2005;Patel et al., 2004). Synthesis and degradation of endocannabinoids are mediated by enzymes that include FAAH and COX2. In the spinal cord, endocannabinoids suppress nociceptive responses in the dorsal horn via pathways that include direct CB1 receptor-mediated and indirect (protein kinase-mediated inhibition) of TRPV1 receptor function (Jeske et al., 2006;Jeske et al., 2009;Pernia-Andrade et al., 2009;Starowicz et al., 2012). We observed that levels of 2-AG were significantly elevated in L6-S2 but not L4-L5 DRGs in the WA stress rats compared to controls. Potential mechanisms include either increased synthesis or decreased degradation of endocannabinoids. Our data supports the latter mechanism, namely, that the expression levels of endocannabinoid degradation enzymes FAAH and COX2 were decreased significantly in L6–S2 but not L4-L5 DRGs in WA stress rats and in corticosterone-injected healthy rats, resulting in an increase in endocannabinoid (2-AG) content in DRGs, which consequently induces down-regulation of the endocannabinoid receptors.
Endocannabinoids including anandamide and 2-AG are synthesized on demand at the cell surface membrane and act locally as paracrine/autocrine factors (Bisogno et al., 2003;Okamoto et al., 2004). Similar effects of glucocorticoids have been reported in other model systems. For example, cortisol-mediated adhesion of synovial fibroblasts is dependent on inhibition of FAAH and elevation of endocannabinoids levels in synovial fibroblasts (Lowin et al., 2012). The molecular mechanism(s) underlying this effect are unresolved but it is noteworthy that putative response elements for glucocorticoids have been identified upstream of the ATG initiation codon in the mouse FAAH gene and activation of these regions demonstrates promoter activity in vitro (Waleh et al., 2002). After L5 spinal nerve ligation, both anandamide and 2-AG increased significantly only in ipsilateral L5 DRG, while CB1 and TRPV1 increased in the ipsilateral uninjured L4 DRG neurons. (Mitrirattanakul et al., 2006). Therefore, it is likely that differential endogenous expression of synthetic/degradation enzymes and receptor targets at the cellular level are likely to play an important role in the region-specific actions of endocannabinoids in DRG neurons, supporting a peripheral action for endocannabinoids but not excluding the likely important role of endocannabinoids in the central nervous system.
Our data supports the interpretation that the differential response to chronic, intermittent stress on the endocannabinoid and endovanilloid pathways in L6-S2 vs L4-L5 DRGs is “hard-wired” into the neurons. This interpretation is based on the results of the in situ experiments examining the effect of corticosterone on L6-S2 vs. L4-L5 DRG neurons obtained from control rats. Repeated administration of CORT in healthy control rats that reproduced the CORT levels observed in the chronic, intermittent WA stressed rats resulted in similar CB1 (down-regulated) and TRPV1 (up-regulated) response observed in L6-S2 but not L4-L5 DRGs in the WA stressed rats. The in vitro studies further support this interpretation. Corticosterone treatment increased TRPV1 and decreased CB1 in L6-S2 DRGs in vitro, which mimicked the responses of these two receptors under chronic stress conditions (Hong et al., 2011). Corticosterone also increased the expression levels of Nav1.7 and Nav1.8 sodium channels in L4-L5 but not L6-S2 DRGs in vitro, supporting a “hard-wired” mechanism in DRG neurons. This differential response may reflect the distinct development fates of these DRGs and/or the bidirectional influence of the target organs, including the colon and bladder compared to the somatosensory distribution (Herrity et al., 2014;La et al., 2011;Christianson et al., 2007). Future studies will need to examine whether stress-response transcription factor binding sites (for example, GR binding regions) demonstrate altered epigenetic regulation, such as enhanced methylation in L4-L5 DRGs which could explain the absence of response in CB1 gene expression to chronic stress.
Our results suggest that chronic intermittent stress-induced somatosensory hyperalgesia is associated with a distinct constellation of changes in pain regulatory pathways compared to those occurring in visceral hyperalgesia. This interpretation is based on the pain response studies which revealed that somatosensory hyperalgesia returned to baseline levels three days after completion of the stress phase. In contrast, visceral hyperalgesia continued to be present 3 days after 10-day WA stress in our study and persisted for several weeks after completion of the stress phase as reported in another study (Bradesi et al., 2005), indicating that chronic stress-induced visceral hyperalgesia lasts much longer than somatosensory hyperalgesia. We examined the hypothesis that reversible activation of voltage-activated sodium channels contributes to the earlier recovery from somatosensory hyperalgesia observed in WA stressed rats because sodium channels have a well-documented role in pain transmission in a variety of pain models (Cummins et al., 2007;Liu & Wood, 2011). While peripheral sensory neurons express nearly all sodium channel subtypes at some levels, there are three subtypes, NaV1.7, NaV1.8, and NaV1.9 which are enriched in peripheral neurons of the trigeminal and dorsal root ganglia (DRG). Relevant to our findings, NaV1.7 channel immunoreactive nerve fibers increased significantly in biopsy specimens of patients with rectal hypersensitivity compared to controls (Yiangou et al., 2007). We observed that both NaV1.7 and NaV1.8 demonstrated transient increase in expression in L4-L5 DRGs that reversed within 3 days after completion of the chronic stress phase that correlated with the timing of the reversible changes in somatosensory hyperalgesia. Distinguishing whether one or both of these sodium channels are causally linked to somatosensory hyperalgesia in the chronic stress model will require targeted gene silencing of NaV1.7 and NaV1.8. Future studies will also need to elucidate the specific signal transduction pathways involved in up-regulation of expression and function in sodium channels in the adult WA stress model and compare these changes with the longer lasting changes observed in the endocannabinoid and endovanilloid pathways. Self-reports from patients with sciatica also showed significant correlation between emotional stress and leg pain suggesting the phenomenon we observed in the WA stress rat model may exist in the human (Grove, 1989).
Highlights.
-
▪
Chronic stress induces both somatosensory and visceral hyperalgesia
-
▪
Changes in TRPV1, endocannabinoid pathway and sodium channels are region-specific in DRG neurons
-
▪
Corticosterone mediates stress-induced alterations of receptor expression
-
▪
Chronic stress-induced visceral pain persists longer than somatosensory pain
Acknowledgments
This work was supported by grant R01DK098205 (J.W.W.) and the University of Michigan Center for Gastrointestinal Research (UMCGR) grant 5P30DK034933 from the US National Institutes of Health. Pilot Feasibility grant F014289 (S.H.) was awarded by the University of Michigan Center for Gastrointestinal Research.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- CB1
cannabinoid receptor 1
- CORT
corticosterone
- COX-2
cyclooxygenase-2
- CRD
colorectal distension
- DRG
dorsal root ganglion
- EMG
electromyography
- GR
glucocorticoid receptor
- FAAH
fatty acid amide hydrolase
- HPA
hypothalamic-pituitary-adrenal
- IBS
Irritable Bowel Syndrome
- Nav
voltage-gated sodium channel
- TRPV1
transient receptor potential vanilloid 1
- TTX
tetrodotoxin
- VMR
visceromotor response
- WA
water avoidance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors disclose no conflict of interest.
References
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164:711–723. doi: 10.1016/j.neuroscience.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagal SK, Chapman ML, Marron BE, Prime R, Storer RI, Swain NA. Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett. 2014;24:3690–3699. doi: 10.1016/j.bmcl.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di M,V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Patel S, Hillard CJ. Endocannabinoids in neuroimmunology and stress. Curr Drug Targets CNS Neurol Disord. 2005;4:657–665. doi: 10.2174/156800705774933023. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain. 2007;131:243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Rocha HA, Dantas BP, Rolim TL, Costa BA, de Medeiros AC. Main ion channels and receptors associated with visceral hypersensitivity in irritable bowel syndrome. Ann Gastroenterol. 2014;27:200–206. [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove GL. Physiologic changes in older skin. Clin Geriatr Med. 1989;5:115–125. [PubMed] [Google Scholar]
- Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrity AN, Rau KK, Petruska JC, Stirling DP, Hubscher CH. Identification of bladder and colon afferents in the nodose ganglia of male rats. J Comp Neurol. 2014;522:3667–3682. doi: 10.1002/cne.23629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Agresta L, Guo C, Wiley JW. The TRPV1 receptor is associated with preferential stress in large dorsal root ganglion neurons in early diabetic sensory neuropathy. J Neurochem. 2008;105:1212–1222. doi: 10.1111/j.1471-4159.2008.05220.x. [DOI] [PubMed] [Google Scholar]
- Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Morrow TJ, Paulson PE, Isom LL, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in tetrodotoxin-sensitive and -resistant sodium channels in dorsal root ganglion neurons in the rat. J Biol Chem. 2004;279:29341–29350. doi: 10.1074/jbc.M404167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280:618–627. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone Mediates Reciprocal Changes in CB 1 and TRPV1 Receptors in Primary Sensory Neurons in the Chronically Stressed Rat. Gastroenterology. 2011;140:627–637. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Xiao Y, Zhu L, Li L, Hu CY, Jiang X, Xu GY. Neonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol. 2013;304:G311–G321. doi: 10.1152/ajpgi.00338.2012. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain. 2009 doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Greenwood-Van MB. Stress-induced pain: a target for the development of novel therapeutics. J Pharmacol Exp Ther. 2014;351:327–335. doi: 10.1124/jpet.114.218065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La JH, Schwartz ES, Gebhart GF. Differences in the expression of transient receptor potential channel V1, transient receptor potential channel A1 and mechanosensitive two pore-domain K+ channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;186:179–187. doi: 10.1016/j.neuroscience.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Suppl 3):S93–S99. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- Lowin T, Zhu W, Dettmer-Wilde K, Straub RH. Cortisol-mediated adhesion of synovial fibroblasts is dependent on the degradation of anandamide and activation of the endocannabinoid system. Arthritis Rheum. 2012;64:3867–3876. doi: 10.1002/art.37684. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, Mackie K, Faull KF, Spigelman I. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126:102–114. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–764. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Makuch W, Osikowicz M, Piscitelli F, Petrosino S, Di M,V, Przewlocka B. Spinal anandamide produces analgesia in neuropathic rats: possible CB(1)- and TRPV1-mediated mechanisms. Neuropharmacology. 2012;62:1746–1755. doi: 10.1016/j.neuropharm.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su OS, Lentz SI, Brosius F, III, Feldman EL. Mouse models of diabetic neuropathy. Neurobiol Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- Tran L, Schulkin J, Greenwood-Van MB. Importance of CRF receptor-mediated mechanisms of the bed nucleus of the stria terminalis in the processing of anxiety and pain. Neuropsychopharmacology. 2014;39:2633–2645. doi: 10.1038/npp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291:203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- Wood JN. Recent advances in understanding molecular mechanisms of primary afferent activation. Gut. 2004;53(Suppl 2):ii9–12. doi: 10.1136/gut.2003.033423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Chessell IP, Bountra C, Chan C, Fertleman C, Smith V, Anand P. Voltage-gated ion channel Nav1.7 innervation in patients with idiopathic rectal hypersensitivity and paroxysmal extreme pain disorder (familial rectal pain) Neurosci Lett. 2007;427:77–82. doi: 10.1016/j.neulet.2007.09.027. [DOI] [PubMed] [Google Scholar]