Summary
Understanding the nature of adjuvants and the immune priming events in autoimmune diseases, such as rheumatoid arthritis, is a key challenge to identify their aetiology. Adjuvants are, however, complex structures with inflammatory and immune priming properties. Synthetic polymers provide a possibility to separate these functions and allow studies of the priming mechanisms in vivo. A well‐balanced polymer, poly‐N‐isopropyl acrylamide (PNiPAAm) mixed with collagen type II (CII) induced relatively stronger autoimmunity and arthritis compared with more hydrophilic (polyacrylamide) or hydrophobic (poly‐N‐isopropylacrylamide‐co‐poly‐N‐tertbutylacrylamide and poly‐N‐tertbutylacrylamide) polymers. Clearly, all the synthesized polymers except the more hydrophobic poly‐N‐tertbutylacrylamide induced arthritis, especially in Ncf1‐deficient mice, which are deficient in reactive oxygen species (ROS) production. We identified macrophages as the major infiltrating cells present at PNiPAAm‐CII injection sites and demonstrate that ROS produced by the macrophages attenuated the immune response and the development of arthritis. Our results reveal that thermo‐responsive polymers with high immune priming capacity could trigger an autoimmune response to CII and the subsequent arthritis development, in particular in the absence of NOX2 derived ROS. Importantly, ROS from macrophages protected against the autoimmune priming, demonstrating a critical regulatory role of macrophages in immune priming events.
Keywords: adjuvant, arthritis, collagen type II, macrophages, poly‐N‐isopropylacrylamide
Introduction
There is an increasing awareness that autoimmune diseases are initiated decades before the clinical onset and that autoimmune priming is the first critical step. It is therefore important to increase our understanding of such early events. Rheumatoid arthritis is one of the most common autoimmune diseases now known to start years or decades before the development of clinically apparent and destructive inflammation in joints. The first signs are the occurrence of autoantibodies, such as rheumatoid factors and anti‐citrullinated protein antibodies. The production of these IgG and IgA autoantibodies are believed to be T‐cell dependent and their appearance is associated with the MHC class II region. The prevailing hypothesis is that MHC class II restricted T cells are triggered by auto‐antigenic peptides bound to certain MHC class II alleles and initiate an autoimmune response leading to B‐cell activation and production of autoantibodies. Even though the identities of such T cells, or the auto‐antigenic peptide(s), are unknown it is most likely that the priming of these T cells is triggered by antigen‐presenting cells activated by inflammatory stimuli, such as adjuvants. The exposure of adjuvants in vivo could be from chronic exposure of pathogens in the gastrointestinal tract or from either smoking or pollutants irritating the lung mucosal tissue.
A similar scenario could be modelled in experimental animals. Immunization with type II collagen (CII) together with adjuvants triggers activation of MHC class II restricted T cells that help B cells to produce pathogenic antibodies. This is a well‐described system, which is suitable to more precisely investigate the nature of the adjuvants that could break this tolerance and to analyse under which conditions an arthritogenic response can be evoked.
Most commonly, very crude adjuvants such as complete Freund's adjuvant (CFA), a suspension of heat‐killed mycobacteria in mineral oil, is used for the CII immunization. CFA not only activates antigen‐presenting cells but also has a range of complex effects, for example, inducing granulomas and apoptosis, and triggering an inflammatory response. We have made some efforts to use better‐defined synthetic polymers as adjuvants. Recently, we demonstrated the adjuvant potential of poly‐N‐isopropylacrylamide (PNiPAAm) ‐based polymers for developing a new arthritis model.1, 2, 3 Without using oil or bacterial components, PNiPAAm mixed with CII induced arthritis in inbred mice and rats. PNiPAAm induces significant antigen‐specific antibody synthesis but without skewing the immune responses toward any particular shift in T helper cell populations. PNiPAAm‐CII immunization induced a robust anti‐CII antibody response, which is independent of Toll‐like receptors. We have also reported a more pronounced adjuvant activity of PNiPAAm in the absence of reactive oxygen species (ROS).4 Generally adjuvant potency of polymers depends on several factors including polymer chemistry, format, charge, chain length and hydrophobic nature.5 Among them, hydrophobic property is the most important factor that could decide the fate of an induced immune response.6 By analysing different variants regarding hydrophobicity, format effect, nature of cells infiltrating the injection site, we could define a variant, PNiPAAm, that was the most effective to prime the immune response rather than just being an inflammatory stimulus. Mixing native CII with PNiPAAm induced a strong autoantibody response and severe arthritis. Interestingly, the response was much higher in Ncf1‐mutated mice, lacking ROS. We know from previous studies that macrophage‐derived ROS is protective in classical collagen‐induced arthritis7, 8 and we show here that ROS derived from macrophages in fact attenuated the priming of an autoimmune response after immunization with CII and PNiPAAm.
Materials and methods
Mice, antigens and antibodies
Age‐matched 7‐ to 8‐week‐old male mice were used in all the experiments. All the mice used were on an arthritis permissive B10.Q/rhd genetic background expressing MHC class II haplotype H‐2q and were determined to be genetically identical using 10K single nucleotide polymorphism typing, except for the targeted loci. Mice with Ncf1 m1j mutation (B10Q.Ncf1 m1j) were described earlier.9 Ncf1 codes for a protein known as p47phox and a natural mutation impair the expression of this gene and totally block the function of the NOX2 complexes responsible for oxidative burst. The B10Q.Ncf1 m1j//m1j .MN + (MN.B10Q.Ncf1) strain has a transgene expressing functional Ncf1 on macrophages using the human CD68 promotor.7, 8 This transgene is used when expressed in a heterozygous manner.
Animals were housed in a climate‐controlled environment with dark and light cycles. Wood shavings and paper were used as enrichment materials in polystyrene cages. Standard food and water were given ad libitum. All the animal experiments were performed and approved under Swedish ethics permit numbers: N310‐07, M107‐07 and N66‐10. Animals were anaesthetized using an isoflurane–air (3 : 1) mixture during all the experimental procedures. Collagen II from bovine articular joints and a rat chondrosarcoma were prepared by pepsin digestion and purified as described earlier.10 Ovalbumin protein grade II was from Sigma Aldrich (St Louis, MO).
Synthesis of polymers
Acrylamide, N‐isopropylacrylamide, N‐tertbutylacrylamide, ammonium persulphate and N,N,N',N'‐tetramethylethylenediamine were purchased from Sigma Aldrich (Darmstadt, Germany). All four polymers (1%, weight/volume) were synthesized through free radical polymerization. For synthesis of polyacrylamide (PAAm), poly‐N‐isopropylacrylamide (PNiPAAm), poly‐N‐isopropylacrylamide‐co‐poly‐N‐tertbutylacrylamide (PNiPAAm‐co‐PNtBAAm) in a 4 : 1 weight ratio and poly‐N‐tertbutylacrylamide (PNtBAAm); 100 μg of respective monomers were weighed and dissolved in 10 ml of degassed water. Since, N‐tertbutylacrylamide did not dissolve in water completely therefore a water : DMSO (4 : 1) mixture was used to dissolve the monomers. All the monomer solutions were purged thoroughly for 15–20 min by nitrogen gas and then ammonium persulphate (0·1%, weight/volume) and TEMED (0·1%, volume/volume) were added for polymerization. All the polymers were purified and characterized before using them for experiments.
Characterization of polymers
Molecular weight of PNiPAAm, PNiPAAm‐co‐PNtBAAm and PNtBAAm were analysed by gel permeation chromatography (Waters, Milford, MA) as described earlier,1, 2, 4 while a viscosity method was used for PAAm due to its insolubility in tetrahydrofuran mobile phase. Hydrophobic character of polymers (log P‐value) was calculated by partition method exploiting the polymer solubility in aqueous (water) and organic solvent (n‐octanol) at room temperature as described.11 As PNiPAAm and PNiPAAm‐co‐PNtBAAm show temperature‐dependent phase transition, their lower critical solution temperatures were determined through turbidity measurement by spectrophotometry as described earlier.12 Binding of polymers with CII was explored through circular dichroism spectroscopy. The circular dichroism spectra were recorded with a JASCO J600A spectropolarimeter (Tokyo, Japan) in the range of 190–250 nm (far UV region).13 In brief, CII 10 μg/ml in PBS and PNiPAAm (0·01%, weight/volume) at different temperatures were taken in a quartz cuvette with 1‐mm light path length. The sample temperature was controlled by a cell holder.
Synthesis of PNiPAAm microgel
For PNiPAAm microgel synthesis, first PNiPAAm‐co‐AAm (4 : 1 weight ratio of monomers) polymer was synthesized through co‐polymerization and later cross‐linked by glutaraldehyde under high‐speed stirring. In brief, 100 mg of co‐polymer was taken in 50 ml paraffin heavy oil with soybean lecithin as an emulsifier. Glutaraldehyde (2%, volume/volume) was added drop‐wise into the solution and the emulsion was stirred at high speed for 4 hr. Newly synthesized PNiPAAm microgel was purified through overnight dialysis and freeze‐dried for further experiments.
Immunohistochemistry
To check the nature of infiltrating cell types at the injection site, PNiPAAm‐CII mixture was injected in mice. Twenty‐four hours post injection, surrounding tissues near the injection site were collected and embedded in Tissue‐Tek® solution for immunohistochemistry. Tissues were cut, fixed, stained for macrophage (using rat anti‐mouse CD11b (M1/70) and intracellular staining of interferon‐γ (IFN‐γ), interleukin‐4 (IL‐4) and IL‐17 of the infiltrating cells. Anti‐mouse IFN‐γ (DB1) and IL‐4 (11B11) antibodies were produced in house, and anti‐IL‐17A antibodies were purchased from eBioscience (San Diego, CA).
Analysis of adjuvant potential of polymers
To test the adjuvant capacity of four different polymers 7–8 week‐old mice were divided into six groups and immunized with PAAm‐CII, PNiPAAm‐CII, PNiPAAm‐co‐PNtBAAm‐CII, PNtBAAm‐CII, CFA‐CII or CII alone. CII emulsified with CFA (Difco, Detroit, MI) and CII alone immunized mice served as positive and negative controls, respectively. CII (1 mg/ml) was mixed with each polymer separately and 200 μg of antigen–polymer mixture (1 : 1) was injected intradermally on day 0 and boosted with 100 μg of respective antigen–polymer/incomplete Freund's adjuvant mixture (1 : 1) on day 21. A similar procedure was adopted for immunization of other mouse strains with PNiPAAm‐CII.
Serological analysis
Serum samples were used for analysis of anti‐CII antibodies using ELISA.14 Affinity‐purified antibody and/or pooled sera from CII‐immunized mice were used as positive controls. Peroxidase‐conjugated goat anti‐mouse IgG antibodies (Southern Biotech, Birmingham, AL) and 2,2'‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) substrate (Roche Diagnostics, Indianapolis, IN) were used as detection system.
Splenocyte culture
For splenocyte culture, B10Q.Ncf1 mice (n = 5) were immunized intradermally with 100 μg of CII and polymers. Ten days after the immunization, splenocytes from these mice were cultured in triplicates at a concentration of 1 × 106 cells per well for 72 hr with negative controls; medium alone or 50 μg/ml ovalbumin (Sigma, St Louis, MO), positive controls; 5 μg/ml of concanavalin A (Sigma, Darmstadt, Germany) or 0·5 μg/ml of anti‐CD3 (clone 17A2), or 50 μg/ml of CII in culture medium. Supernatants of cultured cells were collected after 72 hr for IFN‐γ measurement using sandwich ELISA technique.
Clinical evaluation of arthritis
Mice were scored blindly for joint inflammation twice per week using an extended scoring system published earlier.15 Inflammation is defined by swelling and redness. In this extended scoring system each inflamed toe or knuckle gives one point, whereas an inflamed wrist or ankle gives five points, resulting in a score of 0–15 (5 toes + 5 knuckles + 1 wrist/ankle) for each paw and 0–60 points for each mouse. Arthritis incidence is calculated by dividing the number of sick mice by total number of mice and mean arthritis score is the average of the arthritis score given according to the scoring system.
Results
Synthesis and characterization of polymers
Polyacrylamide (PAAm), PNiPAAm, PNiPAAm‐co‐PNtBAAm and PNtBAAm were synthesized through free radical polymerization (Fig. 1a). By increasing the N‐substitution by alkyl groups, the hydrophobicity of polymers was increased linearly as determined by partition method (Table 1). Out of the four polymers synthesized, PAAm (log P = 0·95) was highly hydrophilic, whereas PNtBAAm (log P = 3·03) was more hydrophobic and had high solubility in octanol. We dissolved PNiPAAm, PAAm and PNiPAAm‐co‐PNtBAAm in water, and PNtBAAm in a water : DMSO mixture (4 : 1). None of the polymers induced tumour necrosis factor‐α synthesis in mice demonstrating absence of systemic toxicity due to polymers (Fig. 1b). Moreover, we did not find any localized toxicity at the site of injection. Hence, all the polymers are biocompatible and not toxic in vivo.
Figure 1.
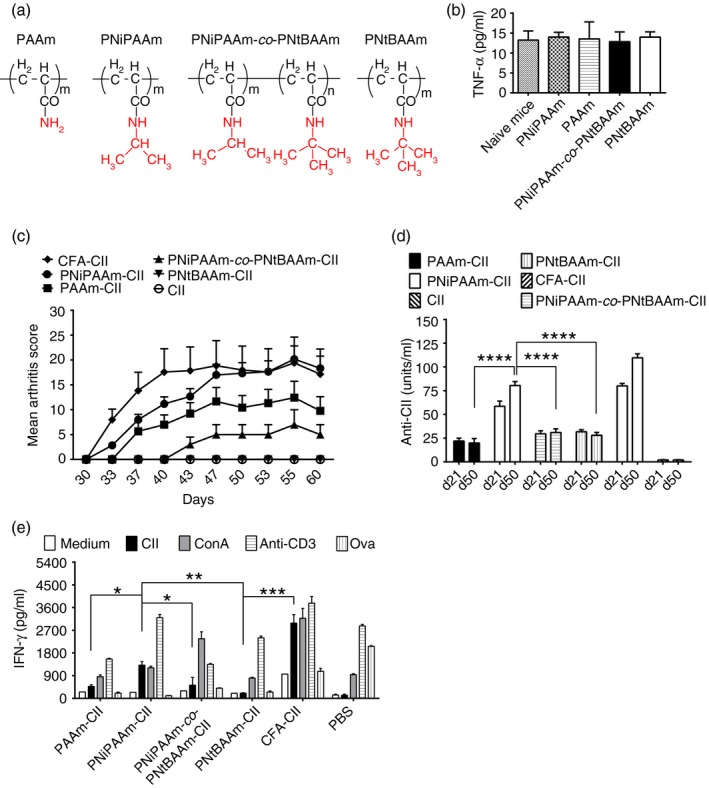
A balance of hydophobicity/hydrophilicity defines the adjuvant property of a polymer. Chemical structure of polymers tested as adjuvants in experimental arthritis are shown (a). Tumour necrosis factor‐α (TNF‐α) analysis at day 21 in serum of mice injected with polymers to assess the systemic biocompatibility of the polymers (b). Arthritis was induced in mice (n = 24 mice per group) using collagen II (CII) and an adjuvant. Mean arthritis score (c) with different polymeric adjuvants mixed with CII in 7‐ to 8‐week‐old B10Q.Ncf1 mice. The maximum score per mouse was 60 points. CII (1 mg/ml) was mixed with each polymer separately and 200 μg of antigen–polymer mixture (1 : 1) was injected intradermally on day 0 and boosted with 100 μg of respective antigen–polymer mixture (1 : 1) on day 21. Only mice that developed arthritis were used for calculations. Anti‐CII antibody levels, before (day 21) and after (day 50) booster immunization of mice (d) were shown. Interferon‐γ (IFN‐γ) (e) concentration in the supernatant of cultured splenocytes re‐stimulated with various stimulants. Error bars indicate ± SEM. *P < 0·05; **P < 0·005 and ***P < 0·0005. n indicates number of mice used in each group.
Table 1.
Physical characterization of polymers
| Polymer | LCSTa (°C) | Molecular weight (D) | Log P b |
|---|---|---|---|
| PAAmc | – | 80 000 (±10 000) | 0·95 |
| PNiPAAmd | ≈32 | 120 000 (±18 000) | 1·22 |
| PNiPAAm‐co‐PNtBAAmd | ≈30 | 95 000 (±12 000) | 1·67 |
| PNtBAAmd | – | 97 000 (±7 500) | 3·03 |
Lower critical solution temperature (LCST) or cloud point of synthesized polymers.
Log P represents experimental partition coefficient of polymers in octanol and water (Kow).
Molecular weight of polymer was determined by viscosity method.
Molecular weight of polymers was determined by gel permeation chromatography. PAAm: Polyacrylamide PNiPAAm: Poly‐N‐isopropylacrylamide PNiPAAm‐co‐PNtBAAm: Poly‐N‐isopropylacrylamide‐co‐poly‐N‐tertbutylacrylamide PNtBAAm: Poly‐N‐tertbutylacrylamide
Hydrophobic and hydrophilic balance promotes adjuvant property
B10Q.Ncf1 (m1J) mice were used to evaluate the hydrophobic/hydrophilic effect on adjuvant potential of synthesized polymers. Ten per cent of mice immunized with PAAm‐CII developed arthritis with a mean maximum score (MMS) of 12 ± 3·2. Further increasing the log P value of the polymer increased arthritis frequency and severity, with the PNiPAAm‐CII group having 75% of mice affected with a more severe disease (MMS: 20 ± 2·6). However, arthritis frequency declined after increasing the log P value further to 1·67 (only 10% of mice in PNiPAAm‐co‐PNtBAAm‐CII group developed arthritis with very low disease severity (MMS: 7 ± 3) (Fig. 1c). Most importantly, arthritis symptoms were completely absent in PNtBAAm‐CII‐immunized mice. In the control groups, 80% (CFA‐CII) and 0% (CII alone) of mice developed arthritis (Fig. 1c).
Antibodies play a pivotal role in arthritis pathogenesis.16 In our study, anti‐CII antibody levels correlated well with the development of clinical disease. Antibody synthesis in the PAAm‐CII group was lower, whereas PNiPAAm‐CII‐immunized mice had the highest antibody titres compared with all the other polymer groups. The PNiPAAm‐co‐PNtBAAm‐CII and PNtBAAm‐CII groups developed significantly lower level of antibodies. The CFA‐CII (positive control) group had the highest level of CII‐specific antibodies, but CII alone (negative control) group developed negligible antibody titres (Fig. 1d). Difference in the adjuvant potential of polymers was further confirmed by in vitro cell proliferation assay by monitoring IFN‐γ production.15 Concentration of IFN‐γ the in PNiPAAm‐CII group was significantly higher than other polymer groups but lower than the positive control group (CFA‐CII) (Fig. 1e). Hence, the highest adjuvant capacity was observed with the polymer having intermediate hydrophobicity (log P value of 1·22).
Binding of PNiPAAm with CII
At room temperature, PNiPAAm binds mainly to the triple helical part of CII, while PAAm did not interact with collagen helix, as confirmed by CD spectroscopy in the far UV region (Fig. 2a). As a negative control PNiPAAm without CII was used, which did not show any signal. When the temperature was increased from 20° to 35°, PNiPAAm shielded CII all around and so did not give any values in the spectrum (Fig. 2b).
Figure 2.
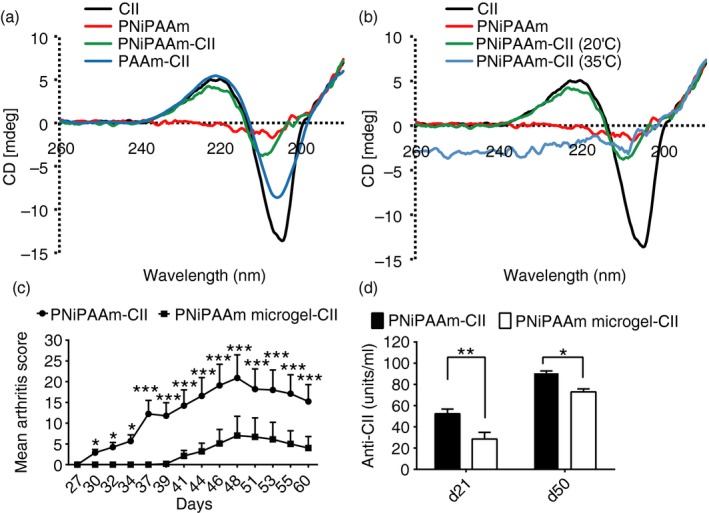
Polymer–collagen II (CII) binding characteristics and adjuvant potential of different formats of the polymer. To assess the effect of temperature‐dependent phase transition of CII, circular dichroism spectra of poly‐N‐isopropylacrylamide (PNiPAAm) and polyacrylamide (PAAm) with CII mixture (a) at 20° (below the lower critical solution temperature (LCST) of PNiPAAm), and 35° (b, above the LCST of PNiPAAm) were recorded. CII and PNiPAAm spectra were used as controls. Spectra were monitored in the far UV (193–250 nm) region using CII (0·01 mg/ml) and JASCO J600A spectropolarimeter. Polymers (0·01%, weight/volume) in 0·02 m phosphate buffer, pH 7·4 were mixed with CII at 20°C. To analyse the format effect on adjuvant property, two different PNiPAAm formats (linear and microgel) were synthesized and tested in 7‐ to 8‐week‐old B10Q.Ncf1 male mice. Mean arthritis score on different days (c) and anti‐CII responses in the sera collected at days 21 and 50 (d) were shown. Two groups of mice, PNiPAAm‐CII (n = 15) and PNiPAAm microgel‐CII (n = 15), were used. Mice were immunized with 100 μg of CII + 100 μg of PNiPAAm in linear or microgel format and boosted on day 21 with 50 μg of respective antigen–polymer format. Error bars denote ± SEM. *P < 0·05; **P < 0·005 and ***P < 0·0005. Represented results are from two independent experiments and all the animals were used for analysis. n indicates the number of mice used in each group. The maximum score per mouse was 60 points.
PNiPAAm format (linear and microgel) affects its adjuvant capacity
To check the format effect of PNiPAAm on its adjuvant potential, we synthesized PNiPAAm‐co‐AAm (4 : 1) and cross‐linked using glutaraldehyde. In the arthritis experiment, 75% of mice immunized with linear PNiPAAm‐CII developed arthritis with high severity (MMS: 20 ± 5·4), whereas only 30% of mice developed arthritis after the injection with PNiPAAm in the microgel format with CII (MMS: 7 ± 4·6) (Fig. 2c). Similarly, the linear form of PNiPAAm induced a significantly greater anti‐CII response compared with the microgel format (Fig. 2d).
ROS produced by macrophages attenuates PNiPAAm‐CII induced autoimmune response and development of arthritis
We found macrophages as the major infiltrating cells present at the PNiPAAm‐CII injection site (Fig. 3a), and the infiltrating cells at the polymer interface showed intracellular staining for the cytokines IL‐4 and IFN‐γ but not IL‐17 (Fig. 3b–d). Presence of a low oxidative environment (reduced ROS levels) significantly enhanced the PNiPAAm‐CII‐induced autoimmune response and the subsequent development of arthritis.4 As macrophages are the major infiltrating cells present at the polymer interface, we used the transgenic mice expressing functional Ncf1 restricted to macrophages (MN.B10Q.Ncf1) to understand the contribution of ROS produced by macrophages in PNiPAAm‐CII‐induced inflammation. Clearly, production of ROS by macrophages reduced the anti‐CII antibody response (Fig. 3e). Consequently, only 10% and 20% of the MN.B10Q.Ncf1 mice developed arthritis after PNiPAAm‐CII or CFA‐CII immunization with low disease severity (MMS: 6·8 ± 2·4 and 1·8 ± 0·6), respectively. On the other hand, 60% and 80% of the transgene negative mice (B10Q.Ncf1) developed arthritis after PNiPAAm‐CII or CFA‐CII injection (MMS: 18·8 ± 5 and 17 ± 4·5), respectively (Fig. 3f).
Figure 3.
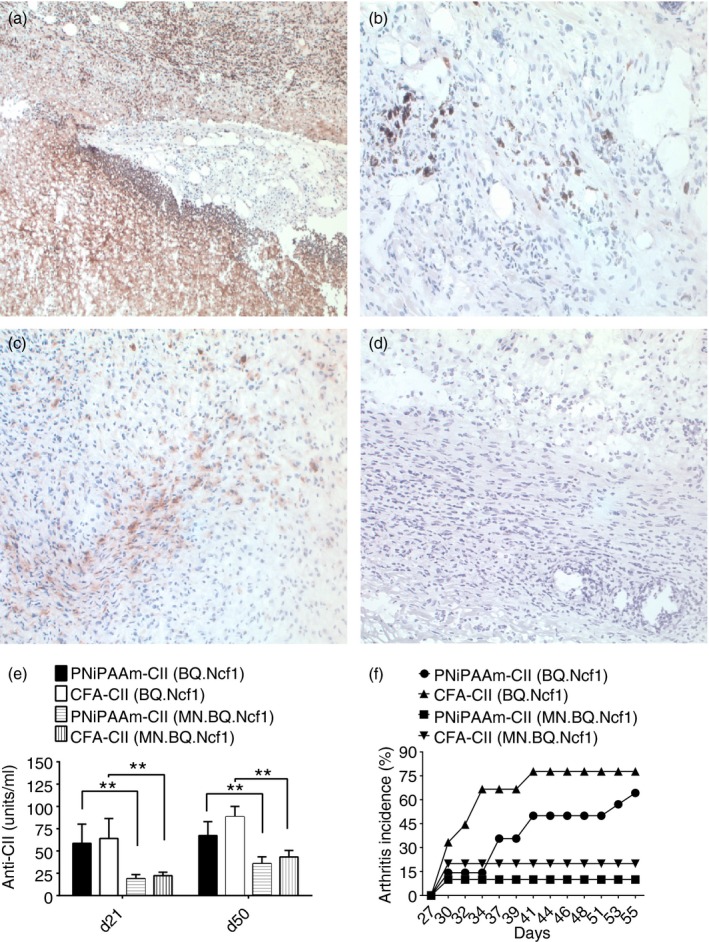
Macrophage specific reactive oxygen species (ROS) attenuates poly‐N‐isopropyla‐crylamide–collagen II (PNiPAAm‐CII) ‐induced arthritis. Immunohistochemistry of surrounding tissues at the PNiPAAm‐CII injection site was shown. Immunostaining of macrophage cells at polymer–tissue interface (a). Intracellular staining for cytokines, interferon‐γ (IFN‐γ) (b), interleukin‐4 (IL‐4) (c) and IL‐17 (d), to understand the nature of the infiltrating cells. All the images were taken at 20 × magnification. Adjuvant potential of PNiPAAm in 7‐ to 8‐week‐old B10Q.Ncf1 (ROS production is reduced in all the cells) and MN.B10Q.Ncf1 (ROS production is reduced in all the cells except macrophages) mice was analysed. Mice were divided into four groups complete Freund's adjuvant (CFA) ‐CII (B10Q.Ncf1, n = 10), CFA‐CII (MN.B10Q.Ncf1, n = 10), PNiPAAm‐CII (B10Q.Ncf1, n = 15) and PNiPAAm‐CII (MN.B10Q.Ncf1, n = 15). Mice were immunized with 100 μg of CII + PNiPAAm/CFA and boosted on day 21 with 50 μg of CII + PNiPAAm/incomplete Freund's adjuvant (IFA). Anti‐CII antibody response (e) and mean arthritis score (f) were shown. The maximum score per mouse was 60 points. Error bars denote ± SEM. *P < 0·05; **P < 0·005 and ***P < 0·0005. Represented results are from two independent experiments and all the animals were used for analysis. n indicates the number of mice used in each group.
Discussion
Adjuvants are crucial molecules, of importance not only for vaccine development but from another perspective as well, because such agents are also critical for the triggering of autoimmune and allergic diseases. We have defined synthetic polymers with properties that enhance antigen presentation and conformational stability of the autoantigen rather than the toxic or inflammatory effects. By using such an adjuvant, PNiPAAm, we could show that autoimmunity to CII is enhanced as well as the subsequent arthritis development, particularly in ROS‐deficient mice. Hence, ROS suppressed autoimmune priming and this effect could be shown to be mediated by macrophages as expression of Ncf1 with the CD68 promoter in Ncf1‐deficient mice immunized with the PNiPAAm‐CII model led to reduction of the autoimmune response and arthritis.
Quantifying the relation between hydrophobic content in a polymeric adjuvant and the elicited immune responses using an experimental arthritis system enabled us to demonstrate a direct correlation between the hydrophobic/hydrophilic nature of polymers with their adjuvant potential. A well‐balanced polymer, PNiPAAm (log P = 1·22) mixed with CII induced significant autoimmune responses and polyarthritis compared with the relatively more hydrophilic PAAm or hydrophobic PNiPAAm‐co‐PNtBAAm and PNtBAAm polymers. However, polymers other than PNtBAAm had various levels of adjuvant capacity. Structurally PNiPAAm has a hydrophilic isopropyl group attached to a hydrophobic amide group and this combination inculcates a precipitating property at body temperature,1 which is optimal for its use of this polymer as a carrier for antigens. On the other hand, PAAm has a primary amino group, which contributes a hydrophilic property that might have inhibited the interactions of this polymer with the antigen‐presenting cells17 at the injection site. Hence, increasing the hydrophobicity (log P = 1·22) might have enabled PNiPAAm to recruit more antigen‐presenting cells and have higher cellular uptake17, 18 with a subsequent induction of more severe arthritis. Most importantly, arthritis symptoms were completely absent in PNtBAAm‐CII‐immunized mice. This could be because of the increased hydrophobic character (log P = 3·03) of this polymer, which might have created an incomprehensible environment for immune cells due to rapid adsorption of different proteins on the hydrophobic surface.18 In addition, there is the possibility of activation through scavenger receptors and complement factor C1q by hydrophobic surfaces, which could lead to clearance of materials rather than initiating the activation of the immune system.19, 20 Hence, a fine balance between hydrophilicity and hydrophobicity could be an important parameter in the adjuvant action of the biodegradable stimuli‐responsive polymers. However, mechanisms of polymer degradation in vivo are not completely deciphered yet, but phagocytes such as macrophages and neutrophils are known to clear polymers from the host.21 Furthermore, the interactive mechanisms by which polymers influence the immune system are far from clear, while depot generation, activation of the complement system and the interactions with specific membrane‐bound pattern recognition receptors, like Toll‐like receptors and C‐type lectin receptors, are likely to be the plausible mechanisms.17, 22
Earlier we demonstrated a single point mutation in the Ncf1 gene (encoding p47phox subunit of the NADPH oxidase complex, a multi‐component electron carrier responsible for ROS production) that regulates arthritis severity and chronicity.9 Macrophages have the highest ROS‐producing capacity among antigen‐presenting cells and the macrophage‐specific ROS was shown not only to play a role in T‐cell selection, maturation and differentiation but also to have a suppressive role in T‐cell activation, which was mediated in an antigen‐dependent fashion.7 Later we have also shown that CD68‐expressing cells (macrophages) can prime T cells and initiate arthritis in the absence of ROS.23 Most importantly, macrophage‐produced ROS was shown to be involved in the induction of regulatory T cells to regulate T‐cell‐mediated inflammation24 and the anti‐inflammatory drug dexamethasone was shown to increase the ROS production and T‐cell suppressive capacity of anti‐inflammatory macrophages.25 In this study, we identified the major infiltrating cells present at the PNiPAAm‐CII injection site are macrophages. Hence, using the transgenic mice expressing functional Ncf1 restricted to macrophages (MN.B10Q.Ncf1), we analysed the contribution of macrophage‐specific ROS on PNiPAAm‐CII‐induced inflammation. Similar to the CII‐CFA group, ROS produced by macrophages attenuated PNiPAAm‐CII‐induced inflammation demonstrating its major suppressive role when polymer was used as an adjuvant. It is of interest to note that both the pro‐inflammatory (type 1) and anti‐inflammatory (type 2) T helper cells were induced after PNiPAAm‐CII immunization in mice1, 4 and macrophage‐produced ROS could have been instrumental in down‐regulating T‐cell responses induced by PNiPAAm‐CII immunization.
In conclusion, we have characterized a polymer adjuvant that enhances immune priming relative to evoking an inflammatory response. Using this adjuvant we could show that ROS from macrophages regulate autoimmune priming and the development of arthritis. This could have implications for understanding the critical immune priming in autoimmune diseases and opening new possibilities for early vaccine studies.
Disclosures
All the authors declare that there is no conflict of interest.
Acknowledgements
We thank Carlos and Kristina Palestro for taking care of the animals. The following foundations provided financial support: Swedish Rheumatism Association, King Gustaf V's 80‐years, the Swedish Foundation for Strategic Research, the Swedish Science Research Council including the VR‐Link (2008‐6007) and VR‐project grant (2009‐2338). AK acknowledges a DBT TATA Innovation Fellowship.
References
- 1. Shakya AK, Kumar A, Nandakumar KS. Adjuvant properties of a biocompatible thermo‐responsive polymer of N‐isopropylacrylamide in autoimmunity and arthritis. J R Soc Interface 2011; 8:1748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shakya AK, Holmdahl R, Nandakumar KS, Kumar A. Characterization of chemically defined poly‐N‐isopropylacrylamide based copolymeric adjuvants. Vaccine 2013; 31:3519–27. [DOI] [PubMed] [Google Scholar]
- 3. Shakya AK, Nandakumar KS. Synthetic polymer as an adjuvant in collagen‐induced arthritis. Curr Protoc Mouse Biol 2014; 4:11–24. [DOI] [PubMed] [Google Scholar]
- 4. Shakya AK, Kumar A, Klaczkowska D, Hultqvist M, Hagenow K, Holmdahl R et al Collagen type II and a thermo‐responsive polymer of N‐isopropylacrylamide induce arthritis independent of Toll‐like receptors: a strong influence by major histocompatibility complex class II and Ncf1 genes. Am J Pathol 2011; 179:2490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shakya AK, Nandkumar KS. Polymers as immunological adjuvants: an update on recent developments. J Biosci Biotechnol 2012; 1:199–210. [Google Scholar]
- 6. Kreuter J, Liehl E, Berg U, Soliva M, Speiser PP. Influence of hydrophobicity on the adjuvant effect of particulate polymeric adjuvants. Vaccine 1988; 6:253–6. [DOI] [PubMed] [Google Scholar]
- 7. Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A 2004; 101:12646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelderman KA, Hultqvist M, Pizzolla A, Zhao M, Nandakumar KS, Mattsson R et al Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest 2007; 117:3020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pizzolla A, Hultqvist M, Nilson B, Grimm MJ, Eneljung T, Jonsson IM et al Reactive oxygen species produced by the NADPH oxidase 2 complex in monocytes protect mice from bacterial infections. J Immunol 2012; 188:5003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grab B, Miles AJ, Furcht LT, Fields GB. Promotion of fibroblast adhesion by triple‐helical peptide models of type I collagen‐derived sequences. J Biol Chem 1996; 271:12234–40. [DOI] [PubMed] [Google Scholar]
- 11. Kuroda K, Caputo GA, DeGrado WF. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chemistry 2009; 15:1123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shakya AK, Sharma P, Kumar A. Synthesis and characterization of thermo‐responsive poly (N‐isopropylacrylamide)–bovine liver catalase bioconjugate. Enzyme Microb Technol 2010; 47:277–82. [Google Scholar]
- 13. Salmaso S, Bersani S, Pennadam SS, Alexander C, Caliceti P. Avidin bioconjugate with a thermoresponsive polymer for biological and pharmaceutical applications. Int J Pharm 2007; 340:20–8. [DOI] [PubMed] [Google Scholar]
- 14. Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collagen‐induced arthritis, using monoclonal anti‐type II collagen antibodies. Arthritis Rheum 1986; 29:400–10. [DOI] [PubMed] [Google Scholar]
- 15. Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell‐deficient mice do not develop type II collagen‐induced arthritis (CIA). Clin Exp Immunol 1998; 111:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rowley MJ, Nandakumar KS, Holmdahl R. The role of collagen antibodies in mediating arthritis. Mod Rheumatol 2008; 18:429–41. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira SA, Gama FM, Vilanova M. Polymeric nanogels as vaccine delivery systems. Nanomedicine 2012; 9:159–73. [DOI] [PubMed] [Google Scholar]
- 18. Singh J, Pandit S, Bramwell VW, Alpar HO. Diphtheria toxoid loaded poly‐(ε‐caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods 2006; 38:96–105. [DOI] [PubMed] [Google Scholar]
- 19. Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature 2009; 462:449–60. [DOI] [PubMed] [Google Scholar]
- 20. Seong SY, Matzinger P. Hydrophobicity: an ancient damage‐associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004; 4:469–78. [DOI] [PubMed] [Google Scholar]
- 21. Shakya AK, Holmdahl R, Nandakumar KS, Kumar A. Polymeric cryogels are biocompatible and their biodegradation is independent of oxidative radicals. J Biomed Mater Res A 2014; 102:3409–18. [DOI] [PubMed] [Google Scholar]
- 22. Sevast'ianov VI, Tseytlina EA. The activation of the complement system by polymer materials and their blood compatibility. J Biomed Mater Res 1984; 18:969–78. [DOI] [PubMed] [Google Scholar]
- 23. Pizzolla A, Gelderman KA, Hultqvist M, Vestberg M, Gustafsson K, Mattsson R et al CD68‐expressing cells can prime T cells and initiate autoimmune arthritis in the absence of reactive oxygen species. Eur J Immunol 2011; 41:403–12. [DOI] [PubMed] [Google Scholar]
- 24. Kraaij MD, Savage ND, van der Kooij SW, Koekkoek K, Wang J, van den Berg JM et al Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci U S A 2010; 107:17686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraaij MD, van der Kooij SW, Reinders ME, Koekkoek K, Rabelink TJ, van Kooten C et al Dexamethasone increases ROS production and T cell suppressive capacity by anti‐inflammatory macrophages. Mol Immunol 2011; 49:549–57. [DOI] [PubMed] [Google Scholar]


