Abstract
Background
Hepatocellular carcinoma (HCC) is characterized by rich vascularization in the tumor, and vascular endothelial growth factor (VEGF) plays important roles in vascularization. The results of the roles of VEGF in predicting efficacy of sorafenib in HCC are conflicting. In this meta-analysis, we aimed to investigate the prognostic and predictive value of VEGF in HCC patients receiving sorafenib.
Material/Methods
PubMed, Embase, and Cochrane library electronic databases were systematically searched for eligible studies. The baseline characteristics were recorded and overall qualities of the eligible studies were assessed by 2 reviewers independently. VEGF levels and data relevant to efficacy of sorafenib were extracted and used for meta-analysis.
Results
The comprehensive search yielded 9 studies that evaluated the relationship between VEGF level and clinical outcome in advanced HCC patients treated with sorafenib. Pooled estimates suggested that high level of VEGF was associated with poor overall survival (HR=1.85; 95% CI: 1.24–2.77; P=0.003) and poor progression-free survival (HR=2.09; 95% CI: 1.43–3.05; P<0.01) in HCC. Mutation of VEGF had a favorable effect on hand-foot skin reaction in HCC patients treated with sorafenib (P<0.05).
Conclusions
High level of VEGF is associated with poor outcomes in HCC patients treated with sorafenib, indicating that VEGF could be used as an indicator of clinical efficacy in patients with HCC. However, more well-designed studies are needed to strengthen our findings.
MeSH Keywords: Prognosis; Carcinoma, Hepatocellular; Receptors, Vascular Endothelial Growth Factor
Background
Hepatocellular carcinoma (HCC) is one of the most common malignancies and has increasing incidence, which causes millions of cancer-related death globally [1]. Due to the low positive rates of diagnosis of HCC, a large proportion of HCC patients lose their opportunity to receive curative-intent therapies, such as resection, radiofrequency ablation, and liver transplantation. The prognosis of patients with advanced HCC with or without prior locoregional treatment is dismal due to lack of effective therapy and the complex nature of liver disease [2–5]. However, with the accumulating recognition of underlying mechanisms of HCC, a number of newly developed molecular targeted agents are emerging and changing the situation.
In recent years, much attention has been focused on revealing the molecular factors or pathways that account for tumor development, growth, and metastases. It is now recognized that angiogenesis is crucial for cancer development, survival, and invasion. The process of formation of new blood vessel in the tumor microenvironment has been the target for therapeutic regimens. Angiogenesis is an elegant process consisting of continuous, sequential periods that finally lead to tumor neovascularization. Angiogenesis contributes to the significant growth of many cancers, including HCC, and the vascular endothelial cells are involved in these steps when the dimension of the tumor exceeds 0.5 mm [6,7]. Vascular endothelial growth factor (VEGF) is a master regulator of angiogenesis in normal and malignant tissues. There are various family members of VEGF and each of them exerts biological functions by binding to different receptors. VEGF plays important roles in prompting proliferation of endothelial cells, thus favoring neovascularization around and within tumor tissues. VEGF participates in several other processes such as recruitment of endothelial cells and activation of receptors related to the proliferation of tumor cells [8,9]. With regards to the critical roles of VEGF in HCC, VEGF-targeted agents that are effective in the treatment of advanced disease have been developed. Sorafenib is a small molecular tyrosine kinase inhibitor that has been used in the treatment of HCC for years. By blocking the synthesis of important cellular factors (e.g., VEGF) in the regulation of angiogenesis and progression of HCC, sorafenib shows effectiveness in clinical application [10–13]. As level of VEGF can be measured in blood samples, several clinical studies questioned whether VEGF could provide sensitive information about HCC response to sorafenib treatment, even adverse events caused by sorafenib. However, the results of these studies do not agree.
In the present meta-analysis, we systematically searched clinical studies related to whether VEGF could predict response of HCC to sorafenib.
Material and Methods
Protocol and registration
The protocol of this review is PRISMA. This study did not have registration information.
Eligibility criteria
Patients: advanced HCC patients, with or without prior treatment; Intervention: Sorafenib in combination with other treatment or not; Comparison: low level of VEGF vs. high level of VEGF; Outcomes: objective responses, progression-free survival, and overall survival.
Information sources and search
The online electronic databases including PubMed, Embase, the Cochrane Library, and CNKI (China National Knowledge Infrastructure) were included and searched for eligible studies for this meta-analysis (date limit: December 1, 2014). The following terms were used for the systematic search: “hepatocellular carcinoma”, “liver cancer”, “HCC”, “VEGF”, “vascular endothelial growth factor”, “response”, “prognosis” and “sorafenib”. For studies without full texts, we contacted the corresponding authors to gain detailed information. We also used Google Scholar to identify relevant studies. The references cited in the reviews were also taken into consideration.
Study selection
For selecting the eligible articles, we used the following criteria: (1) level of VEGF in the blood sample of patients with HCC was detected by RT-PCR (reverse transcription-polymerase chain reaction) or ELISA (enzyme-linked immunosorbent assay); (2) reporting data on treatment efficacy and survival; (3) only the most recent article investigating the same population published by the same author was included. Two well-trained reviewers were involved in the process of study selection. Any disagreement about inclusion was solved by discussion.
Data extraction and quality assessment
Baseline characteristics of each included study, such as title, author, year of publication, journal, numbers of patients, age, sex, intervention, definition of cut-off value, and primary and secondary endpoints, were extracted. If any type of data mentioned above was not available (NA) from the primary study, data were recorded as NA. As most of the included articles were observational studies, quality score or quality-related criteria for inclusion was not applied.
Statistical analysis and synthesis of results
Eligible studies with survival data were included for the synthesis of survival results, and hazard ratio (HR) and its 95% confidence intervals (95% CI) in overall survival (OS) and disease-free survival (DFS) were used for the meta-analysis of survival. If the HR and its 95% CI could not be obtained from the primary study, the methods reported in the study of Parmar et al. was introduced [14], or the calculator provided by the RevMan 5.3 was used to gain missing data.
To detect heterogeneity between the included studies, the χ2 tests were performed based on the Peto’s method [15], and inconsistency value (I2) was calculated. The fixed-effects model was applied when there was no significant heterogeneity, whereas the random-effects model was used if the heterogeneity between HRs was significant. The pooled effect of VEGF level on predicting response to sorafenib was defined as statistically significant if the p value was less than 0.05. All steps of calculation of meta-analysis were performed by RevMan 5.3 (The Cochrane Collaboration).
Results
Study selection and characteristics
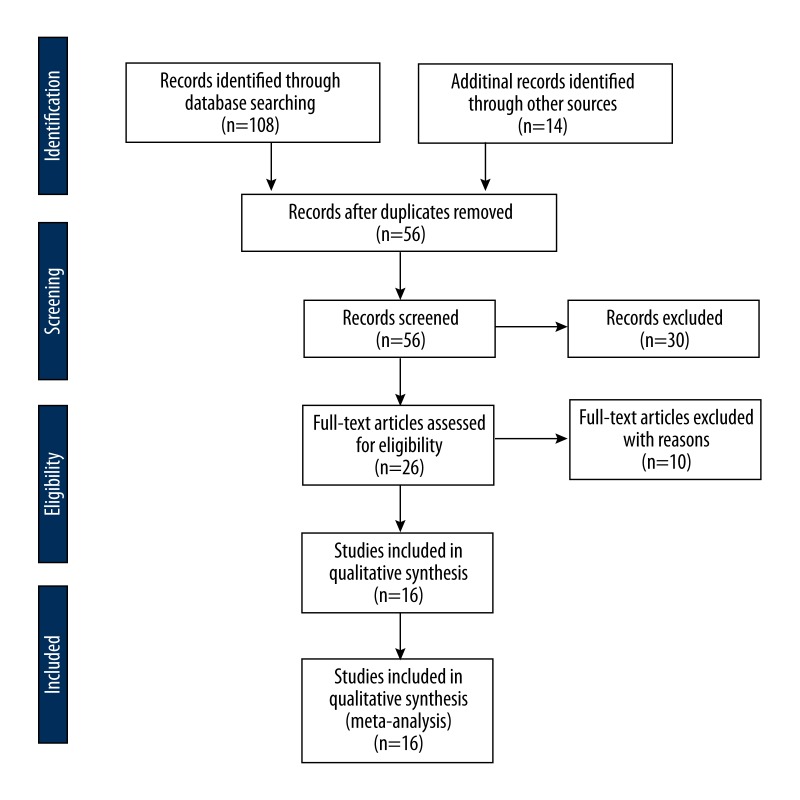
After the systematic search, 9 observational studies [16–24] involving 1202 patients with HCC were eligible for inclusion (Figure 1). The ages of participants had a wide range, from 21 years to 91 years. The publication year ranged from 2011 to 2014. Two studies reported the role of VEGF SNP for predicting response to sorafenib in advanced HCC patients. One study reported data about VEGFR in predicting response to sorafenib. Three trials reported the prognostic value of VEGF level on survival in patients treated with sorafenib. Seven of the 9 studies reported that high level of VEGF was associated with poor efficacy of sorafenib, and the other 2 studies were conference abstracts and did not provide enough information. The detailed characteristics of the included studies are presented in Table 1.
Figure 1.
Flow chart of the selection of eligible studies.
Table 1.
Baseline characteristics of the included studies.
| Study | Year | n | Age | Sex (M/F) | Patients | Intervention | Endpoints | Samples | Test time |
|---|---|---|---|---|---|---|---|---|---|
| Josep et al. | 2012 | 602 | Sorafenib: 64.9±11.2; Control: 66.3±10.2 |
524/78 | Advanced, measurable HCC | Sorafenib: 400 mg, bid | OS, TTP | Blood | Baseline, 12 weeks after treatment |
| Andrea et al. | 2014 | 44 | 67.7±10.1 | 38/6 | HCC | Sorafenib: 800mg/d | OS, RR | Blood | Baseline, 16 weeks after treatment |
| Lee et al. | 2013 | 59 | 57 (37–75) | 52/7 | HCC | Sorafenib: 400 mg, bid | ORR, VEGF | Blood, tissue | NR |
| Scartozzi et al. | 2014 | 148 | 69 (41–86) | 130/18 | HCC | Sorafenib: 400 mg, bid | VEGF SNPs | Blood | Baseline, every 3 weeks |
| Tsuchiya et al. | 2012 | 47 | 70±9 | 38/9 | Advanced, inoperable HCC | Sorafenib | Plasma VEGF; RR | Blood | Baseline, 2 week after, and every month until discontinuation of sorafenib |
| Chen et al. | 2013 | 54 | 45 (21–71) | 46/8 | Advanced HCC | Sorafenib: 400 mg, bid | Treatment response, survival and TTP | Tissue | Every 4 weeks |
| Miyahara et al. | 2013 | 126 | 68 (36–91) | 105/15 | Advanced HCC | Sorafenib: 400/200 mg, bid | RR, OS, PFS, VEGF | Blood | NR |
| Charles | 2011 | 37 | NR | 34/3 | Advanced HCC | Sunitinib: 50 mg/day | ORR | Blood | 4 weeks |
| Tsuchiya et al. | 2014 | 63 | 70 (40–85) | 53/10 | Advanced, inoperable HCC | Sorafenib: 800/400/ 200 mg in 28/28/7 patients | OS, RR VEGF | Blood | Baseline, 1 month after starting sorafenib, and every 3 months thereafter |
n – number of patients; M – male; F – female; HCC – hepatocellular carcinoma; OS – overall survival; TTP – time to progression; ORR – objective response rate; NR – not reported; VEGF – vascular endothelial growth factor; PFS – progression free survival; SNPs – single nucleotide polymorphisms.
Results of meta-analysis
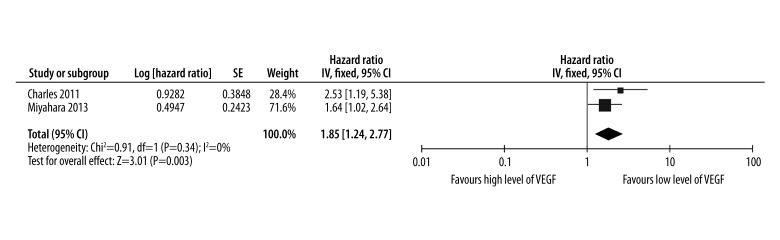
The cut-off values of defining high level of VEGF were 54.9 pg/ml and 68.6 pg/ml in the studies of Charles and Miyahara, respectively. The pooled results of the OS are shown in Figure 2. Overall, the combined HR for the included studies assessing high level of VEGF on OS was 1.85 (95% CI: 1.24–2.77), indicating that high level of VEGF was associated with poor response to sorafenib for HCC. Significant heterogeneity was not observed among the included studies. High level of VEGF had a statistically significant effect on OS in HCC patients treated with sorafenib (P=0.003).
Figure 2.
Levels of VEGF for predicting whether overall survival could be improved by treatment of sorafenib in HCC.
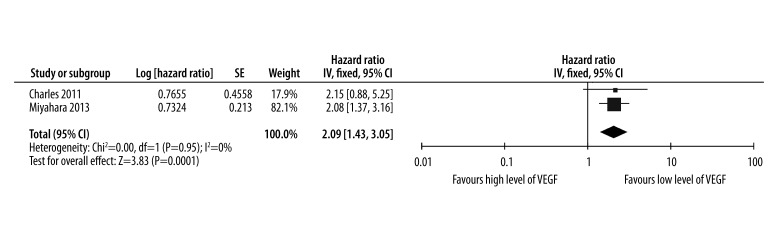
The estimated results of the PFS are presented in Figure 3. The pooled HR for eligible studies evaluating the prognostic role of high level of VEGF on PFS was 2.09 (95% CI: 1.43–3.05), indicating that high level of VEGF was associated with poor efficacy of sorafenib for HCC. The I2 value was 0, indicating that the heterogeneity was not significant between the included studies. High level of VEGF had a statistically significant effect on PFS in HCC patients treated with sorafenib (P<0.01).
Figure 3.
Levels of VEGF for predicting whether progression free survival could be prolonged by administration of sorafenib in HCC.
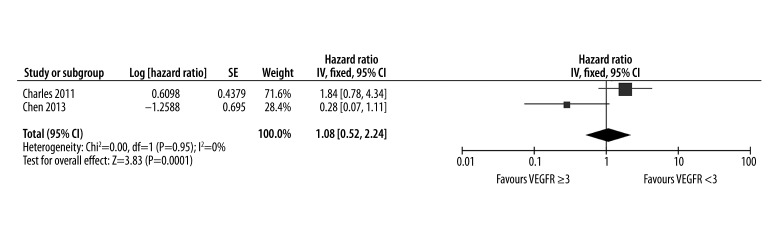
We also evaluated the effect of VEGFR2 on predicting efficacy of sorafenib in patients with HCC. The pooled results of the PFS are shown in Figure 4. Overall, the combined HR of VEGF on PFS was 1.08 (95% CI: 0.52–2.24), indicating that high level of VEGFR2 failed to reflect the response to sorafenib for HCC. High level of VEGFR2 did not have a statistically significant effect on PFS in HCC patients treated with sorafenib (P=0.83).
Figure 4.
Response of VEGFR2 after administration of sorafenib for predicting progression free survival in HCC.
Only 1 study [18] reported data on the effect of VEGF SNPs on incidence of hand-foot skin reaction (HFSR). The pooled results of the VEGF SNP for prediction of HFSR are shown in Table 2. Overall, VEGF SNPS were associated with high OR for grade 2 or 3 HFSR in HCC patients treated with sorafenib. Mutation of VEGF had a statistically significant effect on incidence of HFSR in HCC patients treated with sorafenib (P<0.05).
Table 2.
VEGF polymorphisms associated with high-grade (grade 2 or 3) hand-foot skin reactions (n=59).
| SNP | Grade 2 or 3 HFSR | Without grade 2 or 3 HFSR | OR | P |
|---|---|---|---|---|
| VEGF 94 | 40 | 19 | 13.30 | 0.005 |
| VEGF 1991 | 39 | 19 | 10.13 | 0.036 |
| VEGF IVS3-28 | 40 | 19 | 6.28 | 0.017 |
VEGF – vascular endothelial growth factor; HFSR – hand-foot skin reaction; OR – odds ratio; SNP – single nucleotide polymorphism.
One study [19] reported VEGF and its genotyping in the prediction of clinical outcome for HCC patients treated with sorafenib; the results showed that VEGF SNPs were significant predictors of PFS and OS (Table 3).
Table 3.
VEGF genetic polymorphisms are associated with different PFS and OS.
| SNPs | Genotype | n | PFS (months) | HR | P | OS (months) | HR | P |
|---|---|---|---|---|---|---|---|---|
| VEGF A | CC | 108 | 5.7 | 1.54 | 0.038 | 16.1 | 2.19 | 0.0004 |
| TT+TC | 40 | 3.4 | 8.6 | |||||
| TT+TC | 116 | 6.1 | 0.64 | 0.046 | 14.7 | 0.57 | 0.017 | |
| CC | 32 | 3.0 | 7.4 | |||||
| CC | 52 | 7.6 | 0.67 | 0.044 | 17.9 | 0.61 | 0.025 | |
| AA+AC | 96 | 4.5 | 12.6 | |||||
| CC+CG | 87 | 6.9 | 1.66 | 0.0096 | 17.0 | 1.91 | 0.0016 | |
| GG | 61 | 4.0 | 9.3 | |||||
| VEGF-C | TT+TC | 51 | 10.1 | 0.57 | 0.0043 | 22.0 | 0.62 | 0.0334 |
| CC | 97 | 4.3 | 13.0 |
VEGF – vascular endothelial growth factor; SNP – single nucleotide polymorphism; PFS – progression free survival; HR – hazard ratio.
Discussion
There has been increasing attention on searching for and proving prognostic indicators for HCC patients because these cellular factors guide clinical therapy and monitoring outcomes. To provide as much specific and accurate evidence as possible, it is essential to study certain markers for predicting treatment outcomes using data from clinical trials. In the present study, we examine the prognostic value of different levels of VEGF on predicting outcome of HCC patients who were treated with sorafenib. In this systematic review and meta-analysis, we included and assessed 9 observational studies involving 1202 patients, comparing efficacy of sorafenib in HCC patients with high and low levels of VEGF, aiming to prove the predictive role of VEGF in monitoring efficacy of sorafenib. Pooled estimates showed that high level of VEGF before treatment correlated with good response to sorafenib in HCC.
Besides evaluating the predictive role of VEGF on efficacy and survival in HCC, several published meta-analysis studied the similar value of VEGF in various types of tumors, including lung cancer [25–29], gastric cancer [30,31], colorectal cancer [32,33], and head and neck squamous cancer [34]. These studies together suggested that VEGF plays important roles in development, growth, metastasis, and angiogenesis of tumors, as well as in affecting clinical outcomes in advanced HCC patients. It is now recognized that VEGF is crucial for tumor angiogenesis. VEGF has already been a primary therapeutic target for curing various tumors. Sorafenib is one of the main monoclonal antibodies against generation of several intracellular factors proved to be critical for cancer development and progression, including VEGF. In recent years, sorafenib has been widely used in the treatment of HCC and it exhibited powerful efficacy. However, whether VEGF could be used to predict the response to sorafenib in patients with advanced HCC it is not clear.
Our results showed that VEGF level could be used to predict response to sorafenib in patients with HCC. These data were in accordance with the results of previously published meta-analyses [35–37] that reported VEGF was a good indicator of prognosis and outcome, although we mainly focused on systematically reviewing the relationship between VEGF and response to sorafenib in HCC patients. Zhan et al. [35] examined the correlation between overexpression of VEGF and the efficacy in HCC patients by searching for relevant articles and analyzing their data. They included 14 studies and the results suggested that high level of VEGF had an unfavorable effect on overall survival, but had no significant impact on disease-free survival. Finally, they concluded that VEGF overexpression indicated a poor prognosis for HCC. Another meta-analysis published in 2009 also examined the relationship between VEGF and clinical outcome in HCC patients [37]. The authors performed a meta-analysis to evaluate the use of VEGF as a predictor of survival in patients treated for HCC. The results showed that high VEGF levels predicted poor overall and disease-free survival, indicating that VEGF levels appeared to have obvious predictive ability for evaluating efficacy in HCC and may be useful for defining prognosis in HCC [37]. However, these studies did not directly estimate the association between levels of VEGF and efficacy of sorafenib. There were some improvements in our study by only including studies that explored the predictive role of VEGF on response to sorafenib in HCC. The results showed that high level of VEGF after administration of sorafenib was associated with poor response to sorafenib. VEGF may be a useful predictor of the response to sorafenib alone or in combination with other therapies in patients with HCC. Monitoring of VEGF changes after treatment was useful to evaluate the efficacy of sorafenib and to predict the prognosis of advanced HCC.
Three of the 9 studies were performed using patients from hospitals in Asia, and the rest were performed in cohorts from Western populations. This raises the question of whether the results could be skewed, because the prevalence of HCC in Asia is undoubtedly higher than in the rest of the world. By controlling the included studies, we found that the estimated value of VEGF for predicting response to sorafenib was similar. This suggested that the results of this study might be applicable in HCC patients both from Asia and Western.
There are several limitations in the present study. The first is the publication bias. Although there was no significant heterogeneity between included studies, it is worth noting that the number of participants in most of the primary studies are limited, the treatments of HCC patients are sorafenib alone or in combination with other regimens, the samples used for detecting levels of VEGF are different, the detection time of VEGF in different studies varies from 2 weeks to a few months, and the cut-off values for considering high level of VEGF are not consistent among the included studies. These factors influence the overall effect of VEGF in predicting efficacy of sorafenib. The second limitation is that not all of the included studies provided sufficient data for the pooled analysis. A total of 3 articles failed to provide useful information when estimating the role of VEGF for prediction of response to sorafenib. The information about VEGFR was available in only 1 study. Two studies reported VEGF SNPs for predicting clinical outcomes after administration of sorafenib. With regards of these facts, bias within and between studies remains a major concern affecting the results of the meta-analysis. We recommend that the PRISMA [38] and the views proposed by McShane et al. [39] should be considered for improving the reporting of prognostic studies of tumors; they mainly include blinded assessment of prognostic markers to patient outcome, prospective study design, trial time period, precise outcome definition, provision of candidate variable list, and adequate description and references for assay methods [37]. Further studies using the above criteria are needed.
Conclusions
In conclusion, our meta-analysis explored the correlation between prognostic value of VEGF level and efficacy of sorafenib in patients with HCC. As demonstrated in our study, it was concluded that high VEGF level after administration of sorafenib was associated with poor survival and poor clinical efficacy, and no significant heterogeneity was found between studies. In order to strengthen the results, more prospective studies with well-designed, better-standardized detection of VEGF are required to estimate the association between VEGF level and the efficacy of sorafenib in patients with HCC.
Footnotes
Source of support: Self financing
Conflict of interest
None.
References
- 1.Hollebecque A, Malka D, Ferte C, et al. Systemic treatment of advanced hepatocellular carcinoma: from disillusions to new horizons. Eur J Cancer. 2015;51:327–39. doi: 10.1016/j.ejca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Niu ZS, Niu XJ, Wang M. Management of hepatocellular carcinoma: Predictive value of immunohistochemical markers for postoperative survival. World J Hepatol. 2015;7:7–27. doi: 10.4254/wjh.v7.i1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsburg M, Zivin SP, Wroblewski K, et al. Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol. 2015;26:330–41. doi: 10.1016/j.jvir.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalogeridi MA, Zygogianni A, Kyrgias G, et al. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015;7:101–12. doi: 10.4254/wjh.v7.i1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangro B. Evidence-based integration of selective internal radiation therapy into hepatocellular carcinoma management. Future Oncol. 2014;10:7–11. doi: 10.2217/fon.14.216. [DOI] [PubMed] [Google Scholar]
- 6.Zhu AX, Duda DG, Sahani DV, et al. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 8.Tseng PL, Tai MH, Huang CC, et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349–57. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- 9.Lamszus K, Ulbricht U, Matschke J, et al. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–405. [PubMed] [Google Scholar]
- 10.Abou-Alfa GK, Amadori D, Santoro A, et al. Safety and Efficacy of Sorafenib in Patients with Hepatocellular Carcinoma (HCC) and Child-Pugh A versus B Cirrhosis. Gastrointest Cancer Res. 2011;4:40–44. [PMC free article] [PubMed] [Google Scholar]
- 11.Di Maio M, Daniele B, Perrone F. Targeted therapies: Role of sorafenib in HCC patients with compromised liver function. Nat Rev Clin Oncol. 2009;6:505–6. doi: 10.1038/nrclinonc.2009.114. [DOI] [PubMed] [Google Scholar]
- 12.Roderburg C, Bubenzer J, Spannbauer M, et al. Long-term survival of a HCC-patient with severe liver dysfunction treated with sorafenib. World J Hepatol. 2010;2:239–42. doi: 10.4254/wjh.v2.i6.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong H, Tang YF, Yao TJ, et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (HCC) Oncologist. 2011;16:1721–28. doi: 10.1634/theoncologist.2011-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Pena CE, Lathia CD, et al. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290–300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inghilesi AL, Gallori D, Antonuzzo L, et al. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J Gastroenterol. 2014;20:786–94. doi: 10.3748/wjg.v20.i3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Chung YH, Kim JA, et al. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136–42. doi: 10.1002/cncr.27705. [DOI] [PubMed] [Google Scholar]
- 19.Scartozzi M, Faloppi L, Svegliati Baroni G, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135:1247–56. doi: 10.1002/ijc.28772. [DOI] [PubMed] [Google Scholar]
- 20.Chen D, Zhao P, Li SQ, et al. Prognostic impact of pERK in advanced hepatocellular carcinoma patients treated with sorafenib. Eur J Surg Oncol. 2013;39:974–80. doi: 10.1016/j.ejso.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Miyahara K, Nouso K, Morimoto Y, et al. Pro-angiogenic cytokines for prediction of outcomes in patients with advanced hepatocellular carcinoma. Br J Cancer. 2013;109:2072–78. doi: 10.1038/bjc.2013.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya K, Asahina Y, Matsuda S, et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer. 2014;120:229–37. doi: 10.1002/cncr.28384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya K, Asahina Y, Muraoka M, et al. Decrease of plasma vegf level within 2 months after sorafenib administration predicts treatment efficacy and prognosis in patients with advanced hepatocellular carcinoma. J Hepatol. 2012;56:S406. [Google Scholar]
- 24.Lee D, Chung YH, Kim JA, et al. Vascular endothelial growth factor may be a useful predictor of response to combined therapy with sorafenib and transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology International. 2014;8:S276–S77. [Google Scholar]
- 25.Delmotte P, Martin B, Paesmans M, et al. VEGF and survival of patients with lung cancer: a systematic literature review and meta-analysis. Rev Mal Respir. 2002;19:577–84. [in French] [PubMed] [Google Scholar]
- 26.Fu ZZ, Sun XD, Li P, et al. Relationship between serum VEGF level and radiosensitivity of patients with nonsmall cell lung cancer among asians: a meta-analysis. DNA Cell Biol. 2014;33:426–37. doi: 10.1089/dna.2013.2249. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H, Shao W, Zhao W. VEGF-C in non-small cell lung cancer: meta-analysis. Clin Chim Acta. 2014;427:94–99. doi: 10.1016/j.cca.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Song N, Liu B, Wu J, et al. Vascular endothelial growth factor (VEGF) -2578C/A and -460C/T gene polymorphisms and lung cancer risk: a meta-analysis involving 11 case-control studies. Tumour Biol. 2014;35:859–70. doi: 10.1007/s13277-013-1119-y. [DOI] [PubMed] [Google Scholar]
- 29.Tu J, Wang S, Zhao J, et al. rs833061 and rs699947 on promoter gene of vascular endothelial growth factor (VEGF) and associated lung cancer susceptibility and survival: a meta-analysis. Med Sci Monit. 2014;20:2520–26. doi: 10.12659/MSM.891394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi WX, Shen Z, Tang LN, et al. The role of anti-VEGF agents in the treatment of advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol. 2014;35:7675–83. doi: 10.1007/s13277-014-2037-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Li JP, Zhou P. Vascular endothelial growth factor (VEGF) +936 C/T gene polymorphism and gastric cancer risk: appraisal of a recent meta-analysis. Int J Biol Markers. 2011;26:274–75. doi: 10.5301/JBM.2011.8829. [DOI] [PubMed] [Google Scholar]
- 32.Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–32. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Z, Ba C, Wang W, et al. Vascular endothelial growth factor (VEGF) gene polymorphisms and colorectal cancer: a meta-analysis of epidemiologic studies. Genet Test Mol Biomarkers. 2012;16:1390–94. doi: 10.1089/gtmb.2012.0266. [DOI] [PubMed] [Google Scholar]
- 34.Kyzas PA, Cunha IW, Ioannidis JP. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in head and neck squamous cell carcinoma: a meta-analysis. Clin Cancer Res. 2005;11:1434–40. doi: 10.1158/1078-0432.CCR-04-1870. [DOI] [PubMed] [Google Scholar]
- 35.Zhan P, Qian Q, Yu LK. Serum VEGF level is associated with the outcome of patients with hepatocellular carcinoma: a meta-analysis. Hepatobiliary Surg Nutr. 2013;2:209–15. doi: 10.3978/j.issn.2304-3881.2013.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan P, Qian Q, Yu LK. Prognostic significance of vascular endothelial growth factor expression in hepatocellular carcinoma tissue: a meta-analysis. Hepatobiliary Surg Nutr. 2013;2:148–55. doi: 10.3978/j.issn.2304-3881.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoenleber SJ, Kurtz DM, Talwalkar JA, et al. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385–92. doi: 10.1038/sj.bjc.6605017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–72. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]