Abstract
Objectives
Excessive daytime sleepiness (EDS) and sudden sleep onset (SOS) episodes are frequent in Parkinson׳s disease (PD). The objectives are to identify clinical characteristics and factors associated with EDS and SOS episodes.
Methods
Clinical demographic data were recorded (N=100, mean age=65.0±10.4). EDS was identified by the Epworth Sleepiness Scale (ESS>10) and SOS episodes were registered. Disease severity was evaluated by the Unified Parkinson׳s Disease Rating Scale (UPDRS, I, II, and III), sleep disturbances by the Parkinson׳s Disease Sleep Scale (PDSS<100), depressive symptoms by the Beck Depression Inventory (BDI>10) and rapid eye movement (REM) sleep behavior disorder (RBD) by the REM sleep behavior scale. Levodopa equivalent dose was measured.
Results: PD patients with EDS (67%) were predominately male (73.1%) and had worse disease severity (UPDRS II and III p= 0.005); SOS episodes (39%) were associated with disease duration, diabetes, sleep disturbances (PDSS Scale), disease severity (UPDRS I, II, III) and RBD symptoms (p<0.05). Stepwise regression analysis showed that EDS was independently associated with motor-symptoms severity (UPDRS III scale, p=0.003). SOS episodes were independently associated with disease duration (p=0.006) and sleep disturbances (PDSS scale, p=0.03): patients had more uncomfortable immobility at night, tremor on waking and snoring or difficult breathing.
Discussion
EDS and or SOS episodes are frequent and manifest a differential pattern in PD. SOS episodes are associated with longer disease duration, diabetes, sleep disturbances and RBD symptoms indicating that these “sleep attacks” are of multifactorial origin and probably influenced by brain structural abnormalities.
Keywords: Parkinson’s disease, Sleepiness, Sudden sleep onset, Sleep attacks, Diabetes
1. Introduction
Parkinson׳s disease (PD) presents with classical motor manifestations that include tremor, rigidity, akinesia and postural instability [1,2]. Several non-motor abnormalities, including sleep alterations, such as, excessive daytime sleepiness (EDS), “sleep attacks” or episodes of sudden onset of sleep (SOS), insomnia, restless legs syndrome and rapid eye movement sleep behavior disorder (RBD) have been described [3,4]. Importantly, EDS and SOS episodes greatly affect patients and caregiver׳s routine and potentially increase the risk of accidents [5]. To date, these alterations remain a challenge to treatment as there is not enough evidence to make a recommendation for the management of these sleep-wake abnormalities [6]. Possibly, sleep and wake abnormalities contribute to the heterogeneous clinical manifestations of PD and to the common daytime oscillations of symptoms. Thus, modifying factors that influence EDS and SOS episodes may contribute to improve therapy.
Previous evidences indicate that EDS and SOS episodes have a different pattern of manifestation in PD. For instance, EDS is frequently found [7]; otherwise, SOS is less common and manifests somewhat similar to narcoleptic events [8]. Of note, the extent and severity of SOS in PD is variable: some patients and caregivers inform long duration episodes with atonia while others report episodes of short duration without motor changes. Reports also suggest two distinct types of events: those of sudden onset without warning and those of slow onset with prodrome drowsiness [9]. Classical cataplectic symptoms as occurs in narcolepsy have not been observed in PD patients. Interestingly, a significant higher narcolepsy score in PD patients has been previously observed [10].
Clarifying whether clinical factors and associated comorbidities influence the manifestation of EDS and SOS episodes in PD may contribute for patient care. The objectives of this study are to characterize the clinical symptoms and to identify factors contributing to the presence of EDS and SOS in PD patients.
2. Material and methods
2.1. Study design
This is an observational cross-sectional study of consecutive patients with PD from an outpatient unit at a University Hospital in the city of Fortaleza, Brazil. The study involved 100 patients recruited among a population of 152 patients: 28 were too old or had difficulty with verbal communication, 10 refused to collaborate and 14 were considered as poor-compliant patients. Evaluations were performed over a period of 12 months (July 2010 to July 2011). Subjects were selected consecutively as part of a large cohort of patients with PD being followed in a longitudinal study [Sleep-For-PD study]. Specific questionnaires were all measured concurrently in a face-to-face interview by two trained medical staff. Patients were excluded if they had any severe comorbidity and were not competent to provide their informed consent. The protocol was analyzed and approved by the Ethics Committee (HU-UFC No. 045.0607).
2.2. Measures
Demographic data, habits and comorbidities were recorded using a standardized questionnaire. Daytime somnolence was assessed by the Epworth Sleepiness Scale (ESS), a questionnaire containing eight items that ask about the expectation of dozing in eight hypothetical situations. An ESS score ≥10 indicates EDS [11]. Patients and family or caregivers were interrogated about the presence of SOS or “sleep attacks”. We used the Parkinson׳s Disease Sleep Scale (PDSS), a 15-item visual analog scale that quantifies several aspects of nocturnal disabilities and sleep problems in PD; this scale has also been validated in Brazil [12]. A PDSS score ≤100 defined troublesome nocturnal symptoms and a cut-off of <5 for each item indicated sleep impairment. Disease severity was evaluated by the Unified Parkinson׳s Disease Rating Scale (UPDRS) Parts I, II, III, IV and V. Depressive symptoms were evaluated by the Beck Depression Inventory (BDI) and were defined as present if the score was BDI ≥10 [12]. The RBD scale that indicates clinically probable RBD was administered to all patients. The Levodopa Equivalent Dose was measured.
2.3. Statistical analysis
Descriptive statistics are presented as mean±standard deviation, range and frequency (% values). Fisher׳s exact test for categorical variables, Mann–Whitney U test for continuous variables and Student׳s t-test for normally distributed data with equal variances were performed to compare between patients regarding the presence/absence of EDS and SOS episodes. Logistic regression analysis examined each factor associated with EDS or with SOS episodes. A forward stepwise multiple regression analysis was later performed: variables with historical evidence of influence on sleepiness and/or with a p<0.10 were all included; a p<0.05 was required for a variable to be retained in the final model. Statistical analysis was carried out using SPSS for Windows, version 16.0. Statistical significance was set at p<0.05.
3. Results
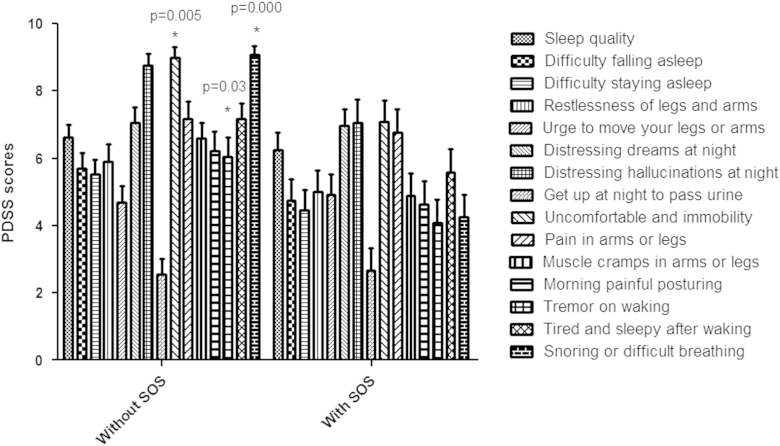
Patient characteristics according to the presence of EDS and to SOS episodes are depicted in Table 1. Individuals with EDS were predominately of male gender and had worse disease severity as evaluated by UPDRS II and III. Patients with SOS episodes had longer disease duration and worse severity of symptoms as evaluated by the UPDRS I, II and III. They also had more symptoms related to RBD (RBD scale). Logistic regression analysis confirmed an association between EDS and symptom severity (UPDRS II and III). Sudden sleep onset episodes were associated with disease duration, diabetes, sleep disturbances (PDSS Scale), disease severity (UPDRS I, II and III) and RBD symptoms (Table 2). Patients with SOS episodes had more EDS (67%, Fisher׳s exact test, p=0.02). Stepwise analysis showed that EDS was independently associated with motor symptoms severity (UPDRS III scale). SOS episodes were independently associated with disease duration and sleep disturbances (PDSS scale) (Table 3). Analysis of sleep disturbances showed that patients with SOS episodes had more night symptoms related to uncomfortable immobility, tremor on waking and snoring or difficult breathing (Fig. 1).
Table 1.
Analysis of factors influencing excessive daytime sleepiness and sudden sleep onset episodes in Parkinson׳s disease patients.
| Variables | All casesN=100 | Without EDSN=33 | With EDSN=67 | Pvalue | Without SOSN=61 | With SOSN=39 | Pvalue |
|---|---|---|---|---|---|---|---|
| Gender M/F | 67/33 | 18/15 | 49/18 | 0.04a,⁎ | 40/21 | 27/12 | 0.27a |
| Age, mean (SD) | 65.0 (10.4) | 63.5 (8.4) | 65.7 (11.2) | 0.34b | 64.5 (10.3) | 65.8 (10.5) | 0.54 |
| Disease duration>5 y, Mean (SD) | 6.8 (5.3) | 19/12 | 31/36 | 0.19a | 37/23 | 13/25 | 0.01a,⁎ |
| LED Mean (SD) | 707.1 (335.2) | 709.7 (408.6) | 705.0 (268.8) | 0.96b | 669.6 (195.3) | 785.4 (524.0) | 0.33b |
| Family history (%) | 28 | 9 | 19 | 0.94a | 21 | 7 | 0.10a |
| Dyskinesia, (%) | 36 | 10 | 26 | 0.65a | 19 | 17 | 0.20a |
| UPDRS I, Mean (SD) | 4.5 (2.8) | 3.6 (2.2) | 4.9 (2.9) | 0.06c | 4.0 (2.7) | 5.4 (2.7) | 0.01c,⁎ |
| UPDRS II, Mean (SD) | 19.5 (9.9) | 12.0 (6.3) | 20.0 (9.0) | 0.000c,⁎⁎ | 14.5 (9.2) | 22.5 (9.1) | 0.000c,⁎⁎ |
| UPDRS III, Mean (SD) | 17.3 (12.6) | 12.4 (8.6) | 22.5 (10.6) | 0.000c,⁎⁎ | 16.0 (12.6) | 24.9 (10.5/ | 0.000c,⁎⁎ |
| BDI, Mean (SD) | 18.2 (12.0) | 18.4 (10.4) | 18.4 (10.4) | 0.84c | 17.8 (11.3) | 19.6 (10.2) | 0.16c |
| PDSS<100 (N, %) | 62 | 60 | 63 | 0.80a | 57 | 70 | 0.25a |
| REMBD, Mean (SD) | 6.2 (2.9) | 5.7 (2.5) | 6.6 (2.8) | 0.15c | 5.7 (2.6) | 7.2 (2.8) | 0.01c,⁎ |
| Visual hallucinations | |||||||
| N (%) | 27 | 7 | 47/20 | 0.62a | 13 | 14 | 0.11a |
| Vivid dreams, N (%) | 57 | 19 | 38 | 0.82a | 34 | 23 | 0.67a |
| Diabetes, N (%) | 18 | 4 | 14 | 0.40a | 7 | 11 | 0.05a |
| Hypertension, N (%) | 45 | 12 | 33 | 0.37a | 24 | 21 | 0.20a |
Abbreviations: y=years; LED=Levodopa Equivalent Dose; UPDRS=Unified Parkinson׳s Disease Rating Scale; BDI=Beck Depression Inventory; PDSS=Parkinson׳s Disease Sleep Scale; REMBD=Rapid eye Movement Sleep Behavior Disorder.
Fisher׳s exact test.
Student׳s t test.
Mann–Whitney test.
p<0.05.
p<0.01.
Table 2.
Logistic regression analysis of factors associated with excessive daytime sleepiness (Epworth Sleepiness Scale>10) and sudden sleep onset episodes.
| Variables |
EDS |
SOS |
||
|---|---|---|---|---|
| OR [CI] | Pvalue | OR [CI] | Pvalue | |
| Gender | 2.55 [1.05––6.20] | 0.03⁎ | 1.74 [0.71–4.23] | 0.22 |
| Age | 1.02 [0.97–1.06] | 0.34 | 1.01 [0.97–1.05] | 0.54 |
| Disease duration | 1.05 [0.96–1.14] | 0.24 | 1.13 [1.03–1.23] | 0.004⁎⁎ |
| LED | 1.00 [0.99–1.00] | 0.96 | 1.00 [0.99–1.00] | 0.33 |
| Dyskinesia | 1.33 [0.54–3.27] | 0.53 | 1.74 [0.75–4.04] | 0.19 |
| Visual hallucinations | 1.45 [0.54–3.93] | 0.45 | 2.10 [0.85–5.19] | 0.10 |
| Vivid dreams | 0.85 [0.35–2.05] | 0.72 | 1.25 [0.54–2.90] | 0.59 |
| Diabetes | 1.78 [0.53–5.99] | 0.34 | 3.08 [1.06–8.91] | 0.03⁎ |
| Arterial hypertension | 1.55 [0.64–3.79] | 0.32 | 1.86 [0.79–4.35] | 0.14 |
| UPDRS I | 1.20 [1.00–1.43] | 0.04⁎ | 1.20 [1.02–1.40] | 0.02⁎ |
| UPDRS II | 1.10 [1.04–1.17] | 0.001⁎⁎ | 1.09 [1.03–1.14] | 0.000⁎⁎ |
| UPDRS III | 1.08 [1.03–1.14] | 0.001⁎⁎ | 1.06 [1.02–1.10] | 0.002⁎⁎ |
| BDI | 0.99 [0.96–1.03] | 0.96 | 1.01 [0.97–1.05] | 0.44 |
| PDSS | 0.99 [0.97–1.00] | 0.30 | 0.98 [0.96–0.99] | 0.01⁎ |
| REMBD | 1.12 [0.96–1.32] | 0.14 | 1.21 [1.03–1.42] | 0.01⁎ |
Abbreviations: LED=Levodopa Equivalent Dose; UPDRS=Unified Parkinson׳s Disease Rating Scale; PDSS=Parkinson׳s Disease Sleep Scale; BDI=Beck Depression Inventory; REMBD=Rapid eye Movement Sleep Behavior Disorder.
p<0.05.
p<0.01.
Table 3.
Multiple logistic regression analysis of factors influencing excessive daytime sleepiness and sudden sleep onset episodes.
|
ESS>10 |
||||
|---|---|---|---|---|
| N=67 | OR | 95% CI | P value | |
| UPDRS III, range | 1.08 | 1.02–1.14 | 0.003⁎⁎ | |
| Controlled for gender, age, disease duration, diabetes and REMBD symptoms | ||||
|
SOS episodes |
||||
| N=39 | OR | 95% CI | P value | |
| Disease duration, range (mean, SD) | 1.13 | 1.03–1.24 | 0.006⁎⁎ | |
| PDSS, range (mean, SD) | 0.98 | 0.96–0.99 | 0.03⁎ | |
| Controlled for gender, age, disease duration, diabetes and REMBD symptoms | ||||
Abbreviations: ESS=Epworth Sleepiness Scale; SOS=Sudden Sleep Onset; PDSS=Parkinson׳s Disease Sleep Scale; REMBD=Rapid eye Movement Sleep Behavior Disorder.
p<0.05.
p<0.01.
Fig. 1.
Patients with sudden sleep onset episodes have more uncomfortable immobility at night, tremor on waking, and snoring and respiratory difficulties at night according to the evaluation of Parkinson׳s Disease Sleep Scale domains.
4. Discussion
Our data confirm that EDS and SOS episodes are frequent in PD. SOS episodes affected nearly 40% of patients and this is in agreement with a recent study involving a large number of PD patients [8]. Several factors such as severity of motor and non-motor symptoms, longer disease duration and the presence of diabetes were associated with SOS episodes. Also, cases with SOS episodes had more sleep disturbances including uncomfortable sensation and immobility at night, tremor on waking, and snoring and sleep related breathing complaints. All this confirms the multifactorial origin of SOS episodes in PD.
Presently, we show that PD patients with SOS have more diabetes and longer disease duration. Previously, increased daytime sleepiness has been shown in diabetes [13]. Compared to individuals without diabetes, patients with diabetes experience more EDS or dozing/sleeping episodes when stopped for a few minutes in traffic [14]. These findings suggest that brain structural abnormalities play a role in the multifactorial origin of SOS in PD. In partial agreement, several studies show that low hypocretin levels in the CSF are present in PD patients [15,16]. Normal levels and no association with sleepiness have also been reported [17,18]. Low hypocretin levels have been shown to correlate with hypothalamic hypocretin cell loss in narcolepsy and other forms of hypersomnia [19]. One cerebral histopathological study has shown that a loss of hypocretin and melanin concentrating hormone cells occurs in parallel with disease progression in PD [20]. All these findings indicate that key structures involved with the regulation of arousal and sleep are compromised in PD, possibly, as part of the widespread damage caused by neurodegeneration that occur not only in PD but also in other parkinsonian syndromes [21].
Interestingly, it has been shown that the hypothalamic hypocretinergic system promote motoneuron discharge and have the capability of modulating the level of motor activity through the final common pathway i.e. inducing changes in the synaptic responses and electrical properties of lumbar motoneurons. In short, the hypothalamic hypocretinergic neurons are capable of modulating the activity of lumbar motoneurons through presynaptic and postsynaptic mechanisms [22]. This is of utmost importance considering that motor behavior alterations are core symptoms in PD. According to Burgess et al. the noradrenergic system acts to synchronize motor and arousal systems. In this model, an excitatory noradrenergic drive maintains postural muscle tone during wakefulness by activating α1 receptors on skeletal motoneurons. Loss of this normal excitatory drive triggers motor inactivity during cataplexy by reducing motoneuron excitation; hypocretin deficiency would cause cataplexy by short-circuiting the noradrenergic drive to skeletal motoneurons. It is suggested that the noradrenergic system functions to couple the brain systems that control postural muscle tone and behavioral arousal state [23]. Considering all these findings, a narcolepsy-like pathophysiology of sleep-wake disturbances and motor abnormalities could coexist in PD.
Furthermore, an independent association between SOS episodes and sleep disturbances such as RBD symptoms was observed. Cumulative evidence shows that RBD precedes the development of PD by many years [24–26]. Previously, a reduction of median cerebellar peduncle that carries key structures from the reticular ascending activating system has been associated with EDS in PD patients [27]. These facts indicate that brain structure abnormalities are on the basis of RBD and SOS in PD.
Controversy remains over the role of sleep apnea in EDS and SOS episodes in PD. Previously, a study has shown that snoring, an indicator of sleep apnea, was the only factor associated with EDS in PD [28]. Recently, CPAP therapy improved daytime sleepiness in patients with PD [29]. Importantly, it is essential to examine factors influencing not only EDS but also the more severe SOS episodes. Potentially, SOS episodes are more threatening and according to our data, they are more related to disease duration and sleep disturbances. Another important issue is to investigate about the presence of RBD that not only contribute for EDS and SOS episodes but is closely related to the presence of sleep apnea. All these issues are important to consider in future attempts to modify EDS and SOS episodes in PD patients. For instance, the effects of reducing overnight immobility and discomfort on vigilance and daytime performance remain to be tested. To date, no evidence has shown that increasing sleep quantity and quality ameliorate EDS or SOS episodes in PD.
In this study, medication did not influence the severity of EDS or the presence of SOS episodes. It is largely recognized that dopamine agonists can precipitate these somewhat narcoleptic events or “sleep attacks” in PD patients [30,31]. The effects of dopamine agonists on sleep have been variable and possibly related to the severity of baseline symptoms [32]. Improvement of sleep symptoms and fatigue have been reported after the use of dopamine agonists [33]. Ropinirole and other non-ergot dopamine D2 receptor agonists cause selective loss of orexin-immunoreactive neurons in culture of rat hypothalamus [34]. An explanation for these findings may be a D2 receptor-mediated presynaptic suppression of glutamatergic excitatory inputs to orexin neurons causing silencing of excitatory activity of orexin neurons and secondary depletion of orexin. This unwanted dopamine agonist effect would further complicate the orexin loss that occurs with PD neurodegeneration [35]. All this evidence not only confirms a disturbance of the orexinergic system and secondary sleep/arousal cycle disruption but also shows a great deal of clinical variability among patients. It is reasonable to consider that EDS and/or SOS are not influenced only by the use of dopaminergic agonists but by the disease process and the several associated cerebral alterations that occur in PD [36,37].
Limitations to this study must be acknowledged. The present data are based on the evaluation of a reduced number of patients. However, the frequency of EDS and SOS episodes are in agreement with a study involving a large number of individuals [8]. Also, this study is based on questionnaires and objective evidence obtained from polysomnography and multiple sleep latency tests would be of interest. Of note, studies about the value of subjective measures of sleepiness have led to the idea that objective and subjective measures have different meaning [38,39]. Furthermore, an association between subjective sleep quality and sleep measures obtained from polysomnography has not been previously found [40]. It has been said that total dopaminergic drug dose rather than the specific dopamine agonist used is the best predictor of daytime sleepiness in PD patients receiving dopamine agonist therapy [41].
In conclusion, we show that EDS and or SOS episodes in PD are frequent and have a differential nature. SOS episodes are associated with longer disease duration, diabetes, sleep disturbances and RBD symptoms. Brain structural abnormalities and hypocretin deficiency probably influence other environmental factors. This survey strongly suggests that SOS is a multifactorial phenomenon. The therapeutic management of EDS and SOS episodes in PD should consider that these symptoms can be severe and unexpected and all aggravating factors must be considered.
Acknowledgments
This study was partially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- 1.Gazewood J.D., Richards D.R., Clebak K. Parkinson disease: an update. Am Fam Phys. 2013;87:267–273. [PubMed] [Google Scholar]
- 2.Colosimo C., Albanese A., Hughes A.J., de Bruin V.M., Lees A.J. Some specific clinical features differentiate multiple system atrophy (striatonigral variety) from Parkinson׳s disease. Arch Neurol. 1995;52:294–298. doi: 10.1001/archneur.1995.00540270090024. [DOI] [PubMed] [Google Scholar]
- 3.Arnulf I., Leu S., Oudiette D. Abnormal sleep and sleepiness in parkinson׳s disease. Curr Opin Neurol. 2008;21:472–477. doi: 10.1097/WCO.0b013e328305044d. [DOI] [PubMed] [Google Scholar]
- 4.Martinez D., Lenz Mdo C. Sleep-related movement disorders. Sleep Sci. 2007;1:6–14. [Google Scholar]
- 5.Arnulf I., Leu-Semenescu S. Sleepiness in Parkinson׳s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S101–104. doi: 10.1016/S1353-8020(09)70792-8. [DOI] [PubMed] [Google Scholar]
- 6.Bruin V.M., Bittencourt L.R., Tufik S. Sleep-wake disturbances in Parkinson׳s disease: Current evidence regarding diagnostic and therapeutic decisions. Eur Neurol. 2012;67:257–267. doi: 10.1159/000335078. [DOI] [PubMed] [Google Scholar]
- 7.Poryazova R., Benninger D., Waldvogel D., Bassetti C.L. Excessive daytime sleepiness in parkinson׳s disease: Characteristics and determinants. Eur Neurol. 2010;63:129–135. doi: 10.1159/000276402. [DOI] [PubMed] [Google Scholar]
- 8.Korner Y., Meindorfner C., Moller J.C., Stiasny-Kolster K., Haja D., Cassel W. Predictors of sudden onset of sleep in Parkinson׳s disease. Mov Disord. 2004;19:1298–1305. doi: 10.1002/mds.20163. [DOI] [PubMed] [Google Scholar]
- 9.Homann C.N., Wenzel K., Suppan K., Ivanic G., Kriechbaum N., Crevenna R. Sleep attacks in patients taking dopamine agonists: review. Br Med J. 2002;324:1483–1487. doi: 10.1136/bmj.324.7352.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happe S., Schrodl B., Faltl M., Muller C., Auff E., Zeitlhofer J. Sleep disorders and depression in patients with Parkinson׳s disease. Acta Neurol Scand. 2001;104:275–280. doi: 10.1034/j.1600-0404.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 11.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Margis R., Donis K., Schonwald S.V., Fagondes S.C., Monte T., Martin-Martinez P. Psychometric properties of the Parkinson׳s disease sleep scale—Brazilian version. Parkinsonism Relat Disord. 2009;15:495–499. doi: 10.1016/j.parkreldis.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros C., Bruin V., Ferrer D., Paiva T., Montenegro R., Jr., Forti A. Excessive daytime sleepiness in type 2 diabetes. Arq Bras Endocrinol Metabol. 2013;57:425–430. doi: 10.1590/s0004-27302013000600003. [DOI] [PubMed] [Google Scholar]
- 14.Hayashino Y., Yamazaki S., Nakayama T., Sokejima S., Fukuhara S. Relationship between diabetes mellitus and excessive sleepiness during driving. Exp Clin Endocrinol Diab. 2008;116:1–5. doi: 10.1055/s-2007-984442. [DOI] [PubMed] [Google Scholar]
- 15.Asai H., Hirano M., Furiya Y., Udaka F., Morikawa M., Kanbayashi T. Cerebrospinal fluid-orexin levels and sleep attacks in four patients with Parkinson׳s disease. Clin Neurol Neurosurg. 2009;111:341–344. doi: 10.1016/j.clineuro.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Drouot X., Moutereau S., Nguyen J.P., Lefaucheur J.P., Creange A., Remy P. Low levels of ventricular csf orexin/hypocretin in advanced pd. Neurology. 2003;61:540–543. doi: 10.1212/01.wnl.0000078194.53210.48. [DOI] [PubMed] [Google Scholar]
- 17.Compta Y., Santamaria J., Ratti L., Tolosa E., Iranzo A., Munoz E. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson׳s disease dementia. Brain. 2009;132:3308–3317. doi: 10.1093/brain/awp263. [DOI] [PubMed] [Google Scholar]
- 18.Drouot X., Moutereau S., Lefaucheur J.P., Palfi S., Covali-Noroc A., Margarit L. Low level of ventricular csf orexin-a is not associated with objective sleepiness in pd. Sleep Med. 2011;12:936–937. doi: 10.1016/j.sleep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M., Kanbayashi T., Sugiura T., Inoue Y. Relationship between clinical characteristics of narcolepsy and csf orexin-a levels. J Sleep Res. 2011;20:45–49. doi: 10.1111/j.1365-2869.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- 20.Thannickal T.C., Lai Y.Y., Siegel J.M. Hypocretin (orexin) cell loss in Parkinson׳s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gama R.L., Tavora D.F., Bomfim R.C., Silva C.E., Bruin V.M., Bruin P.F. Morphometry mri in the differential diagnosis of parkinsonian syndromes. Arq Neuropsiquiatr. 2010;68:333–338. doi: 10.1590/s0004-282x2010000300001. [DOI] [PubMed] [Google Scholar]
- 22.Yamuy J., Fung S.J., Xi M., Chase M.H. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess C.R, Peever J.H. A noradrenergic mechanism functions to couple motor behavior with arousal state. Curr Biol. 2013;23:1719–1725. doi: 10.1016/j.cub.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Schenck C.H., Boeve B.F., Mahowald M.W. Delayed emergence of a Parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Postuma R.B., Gagnon J.F., Vendette M., Fantini M.L., Massicotte-Marquez J., Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic rem sleep behavior disorder. Neurology. 2009;72:1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boot B.P., Boeve B.F., Roberts R.O., Ferman T.J., Geda Y.E., Pankratz V.S. Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study. Ann Neurol. 2012;71:49–56. doi: 10.1002/ana.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gama R.L., Tavora D.G., Bomfim R.C., Silva C.E., de Bruin V.M., de Bruin P.F. Sleep disturbances and brain mri morphometry in Parkinson׳s disease, multiple system atrophy and progressive supranuclear palsy—a comparative study. Parkinsonism Relat Disord. 2010;16:275–279. doi: 10.1016/j.parkreldis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Braga-Neto P., da Silva-Junior F.P., Sueli Monte F., de Bruin P.F., de Bruin VM. Snoring and excessive daytime sleepiness in Parkinson׳s disease. J Neurol Sci. 2004;217:41–45. doi: 10.1016/j.jns.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Neikrug A.B., Liu L., Avanzino J.A., Maglione J.E., Natarajan L., Bradley L. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014;37:177–185. doi: 10.5665/sleep.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frucht S., Rogers J.D., Greene P.E., Gordon M.F., Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology. 1999;52:1908–1910. doi: 10.1212/wnl.52.9.1908. [DOI] [PubMed] [Google Scholar]
- 31.Montastruc J.L., Brefel-Courbon C., Senard J.M., Bagheri H., Ferreira J., Rascol O. Sleep attacks and anti-Parkinsonian drugs: a pilot prospective pharmacoepidemiologic study. Clin Neuropharmacol. 2001;24:181–183. doi: 10.1097/00002826-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Ray Chaudhuri K., Martinez-Martin P., Rolfe K.A., Cooper J., Rockett C.B., Giorgi L. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson׳s disease. Eur J Neurol. 2011 doi: 10.1111/j.1468-1331.2011.03442.x. [DOI] [PubMed] [Google Scholar]
- 33.Ray Chaudhuri K., Martinez-Martin P., Antonini A., Brown R.G., Friedman J.H., Onofrj M. Rotigotine and specific non-motor symptoms of Parkinson׳s disease: Post hoc analysis of recover. Parkinsonism Relat Disord. 2013;19:660–665. doi: 10.1016/j.parkreldis.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Michinaga S., Hisatsune A., Isohama Y., Katsuki H. An anti-Parkinson drug ropinirole depletes orexin from rat hypothalamic slice culture. Neurosci Res. 2010;68:315–321. doi: 10.1016/j.neures.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Haq I.Z., Naidu Y., Reddy P., Chaudhuri K.R. Narcolepsy in Parkinson׳s disease. Expert Rev Neurother. 2010;10:879–884. doi: 10.1586/ern.10.56. [DOI] [PubMed] [Google Scholar]
- 36.Moller J.C., Rethfeldt M., Korner Y., Stiasny-Kolster K., Cassel W., Meindorfner C. Daytime sleep latency in medication-matched Parkinsonian patients with and without sudden onset of sleep. Mov Disord. 2005;20:1620–1622. doi: 10.1002/mds.20630. [DOI] [PubMed] [Google Scholar]
- 37.Rissling I., Korner Y., Geller F., Stiasny-Kolster K., Oertel W.H., Moller J.C. Preprohypocretin polymorphisms in parkinson disease patients reporting sleep attacks. Sleep. 2005;28:871–875. doi: 10.1093/sleep/28.7.871. [DOI] [PubMed] [Google Scholar]
- 38.Anderson C., Platten C.R., Horne J.A. Self-reported ‘sleep deficit’ is unrelated to daytime sleepiness. Physiol Behav. 2009;96:513–517. doi: 10.1016/j.physbeh.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Unruh M.L., Sanders M.H., Redline S., Piraino B.M., Umans J.G., Chami H. Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52:305–313. doi: 10.1053/j.ajkd.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medeiros C.A., Carvalhedo de Bruin P.F., Lopes L.A., Magalhaes M.C., de Lourdes Seabra M., de Bruin V.M. Effect of exogenous melatonin on sleep and motor dysfunction in parkinson׳s disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254:459–464. doi: 10.1007/s00415-006-0390-x. [DOI] [PubMed] [Google Scholar]
- 41.Razmy A., Lang A.E., Shapiro C.M. Predictors of impaired daytime sleep and wakefulness in patients with parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol. 2004;61:97–102. doi: 10.1001/archneur.61.1.97. [DOI] [PubMed] [Google Scholar]