Abstract
We examined nutrient transport in the intestines of mice exposed to chronic low-LET 137Cs gamma rays. The mice were whole-body irradiated for 3 days at dose rates of 0, 0.13 and 0.20 Gy/h, for total dose delivery of 0, 9.6 or 14.4 Gy, respectively. The mice were fed either a control diet or a diet supplemented with high levels of vitamins A, C and E. Our results showed that nutrient transport was perturbed by the chronic irradiation conditions. However, no apparent alteration of the macroscopic intestinal structures of the small intestine were observed up to day 10 after initiating irradiation. Jejunal fructose uptake measured in vitro was strongly affected by the chronic irradiation, whereas uptake of proline, carnosine and the bile acid taurocholate in the ileum was less affected. D-glucose transport did not appear to be inhibited significantly by either 9.6 or 14.4 Gy exposure. In the 14.4 Gy irradiated groups, the diet supplemented with high levels of vitamins A, C and E increased intestinal transport of fructose compared to the control diet (day 10; t test, P = 0.032), which correlated with elevated levels of vitamins A, C and E in the plasma and jejunal enterocytes. Our earlier studies with mice exposed acutely to 137Cs gamma rays demonstrated significant protection for transport of fructose, glucose, proline and carnosine. Taken together, these results suggest that high levels of vitamins A, C and E dietary supplements help preserve intestinal nutrient transport when intestines are irradiated chronically or acutely with low-LET gamma rays.
INTRODUCTION
A variety of radiation exposure conditions are possible in the event of an accidental radiological emergency or terrorist attack. The ensuing radiation exposures received by victims and emergency responders may be delivered chronically over a period of hours and days, or acutely in a matter of minutes. The radiation exposures may arise externally from radiation sources outside the body or internally from ingested radionuclides. The gastrointestinal (GI) injuries that arise in casualties from such radiation exposures can lead to temporary or persistent debilitation or death. While the implications of death are apparent and catastrophic, the economic and social implications of temporary debilitation can also be enormous. Accordingly, development of medical products capable of ameliorating radiation-induced reductions of important GI functions such as nutrient transport is warranted.
In humans, intestinal irradiation increases the risk of chronic diarrhea and nutrient malabsorption by 40% (1). Thus, radiation exposure can have a deleterious effect on the ability of the small intestine to absorb nutrients. For almost all nutrients, transport through the apical membrane of the enterocyte is the limiting step (2). Sugars, amino acids, minerals, water-soluble vitamins and even some fats require apical transporters to cross the hydrophobic apical membrane. Many transporters are secondarily active and dependent on the electrochemical gradient of Na+. The main product of carbohydrate digestion, D-glucose, and a significant product of meat digestion, L-proline, are nutrients absorbed by Na+-dependent carriers in the proximal intestine. Likewise, uptake of taurocholate, a bile acid essential for fat digestion and absorption, occurs via a Na+-dependent transporter found only in the distal small intestine. Finally, carnosine, a dipeptide found mainly in milk and a representative substrate of the broadly specific H+-dependent oligopeptide carrier, is also ultimately dependent on the Na+ gradient. In contrast to these nutrients, D-fructose, the main fruit sugar, is absorbed specifically by a member of the facilitative GLUT family of glucose transporters. The D-fructose transporter GLUT5 can only mediate the downhill passive transport of fructose across the intestinal apical membrane into the cytosol. Most of these nutrients are then metabolized in the cytosol, thereby increasing the downhill chemical gradient for absorption. The nonmetabolizable glucose analog, 3-O-methylglucose, is absorbed by the same transporter mediating D-glucose uptake, and their comparative uptake rates are often used to distinguish effects on membrane transport from those on glucose metabolism in the cytosol (3). Our studies have shown that transport rates of these nutrients are affected differently when mice are exposed to acute doses of low-LET 137Cs gamma rays (4, 5). However, the effect of chronic irradiation on nutrient transport is not known and agents to prevent or mitigate such reductions have not been assessed.
Developing radioprotectors that protect the GI tract and/or mitigate GI damage caused by chronic irradiation is a challenge. In this scenario, protracted administration of the radioprotector is required, highlighting the need for radioprotectors with low chemotoxicity even when administered over days to weeks (6). Another important consideration is availability. While specialized radioprotectors can be manufactured and stockpiled for use by emergency responders, the immediate availability of a fresh supply of such agents to a potentially large number of victims in a mass casualty radiation event is a complex undertaking. The shortest time for such availability is generally considered to be more than one day. Therefore, identifying radioprotectors that are already in the hands of the general public, without the need for stockpiling, is desirable.
The chemotoxicity of synthetic radioprotectors often limits their use in humans (7). Weiss and Landauer (6) have suggested the use of naturally occurring antioxidants and related agents. Naturally occurring antioxidants are often less effective radioprotectors than synthetic thiols, however, they may provide a longer window of protection against effects caused by low dose, low-dose-rate exposures. In this context, it is worth noting our early studies on protection against the deleterious effects of chronic exposure to both low- and high-linear energy transfer (LET) radiations in mouse testes by vitamin A (8). Furthermore, the combination of vitamin A and soybean oil yielded dose modification factors as high as 5 against low-LET radiation and about 2 against high-LET alpha particles (8). Also of interest are the radioprotective properties of vitamin C, which have been studied by us and others (9–17). Vitamin C mitigates radiation-induced damage even when administered after irradiation (15) and patients have benefited from taking vitamin C after abdominal radiotherapy (9). Of particular interest in relationship to GI function is that vitamin C has been shown to protect against radiation-induced cardiac endothelial damage, which causes selective impairment of endothelial-dependent vasodilation (18). Therefore, vitamin C should also reduce intestinal endothelial damage that occurs at high radiation doses (19). Vitamin E has also been shown to confer protection against ionizing radiation. Of particular relevance to nutrient transport, vitamin E protects the intestine from physiological damage caused by sublethal doses (20). Vitamin E has been reported to maintain jejunal, ileal and colonic fluid absorption in irradiated rats (21). For lethal radiation doses, injected vitamin E provided better protection than dietary vitamin E (22), although small bowel crypt cell numbers, mucosal height and goblet cell numbers were significantly protected from radiation effects by dietary vitamin E (23). More recently, the vitamin E analogs, δ-tocotrienol (DT3) and γ-tocotrienol (GT3), have been shown to protect the hematopoietic/immune system and GI tract against injury after total-body irradiation (TBI) in mice (24–26).
Mixtures of radioprotectors offer a means to improve protection. Vitamin C and E supplements reduced bleeding and diarrhea in patients with chronic radiation proctitis due to previous pelvic irradiation (27). A combination of vitamins A, C and E reduced bone marrow toxicity caused by radioimmunotherapy with 131I-labeled antibodies (10). Furthermore, we have demonstrated a diet enriched in vitamins A, C and E (vitamin ACE) protects intestinal transport of these nutrients under acute irradiation conditions (4).
The current study extends the above findings by measuring nutrient transport after 3-day chronic whole-body irradiation with 137Cs gamma rays. While there is literature on chronic irradiation affecting the intestinal cell cycle (28–32), there is a dearth of information on its effects on intestinal nutrient transport. Accordingly, we tested several hypotheses: 1. That intestinal absorptive function, which has been shown to be markedly affected by acute gamma irradiation, can also be perturbed by chronic gamma irradiation; 2. That different classes of nutrients are differentially affected by chronic gamma irradiation; 3. That radiation effects on post-absorptive nutrient metabolism may have a deleterious effect on uptake; and 4. That a vitamin ACE dietary supplement previously shown to protect nutrient absorption from acute irradiation (4), can also protect nutrient transport under chronic irradiation conditions, thereby enabling irradiated intestinal epithelial cells to preserve their function of intestinal nutrient absorption in vivo.
MATERIALS AND METHODS
Animals
Swiss Webster juvenile male mice, 30–45 g, 7–8 weeks of age, were purchased from Taconic Biosciences, Inc. (Germantown, NY). The experiments were conducted in the New Jersey Medical School Comparative Medicine Resources facility, which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care under a protocol approved by the Institutional Animal Care and Use Committee (IACUC), University of Medicine and Dentistry of New Jersey (UMDNJ, now Rutgers University).
Animal Diets and Acclimation
To facilitate direct comparisons with our data on acute irradiation published previously (4), the same animal diets were used. The control diet was a pelleted AIN-76A rodent diet (Research Diets, New Brunswick, NJ). This standard rodent diet contains their recommended daily levels of vitamins A and E, and no vitamin C (synthesized endogenously). AIN-76A is free of soy that includes phytoestrogens and other potential radioprotectors; the absence of these natural radioprotectors is important to avoid potential masking of the radioprotective effects of the study vitamins. The experimental vitamin ACE diet consisted of AIN-76A heavily enriched with vitamin A (400 IU/g retinyl acetate; 100-fold that of control), vitamin C (29 mg/g L-ascorbate phosphate; vs. none in control), vitamin E (12.5 IU/g α-tocopherol acetate; 250-fold that of control). Details regarding the composition of the diets are provided in Supplementary Table S1 (http://dx.doi.org/10.1667/RR14034.1.S1). All mice were acclimated to the laboratory environment and provided the control diet for a period of 11–14 days. Control mice were then continued on the control diet, whereas the test animals were provided the vitamin ACE diet beginning 4 days prior to initiating chronic irradiation with 137Cs gamma rays (see below).
Animal Irradiator
A low dose rate JL Shepherd Model 28-10 irradiator (JL Shepherd, San Fernando, CA) with a 1.5 TBq (40 Ci) 137Cs source was fitted with a 12 h light–dark cycle and positive airflow ventilator (Fig. 1A and B). Mice were housed within the irradiator in modified duplex cages (Thoren Caging Systems Inc., Hazelton, PA). Cage modifications minimized the use of metal and allowed ad libitum access to feed and water while in the irradiator (Fig. 1C and D). The absorbed dose rates (up to 0.20 Gy/h) received by the animals in the irradiator were measured with a calibrated ionization chamber (Radcal Corporation, Monrovia, CA) under simulated caging conditions. The resultant dose rate calibration curve was used for precise placement of cages in the chamber to deliver the desired cumulative dose. The partitioned side-by-side chambers allowed for the discriminate feeding of the control diet and the vitamin ACE diet during exposure to isodoses of 662 keV 137Cs gamma rays, which create secondary electrons with average LET ~0.9 keV/μm in liquid water.
FIG. 1.
Low-dose-rate gamma irradiator and caging system. Panel A: JL Shepherd Model 28A cabinet irradiator. A 137Cs source is mounted on top of the irradiator and the beam projects downward into the cabinet. A ventilation fan is located on the lower left side. The shielded door is on the right side. Panel B: Scissors jack used to achieve reproducible placement of mouse cages within the irradiator. Three cages were stacked (not shown). Panel C: Mouse cage with tandem living quarters to accommodate two different diets. Animal diets and water bags with nipples were constrained along the back walls of the cage with stainless steel mesh. Amounts of each material were minimized to reduce absorption and scatter of the 662 keV gamma rays. Panel D: Custom-made plastic cage tops were also designed to minimize absorption and scatter of gamma rays.
Experimental Design
The experiments were divided into 15 separate trials with 8–12 mice per trial. Mice were received by the Comparative Medicine Resource Center (CMRC) at the New Jersey Medical School Cancer Center and housed 2 per sterilized cage with filtered, positive airflow. Control diet and water were provided ad libitum. Cage facilities were maintained in a climate-controlled room also with 12 h light–dark cycle. Mice were weighed the day after arrival, stratified by weight, then randomly assigned to 1 of 6 study groups. A 2 × 3 factorial experimental design was used with 2 diets, 3 radiation-absorbed doses and 4 tissue harvest time periods. Mice were randomized based on body weight. This resulted in an overall body weight mean ranging from 37.2–37.6 g with n = 24 per diet/dose group. Each diet/dose group was again randomly selected for tissue harvest on day 3, 5, 8 and 10 after initiating a 3-day chronic irradiation (Fig. 2). These days were selected based on our previous studies, which determined that the nadir for nutrient transport occurs on day 8 after acute irradiation (4, 5). Due to mortality in the day 8 and 10 sacrifice groups, 6 additional mice of similar weight were added to adjust for the decrease in available viable tissues for assay of nutrient transport on day 8 and 10 (total n = 30).
FIG. 2.
Design of experiments wherein mice were chronically irradiated with low-LET gamma rays and sacrificed at various times after initiation of a 3-day irradiation period at constant dose rate. Mice were fed the control or vitamin ACE diet, irradiated and sacrificed day 3, 5, 8 and 10 after initiation of chronic irradiation.
Absorbed doses of 6.8 (data not shown; no effect on transport), 9.6 and 14.4 Gy were delivered chronically during 72 h of exposure to the low-LET 137Cs source (Fig. 1A). The corresponding dose rates were 0.094, 0.13 and 0.20 Gy/h, respectively. Prior to irradiation, each diet/dose mice pair was transferred from the CMRC ventilated cages to the modified duplex cages (Fig. 1C and D). Nonirradiated (0 Gy) mice were also placed in duplex cages. Mouse weight, diet and water consumption were measured before irradiation, at the conclusion of the 3-day irradiation period and at regular intervals throughout the study period. As detailed below, on predetermined days after initiation of the 3-day irradiation, animals were sacrificed and the small intestine was removed for analysis.
Intestinal Nutrient Uptake
The rates of intestinal nutrient uptake were determined as described previously (4, 33, 34). Briefly, the small intestine was removed from anesthetized mice and 1 cm everted segments of the small intestine were individually mounted on steel rods. Since active transport of bile acids occurs primarily in the distal ileum, only that segment was used for taurocholate uptake. The intestinal segments were preincubated in a KRb1 buffer. Then for glucose uptake, the tissue was incubated in KRb2 buffer containing 50 mM D-glucose, 380 μM D-[14C]glucose and 0.2 μM L-[3H]glucose. Fructose uptake was measured in 50 mM D-fructose, 1.6 μM D-[14C]fructose and 0.2 μM L-[3H]glucose. Uptake of the nonmetabolizable glucose analog, 3-O-methyl-D-glucose, was measured in 25 mM 3-O-methyl-D-glucose, 1.2 μM D-[14C]3-O-methyl-glucose and 0.2 μM of L-[3H]glucose. L-[3H]glucose was used to correct for adherent fluid and passive diffusion of glucose, fructose and methyl-glucose (4). For L-proline uptake, the segment was incubated in 50 mM L-proline, 0.2 μM L-[3H]proline and 20 μM [14C]inulin-carboxyl (3.9 μCi/ml); for L-carnosine, the segment was incubated in 25 mM L-carnosine, 0.9 μM L-[3H]carnosine and 20 μM [14C]inulin-carboxyl (3.9 μCi/ml); for taurocholic acid, the segment was incubated in 0.6 mM taurocholic acid, 0.07 μM [3H]taurocholic acid and 20 μM [14C]inulin-carboxyl (3.9 μCi/ml). In the cases of proline, carnosine, and taurocholate, [14C]inulin-carboxyl was used to correct for adherent fluid. Activity of 3H and 14C in each segment was determined by dual-label liquid scintillation counting. Quench corrections were determined using the Beckman LS 6500 software (Beckman Coulter Inc., Fullerton, CA) and counting samples with a range of intestinal tissue weights, each spiked with the same amount of 3H alone, 14C alone or 3H and 14C. Uptakes of nutrients are expressed as nM min −1 cm −1 of intestine.
Intestinal Absorption of Vitamins
In a separate study, mice were fed the control diet or the vitamin ACE-enriched diet (n = 6 for 5 days). Intestines were harvested for determination of retinyl esters, retinol, tocopherol esters and tocopherols as described above. Sections (3 cm) of the proximal jejunum and distal ileum were cut, weighed, flash frozen in liquid nitrogen and stored at −80°C. Adjacent intestinal jejunal and ileal sections were also harvested for determination of ascorbic acid and dehydro-ascorbic acid concentrations. A 4 cm length was sectioned and placed into a tared cryovial and weighed. One ml of 5% metaphosphoric acid was added to the vial and the sample homogenized with a microprobe blade homogenizer to release cellular content and stabilize the ascorbic acid. The sample was flash frozen in liquid nitrogen then transferred to −80°C.
Whole blood was collected by cardiac puncture into a K2EDTA-treated (anticoagulant) 1 ml syringe. The collected whole blood was centrifuged at 4°C for 5 min and the plasma supernatant transferred to a cryovial. Plasma from 2 mice was pooled from each diet study group. For vitamin C analysis, a 50 μl aliquot was transferred to a separate cryovial containing 100 μl of a 10% metaphosphoric acid solution followed by 10 s of vigorous vortexing to precipitate proteins and stabilize the ascorbic acid. The vortexed sample was allowed to stand at room temperature for 2 min before being frozen at −80°C until analysis. The remaining volume of plasma was preserved at −80°C for analysis of retinyl esters, retinol, tocopherol esters and tocopherols. Frozen tissues and plasma were submitted to Biomedical Research, Our Lady of Mercy Medical Center (Bronx, NY) for analysis.
Statistics
Uptake values were expressed as means ± standard errors unless otherwise stated. The number of mice used is denoted by n. One-way analysis of variance (ANOVA) (dose or diet effect) and two-way ANOVA (interaction of diet and dose) with general linear model analysis (GLM) for unbalanced data (i.e., differing sample sizes) were used to determine the statistical significance of diet, dose and the interaction of diet and dose on each harvest day. Mean differences were considered significant atP < 0.05. Pairwise comparisons were done subsequent to two-way ANOVA with GLM. Statistical evaluations were performed using SAS (Statistical Analysis Systems, Cary, NC). A Kaplan-Meier analysis of the mortality data was performed using the survival log-rank analysis tool in SigmaPlot v12.5 (Systat® Software Inc., San Jose, CA). Animals removed from a group for analysis of intestinal tissue were censored.
Histology
Mouse jejunum was fixed in fresh 4% paraformaldehyde in PBS (pH 7.35) overnight at room temperature and then embedded in paraffin. Sections (0.5 μm) were hematoxylin and eosin (H&E) stained with an Automat Autostainer XL (Leica Microsystems Inc., Bannockburn, IL). Examinations of villi were performed at 10× and 20× magnification with a BX60 microscope (Olympus, Melville, NY). All images were captured using a SPOT RT-SE color digital camera and SPOT imaging software was used for image acquisition and processing (Diagnostic Instruments, Sterling Heights, MI).
RESULTS
Dietary Consumption, Body Weight, Mortality of Mice
Diet had no significant impact on the rate of ingestion of water, however, the irradiated animal groups ingested about 50% less water than the unirradiated groups (P < 0.001; Supplementary Fig. S1; http://dx.doi.org/10.1667/RR14034.1.S1). Similarly, diet had no effect on the rate of food intake, but the irradiated groups consumed comparatively less (P < 0.0001) food than the unirradiated diet groups during the 3-day irradiation period (Supplementary Fig. S2A; http://dx.doi.org/10.1667/RR14034.1.S1). Notably, during the 3-day irradiation period, there was a reduction in dietary consumption rates for all mice, but the magnitude of reduction was less in the unirradiated mice. This reduction in rate of food intake may be solely due to the unique characteristics of housing in the irradiator chamber during the 3-day irradiation period. While the 9.6 Gy irradiated group experienced dietary consumption recovery after exposure, the 14.4 Gy irradiated group did not. This effect of radiation-absorbed dose was most notable on day 8 and 10 after the chronic irradiation regimen was initiated.
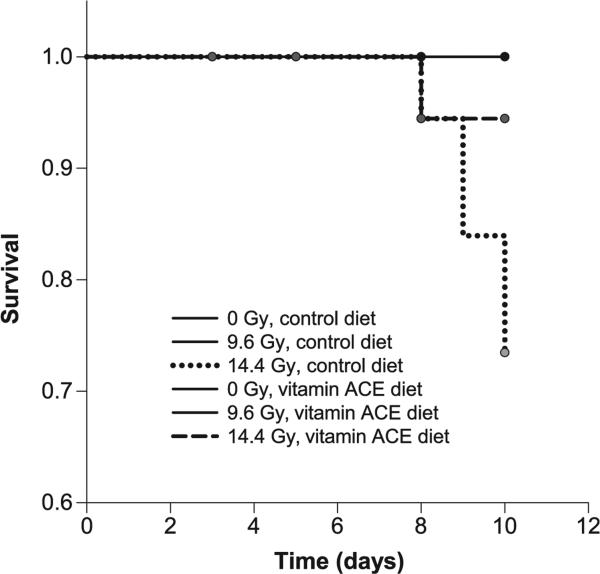
A significant reduction in body weight of 14.4 Gy irradiated animals was observed on day 8 and 10 (P = 0.0049 and P < 0.0001, respectively) (Supplementary Fig. S2B; http://dx.doi.org/10.1667/RR14034.1.S1). These changes in mean body weights were similar between the control diet and vitamin ACE diet groups. Kaplan-Meier survival log-rank analysis indicated that the difference in survival between the control diet and vitamin ACE diet groups that received 14.4 Gy irradiation was almost significant (P = 0.055) (Fig. 3).
FIG. 3.
Kaplan-Meier plot of animal survival as a function of time after initiating chronic irradiation of the mice. Animals were fed either the control or vitamin ACE diet according the schedule shown in Fig. 2. Absorbed doses of 0, 9.6 and 14.4 Gy were delivered over the 3-day irradiation period. Animals were censored when their intestines were harvested (day 3, 5, 8 and 10 indicated with filled circles) for analysis of the principal end point, nutrient uptake, thereby progressively reducing the number of animals at risk for assessment of survival. No animals were followed after day 10, the final harvest day for analysis of nutrient uptake.
Intestinal Weights and Length, and Mucosal Morphology
At various times after initiating irradiation, the animals were sacrificed and the small intestines were collected for measurements of intestinal weight, intestinal length, and nutrient transport. No significant changes were noted on day 3, 5 and 8 after initiating the irradiation (Supplementary Table S2; (http://dx.doi.org/10.1667/RR14034.1.S1). There was a significant modest trend (P = 0.027) indicating a reduction in intestinal weight with increasing radiation dose and time after initiation of the 3-day chronic irradiation.
Cross sections of preserved proximal jejunum from both experiments were H&E stained. Light micrographs of the mucosa showed no instances of shedding of villus cells or of villi sections devoid of enterocytes (Supplementary Fig. S3; http://dx.doi.org/10.1667/RR14034.1.S1). This mucosal morphology confirms the data in Supplementary Table S2 (http://dx.doi.org/10.1667/RR14034.1.S1), which shows only modest changes in total intestinal weight of mice irradiated at 14.4 Gy and sacrificed 8 days after initiation of the chronic irradiation protocol.
Intestinal Absorption of Dietary Vitamins
Vitamin A was provided in the diet in the form of retinyl acetate. The proximal and distal jejunum showed robust increases in the tissue concentration of retinol and four retinyl esters for mice fed the vitamin ACE diet (Fig. 4A and B). The tissue concentrations in descending order were retinyl-palmitate (C16:0), retinyl-stearate (C18:0), retinyloleate (C18:1), retinol and retinyl-linoleate (C18:2). The composition in the blood plasma was slightly different from that in the tissue, with retinol levels increasing and retinylpalmitate decreasing (Fig. 4C).
FIG. 4.
Retinoid concentrations in murine proximal jejunum (panel A), distal jejunum (panel B) and plasma (panel C), after 5 days on the vitamin ACE-enriched or control diet. Results are means 6 SE for tissue (n = 6) and for pooled plasma samples (n = 3). Marked increases in retinoid concentration were found in the intestines of mice on the vitamin ACE diet compared with control diet-fed mice.
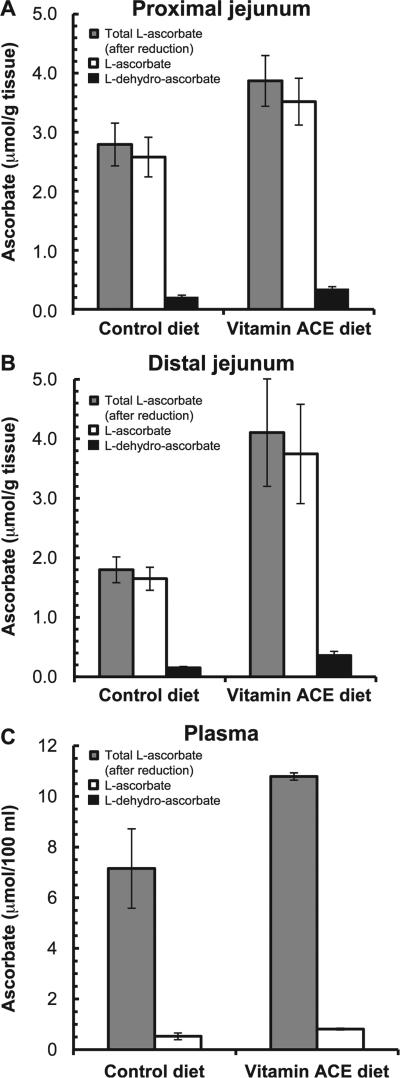
Vitamin C was provided in the diet in the form of L-ascorbic acid phosphate. Vitamin C is produced endogenously in the mouse gut and dietary vitamin C is not absorbed well (35). Tsao et al. showed that large increases in dietary vitamin C results in only small increases in vitamin C concentrations in brain, kidney, muscle, liver and spleen of mice (36). These findings help explain the similar concentrations of vitamin C observed in the intestinal tissues and in the plasma of our control diet and vitamin ACE-augmented groups (Fig. 5). Vitamin C (L-ascorbate) is highly oxidizable, which is why a small amount is found in the form of L-dehydro-ascorbate. Total ascorbate is the sum of both forms.
FIG. 5.
Ascorbic acid concentrations in murine proximal jejunum (panel A), distal jejunum (panel B) and plasma (panel C) after 5 days on the vitamin ACE-enriched diet or control diet. Results are means ± SE for tissue (n = 6) and for pooled plasma samples (n = 3). A greater amount of ascorbic acid was found in the intestines of mice on the vitamin ACE diet than in the control diet-fed mice.
Vitamin E was provided in the diet in the form of α-tocopherol-acetate. In the vitamin ACE diet group, both the proximal and distal regions of the jejunum had high concentrations of α-tocopherol-acetate and α-tocopherol. The concentration of hydrolyzed form, α-tocopherol, was about twofold higher than that of α-tocopherol-acetate in the jejunum (Fig. 6A and B). Only very low concentrations of the converted form, γ-tocopherol, were detected in the same tissues. Other forms of vitamin E may also have been present but were not measured. In the plasma, levels of α-tocopherol-acetate and of γ-tocopherol were low and very low, respectively, whereas concentrations of α-tocopherol were elevated (Fig. 6C).
FIG. 6.
Tocopherol concentrations in murine proximal jejunum (panel A), distal jejunum (panel B) and plasma (panel C) after 5 days on the vitamin ACE-enriched diet or control diet. Results are means ± SE for tissue (n = 6) and for pooled plasma samples (n = 3). Marked increases in tocopherol concentrations were found in the intestines of mice on the vitamin ACE diet compared with control diet-fed mice.
Intestinal Nutrient Uptakes
Sugar transport, including D-glucose, D-methyl-glucose and D-fructose, was measured in the proximal jejunum (Fig. 7). Chronic gamma irradiation for 3 days caused significant dose-dependent reductions in D-glucose transport on day 3 (P = 0.011), but not on day 5, 8 and 10 (P = 0.065, P = 0.13 and P = 0.54, respectively) after initiating the irradiation protocol (Fig. 7A). Vitamin ACE supplementation did not significantly affect D-glucose transport on day 5, 8 and 10 (P = 0.35, P = 0.46 and P = 0.71, respectively), but did on day 3 (P = 0.041). The interaction between dose and diet was insignificant for D-glucose uptake on all days. Overall, 3-O-methyl-D-glucose transport does not appear to be dependent on both radiation dose [except at day 8 (P = 0.017)] and vitamin ACE supplementation (P > 0.05; Fig. 7B). In contrast, fructose transport was consistently highly sensitive to the 3-day chronic low-LET gamma irradiation in a dose-dependent manner, on day 5, 8 and 10 (P = 0.015, P = 0.0003 and P < 0.0001, respectively) after irradiation was initiated (Fig. 7C). The 14.4 Gy exposure reduced fructose transport by ~43%, ~42% and ~75% on day 5, 8 and 10, respectively. Vitamin ACE supplementation appeared to protect intestinal fructose transport against radiation-induced reductions when measured 8 and 10 days after irradiation was initiated (Fig. 7C) with 1.7- and 3.9- fold increases in transport for the vitamin ACE-treated animals compared to their diet-matched controls. However, the interaction between diet and dose was not significant when tested with the GLM model (P = 0.16 and 0.48 for day 8 and 10, respectively). Pairwise tests after GLM analysis also did not yield significant differences between the control and vitamin ACE diets (Fig. 7C, letters above bars). However, paired t tests between the 14.4 Gy irradiation data for the control and vitamin ACE diet groups yielded P = 0.066 and 0.032 on day 8 and 10, respectively.
FIG. 7.
Intestinal uptake of D-glucose (panel A), 3-O-methyl-D-glucose (panel B) and D-fructose (panel C) in the proximal jejunum of mice at various days after initiation of 3-day chronic irradiation of mice with 137Cs gamma rays. Total absorbed doses of 0, 9.6 and 14.4 Gy were administered. Mice were fed the control diet (open bars) or vitamin ACE diet (filled bars). Results are means ± SE of 6 independent experiments. Bars marked by the same letters do not differ significantly [e.g., day 8 fructose uptake for control diet 14.4 Gy (open bar annotated with “c”) is not significantly different (P > 0.05) than vitamin ACE diet 14.4 Gy (filled bar annotated with “b,c”) when compared pairwise after two-way ANOVA GLM]. Bars marked by different letters indicate significant difference [P < 0.05; e.g., control diet day 10 fructose uptake for 14.4 Gy irradiation (open bar annotated with “b”) is significantly different (P < 0.05) than 0 Gy (open bar annotated with “a”)]. A separate pairwise t test of the 14.4 Gy irradiated, vitamin ACE-fed vs. control diet-fed group was significant (denoted by “*”). Absence of letters within a given plot indicates lack of statistical difference between any bars (i.e., no effect of dose or diet). Error bars depict standard errors.
Transport of the amino acid proline and the dipeptide carnosine were also measured in the proximal part of the jejunum. Carnosine transport was reduced in a dose-dependent manner on day 5 (P = 0.073) and 10 (P = 0.047) after initiation of the 3-day chronic irradiation (Fig. 8A). Proline transport was similarly reduced on day 8 and 10 (P = 0.14 and 0.0025, respectively; Fig. 8B). Vitamin ACE supplementation appeared to protect carnosine and proline transport on day 8 and 10. GLM analysis indicated that the interaction of dose and diet was not significant, however, the carnosine data for the 14.4 Gy irradiated groups on day 8 indicates significant protection (P = 0.045). Finally, taurocholate uptake was measured in the distal part of the jejunum. The taurocholate uptake was substantially, but not significantly (P = 0.11, according to two-way GLM analysis), reduced by chronic gamma irradiation at day 8 after its initiation (Fig. 8C). Yet, pairwise comparison yielded a significant (P = 0.011) reduction in taurocholate transport in the 14.4 Gy irradiated group relative to the unirradiated group. The vitamin ACE diet had apparent protective effect for taurocholate transport on day 8, but not at a statistically significant level.
FIG. 8.
Uptake of carnosine in the proximal jejunum (panel A), proline in the proximal jejunum (panel B), and taurocholate in the distal jejunum (panel C), of mice at various days after initiation of 3-day chronic irradiation of mice with 137Cs gamma rays. Total absorbed doses of 0, 9.6 and 14.4 Gy were administered. Mice were fed the control diet (open bars) or vitamin ACE diet (filled bars). Results are means ± SE of 6 independent experiments. Bars marked by same letters do not differ significantly [e.g., day 10 carnosine uptake for control diet 14.4 Gy (open bar annotated with “a,b”) is not significantly different (P > 0.05) than vitamin ACE diet 14.4 Gy (filled bar annotated with “b”) when compared pairwise after two-way ANOVA GLM]. Bars marked by different letters indicate significant difference (P < 0.05) [e.g., day 8 TAD uptake for control diet 14.4 Gy (open bar annotated with “b”) is significantly different (P < 0.05) than control diet 0 Gy (open bar annotated with “a”)]. Absence of letters within a given plot indicates lack of statistical difference between any bars (i.e., no effect of dose or diet). Error bars depict standard errors.
DISCUSSION
These studies extend and expand on our earlier studies that investigated reductions in intestinal nutrient transport after acute whole-body exposure to 137Cs gamma rays and the capacity of a vitamin ACE-enriched diet to protect against these reductions (4, 5). Accordingly, the focus of the current discussion is to compare our findings for chronic versus acute whole-body irradiation.
Chronic Irradiation and Intestinal Nutrient Transport
Our preliminary measurements of intestinal transport of sugars after a 3-day chronic irradiation with 6.8 Gy (0.094 Gy/h) showed that, unlike our findings for a 7 Gy acute irradiation (5), this absorbed dose did not affect their transport (data not shown). However, increasing the absorbed dose rate to 0.13 and 0.20 Gy/h, and the corresponding absorbed dose to 9.6 and 14.4 Gy, was sufficient to reduce transport of some sugars. While D-glucose transport is comparatively resistant to acute (5) and chronic gamma irradiation at these higher doses, D-fructose transport is sensitive to both regimens (5). Our data also show that intestinal transport of the amino acid proline, the dipeptide carnosine and the bile acid taurocholate are each mildly sensitive to both acute (4) and chronic irradiation. Bile acid malabsorption is a cause of gastrointestinal symptoms after radiotherapy (37), primarily because the bile acid transporters are localized in the distal end of the small intestine, an anatomical location frequently subjected to pelvic radiotherapy. The clear trend as indicated by comparing transport among nutrients, is that there is a slight but consistent reduction in transport of all nutrients, either by day 8 or 10. This suggests that chronic irradiation does not necessarily target specific nutrients, but virtually all nutrients, and further suggests that these radiation effects are mediated by a mechanism affecting most nutrient transport. The results in Supplementary Table S2 (http://dx.doi.org/10.1667/RR14034.1.S1) and our uptake measurements, suggest that there is a small (~20%) reduction in intestinal weight and in intestinal absorptive mucosa. Reduced mucosal mass would mean a decreased number of absorptive cells, affecting all transporter activity (38).
Intestinal nutrient transport is a major function of intestinal enterocytes that have already undergone the 48–72 h differentiation process from stem cells in the crypt region (39). The reason why intestinal malabsorption is affected more strongly on day 8–10 after initiating irradiation than on day 3–5 is that the relatively radioresistant mature absorptive cells are still functional within 3–5 days. Since most of these cells are eventually exfoliated after day 5, new absorptive cells must replace and take over their function. When the rate of replacement is not compromised by radiation exposure, the rate of cell proliferation in the crypt balances that of cell exfoliation at the villus tips. Exposure to ionizing radiation, however, disrupts stem cell proliferation and stem cell survival in the crypts, and in fact the number of surviving crypts can be expressed as a function of radiation dose (40). After acute exposure to greater than 8 Gy of gamma rays, the number of surviving crypts begins to decrease as a function of radiation dose, eventually reducing the amount of descendant absorptive cells (41). Substantially higher doses are tolerated when the intestines are irradiated chronically (28, 41, 42). In the current studies, even at 14.4 Gy, a sufficient number of crypt cells are able to proliferate and differentiate while being irradiated chronically such that intestinal mass is not drastically affected and absorptive function is only modestly reduced for most transporters. Using fructose transport as a more sensitive marker of organ dysfunction, we found that acute irradiation with 10 Gy reduces fructose uptake by ~85% at day 8 postirradiation (5), whereas a 3-day chronic irradiation requires 14.4 Gy to reduce it by about the same amount (~90%).
Protection of Nutrient Transport with Vitamins A, C and E
A dietary supplement of vitamins A, C and E afforded some protection against reductions in fructose transport caused by chronic gamma irradiation in the current study. A t test of the day 10 data (P = 0.032) lends significance to the 3.9-fold increase in intestinal transport of fructose for the vitamin ACE-treated animals compared to their diet-matched controls. This finding is tempered by the GLM statistical analysis, which failed to demonstrate statistical significance. Similarly, there was some protection against radiation-induced reductions in carnosine, proline and taurocholate. However, it is not clear why the vitamin ACE supplement afforded generally more robust protection against reductions in transport of these nutrients that were caused by acute irradiation (5). The supplemented vitamins were bioavailable in both protocols as evidenced by the high concentrations of vitamins A, C and E found in the intestinal mucosa and blood; the mechanisms underlying intestinal absorption of these vitamins is well described (43–45). Therefore, our finding suggests that dietary supplementation with high concentrations of these antioxidant vitamins reduces the detrimental effect of the bolus of radical species produced by acute irradiation, whereas protection of moderate significance above the endogenous capacity is afforded when the radical species are produced chronically. It is also possible that the lower significance of the protection observed in the chronic irradiation studies may simply be due to the higher degree of variability in the nutrient transport measurements associated with the experimental protocol required for this irradiation protocol.
Effects on Clinical Parameters and Intestinal Morphology
During the 3-day irradiation period the unirradiated mice were housed in the same modified duplex cages as the irradiated mice, but their cages were placed outside the irradiator. Reduced dietary intake in both the unirradiated and irradiated animals during the 3-day period was attributed to the modified duplex cages for chronic irradiation. This dietary reduction, coupled with the decreased dietary intake by the 14.4 Gy irradiated animals relative to control animals from day 3 to 10, likely contributed to the ~25% reduction in body weight among the 14.4 Gy irradiated vitamin ACE-fed group relative to the control groups on day 10. Important to the current studies on nutrient transport, our earlier studies in calorie-restricted mice indicate that acute (2 days–2 weeks) reductions in daily food consumption by up to 30% have no effect on intestinal nutrient transport (46).
There was a reduction in survival of the 14.4 Gy control group, however the vitamin ACE diet appears to afford some degree of protection against the lethal effects caused by this chronic irradiation. This finding is supported by the studies of Blumenthal et al., who found that a dietary supplement of vitamins A, C and E (administered in diet at 10× the daily requirement) increased the maximum tolerated dose of 131I-labeled radiolabeled antibody administered to mice (131I-MN-14) (10). Other studies have shown that antioxidant diet supplementation can be an effective treatment to reduce radiation death in rodents (47, 48). Despite the untoward effects observed for animal survival, even 14.4 Gy irradiation caused relatively little morphological change in the intestine of mice eight days after initiating irradiation. The same observation was made on day 8 after acute 10 Gy irradiation of mice (5).
Implications
In the context of radiation emergencies, dietary supplementation with high levels of vitamins A, C and E might be considered in addition to supportive care that could include glutamine, antibiotics, probiotics and electrolytes. However, in a radiation emergency situation, hematopoietic injury would probably be the major concern and the effect of antioxidant vitamins during chronic exposures that would result in hematopoietic injury needs further study.
It is also interesting to note that the high radiosensitivity of intestinal transport of D-fructose may have some implications for the nutritional design of diets used for individuals whose intestines have been exposed to high doses of ionizing radiation delivered acutely or chronically. For example, about 50% of the calories contained in emergency rations are derived from carbohydrates, and a substantial fraction of them are from sugars. Sugars are added in various forms including sucrose, high fructose corn syrup and glucose, depending on the manufacturer of the emergency rations. Our data suggest that fructose and sucrose (broken down in the duodenal villi into its constituent monosaccharides, glucose and fructose) might be minimized as ingredients in these products when considering the nutritional needs of radiation victims. Furthermore, our data show that glucose transport is resistant to ionizing radiation, suggesting that glucose may be preferred as the main sugar ingredient for such emergency rations. This may also be relevant for formulating the diets of radiation therapy patients that experience radiation enteritis, a condition that sometimes leads to malnutrition (49). Further studies are warranted to ascertain the optimum nutritional intake for irradiated individuals.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the staff at the NJMS Comparative Resource Facility and Radiation Safety for their assistance with these studies, Dr. D. Lagunoff for assistance with the histology, Dr. E. Norkus for assaying the vitamin content in tissues and Dr. M. Ricci at Research Diets for help in formulating the diets. This study was supported in part by grant no. RC1 AI078518 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health (NIH). The laboratory of Dr. Ferraris also received support from the National Science Foundation (grant no. IOS-1121049) and the NIH (grant no. RDK075617A), and the laboratory of Dr. Howell received support from the NIH (grant no. RO1 CA83838).
Footnotes
SUPPLEMENTARY INFORMATION
Table S1. Compositions of control diet and vitamin A, C and E supplemented diet.
Table S2. Intestinal lengths and weights.
Fig. S1. Average daily water consumption during the 3-day chronic gamma irradiation period.
Fig. S2. Effect of diet and radiation dose on dietary intake and body weight as a function of time before and after initiating irradiation.
Fig. S3. Light micrograph of an H&E-stained cross section of proximal jejunum of mice fed with control or vitamin ACE diet and sacrificed on day 8 after initiation of the chronic irradiation protocol.
REFERENCES
- 1.Letschert JG. The prevention of radiation-induced small bowel complications. Eur J Cancer. 1995;31A:1361–5. doi: 10.1016/0959-8049(95)00179-m. [DOI] [PubMed] [Google Scholar]
- 2.Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 3.Tharabenjasin P, Douard V, Patel C, Krishnamra N, Johnson RJ, Zuo J, et al. Acute interactions between intestinal sugar and calcium transport in vitro. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1–12. doi: 10.1152/ajpgi.00263.2013. [DOI] [PubMed] [Google Scholar]
- 4.Roche M, Kemp FW, Agrawal A, Attanasio A, Neti PV, Howell RW, et al. Marked changes in endogenous antioxidant expression precede vitamin A-, C-, and E-protectable, radiation-induced reductions in small intestinal nutrient transport. Free Radic Biol Med. 2011;50:55–65. doi: 10.1016/j.freeradbiomed.2010.10.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roche M, Neti PV, Kemp FW, Agrawal A, Attanasio A, Douard V, et al. Radiation-induced reductions in transporter mRNA levels parallel reductions in intestinal sugar transport. Am J Physiol Regul Integr Comp Physiol. 2010;298:R173–82. doi: 10.1152/ajpregu.00612.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 7.Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect. 1997;105(Suppl 6):1473–8. doi: 10.1289/ehp.97105s61473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harapanhalli RS, Narra VR, Yaghmai V, Azure MT, Goddu SM, Howell RW, et al. Vitamins as radioprotectors in vivo. II. Protection by vitamin A and soybean oil against radiation damage caused by internal radionuclides. Radiat Res. 1994;139:115–22. [PubMed] [Google Scholar]
- 9.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 10.Blumenthal RD, Lew W, Reising A, Soyne D, Osorio L, Ying Z, et al. Anti-oxidant vitamins reduce normal tissue toxicity induced by radio-immunotherapy. Int J Cancer. 2000;86:276–80. doi: 10.1002/(sici)1097-0215(20000415)86:2<276::aid-ijc19>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Narra VR, Harapanhalli RS, Howell RW, Sastry KSR, Rao DV. Vitamins as radioprotectors in vivo. I. Protection by vitamin C against internal radionuclides in mouse testes: Implications to the mechanism of the Auger effect. Radiat Res. 1994;137:394–9. [PubMed] [Google Scholar]
- 12.Narra VR, Howell RW, Sastry KSR, Rao DV. Vitamin C as a radioprotector against 131I in vivo. J Nucl Med. 1993;34:637–40. [PubMed] [Google Scholar]
- 13.O'Connor MK, Malone JF, Moriarty M, Mulgrew S. A radioprotective effect of vitamin C observed in Chinese hamster ovary cells. Br J Radiol. 1977;50:587–91. doi: 10.1259/0007-1285-50-596-587. [DOI] [PubMed] [Google Scholar]
- 14.Okunieff P. Interactions between ascorbic acid and the radiation of bone marrow, skin, and tumor. Am J Clin Nutr. 1991;54:1281S–3S. doi: 10.1093/ajcn/54.6.1281s. [DOI] [PubMed] [Google Scholar]
- 15.Sarma L, Kesavan PC. Protective effects of vitamins C and E against c-ray-induced chromosomal damage in mouse. Int J Radiat Biol. 1993;63:759–64. doi: 10.1080/09553009314552161. [DOI] [PubMed] [Google Scholar]
- 16.Ueno A, Vannais D, Lenarczyk M, Waldren CA. Ascorbate, added after irradiation, reduces the mutant yield and alters the spectrum of CD59-mutations in A(L) cells irradiated with high LET carbon ions. J Radiat Res. 2002;43:S245–9. doi: 10.1269/jrr.43.s245. [DOI] [PubMed] [Google Scholar]
- 17.Waldren CA, Vannais DB, Ueno AM. A role for long-lived radicals (LLR) in radiation-induced mutation and persistent chromosomal instability: counteraction by ascorbate and RibCys but not DMSO. Mutat Res. 2004;551:255–65. doi: 10.1016/j.mrfmmm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.On YK, Kim HS, Kim SY, Chae IH, Oh BH, Lee MM, et al. Vitamin C prevents radiation-induced endothelium-dependent vasomotor dysfunction and de-endothelialization by inhibiting oxidative damage in the rat. Clin Exp Pharmacol Physiol. 2001;28:816–21. doi: 10.1046/j.1440-1681.2001.03528.x. [DOI] [PubMed] [Google Scholar]
- 19.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JF, Landauer MR, Gunter-Smith PJ, Hanson WR. Effect of radioprotective agents on survival after acute intestinal radiation injury. In: Dubois A, King GL, Livengood DR, editors. Radiation and the gastrointestinal tract. CRC Press; Boca Raton, FL: 1995. pp. 183–99. [Google Scholar]
- 21.Empey LR, Papp JD, Jewell LD, Fedorak RN. Mucosal protective effects of vitamin E and misoprostol during acute radiation-induced enteritis in rats. Dig Dis Sci. 1992;37:205–14. doi: 10.1007/BF01308173. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–5. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 23.Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol). Ann Surg. 1995;222:504–8. doi: 10.1097/00000658-199522240-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XH, Ghosh SP, Ha CT, Fu D, Elliott TB, Bolduc DL, et al. Delta-tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat Res. 2013;180:649–57. doi: 10.1667/RR13398.1. [DOI] [PubMed] [Google Scholar]
- 25.Berbee M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulkarni S, Ghosh SP, Satyamitra M, Mog S, Hieber K, Romanyukha L, et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat Res. 2010;173:738–47. doi: 10.1667/RR1824.1. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy M, Bruninga K, Mutlu EA, Losurdo J, Choudhary S, Keshavarzian A. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol. 2001;96:1080–4. doi: 10.1111/j.1572-0241.2001.03742.x. [DOI] [PubMed] [Google Scholar]
- 28.Quastler H, Bensted JPM, Lamerton LF, Simpson SM. Adaptation to continuous irradiation: observations on the rat intestine. Br J Radiol. 1959;32:501–12. [Google Scholar]
- 29.Fry RJM, Lesher S, Sallese A, Staffeldt E. The generatrion cycle of duodenal crypt cells of mice exposed to 220 roentgens of cobalt-60 irradiation per day. Radiat Res. 1963;19:628–35. [PubMed] [Google Scholar]
- 30.Wimber DE, Lamerton LF. Cell population kinetics in the intestine of continuously irradiated mice, using double-labelling autoradiography. Radiat Res. 1966;28:694–700. [PubMed] [Google Scholar]
- 31.Lesher S, Lamerton LF, Sacher GA, Fry RJ, Steel GG, Roylance PJ. Effect of continuous gamma irradiation of the generation cycle of the duodenal crypt cells of the mouse and rat. Radiat Res. 1966;29:57–70. [PubMed] [Google Scholar]
- 32.Rijke RP, Plaisier H, Hoogeveen AT, Lamerton LF, Galjaard H. The effect of continuous irradiation on cell proliferation and maturation in small intestinal epithelium. Cell Tissue Kinet. 1975;8:441–53. doi: 10.1111/j.1365-2184.1975.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 33.Karasov WH, Diamond JM. Adaptive regulation of sugar and amino acid transport by vertebrate intestine. Am J Physiol. 1983;245:G443–62. doi: 10.1152/ajpgi.1983.245.4.G443. [DOI] [PubMed] [Google Scholar]
- 34.Ferraris RP, Casirola DM, Vinnakota RR. Dietary carbohydrate enhances intestinal sugar transport in diabetic mice. Diabetes. 1993;42:1579–87. doi: 10.2337/diab.42.11.1579. [DOI] [PubMed] [Google Scholar]
- 35.Michels AJ, Frei B. Myths, artifacts, and fatal flaws: identifying limitations and opportunities in vitamin C research. Nutrients. 2013;5:5161–92. doi: 10.3390/nu5125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsao C, Leung PY, Young M. Effect of dietary ascorbic acid intake on tissue vitamin C in mice. J Nutri. 1987;117:291–7. doi: 10.1093/jn/117.2.291. [DOI] [PubMed] [Google Scholar]
- 37.Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007;8:1007–17. doi: 10.1016/S1470-2045(07)70341-8. [DOI] [PubMed] [Google Scholar]
- 38.Ferraris RP, Villenas SA, Hirayama BA, Diamond J. Effect of diet on glucose transporter site density along the intestinal crypt-villus axis. Am J Physiol. 1992;262:G1060–8. doi: 10.1152/ajpgi.1992.262.6.G1060. [DOI] [PubMed] [Google Scholar]
- 39.Ferraris RP. Dietary and developmental regulation of intestinal sugar transport. Biochem J. 2001;360:265–76. doi: 10.1042/0264-6021:3600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potten CS. The cell kinetic mechanism for radiation-induced cellular depletion of epithelial tissue based on hierarchical differences in radiosensitivity. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40:217–25. doi: 10.1080/09553008114551101. [DOI] [PubMed] [Google Scholar]
- 41.Potten CS, Hendry JH. Radiation and gut. Amsterdam: Elsevier. 1995 [Google Scholar]
- 42.Krebs JS, Leong GF. Effect of exposure rate on the gastrointestinal LD50 of mice exposed to 60Co gamma rays or 250 kVp x-rays. Radiat Res. 1970;42:601–13. [PubMed] [Google Scholar]
- 43.Rigotti A. Absorption, transport, and tissue delivery of vitamin E. Mol Aspects Med. 2007;28:423–36. doi: 10.1016/j.mam.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu Rev Physiol. 2004;66:419–46. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 45.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 46.Ferraris RP, Cao QX, Prabhakaram S. Chronic but not acute energy restriction increases intestinal nutrient transport in mice. J Nutr. 2001;131:779–86. doi: 10.1093/jn/131.3.779. [DOI] [PubMed] [Google Scholar]
- 47.Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 2010;173:462–8. doi: 10.1667/RR1716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto T, Kinoshita M, Shinomiya N, Hiroi S, Sugasawa H, Matsushita Y, et al. Pretreatment with ascorbic acid prevents lethal gastrointestinal syndrome in mice receiving a massive amount of radiation. J Radiat Res. 2010;51:145–56. doi: 10.1269/jrr.09078. [DOI] [PubMed] [Google Scholar]
- 49.Webb GJ, Brooke R, De Silva AN. Chronic radiation enteritis and malnutrition. J Dig Dis. 2013;14:350–7. doi: 10.1111/1751-2980.12061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.