Abstract
BACKGROUND & AIMS
Severe burn injury has been demonstrated to delay gastric emptying. The aim of this study was to investigate effects and cellular mechanisms of auricular electroacupuncture (AEA) at the acupoints innervated by the auricular branch of vagus nerve (ABVN) on burn-induced gastric dysmotility in rats.
METHODS
Propranolol (β-adrenoceptor antagonist) was injected intraperitoneally after the rats underwent burn injury. All experiments were performed six hours following burn/sham burn injury. AEA was performed at bilateral auricular acupoints for 45min. Electrocardiogram was recorded for 30min. Plasma hormones were measured; cyclooxygenase (COX)-2 expressions in gastric tissue were measured using western blotting and real time RT-PCR.
RESULTS
1) Burn injury delayed gastric emptying (P=0.006) and AEA increased gastric emptying by 49% (P=0.045). 2) Burn injury evoked a significant elevation in plasma noradrenaline, which was suppressed by AEA. 3) Burn injury significantly increased protein and mRNA expressions of COX-2 in gastric fundus and antrum. AEA suppressed burn-induced increase in protein expressions but not mRNA expressions of COX-2.
CONCLUSIONS
Burn injury delays gastric emptying by up-regulating COX-2 attributed to sympathetic overactivity. AEA improves burn-induced delay in gastric emptying, possibly mediated via the sympathetic-COX-2 pathway.
Keywords: auricular electroacupuncture, vagal nerve stimulation, gastric emptying, COX-2, autonomic function
Graphical abstract

Introduction
Following severe burn injury, gastric dysmotility is a frequently encountered problem deterring early oral and/or enteral feeding in patients (1). This was verified in our rodent model of scald burn injury (2, 3). Early enteral resuscitation following burn injury is strongly recommended for its known clinical benefits (4, 5); however, it may be delayed due to burn-induced delayed gastric emptying. Accordingly, novel therapies that may improve gastric emptying and further reduce vomiting are recommended.
Acupuncture has been used as one of alternative and complementary treatments for functional gastrointestinal disorders for many years. Our previous studies demonstrated prokinetic effects of acupuncture on gut motility in both animals (6, 7) and humans (8). The application of acupuncture on specific acupoints at the auricle (Auricular acupuncture) was shown to improve gastrointestinal symptoms in clinical studies; it significantly reduced postoperative vomiting in patients (9, 10) and might be used as a complementary therapy for constipation (11). However, little is known about the effects of auricular acupuncture on gastric motility.
Recent studies have suggested an auriculo-vagal afferent pathway involved in the effects of auricular acupuncture on visceral organs (12, 13). Certain auricular acupoints are innervated with the auricular branch of vagus nerve (ABVN) and thus auricular electroacupuncture (AEA, electrical stimulation at auricular acupoints) is equivalent to auricular vagal nerve stimulation (AVNS). Accordingly, AEA or AVNS is expected to alter autonomic functions; it may alter vagal nerve function by direct electrical stimulation on the nerve and may also alter sympathetic nerve function via the vagal afferent pathway. It is well known that gastrointestinal motility is enhanced by vagal activation and/or sympathetic suppression and inhibited by vagal suppression and/or sympathetic activation. Therefore, AEA or AVNS is expected to alter gastric motility functions.
Numerous studies revealed the regulatory effects of prostaglandins and COX-2 in gastrointestinal motility. Prostaglandins work as local regulatory agents mediating both amplitude and frequency of muscle contraction in the gut (14, 15). COX-2, an inducible enzyme to catalyze the synthesis of prostaglandins, was reported to mediate gastrointestinal motility under various pathological conditions, including inflammation (16), post-operative ileus (17) and burn injury (2, 14). It was however, unknown whether COX-2 would play a role in burn-induced dysmotility.
Sympathetic overactivity is common in burn and propranolol, a β-blocker, has been consistently shown to have a therapeutic effect on burn by altering cardiac and metabolic functions and etc (18–20). The sympathetic system is also well-known to suppress gastrointestinal motility. However, it was unknown whether sympathetic overactivity would play a role in burn-induced delay in gastric emptying. Accordingly, it was of interest to study whether propranolol could block burn-induced delay in gastric emptying.
The aim of this study was to investigate possible ameliorating effect of AEA or AVNS on burn-induced gastric dysmotility and mechanisms involving autonomic function and COX-2 in a rodent model of thermal injury.
Materials and Methods
Animals
Adult male Sprague-Dawley rats weighing 300–350g were housed under controlled conditions of 22°C and a 12-hour light/dark cycle. All procedures performed on animals were approved by the Animal Care and Use Committee of the University of Texas Medical Branch.
Fifty three rats were randomly assigned to 6 groups: sham burn (n=10), burn (n=10), AEA+burn (n=10), sham-EA1+burn (n=9), sham-EA2+burn (n=6) and propranolol+burn (n=8). Following 24 hour fast, burn injury was inflicted. Then six hours after sham or burn injury, a solid meal was offered and AEA was performed for 45 minutes. All animals were sacrificed at 90 minutes after meal; stomachs and blood were collected for mechanistic analyses.
Thermal injury
A full-thickness (3rd degree) scald burn was inflicted according to the Walker-Mason burn model(21). Following 24 hour fast, animals were anesthetized with 5% isoflurane and received one time intramuscular injection of buprenorphine (0.1mg/kg, Reckitt Benckiser Healthcare) as analgesia as previously prescribed (2, 3). The dorsal and ventral skin surface was shaved; the animals were then placed into a mold with a rectangle opening. The exposed skin was immersed in 95°C water. A 60% total body surface area (TBSA) scald burn or sham burn was inflicted by scalding the dorsum for 10s and ventral surface for 2s. A fluid resuscitation was given immediately after burn by intraperitoneal administration of lactated Ringer’s solution (4ml/kg/%TBSA/24h). Sham burn rats received identical treatment except that the water for scald was at 25°C. Animals were then placed back to their individual cages without food and water supply.
Drug administration
According to the method previously described (3) (22), a β-adrenoreceptor antagonist, propranolol (10mg/kg; Sigma) was injected at two time points, one immediately after burn injury and the other at six hours following burn injury. The drugs were dissolved in 0.5ml 10% DMSO and administrated intraperitoneally. In the corresponding control session, an equal volume of vehicle was administrated.
Gastric Emptying
Six hours following sham or burn injury, pre-weighed regular chow pellets (1.5–2.0g) were given to animals. All animals consumed the food within 5–10min. At ninety minutes after the meal, the animals were sacrificed by decapitation under deep anesthesia with 5% isoflurane. The stomach was collected and cut opened, gastric content was scraped out and dried for 48 hours. A well-established formula was used to calculate gastric emptying (23): Gastric emptying (%) = (1−dried weight of food recovered from stomach/weight of food intake) ×100.
AEA/AVNS Treatment
Immediately after food consumption, animals were subjected to AEA for 45min. AEA was applied at bilateral auricular concha using an electrical stimulator (PulseMaster A300, WPI, Sarasota, USA). Stimulation parameters were set according to our previous studies (pulse train: train on time was 2 s, off time was 3 s, pulse frequency was 25 Hz, pulse width was 0.5 ms and amplitude was 0.3–0.4mA) (24). The stimulus amplitude was set as the ear twitch is detectable but bearable by the animal. Two sham-EA groups were set as follows: needles were inserted into non-acupoints at the tail of the animal and connected to stimulator with power off (sham-EA1) or power on (sham-EA2).
Measurement of plasma hormones
Norepinephrine (NE) and pancreatic polypeptide (PP) were measured to assess autonomic function as the plasma NE level is proportional to the sympathetic activity(25), while plasma PP level reflects vagal activity (26). The plasma was obtained from blood collected immediately after decapitation and analyzed within 3 weeks. Quantitative enzyme immunoassay was used to analyze the plasma level of NE (LDN, Nordhorn, Germany) and PP (Novatein Biosciences, Cambridge, MA).
COX-2 Expression
Gastric fundus and antrum were collected and stored at −80°C for COX-2 analysis.
Western blot analysis
Western blot was used to measure the protein expression of COX-2 using a standard method prescribed previously (27). In short, the gastric fundus and antrum were homogenized on ice in lysis buffer supplemented with 1/100 proteinase inhibitor cocktail (Sigma Aldrich, St Louis, MO). The lysis buffer was made with following ingredients: 20 mM Tris-HCL (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 ug/mL leupeptin. Equal amount of total proteins were separated on 4%–12% Bis-Tris NuPAGE Gels (Life Technologies, Carlsbad, CA). Then they were transferred to nitrocellulose membranes (BioRad, Hercules, CA) and blocked for 1 hour in blocking buffer (LI-COR Biosciences) at room temperature. The membranes were incubated with COX-2 antibody (1/500 dilution, Cayman Chemical, Ann Arbor, MI) overnight at 4°C. Then Alexa Fluor 680 goat anti-rabbit IgG (1/5000 dilution, Invitrogen) was applied for 1 hour at room temperature. The analysis was finalized by Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Real-time PCR
The extraction of total RNA was performed using Qiagen RNeasy kit (Qiagen, Valencia, CA). Reversely transcription (RT) PCR was performed using SuperScript III First-Strand Synthesis System (Invitrogen). Equal quantity of total RNA (1μg) was used. The Real-time quantitative PCR assay for COX-2 mRNA expression was performed in a StepOne Plus thermal cycler (Applied Biosystems, Foster City, CA), using TaqMan primers and probes specific for rat COX-2 gene (Applied Biosystems). Gene 18s rRNA was used as endogenous reference. The relative quantification of COX-2 mRNA expression was performed in an optical 96-well reaction plate; Seven μl (2μl for 18s) diluted cDNA (1:5) of each sample was assayed in triplicate. The relative quantitation of COX-2 gene was obtained using the comparative Ct (ΔΔ CT) method and normalized with 18s reference gene.
Statistics
All data are presented as mean ± standard error of mean. Student’s t-test and One-way ANOVA followed by post hoc analysis were used for comparisons between means. P value <0.05 was accepted as statistically significant.
Results
Effects of AEA on burn-induced delay in gastric emptying
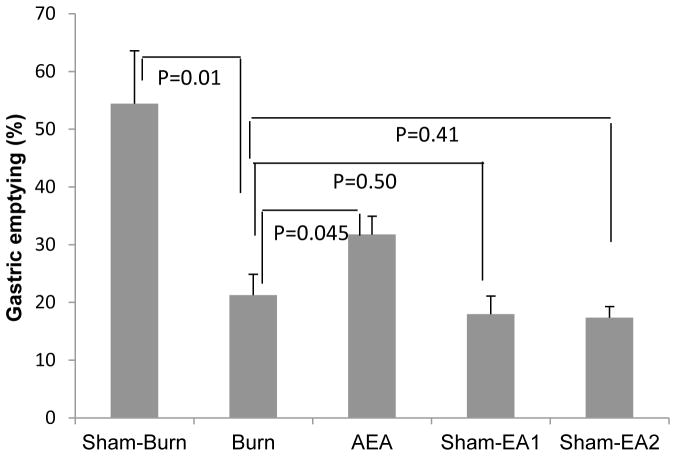
The cutaneous thermal injury substantially delayed gastric emptying (P=0.01 vs. sham-burn) and the AEA treatment accelerated gastric emptying by 49% (P=0.045 vs. burn) in burned rats (Fig. 1). Whereas, burned rats treated with either Sham-EA1 or Sham-EA2 showed no increase in gastric emptying (P=0.50 and 0.41, respectively vs. burn). No difference in gastric emptying was noted between these two Sham-EA groups. Then in subsequent experiments, only sham-EA1 was tested.
Figure 1.
Effects of AEA on gastric emptying in burned rats. Burn injury decreased the percentage of gastric emptying; this was improved with AEA
COX-2 mechanisms involved in the prokinetic effects of AEA
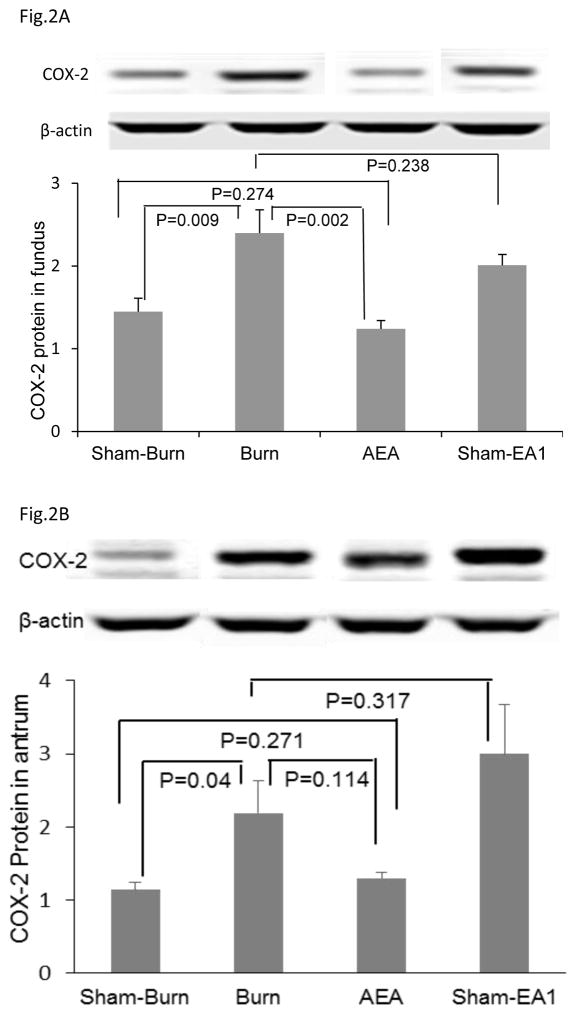
The cutaneous thermal injury substantially increased expressions of COX-2 protein and mRNA in both gastric fundus and gastric antrum (Fig. 2A and 2B). In the fundus, the COX-2 protein expression was substantially increased with the burn injury and this increase was almost completely suppressed with AEA (Fig. 2A). In the antrum, however, it was also increased with the burn injury; however, this increase was not as substantial as in the funds and was not significantly suppressed with SEA (Fig. 2B). Sham-EA showed no effects on the burn-induced increase in COX2 protein expression neither in the fundus, nor in the antrum.
Figure 2.
Effects of AEA on protein expressions of COX-2 A: In fundus, AEA treatment significantly improved burn-induced increase in COX-2 protein expression, but such ameliorative effect was not showed with the treatment of sham-EA1. B: In antrum, AEA treatment prevented the burn-induced increase in COX-2 protein expression.
The COX-2 mRNA expressions in the fundus and the antrum in the burn rats were not altered with either TEA or sham-TEA.
Autonomic mechanisms involved in the prokinetic effects of AEA
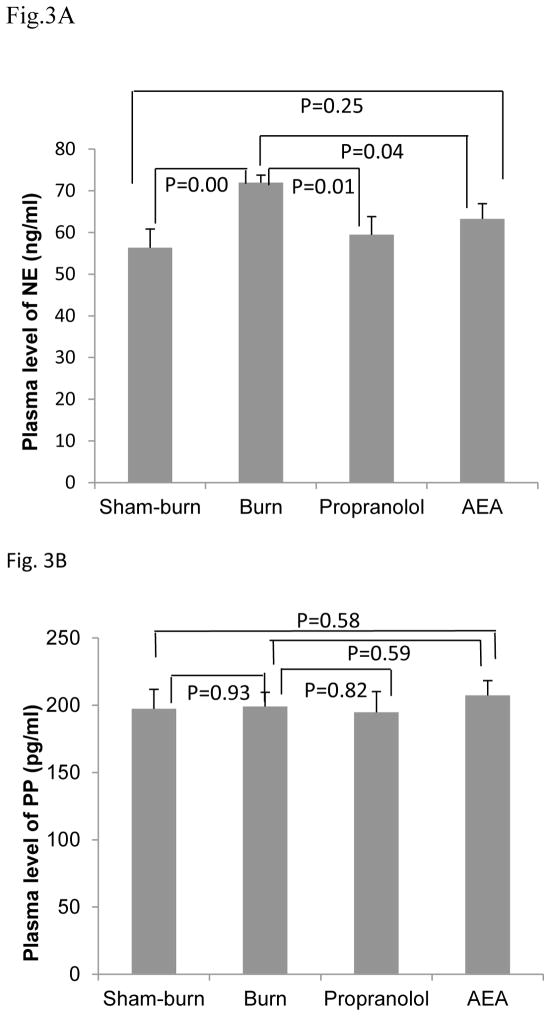
We investigated the alteration of autonomic function in burned rats and observed a significant increase in plasma NE as compared to sham burn rats (P=0.005) (see Fig. 3A). There was no difference in plasma PP levels between the burn and sham-burn groups (Fig. 3B).
Figure 3.
Effects of AEA on plasma levels of NE and PP in burned rats. (A) Burn injury significantly increased the plasma NE, but this effect was suppressed with both AEA and propranolol. (B) Neither burn injury, nor AEA/propranolol showed any effect on plasma PP.
Interestingly, the AEA treatment significantly suppressed the burn-induced increase in plasma NE (P=0.04 vs. Burn, Fig. 3A) but showed no effects on plasma PP in burned rats (P=0.59 vs. Burn) (Fig. 3B). The same effects were noted with the β-adrenoreceptor antagonist, propranolol: it suppressed plasma NE but had no effect on plasma PP in burn rats.
Discussion
This study demonstrated for the first time that auricular electroacupuncture or auricular vagal nerve stimulation improved burn-induced delay in gastric emptying, suppressed burned-induced increase in COX-2 protein expression in the stomach and inhibited burned-induced increase in NE, suggesting an autonomic-COX-2 mechanism.
Previous studies reported antiemetic effects of auricular acupuncture in the postoperative patients (9, 10). Others showed that the effects of auricular acupuncture on gastric motility were even comparable to that of a prokinetic drug (28). Compared to body acupuncture, AEA is more convenient without undressing patients or affecting patients’ activity. According to our current results, AEA represents itself as a feasible effective treatment for burn-induced delay in gastric emptying.
Our choice of auricular acupoints for stimulation was based on the innervation of auricle, described in previous studies (29, 30). Stimulation of the ear or external auditory canal is known to have direct effects on visceral organs mediated via the autonomic pathway (31, 32). Most of the auricular acupoints for the therapy of visceral diseases are located at the auricular concha, which is mainly innervated by the auricular branch of vagus nerve. In the present study, we selected the bilateral auricular concha for application of electrical stimulation and revealed a prokinetic effect of AEA on gastric motility in burned rats.
COX-2 was previously reported to play an important role in the regulation of gastrointestinal motility (2, 14) (16) (17). The present study demonstrated that burn increased expression of COX-2 in the stomach, especially in the fundus and verified the contribution of COX-2 in burn-induced delay in gastric emptying via the administration of a selective COX-2 inhibitor. This study also demonstrated the interaction between the sympathetic system and COX-2, as β-blockade treatment normalized the overexpressed COX-2 in gastric tissues of burned rats. This was consistent with previous studies (33, 34). Taken together, these findings suggest that sympathetic hyperactivity may mediate burn-induced delay in gastric emptying either directly or via the COX-2 pathway.
To reveal the mechanism of AEA on burn-induced delayed gastric emptying, we detected the response of autonomic nervous system to AEA. Interestingly, AEA decreased the burn-induced increase in plasma NE but showed no effects on plasma PP. These unexpected results might be explained by the auriculovagal afferent pathway proposed in previous studies (35); Electrical stimulation at the auricular acupoints innervated by the auricular branch vagus nerve activated the neurons in the nucleus of the solitary tract (NTS) via projections from the auricular vagal nerve to NTS (36, 37), which in turns inhibited sympathetic efferent activity via the extensive connections between the NTS with other central nuclei (38–40).
AEA reduced the protein expression of COX-2 in the fundus but not so much in the antrum in comparison with sham-AEA. However, in comparison with the control without burn, AEA seemed to prevent increase in COX-2 in the antrum. Further studies are needed to investigate why COX2 is more involved in the fundus but less involved in the antrum in burn-induced gastric dysmotility. AEA showed no effects on the mRNA expression of COX-2. Such a difference in protein and mRNA expressions of COX-2 may be explained by the duration of AEA treatment and/or timing in the collection of gastric tissue for analysis; at the time of tissue collection, the effect of TEA on COX-2 mRNA might have already diminished and started to recover to normal or a longer stimulation time might be necessary for TEA to alter the mRNA expressions. Further studies are needed. Since the interaction of the sympathetic system and COX-2 was documented (33, 34), these findings may suggest that the accelerative effect of AEA on gastric emptying in burned rats is mediated via the sympathetic–COX-2 pathway.
In summary, auricular electroacupuncture or auricular vagal nerve stimulation improves burn-induced delay in gastric emptying by down-regulating COX-2 via the sympathetic inhibition.
Acknowledgments
Grant support: National Institutes of Health, CA149956
Abbreviations used in this paper
- AEA
auricular electroacupuncture
- COX
Cyclooxygenase
- IL
interleukin
- NE
norepinephrine
- PP
pancreatic polypeptide
Footnotes
Disclosures: all the authors disclosed no conflicts.
Author Contributions: Dr. Li: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis. Dr. Yin: technical support, study design and manuscript preparation. Dr. Zhang: acquisition of data. Dr. Winston and Dr. Shi: acquisition of data and critical revision of the manuscript for important intellectual content. Dr. Chen: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding and study supervision.
References
- 1.Nguyen NQ, Ng MP, Chapman M, Fraser RJ, Holloway RH. The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care. 2007;11:R16. doi: 10.1186/cc5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira HM, Sallam HS, Espana-Tenorio J, et al. Gastric and small bowel ileus after severe burn in rats: the effect of cyclooxygenase-2 inhibitors. Burns. 2009;35:1180–1184. doi: 10.1016/j.burns.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Sallam HS, Oliveira HM, Liu S, Chen JD. Mechanisms of burn-induced impairment in gastric slow waves and emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R298–305. doi: 10.1152/ajpregu.00135.2010. [DOI] [PubMed] [Google Scholar]
- 4.Khorasani EN, Mansouri F. Effect of early enteral nutrition on morbidity and mortality in children with burns. Burns. 2010;36:1067–1071. doi: 10.1016/j.burns.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Lu G, Huang J, Yu J, et al. Influence of early post-burn enteral nutrition on clinical outcomes of patients with extensive burns. J Clin Biochem Nutr. 2011;48:222–225. doi: 10.3164/jcbn.10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol. 2008;295:G614–620. doi: 10.1152/ajpgi.90322.2008. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang H, Yin J, Wang Z, Pasricha PJ, Chen JD. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. Am J Physiol Gastrointest Liver Physiol. 2002;282:G390–396. doi: 10.1152/ajpgi.00272.2001. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Hou X, Zha H, Gao Z, Zhang Y, Chen JD. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci. 2006;51:2154–2159. doi: 10.1007/s10620-006-9412-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Kim CW, Kim KS. Clinical observations on postoperative vomiting treated by auricular acupuncture. Am J Chin Med. 2003;31:475–480. doi: 10.1142/S0192415X03001156. [DOI] [PubMed] [Google Scholar]
- 10.Sahmeddini MA, Fazelzadeh A. Does auricular acupuncture reduce postoperative vomiting after cholecystectomy? J Altern Complement Med. 2008;14:1275–1279. doi: 10.1089/acm.2008.0264. [DOI] [PubMed] [Google Scholar]
- 11.Li MK, Lee TF, Suen KP. A review on the complementary effects of auriculotherapy in managing constipation. J Altern Complement Med. 2010;16:435–447. doi: 10.1089/acm.2009.0348. [DOI] [PubMed] [Google Scholar]
- 12.He W, Wang X, Shi H, et al. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med. 2012;2012:786839. doi: 10.1155/2012/786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Yin J, Chen JD. Ameliorating effects of auricular electroacupuncture on rectal distention-induced gastric dysrhythmias in rats. PLoS One. 2015;10:e0114226. doi: 10.1371/journal.pone.0114226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan HT, Chen JD. Roles of nitric oxide and prostaglandins in pathogenesis of delayed colonic transit after burn injury in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1316–1324. doi: 10.1152/ajpregu.00733.2004. [DOI] [PubMed] [Google Scholar]
- 15.Sanders KM. Role of prostaglandins in regulating gastric motility. Am J Physiol. 1984;247:G117–126. doi: 10.1152/ajpgi.1984.247.2.G117. [DOI] [PubMed] [Google Scholar]
- 16.Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol. 2004;557:191–205. doi: 10.1113/jphysiol.2004.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz NT, Kalff JC, Turler A, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 18.Ali A, Herndon DN, Mamachen A, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care. 2015;19:217. doi: 10.1186/s13054-015-0913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256:402–411. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011;149:231–239. doi: 10.1016/j.surg.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker HL, Mason AD., Jr A standard animal burn. J Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Park DS, Seo BK, Baek YH. Analgesic effect of electroacupuncture on inflammatory pain in collagen-induced arthritis rats: mediation by alpha2- and beta-adrenoceptors. Rheumatol Int. 2012 doi: 10.1007/s00296-012-2369-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R427–432. doi: 10.1152/ajpregu.00499.2004. [DOI] [PubMed] [Google Scholar]
- 24.Yin J, Chen J, Chen JD. Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying, and impaired accommodation in diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:G563–570. doi: 10.1152/ajpgi.00252.2009. [DOI] [PubMed] [Google Scholar]
- 25.Esler M, Willett I, Leonard P, et al. Plasma noradrenaline kinetics in humans. J Auton Nerv Syst. 1984;11:125–144. doi: 10.1016/0165-1838(84)90071-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz TW. Pancreatic polypeptide: a unique model for vagal control of endocrine systems. J Auton Nerv Syst. 1983;9:99–111. doi: 10.1016/0165-1838(83)90134-0. [DOI] [PubMed] [Google Scholar]
- 27.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G99–G108. doi: 10.1152/ajpgi.00379.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borozan S, Petkovic G. Ear acupuncture has a hypotonic effect on the gastrointestinal tract. Vojnosanit Pregl. 1996;53:31–33. [PubMed] [Google Scholar]
- 29.Gao XY, Zhang SP, Zhu B, Zhang HQ. Investigation of specificity of auricular acupuncture points in regulation of autonomic function in anesthetized rats. Auton Neurosci. 2008;138:50–56. doi: 10.1016/j.autneu.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 31.Engel D. The gastroauricular phenomenon and related vagus reflexes. Arch Psychiatr Nervenkr. 1979;227:271–277. doi: 10.1007/BF00367396. [DOI] [PubMed] [Google Scholar]
- 32.Thakar A, Deepak KK, Kumar SS. Auricular syncope. J Laryngol Otol. 2008;122:1115–1117. doi: 10.1017/S0022215107000758. [DOI] [PubMed] [Google Scholar]
- 33.Gonzales R, Sherbourne CD, Goldyne ME, Levine JD. Noradrenaline-induced prostaglandin production by sympathetic postganglionic neurons is mediated by alpha 2-adrenergic receptors. J Neurochem. 1991;57:1145–1150. doi: 10.1111/j.1471-4159.1991.tb08272.x. [DOI] [PubMed] [Google Scholar]
- 34.Ueda F, Ideguchi K, Taniguchi N, Kimura K. Adrenergic regulation of prostaglandin biosynthesis in cultured rabbit gastric epithelial cells. Jpn J Pharmacol. 1994;65:113–120. doi: 10.1254/jjp.65.113. [DOI] [PubMed] [Google Scholar]
- 35.He W, Wang X, Shi H, et al. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med. 2012:786839. doi: 10.1155/2012/786839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao XY, Li YH, Liu K, et al. Acupuncture-like stimulation at auricular point Heart evokes cardiovascular inhibition via activating the cardiac-related neurons in the nucleus tractus solitarius. Brain Res. 1397:19–27. doi: 10.1016/j.brainres.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 37.Mei ZG, Zhu B, Li YH, Rong PJ, Ben H, Li L. Responses of glucose-sensitive neurons and insulin-sensitive neurons in nucleus tractus solitarius to electroacupuncture at auricular concha in rats. Zhongguo Zhen Jiu. 2007;27:917–922. [PubMed] [Google Scholar]
- 38.Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 219:48–61. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichinose TK, Minic Z, Li C, O’Leary DS, Scislo TJ. Activation of NTS A(1) adenosine receptors inhibits regional sympathetic responses evoked by activation of cardiopulmonary chemoreflex. Am J Physiol Regul Integr Comp Physiol. 303:R539–550. doi: 10.1152/ajpregu.00164.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scislo TJ, Augustyniak RA, Barraco RA, Woodbury DJ, O’Leary DS. Activation of P2x-purinoceptors in the nucleus tractus solitarius elicits differential inhibition of lumbar and renal sympathetic nerve activity. J Auton Nerv Syst. 1997;62:103–110. doi: 10.1016/s0165-1838(96)00116-6. [DOI] [PubMed] [Google Scholar]