Abstract
Excessive weight gain in adults is associated with a variety of negative health outcomes. Unfortunately, dieting, exercise, and pharmacological interventions have had limited long-term success in weight control and can result in detrimental side effects, including accelerating age-related cancellous bone loss. We investigated the efficacy of using hypothalamic leptin gene therapy as an alternative method for reducing weight in skeletally-mature (9-month-old) female rats and determined the impact of leptin-induced weight loss on bone mass, density, and microarchitecture, and serum biomarkers of bone turnover (CTx and osteocalcin). Rats were implanted with cannulae in the 3rd ventricle of the hypothalamus and injected with either recombinant adeno-associated virus encoding the gene for rat leptin (rAAV-Leptin, n=7) or a control vector encoding green fluorescent protein (rAAV-GFP, n=10) and sacrificed 18 weeks later. A baseline control group (n=7) was sacrificed at vector administration. rAAV-Leptin-treated rats lost weight (−4±2%) while rAAV-GFP-treated rats gained weight (14±2%) during the study. At study termination, rAAV-Leptin-treated rats weighed 17% less than rAAV-GFP-treated rats and had lower abdominal white adipose tissue weight (−80%), serum leptin (−77%), and serum IGF1 (−34%). Cancellous bone volume fraction in distal femur metaphysis and epiphysis, and in lumbar vertebra tended to be lower (p<0.1) in rAAV-GFP-treated rats (13.5-months-old) compared to baseline control rats (9-months-old). Significant differences in cancellous bone or biomarkers of bone turnover were not detected between rAAV-Leptin and rAAV-GFP rats. In summary, rAAV-Leptin-treated rats maintained a lower body weight compared to baseline and rAAV-GFP-treated rats with minimal effects on bone mass, density, microarchitecture, or biochemical markers of bone turnover.
Keywords: rAAV-Leptin, microcomputed tomography, dual energy absorptiometry, white adipose tissue
Introduction
Insidious excessive weight gain is common in adults and is associated with increased risk for various chronic diseases, including heart disease, chronic obstructive pulmonary disease, type 2 diabetes, osteoarthritis, and certain cancers (Franssen, et al. 2008; Low, et al. 2009; Magliano 2008). Weight loss, whether induced by caloric restriction alone or in combination with exercise and/or pharmaceutical intervention, may attenuate or reverse the health risks associated with excessive weight gain. Unfortunately, the long-term efficacy of conventional weight loss interventions is generally poor and many individuals weight-cycle through repetitive bouts of weight loss followed by rapid weight regain (Elfhag and Rossner 2005; Wu, et al. 2009; Yaskin, et al. 2009).
The adipokine leptin plays an essential role in energy homeostasis (Rosenbaum and Leibel 2014) and adult-onset weight gain is closely associated with an increase in circulating leptin and development of leptin resistance (Carter, et al. 2013; Knobelspies, et al. 2010; Morris and Rui 2009; Scarpace and Tumer 2001). As a consequence, avoiding leptin resistance may be essential to achieving life-long weight control. However, the mechanisms mediating leptin resistance are incompletely understood. To date, studies suggest that high blood leptin concentrations result in saturation of leptin transport across the blood brain barrier (blood brain barrier resistance) (Banks and Lebel 2002) and/or down regulation of leptin signaling in the hypothalamus due to constitutive expression of suppressor of cytokine signaling (hypothalamic resistance) (Bjorbaek, et al. 1998).
Delivery of leptin directly into the hypothalamus by gene therapy normalizes body weight and extends lifespan in morbidly obese leptin-deficient ob/ob mice in the absence of detectable circulating leptin (Boghossian, et al. 2007; Dhillon, et al. 2000). Additionally, hypothalamic leptin gene therapy slows weight gain in rodents capable of producing leptin (Boghossian, et al. 2005). These findings suggest that increasing leptin levels directly in the hypothalamus, in addition to bypassing blood brain barrier-mediated leptin resistance, overcomes hypothalamic leptin resistance. However, it is less clear whether this approach can induce weight loss and/or maintain lower body weight in aging rodents with normal circulating levels of the hormone (Shapiro, et al. 2008).
In humans, calorie restriction-induced weight loss is often associated with bone loss, leading to increased risk for osteoporosis (Lee, et al. 2010b; Shapses and Riedt 2006). Importantly, weight regain does not restore bone and, as a consequence, weight cycling is especially deleterious to the skeletal system (Lee et al. 2010b; Villalon, et al. 2011). Because osteoporotic fractures are associated with decreased quality of life and increased mortality (Cauley 2013), there is strong incentive to develop weight loss strategies that preserve bone mass.
Leptin is a candidate factor for coupling bone metabolism to energy availability. Leptin is required for normal skeletal growth, maturation and turnover (Arounleut, et al. 2013; Gat-Yablonski and Phillip 2008; Turner, et al. 2013b; Turner, et al. 2014). Weight loss results in decreased leptin levels (Hamann and Matthaei 1996), reduced bone accrual during growth (Devlin, et al. 2010), and accelerated age-related bone loss (Talbott, et al. 2001; Turner and Iwaniec 2011). Importantly, leptin treatment attenuates the inhibitory effects of caloric restriction on bone growth (Gat-Yablonski, et al. 2004; Goldstone, et al. 2002). Furthermore, leptin administered at supraphysiological levels has been reported to maintain bone mineral density despite inducing weight loss (Stunes, et al. 2012).
Taken together, the above findings suggest the respective skeletal changes accompanying caloric restriction-induced weight loss and leptin-induced weight loss differ. Specifically, low hypothalamic leptin levels induced by caloric restriction lead to an adaptive response similar to starvation and result in rapid bone loss. In contrast, elevating hypothalamic leptin levels decreases appetite and increases energy expenditure (Friedman 2010) leading to weight loss with minimal impact on the skeleton. Although suggestive, the aforementioned conclusions are based on short-term studies where leptin was administered subcutaneously to growing rats with relatively low levels of circulating leptin. It is less clear whether similar benefits can be achieved in skeletally mature animals exhibiting weight gain as well as age-related bone loss. As such, the present study was designed to determine the long-term efficacy of increased hypothalamic leptin in reducing adult onset-associated weight gain and the impact this intervention has on bone mass, density and architecture.
Material and Methods
Animals
Nine-month-old female Sprague Dawley rats (Harlan; Indianapolis, IN) were used in the experiment. The rats were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the experimental protocol was approved (IACUC #D642) by the Institutional Animal Care and Use Committee at the University of Florida (Gainesville, FL).
Experimental Design
The rats were randomized by weight into 3 groups: baseline control (n = 7), rAAV-GFP control (n = 10), and rAAV-Leptin (n = 7). The baseline group (body weight; 289 ± 7g, mean ± SE) was sacrificed at vector administration. The rAAV-GFP and rAAV-Leptin groups were sacrificed at 18 weeks post-vector administration. All rats were housed individually in a temperature- (21–23°C) and light-controlled (lights on 8:00 – 18:00 hours) room under specific pathogen-free conditions. Food and water were available ad libitum to all animals. The rats were weighed and food consumption determined weekly.
Construction and Packaging of rAAV Vectors
The rAAV-Leptin and rAAV-GFP vectors were constructed and packaged as described previously (Beretta, et al. 2002). In brief, the vector pTR-CBA-Ob EcoRI fragment of pCR-rOb (a gift from Dr. Roger H. Unger, University of Texas Southwestern Medical Center, Dallas, TX) containing rat leptin cDNA was subcloned into rAAV vector plasmid pAAVβGEnh after deleting the EcoRI fragment carrying the β-glucoronidase cDNA sequence (Dhillon 2000; Dhillon et al. 2000; Dhillon, et al. 2001a; Zolotukhin, et al. 1999). The control vector, rAAV-GFP, was similarly constructed to encode the GFP gene (Dhillon 2000; Dhillon et al. 2001a; Dube, et al. 2002; Zolotukhin et al. 1999).
Vector Administration
For vector administration, the rats were anesthetized with 2–3% isoflurane delivered in oxygen and stereotaxically implanted with a permanent cannula in the 3rd cerebroventricle. The coordinates employed for cannula placement were based on the rat brain atlas. After 1 week of recovery, rats were injected once intracerebroventricularly with either rAAV-GFP (5 μL, 8.25 X 1011 virus particles) or rAAV-Leptin (5 μL, 7.7 X. 1011 virus particles).
Tissue Collection and Analyses
Rats were fasted overnight prior to tissue collection. For tissue collection, the rats were anesthetized with 2–3% isoflurane delivered in oxygen. Blood was collected from abdominal aorta and serum stored at −20°C for analysis of leptin, insulin-like growth factor 1 (IGF1), growth hormone, glucose, adiponectin, collagen type 1 cross-linked C-telopeptide (CTx), and osteocalcin. Death was induced by decapitation. Hypothalami were excised and stored in RNAlater (Ambion, Austin, TX) for analysis of leptin and NPY mRNA levels. Abdominal white adipose tissue (WAT) was excised and weighed and samples of WAT were stored in RNAlater for analysis of leptin and IGF1 mRNA levels. Femora and 2nd lumbar vertebrae were collected and stored in 70% ethanol for analysis of bone mass, density, and architecture.
Serum Chemistry
Serum glucose was measured using Autokit Glucose (Wako, Richmond, VA). Serum leptin was assayed using the rat leptin radioimmunoassay kit from Linco Research, Inc. (St Louis, MO) according to the manufacturer’s instructions. Serum IGF1 was measured with a radioimmunoassay using a polyclonal antibody to IGF1 after separation of IGF-binding proteins by acid ethanol extraction. Serum osteocalcin was measured using rat Gla-osteocalcin high sensitive EIA kit (Clontech, Mountain View, CA). Serum CTx was measured using rat CTx-I ELISA kit (Novateinbio, Cambridge, MA). Serum adiponectin was measured using rat total adiponectin Quantikine ELISA kit (R&D Systems, Minneapolis, MN). Serum growth hormone was measured using a rat/mouse growth hormone ELISA kit (EMB Millipore, Billerica, MA).
RNA analysis
Tissue samples were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). Total cellular RNA was isolated according to manufacturer’s protocol. RNA quantity was determined spectrophotometrically, and RNA quality was evaluated via formaldehyde agarose gel electrophoresis.
Real-Time PCR
cDNA for RT-PCR was synthesized using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). 1 μg of total RNA was reverse transcribed using random hexamer primers and SuperScript II reverse transcriptase according to manufacturer’s protocol. Real-time PCR primers that are specific for rat leptin (forward: 5′-CCTGTGGCTTTGGTCCTATCTG-3′, reverse: 5′-AGGCAAGCTGGTGAGGATCTG-3′), NPY (forward′ 5′-GCCATGATGCTAGGTAACAAACG-3′, reverse: 5′-GTTTCATTTCCCATCACCACATG-3′), IGF1 (forward: 5′-CCGGACCAGAGACCCTTTG-3′, reverse: 5′-CCTGTGGGCTTGTTGAAGTAAAA-3′), and 18S ribosomal RNA (18S) (forward: 5′-GGACCAGAGCGAAAGCATTTGC-3′, reverse: 5′-CGCCAGTCGGCATCGTTTATG-3′) were synthesized by Operon Biotechnologies (Huntsville, AL). Real-time PCR reactions were performed using DyNAmo HS SYBR Green qPCR kit (New England Biolabs, Ipswich, MA). A standard curve that was generated from serial dilutions of purified plasmid DNA that encoded the respective genes was used to measure mRNA transcript copy number. mRNA data represent normalized copy number using 18S ribosomal RNA gene.
Dual Energy X-ray Absorptiometry
Total femur bone mineral content (BMC, g), area (cm2), and bone mineral density (BMD, g/cm2) were measured ex vivo using dual energy absorptiometry (DXA; Piximus 2, Lunar Corp., Madison, WI).
Microcomputed Tomography
Microcomputed tomography (μCT) was used for nondestructive high resolution 3-dimensional evaluation of cortical and cancellous bone volume and architecture. Midshaft and distal femora and 2nd lumbar vertebrae were scanned in 70% ethanol using a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland) at a voxel size of 16 × 16 × 16 μm (55 kVp x-ray voltage, 145 μA intensity, and 200 ms integration time). Filtering parameters sigma and support were set to 0.8 and 1, respectively. Bone segmentation was conducted at a threshold of 245 (scale, 0–1000) determined empirically. Cortical bone in the mid femur diaphysis and cancellous bone in the distal femur metaphysis and epiphysis were evaluated. Automated contouring was used to delineate cortical bone from non-bone. Following, all cortical slices were examined visually for inclusion of cancellous struts originating from the endocortex (extremely rare at this site) and manually removed when present. Twenty consecutive slices (320 μm) of bone were evaluated and cross-sectional volume (cortical and marrow volume, mm3), cortical volume (mm3), marrow volume (mm3), and cortical thickness (μm) were measured. Polar moment of inertia (IPolar) was determined as a surrogate measure of bone strength in torsion. For the femoral metaphysis, 75 consecutive slices (1,200 μm) of cancellous bone, 150 slices (2400 μm) proximal to the growth plate, were evaluated. The entire cancellous compartment (60 ± 1 slices, 960 ± 16 μm, mean ± SE) was assessed in the femoral epiphysis. Analysis of lumbar vertebra included the entire region of cancellous bone between the cranial and caudal growth plates (282 ± 2 slices, 4,512 ± 36 μm). Manual contouring was used to delineate cancellous from cortical bone in the femur metaphysis, femur epiphysis, and vertebral body. Direct cancellous bone measurements included cancellous bone volume fraction (bone volume/tissue volume, %), connectivity density (mm−3), trabecular thickness (μm), trabecular number (mm−1), and trabecular spacing (μm) (Thomsen, et al. 2005).
Statistical Analysis
Mean responses were compared between three groups (baseline, rAAV-GFP, and rAAV-Leptin) using analysis of variance (ANOVA), while two-group comparisons were made using t-tests. A modified F test was used when the assumption of equal variance was violated, with Welch’s two-sample t-test used for two-group comparisons (Welch 1951). The Kruskal-Wallis nonparametric test was used when only the normality ANOVA assumption was violated, in which case the Wilcoxon-Mann-Whitney test was used for two-group comparisons. The required conditions for valid use of t-tests and ANOVA were assessed using Levene’s test for homogeneity of variance, plots of residuals versus fitted values, normal quantile plots, and the Anderson-Darling test of normality. Longitudinal data on weekly measurements of body weight (weeks 0 to 18) and food intake (weeks 1 to 18) were analyzed using linear mixed models to account for correlated data. To accommodate different slopes for the rAAV-GFP and rAAV-Leptin groups and varying slope coefficients across time for these groups, the data on body weight were modeled using a random intercept linear spline model with a single knot at week 4. The random intercept, random slope mixed model for food intake allowed for different marginal intercepts and slopes between the two groups. The Benjamini and Hochberg method for maintaining the false discovery rate at 5% was used to adjust for multiple comparisons (Benjamini and Hochberg 1995). Differences were considered significant at p ≤ 0.05. Data are presented as mean ± SE. Data analysis was performed using R version 2.12 (Team 2010).
Results
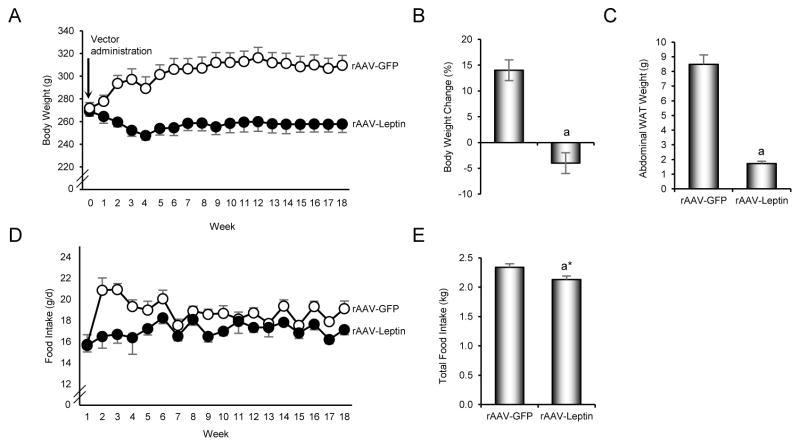
The effects of hypothalamic rAAV-Leptin gene therapy on body weight, abdominal WAT weight, and food intake are shown in Figure 1. rAAV-Leptin-treated rats lost weight (-4 ± 2%) during the 18 week duration of study; the estimated decrease in mean weight from weeks 0 to 18 was 8 ± 3 grams (p = 0.006; 95% CI: 2 grams, 13 grams). The weight loss in the rAAV-Leptin group occurred during the initial 4 weeks post-vector administration (estimated slope: −3.6, p < 0.0001; 95% CI: −5.2, −2.1) with weight increasing thereafter (estimated slope: 0.5, p = 0.01; 95% CI: 0.12, 0.85) (Figure 1A and B). In contrast, control rAAV-GFP-treated rats gained weight (14 ± 2%) during the study; the estimated increase in mean weight from weeks 0 to 18 was 40 ± 2 grams (p = 0.006; 95% CI: 36, 45). The increase in weight was greater during the first 4 weeks following vector administration (estimated slope: 7.7, p < 0.0001; 95% CI: 6.4, 9.0) than during the subsequent 13 weeks (estimated slope: 0.7, p < 0.0001; 95% CI: 0.39, 1.01). The rate of change in body weight (i.e., slope) differed between rAAV-GFP-treated and rAAV-Lep-treated rats during the first 4 weeks of treatment (p < 0.0001) but not during the subsequent 13 weeks. At study termination, rAAV-Leptin-treated rats weighed 17% less (53 ± 10 grams; p < 0.0001; 95% CI: 32 grams, 74 grams) than the rAAV-GFP-treated rats. This difference in body weight was associated with a pronounced reduction in abdominal WAT; WAT weight was 80% lower in rAAV-Leptin-treated compared to rAAV-GFP-treated rats (Figure 1C).
Figure 1.
Effects of increased hypothalamic leptin via rAAV-Leptin therapy on body weight over time (A), body weight change (B), abdominal white adipose tissue (WAT) weight (C), food intake over time (D), and cumulative food intake (E). Values are mean ± SE, n=7–10/group. aDifferent from rAAV-GFP, p ≤ 0.05. a*Different from rAAV-GFP, p < 0.1.
Food consumption was lower in rAAV-Leptin-treated rats compared to rAAV-GFP-treated rats (Figure 1D). Although significant differences in food intake were not detected between the two groups at 1 week post-vector administration, the estimated difference in mean food intake between the two groups during weeks 2 to 4 was 2.4 g/d (95% CI: 1.6, 3.3; p<0.0001); food intake averaged 17.1 g/d (95% CI: 16.2, 17.9) in the leptin group and 19.5 g/d (95% CI: 18.8, 20.2) in the GFP group (p<0.0001). For weeks 5 to 18, the difference in food intake was still significant (p=0.002), but the estimated difference dropped to 1.3 g/d (95% CI: 0.5, 2.1); food intake averaged 17.2 g/d (95% CI: 16.4, 17.9) in the leptin group and 18.5 g/d (95% CI: 17.86, 17.90) in the GFP group. Total food intake over the 18 week duration was 9% lower (p=0.071) in the rAAV-Leptin group compared to the rAAV-GFP group (Figure 1E).
The effects of rAAV-Leptin gene therapy on leptin and NPY mRNA expression in hypothalamus and leptin and IGF1 mRNA expression in abdominal WAT are shown in Figure 2. As expected, rAAV-Leptin-treated rats had higher hypothalamic leptin mRNA levels than rAAV-GFP-treated rats (Figure 2A). Significant differences in NPY mRNA expression in hypothalamus were not detected with treatment (Figure 2B). Significant differences were also not detected with treatment for leptin and IGF1 mRNA expression in abdominal WAT (Figure 2C and D).
Figure 2.

Effects of increased hypothalamic leptin via rAAV-Leptin therapy on leptin (A) and NPY (B) mRNA expression in hypothalamus, and leptin (C) and IGF1 (D) mRNA expression in abdominal white adipose tissue. Values are mean ± SE, n=7–10/group. aDifferent from rAAV-GFP, p ≤ 0.05.
The effects of rAAV-Leptin gene therapy on serum leptin, IGF1, growth hormone, glucose, adiponectin, CTx, and osteocalcin are shown in Figure 3. Serum leptin and IGF1 concentrations were lower in rAAV-Leptin-treated rats compared to rAAV-GFP-treated rats (Figure 3A and B). Significant differences between rAAV-Leptin-treated and rAAV-GFP-treated rats were not detected for growth hormone (Figure 3C), glucose (Figure 3D), or adiponectin (Figure 3E). Furthermore, significant differences between the 2 treatment groups were not detected for CTx, a marker of bone resorption (Figure 3F) or osteocalcin, a marker of bone turnover (Figure 3G).
Figure 3.
Effects of increased hypothalamic leptin via rAAV-Leptin therapy on serum leptin (A), IGF1 (B), growth hormone (C), glucose (D), adiponectin (E), CTx (F), and osteocalcin (G). Values are mean ± SE, n=7–10/group. aDifferent from rAAV-GFP, p ≤ 0.05.
The effects of rAAV-Leptin gene therapy on femur area, BMC, and BMD are shown in Figure 4. Bone area tended to be higher in rAAV-GFP-treated rats (13.5 months old) compared to baseline control rats (9 months old; p = 0.072) and rAAV-Leptin-treated rats (13.5 months old; p = 0.056). Significant differences in bone area were not detected between rAAV-Leptin-treated rats and baseline control rats. Significant differences in either BMC or BMD were not detected among baseline, rAAV-GFP, and rAAV-Leptin rats.
Figure 4.
Effects of increased hypothalamic leptin via rAAV-Leptin therapy on femur area (A), bone mineral content (B), and bone mineral density (C). Values are mean ± SE, n=7–10/group. a*Different from Baseline, p < 0.1. b*Different from rAAV-GFP, p < 0.1.
The effects of rAAV-Leptin gene therapy on cortical bone in the femoral diaphysis and cancellous bone in the femoral metaphysis and epiphysis are shown in Table 1.
Table 1.
Effects of hypothalamic rAAV-Leptin therapy on cortical bone architecture in the femoral diaphysis and cancellous bone volume fraction (bone volume/tissue volume) and cancellous bone architecture in distal femur metaphysis and epiphysis.
| Endpoint | Baseline | rAAV-GFP | rAAV-Leptin | FDR-adjusted p-value |
|---|---|---|---|---|
| Femur Diaphysis (cortical bone) | ||||
| Cross-sectional volume (mm3) | 3.09 ± 0.08 | 3.29 ± 0.06 | 3.05 ± 0.06b* | 0.072 |
| Cortical volume (mm3) | 2.01 ± 0.04 | 2.16 ± 0.02a | 2.05 ± 0.04b* | 0.056 |
| Marrow volume (mm3) | 1.08 ± 0.04 | 1.12 ± 0.04 | 1.00 ± 0.04 | 0.182 |
| Cortical thickness (μm) | 724 ± 8 | 753 ± 7 | 748 ± 13 | 0.135 |
| Ipolar (mm) | 14.60 ± 0.73 | 16.65 ± 0.49a* | 14.46 ± 0.58b* | 0.071 |
| Distal Femur Metaphysis (cancellous bone) | ||||
| Bone volume/tissue volume (%) | 28.0 ± 3.4 | 18.1 ± 2.3a* | 20.9 ± 1.5 | 0.072 |
| Connectivity density (1/mm3) | 92.9 ± 5.6 | 59.3 ± 7.4a | 70.6 ± 5.6a* | 0.052 |
| Trabecular number (1/mm) | 4.6 ± 0.2 | 3.8 ± 0.2a* | 4.0 ± 0.1a* | 0.058 |
| Trabecular thickness (μm) | 78 ± 4 | 69 ± 3 | 70 ± 2 | 0.195 |
| Trabecular spacing (μm) | 214 ± 11 | 263 ± 15a* | 245 ± 8a* | 0.087 |
| Distal Femur Epiphysis (cancellous bone) | ||||
| Bone volume/tissue volume (%) | 42.4 ± 1.0 | 37.1 ± 1.3a* | 38.3 ± 0.7a | 0.056 |
| Connectivity density (1/mm3) | 39.0 ± 2.1 | 35.9 ± 1.5 | 34.8 ± 2.0 | 0.378 |
| Trabecular number (1/mm) | 3.9 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 0.364 |
| Trabecular thickness (μm) | 108 ± 1 | 102 ± 2 | 102 ± 2 | 0.108 |
| Trabecular spacing (μm) | 241 ± 9 | 256 ± 5 | 257 ± 8 | 0.328 |
Data are mean ± SE; n = 7 – 10/group
Different from Baseline, p ≤ 0.05.
Different from Baseline, p < 0.1
Different from rAAV-GFP, p < 0.1
Femur diaphysis
13.5-month-old rAAV-GFP-treated rats had greater cortical volume and tended to have greater polar moment of inertia (p = 0.072) than 9-month-old baseline control rats. Cross-sectional volume, cortical volume, and polar moment of inertia also tended to be greater (p = 0.058, p = 0.071, and p = 0.056, respectively) in rAAV-GFP-treated rats compared to rAAV-Leptin-treated rats. Significant differences between 13.5-month-old rAAV-Leptin-treated rats and 9-month-old baseline control rats were not detected for any of the cortical endpoints evaluated. Significant differences in marrow volume and cortical thickness were not detected among the 3 groups.
Distal femur metaphysis
13.5-month-old rAAV-GFP-treated rats had lower connectivity density and tended to have lower cancellous bone volume fraction (p = 0.071) and trabecular number (p = 0.056) and greater trabecular spacing (p = 0.072) than 9-month-old baseline control rats. 13.5-month-old rAAV-Leptin-treated rats tended to have lower connectivity density (p = 0.060) and trabecular number (p = 0.072) and greater trabecular spacing (p = 0.084) than 9-month-old baseline control rats. Significant differences between rAAV-GFP and rAAV-Leptin rats were not detected for any of the cancellous endpoints evaluated. Significant differences in trabecular thickness were not detected among the 3 groups.
Distal femur epiphysis
Cancellous bone volume fraction tended to be lower (p = 0.056) in 13.5-month-old rAAV-GFP-treated rats and was lower in 13.5-month-old rAAV-Leptin-treated rats than in 9-month-old baseline control rats. Significant differences in cancellous bone volume fraction were not detected between rAAV-GFP-treated and rAAV-Leptin-treated rats. Significant differences in connectivity density, trabecular number, trabecular thickness, and trabecular spacing were not detected among the 3 groups.
Lumbar vertebra
The effects of rAAV-Leptin gene therapy on cancellous bone in the lumbar vertebra are shown in Table 2. 13.5-month-old rAAV-GFP-treated rats tended to have lower cancellous bone volume fraction (p = 0.071) and trabecular number (p = 0.072) and greater trabecular spacing (p = 0.072) than 9-month-old baseline control rats. Significant differences in bone volume fraction, trabecular number, or trabecular spacing were not detected between rAAV-GFP-treated and rAAV-Leptin-treated rats. Significant differences among the 3 groups were not detected for connectivity density or trabecular thickness.
Table 2.
Effects of hypothalamic rAAV-Leptin therapy on cancellous bone volume fraction (bone volume/tissue volume) and cancellous bone architecture in lumbar vertebra.
| Endpoint | Baseline | rAAV-GFP | rAAV-Leptin | FDR-adjusted p-value |
|---|---|---|---|---|
| Lumbar Vertebra (cancellous bone) | ||||
| Bone volume/tissue volume (%) | 36.5 ± 1.9 | 28.9 ± 2.05a* | 32.7 ± 1.8 | 0.087 |
| Connectivity density (1/mm3) | 50.4 ± 2.7 | 52.1 ± 3.1 | 56.0 ± 4.8 | 0.625 |
| Trabecular number (1/mm) | 4.1 ± 0.1 | 3.6 ± 0.13a* | 3.9 ± 0.1 | 0.090 |
| Trabecular thickness (μm) | 87 ± 2 | 79 ± 2 | 82 ± 3 | 0.182 |
| Trabecular spacing (μm) | 236 ± 9 | 273 ± 11a* | 252 ± 8 | 0.087 |
Data are mean ± SE; n = 7 – 10/group
Different from Baseline, p < 0.1
Discussion
The long-term effects of hypothalamic rAAV-Leptin gene therapy on energy balance and bone metabolism were evaluated in skeletally mature (9-month-old) female rats. Consistent with previous studies (Boghossian et al. 2005), rAAV-Leptin-treated rats lost weight while rAAV-GFP-treated rats gained weight during the 18 week duration of study. The respective changes in weight occurred during the first few weeks following vector administration, stabilizing thereafter. At study termination, rAAV-Leptin-treated rats weighed less than rAAV-GFP-treated rats and had lower abdominal WAT weight, serum leptin, and serum IGF1. In contrast, rAAV-Leptin treatment had minimal long-term effects on bone mass, density, and architecture or serum markers of bone turnover.
In the present study, the initial reduction in body weight following leptin gene therapy was associated with lower (−12.3%) energy intake compared to rAAV-GFP treatment. The initial increase in energy intake in rAAV-GFP-treated rats following vector administration likely represents a rebound in food consumption following a surgery-induced reduction in food intake. In support, historical data indicate that age-matched ad lib-fed rats consume ~18 g/d of diet (Iwaniec, et al. 2011). In contrast, an attenuated rebound in food intake was apparent in rAAV-Leptin treated rats. Once weight stabilized at a lower level, energy intake continued to be lower (−7%) in the rAAV-Leptin-treated rats than in rAAV-GFP-treated rats. These findings are consistent with previous long-duration gene therapy studies (Dhillon et al. 2001a; Torto, et al. 2006). The regulatory actions of leptin to reduce energy intake are mediated through a hypothalamic relay involving activation of leptin receptors on orexigenic NPY and anorectic proopiomelanocortin-expressing neurons (Forbes, et al. 2001; Schwartz, et al. 1996b). Morbidly obese leptin-deficient ob/ob mice are hyperphagic and express increased hypothalamic mRNA levels for NPY (Schwartz, et al. 1996a) and these abnormalities are reversed following leptin treatment (Duan, et al. 2007). At study termination, hypothalamic leptin gene expression was elevated in the rAAV-Leptin gene therapy group but significant differences in hypothalamic NPY gene expression were not observed between treatment groups. It is possible, however, that reduced NPY expression was associated with initial weight loss following vector administration (Bagnasco, et al. 2002; Beretta et al. 2002).
Genetic obesity in leptin-deficient ob/ob mice is reversed following administration of leptin (Levin, et al. 1996). In contrast, normal rats and mice exhibit age-related weight gain in spite of increased leptin levels. The failure of increased serum leptin to prevent further weight gain is generally attributed to blood brain barrier leptin resistance and/or hypothalamic leptin resistance (Banks, et al. 1999; Banks, et al. 1996; Bluher and Mantzoros 2004; Bray 2004; Burguera, et al. 2000; Frederich, et al. 1995; Sahu 2002; Van Heek, et al. 1997). Gene therapy bypasses blood brain barrier leptin resistance by direct introduction of leptin into the hypothalamus. The present study demonstrates the long-term efficacy of this approach in inducing weight loss and maintaining lower body weight in skeletally mature female rats. Factors such as diet composition and physical activity may impact the efficacy of hypothalamic leptin gene therapy in preventing excessive weight gain (Dube et al. 2002; Dube, et al. 2008; Shapiro et al. 2008). Nevertheless, our findings in rats consuming normal diet suggest that increasing hypothalamic leptin levels overcomes leptin resistance to prevent adult-onset weight gain.
The elevated leptin mRNA levels that we detected in the hypothalamus 18 weeks following administration of rAAV-Leptin contrast with the notable reduction in serum leptin. The 77% reduction in serum leptin observed in the present study was associated with a similar decrease (−80%) in abdominal WAT weight. This finding is consistent with the concept that leptin levels reflect fat stores (Hickey, et al. 1996). It is notable that the magnitude of lowering WAT weight (−80%) following rAAV-Leptin treatment greatly exceeded lowering body weight (−17%). This is consistent with previous reports indicating that rAAV-Leptin treatment preferentially reduces adipose tissue while preserving lean tissue (Dhillon, et al. 2001b). Furthermore, leptin administration has been shown to increase muscle mass in leptin–deficient ob/ob mice (Bartell, et al. 2011) and aged C57BL6 mice (Hamrick, et al. 2010).
rAAV-Leptin treatment lowered serum IGF1 levels. IGF1 is an important mediator of the anabolic actions of growth hormone. A primary target of growth hormone is the liver, where the hormone stimulates IGF1 secretion through the activation of the hepatic growth hormone receptor. In turn, IGF1 modifies growth hormone secretion through a negative-feedback loop (Clemmons 2007). Increased IGF1 levels and decreased growth hormone secretion are associated with obesity (Garten, et al. 2012), whereas decreased IGF1 levels are associated with caloric restriction (Mitterberger, et al. 2011; Rasmussen, et al. 1995). Thus, the observed decrease in serum IGF1 could reflect an increase in growth hormone secretion. However, we did not detect a change in growth hormone levels with treatment, a finding consistent with the lack of an effect of caloric restriction on growth hormone levels reported in obese subjects in some human studies (Mitterberger et al. 2011; Rasmussen et al. 1995).
A substantial proportion of serum IGF1 is nonhepatic in origin (Liu, et al. 2000). Adipocytes produce IGF1 (D’Esposito, et al. 2012), potentially contributing to reduced growth hormone secretion associated with obesity. Leptin gene therapy did not impact IGF1 mRNA levels in WAT but the dramatic decrease in WAT following gene therapy may have contributed to the reduction in serum levels of this growth factor.
The decrease in serum IGF1 following rAAV-Leptin administration in normal rats contrasts with the response of ob/ob mice to leptin. Whereas, acute intravenously administered leptin had no effect on serum IGF1 (Burcelin, et al. 1999), chronic intracerebroventricular leptin administration to ob/ob mice was reported to increase serum IGF1 levels (Bartell et al. 2011). The latter long-term response may be related to normalization of growth hormone secretion in ob/ob mice (Luque, et al. 2007; Sinha, et al. 1975).
Elevated blood leptin is a risk factor for cardiovascular disease, diabetic kidney disease and several cancers (Cheung, et al. 2010; Cleary, et al. 2010; Hoda, et al. 2012; Hou and Luo 2011; Nishimura, et al. 2012) and high serum IGF1 levels are associated with increased cancer risk (Chaves and Saif 2011; Cleary, et al. 2009; Ribeiro, et al. 2004). Thus, reduction in serum leptin and IGF1, in addition to weight loss, may be a positive side effect of increasing hypothalamic leptin (Lee, et al. 2010a; Moore, et al. 2008; Olivo-Marston, et al. 2009; Wu, et al. 2010).
Leptin increases bone nodule formation in vitro (Gordeladze, et al. 2002) and increases bone formation when delivered to ob/ob mice by either intermittent subcutaneous administration (Turner et al. 2013b) or intracerebroventricular infusion (Bartell et al. 2011). Furthermore, hypothalamic leptin gene therapy increases osteoblast-lined bone perimeter and serum osteocalcin, and normalizes bone architecture in ob/ob mice without increasing serum leptin (Turner et al. 2013b). Although ob/ob mice have reduced osteoblast number and activity, the effects of leptin deficiency on bone metabolism appear to be partially compensated for by factors related to development of morbid obesity. Specifically, normalizing weight gain in ob/ob mice by dietary restriction and thermoneutral housing accentuated their skeletal phenotype (Turner et al. 2014), characterized by reduced bone growth and turnover, low total bone mass and mild osteopetrosis.
In contrast to the notable effects of leptin to increase bone formation in leptin-deficient mice, increased leptin levels associated with moderate weight gain had no effect on bone metabolism in skeletally mature rats (Turner and Iwaniec 2010). Similarly, prevention of weight gain in skeletally mature rats by mild caloric restriction had no negative impact on bone formation (Turner and Iwaniec 2010). In the present long-term study, reduced serum leptin induced by hypothalamic leptin gene therapy was not associated with altered serum markers of bone turnover or accelerated age-related bone loss. Taken together, these findings suggest that the bone anabolic effects of leptin are most pronounced at low serum concentrations of the hormone and as a consequence hyperleptinemia does not confer additional positive skeletal benefits.
Bone mass in growing rodents is tightly coupled to body size (Iwaniec, et al. 2009). Cessation of linear bone growth occurs in rats as a consequence of formation of bone bridges that penetrate the growth plate, rendering further growth unfeasible (Martin, et al. 2003). The time table for linear growth cessation varies among bones but growth generally ceases in female Sprague Dawley rats by 8 months of age. As in adult humans, periosteal bone formation continues in long bones of skeletally mature rats at a very slow rate throughout life (Briot, et al. 2010; Stathopoulos, et al. 2011). Weight typically increases in rodents following skeletal maturity but the impact of weight changes on the skeleton has not been thoroughly explored (Iwaniec and Turner 2013). In the present study, bone area measured by DXA and cortical bone volume measured by μCT exhibited an upward trend in rAAV-GFP rats whereas these parameters remained at baseline control levels in rAAV-Leptin rats. The strong association between bone size and body weight typically observed in WT mice are preserved in leptin-deficient ob/ob mice (Iwaniec et al. 2009), a finding suggesting that increased bone size in aging rAAV-GFP rats is due to their increased body weight.
As expected, age-related cortical bone loss was not observed in the present study. In humans, a negative intracortical bone remodeling balance contributes to age-related bone loss (Seeman 2013). A limitation of rodents is that they do not exhibit intracortical bone remodeling. As a consequence, age-related cortical bone loss is limited to resorption occurring on the endocortical bone surface. Bone loss associated with aging at this site is slow and compensated for by addition of bone onto the periosteal surface (Turner, et al. 2013a).
As in adult humans, cancellous bone remodeling continues throughout adult life in skeletally mature rats (Iwaniec and Turner 2013). An imbalance between bone formation and bone resorption is responsible for persistent bone loss during aging in both species. The trend for age-related cancellous bone loss observed in femur and lumbar vertebra in the present study is consistent with previous results where significant bone loss is noted between 8 months and 2 years of age (Turner et al. 2013a; Turner, et al. 2001). Caloric restriction accelerates age-related cancellous bone loss (Bodnar, et al. 2012; Mardon, et al. 2008a; Mardon, et al. 2008b), a finding consistent with the hypothesis that a decrease in leptin levels in the context of low energy availability contributes to bone loss. Leptin treatment, in spite of drastically reducing body weight in growing ob/ob mice, results in increased longitudinal bone growth, increased bone formation, increased bone resorption, a net increase in bone mass and normalization of bone microarchitecture (Bartell et al. 2011; Hamrick, et al. 2005; Iwaniec, et al. 2007; Turner et al. 2013b).
In summary, hypothalamic leptin gene therapy, in spite of inducing weight loss, had minimal effects on bone mass, density, microarchitecture or biochemical markers of bone turnover. The lack of an effect of rAAV-Leptin gene therapy on bone in skeletally mature rats contrasts with the negative skeletal effects associated with similar weight loss induced by caloric restriction (Turner and Iwaniec 2011). There has been remarkable recent progress in gene therapy, including treatment of diseases of the CNS (Kantor, et al. 2014; Leone, et al. 2012). Our findings suggest that interventions, including hypothalamic leptin gene therapy, targeted toward increasing hypothalamic leptin levels have the potential to overcome leptin resistance and lower body weight without negatively impacting the skeleton.
Acknowledgments
Funding: This work was supported by NIH AR 060913 and DOD W81XWH-04-1-0701.
Footnotes
Declaration of interest: The authors have nothing to declare.
Literature Cited
- Arounleut P, Bowser M, Upadhyay S, Shi XM, Fulzele S, Johnson MH, Stranahan AM, Hill WD, Isales CM, Hamrick MW. Absence of functional leptin receptor isoforms in the POUND (Lepr(db/lb)) mouse is associated with muscle atrophy and altered myoblast proliferation and differentiation. PLoS One. 2013;8:e72330. doi: 10.1371/journal.pone.0072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco M, Dube MG, Kalra PS, Kalra SP. Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology. 2002;143:4409–4421. doi: 10.1210/en.2002-220505. [DOI] [PubMed] [Google Scholar]
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Lebel CR. Strategies for the delivery of leptin to the CNS. J Drug Target. 2002;10:297–308. doi: 10.1080/10611860290031895. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, She JX, Della-Fera MA, Baile CA. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–1720. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the flase discover rate: a practical and powerful approach to multiple testing. J Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Beretta E, Dube MG, Kalra PS, Kalra SP. Long-term suppression of weight gain, adiposity, and serum insulin by central leptin gene therapy in prepubertal rats: effects on serum ghrelin and appetite-regulating genes. Pediatr Res. 2002;52:189–198. doi: 10.1203/00006450-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Bluher S, Mantzoros CS. The role of leptin in regulating neuroendocrine function in humans. J Nutr. 2004;134:2469S–2474S. doi: 10.1093/jn/134.9.2469S. [DOI] [PubMed] [Google Scholar]
- Bodnar M, Skalicky M, Viidik A, Erben RG. Interaction between exercise, dietary restriction and age-related bone loss in a rodent model of male senile osteoporosis. Gerontology. 2012;58:139–149. doi: 10.1159/000329113. [DOI] [PubMed] [Google Scholar]
- Boghossian S, Lecklin A, Torto R, Kalra PS, Kalra SP. Suppression of fat deposition for the life time with gene therapy. Peptides. 2005;26:1512–1519. doi: 10.1016/j.peptides.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Boghossian S, Ueno N, Dube MG, Kalra P, Kalra S. Leptin gene transfer in the hypothalamus enhances longevity in adult monogenic mutant mice in the absence of circulating leptin. Neurobiol Aging. 2007;28:1594–1604. doi: 10.1016/j.neurobiolaging.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Briot K, Kolta S, Fechtenbaum J, Said-Nahal R, Benhamou CL, Roux C. Increase in vertebral body size in postmenopausal women with osteoporosis. Bone. 2010;47:229–234. doi: 10.1016/j.bone.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Kamohara S, Li J, Tannenbaum GS, Charron MJ, Friedman JM. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes. 1999;48:1264–1269. doi: 10.2337/diabetes.48.6.1264. [DOI] [PubMed] [Google Scholar]
- Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes. 2000;49:1219–1223. doi: 10.2337/diabetes.49.7.1219. [DOI] [PubMed] [Google Scholar]
- Carter S, Caron A, Richard D, Picard F. Role of leptin resistance in the development of obesity in older patients. Clin Interv Aging. 2013;8:829–844. doi: 10.2147/CIA.S36367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68:1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves J, Saif MW. IGF system in cancer: from bench to clinic. Anticancer Drugs. 2011;22:206–212. doi: 10.1097/CAD.0b013e32834258a1. [DOI] [PubMed] [Google Scholar]
- Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatr Nephrol. 2010;25:711–724. doi: 10.1007/s00467-009-1427-z. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–213. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- Cleary MP, Ray A, Rogozina OP, Dogan S, Grossmann ME. Targeting the adiponectin:leptin ratio for postmenopausal breast cancer prevention. Front Biosci (Schol Ed) 2009;1:329–357. doi: 10.2741/S30. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Value of insulin-like growth factor system markers in the assessment of growth hormone status. Endocrinol Metab Clin North Am. 2007;36:109–129. doi: 10.1016/j.ecl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- D’Esposito V, Passaretti F, Hammarstedt A, Liguoro D, Terracciano D, Molea G, Canta L, Miele C, Smith U, Beguinot F, et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia. 2012;55:2811–2822. doi: 10.1007/s00125-012-2629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H. Effects of recombinant adeno-associated virus encoding leptin on body weight regulation and energy homeostasis. University of Florida; 2000. p. 171. [Google Scholar]
- Dhillon H, Ge Y, Minter RM, Prima V, Moldawer LL, Muzyczka N, Zolotukhin S, Kalra PS, Kalra SP. Long-term differential modulation of genes encoding orexigenic and anorexigenic peptides by leptin delivered by rAAV vector in ob/ob mice. Relationship with body weight change. Regul Pept. 2000;92:97–105. doi: 10.1016/s0167-0115(00)00155-5. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Mol Ther. 2001a;4:139–145. doi: 10.1006/mthe.2001.0427. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, Muzyczka N, Kalra PS. Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul Pept. 2001b;99:69–77. doi: 10.1016/s0167-0115(01)00237-3. [DOI] [PubMed] [Google Scholar]
- Duan J, Choi YH, Hartzell D, Della-Fera MA, Hamrick M, Baile CA. Effects of subcutaneous leptin injections on hypothalamic gene profiles in lean and ob/ob mice. Obesity (Silver Spring) 2007;15:2624–2633. doi: 10.1038/oby.2007.314. [DOI] [PubMed] [Google Scholar]
- Dube MG, Beretta E, Dhillon H, Ueno N, Kalra PS, Kalra SP. Central leptin gene therapy blocks high-fat diet-induced weight gain, hyperleptinemia, and hyperinsulinemia: increase in serum ghrelin levels. Diabetes. 2002;51:1729–1736. doi: 10.2337/diabetes.51.6.1729. [DOI] [PubMed] [Google Scholar]
- Dube MG, Torto R, Kalra SP. Increased leptin expression selectively in the hypothalamus suppresses inflammatory markers CRP and IL-6 in leptin-deficient diabetic obese mice. Peptides. 2008;29:593–598. doi: 10.1016/j.peptides.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Forbes S, Bui S, Robinson BR, Hochgeschwender U, Brennan MB. Integrated control of appetite and fat metabolism by the leptin-proopiomelanocortin pathway. Proc Natl Acad Sci U S A. 2001;98:4233–4237. doi: 10.1073/pnas.071054298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Friedman JM. A tale of two hormones. Nat Med. 2010;16:1100–1106. doi: 10.1038/nm1010-1100. [DOI] [PubMed] [Google Scholar]
- Garten A, Schuster S, Kiess W. The insulin-like growth factors in adipogenesis and obesity. Endocrinol Metab Clin North Am. 2012;41:283–295. v–vi. doi: 10.1016/j.ecl.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Gat-Yablonski G, Ben-Ari T, Shtaif B, Potievsky O, Moran O, Eshet R, Maor G, Segev Y, Phillip M. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology. 2004;145:343–350. doi: 10.1210/en.2003-0910. [DOI] [PubMed] [Google Scholar]
- Gat-Yablonski G, Phillip M. Leptin and regulation of linear growth. Curr Opin Clin Nutr Metab Care. 2008;11:303–308. doi: 10.1097/MCO.0b013e3282f795cf. [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Howard JK, Lord GM, Ghatei MA, Gardiner JV, Wang ZL, Wang RM, Girgis SI, Bailey CJ, Bloom SR. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem Biophys Res Commun. 2002;295:475–481. doi: 10.1016/s0006-291x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes. 1996;104:293–300. doi: 10.1055/s-0029-1211457. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, Zhou L, Isales CM, Mi QS. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010;400:379–383. doi: 10.1016/j.bbrc.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, Houmard JA, Marks RH, Caro JF. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- Hoda MR, Theil G, Mohammed N, Fischer K, Fornara P. The adipocyte-derived hormone leptin has proliferative actions on androgen-resistant prostate cancer cells linking obesity to advanced stages of prostate cancer. J Oncol. 2012;2012:280386. doi: 10.1155/2012/280386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905–913. doi: 10.1111/j.1440-1681.2011.05619.x. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28:1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Boghossian S, Trevisiol CH, Wronski TJ, Turner RT, Kalra SP. Hypothalamic leptin gene therapy prevents weight gain without long-term detrimental effects on bone in growing and skeletally mature female rats. J Bone Miner Res. 2011;26:1506–1516. doi: 10.1002/jbmr.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Dube MG, Boghossian S, Song H, Helferich WG, Turner RT, Kalra SP. Body mass influences cortical bone mass independent of leptin signaling. Bone. 2009;44:404–412. doi: 10.1016/j.bone.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniec UT, Turner RT. Animal Models for Osteoporosis 2013 [Google Scholar]
- Kantor B, McCown T, Leone P, Gray SJ. Clinical applications involving CNS gene transfer. Adv Genet. 2014;87:71–124. doi: 10.1016/B978-0-12-800149-3.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobelspies H, Zeidler J, Hekerman P, Bamberg-Lemper S, Becker W. Mechanism of attenuation of leptin signaling under chronic ligand stimulation. BMC Biochem. 2010;11:2. doi: 10.1186/1471-2091-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010a;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, Harris TB, Newman AB, Health ABCS. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010b;65:78–83. doi: 10.1093/gerona/glp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, Wang DJ, Assadi M, Goldfarb O, Goldman HW, et al. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4:165ra163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci U S A. 1996;93:1726–1730. doi: 10.1073/pnas.93.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141:4436–4441. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- Luque RM, Huang ZH, Shah B, Mazzone T, Kineman RD. Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Physiol Endocrinol Metab. 2007;292:E891–899. doi: 10.1152/ajpendo.00258.2006. [DOI] [PubMed] [Google Scholar]
- Magliano M. Obesity and arthritis. Menopause Int. 2008;14:149–154. doi: 10.1258/mi.2008.008018. [DOI] [PubMed] [Google Scholar]
- Mardon J, Habauzit V, Trzeciakiewicz A, Davicco MJ, Lebecque P, Mercier S, Tressol JC, Horcajada MN, Demigne C, Coxam V. Influence of high and low protein intakes on age-related bone loss in rats submitted to adequate or restricted energy conditions. Calcif Tissue Int. 2008a;82:373–382. doi: 10.1007/s00223-008-9125-6. [DOI] [PubMed] [Google Scholar]
- Mardon J, Zangarelli A, Walrand S, Davicco MJ, Lebecque P, Demigne C, Horcajada MN, Boirie Y, Coxam V. Impact of energy and casein or whey protein intake on bone status in a rat model of age-related bone loss. Br J Nutr. 2008b;99:764–772. doi: 10.1017/S0007114507837469. [DOI] [PubMed] [Google Scholar]
- Martin EA, Ritman EL, Turner RT. Time course of epiphyseal growth plate fusion in rat tibiae. Bone. 2003;32:261–267. doi: 10.1016/s8756-3282(02)00983-3. [DOI] [PubMed] [Google Scholar]
- Mitterberger MC, Mattesich M, Klaver E, Piza-Katzer H, Zwerschke W. Reduced insulin-like growth factor-I serum levels in formerly obese women subjected to laparoscopic-adjustable gastric banding or diet-induced long-term caloric restriction. J Gerontol A Biol Sci Med Sci. 2011;66:1169–1177. doi: 10.1093/gerona/glr149. [DOI] [PubMed] [Google Scholar]
- Moore T, Carbajal S, Beltran L, Perkins SN, Yakar S, Leroith D, Hursting SD, Digiovanni J. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 2008;68:3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Soda T, Nakazawa S, Yamanaka K, Hirai T, Kishikawa H, Ichikawa Y. Serum adiponectin and leptin levels are useful markers for prostate cancer screening after adjustments for age, obesity-related factors, and prostate volume. Minerva Urol Nefrol. 2012;64:199–208. [PubMed] [Google Scholar]
- Olivo-Marston SE, Hursting SD, Lavigne J, Perkins SN, Maarouf RS, Yakar S, Harris CC. Genetic reduction of circulating insulin-like growth factor-1 inhibits azoxymethane-induced colon tumorigenesis in mice. Mol Carcinog. 2009;48:1071–1076. doi: 10.1002/mc.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MH, Juul A, Kjems LL, Skakkebaek NE, Hilsted J. Lack of stimulation of 24-hour growth hormone release by hypocaloric diet in obesity. J Clin Endocrinol Metab. 1995;80:796–801. doi: 10.1210/jcem.80.3.7533771. [DOI] [PubMed] [Google Scholar]
- Ribeiro R, Lopes C, Medeiros R. Leptin and prostate: implications for cancer prevention--overview of genetics and molecular interactions. Eur J Cancer Prev. 2004;13:359–368. doi: 10.1097/00008469-200410000-00002. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223:T83–96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Tumer N. Peripheral and hypothalamic leptin resistance with age-related obesity. Physiol Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte D, Jr, Woods SC, Seeley RJ, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996a;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996b;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013;68:1218–1225. doi: 10.1093/gerona/glt071. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Matheny M, Zhang Y, Tumer N, Cheng KY, Rogrigues E, Zolotukhin S, Scarpace PJ. Synergy between leptin therapy and a seemingly negligible amount of voluntary wheel running prevents progression of dietary obesity in leptin-resistant rats. Diabetes. 2008;57:614–622. doi: 10.2337/db07-0863. [DOI] [PubMed] [Google Scholar]
- Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha YN, Salocks CB, Vanderlaan WP. Prolactin and growth hormone secretion in chemically induced and genetically obese mice. Endocrinology. 1975;97:1386–1393. doi: 10.1210/endo-97-6-1386. [DOI] [PubMed] [Google Scholar]
- Stathopoulos KD, Katsimbri P, Atsali E, Metania E, Zoubos AB, Skarantavos G. Age-related differences of bone mass, geometry, and strength in treatment-naive postmenopausal women. A tibia pQCT study. J Clin Densitom. 2011;14:33–40. doi: 10.1016/j.jocd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Stunes AK, Westbroek I, Gordeladze JO, Gustafsson BI, Reseland JE, Syversen U. Systemic leptin administration in supraphysiological doses maintains bone mineral density and mechanical strength despite significant weight loss. Endocrinology. 2012;153:2245–2253. doi: 10.1210/en.2011-1848. [DOI] [PubMed] [Google Scholar]
- Talbott SM, Cifuentes M, Dunn MG, Shapses SA. Energy restriction reduces bone density and biomechanical properties in aged female rats. J Nutr. 2001;131:2382–2387. doi: 10.1093/jn/131.9.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A languare and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W. Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc. 2005;218:171–179. doi: 10.1111/j.1365-2818.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP. Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 2006;14:1312–1319. doi: 10.1038/oby.2006.149. [DOI] [PubMed] [Google Scholar]
- Turner RT, Iwaniec UT. Moderate weight gain does not influence bone metabolism in skeletally mature female rats. Bone. 2010;47:631–635. doi: 10.1016/j.bone.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Iwaniec UT. Low dose parathyroid hormone maintains normal bone formation in adult male rats during rapid weight loss. Bone. 2011;48:726–732. doi: 10.1016/j.bone.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Iwaniec UT, Andrade JE, Branscum AJ, Neese SL, Olson DA, Wagner L, Wang VC, Schantz SL, Helferich WG. Genistein administered as a once-daily oral supplement had no beneficial effect on the tibia in rat models for postmenopausal bone loss. Menopause. 2013a;20:677–686. doi: 10.1097/gme.0b013e31827d44df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013b;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res. 2001;16:589–594. doi: 10.1359/jbmr.2001.16.3.589. [DOI] [PubMed] [Google Scholar]
- Turner RT, Philbrick KA, Wong CP, Olson DA, Branscum AJ, Iwaniec UT. Morbid obesity attenuates the skeletal abnormalities associated with leptin deficiency in mice. J Endocrinol. 2014;223:M1–15. doi: 10.1530/JOE-14-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalon KL, Gozansky WS, Van Pelt RE, Wolfe P, Jankowski CM, Schwartz RS, Kohrt WM. A losing battle: weight regain does not restore weight loss-induced bone loss in postmenopausal women. Obesity (Silver Spring) 2011;19:2345–2350. doi: 10.1038/oby.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BL. On the comparison of several mean values: an alternative approach. Biometrika. 1951;38:330–336. [Google Scholar]
- Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10:313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N, Chen X, Mendoza A, Hong SH, Khanna C, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaskin J, Toner RW, Goldfarb N. Obesity management interventions: a review of the evidence. Popul Health Manag. 2009;12:305–316. doi: 10.1089/pop.2008.0049. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]