Abstract
The cytoarchitecture and cortical connections of the ventral motor region are investigated using Nissl, and NeuN staining methods and the fluorescent retrograde tract tracing technique in the rhesus monkey. On the basis of gradual laminar differentiation, it is shown that the ventral motor region stems from the ventral proisocortical area (anterior insula and dorsal Sylvian opercular region). The cytoarchitecture of the ventral motor region is shown to progress in three lines, as we have recently shown for the dorsal motor region. Namely, root (anterior insular and dorsal Sylvian opercular area ProM), belt (ventral premotor cortex) and core (precentral motor cortex) lines. This stepwise architectonic organization is supported by the overall patterns of corticocortical connections. Areas in each line are sequentially interconnected (intralineal connections) and all lines are interconnected (interlinear connections). Moreover, root areas, as well as some of the belt areas of the ventral and dorsal trend are interconnected. The ventral motor region is also connected with the ventral somatosensory areas in a topographic manner. The root and belt areas of ventral motor region are connected with paralimbic, multimodal and prefrontal (outer belt) areas. In contrast, the core area has a comparatively more restricted pattern of corticocortical connections. This architectonic and connectional organization is consistent in part, with the functional organization of the ventral motor region as reported in behavioral and neuroimaging studies which include the mediation of facial expression and emotion, communication, phonic articulation, and language in human.
Keywords: Cerebral Cortex, Frontal Lobe, Limbic System, Motivation, Motor Behavior, Orofacial Movement
1.0 INTRODUCTION
In a recent study, we have shown that the dorsal motor and premotor regions are related architectonically and connectionally with the rostral cingulate proisocortical areas and the cingulate motor regions in a stepwise manner (Morecraft et al., 2012). Moreover, it was shown that the dorsal motor, premotor and cingulate motor regions can be grouped in a tripartite manner by virtue of their interconnections. While studying the architectonics and connections of the ventral somatosensory areas, it was shown that these areas are sequentially related to the ventral motor, ventral premotor and the insular regions (Cipolloni and Pandya, 1999). A great deal of interest has also been focused on the ventral precentral, premotor and prefrontal areas using neuroanatomical, electrophysiological, behavioral and neuroimaging methodologies (e.g., Goldschlak et al, 1984; Matelli et al., 1986; Barbas and Pandya 1987, 1989; Rizzolatti et al., 1987; Gentilucci et al., 1989; Murray and Sessle, 1992; Morecraft et al., 2001, 2014; Petrides and Pandya, 2002; Simonyan and Jürgens, 2002, 2005; Wang et al., 2002; Hatanaka et al., 2005; Jürgens and Ehrenreich, 2007; Frey et al., 2008, 2014; Gerbella et al., 2010, 2011, 2015; Umarova et al., 2010; Arce et al., 2013; Margulies and Petrides, 2013; Bonini et al., 2014). This has largely been due to the important role that this brain region has in communication, articulation and orofacial movements including facial expression.
In the present study, we have investigated the hypothesis that, unlike the dorsal motor and premotor areas which have been shown to be related to the anterior cingulate proisocortex (Morecraft et al., 2012), the ventral motor and premotor areas may have a closer relationship with the ventral proisocortical areas, that is, area ProM (PrCo) and rostral insula. Also, we investigated the possibility that the ventral motor and premotor areas are organized into a tripartite manner, that is, into a root, belt and core pattern much like we have found for the dorsal motor and premotor areas (Morecraft et al., 2012). We therefore have re-examined the architecture and connections of the ventral proisocortical, as well as the ventral motor and premotor areas using Nissl and NeuN stained material and the fluorescent retrograde tract tracer technique respectively. As will be demonstrated, our study indicates that the ventral motor and premotor areas are structurally related to the insular cortex in a stepwise manner, and like the dorsal motor and premotor areas, are also organized in a tripartate manner.
2.0 MATERIAL AND METHODS
Cortical architecture and connections of the ventral motor and premotor areas as well as the rostral Sylvian opercular and insular cortices were investigated in 10 rhesus monkeys (Macaca mulatta). Five monkeys were used to investigate the pattern of cortical cytoarchitecture, and 5 animals were used to study the corticocortical connections (9 different injection sites). All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of South Dakota and followed the guidelines for the ethical treatment of animals outlined by the United States Department of Agriculture (USDA) and the National Institutes of Health. All monkeys were housed and cared for in a USDA and Association for Assessment and Accreditation of Laboratory Animal Care approved and inspected facility. Fluorescent retrograde tracing (FRT) injections were used in multiple experimental combinations in this study to minimize the number of animals needed to accomplish the study aims and strengthen our understanding of the spatial relationships of the potentially different cortical connectional patterns in the same experimental brain. Thus, in 4 of the 5 animals used for our connectional evaluation, we injected both diamidino-yellow (DY) and Fast Blue (FB) into different targets of the same cortical hemisphere. These paired experiments included injection Cases 1 and 2 (SDM66), Cases 3 and 4 (SDM69), Cases 5 and 7 (SDM40) and Cases 6 and 8 (SDM39). The surgical and experimental procedures for the 3 animals processed for Nissl substance at Boston University were approved by the Boston University Institutional Animal Care and Use Committee.
2.1 Neurosurgical and Cortical Injection Procedures
All surgical procedures were performed using sterile methods and have been described in detail (Morecraft et al., 2012). Breifly, each monkey was immobilized with atrophine (0.5mg/kg) and ketamine hydrochloride (10mg/kg). The animals were then intubated and anesthetized with isofluorane inhalation (1.5–2% with surgical grade air/oxygen) except for Case 8, which was anesthetized with pentobarbitol. The monkey was then placed into a head holding device and then Mannitol (1.0–1.5g/kg) was administered intravenously by infusion line drip.
A skin incision and frontoparietal craniotomy was made over the lateral surface of the cerebral hemisphere. The lateral cortical surface of interest was then exposed by making a cruciform or U-shaped dural flap. In the case of neurosurgical exposure of the insula, a fine tipped syringe was used to slowly infuse a 0.9% solution of sterile saline below the arachnoid matter overlying the lateral fissure. This process created a small space, or opening in the lateral fissure. Cottonioid padding was then inserted between the frontal and temporal operculum to increase this space, facilitating clear exposure of the insular cortex in the depths of the lateral fissure.
With the aid of a surgical microscope, an injection of the retrograde neural tracer FB (at a concentration of 3–5% in 0.9% phosphate buffer at pH 7.4) and DY (at a concentration of 3–4% in 0.9% phosphate buffer at pH 7.4) was made into predetermined cortical targets. Pressure injections (0.3–0.4 µL per penetration) of each tracer were made using a Hamilton microsyringe with a cannule tip inserted 2–3 mm below the cortical surface as described previously (Morecraft et al., 2012). Only one penetration was made in the intended cytoarchitectonic region in all Cases with the exception of Case 9 (SDM8), which received 3 closely spaced injections of DY into the physiologically-defined face representation of the primary motor cortex (M1). Following injections of tract tracing compound in all Cases, the dura was closed and the bone flap was replaced and anchored. Subsequently the muscle and skin were closed in layers and the animal monitored postoperatively. Bicillin LA (300,000–750,000 units) was used as prophylaxis antibiotic and buprenorphine (0.01 mg/kg) was used as a post-operative analgesic.
2.2 Intracortical Microstimulation Procedure
In all experiments, excluding the insula injection Case, the ventral premotor cortex and ventral precentral motor cortex was physiologically mapped using a tungsten electrode (impedance 0.5–1.5 MΩ) as previously described in detail (Morecraft et al., 2012). Briefly, an initial stimulation was attempted 100 µm below the pia and then the electrode was advanced at 500 µm intervals at which the stimulation process was repeated at each depth until movement was clearly detected. Movements were evoked using a train duration of 50 ms and pulse duration of 0.2 msec delivered at 330 Hz (Huntley and Jones 1991; Morecraft et al., 2012, 2013). Current intensity ranged between 1 and 90 µA. Threshold currents were determined and the evoked movements were recorded if noted by at least 2 observers. In all microstimulation experimentals conducted in the present study, orofacial movements were evoked and injections of retrograde tracer were carefully made below the cortical surface.
2.3 Histological Procedures
After the FRT injection was made, each monkey survived for a period of 19–33 days to allow for axon transport of each tracer, then was deeply anesthetized with an overdose of pentobarbital (50mg/kg or more). The monkey was then perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB), 10% sucrose in 0.1M PB, and finally 30% sucrose in 0.1M PB. All solutions were adjusted to a pH of 7.2–7.4. The brain was removed and cryoprotected in 30% sucrose in 0.1M PB for approximately 3 days at 4° C. Subsequently, the cortex was frozen with pulverized dry ice then sectioned in the coronal plane on a sliding microtome at a thickness of 50 µm. Tissue sections were collected in chilled 0.1M PB in an organized manner to form 10 series of tissue sections, with each series containing evenly spaced sections that were 500 µm apart through the entire brain. Tissue sections that were to be used for fluorescent analysis were immediately mounted on gelatin-subbed slides and dried overnight in a refrigerated environment. After an appropriate drying period, the sections were coverslipped using Deepex (Sigma, St. Louis, MO, USA) and stored at 4° C. In another complete series of tissue sections, each cortical section was mounted on subbed slides, dried overnight at room temperature, defatted and stained for Nissl substance using thionin and coverslipped with Permount (Fisher Chemicals, Denver, CO, USA). The Nissl stained tissue sections were stored at room temperature.
For additional microscopic cytoarchitectonic analyses, the brains of three non-operated animals were embedded in paraffin and then prepared with Nissl stain. The same brains were used in our previous studies designed to examine the transitional nature of cytoarchitectonic organization in the ventrolateral parietal region (Cipolloni and Pandya, 1999), posterior cingulate and adjacent dorsal parietal region (Morecraft et al., 2004), and rostral cingulate and adjacent dorsal frontal motor region (Morecraft et al., 2012). The brains of two additional animals prepared for the immunohistochemical detection of NeuN were also used in the present study. The same NeuN preparations were used in our previous study on the transitional nature of cytoarchitectonic organization of the anterior cingulate and dorsal frontal motor region, and the immunohistochemical procedures are described in detail in that report (Morecraft et al., 2012).
2.4 Data Analysis
The locations of labeled neurons and injection sites were plotted by charting each individual coronal tissue section using epiflourescent illumination on a Olympus microscope (BX-51 or BX-60) attached to a computer-controlled MAC 2000 motorized microscope stage (Ludl Electronic Products, Hawthorne, NY, USA) joined to a Neurolucida neuroanatomical data collection and analysis system (Microbrightfield Inc., Colchester, VT, USA). In experimental Case 9 (SDM8), the injection site and labeled boutons were plotted using a Hewlett Packard X-Y plotter (HP-7045). Nissl stained sections were used to map the cytoarchitectonic organization on individual chartings of each tissue section. The individual chartings of the coronal sections were then used to reconstruct an image showing the location of each injection site and topography of labelled cell regions on the lateral, medial and ventral surface of the hemisphere that was generated from metrically calibrated photographs of each brain surface. For the intracortical microstimulation experiments, digital photographs depicting electrode position relative to sulcal landmarks, vessels and a metric grid were used as reference points to superimpose stimulation mapping data onto the image of the hemispheric surface to determine the regional somatotopic organization.
Adobe Illustrator and Adobe PhotoShop (Adobe Systems, San Jose, CA, USA) were used to prepare illustrations. Specifically, line drawings of each cortical surface and reconstructed data were created using Adobe Illustrator to demonstrate the cortical locations of the injection sites and retrograde neuronal labeling. The topography of labeling within the sulci depths was illustrated and presented as pullouts, by forming and unfolded flattened image of the cortex forming the depths of each sulci. In addition, representative coronal levels from plotted tissue sections were chosen for line drawing illustrations to demonstrate the relative intensities of the projection and labeling patterns on the cortical surface, and in the depths of the sulci. Photographic montages were created using digital photo images of the injection sites and representative examples of retrogradely labeled neurons acquired using a SpotFlex 64Mp digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) interfaced with a Pentium 4 Dell Computer (Dell Computer Corp., Round Rock, TX, USA).
3.0 RESULTS
3.1 Architecture of the Rostral Insular, Opercular, Ventral Premotor and Ventral Motor Regions
The rostral portion of the insular cortex (insular proisocortex) is continuous with the orbital proisocortex on the one hand (Sanides, 1968, 1972; Galaburda and Pandya, 1983; Mesulam and Mufson, 1982a, 1985), and with the rostral portion of the dorsal Sylvian operculum on the other (Cipolloni and Pandya, 1999). The dorsal Sylvian operculum and the adjacent areas contain several architectonic fields including area ProM, the gustatory area, pre-second somatosensory area (pre-SII), area PV, second somatosensory cortex (second somatosensory area proper, or SII), as well as the precentral portions of areas 3, 1 and 2 (Fig. 1) (Vog and Vogt, 1919; Roberts and Akert, 1963; Sanidies, 1972; Jones and Burton, 1976; Barbas and Pandya, 1987; Preuss and Goldman-Rakic, 1991; Krubitzer et al., 1995; Cipolloni and Pandya, 1999; Disbrow et al., 2003). Although several studies have identified these architectonic areas, we will describe the architectonic features to see if there is progressive laminar differentiation from the insular and opercular proisocortex (insular-opercular region) to the ventral premotor and ventral motor regions.
Figure 1.
Schematic representation of lateral (upper diagram) and medial (lower diagram) surfaces of the cerebral cortex of the rhesus monkey (Macaca mulatta) modified according to Morecraft et al., 2004. The architectonic areas depicted in these diagrams are based on the findings of several investigators: occipital (Paxinos et al., 2000; Pandya and Yeterian, 2010), parietal (Pandya and Seltzer, 1982), inferotemporal and superior temporal sulcus (Seltzer and Pandya, 1978; Desimone and Ungerleider, 1986), superior temporal gyrus and supratemporal plane (Pandya and Sanides, 1973; Krubitzer and Kaas, 1990), insular, parietotemporal opercular and frontotemporal opercular (Roberts and Akert, 1963, Jones and Burton, 1976; Mesulam and Mufson, 1982a; Krubitzer and Kaas, 1990; Krubitzer et al., 1995), lateral premotor (Barbas and Pandya 1987), prefrontal (Petrides and Pandya, 1994), cingulate (Morecraft and Van Hoesen 1992, 1998; Morecraft et al., 2004); and parahippocampal (Blatt et al., 2003) regions. The organization and location of the supplementary motor cortex (MII) is based on the observations of Morecraft and colleagues (McNeal et al., 2010; Morecraft et al., 2015a,b) and Luppino and co-workers (1993) and the pre-SMA region by Matelli and colleagues (Matelli et al. 1985, Luppino et al., 1993).
The rostral part of the insula is proisocortical type of cortex. The cytoarchitecture of this region has been described by Mesulan and Mufson (1982a), Galaberda and Pandya (1983), and more recently by Evrard and colleagues (Evrard et al., 2014). This proisocortex has a simple laminar arrangement that is characterized by an outer and inner stratum (Fig. 2A). The inner stratum is more prominent and layers V and VI are virtually fused. The outer stratum (layers II and III) is characterized by a poorly defined layer II, with more loosely spaced small and medium sized pyramidal neurons. Layer IV is virtually absent. Area ProM is proisocortical in nature and situated in the rostral portion of the upper bank of the Sylvian fissure in the outer lip and in the adjacent convexity cortex (Fig. 1). It lacks layer IV neurons and its infragranular layers V and VI are more prominent than are the supragranular layers (Fig. 2B). This area corresponds to area 6bβ of Vogt and Vogt (1919), area PrCO of Roberts and Akert (1963) and area pK of Jones and Burton (1976). Dorsally area ProM extends up to the inferior precentral dimple. Located dorsal to area ProM is a group of ventral premotor areas (Fig. 1). These areas have been designated as area 6bα and 6aα by Vogt and Vogt (1919), and area 6Vb and 6Va by Barbas and Pandya (1987). There is an additional area that lies in the caudal bank of the inferior limb of the arcuate sulcus. This has been designated as area 44 by Petrides and Pandya (Petrides and Pandya, 1994, Petrides et al., 2012).
Figure 2.
Bright-field photomicrographs of NeuN stained tissue sections showing the cytoarchitecture of the rostral insula, ventral lateral premotor, and ventral precentral regions. A, proisocortical insular cortex. B, area ProM. C, area 6Vb. D, area 6Va. E, area 4. Scale bar in panel A = 1 mm and applies to all panels. The asterisk in panels C and D show the location of an incipient layer IV. For abbreviations, see list.
Architectonically, areas 6Vb and 6Va resemble area 6 dorsal motor region with some differences. There is a presence of an incipient layer IV in the ventral premotor areas (Fig. 2C, D). Both the upper cortical strata (layers I-III) and lower cortical strata (layers V and VI) contain mostly medium sized pyramidal neurons. The difference between areas 6Vb and 6Va is that in area 6Va there is a further accumulation of neurons in the upper strata layers and evidence of scattered large pyramidal neurons in the lower cortical strata. Area 44, which occupies the caudal bank of the lower limb of the arcuate sulcus has a better developed layer IV than areas 6Va and 6Vb. Area 44 also has scattered large pyramidal neurons in layers III and V. Caudal to the ventral portion of area 6 is the ventral division of area 4 which extends into the rostral bank of the central sulcus (Fig. 1). The ventral portion of area 4 extends dorsally up to the level of the spur of the arcuate sulcus and remains above the inferior precentral dimple, that is, above the precentral portion of area 3. The architecture of area 4 resembles that of dorsal area 4 but the Betz cells in this region are not as prominent as noted in the dorsal portion of area 4 (Fig. 2E).
In summary, just as the dorsal premotor and motor areas can be followed from the cingulate proisocortical regions, the ventral premotor and motor areas can be traced architectonically from proisocortex of the anterior insula and opercular area ProM. It should be pointed out that the precursor to area ProM is the rostral insular proisocortex. On the basis of gradational laminar changes, three architectonic lines can be traced from area ProM. One line (root) occupies the dorsal Sylvian operculum consisting of area pre-SII type of cortex that is characterized by prominent infragranular neurons, and area PV/SII that has a comparatively more marked layer IV (Krubitzer et al., 1995; Pandya and Cippoloni, 1999; Disbrow et al., 2003). A second line (belt) arises from area ProM to ventral area 6 which is characterized by small and medium sized pyramidal neurons in layers III and V and incipient layer IV. This line progresses dorsally in area 6Va in which there is an increase of moderate sized pyramidal cells in layer III, and a more prominent layer IV (Barbas and Pandya, 1987). The third component of the belt line resides in the caudal bank of the inferior limb of the arcuate sulcus (area 44) that is characterized by the presence of large pyramidal neurons in layer III and layer V, and a dysgranular layer IV (Petrides and Pandya, 1994, 2002. A third line (core) emanates more dorsally and caudally to precentral area 4 which is characterized as agranular cortex with large pyramidal neurons in layer III, and larger pyramidal neurons (Betz cells) in layer V (Sanides, 1969, 1972; Pandya et al., 2015). Finally, it should be emphasized that the architectonic description of the dorsal Sylvian opercular area, ventral premotor and precentral areas has been available for more than one hundred years. Our revisit to the architectonic description of these areas have two purposes: one is to see if one can identify systematic changes in the laminar patterns from the proisocortical region leading to the premotor and motor isocortical regions, and secondly, is to correlate the architectural changes with the cortical connectivity patterns of these distinct areas.
3.2 Connectional Analyses of the Rostral Insular, Anterior Opercular, Ventral Premotor and Ventral Motor Regions
The corticocortical connections from 9 experimental Cases with injections of fluorescent retrograde tracers (FRT) placed within the anterior insula, frontal operculum, ventral premotor and ventral precentral cortex are described (Figs. 3, 4). First we describe the insula injection Cases followed by opercular (ProM), ventral premotor (area 6Vb) and ventral precentral (area 4) Cases (Figs. 5–12). We have also included two control Cases in which the injection site is located in the precentral somatosensory cortex (areas 1–2) to demonstrate the commonalities of ventral frontal connections as well as the lack of ventral somatosensory connections with the dorsal motor region, including the absence of connections with the medial wall of the frontal cortex and anterior cingulate cortex. Since a major goal of the present study was to investigate the overall trend of the corticiocortical connections that characterize these different regions, proceeding from the insula through the opercular and ventrolateral motor regions, we elected to make a small injection in predetermined parts of each major cytoarchitectonic area. The results of these findings are described below.
Figure 3.
Composite diagram to show the location of the Fast blue (FB) and diamidino-yellow (DY) injection site in Cases 1 through 9 (C1-C9) located in the insula, ventral lateral premotor and ventral precentral motor regions. For abbreviations, see list.
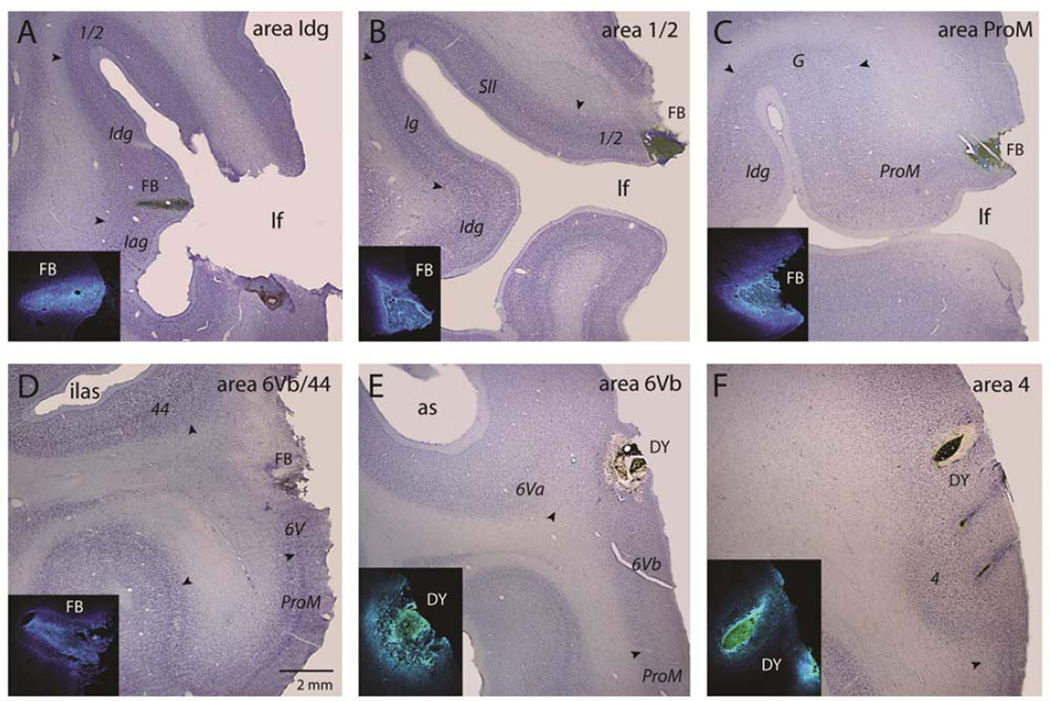
Figure 4.
Low power photomicrographs of showing the location of FRT injections in Nissl stained tissue preparations in selected Cases. The inset in each panel is a higher power fluorescent image of the injection site. A, Case 1 (SDM66 FB). B, Case 4 (SDM69 FB). C, Case 5 (SDM40 FB). D, Case 6 (SDM39 FB). E, Case 8 (SDM39 DY). F, Case 9 (SDM8 DY). Scale bar = 1mm and applies to all Nissl stained images. For abbreviations, see list.
Figure 5.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the anterior insula and cortical distribution of labeled neurons (black dots) in Case 1.
Figure 12.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the ventral region of the precentral cortex (area 4, orofacial representation of M1) and the cortical distribution of labeled neurons (black dots) in Case 9. Also shown are 5 representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the cortical distribution of labeled neurons.
In Case 1, an injection of FB was located at the junction of the agranular and dysgranular insula (Figs. 3, 4A, 5, 13A), whereas in Case 2, the DY injection site was located in the anterior part of dysgranular insula (Figs. 3, 6). Overall the distribution of labeled neurons was similar except for some differences. Locally, labeled neurons were observed rostrally up to the agranular insula and caudally into the granular insula. Distinct clusters of neurons were observed in area ProM, in the rostral portion of the frontal operculum, and in the ventral portion of area 6 (6Vb). The frontal and pericentral operculum also displayed significant numbers of neurons in the pre-SII type cortex, area PV and the rostral portion of SII. In the orbital frontal cortex there were discrete clusters of labeled cells in the lateral portion of area 11 and in the medial portion of area 13 as well as in the orbital proisocortex. Additionally, a distinct field of labeled neurons occurred in the caudal portion of area 47/12.
Figure 13.
Photomicrographs showing representative examples of FRT labeled cells in six selected Cases. A) Fast blue labeled cells in the perirhinal cortex (area 35) following an injection of FB in anterior dysgranular insula in Case 1. B) Fast blue labeled cells in prefrontal area 46 following an injection of FB in the precentral extension of areas 1–2 in Case 4. C) Fast blue labeled cells in the dysgranular insula following an injection of FB in ProM in Case 5.D) Fast blue labeled cells in prefrontal area 47/12 following an injection of FB in the ventral and rostral portion of area 6Vb in Case 6. E) Diamidino-yellow labeled cells in somatosensory area 2 following an injection of DY into dorsal part of area 6Vb in Case 7. F) Diamidino-yellow labeled cells in the rostral part of the supplementary motor cortex (MII or area 6m) following an injection of DY into the ventral part of MI (area 4) in Case 9. Scale bar in A = 200 µm and also applies to panels B, C, D and E. Scale bar in F = 100 µm.
Figure 6.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the anterior insula and cortical distribution of labeled neurons (black dots) in Case 2. Also shown are 5 representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the cortical distribution of labeled neurons.
On the medial surface in both Cases there were labeled neurons involving areas 24a and 24b on the gyral portion of cortex and area 24c in the lower bank of the cingulate sulcus. Some neurons were also observed in the rostral part of areas 23a and 23b. In the more caudal injection site (Case 2, DY) labeled neurons were also found in the ventral portion of area 46, as well as in areas 3, 1, PFop and PF of the ventral parietal region, and in area IPd of the intraparietal sulcus (Fig. 6).
In the temporal lobe labeled neurons were observed in the ventral portion of the temporal polar proisocortex which extended into the rostral portion of the superior temporal sulcus as well as gyrus. Further medially into the ventral portion of the temporal lobe, labeled neurons were found in the rostral portion of area TL (TLr), perirhinal cortex (area 35) and rostral portion of the entorhinal cortex (Fig. 13A). In the rostral part of the parahippocamopal gyrus, a few labeded neurons were found in areas TF, TL and TH. Labeled neurons were also located in the region of the gustatory cortex as well as in the peri-amygdaloid cortical region. In the FB injection located rostrally in the agranular/dysgranular insula (Case 1, Fig. 5), a few labeled cells were found in area CA1 of the hippocampus.
In Cases 3 and 4, an FRT injection of DY and an injection of FB respectively, was placed in the precental opercular region involving areas 1 and 2, near the lateral border of SII (Figs. 3, 4B, 7). The inclusion of these experiments was to serve as control. Cases which show the absence of precentral somatosensory cortex connections with the dorsal motor region and medial wall of the hemisphere. The results of both injection sites were similar. The resulting labeling occurred mainly in the dysgranular insula as well as dorsal Sylvian operculum and area ProM rostrally. There were also labeled neurons in the gustatory cortex, preSII-type of cortex, as well as the region of PV/SII proper and adjacent area PFop. The labeling spread into the ventral precentral gyrus areas 3, 1, and 2 and extended into areas 1, 2, PF and PFop of the rostral ventral parietal cortex. Also labeling was noted in frontal areas 6Vb, 6Va, 44, 47/12 and the ventral region of area 46. On the orbital surface small clusters of labeled cells we found in areas 11, 13 and 47/12. No labeled neurons were found in the dorsal motor and premotor region or on the medial surface of the hemisphere.
Figure 7.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the precentral extension of are 2 and cortical distribution of labeled neurons (black dots) in Case 3.
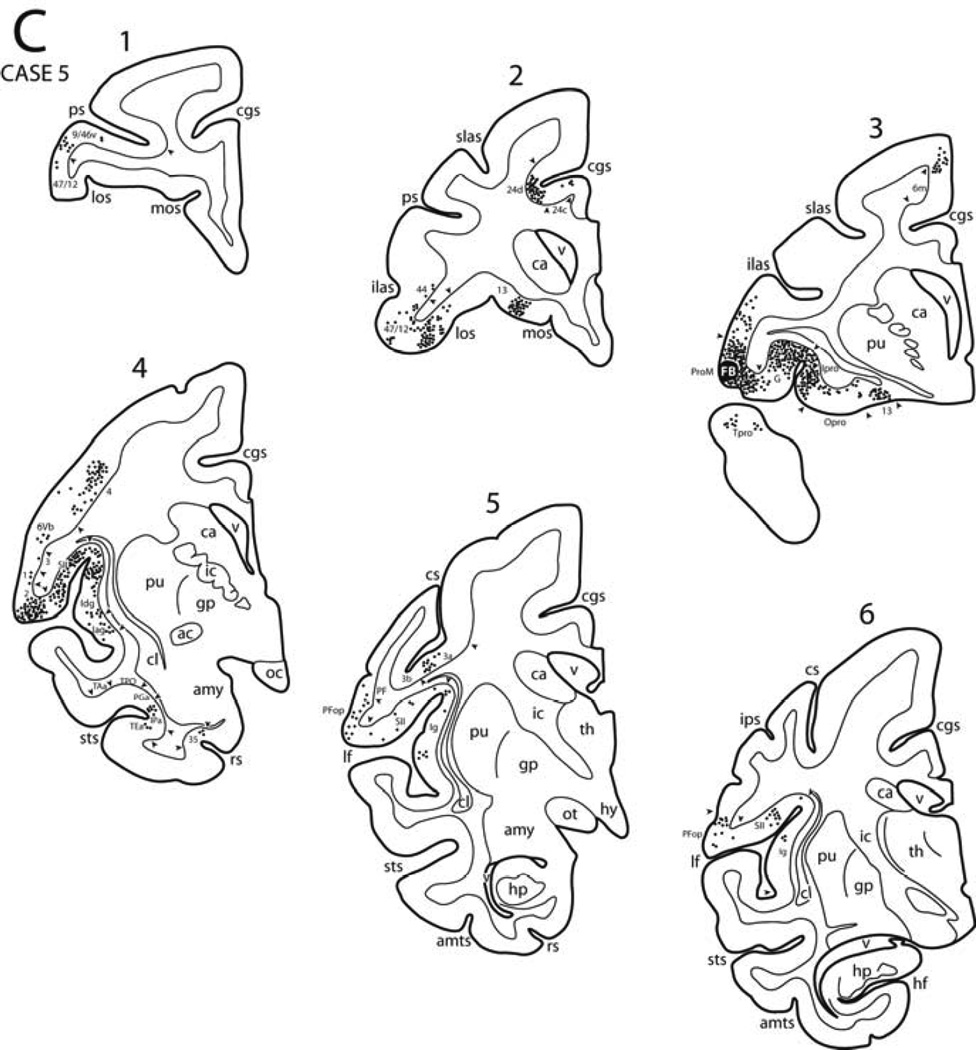
In Case 5, an injection of FB was placed in the ventral part of the lateral premotor region. Specifically, the injection site involved the anterior most-part of the frontal operculum (Figs. 3, 4C, 8, 13C). Sanides (1968) and Barbas and Pandya (1987) used the term ProM to identify this opercular proisocortical motor area (Fig. 1). The resulting labeled neurons were observed mainly in the ventral parts of the prefrontal and premotor areas, and in pre- and postcentral gyri. In addition, labeled neurons were observed in the dorsal Sylvian operculum, insula, ventral prefrontal cortex, orbitofrontal cortex, perirhinal cortex, anterior part of the cingulate region and superior frontal lobule.
Figure 8.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in opercular area ProM and the cortical distribution of labeled neurons (black dots) in Case 5. Also shown are 6 representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the cortical distribution of labeled neurons.
Specifically, in this Case labeled neurons were seen in agranular, dysgranular and granular parts of the insula except for its caudal-most region (Figs. 8, 13C). Some neurons were also present in the gustatory area in the frontal operculum. A substantial number of labeled neurons were observed in the dorsal part of the Sylvian operculum and in the immediately adjacent cortex. These included the rostral part of the operculum (pre-SII type cortex) and areas PV and SII proper caudally, as well the as pre- and postcentral parts of areas 1, 2 and 3. In the temporal lobe a cluster of labeled neurons occupied a small region in the dorsolateral part of temporal proisocortex and rostral part of the superior temporal sulcus. Dorsal to the injection site significant numbers of labeled neurons occurred in the ventral parts of area 6 (i.e., areas 6Va and 6Vb) and orofacial part of area 4. Within the adjacent portion of the central sulcus, a discrete cluster of labeled neurons was noted in areas 3a abd 3b. Rostral to the injection site neurons were noted in the opercular part of area 47/12 and distinct clusters were found in the orbitofrontal cortex involving areas 47/12 and 13 including orbital proisocortex and area 11. A few labeled neurons were found in the ventral portion of area 9/46 of the prefrontal cortex. On the medial surface, two groups of labeled neurons were present in the depths of the cingulate sulcus corresponding to the rostral, orofacial region of area 24c/d (M3) at the level of the genu of the corpus callosum, and the rostral, orofacial region of area 23c/d (M4) a the level of the anterior commissure. A few labeled neurons were found in mid-cingulate region and the rostral, orofacial part of area 6m of the supplementary motor cortex (MII).
In Case 6, a FB injection was placed in the ventral portion of area 6 (Figs. 3, 4D, 9, 13D). This injection involved the ventral most portion of area 6Vb, adjacent part of area 44 in the lower limb of the arcuate sulcus, and the gyral portion of area ProM. Like the above Case, labeled neurons were observed in the rostral portion of the insula, mainly in its agranular and dysgranular portions. Labeled neurons were present in the dorsal Sylvian operculum which included the gustatory area, area ProM, the pre-SII type of cortex, and PV/SII proper. Labeled neurons also extended into the caudal portion of the opercular region including areas PFop and PGop. Above the Sylvian operculum, labeled neurons were found in the caudal portion of the ventral premotor area 6Vb and in the precentral portions of areas 3, 1 and 2. Like the previous Case, labeled neurons were observed in the postcentral gyrus which included areas 1, 2, and PF. Additionally, labeling was noted in area PFG. Above the injection site labeled neurons were seen in area 6Va and in the lower, orofacial part of area 4, including its gyral and sulcal regions. Rostrally labeled neurons were noted in the ventral prefrontal region area 47/12 as well as the ventral region of area 46. On the orbitofrontal surface, distinct clusters of labeled neurons were observed in areas 47/12, 11, 13 and the orbital proisocortex. On the medial surface of the hemisphere distinct patches were noted in the cingulate sulcus. One such locus was in the rostral and middle parts of areas 24c and 24d (M3) above and caudal to the genu of the corpus callosum, and the other were located in the rostral, orofacial region of areas 23c and 23d (M4) at the level of the anterior commissure. Like the previous case, a few neurons were also seen in the caudal portion of area 24.
Figure 9.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the ventral and rostral region of area 6Vb and cortical distribution of labeled neurons (black dots) in Case 6.
In Case 7, a DY injection was placed in the dorsal part of area 6Vb (Figs., 3, 10, 13E). In this Case the basic patterns of labeled neurons were similar to that of the previous Case with some differences as well. Labeled neurons in the insula were less in number and limited to small patchy clusters in Idg and Ig. In the dorsal Sylvian operculum the labeled neurons were observed in area ProM, gustatory area, pre-SII type cortex, and PV/SII proper. There were also labeled neurons in the precentral part of areas 3, 1 2 as well as the postcentral parts of areas 1 and 2. A significant number of labeled neurons were found in areas PFop, PF, PFG and PGop of the rostral inferior parietal lobule. Around the injection site substantial numbers of labeled neurons were present in areas 6Va and 6Vb, in the lower (orofacial) part of area 4, and in the depth of the central sulcus involving areas 4, 3a and 3b. Rostral to the injection site neurons were found in the orbitofrontal and opercular parts of area 47/12 and a small cluster of neurons were found in the ventral region of area 46. Like Case 5, two groups of labeled neurons were found in the depths of the cingulate sulcus corresponding to the rostral, orofacial part of the caudal cingulate motor cortex (M4 or areas 23c/d) and rostral, orofacial part of the rostral cingulate motor cortex (M3 or areas 24c/d). A few labeled neurons were also located in the mid-cingulate gyrus (caudal part of area 24). Finally, a concentrated patch of labeled cells also was found in the rostral, orofacial part of area 6m (MII).
Figure 10.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the dorsal of area 6Vb and cortical distribution of labeled neurons (black dots) in Case 7.
In Case 8, a DY injection was placed in the ventral premotor area 6Vb which extended into the adjacent caudal bank of the arcuate sulcus to involve area 44 (Figs., 3, 4E, 8, 13D). In this Case, the basic patterns of labeled neurons were similar to those of Case 7. Thus, labeled neurons were noted in the insula, dorsal Sylvian operculum including area ProM, gustatory area, pre-SII type of cortex, and area PV/SII proper. Labeled neurons were also noted in the ventral portion of the precentral area 3 and areas 1–2. A significant number of labeled neurons were found in the postcentral gyral areas 1 and 2, area PF and area PFG. Both banks of the rostral portion of the intraparietal sulcus, including the vestibular area, the dorsal portion of area 2, and areas PEa and POa of the intraparietal sulcus also displayed labeled neurons. A few labeled cells occupied area PE of the superior parietal lobule. Around the injection site, a substantial number of labeled neurons were seen in dorsal and ventral areas including the ventral portion of area 6 and in the gyral portion of area ProM. Also, labeled neurons were observed in the middle (arm) and lower (orofacial) parts of area 4 of the precentral gyrus and the adjacent portions of the precentral sulcus. Neurons were also present in the mid-portion of the caudal bank of the central sulcus in areas 3 and 1. In the prefrontal cortex, labeled neurons were found in areas 47/12, 9/46 as well as the rostral and mid-portions of ventral bank of the principle sulcus involving areas 46 and 9/46. Labeling in area 47/12 spread ventrally to involve the lateral region of orbitofrontal cortex. Like the Case 7, distinct clusters of labeled cells were observed in and around the cingulate sulcus. These included M3 and fthe rostral (orofacial) region of M4 as well as the midportion (arm) part of MII. Finally, a few labeled neurons were also present in areas 24a and 24b of the cingulate gyrus.
In Case 9, 3 closely spaced DY injections were placed into the lower part of the precentral gyrus in an area that was physiologically characterized as the orofacial representation of the primary motor cortex (MI) (Figs., 3, 4F, 12, 13F). Architectonically, the injection site involved area 4, and possibly the most caudal part of area 6Vb. Unlike the previous Cases, only a few labeled neurons were noted in the rostral part of insular cortex. Within the dorsal Sylvian operculum labeled neurons were noted in area ProM, the gustatory area and in pre-SII type cortex. Light labeling spread rostrally to involve the inferior lateral and opercular portion of area 47/12. Labeled neurons were present in the precentral portions of areas 3, 1 and 2. In the postcentral region, labeled neurons were found in areas 1, 2 and PF as well as SII and area PFop of the dorsal Sylvian opercular region A significant number of labeled neurons were observed in the ventral portion of area 6 (areas 6Vb and 6Va) adjacent to the injection site, and rostrally in area 6 by the arcuate sulcus, but not within its caudal bank. Distinct clusters of labeled neurons were noted in areas 4, 3a, 3b, and 1 in the central sulcus adjacent to the injection site. On the medial surface a distinct patch of labeled neurons were found in the depths of the anterior portion of the cingulate sulcus, corresponding to the rostral, orofacial part of the cingulate motor cortex (M3 or areas 24c and d). In addition, a second patch was seen posteriorly in the depth of the cingulate sulcus in a location corresponding to the rostral, orofacial portion of the caudal cingulate motor cortex (area 23c of M4). Unlike the previous two Cases, no labeled neurons were found in cortex lining the medial surface of the cingulate gyrus. Finally, a dense patch of labeled neurons also occurred in the dorso-medial portion of the superior frontal gyrus corresponding to the rostral (orofacial) portion of MII.
4.0 DISCUSSION
The ventral motor and premotor areas in the monkey cerebral cortex contain predominatly face, head and neck representations (e.g., Woolsey, 1952, 1958; Luschei and Goodwin, 1975; Larson et al., 1980; McGuinness et al., 1980; Murray and Sessle, 1992; Yao et al., 2001). Recent anatomical and physiological studies have shown that the more dorsal part of the ventral premotor area is related to hand/digit movement (e.g., Dum and Strick, 2005; Hoshi and Tanji, 2007; Schmidlin et al., 2008; Gharbawie et al., 2011; Gerbella et al., 2015). Furthermore, Rizzolatti and colleagues have shown that the ventral premotor region contains “mirror” neurons (Fabbri-Destro and Rizzolatti, 2008; Casile, 2013) and other investigators have noted that the ventral premotor cortex is involved in a whole body movement (Graziano et al., 2002). Previous investigation of the dorsal motor region, on the basis of cytoarchitecture and connections, has shown that a close relationship exists between the medial proisocortical area (i.e., anterior cingulate region), the surrounding rostral cingulate motor areas, and the adjacent supplementary motor area (Morecraft et al., 2012). It was also found that this architectonic and connectional relationship extends from the supplementary motor region to involve the dorsal premotor and dorsal precentral motor regions. The question arises as to how the ventral motor regions are organized in terms of their structural relationship to the ventral proisocortex (i.e., anterior insular region and rostral Sylvian opercular area - area ProM). Like the dorsal motor region, the ventral precentral and premotor regions may be related in a stepwise manner to the insular and opercular proisocortical regions. Indeed, the results of the present study show that a stepwise structural progression can be traced from the insular and opercular proisocortex to the ventral premotor and motor regions. We will first discuss the architectural progression of the insular and opercular proisocortex cortex leading to the premotor and precentral motor cortex, and subsequently will discuss the interconnections of these areas.
4.1 Architectonic Characteristics of the Ventral Motor System
It seems that the ventral premotor and precentral regions can be traced from the insular proisocortex and the frontal opercular area ProM on the basis of laminar differentiation (Sanides, 1970; Barbas and Pandya, 1987). The rostral insular region and opercular area ProM are characterized by having prominent neurons in lower strata (layers V/VI) and a relatively sparse number of neurons in the upper strata (layers II and III), as well as an absence of layer IV neurons. From area ProM various lines of architectonic differentiation seem to occur. One line leads to the ventral premotor areas, a second line culminates in the ventral precentral area, and the third line in the dorsal Sylvian opercular cortex (pre-SII type of cortex). Adjacent, and dorsal to area ProM is the ventral premotor area 6V. This area has architectonic features that resemble dorsal area 6 (Morecraft et al., 2012) with one major difference. Specifically, in area 6V there is the presence of an incipient layer IV. Ventral area 6 has been divided into two major divisions, a ventral area 6Vb and more dorsal area 6Va. In premotor area 6Vb there is the presence of small and medium sized pyramidal cells in layers III and V, along with an incipient layer IV. More dorsally, in area 6Va layers III and V are more prominent, and there is the presence of occasional large pyramidal neurons (Betz cell type) in layer V (e.g., Barbas and Pandya, 1987). Ventral area 6 extends caudally up to area 4. Rostrally, ventral area 6 extends to dysgranular area 44 in the lower limb of the arcuate sulcus. Dorsally, are 6Va extends up to the level of the spur of the arcuate sulcus. Area 44 has a dysgranular cortex with prominent layer III pyramidal neurons and the presence of large pyramidal neurons in layer V (Petrides and Pandya, 1994). From area ProM a second architectonic line can be traced above the inferior precentral dimple (ventral area 4) which extends up to the level of the arcuate spur in the ventral part of the precentral gyrus and the adjoining anterior bank of the central sulcus. Ventral area 4 is agranular, and is characterized by having large pyramidal neurons in layer V (Betz cells). These Betz cells however, are not as large as those that occur in the dorsal portion of area 4 (Sanides, 1972). The third line of architectonic differentiation can be traced in the dorsal Sylvian operculum caudally, that leads to the pre-SII-type of cortex which has prominent infragranular layers and few layer IV granular neurons. In turn, the pre-SII type of cortex leads to the PV-SII type of cortex which has more granular cells in layer IV along with the prominent infragranular emphasis (Cipolloni and Pandya, 1999; Pandya et al., 2015). Thus, it seems that the ventral portion of the premotor and precentral regions, and the dorsal Sylvian opercular region show sequential architectonic changes from area ProM in three lines. These architectonic observations are based on the qualitative changes in the laminae in an orderly fashion from the proisocortex (Fig. 14A, also see Results, section 3.1).
Fig. 14.
A) Summary diagram illustrating the general plan of architectonic progression in each line (root, see green arrows; belt see blue arrows; core, see red arrow). B) Summary diagram showing the sequential intralinear connections of each line. The root line interconnections are illustrated by the bidirectional sequence of green arrows. The belt line of interconnections are shown by the bidirectional blue lines, and the core line interconnections by the bidirectional red arrow. C). Summary diagram depicting the interlineal connections of the root, belt and core lines. Note that each line is interconnected with 2 other lines. For example, the root line is interconnected with the belt and core lines while the belt line is interconnected with the core and root lines. Finally the core line is interconnected with both belt and root lines.
4.2 Cortical Connections of the Ventral Proisocortical, Premotor and Precentral Regions
Previous experimental studies by Mesulam and Mufson (1982b, c) have shown that the rostral insular area has widespread cortical connections which include reciprocal connections with the opercular area ProM (area PrCO), which is in agreement with our observations (Fig. 15A). According to the present study, area ProM (which is proisocortical in nature) (Case 5) receives projections from the insula (areas Iag, Idg and Ig) as well as the dorsal Sylvian opercular areas, the pre-SII type of cortex, precentral portions of areas 3, 1, 2 and area PV/SII (Fig. 15B). Moreover, area ProM receives connections from the ventral portion of area 6 (area 6Vb), area 47/12 and the ventral portion of area 46, as well as the orbital proisocortex (Pro) and surrounding areas 13 and 11. Other areas projecting to area ProM are the precentral portion of areas 3, 1 and 2, the ventral portion of area 4, the adjacent portion of areas 3a and 3b in the central sulcus, with less from the ventral portion of the post-central gyrus (areas 1 and 2). Area ProM also receives afferents from proisocortical areas M3 (orofacial region of the rostral cingulate motor cortex) and area 24 of the cingulate gyrus. Finally, ProM receives input from the orofacial parts of M4 and MII (see Morecraft et al., 2001, 2014 for orofacial representations).
Fig. 15.
Summary diagrams showing the cortical afferents to the rostral insula (A), opercular area ProM (B), area 6VB (C), and ventral area 4 (D).
The ventral portion of premotor area 6 (ventral part of area 6Vb) (Case 6), like area ProM, receives connections from the insular cortex (areas Iag, Idg and Ig) as well as the dorsal Sylvian opercular areas ProM, the pre-SII type cortex, and the precentral portions of areas 3, 1, 2, including area PV/SII (Fig. 15C). This portion of area 6 also receives connections from the rostral portion of area 46 as well as areas 45, 47/12, 13 and 11 of the prefrontal cortex. Unlike area ProM, the ventral portion of the premotor area 6 also receives substantial projections from the ventral parietal cortex including areas 3, 1, 2, PF, PFG, PGop and POa. Another group of projections to area 6Vb is from area 6Va, and the ventral portion of area 4. Finally, like area ProM, this cortex also receives projections from cingulate motor areas M3 and M4 (the respective orofacial representations - Morecraft et al., 2001, 2014), as well as the gyral part of area 24, and the rostral part of MII.
The dorsal portion of area 6Vb (Cases 7 and 8) like areas ProM and the more ventral part of area 6Vb, receives projections from the insula and from the dorsal Sylvian opercular cortex, including areas ProM, the pre-SII type of cortex, the precentral portions of areas 3, 1, 2, as well as area PV/SII. The dorsal portion of area 6Vb (that is adjacent to area 6Va) also receives projections from other portions of ventral area 6 as well as from prefrontal area 46 and area 47/12 in the orbitofrontal cortex. The other afferents to dorsal area 6Vb are from areas 3, 1, 2, PF and PFG of the post-central gyrus. Like areas ProM and the ventral part of area 6Vb, the dorsal part of area 6Vb receives significant afferents from the ventral portion of area 4 as well as the adjacent portion of area 3a in the depth of the central sulcus. This part of area 6Vb also receives projections from the orofacial representations in MII, M3 and M4 as well as the gyral portion of area 24.
The ventral portion of area 4 (the precentral orofacial representation) (Case 9) receives comparatively fewer projections from the anterior insula and the dorsal Sylvian opercular areas ProM, the pre-SII type of cortex, and area PV/SII (Fig. 15D). This part of area 4 also receives projections from both the dorsal and ventral portions of adjacent area 6, and prefrontal area 47/12. In addition, the ventral portion of area 4 receives projections from the precentral portions of areas 3, 1 and 2 as well as portions of areas 3a, 3b, 1, 2 and PF of the ventral region of the postcentral gyrus. Like the ventral premotor areas and area ProM, the ventral area 4 receives projections from the rostral part of MII, and from the rostral portion of areas M3 and M4 (orofacial representations). Overall, this projection pattern is in agreement with previous studies which have examined the cortical connections of the ventral precentral orofacial region (e.g., Pandya and Vignolo, 1971; Godschalk et al., 1984; Morecraft et al., 1996; Tokuno et al., 1997)
Thus, it seems that, like the dorsal motor proiso- and isocortical areas (Morecraft et al., 2012), the ventral motor proiso- and isocortical areas (area ProM and the ventral portion of area 6) have widespread connections with the prefrontal and parietal cortices as well as with the ventral portions of areas 3, 4, and the rostral portions of MII and the two cingulate motor areas (Fig. 15). In contrast, the ventral portion of precentral motor area 4, like the medial and dorsal portion of the precentral area 4, has restricted connections with areas 6, ProM, 3, 1, 2 and SII, as well the rostral parts of MII and the cingulate motor areas.
4.3 Comparisons of Ventral Motor Trend Connectivity with the Dorsal Motor Trend and Cortical Sensory Systems
The present architectonic and connectional observations suggest that, like the somatosensory cortex (Cipolloni and Pandya, 1999; Morecraft et al., 2004), motor and premotor areas can be viewed as organized according to dorsal and ventral trends. In our previous paper (Morecraft et al., 2012), it was shown that the dorsal motor trend progresses from the cingulate proisocortex in a stepwise manner leading to the cingulate and supplementary motor areas and dorsal premotor and precentral motor areas. Likewise, the ventral motor trend emanates from rostral insular and area ProM proisocortical areas, and leads to the dorsal Sylvian opercular area, as well as ventral premotor and precentral cortical regions (Fig. 14A). In both dorsal and ventral motor trends, the progressive laminar changes can be traced from an emphasis on the infragranular layers to the supragranular layers. The very caudal part of area 6 could represent the intermediate step between area ProM and precental motor cortex, and in fact there is some evidence from connections to support this view. We would like to point out that the precentral motor cortex has unique characteristics along with the emphasis of the layer III neurons, there is more prominent pyramidal neurons in layer V, culminating with the presence of the large Betz cells. Indeed, the overall architectonic trends parallel the connectional patterns of the subregions within these trends. Thus, it seems that in both trends proisocortical areas have widespread connections that include not only motor and sensory areas but also prefrontal, temporal and parietal association, as well as limbic and multimodal areas (Fig. 15A, B). Area 4, the primary motor cortex, on the other hand, has much more restricted connections with motor, sensory, premotor and parietal cortical regions (Fig. 15D). The premotor areas have intermediate types of connections, in that they have more limited connections with multimodal and ventromedial limbic regions (Fig. 15C). All areas, starting from the proisocortical region in both, dorsal and ventral trends have specialized connections with motor and premotor, as well as supplementary motor, cingulate motor, area ProM and insular areas. This pattern of connectivity resembles that of the dorsal and ventral parts of the cortical somatosensory system (Cipolloni and Pandya, 1999; Morecraft, et a., 2004). In both of these studies, it was further suggested that cortical somatosensory areas are organized in a tripartite manner, that is, into root, core and belt lines. The core area, that is the primary somatosensory region, is surrounded by belt and root areas. It is also suggested that, in such an organization, a given core area is connected with the adjacent core areas, as well as belt and root areas. Likewise, the belt area is connected with adjacent belt areas, in addition to core and root areas. The root area, similarly, is shown to be connected with the adjacent root areas as well as the core and belt areas.
The present study, as well as the recent study of Morecraft and colleagues (Morecraft et al., 2012), shows that a similar connectional organization is true for the motor and premotor cortical regions of both (dorsal and ventral) trends. Thus, in the dorsal trend, cingulate motor areas (M3 and M4) and MII are considered as root areas whereas the dorsal premotor area 6 is considered as a belt area, and adjacent dorsal area 4 is equivalent to a core region (Morecraft et al., 2012). The connectional observations show that the root areas are interconnected (cingulate and supplementary motor areas) as well as the dorsal premotor areas 6DC and 6DR (belt) and dorsal precentral area 4 (core). The belt area (dorsal premotor area 6), is connected with belt and root areas (supplementary and cingulate motor areas) as well as with the core area (area 4). Finally, the dorsomedial part of area 4, a core region, is shown to be connected with the lateral part of area 4, a core area, as well as with the neighboring root area (cingulate and supplementary motor areas) and belt area (dorsal area 6).
According to the present study, in the ventral trend the root area ProM is connected with other root areas, that is, the pre-SII type of cortex as well as with area SII/PV and insular cortical regions (Fig. 14B). Area ProM is also connected with the ventral portion of area 6 (belt) and the ventral portion of area 4 (core). Likewise, the ventral belt areas (areas 6Va and 6Vb) are connected with other parts of the belt region (area 44) as well as to root areas, that is, area ProM, the pre-SII type of cortex, area SII/PV and the insula (Fig. 14B,C). Belt areas are also connected with the core area, that is, the ventral portion of area 4. Ventral core area 4 is connected with the other portions of ventral area 4 as well as with root areas ProM, the pre-SII type of cortex and area SII/PV on the one hand, and with the ventral portion of area 6, a belt region, on the other hand.
Thus, it seems that precentral and premotor areas are organized not only in a dorsoventral trend, but areas in both trends show a root, belt and core type of organization. It should be pointed out that although the root, belt and core areas are strongly interconnected within each trend, there are interconnections between the two trends. Thus, it seems that the root areas of both trends (MII, M3, M4 and area ProM as well as cingulate and insular proisocortices) show definite interconnections. Likewise, there are few but definite connections between the dorsal and ventral belt areas.
Another interesting aspect of our study is that the precentral motor core, belt and root areas are also connected with a somatosensory core area 3, belt areas 1, 2 and PE, and root areas SII and SSA of the postcentral gyrus. It has been suggested that cortical somatosensory and somatomotor areas are integral parts of one major system, that is, the somatosensorimotor system (Woolsey, 1958). Therefore, it is quite fitting that precentral, premotor and postcentral cortices are interrelated. Finally, we would like to point out that a dual trend organization in the motor system suggests that the dorsal trend begins from the cingulate proisocortex whereas the ventral trend emanates from the insular proisocortex (Sanides, 1969). The dorsal trend consists of limb and trunk representations and is primarily involved in locomotion, posture, motivation, and exploratory activities (Lawrence and Kuypers, 1968a, b). In contrast, the ventral trend is mainly related to the head, neck and face, thus subserving facial expression, emotion and communication (language in humans). A similar dual pattern of organization has been shown in the visual system (Ungerlider and Mishkin, 1982; Yeterian and Pandya, 2010; Pandya et al., 2015). The dorsal visual stream consists of areas dealing with peripheral visual fields and is thought to play a role in the visual spatial and attention aspects of visual function (Ungerlider and Mishkin, 1982; Colby and Duhamel, 1991, 1998; Anderson et al., 2005; Galletti et al., 2001, 2005; Ungerlider et al., 2008). The ventral visual stream consists of central visual field areas and is involved in object identification, discrimination, pattern recognition and memory (e.g., Mishkin et al., 1983; Cirillo et al., 1989). Likewise for the auditory system, a dual pattern of organization has been suggested, (albeit with some reservation) in the nonhuman primate (Rauschecker, 1997, 1998; Rauschecker and Tian 2000, Kaas and Hackett, 1999, 2000) as well as in the human (Clarke et al., 2002). Accordingly the caudal auditory area is considered to be involved preferentially in sound localization while the rostral areas are thought to be devoted mainly to sound recognition and memory (Colombo et al., 1990, 1996; Tian et al., 2001; Pandya et al., 2015).
Within this dual trend each sensory system is shown to have a tripartite organization. Thus, as mentioned above, the somatosensory system has been demonstrated to be organized in a tripartite manner (Cipolloni and Pandya, 1999; Morecraft, et al., 2004). Likewise the auditory system has also been shown to be organized in a tripartite manner (Galaburda and Pandya, 1983; Seltzer and Pandya, 2009). Recent observations indicate that the visual system is organized also in a tripartite manner (Yeterian and Pandya, 2010; Pandya et al., 2015). Thus, it seems that dual trends and tripartite type of organizations are common principles of sensorimotor systems of the cerebral cortex.
It is suggested that both in the auditory and the somatosensory systems, the belt areas in each trend are surrounded by outer belt (multimodal) areas (Pandya and Yeterian 1990; Cippiloni and Pandya, 1999; Pandya et al., 2015). Thus, for example, in the auditory system the outer belt consists of the insular cortex on the one hand and the multimodal cortex of the superior temporal sulcus on the other hand. It is shown that these outer belt areas are reciprocally connected with root and belt areas. In the motor system, prefrontal and posterior parietal association areas are connected with the premotor areas and can be considered as outer belt regions. In addition, the root areas (MII, M3, M4 and ProM) are connected with frontal, parietal and temporal areas as well as with insular and ventromedial limbic areas. These areas are also viewed as part of the outer belt regions. Thus, it seems that this concept of an outer belt or multimodal-limbic envelop is true for the motor and premotor regions and may be part of the overall proposed organization of the cortical sensorimotor areas (Pandya et al., 2015). It is important to note with regard to the present ventral motor trend, that the rostral insula is considered a root area (limbic, proiscortical area Iag). In contrast the middle and caudal part of the insula is characterized functionally and connectionally as multimodal (e.g., this part of the insula has connections with limbic, visceral, olfactory, gustatory, auditory, and somatosensory regions) and hence, it is referred to as part of the outer belt concept.
It has become increasingly clear that the motor activity emerging from the precentral gyrus requires that other cortical regions also play significant roles in order that motor behavior becomes meaningful. The preferential ties of the ventral premotor and precentral areas with insular and opercular areas perhaps are more tuned toward such activity as appropriate facial expression, gestures and mirroring as well as understanding the emotional and phonemic aspects of communication and language. Such proposed functional relationships to the motor activity emerging from the precentral motor areas remain to be proven. However, it is clear that motor activity, whether it has to do with locomotion, exploration, facial expression or language, definitely requires the input from varied sources. The fact that motor areas on the convexity have extensive connections with cingulate and insular proisocortical areas speaks of a commonality of function in that the motor areas on the convexity support voluntary motor function while the cingulate and insular regions may support the emotional and motivational component for motor function (Morecraft and Van Hoesen, 1998, 2003; Morecraft et al., 2001, 2005, 2014) Also, it is worthwhile to note that there are ample interconnections between the ventral and dorsal motor trends which may provide for proper harmony in complex and integrated motor behaviors.
4.4 Summary and Conclusions
In conclusion, although the specific aim of the present study was to expand upon the organization of the ventral motor region using an architectural and connectional approach, we have also combined some of our other observations dealing with the architecture and connections of the dorsal motor region (Morecraft et al., 2012) in order to provide an overall concept of the cortical motor system in general. Thus, we have shown that the precentral and premotor areas are related to dorsal (cingulate) and ventral (area ProM and insular regions) in a stepwise manner. We have viewed that the motor system has been organized from the dorsal and ventral trends: the dorsal trend being related to the cingulate proisocortical region while the ventral trend is related to area ProM and the insular proisocortical regions. We have further suggested that each trend is organized into root, belt and core lines. The core line is primary motor in nature, while the belt areas consist of premotor areas. The root areas (medial and ventral proisocortical areas) are part of the limbic and paralimbic regions. We have also suggested that core areas are surrounded by belt and root areas and, in turn, belt and root areas are surrounded by a multimodal-limbic outer belt. We have suggested that such an organization exists for the cortical areas of all sensory modalities as well. Although several studies have advanced different proposals regarding the organization of the sensorimotor system of the cerebral cortex, we attempt to provide an underlying principle of organization of the cerebral cortex based on cytoarchitecture and connectivity. Using such a framework, we hope future investigations can be conducted to provide further insight into the coherent functional organization of the cerebral cortex in general, and the cortical sensorimotor system specifically.
Figure 11.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site (irregular black sphere) in the rostral and dorsal region of area 6Vb and cortical distribution of labeled neurons (black dots) in Case 8. Also shown are 7 representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the cortical distribution of labeled neurons.
Highlights.
-
-
Cytoarchitecture and cortical connections of the anterior insula and ventral motor cortex were investigated.
-
-
Stepwise architectonic changes were paralleled by stepwise changes in cortical connections.
-
-
Widespread cingulate connections were followed by progressively more restricted connections.
-
-
These findings enhance our understanding of the primate cortical limbic-motor interface.
Acknowledgement
Grant Sponsor: National Institutes of Health; Grant numbers: NS 046367 and NS 33003 (to RJM).
LIST OF ABBREVIATIONS
- A
arm
- ac
anterior commissure
- amts
anterior medial temporal sulcus
- amy
amygdala
- apos
anterior parieto-occipital sulcus
- as
arcuate spur
- ca
caudate nucleus
- CA1
Cornu Ammonis 1 subfield according to Lorente de Nó (1934)
- cc
corpus callosum
- ccg
genu of the corpus callosum
- ccs
splenium of the corpus callosum
- cf
calcarine fissure
- cl
claustrum
- cgs
cingulate sulcus
- cs
central sulcus
- D
digit
- DY
diamidino yellow
- F
face
- FB
fast blue
- FRT
fluorescent retrograde tracer
- gp
globus pallidus
- hf
hippocampal fissure
- hp
hippocampus
- hy
hypothalamus
- Iag
insula, agranular sector
- ic
internal capsule
- Idg
insula, dysgranular sector
- Ig
insula, granular sector
- ilas
inferior limb of the arcuate sulcus
- ios
inferior occipital sulcus
- Ipro
insula proisocortex
- iprs
inferior precentral sulcus
- ips
intraparietal sulcus
- L
leg
- lf
lateral fissure
- los
lateral orbital sulcus
- ls
lunate sulcus
- MI
primary motor cortex (area 4)
- MII
supplementary motor cortex (area 6m)
- M3
rostral cingulate motor cortex (areas 24c and 24d)
- M4
caudal cingulate motor cortex (areas 23c and 23d)
- mos
medial orbital sulcus
- oc
optic chiasm
- OFC
orbitofrontal cortex
- olf
olfactory sulcus
- ot
optic tract
- ots
occipital temporal sulcus
- paAc
para-auditory cortex caudal
- paAlt
para-auditory cortex lateral
- paAr
para-auditory cortex rostral
- paI
parainsular cortex
- pAMYc
peri-amygdaloid cortex
- ParaSub
parasubiculum
- pmts
posterior middle temporal sulcus
- POC
primary olfactory cortex
- poms
medial parieto-occipital sulcus
- preSMA
pre-supplementary motor cortex
- PreSub
presubiculum
- Pro
proisocortex
- proA
proauditory cortex
- ProM
proisocortical motor cortex
- ProStr
area prostriata
- ProSub
prosubiculum
- ps
principal sulcus
- pu
putamen
- reit
retroinsular temporal area
- reipt
retroinsular parietal temporal area
- rf
rhinal fissure
- RI
retroinsular area
- ros
rostral sulcus
- SI
primary somatosensory cortex
- SII
secondary somatosensory cortex
- sbps
subparietal sulcus
- slas
superior limb of the arcuate sulcus
- spcs
superior precentral sulcus
- spocs
superior postcentral sulcus
- SSA
supplementary somatosensory cortex (area PEci)
- STG
superior temporal gyrus
- stn
subthalamic nucleus
- sts
superior temporal sulcus
- Sub
subiculum
- th
thalamus
- TMA
transitional motor area
- Tpro
temporal proisocoretex
- TSA
transitional sensory area
- v
ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen RA. Encoding of intention and spatial location in the posterior parietal cortex. Cereb Cortex. 1995;5:457–469. doi: 10.1093/cercor/5.5.457. [DOI] [PubMed] [Google Scholar]
- Arce FI, Lee JC, Ross CF, Sessle BJ, Hatsopoulos NG. Directional information from neuronal ensembles in the primate orofacial sensorimotor cortex. J Neurophysiol. 2013;110:1357–1369. doi: 10.1152/jn.00144.2013. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Bonin G, Bailey P. The neocortex of macaca mulatta. Urbana: University of Illinois Press; 1947. [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G. Ventral premotor neurons encoding representations of action during self and others’ in action. Curr Biol. 2014;24:1611–1614. doi: 10.1016/j.cub.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Casile A. Mirror neurons (and beyond) in the macaque brain: an overview of 20 years of research. Neurosci Lett. 2013;540:3–14. doi: 10.1016/j.neulet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Cirillo RA, Horel JA, George PJ. Lesions of the anterior temporal stem and the performance of delayed match-to-sample and visual discriminations in monkeys. Behavioral Brain Res. 1989;34:55–69. doi: 10.1016/s0166-4328(89)80090-7. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J Comp Neurol. 1999;403:431–458. [PubMed] [Google Scholar]
- Clarke S, Thiran AB, Maeder P, Adriani M, Vernet O, Regli L, Cuisenaire O, Thiran JP. What and where in human audition: selective defcits following focal hemispheric lesions. Exp Brain Res. 2002;147:8–15. doi: 10.1007/s00221-002-1203-9. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29:517–537. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Colombo M, D’Amato MR, Rodman HR, Gross CG. Auditory association cortex lesions impair auditory short-term memory in monkeys. Science. 1990;247:336–338. doi: 10.1126/science.2296723. [DOI] [PubMed] [Google Scholar]
- Colombo M, Rodman HR, Gross CG. The effects of superior temporal cortex lesions on the processing and retention of auditory information in monkeys (Cebus apella) J Neurosci. 1996;16:4501–4517. doi: 10.1523/JNEUROSCI.16-14-04501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padburg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Evrard HC, Logothetis NK, Craig AD. Modular architectonic organization of the insula in the macaque monkey. J Comp Neurol. 2014;522:64–97. doi: 10.1002/cne.23436. [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiol. 2008;23:171–179. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Mackey S, Petrides M. Cortico-cortical connections of areas 44 and 45B in the macaque monkey. Brain Lang. 2014;131:36–55. doi: 10.1016/j.bandl.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- Galletti C, Gamberini M, Kutz DF, Fattori P, Luppino G, Matelli M. The cortical connections of area V6: an occipitoparietal network processing visual information. Eur J Neurosci. 2001;13:1572–1588. doi: 10.1046/j.0953-816x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- Galletti C, Gamberini M, Kutz DF, Baldinotti I, Fattori P. The relationship between V6 and PO in macaque extrastriate cortex. Eur J Neurosci. 2005;21:959–970. doi: 10.1111/j.1460-9568.2005.03911.x. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Somatotopic representation in inferior area 6 of the macaque monkey. Brain Behav Evol. 1989;33:118–121. doi: 10.1159/000115912. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb Cortex. 2010;20:141–68. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct Funct. 2011;216:43–65. doi: 10.1007/s00429-010-0293-6. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Borra E, Mangiaracina C, Rozzi S, Luppino G. Corticostriate Projections from Areas of the “Lateral Grasping Network”: Evidence for Multiple Hand-Related Input Channels. Cereb Cortex. 2015 Jun 17; doi: 10.1093/cercor/bhv135. pii: bhv135. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Kuypers HG, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: an anatomical and electrophysiological study. Exp Brain Res. 1984;56:410–424. doi: 10.1007/BF00237982. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokuno H, Nambu A, Inoue T, Takada M. Input-output organization of jaw movement-related areas in monkey frontal cortex. J Comp Neurol. 2005;492:401–425. doi: 10.1002/cne.20730. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J Neurophysiol. 1991;66:390–413. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in the cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976;168:197–248. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Res. 2007;1148:90–95. doi: 10.1016/j.brainres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Kaas J. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Larson CR, Byrd KE, Garthwaite CR, Luschei ES. Alterations in the pattern of mastication after ablations of the lateral precentral cortex in rhesus monkeys. Exp Neurol. 1980;70:638–651. doi: 10.1016/0014-4886(80)90189-2. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal ablations. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA proper) and area 6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goodwin GM. Role of monkey precentral cortex in control of voluntary jaw movements. J Neurophysiol. 1975;38:146–157. doi: 10.1152/jn.1975.38.1.146. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Petrides M. Distinct parietal and temporal connectivity profiles of ventrolateral frontal areas involved in language production. J Neurosci. 2013;33:16846–16852. doi: 10.1523/JNEUROSCI.2259-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of macaque monkey. Behav Brain Res. 1985;18:125–137. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- McGuinness E, Sivertsen D, Allman JM. Organization of the face representation in macaque motor cortex. J Comp Neurol. 1980;193:591–608. doi: 10.1002/cne.901930302. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I: Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982c;212:38–52. doi: 10.1002/cne.902120104. 1982. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 4. New York: Plenum Press; 1985. pp. 179–226. [Google Scholar]
- Mishkin M, Ungerlider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Convergence of Limbic Lobe Input to the Cingulate Motor Cortex in the Rhesus Monkey. Brain Research Bulletin. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Schroeder CM, Keifer J. Organization of face representation in the cingulate cortex of the rhesus monkey. NeurpReport. 1996;7:1343–1348. doi: 10.1097/00001756-199605310-00002. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney M, Pandya DN. Cytoarchitecture and Cortical Connections of the Posterior Cingulate and Adjacent Somatosensory Fields in the Rhesus Monkey. Journal of Comparative Neurology. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Functional neuroanatomy of limbic structures and some relationships with prefrontal cortex. In: Fogel BS, Schiffer RD, Rao SM, editors. Neuropsychiatry. 2nd ed. Philadelphia: Williams and Wilkins; 2003. pp. 294–327. [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing W. The Motor Cortex and Facial Expression: New Insights from Neuroscience. The Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521:4205–4235. doi: 10.1002/cne.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Solon-Cline KM, Ge J, Darling WG. Cortical innervation of the hypoglossal nucleus in the non-human primate (Macaca mulatta) J Comp Neurol. 2014;522:3456–3484. doi: 10.1002/cne.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, Rotella DL, Darling WG. Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury. J Comp Neurol. 2015a;523:669–697. doi: 10.1002/cne.23703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, Rotella DL, Darling WG. Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the nonlesioned hemisphere in monkey (macaca mulatta) J Comp Neurol. in press doi: 10.1002/cne.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: afferent cortical input and comments on the claustrum. J Comp Neurol. 1982b;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Murray GM, Sessle BJ. Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. J Neurophysiol. 1992;67:747–758. doi: 10.1152/jn.1992.67.3.747. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Petrides M, Seltzer B, Cipolloni PB. Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept. Oxford University Press; 2015. [Google Scholar]
- Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z Anat Entwicklungsgesch. 1973;139 doi: 10.1007/BF00523634. 127-16.1. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of the posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Res. 1971;26:217–233. [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Prefrontal cortex in relation to other cortical areas in rhesus monkey: architecture and connections. Prog Brain Res. 1990;85:63–94. doi: 10.1016/s0079-6123(08)62676-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The rhesus monkey brain in stereotaxic coordinates. New York: Academic Press; 2000. [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Amsterdam: Elsevier; 1994. pp. 17–58. [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Processing of complex sounds in the auditory cortex of cat, monkey, and man. Acta Otolaryngol Suppl. 1997;532:34–38. doi: 10.3109/00016489709126142. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Parallel processing in the auditory cortex of primates. Audiol Neurootol. 1998;3:86–103. doi: 10.1159/000013784. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing “what” and “where” in auditory cortex. Proc Natl Acad Sci. 2000;97:11800–11806. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in macaque nonprimary auditory cortex. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Gentilucci M, Fogassi L, Luppino G, Matelli M, Ponzoni-Maggi S. Neurons related to goal-directed motor acts in inferior area 6 of the macaque monkey. Exp Brain Res. 1987;67:220–224. doi: 10.1007/BF00269468. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. 2014;94:655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- Roberts TS, Akert K. Insular and opercular cortex and its thalamic projection in Macaca mulatta. Schweitz Arch Neurol Neurochir Psychiatr. 1963;92:1–43. [PubMed] [Google Scholar]
- Sanides F. The architecture of the cortical taste nerve areas in the squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968;8:97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- Sanides F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann NY Acad Sci. 1969;167:404–423. [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna, editors. The primate brain, advances in primatology. Vol. 1. New York: Appleton-Century-Crofts; 1972. pp. 137–208. [Google Scholar]
- Schmidlin E, Brochier T, Maier MA, Kirkwood PA, Lemon RN. Pronounced reduction of digit motor responses evoked from macaque ventral premotor cortex after reversible inactivation of the primary motor cortex. J Neurosci. 2008;28:5772–5783. doi: 10.1523/JNEUROSCI.0944-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Posterior cingulate and retrosplenial cortex connections of the caudal superior temporal region in the rhesus monkey. 2009;195:325–334. doi: 10.1007/s00221-009-1795-4. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Cortico-cortical projections of the motorcortical larynx area in the rhesus monkey. Brain Res. 2002;949:23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Afferent cortical connections of the motor cortical larynx area in the rhesus monkey. Neurosci. 2002;130:133–149. doi: 10.1016/j.neuroscience.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in the monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Takada M, Nambu A, Inase M. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J Comp Neurol. 389:34–48. doi: 10.1002/(sici)1096-9861(19971208)389:1<34::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Galkin TW, Desimone R, Gattass R. Cortical connections of area V4 in the macaque. Cereb Cortex. 2008;18:477–499. doi: 10.1093/cercor/bhm061. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Umarova RM, Saur D, Schnell S, Kaller CP, Vry MS, Glauche V, Rijntjes M, Hennig J, Kiselev V, Weiller C. Structural connectivity for visuospatial attention: significance of ventral pathways. Cereb Cortex. 2010;20:121–129. doi: 10.1093/cercor/bhp086. [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeine Ergebnisse unserer Hirnforschung. J Psych Neurol. 1919;25:221–473. [Google Scholar]
- Wang Y, Shima K, Isoda M, Sawamura H, Tanji J. Spatial distribution and density of prefrontal cortical cells projecting to three sectors of premotor cortex. NeuroReport. 2002;13:1341–1344. doi: 10.1097/00001756-200207190-00025. [DOI] [PubMed] [Google Scholar]
- Woolsey CN. Organization of sensory and motor areas. In: Harlow HF, Woolsey CN, editors. Biological and biochemical bases of behavior. Madison, Wisconsin: University of Wiscons; in Press. 1958. pp. 63–81. [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Yao D, Yamamura K, Narita N, Martin RE, Murray GM, Sessle BJ. Neuronal activity patterns in primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol. 2001;87:2531–2541. doi: 10.1152/jn.2002.87.5.2531. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys. J Comp Neurol. 2010;518:3725–3751. doi: 10.1002/cne.22420. [DOI] [PubMed] [Google Scholar]