Abstract
BACKGROUND AND OBJECTIVES
Chronic rhinosinusitis (CRS) is a commonly diagnosed disease that has a significant impact on a child’s quality of life. However, no useful biomarker is available to identify antibiotic-responsive CRS. We determined the significance of eosinophil-related markers and total IgE levels in childhood CRS with regard to antibiotic response.
DESIGN AND SETTING
This was a case-control study of patients admitted to Uijeongbu St. Mary’s Hospital between November 2010 and November 2011 and diagnosed with CRS.
PATIENTS AND METHODS
In this analytical cross-sectional study, we identified children whose symptoms and radiologic abnormalities did not resolve after 12 weeks despite appropriate antibiotics (non-responder CRS group), children whose symptoms and radiologic abnormalities resolved after 12 weeks with appropriate antibiotics (responder CRS group), and healthy controls selected from clinic patients. Skin prick tests were performed along with serum total IgE, total eosinophil count (TEC), serum eosinophil cationic protein (ECP) level, and ImmunoCAP analysis for common allergens.
RESULTS
This study included 36 responders, 22 nonresponders and 22 healthy controls. The prevalence of allergic diseases, atopy, and a family history of allergic diseases were significantly higher in the non-responder group than in the responder and control groups. TEC, ECP, and total IgE levels were significantly higher in the non-responder group than in the responder and control groups (all P<.05). Multiple linear regression analysis showed that no response to appropriate antibiotics and TEC was positively associated with ECP concentration.
CONCLUSION
These findings suggest that there is a high prevalence of allergic diseases in the non-responder group, that the TEC and ECP levels in the non-responder group are significantly higher than those in the responder group and controls, and that no response to antibiotics may be due to eosinophilic inflammation. The measurement of serum ECP may be useful in monitoring the progress of childhood CRS with regard to antibiotic response.
Rhinosinusitis (RS) is one of the most commonly diagnosed diseases worldwide, affecting 15–20% of the pediatric population every year.1 It is characterized by inflammation of the nasal and paranasal sinus mucosa.2 The diagnosis is based upon the presence of typical symptoms and clinical examination findings. There are medications available that may relieve symptoms related to RS and its comorbidities such as allergic rhinitis (AR).
Several studies suggest a close correlation between AR and acute3 and chronic RS.4,5 Underlying conditions, including allergic diseases and specifically allergic rhinitis, are not only predisposing factors for CRS, but are also associated with more severe diseases and decreased rate of success following antibiotic therapy, if the “allergic components” are not contemporarily treated. 6,7 Therefore, allergy biomarkers, such as TEC, ECP and total serum IgE levels may predict patient response to antibiotic therapy. We assessed disease-specific marker at initial presentation. The aim of the present study was to determine the significance of eosinophil related markers and total IgE levels in childhood CRS with regard to antibiotic response.
MATERIALS AND METHODS
Participants
This study included patients younger than 15 years of age who presented with typical RS symptoms at the Department of Pediatrics, Uijeongbu Saint Mary’s Hospital, The Catholic University of Korea in Uijeongbu, South Korea, between November 2010 and November 2011. Healthy controls volunteered to participate in this study. All patients were evaluated according to the diagnostic criteria developed by consensus reports of the Joint Task Force on Practice Parameters5 and the Subcommittee on the Management of Sinusitis and Committee on Quality Improvement.7
RS was defined as the presence of two or more of the following symptoms that lasted at least 10 days: anterior and/or posterior mucopurulent drainage, nasal congestion or blockage, cough, facial pain, pressure, or dullness, or a reduced or absent sense of smell.5 Subjects who did not show anterior and/or posterior mucopurulent drainage were excluded in order to confirm the diagnosis of bacterial RS. An empirical regimen of amoxicillin (90 mg/kg) plus clavulanic acid (6.4 mg/kg) and second or third generation of cephalosporin with adjunctive therapy such as saline irrigation and nasal corticosteroids was given to all RS subjects. The RS subjects were followed up every 4 weeks. The subjects who required at least 12 weeks of antibiotics were considered to have CRS4 and thus recruited into the study. Antibiotic-responsive CRS was defined as CRS subjects with no residual respiratory symptoms and signs with improvement of radiologic abnormalities after 12 weeks of antibiotic treatment (responder CRS group). CRS that was non-responsive to antibiotic treatment was defined as CRS subjects with persistent residual respiratory symptoms and signs or radiologic abnormalities despite 12 weeks of antibiotic treatment (non-responder CRS group). All the patients older than 24 months of age in the non-responder group received anterior nasal endoscopy to evaluate the nasal cavity and associated structures. Gastrointestinal diseases, ciliary dyskinesia, bronchiectasis, or anatomical problems of the nose were diagnosed by confirmative tests, including radiological tests, respiratory mucosal biopsies, and 24-hour pH monitoring, when necessary. Nasal corticosteroid was prescribed and normal saline irrigation was recommended as adjunctive treatments to both responder and non-responder CRS subjects. Patients with a history of recurrent acute RS, history of prematurity, hematologic malignancy or immunologic deficiency were excluded. A respiratory healthy control group was selected from patients who visited the well baby clinic and those who visited the hospital for minor surgery without any history of medication. The following medications were not permitted in the time frame specified prior to entering the study: nasal and oral decongestants: 24 h; nasal or oral antihistamines: 72 h; nasal or inhaled corticosteroids, leukotriene receptor antagonists, 5-lipoxygenase inhibitors, methylxanthines, non-prescription drugs: 7 days; short course oral corticosteroids: 12 weeks; chronic oral corticosteroids: 6 months.8 Written informed consent was obtained from guardians or parents. The Institutional Review Board at The Catholic University approved this study.
Demographic and clinical data were recorded and assessed including the presence of physician-diagnosed allergic diseases, known anatomic problems of the nose, and family history of allergies. Allergic diseases were diagnosed based on skin prick tests and specific IgE assays in addition to patient history and physical examination.
Clinical parameters
Allergic skin-prick testing was performed with 8 common aeroallergens: house dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae), birch, mugwort, Alternaria alternata, dog, cat, and cockroach at the beginning of the study. Histamine at a concentration of 10 mg/mL was used as a positive control. A positive response to an allergen was defined as a wheal equal to or greater than that of the positive control. Total and specific IgE levels were measured using the Pharmacia CAP assay (Uppsala, Sweden). A specific IgE test was performed with six allergens common in Korea: Dermatophagoides pteronyssinus, Dermatophagoides farinae, egg whites, cow milk, German cockroach, and Alternaria alternata. A level ≥0.35 IU/mL was considered positive, as specified by the manufacturer. Atopy was defined as sensitization to one or more inhalant allergens in the above tests with clinical features of allergic diseases. We performed a stool exam to rule out parasite infestation in patients with eosinophilia.
Blood eosinophil markers and total IgE levels
To find a disease-specific marker, we assessed serum total IgE, aeroallergen specific IgE, total peripheral blood eosinophil counts (TEC), and serum eosinophil cationic protein (ECP) levels in children with CRS at initial presentation. The number of peripheral blood eosinophils was counted with blood samples containing EDTA using an automated hematology analyzer (Model: XE2100 D, Sysmex, Japan). Serum total IgE level was measured using ImmumoCAP tests (Phadia AB, Uppsala, Sweden) according to the manufacturer’s instructions. Serum ECP level was measured using a commercially available fluoroimmunoassay kit (Phadia AB, Uppsala, Sweden), which had a detection limit of less than 2.0 μg/L. Blood sample collection, serum preparation, and serum ECP measurement were performed at the beginning of the study according to the manufacturer’s instructions.
Imaging studies
Waters’ view x-rays were performed in all responder CRS patients at the beginning of the study to determine the radiopacity in the paranasal sinuses. A lateral X-ray view of the skull was obtained to assess adenoid hypertrophy when clinically indicated. Anatomic anomalies of the nose were diagnosed by performing confirmative tests, including radiological tests, in all patients in the non-responder CRS group. Waters’ view x-rays were performed in all CRS patients at the end of the study to determine the clearance of radiopacity in the paranasal sinuses. Coronal CT scans in bone window settings were performed at the end of the study to determine in all the CRS patients who showed abnormalities in Waters’ view x-ray to confirm the presence of abnormal findings in the paranasal sinuses.9
Determination of response to antibiotics
Response to antibiotic therapy was determined by complete clearing of the initial radiologic abnormalities or, in cases of mucosal thickening, by a significant decrease in thickness to <6 mm within the maxillary sinuses that was associated with improvement of the clinical signs and symptoms of RS.10
Statistical analysis
All continuous variables are presented as means and standard deviations. ECP was approximately normally distributed, and therefore analysis of variance (ANOVA) was used for analysis. TEC and total IgE were not normally distributed, and therefore the Kruskal–Wallis and Mann–Whitney U two-tailed tests were used for analysis. The chi-square test was used to examine differences between categorical variables between groups. The Spearman test was used for correlation analysis. To examine independent correlates of serum ECP concentrations, multiple regression analysis was conducted with ECP level as the dependent variable. All statistical analyses were performed with IBM SPSS Statistics for Windows, Ver. 21.0 (IBM Corp., Armonk, NY, USA). All statistical tests were two-sided and statistical significance was determined at a P value <.05.
RESULTS
A total of 36 non-responder patients, 22 responder patients, and 22 controls were included in the final analysis.
Characteristics of study participants
Eighty subjects including 36 non-responder CRS patients, 22 responder CRS patients, and 22 controls were recruited and included in the final analysis. The clinical characteristics of each group are shown in Table 1. There were no significant differences by age or sex. The prevalence of allergic diseases such as asthma, allergic rhinitis, and atopic dermatitis and a family history of allergies were significantly higher in the non-responder CRS group as compared to the responder CRS and control groups. The control subjects were found to have no atopy by skin prick or blood tests. There were 24 (77.4%) atopic patients in the nonresponder group and 9 (45.0%) atopic patients in the responder group. Saline irrigation and nasal corticosteroids were not successful in either the atopic and nonatopic non-responder groups (data not shown). No patients in the non-responder group received surgery.
Table 1.
Clinical characteristics of subjects.
| Non-responder CRS group (n=36) | Responder CRS group (n=22) | Control (n=22) | P value | |
|---|---|---|---|---|
|
| ||||
| Age, y | 5.8 (3.0) | 5.6 (2.7) | 4.3 (1.8) | .089 |
| Sex (M/F) | 25/11 | 13/9 | 14/8 | .716 |
| Allergic rhinitis | 14/30 | 5/18 | 0/21 | <.001 |
| Asthma | 9/30 | 3/18 | 0/22 | <.001 |
| Atopic dermatitis | 13/30 | 10/17 | 2/22 | .004 |
| Atopya | 24/31 | 9/20 | 0/22 | <.001 |
| Der f or Der p positivity | 21/31 | 6/19 | 0/20 | <.001 |
| Family history of allergies | 21/30 | 2/17 | 6/22 | <.001 |
Values are expressed as actual numbers or mean (standard deviation).
Atopy: Positivity to ImmunoCAP or skin prick test.
CRS, chronic rhinosinusitis.
Comparison of serum eosinophil cationic protein levels, total eosinophil count, serum total IgE in the three groups
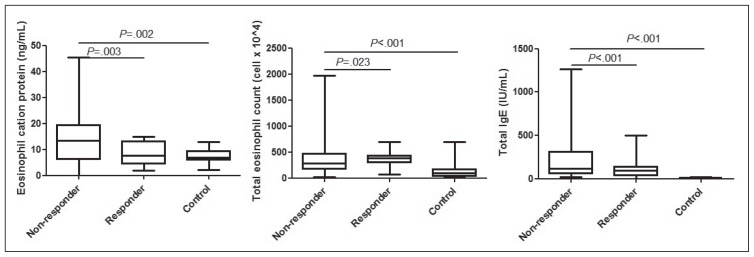
All the biomarkers of allergic sensitization, i.e. total serum IgE, total eosinophil count and ECP levels were significantly elevated (P<.001, P=.006 and P<.001, respectively) in children whose symptoms (and radiologic abnormalities) did not resolve after antibiotics (Figure 1). After CRS was stratified by atopy, atopic non-responders had higher ECP and total IgE levels than the atopic responders (P=.007 and P=.011, respectively) (Table 2). No significant differences were observed in ECP and TEC between the non-atopic non-responders and the non-atopic responders, except total IgE levels (Table 2). There were 2 patients who were sensitized to birch and mugwort in our study and they were enrolled in November, which is not during seasonal allergy period in Korea (data not shown).
Figure 1.
Comparison of eosinophil cationic protein levels (A), total eosinophil count (B), and total IgE (C) in the three groups. The bottom and top of the boxes are the first and third quartiles, and the band inside the box is always the second quartile (the median). The ends of the whiskers represent the minimum and maximum of all of the data.
Table 2.
Comparisons between the atopic responders and the atopic non-responders and between the non-atopic responders and the non-atopic non-responders in total eosinophil counts, eosinophil cationic protein levels, and total IgE.
| Atopic CRS | P value | Non-atopic CRS | P value | |||
|---|---|---|---|---|---|---|
| Non-responders (n=24) | Responders (n=10) | Non-responders (n=7) | Responders (n=11) | |||
|
| ||||||
| ECP (μg/L) | 18.9 (10.7) | 9.1 (3.7) | .007 | 12.7 (8.4) | 8.5 (4.6) | .341 |
| TEC (cells/mm3) | 350.0 (250.0–710.0) | 335.0 (250.0–452.5) | .691 | 240.0 (150.0–570.0) | 350.0 (200.0–460.0) | .586 |
| Total IgE (IU/mL) | 365.0 (165.3–840.0) | 105.5 (29.9–272.3) | .011 | 45.0 (22.7–60.7) | 0.0 (0.0–18.0) | .028 |
Values for ECP are expressed as mean (standard deviation), TEC and total IgE are expressed as median (interquartile range).
Atopy: Positivity to ImmunoCAP or skin prick test.
CRS, chronic rhinosinusitis; ECP, eosinophil cationic protein; TEC, total eosinophil count. Atopic non-responders had higher ECP and total IgE levels than the atopic responders (P=.007 and P=.011, respectively).
Relationships between serum eosinophil cationic protein levels and total eosinophil counts, between total eosinophil count and IgE, and between serum eosinophil cationic protein levels and IgE in the non-responder chronic rhinosinusitis group
Independent correlates of serum ECP concentrations and multiple regression analysis was conducted with ECP level as the dependent variable. In addition, significant correlations were found in the non-responder CRS group between ECP and total eosinophil counts (P=.003), total eosinophil count and total IgE (P=.016) and ECP and total IgE (P=.026). Multiple regression analysis showed that no response to antibiotic therapy and total eosinophil counts were positively associated with serum ECP concentrations (Table 3). No correlations were found in the responder CRS group or in the controls between ECP, TEC, and total IgE (data not shown).
Table 3.
Results of multiple regression analysis assessing relationships between eosinophil cationic protein concentration and clinical variables.a
| Variable | β | SE | P value |
|---|---|---|---|
|
| |||
| Sex (male) | −3.003 | 2.513 | .239 |
| Age (year) | −0.171 | 0.434 | .696 |
| History of allergic disease | 3.028 | 3.081 | .332 |
| Non-responder patients (yes) | 6.118 | 2.928 | .043 |
| Total eosinophil count | 0.013 | 0.004 | .005 |
| Total IgE | 0.0008 | 0.004 | .837 |
| Atopyb | 1.376 | 2.754 | .620 |
Multiple linear regression analysis included sex, age, past history of allergic disease, non-responder patients (yes), total eosinophil count, total IgE, and atopy.
Atopy: Positivity to ImmunoCAP or skin prick test.
Radiologic findings
Coronal CT scans in bone window settings obtained in the non-responder CRS group revealed mucosal thickening in all patients, a finding suggestive of mucosal inflammation in the paranasal sinuses. No subjects were found to have anatomical abnormalities on coronal CT images.
DISCUSSION
In this study, we found that TEC, ECP, and total IgE levels in the non-responder CRS group were significantly higher than those in the responder CRS group and the controls. Additionally, non-responder CRS patients with positive biomarkers of allergic sensitization who showed no response to appropriate antibiotics should be treated with additional treatments such as antihistamines, oral or inhaled corticosteroids.
The high prevalence of allergic diseases and atopy in the CRS patients, especially in the non-responder CRS group, in the present study is in accordance with previous studies which reported that nasal allergic inflammation may favor viral infections in the upper airways, leading to acute and eventually chronic sinusitis.11,12
There are several possible explanations to account for the group of CRS patients that did not respond to appropriate antibiotic therapy. First, the non-responder CRS patients may have been infected with microbes that protect against antibiotics using mechanisms such as biofilms in the mucosal linings of the sinuses.13 However, if saline irrigation and nasal corticosteroids were successful in the treatment of the non-responder CRS group, the hypothesis that biofilm formation is involved in the failure of antibiotic treatment is unlikely, while it is possible that ECP might be released from eosinophils in the nasal mucosa and stimulate the release of TGF-α in atopic children with CRS.14 TGF-α synthesis and release is known to be inhibited by corticosteroids.14 However, in our study, no evidence of remodeling of the sinus extracellular matrix was seen by CT scan in the non-responder CRS group.14 Second, it is speculated that the inflammatory response to bacteria is responsible for the pathogenesis of CRS rather than the action of microbes in adults.15 The finding of a sinus mucosal infiltrates of eosinophils, plasma cells, and lymphocytes suggests a process of “bacterial allergy.”16 In clinical practice, it is possible that the spectrum of the illness ranges from infectious etiologies such as bacterial infection to exclusively noninfectious inflammatory conditions such as AR.17 Therefore, having concomitant AR could be a reasonable explanation to account for how those with CRS did not respond to appropriate antibiotic therapy. This is reflected by the finding in the present study that non-responder CRS patients had a significantly higher prevalence of allergic disease and atopy compared with those in the responder CRS patients and controls. ECP levels in the serum and nasal cavity are known to reflect ongoing eosinophilic nasal inflammation with a significant correlation between serum and nasal cavity levels of ECP.18–20 A previous study demonstrated that an allergen challenge of the nose led to a significant increase in maxillary sinus eosinophils and ECP level, suggesting that they can be used to monitor maxillary sinus inflammation. 13 Although the present study did not measure eosinophil count or ECP level in the maxillary sinus or the nasal cavity, we observed a significant increase in the serum ECP levels of the non-responder CRS patients as compared with the responder CRS patients and the controls. The high serum ECP levels in the non-responder CRS patients may be a result of the fact that most of these patients had concomitant AR and an already high sensitization to common inhalant allergens such as house dust mite. This hypothesis is supported by the finding in the present study that higher ECP levels were observed in the atopic non-responders than in the atopic responders and by the multivariate linear regression analysis showing serum ECP levels were positively associated with no response to appropriate antibiotic therapy. Within the non-atopic CRS group, non-responders showed a higher level of ECP than the responder group, although they failed to achieve statistical significance. Further study will be needed to elucidate the relationship between ECP and antibiotics response.
Study patients with CRS were classified into the non-responder group and the responder group with respect to antibiotic response and improvement of radiologic abnormality based on a previous study.21 The reason for utilizing this classification is that we aimed to determine whether serum ECP, which is released from activated eosinophils during inflammation,22 can be used to differentiate antibiotic-responsive CRS patients from those that are non-responsive to antibiotic treatment. Based on the findings of the present study, we speculate that ECP may be a more useful surrogate marker of vigorous eosinophilic inflammation than TEC. Further, if the ECP level is high in patients with CRS, clinicians can start early treatment with alternative options such as nasal steroids, saline nasal irrigation, and antileukotrienes to reduce nasal eosinophilic inflammation in addition to antibiotic therapy for bacterial infection. Even though ECP is costlier than total IgE or TEC, ECP can predict antibiotics response more accurately than TEC or total IgE, and the cost can be neutralized by the appropriate prescription of antibiotics.
Our study had limitations. First, much to our regret, we measured serum ECP level only once at the beginning of the study. If we had also measured ECP level at the end of the study and observed changes in ECP level between groups, we could have presented more convincing results. Second, we did not measure TGF-α level in our subjects, nor perform nasal mucosal biopsy; however, coronal CT scans were performed in all nonresponder CRS patients to determine the presence of abnormal findings, suggestive of mucosal inflammation in the paranasal sinuses. Lastly, we did not differentiate between perennial or seasonal allergic rhinitis.
In conclusion, childhood CRS that is not responsive to appropriate antibiotic therapy is often caused by eosinophilic inflammation reflected by elevated serum ECP levels. Monitoring serum ECP concentration may be useful for identifying eosinophil-mediated sinus inflammation that is not likely to respond to antibiotics. Clinicians should take this into account when making treatment plans for children with CRS.
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Zacharisen M, Casper R. Pediatric sinusitis. Immunol Allergy Clin North Am. 2005;25:313–32. vii. doi: 10.1016/j.iac.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.DeMuri GP, Wald ER. Clinical practice. Acute bacterial sinusitis in children. N Engl J Med. 2012;367:1128–34. doi: 10.1056/NEJMcp1106638. [DOI] [PubMed] [Google Scholar]
- 3.Savolainen S. Allergy in patients with acute maxillary sinusitis. Allergy. 1989;44:116–22. doi: 10.1111/j.1398-9995.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 4.Steinke JW, Borish L. The role of allergy in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2004;24:45–57. doi: 10.1016/S0889-8561(03)00108-5. [DOI] [PubMed] [Google Scholar]
- 5.Slavin RG, Spector SL, Bernstein IL, Kaliner MA, Kennedy DW, Virant FS, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S13–47. doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan HH, Fornelli R, Ortiz AO, Rodman S. Correlation of allergy and severity of sinus disease. Am J Rhinol. 1999;13:345–7. doi: 10.2500/105065899781367500. [DOI] [PubMed] [Google Scholar]
- 7.Mori F, Barni S, Pucci N, Rossi ME, Orsi Battaglini C, Novembre E. Upper airways disease: role of corticosteroids. Int J Immunopathol Pharmacol. 2010;23:61–6. [PubMed] [Google Scholar]
- 8.Murdoch RD, Bareille P, Ignar D, Miller S, Gupta A, Boardley R, Zieglmayer P, Zieglmayer R, Lemell P, Horak F. The improved efficacy of a fixed dose combination of fluticasone furoate and levocabastine relative to the individual components in the treatment of allergic rhinitis. Clin Exp Allergy. 2015 Apr 21; doi: 10.1111/cea.12556. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Clinical practice guideline: management of sinusitis. Pediatrics. 2001;108:798–808. doi: 10.1542/peds.108.3.798. [DOI] [PubMed] [Google Scholar]
- 10.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 11.Dohlman AW, Hemstreet MP, Odrezin GT, Bartolucci AA. Subacute sinusitis: are antimicrobials necessary? J Allergy Clin Immunol. 1993;91:1015–23. doi: 10.1016/0091-6749(93)90214-z. [DOI] [PubMed] [Google Scholar]
- 12.Stokken J, Gupta A, Krakovitz P, Anne S. Rhinosinusitis in children: a comparison of patients requiring surgery for acute complications versus chronic disease. Am J Otolaryngol. 2014;35:641–6. doi: 10.1016/j.amjoto.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Young LC, Stow NW, Zhou L, Douglas RG. Efficacy of medical therapy in treatment of chronic rhinosinusitis. Allergy Rhinol (Providence) 2012;3:e8–e12. doi: 10.2500/ar.2012.3.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bystrom J, Patel SY, Amin K, Bishop-Bailey D. Dissecting the role of eosinophil cationic protein in upper airway disease. Curr Opin Allergy Clin Immunol. 2012;12:18–23. doi: 10.1097/ACI.0b013e32834eccaf. [DOI] [PubMed] [Google Scholar]
- 15.Lotvall J, Ekerljung L, Lundback B. Multi-symptom asthma is closely related to nasal blockage, rhinorrhea and symptoms of chronic rhinosinusitis-evidence from the West Sweden Asthma Study. Respir Res. 2010;11:163. doi: 10.1186/1465-9921-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foreman A, Boase S, Psaltis A, Wormald PJ. Role of bacterial and fungal biofilms in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2012;12:127–35. doi: 10.1007/s11882-012-0246-7. [DOI] [PubMed] [Google Scholar]
- 17.Ramadan HH. Chronic rhinosinusitis in children. Int J Pediatr. 2012;2012:573942. doi: 10.1155/2012/573942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Meng J, Qiao X, Liu Y, Liu F, Zhang N, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:1061–8. doi: 10.1016/j.jaci.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Mortuaire G, Gengler I, Vandenhende-Szymanski C, Delbeke M, Gatault S, Chevalier D, et al. Immune profile modulation of blood and mucosal eosinophils in nasal polyposis with concomitant asthma. Ann Allergy Asthma Immunol. 2015;114:299–307.e2. doi: 10.1016/j.anai.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Kim KS, Won HR, Park CY, Hong JH, Lee JH, Lee KE, et al. Analyzing serum eosinophil cationic protein in the clinical assessment of chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27:e75–80. doi: 10.2500/ajra.2013.27.3901. [DOI] [PubMed] [Google Scholar]
- 21.Serrano CD, Valero A, Bartra J, Roca-Ferrer J, Munoz-Cano R, Sanchez-Lopez J, et al. Nasal and bronchial inflammation after nasal allergen challenge: assessment using noninvasive methods. J Investig Allergol Clin Immunol. 2012;22:351–6. [PubMed] [Google Scholar]
- 22.Wald ER, Byers C, Guerra N, Casselbrant M, Beste D. Subacute sinusitis in children. J Pediatr. 1989;115:28–32. doi: 10.1016/s0022-3476(89)80324-5. [DOI] [PubMed] [Google Scholar]