Abstract
The interstitial lung diseases (ILDs) are a diverse group of disorders characterized by a varying combination of inflammation and fibrosis of the pulmonary parenchyma. Treatment and prognosis of ILD typically depend on the underlying ILD subtype, highlighting the importance of accurate classification and diagnosis. Besides a thorough history and clinical examination, the protocol should include a 6-minute walk test, chest radiography, high-resolution computed tomography, biochemical analysis, pulmonary function tests, blood gas analysis, bronchoalveolar lavage, and, when necessary, a lung biopsy. The final diagnosis of ILD entities requires dynamic interaction between clinicians, radiologists and pathologists to reach a clinico-radiologic-pathologic diagnosis, the gold standard no longer being the histology but rather a multidisciplinary approach.
Keywords: interstitial lung disease, pulmonary fibrosis, usual interstitial pneumonia, nonspecific interstitial pneumonia, diagnosis
Introduction
The term interstitial lung disease (ILD) refers to a group of disorders that are characterized by a varying combination of inflammation and fibrosis involving the space between the epithelial and endothelial basement membranes [1,2]. Treatment and prognosis of ILD typically depend on the underlying ILD subtype, highlighting the importance of accurate classification and diagnosis [3]. The ILDs are classified together due to their similar clinical, radiologic, physiologic, and/or pathologic manifestations [4], and currently an etiology-based classification system based on multidisciplinary diagnosis is the cornerstone of ILD management [3]. Main categories include ILD related to environmental exposures, connective tissue disease (CTD)-related ILD, sarcoidosis, and the idiopathic interstitial pneumonias (IIPs) [e.g., idiopathic pulmonary fibrosis (IPF), characterized by the morphologic pattern of usual interstitial pneumonia (UIP), and nonspecific interstitial pneumonia (NSIP)] [3]. The 2002 American Thoracic Society/European Respiratory Society (ARS/ERS) Classification [5] has been recently updated, distinguishing major IIPs from rare IIPs [6,7]. The detailed classification together with specific examples for each category are presented in Table I.
Table I.
American Thoracic Society/European Respiratory Society classification of interstitial lung disease.
| Exposure-related | hypersensitivity pneumonitis, pneumoconiosis | |
| CTD-related | rheumatoid arthritis, systemic sclerosis, polymyositis, dermatomyositis, systemic lupus erythematosus, mixed connective tissue diseases | |
| Sarcoidosis | ||
| Idiopathic | Major | Chronic fibrosing: IPF, NSIP Smoking related: RB-ILD, DIP Acute/subacute: COP, AIP |
| Rare | LIP, PPFE | |
| Other | vasculitis, diffuse alveolar hemorrhage, Langerhans cell histiocytosis, eosinophilic pneumonia, neurofibromatosis, lymphangioleiomyomatosis | |
AIP - acute interstitial pneumonia; COP - cryptogenic organizing pneumonia; CTD - connective tissue disease; DIP - desquamative interstitial pneumonia; IPF - idiopathic pulmonary fibrosis; LIP - lymphoid interstitial pneumonia; NSIP - nonspecific interstitial pneumonia; PPFE - pleuro-parenchymal fibroelastosis; RB-ILD - respiratory bronchiolitis-associated interstitial lung disease.
Although 50%–60% of patients with IIP receive a diagnosis of UIP, NSIP is the second most common cause of IIP, accounting for 14%–36% of cases [8]. NSIP was accepted as a subtype only in 2008 [4,9]. Distinction of idiopathic NSIP from IPF is important because NSIP has a considerably better prognosis, the 5-year survival being approximately 80% compared to approximately 30% for IPF [10].
The annual incidence of IPF in the USA is estimated at 6.8–8.8 per 100.000, while in the UK the annual incidence is between 0.22–7.4 per 100.000 [7]. It occurs predominantly in older adults, with a prevalence of 20.2 men per 100.000 and 13.2 women per 100.000. It has been estimated that approximately 40.000 new patients will be diagnosed with IPF each year in Europe [11]. A recent study analyzing a group of 178 cases with ILD from a tertiary care hospital in Romania showed a frequency of 23% for IPF and 16% for CTD-related ILD. The lower occurrence of IPF, as compared to the general European and US incidence, seems to be related to the under-referral and under-diagnosis of the disease [12].
This review aims to provide a comprehensive overview on ILD diagnosis, with special focus on Idiopathic Pulmonary Fibrosis and Nonspecific Interstitial Pneumonia. Establishing an accurate diagnosis is challenging and requires an integrated multidisciplinary approach involving pulmonologists, radiologists and pathologists. In addition to a thorough history and clinical examination, the protocol should include a 6-minute walk test (6MWT), chest radiograph, high-resolution computed tomography (HRCT), biochemical analysis, pulmonary function tests (PFT), blood gas analysis, bronchoalveolar lavage (BAL), tuberculin test, transthoracic echocardiography, an electrocardiogram and, when necessary, a lung biopsy. Other biochemical tests may be added in a suggestive clinical context [2].
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, life-threatening, progressively fibrosing idiopathic interstitial pneumonia [13], which impairs functional status and quality of life [4,14,15]. It is an usual interstitial pneumonia of unknown cause: no chronic occupational or environmental exposure, clinical evidence of CTD, or history of radiation therapy and drug exposure known to cause ILD (nitrofurantoin, methotrexate, cyclophosphamide, or any of a variety of chemotherapeutic drugs) [16,17]. When assessed by expert clinicians and radiologists, in the presence of typical clinical and HRCT features, the sensitivity and specificity for the diagnosis of IPF range 60–80% [18,19].
The revised diagnostic criteria for IPF are: exclusion of other known causes of ILD; presence of a UIP pattern on HRCT scans in individuals not subjected to surgical lung biopsy (SLB); and specific combinations of HRCT and SLB pattern in individuals subjected to SLB [13]. In the absence of SLB, all the major criteria and at least 3 of the 4 minor criteria showed in Table II. are required for a diagnosis of IPF [20].
Table II.
American Thoracic Society/European Respiratory Society Criteria for Diagnosis of IPF in the Absence of Surgical Lung Biopsy.
| Major criteria | Exclusion of other known causes of interstitial lung disease (toxic effects of certain drugs, environmental exposures, and connective tissue diseases) Abnormal results of PFT, including evidence of restriction (reduced vital capacity, often with an increased FEV1/FVC ratio) and impaired gas exchange (decreased Pao2 with rest or exercise, or decreased diffusing capacity of lung for carbon monoxide) Bibasilar reticular abnormalities with minimal ground-glass opacities at HRCT TBLB or BAL shows no features to support an alternative diagnosis |
| Minor criteria | Age >50 years Insidious onset of otherwise unexplained dyspnea on exertion Duration of illness > 3 months Bibasilar inspiratory crackles (dry or “Velcro” type) |
BAL - bronchoalveolar lavage; FEV1 - forced expiratory volume in 1 second; FVC - forced vital capacity; HRCT - high-resolution computed tomography; Pao2 - arterial partial pressure of oxygen; PFT – pulmonary function tests; TBLB - transbronchial lung biopsy.
Although recent advances have been made in the understanding of the underlying pathogenesis of IPF, no medical therapy has been shown to improve respiratory symptoms or functional status, or to prolong survival [14]. The hypothesis based on the presence of an inflammatory process has been abandoned due to the lack of efficacy of anti-inflammatory therapy in the treatment of IPF and lung biopsy results without inflammation [21]. The recurrent or persistent alveolar epithelial injury with dysregulated repair was proposed instead to explain disease development [7].
The two patterns of decline in lung function described in IPF are represented by a slow and insidious or by an episodic and more severe evolution. When an episode of decline progresses so fast that it demands clinical attention, the term acute exacerbation of IPF has been applied [22]. A consensus paper has suggested the following criteria for acute exacerbation: unexplained worsening or development of dyspnoea within a month; development of new bilateral ground-glass opacities or consolidation on CT scan; and absence of an alternative explanation such as heart failure, infection or pulmonary embolism [23].
Usual Interstitial Pneumonia
Usual interstitial pneumonia (UIP) is the most common histopathological form of diffuse lung fibrosis occurring in older adults, first described by Liebow in 1969 [16]. Clinical conditions associated with a histopathological pattern of UIP are: IPF, CTD, drug toxicity, chronic hypersensitivity pneumonitis, asbestosis, familial idiopathic pulmonary fibrosis, Hermansky–Pudlak syndrome [13].
The high-resolution computed tomography imaging features of UIP are characterized by reticular opacities, often associated with traction bronchiectasis, honeycombing manifested as mainly subpleural cluster cystic airspaces, with well-defined thickened walls, and the absence of additional radiological features considered incompatible with a diagnosis of IPF [24,25]. Ground-glass opacities are common, but less extensive than the reticulation, with a characteristically patchy basal and peripheral distribution [13,26].
The UIP pattern of fibrosis is histologically characterized by two key features: spatial heterogeneity, which refers to a patchy distribution of dense parenchymal scar alternating with areas of less affected or normal parenchyma; temporal heterogeneity, which reflects different stages in the evolution of fibrosis, a combination of old and active lesions [10,16].
Nonspecific Interstitial Pneumonia
Nonspecific interstitial pneumonia (NSIP) is a chronic interstitial lung disease characterized by relatively homogeneous expansion of the alveolar walls by inflammation and/or fibrosis. NSIP may be idiopathic, but more commonly occurs as a manifestation of CTD; it can be associated with toxic effects of drugs, occupational exposure, hypersensitivity pneumonitis, infections, chronic aspiration, granulomatous disease or chronic interstitial lung disease complicating diffuse alveolar damage [8,10,27].
NSIP is characterized by spatial and temporal homogeneity of parenchymal lung involvement [4]. The histology ranges from a predominantly inflammatory process (cellular NSIP) to predominant fibrosis (fibrotic NSIP) [10]. The diagnosis of NSIP without biopsy is highly inaccurate, with ~50% of cases being missed [27]. Ground-glass attenuation alone or mixed with consolidation is the most common CT feature. The distribution of ground-glass opacities is often symmetric with involvement of the middle and lower zones or the lower zones alone, with possible sparing of the subpleural lung [28]. Cellular NSIP is much less common than fibrotic NSIP [8]. However, patients with NSIP and only ground-glass opacities on HRCT typically have cellular NSIP [10].
Connective Tissue Disease
Involvement of the respiratory system is common in connective tissue disease (CTD) and results in significant morbidity and mortality [29]. In general, the features of pulmonary fibrosis owing to CTD are similar to those of idiopathic interstitial pneumonias [30]. All components of the lung can be affected, including the interstitium, the large and small airways, the pleura and the pulmonary vasculature. A combination of patterns is frequently seen [31]. The lung disease associated with CTD may precede the clinical presentation of the collagen disease, sometimes by more than 5 years [29]. ILD can occur in all of the CTDs, with the highest frequency observed in systemic sclerosis and the lowest in systemic lupus erythematosus [31,32]. Overall, CTD-related ILDs are more often associated with NSIP than UIP as compared to idiopathic interstitial penumonias. NSIP is seen more frequently in systemic sclerosis, polymyositis, dermatomyositis, and mixed connective tissue diseases, while rheumatoid arthritis is more frequently associated with UIP [33]. In CTD, ground-glass opacities may reflect inhomogeneous lung ventilation or perfusion as well as infection or drug toxicity [34].
Clinical presentation
The clinical presentations of idiopathic interstitial pneumonias and CTD-related ILD are similar, with the typical patient presenting with insidious onset of dyspnea, sometimes associated with a nonproductive cough, and having basal end-inspiratory dry rales on auscultatory examination [21,22,33,35]. Asymptomatic, early lung fibrosis has been increasingly recognized and reported in individuals with familial pulmonary fibrosis, especially in those with a history of smoking [36]. It is well recognized that symptoms precede diagnosis by a median of 1 to 2 years [37], and radiographic evidence of disease may even precede symptoms, suggesting “subclinical” periods of disease [36,37]. Patients may eventually develop cor pulmonale, death usually coming as consequences of respiratory insufficiency and anoxia [33].
There is a strong association between gastro-esophageal reflux and IPF. Although a causal relationship is unclear, it has been hypothesized that gastroesophageal reflux may be a risk factor for microaspiration, which may be important in the pathogenesis and natural history of IPF [36].
In general, patients with NSIP experience slowly develop shortness of breath. Other common symptoms include nonproductive cough, fatigue, malaise, anorexia, and weight loss; low grade fever has also been reported. Clubbing was reported in 10% of the patients with NSIP and in 65% of IPF cases [38,39].
6 Minute Walk Test
The 6 Minute Walk Test (6MWT) is a simple and easily performed assessment of a subject’s submaximal functional capacity. It measures the distance a patient can walk on a flat surface over a period of six minutes and has become the most widely used exercise test, given its ease of administration and reproducibility [14,36].
For patients with IPF, the 6MWT appears to be a valid reflection of global functional capacity and it is frequently used clinically to assess changes in disease status over time [14]. Several studies suggested that the 6MWT is a reliable and responsive measure of exercise tolerance in patients with IPF and that the minimal clinically important difference (the smallest change in distance that patients can perceive as different from the previous test and that would mandate a change in management) in 6-minute-walk distance is between 24 and 45 meters [14,15,36].
High-Resolution Computed Tomography
High-Resolution Computed Tomography (HRCT), nowadays usually performed with a multidetector CT scanner, is currently the primary imaging modality for the detection, diagnosis and follow-up of ILD, especially when typical CT patterns of the disease are present. When CT signs are atypical, a diagnosis may only be possible after thorough clinico-radiologic correlation and, in case of discordance, a lung biopsy may be indicated, with a further multidisciplinary approach [15,40].
The ability to diagnose UIP with HRCT has been well established, but the ability to diagnose NSIP with HRCT has consistently proved more challenging [8,41–43]. Several studies have been evaluating the accuracy of HRCT in the diagnosis of UIP and NSIP, in patients with biopsy-proven disease. For UIP, the sensitivity and specificity range between 48–78% and 70–95%, respectively [44,45], whereas in NSIP these vary around 70% and 60% [28]. Sensitivity and specificity for diagnosis seem to be lower in patients with concurrent emphysema [40,46,47].
CT patterns that are atypical for UIP include micronodules, mosaic attenuation/air trapping, nonhoneycomb cysts, more extensive ground-glass opacities than reticular abnormality, consolidation, or a peribronchovascular-predominant distribution [48]. The key CT features that favor the diagnosis of NSIP over UIP are homogeneous lung involvement without an obvious apicobasal gradient, extensive ground-glass abnormalities, a finer reticular pattern, and micronodules [20].
Pulmonary function tests and laboratory tests
The British Thoracic Society guidelines [49] recommend the following tests: a complete blood cell count with differential leukocyte counts, renal function tests, electrolytes, liver function tests, and urinalysis (including dipstick). Other tests, including anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, erythrocyte sedimentation rate, angiotensin-converting enzyme, a 24-h urine collection, and IgG antibodies, are meant to rule out an underlying CTD [2].
PFT are simple, widely available, and often used in the evaluation and management of patients with ILD, particularly IPF and NSIP [1]. PFT should include spirometry, diffusing capacity for carbon monoxide (DlCO), and arterial blood gas study [2]. Potential clinical applications include: 1) aid in the diagnosis of ILD, although the pattern of abnormality is nonspecific; 2) provide a rough estimate of histologic severity but do not provide definitive quantification of histologic fibrosis or inflammation; 3) baseline PFTs may provide an estimate of prognosis; 4) serial PFTs provide valuable information in determining disease progression and response to therapy. Forced vital capacity (FVC) and DlCO are the most valuable serial measurements [1].
PFT most consistently reveals restrictive ventilatory pattern, with decreased total lung capacity, FVC, and DlCO. Functional decline may be episodic and unpredictable, with long periods of apparent stability. With each decline, patients may stabilize but never experience improvement in measured function [22].
In IPF, gas exchange is impaired with an increased alveolar-arterial gradient for partial pressure of oxygen. Even when the resting partial arterial pressure of oxygen is normal, an exercise-induced decrease in arterial oxygen saturation is commonly observed [17].
Several epithelial or macrophage-related proteins, including surfactant protein SP-A and SP-D, Krebs von den Lungen-6 (KL-6), C-C chemokine, and matrix metalloproteinase, appear elevated in both blood and BAL fluid and have been associated with an adverse outcome in patients with IPF [50]. Fibrocytes, mesenchymal cell progenitors involved in tissue repair and fibrosis, show elevated circulating levels in IPF and increase further during acute exacerbations [36].
Bronchoalveolar lavage
The bronchoalveolar lavage (BAL) has gained widespread acceptance as a procedure that can be performed safely to retrieve respiratory secretions for the examination of cellular and acellular components. Ideally, the percentage of BAL fluid that is retrieved should be >30% for a patient with ILD [51]. Because instilled fluid during BAL may interfere with the interpretation of HRCT images, the HRCT should always be carried out before the bronchoscopy with BAL [2].
Veeraraghavan et al [52] suggested that BAL findings do not help discriminate between UIP and NSIP in patients presenting with clinical features of idiopathic interstitial pneumonia and they have no prognostic value, once the histopathological distinction between the two diseases has been made [53]. However, a more recent retrospective study, demonstrated that BAL lymphocytosis in patients who appear to have fibrotic idiopathic pulmonary fibrosis suggests that the diagnosis is rather NSIP, than IPF [51]. The most important application of BAL is to increase the index of suspicion for alternative disorders in patients with suspected IPF, including hypersensitivity pneumonitis and NSIP, by the demonstration of lymphocytosis (>30%) [13]. According to the ATS/ERS 2011 guidelines, BAL is not needed in the majority of patients for diagnosis, except for some situations when it could rule out alternative causes of ILD [25].
Biopsy
A surgical lung biopsy (SLB) is performed only when no confident diagnosis can be made based on other available information and when a specific histological diagnosis is thought to add important value to prognosis or therapy [2]. Specimens can be obtained by video-assisted thoracoscopic surgery or by thoracotomy. The location where the lung biopsy is taken should be guided by the most affected sites on HRCT, although areas with scarred or honeycombed lung must be avoided [2]. Recent studies show an incidence of post-SLB complications of 16–71% [54], and an overall postoperative mortality of 4.5–6% [54,55]. Conditions that lead to higher risks of SLB include: older age, immunocompromised conditions, DLCO<50% predicted value, requiring mechanical ventilation, pulmonary hypertension or attempted surgery for coexistent lung neoplasia [18,56].
Transbronchial lung biopsy (TBLB) is not recommended based on its low-quality evidence, but this point is controversial. Using this method, tiny specimens representing the centrilobular lung parenchyma may be obtained with a low incidence of side effects - mainly pneumothorax [11]. TBLB may not be sufficient for the definite pathological diagnosis due to the amount and site of biopsies. A retrospective study on 21 IPF cases found that only 32% of patients could be accurately diagnosed with tissue obtained by TBLB, compared to 95.4% by SLB [57].
Conclusions
The American Thoracic Society/European Respiratory Society guidelines emphasize that the reference standard in the diagnosis of interstitial lung disease is no longer the histology. The final diagnosis of interstitial lung disease requires multidisciplinary meetings between clinicians, radiologists and pathologists to reach a consensus clinico-radiologic-pathologic diagnosis, which represents the current international gold standard.
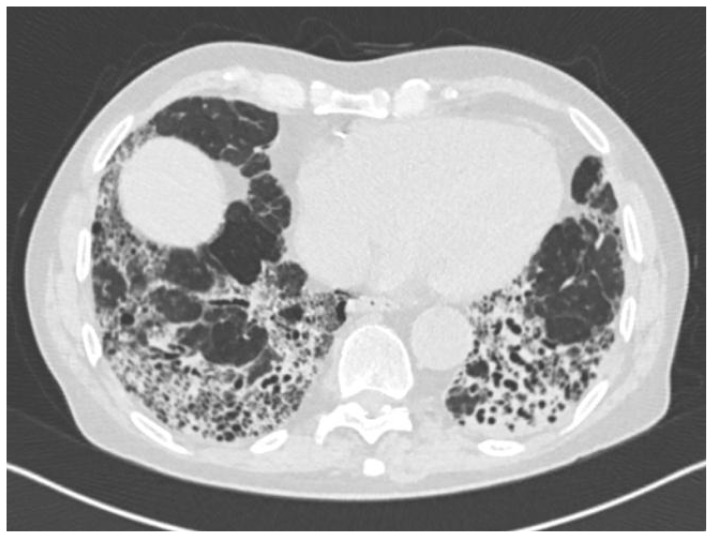
Figure 1.
76-year-old patient with UIP pattern on HRCT: bilateral reticular opacities, due to intra- and interlobular septal thickening, associated with traction bronchiectasis and honeycombing, predominantly in the postero-bazal and subpleural regions of segment 10.
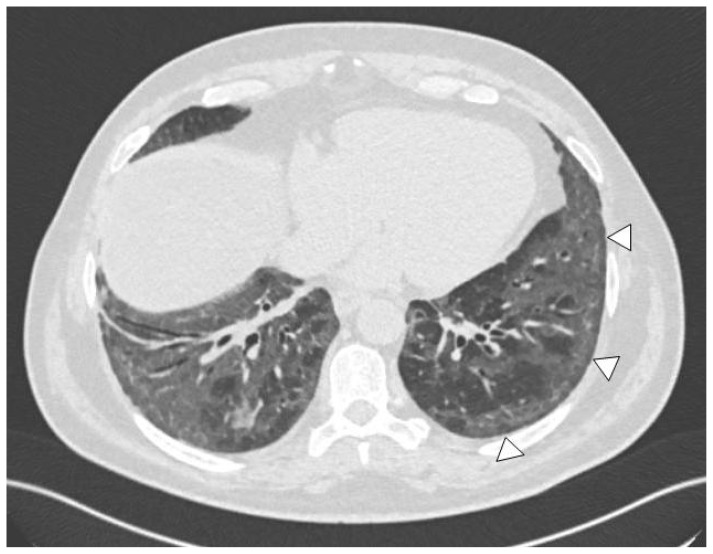
Figure 2.
58-year-old patient with (cellular) NSIP pattern on HRCT: symmetric ground-glass opacities in both lung bases, with sparing of the subpleural regions (arrowheads).
References
- 1.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):315–321. doi: 10.1513/pats.200602-022TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deconinck B, Verschakelen J, Coolen J, Verbeken E, Verleden G, Wuyts W. Diagnostic workup for diffuse parenchymal lung disease: schematic flowchart, literature review, and pitfalls. Lung. 2013;191(1):19–25. doi: 10.1007/s00408-012-9433-5. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson CJ, Collard HR. Update on the diagnosis and classification of ILD. Curr Opin Pulm Med. 2013;19(5):453–459. doi: 10.1097/MCP.0b013e328363f48d. [DOI] [PubMed] [Google Scholar]
- 4.Larsen BT, Colby TV. Update for pathologists on idiopathic interstitial pneumonias. Arch Pathol Lab Med. 2012;136(10):1234–1241. doi: 10.5858/arpa.2012-0225-RA. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 6.ATS/ERS committee on idiopathic interstitial pneumonias. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmucci S, Roccasalva F, Puglisi S, Torrisi SE, Vindigni V, Mauro LA, et al. Clinical and radiological features of idiopathic interstitial pneumonias (IIPs): a pictorial review. Insights Imaging. 2014;5(3):347–64. doi: 10.1007/s13244-014-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kligerman SJ, Groshong S, Brown KK, Lynch DA. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics. 2009;29(1):73–87. doi: 10.1148/rg.291085096. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Hunninghake G, King TE, Jr, Lynch DA, Colby TV, Galvin JR, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177(12):1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 10.Silva CI, Müller NL. Idiopathic interstitial pneumonias. J Thorac Imaging. 2009;24(4):260–273. doi: 10.1097/RTI.0b013e3181c1a9eb. [DOI] [PubMed] [Google Scholar]
- 11.Poletti V, Ravaglia C, Buccioli M, Tantalocco P, Piciucchi S, Dubini A, et al. Idiopathic pulmonary fibrosis: diagnosis and prognostic evaluation. Respiration. 2013;86(1):5–12. doi: 10.1159/000353580. [DOI] [PubMed] [Google Scholar]
- 12.Strâmbu I, Belaconi I, Stoicescu I, Ioniţă D, Cojocaru F, Niţă C, et al. Interstitial lung diseases: an observational study in patients admitted in “Marius Nasta” Institute of Pulmonology Bucharest, Romania, in 2011. Pneumologia. 2013;62(4):206–211. [PubMed] [Google Scholar]
- 13.Wells AU. Managing diagnostic procedures in idiopathic pulmonary fibrosis. Eur Respir Rev. 2013;22(128):158–162. doi: 10.1183/09059180.00001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swigris JJ, Wamboldt FS, Behr J, du Bois RM, King TE, Raghu G, Brown KK. The 6 minute walk in idiopathic pulmonary fibrosis: longitudinal changes and minimum important difference. Thorax. 2010;65(2):173–177. doi: 10.1136/thx.2009.113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183(9):1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 16.Smith M, Dalurzo M, Panse P, Parish J, Leslie K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J Clin Pathol. 2013;66(10):896–903. doi: 10.1136/jclinpath-2013-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, et al. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc. 2014;89(8):1130–1142. doi: 10.1016/j.mayocp.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Luo Q, Han Q, Chen X, Xie J, Wu L, Chen R. The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study. J Thorac Dis. 2013;5(3):283–288. doi: 10.3978/j.issn.2072-1439.2013.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172(4):488–493. doi: 10.1164/rccm.200412-1756OC. [DOI] [PubMed] [Google Scholar]
- 20.Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595–615. doi: 10.1148/rg.273065130. [DOI] [PubMed] [Google Scholar]
- 21.Kekevian A, Gershwin ME, Chang C. Diagnosis and classification of idiopathic pulmonary fibrosis. Autoimmun Rev. 2014;13(4–5):508–512. doi: 10.1016/j.autrev.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med. 2012;136(6):591–600. doi: 10.5858/arpa.2011-0511-OA. [DOI] [PubMed] [Google Scholar]
- 23.Churg A, Wright JL, Tazelaar HD. Acute exacerbations of fibrotic interstitial lung disease. Histopathology. 2011;58(4):525–50. doi: 10.1111/j.1365-2559.2010.03650.x. [DOI] [PubMed] [Google Scholar]
- 24.Du Bois RM. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;124:141–146. doi: 10.1183/09059180.00000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DS, Collard HR, King TE. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tafti SF, Mokri B, Mohammadi F, Bakhshayesh-Karam M, Emami H, Masjedi MR. Comparison of clinicoradiologic manifestation of nonspecific interstitial pneumonia and usual interstitial pneumonia/idiopathic pulmonary fibrosis: a report from NRITLD. Ann Thorac Med. 2008;3(4):140–145. doi: 10.4103/1817-1737.43081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mink SN, Maycher B. Comparative manifestations and diagnostic accuracy of high-resolution computed tomography in usual interstitial pneumonia and nonspecific interstitial pneumonia. Curr Opin Pulm Med. 2012;18(5):530–534. doi: 10.1097/MCP.0b013e3283568026. [DOI] [PubMed] [Google Scholar]
- 29.Lynch DA. Lung disease related to collagen vascular disease. J Thorac Imaging. 2009;24(4):299–309. doi: 10.1097/RTI.0b013e3181c1acec. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JH, Misumi S, Sahin H, Brown KK, Newell JD, Lynch DA. Computed tomographic features of idiopathic fibrosing interstitial pneumonia: comparison with pulmonary fibrosis related to collagen vascular disease. J Comput Assist Tomogr. 2009;33(3):410–415. doi: 10.1097/RCT.0b013e318181d551. [DOI] [PubMed] [Google Scholar]
- 31.de Lauretis A, Veeraraghavan S, Renzoni E. Review series: Aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011;8(1):53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 32.Marigliano B, Soriano A, Margiotta D, Vadacca M, Afeltra A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun Rev. 2013;12(11):1076–1084. doi: 10.1016/j.autrev.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Kocheril SV, Appleton BE, Somers EC, Kazerooni EA, Flaherty KR, Martinez FJ, et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 2005;53(4):549–557. doi: 10.1002/art.21322. [DOI] [PubMed] [Google Scholar]
- 34.Marten K, Dicken V, Kneitz C, Höhmann M, Kenn W, Hahn D, Engelke C. Interstitial lung disease associated with collagen vascular disorders: disease quantification using a computer-aided diagnosis tool. Eur Radiol. 2009;19(2):324–332. doi: 10.1007/s00330-008-1152-1. [DOI] [PubMed] [Google Scholar]
- 35.Lynch DA, Travis WD, Müller NL, Galvin JR, Hansell DM, Grenier PA, et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236(1):10–21. doi: 10.1148/radiol.2361031674. [DOI] [PubMed] [Google Scholar]
- 36.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 37.Nagai S, Nagao T, Kitaichi M, Izumi T. Clinical courses of asymptomatic cases with idiopathic pulmonary fibrosis and a histology of usual interstitial pneumonia. Eur Respir J. 1998;11:131s. [Google Scholar]
- 38.Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162(6):2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 39.Fujita J, Yamadori I, Bandoh S, Mizobuchi K, Suemitsu I, Nakamura Y, et al. Clinical features of three fatal cases of nonspecific interstitial pneumonia. Intern Med. 2000;39:407–411. doi: 10.2169/internalmedicine.39.407. [DOI] [PubMed] [Google Scholar]
- 40.Verschakelen JA. The role of high-resolution computed tomography in the work-up of interstitial lung disease. Curr Opin Pulm Med. 2010;16(5):503–510. doi: 10.1097/MCP.0b013e32833cc997. [DOI] [PubMed] [Google Scholar]
- 41.Elliot TL, Lynch DA, Newell JD, Jr, Cool C, Tuder R, Markopoulou K, et al. High-resolution computed tomography features of nonspecific interstitial pneumonia and usual interstitial pneumonia. J Comput Assist Tomogr. 2005;29:339–345. doi: 10.1097/01.rct.0000162153.55253.d3. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald SLS, Rubens M, Hansell DM, Copley SJ, Desai SR, du Bois RM, et al. Nonspecific interstitial pneumonia and usual interstitial pneumonia: comparative appearances and diagnostic accuracy of thin-section CT. Radiology. 2001;221:600–605. doi: 10.1148/radiol.2213010158. [DOI] [PubMed] [Google Scholar]
- 43.Tsubamoto M, Muller NL, Johkoh T, Ichikado K, Taniguchi H, Kondoh Y, et al. Pathologic subgroups of nonspecific interstitial pneumonia: differential diagnosis from other idiopathic interstitial pneumonias on high-resolution computed tomography. J Comput Assist Tomogr. 2005;29:793–800. doi: 10.1097/01.rct.0000182853.90520.84. [DOI] [PubMed] [Google Scholar]
- 44.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116:1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 45.Hunninghake GW, Zimmerman MB, Schwartz DA, King TE, Jr, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 46.Johkoh T. Nonspecific interstitial pneumonia and usual interstitial pneumonia: is differentiation possible by high-resolution computed tomography? Semin Ultrasound CT MR. 2014;35(1):24–28. doi: 10.1053/j.sult.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Akira M, Inoue Y, Kitaichi M, Yamamoto S, Arai T, Toyokawa K. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: Thin-section CT findings. Radiology. 2009;251:271–279. doi: 10.1148/radiol.2511080917. [DOI] [PubMed] [Google Scholar]
- 48.Lynch DA, Huckleberry JM. Usual interstitial pneumonia: typical and atypical high-resolution computed tomography features. Semin Ultrasound CT MR. 2014;35(1):12–23. doi: 10.1053/j.sult.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Wells A, Hirani N on behalf of the British Thoracic Society Interstitial lung disease guideline group, a subgroup of the British Thoracic Society Standards of Care Committee, in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Interstitial lung disease guidelines: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63:v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 50.Hansell DM. Classification of diffuse lung diseases: why and how. Radiology. 2013;268(3):628–640. doi: 10.1148/radiol.13120908. [DOI] [PubMed] [Google Scholar]
- 51.Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J. 2011;38(4):761–769. doi: 10.1183/09031936.00069509. [DOI] [PubMed] [Google Scholar]
- 52.Veeraraghavan S, Latsi PI, Wells AU, Pantelidis P, Nicholson AG, Colby TV, et al. BAL findings in idiopathic nonspecific interstitial pneumonia and usual interstitial pneumonia. Eur Respir J. 2003;22:239–244. doi: 10.1183/09031936.03.00105202. [DOI] [PubMed] [Google Scholar]
- 53.Shin KM, Lee KS, Chung MP, Han J, Bae YA, Kim TS, Chung MJ. Prognostic determinants among clinical, thin-section CT, and histopathologic findings for fibrotic idiopathic interstitial pneumonias: tertiary hospital study. Radiology. 2008;249(1):328–337. doi: 10.1148/radiol.2483071378. [DOI] [PubMed] [Google Scholar]
- 54.Kreider ME, Hansen-Flaschen J, Ahmad NN, Rossman MD, Kaiser LR, Kucharczuk JC, et al. Complications of videoassisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Lettieri CJ, Veerappan GR, Helman DL, Mulligan CR, Shorr AF. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127:1600–1605. doi: 10.1378/chest.127.5.1600. [DOI] [PubMed] [Google Scholar]
- 56.Hodnett PA, Naidich DP. Fibrosing interstitial lung disease. A practical high-resolution computed tomography-based approach to diagnosis and management and a review of the literature. Am J Respir Crit Care Med. 2013;188(2):141–149. doi: 10.1164/rccm.201208-1544CI. [DOI] [PubMed] [Google Scholar]
- 57.Berbescu EA, Katzenstein AL, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129:1126–1131. doi: 10.1378/chest.129.5.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]