Abstract
The immediate effect within minutes to hours of personal exposure to ambient fine particulate matter (PM2.5) on cardiac autonomic function is limited, particularly at night. Our study aimed to assess the lagged association between personal exposure to PM2.5 and nocturnal heart rate variability (HRV). Repeated measures panel study among 21 community adults recruited from a local health clinic during the period of March 1, 2004 to August 31, 2004, in Boston, Massachusetts, in the United States. Ambulatory electrocardiogram (ECG) and continuous monitoring of personal exposure to PM2.5 and were measured for up to two consecutive days. We calculated 5-min time-specific average PM2.5 exposure for each participant. Mixed effects models were fit for 5-min standard deviation of normal-to-normal intervals (SDNN) and 5-min heart rate (HR) in relation to 5-min PM2.5 exposure lagged in 5-min intervals up to 4 hours. We found an 8.4% decrease in nocturnal SDNN (95% CI: −11.3% to −5.5%) and a 1.9% increase in nighttime HR (95% CI: 1.1% to 2.7%) for an interquartile range (IQR) increase in PM2.5 (13.6 µg/m3), after adjusting for confounders. Significant decreases in nocturnal SDNN associated with PM2.5 exposure occurred within 2.5 hours. The largest decrease in nocturnal SDNN of −12.8% (95% CI: −16.4 to −9.1%) that was associated with PM2.5 exposure was found with a lag of 25 minutes. Rapid changes in nocturnal HRV associated with personal PM2.5 exposure occurred within the previous 2.5 hours, with the largest effects at 25 minutes, suggesting immediate cardiac autonomic effects of fine particulate exposure.
Keywords: lag, air pollution, night HRV, epidemiology
In 2012, the U.S. Environmental Protection Agency (EPA) strengthened the annual National Ambient Air Quality Standard (NAAQS) for ambient fine particulate matter of less than 2.5 µm in mean aerodynamic diameter (PM2.5) to 12 micrograms per cubic meter (µg/m3) from 15 µg/m3 in 1997 to protect public health. A body of epidemiological evidence has demonstrated the association between exposure to PM2.5 and cardiovascular events including ischemic heart disease (IHD), myocardial infarction (MI), stroke, arrhythmia, and heart failure exacerbation.1–3 Cardiac autonomic function, mainly indicated by heart rate variability (HRV), has been suggested as one of the hypothesized mechanisms linking PM exposure with adverse cardiac events4–10. These epidemiologic studies showed heterogeneous time-lagged effects of PM2.5 exposure on reduced HRV. However, to our knowledge, studies have not yet explored the very acute effects of personal PM2.5 exposure within minutes to hours on HRV. To provide further insights into the time course of the very acute effects of PM2.5 exposure on cardiac autonomic function, the present study was designed to investigate the association between personal exposure to PM2.5 lagged in 5-min intervals up to 4 hours, and nocturnal HRV in a community-based general adult population; as nocturnal HRV better captured cardiac autonomic effects of personal PM2.5 exposure in occupationally exposed11 and general population adults.12
Methods
This repeated-measures panel study was designed to investigate the time course of the effects of personal PM2.5 exposure on cardiac autonomic dysfunction. The study population has been described previously.12,13 Briefly, the study participants consisted of 21 adults, who did not have overt evidence of heart disease, that were recruited from a local health clinic in an inner city neighborhood of Boston, Massachusetts, during the period of March 1, 2004 to August 31, 2004. Each participant completed a modified American Thoracic Society (ATS) questionnaire,14 which also included information on socio-demographic factors (age, gender, and smoking status) and medication use. Information on medication use included statins, non-steroidal anti-inflammatory drugs (NSAIDs), antihypertensive medications (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), or aspirin which were taken at least once during the study period. All participants completed continuous monitoring of ECG and personal PM2.5 up to two consecutive days. The study was approved by the Institutional Review Boards of the Harvard School of Public Health and the Upham’s Corner Neighborhood Health Center. All subjects gave written informed consent prior to participate in the study.
We used nocturnal HRV and nocturnal HR, for a period of 7 hours from 00:00 am to 07:00 am, as measures of cardiac autonomic response to PM2.5 exposure.11,12 The ECG of each individual was measured continuously for up to two 24-hr periods using a five-lead ECG Holter monitor, Dynacord 3-Channel Model 423 (Raytel Cardiac Services, Windsor, Connecticut). Of the 21 individuals, 18 subjects were monitored entire period and three individuals did not have nocturnal HRV (Figure 1). The ECG monitoring protocol has been provided previously.12 Briefly, the holter monitor was calibrated 15 minutes before placing electrodes. Separate electrodes were placed on the participant’s skin, and if necessary, the area was shaved for proper adhesion, and the leads were periodically checked by study staff. Each 24 hour recording was sent to Raytel Cardiac Services for processing and analysis using a StrataScan 563 (DelMar Avionics, Irvine, California) and then screened to correct data artifacts. A trained professional with no exposure information performed all analyses and edited all normal or abnormal findings based on standard criteria. The mean of SD of normal-to-normal intervals (SDNN, in milliseconds), as a time-domain HRV measure, and the mean heart rate (HR, in beats per minute) were calculated in standard 5-minute segments throughout the entire recording and matched with the corresponding personal 5-min PM2.5 intervals.
Figure 1.
Overview of study design and measurements
Continuous real-time personal PM2.5 concentrations were obtained for each individual with the TSI SidePak Model AM510 Personal Aerosol Monitor, which uses light-scattering technology to determine mass concentration (TSI Inc., Shoreview, MN). Each SidePak was fit with a PM2.5 inlet impactor. The air sample was drawn through a 1.2 meter long Tygon tube into the impaction inlet at a flow rate of 1.7 L/min, adjusted using a DryCal DC-Lite Primary Air Flow Meter (BIOS, Butler, New Jersey, USA). The PM2.5 monitor was placed in a padded pouch, with the inlet tubing secured in the participant’s breathing zone, and attached to each participant. Participants were instructed to wear their monitors throughout the day and to place the monitor by their bed when sleeping at night. The monitor collected PM2.5 concentrations every 10 seconds and reported 5-minute averages.
We analyzed data using PROC MIXED in the SAS statistical package version 9.4 (SAS Institute Inc, Cary, North Carolina, USA). We treated exposure (PM2.5) and outcome variables (nocturnal SDNN and nocturnal HR) as repeated measurements in 5-min segments. Dependent variables, 5-min nocturnal SDNN and 5-min nocturnal HR, were log10-transformed to improve normality and stabilize the variance. Linear mixed effects models with random intercepts and unstructured covariance were used to estimate the percent changes as (10[β×IQR]−1)×100%, where β is the estimated regression coefficient and IQR is the interquartile range, in 5-min SDNN and 5-min HR for IQR increase in 5-min PM2.5. To assess lagged effects of PM2.5, we used 5-min lags up to 4 hours (total 48 lags calculated by 12 lags per hour × 4 hour) of PM2.5 matched on the time of continuous ECG for each subjects. In these lag models, one lag indicates a 5-min separation between the exposure and the outcome. We adjusted each model for age, gender, smoking status (current smoker and non-smoker), use of statins, NSAIDs, antihypertensive medications (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), and aspirin. Results are given as estimated percent changes with their 95% confidence intervals (CIs) in 5-min HRV and 5-min HR per IQR increase in 5-min PM2.5 exposure (13.6 µg/m3).
Results
The demographic and clinical characteristics of each study participant are shown in Table 1. Subjects were on average 44 years of age; 81% were female, 29% were current smokers, and 48% took regular medications including any of statin (10%), NSAIDs (29%), antihypertensive medications (43%), or aspirin (14%). Of the 21 participants, 15 participants (71%) had one or more diseases including diabetes (9.5%), chronic bronchitis (9.5%), asthma (33.3%) or hypertension (57.1%).
Table 1.
Characteristics of the study subjects (N=21)
| SDNN (msec, mean±SD) |
Heart rate (bpm, mean±SD) |
PM2.5 (µg/m3, mean±SD) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Gender | Race | Smoker | Statin | NSAIDs | Aspirin | AHT† | Entire recording |
Night | Entire recording |
Night | Entire recording |
Night |
| 1 | 21 | F | B | N | - | - | - | - | 69.7±29.7 | 74.4±31.8 | 83.3±15.2 | 71.0±9.2 | 15.5±5.7 | 10.0±2.6 |
| 2 | 23 | M | B | N | - | - | - | - | 70.3±32.4 | 84.1±39.5 | 73.6±13.9 | 58.6±4.8 | 9.5±9.4 | 6.6±2.3 |
| 3 | 23 | F | B | N | - | - | - | - | 67.0±.25.5 | 74.0±24.3 | 82.9±15.7 | 69.7±5.7 | 18.1±4.2 | 20.5±4.1 |
| 4 | 25 | F | B | N | - | - | - | - | 68.7±23.2 | 77.8±26.2 | 71.6±10.8 | 57.3±4.7 | 12.8±24.4 | 6.4±1.3 |
| 5 | 26 | F | B | N | - | - | - | - | 102.5±24.7 | - | 66.2±10.0 | - | 8.8±15.6 | 4.1±0.6 |
| 6 | 33 | F | B | S | - | - | - | - | 28.6±12.0 | 35.9±13.2 | 101.2±13.4 | 86.8±5.5 | 52.4±97.3 | - |
| 7 | 39 | F | H | N | - | - | - | + | 41.0±25.4 | 57.9±19.7 | 74.7±10.1 | 66.4±5.7.3 | 10.3±13.9 | 4.1±3.5 |
| 8 | 41 | F | B | N | - | - | - | + | 54.0±22.0 | 60.0±27.8 | 79.3±14.6 | 63.7±8.5 | 6.4±4.7 | 6.3±1.5 |
| 9 | 43 | F | B | S | - | - | - | + | 41.3±19.4 | - | 88.1±13.0 | - | 175.8±88.9 | - |
| 10 | 45 | F | H | N | - | - | - | - | 57.4±21.0 | 58.4±22.4 | 84.1±12.3 | 81.0±11.3 | 4.6±6.3 | 0.4±0.3 |
| 11 | 45 | F | B | N | - | - | - | - | 46.9±16.4 | 52.6±17.4 | 82.9±12.6 | 72.1±5.9 | 6.1±8.1 | 3.2±0.6 |
| 12 | 45 | F | H | N | - | + | - | - | 42.7±17.6 | 54.7±25.5 | 83.6±12.6 | 66.0±4.3 | 18.8±72.4 | 2.1±0.6 |
| 13 | 46 | F | B | N | - | - | - | - | 54.0±20.6 | 52.9±23.3 | 83.4±12.8 | 76.3±19.3 | 13.4±48.2 | 3.5±1.3 |
| 14 | 47 | F | B | N | - | - | - | - | 29.9±11.6 | 34.2±13.3 | 98.2±10.1 | 87.3±7.9 | 9.7±8.6 | 6.6±4.6 |
| 15 | 51 | M | H | S | - | + | + | + | 28.4±17.9 | 55.4±19.3 | 96.4±10.8 | 79.2±6.4 | 24.9±50.7 | 21.9±12.6 |
| 16 | 54 | F | B | N | - | + | + | + | 34.5±14.2 | 37.4±16.8 | 94.7±12.4 | 84.4±10.0 | 63.5±58.7 | 9.3±6.6 |
| 17 | 55 | M | B | S | - | - | - | + | 36.8±29.4 | 55.2±55.5 | 78.4±7.6 | 75.0±7.4 | 64.7±86.7 | 49.7±22.9 |
| 18 | 61 | F | B | N | + | + | - | + | 54.5±23.6 | 56.9±32.1 | 79.5±10.6 | 66.9±4.3 | 12.6±27.9 | 7.2±0.9 |
| 19 | 66 | F | H | N | - | + | - | + | 50.4±19.7 | 48.1±25.8 | 70.1±6.5 | 69.5±6.4 | 13.5±5.2 | 12.3±2.6 |
| 20 | 68 | F | B | S | + | + | + | + | 34.3±10.3 | - | 76.1±6.1 | - | 26.7±51.3 | - |
| 21 | 69 | M | W | S | - | - | - | - | 30.8±11.9 | 27.5±10.9 | 82.4±6.4 | 85.8±5.1 | 218.6±251.4 | 12.8±30.1 |
| Mean | 44 | 49.6±27.2 | 55.2±30.0 | 82.8±14.8 | 73.4±12.3 | 29.8±77.7 | 10.7±15.0 | |||||||
F, female; M, male; B, non-Hispanic black; H, Hispanic; W, white; N, non-smoker; S, current smoker; AHT, anti-hypertensive medication.
Antihypertensive medication includes any of beta blocker, calcium channel blocker or ACE inhibitor/angiotensin receptor blockers.
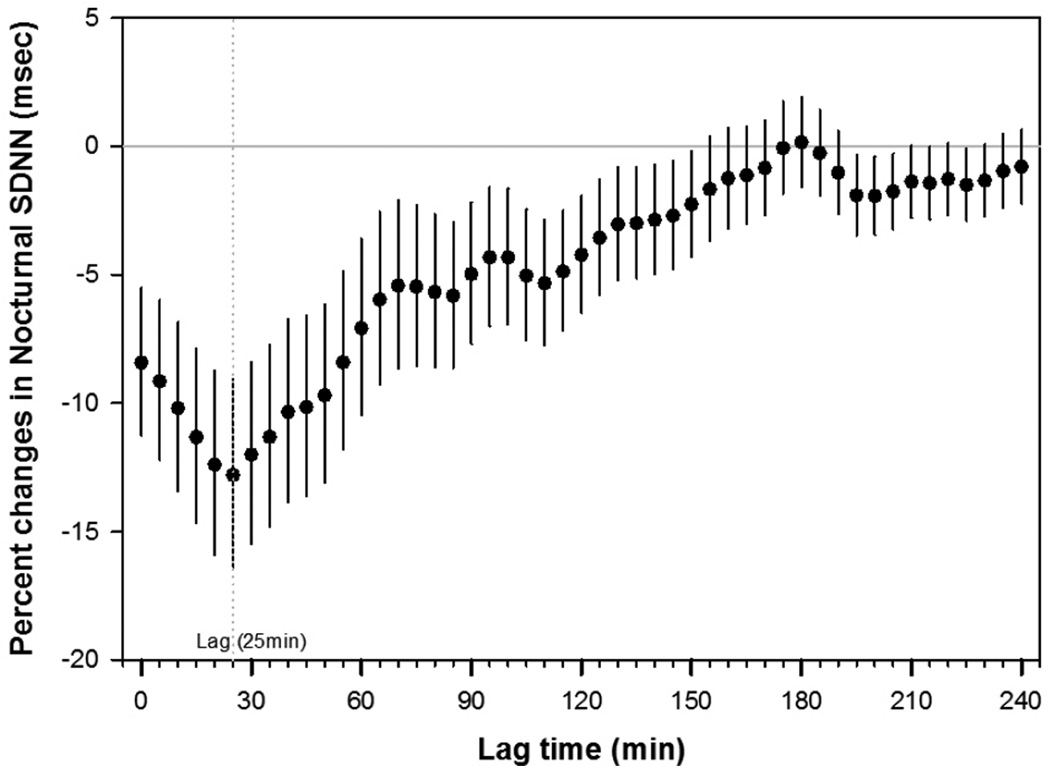
Figure 2 shows adjusted lag associations of nocturnal SDNN and nocturnal HR with an IQR (13.6 µg/m3) increase of personal PM2.5. Greater decreases in nocturnal SDNN associated with PM2.5 exposure occurred within 2.5 hours: the largest decrease in nocturnal SDNN of −12.80% (95% CI: −16.36% to −9.09%) and the largest increase in nocturnal HR of 2.27% (95% CI: 1.22% to 3.33%) associated with PM2.5 exposure were found with a lag of 25 minutes and 20 minutes, respectively. When we adjusted for multiple comparisons using a Bonferroni correction and false discovery rate (FDR) estimation, strong and consistent associations were found within the lags of 25 minutes of PM2.5 exposure (Supplement Table 1).”
Figure 2.
Adjusted percent changes and 95% CIs in nocturnal SDNN and nocturnal HR for an IQR increase in PM2.5 (13.6 µg/m3). Models were adjusted for age, gender, smoking status, use of statin, use of NSAIDs, use of hypertension medication (beta blocker, calcium channel blocker or ACE inhibitors/angiotensin receptor blockers), and use of aspirin. Circle black/white symbols indicate the effect estimate.
Results were similar when we additionally adjusted for ethnicity since ethnic differences in HRV has been reported15: the largest decrease in nocturnal SDNN of −13.41% (95% CI: −17.11% to −9.55%) and the largest increase in nocturnal HR of 2.36% (95% CI: 1.31% to 3.42%) associated with PM2.5 exposure were found with a lag of 25 minutes. Results were also similar when the analysis was additionally adjusted for diabetes: the largest decrease in nocturnal SDNN of −12.47% (95% CI: −16.10% to −8.69%) and the largest increase in nocturnal HR of 2.29% (95% CI: 1.23% to 3.36%) associated with PM2.5 exposure were found with a lag of 25 minutes.
We also analyzed the lagged associations of nocturnal HRV with PM2.5 classified by three groups: low exposure (<12 µg/m3) based on a new annual NAAQS, medium exposure (12 to 24 µg/m3), and high (≥ 24 µg/m3) exposure. Similar patterns of associations of nocturnal HRV with 5-min lagged PM2.5 exposure were found; particularly, subjects with high and medium PM2.5 exposure had 37.1% (95% CI: −46.0% to −26.7%) and 23.8% (95% CI: −29.7% to −17.5%) lower nocturnal SDNN than individuals with the low PM2.5 exposure with a lag of 25 minutes (Supplement Table 2).
Discussion
In this community-based repeated measures panel study, we found lagged associations between personal exposure to PM2.5 and rapid changes in cardiac autonomic function. Greater reductions in nocturnal HRV associated with PM2.5 exposure occurred within 2.5 hours: the largest decrease in nocturnal SDNN associated with PM2.5 exposure was found with a lag of 25 minutes, suggesting very rapid responses.
Although, to our knowledge, there is no previous study that assessed the short-term lag association between personal PM2.5 exposure and nocturnal HRV in individuals, who were assumed to be asleep at night and hence limiting the influence of physical activity and mental stress on HRV, a body of epidemiologic evidence suggests that particulate air pollution has been associated with cardiac autonomic dysfunction.4–10, 16–18 But these studies have shown various lagged effects of PM2.5 exposure that occurred within hours or same day of exposure4,5, 8–10 or longer time lags for more than one day16–18 using data from fixed ambient monitoring sites, which may lead to exposure misclassification, other than individual level. Only a few studies monitored individual-level PM2.5 and found acute effects on HRV.5,7,19 In community-dwelling adults from the APACR (Air Pollution and Cardiac Risk and its Time Course) study,5 the effects of personal PM2.5 on decreased cardiac autonomic modulation (CAM) measures occurred acutely, with the strongest effects occurring between 4 and 6 hours within 6 hours after elevated PM2.5 exposure, but potential confounders such as medication use were not considered in the models. In an occupational cohort of boilermaker welders, a biphasic cardiac autonomic response with acute (2 hours) and delayed (9–13 hours) was associated with a decline in SDNN index (SDNNi), the mean of the SDNN for all 5-min segments.7 That study may not generalizable, however, because only young male workers who may be exposed to relatively high levels of occupational PM2.5 (mean: 1.12 mg/m3) were studied, and the composition of welding fume particles may not be similar to urban ambient air. In a controlled human exposure chamber study,19 decreases in HRV were observed following 2 hour exposures to concentrated ambient particles (CAPs) in concentrations of 5–200µg/m3 among elderly participants, but not among young volunteers. Because physical activity and mental stress could influence HRV,20 young volunteers exercised intermittently (alternating 15 minutes of rest and 15 minutes of moderate exercise on a cycle ergometer) during the exposure to assess the effects of particulate exposure. In the current population, there was a relatively weak association between personal PM2.5 and daytime HRV compared to nocturnal HRV associations with PM2.5 exposure.12
The mechanisms responsible for the acute cardiovascular effect of the PM exposure are not fully elucidated, but it may be biologically plausible, as particles could stimulate afferent nerves in the lungs which influence the autonomic nervous system.21 In a human study of healthy volunteers, inhaled particles rapidly pass into the systemic circulation within minutes: detected in blood at 1 minute, reached a maximum concentration between 10 and 20 minutes.22 In the systemic circulation, particles can cause oxidative stress directly or via acute pulmonary inflammation through the generation of reactive oxygen species (ROS) and proinflammatory cytokines.23 These products may play a role in decreased HRV, reflecting the influence of both sympathetic and parasympathetic system. The acute effect of PM exposure observed in this study may occur via direct mechanisms involving direct activation of pulmonary neural reflex arcs.2 In addition, impaired cardiac autonomic function, which is assessed by decreased HRV, is one of the most studied potential mechanisms that link fine particulate air pollution and cardiac diseases as previous studies have shown the acute, within hours, adverse cardiac effects of fine particulate air pollution including myocardial infarction,24 prolonged ventricular repolarization,25 ST segment depression, an ECG marker of myocardial ischemia,8,26 and cardiac arrhythmia.27
The major limitation of this panel study includes the relatively small number of study participants and predominantly female population, limiting generalizability. To maximize statistical power in the small number of subjects, we used repeated measurements and adopted linear mixed effects models to detect the associations between personal PM2.5 and nocturnal HRV. Another limitation is that we are unable to provide information on which constituents of PM2.5 result in the observed detrimental cardiac autonomic effects.
Supplementary Material
Acknowledgement
The authors would like to thank Dr. Jee Young Kim and Ms. Li Su for their help in primary data collection and analyses. This study was supported by the U.S. Environmental Protection Agency (EPA) STAR (Science to Achieve Results) grant #RD-83083801 and National Institutes of Health (NIH) grant ES000002.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None declared.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 3.Dockery DW, Stone PH. Cardiovascular risks from fine particulate air pollution. N Engl J Med. 2007;356:511–513. doi: 10.1056/NEJMe068274. [DOI] [PubMed] [Google Scholar]
- 4.Hampel R, Breitner S, Schneider A, Zareba W, Kraus U, Cyrys J, Geruschkat U, Belcredi P, Muller M, Wichmann HE, Peters A. Acute air pollution effects on heart rate variability are modified by SNPs involved in cardiac rhythm in individuals with diabetes or impaired glucose tolerance. Environ Res. 2012;112:177–185. doi: 10.1016/j.envres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 5.He F, Shaffer ML, Li X, Rodriguez-Colon S, Wolbrette DL, Williams R, Cascio WE, Liao D. Individual-level PM(2).(5) exposure and the time course of impaired heart rate variability: the APACR Study. J Expo Sci Environ Epidemiol. 2011;21:65–73. doi: 10.1038/jes.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ Health Perspect. 2010;118:324–330. doi: 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallari JM, Fang SC, Eisen EA, Schwartz J, Hauser R, Herrick RF, Christiani DC. Time course of heart rate variability decline following particulate matter exposures in an occupational cohort. Inhal Toxicol. 2008;20:415–422. doi: 10.1080/08958370801903800. [DOI] [PubMed] [Google Scholar]
- 8.Lanki T, Hoek G, Timonen KL, Peters A, Tiittanen P, Vanninen E, Pekkanen J. Hourly variation in fine particle exposure is associated with transiently increased risk of ST segment depression. Occup Environ Med. 2008;65:782–786. doi: 10.1136/oem.2007.037531. [DOI] [PubMed] [Google Scholar]
- 9.Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 10.Chan CC, Chuang KJ, Shiao GM, Lin LY. Personal exposure to submicrometer particles and heart rate variability in human subjects. Environ Health Perspect. 2004;112:1063–1067. doi: 10.1289/ehp.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavallari JM, Eisen EA, Chen JC, Fang SC, Dobson CB, Schwartz J, Christiani DC. Night heart rate variability and particulate exposures among boilermaker construction workers. Environ Health Perspect. 2007;115:1046–1051. doi: 10.1289/ehp.10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MS, Eum KD, Fang SC, Rodrigues EG, Modest GA, Christiani DC. Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. Int J Cardiol. 2014;176:166–170. doi: 10.1016/j.ijcard.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JY, Prouty LA, Fang SC, Rodrigues EG, Magari SR, Modest GA, Christiani DC. Association between fine particulate matter and oxidative DNA damage may be modified in individuals with hypertension. J Occup Environ Med. 2009;51:1158–1166. doi: 10.1097/JOM.0b013e3181b967aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 15.Hall MH, Middleton K, Thayer JF, Lewis TT, Kline CE, Matthews KA, Kravitz HM, Krafty RT, Buysse DJ. Racial differences in heart rate variability during sleep in women: the study of women across the nation sleep study. Psychosom Med. 2013;75:783–790. doi: 10.1097/PSY.0b013e3182a7ec5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Hartog JJ, Lanki T, Timonen KL, Hoek G, Janssen NA, Ibald-Mulli A, Peters A, Heinrich J, Tarkiainen TH, van Grieken R, van Wijnen JH, Brunekreef B, Pekkanen J. Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease. Environ Health Perspect. 2009;117:105–111. doi: 10.1289/ehp.11062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler A, Zanobetti A, Gold DR, Schwartz J, Stone P, Suh HH. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect. 2006;114:560–566. doi: 10.1289/ehp.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SK, O'Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl. 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 20.Binici Z, Mouridsen MR, Kober L, Sajadieh A. Decreased nighttime heart rate variability is associated with increased stroke risk. Stroke. 2011;42:3196–3201. doi: 10.1161/STROKEAHA.110.607697. [DOI] [PubMed] [Google Scholar]
- 21.Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109(Suppl 4):579–584. doi: 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(Suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Shaffer ML, Rodriguez-Colon S, He F, Li X, Wolbrette DL, Yanosky J, Cascio WE. Acute adverse effects of fine particulate air pollution on ventricular repolarization. Environ Health Perspect. 2010;118:1010–1015. doi: 10.1289/ehp.0901648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He F, Shaffer ML, Rodriguez-Colon S, Bixler EO, Vgontzas AN, Williams RW, Wu R, Cascio WE, Liao D. Acute effects of fine particulate air pollution on ST segment height: a longitudinal study. Environ Health. 2010;9:68. doi: 10.1186/1476-069X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He F, Shaffer ML, Rodriguez-Colon S, Yanosky JD, Bixler E, Cascio WE, Liao D. Acute effects of fine particulate air pollution on cardiac arrhythmia: the APACR study. Environ Health Perspect. 2011;119:927–932. doi: 10.1289/ehp.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.