Abstract
Progesterone promotes differentiation coupled to proliferation and pro-survival in the breast, but inhibits estrogen-driven growth in the reproductive tract and ovaries. Herein, it is demonstrated, using progesterone receptor (PR) isoform-specific ovarian cancer model systems, that PR-A and PR-B promote distinct gene expression profiles that differ from PR-driven genes in breast cancer cells. In ovarian cancer models, PR-A primarily regulates genes independently of progestin, while PR-B is the dominant ligand-dependent isoform. Notably, FOXO1 and the PR/FOXO1 target-gene p21 (CDKN1A) are repressed by PR-A, but induced by PR-B. In the presence of progestin, PR-B, but not PR-A, robustly induced cellular senescence via FOXO1-dependent induction of p21 and p15 (CDKN2B). Chromatin immunoprecipitation (ChIP) assays performed on PR-isoform specific cells demonstrated that while each isoform is recruited to the same PRE-containing region of the p21 promoter in response to progestin, only PR-B elicits active chromatin marks. Overexpression of constitutively active FOXO1 in PR-A-expressing cells conferred robust ligand-dependent upregulation of the PR-B target genes GZMA, IGFBP1, and p21, and induced cellular senescence. In the presence of endogenous active FOXO1, PR-A was phosphorylated on Ser294 and transactivated PR-B at PR-B target genes; these events were blocked by the FOXO1 inhibitor (AS1842856). PR isoform-specific regulation of the FOXO1/p21 axis recapitulated in human primary ovarian tumor explants treated with progestin; loss of progestin sensitivity correlated with high AKT activity.
Keywords: Ovarian cancer, progesterone receptor, isoforms, progestins, Ser294, FOXO1, p21, transactivation, cellular senescence, granzyme A, AKT
Introduction
Approximately 1.3% of women will be diagnosed with ovarian cancer in their lifetime.1 Increased ovarian cancer risk is associated with progesterone deficiencies during infertility or with increasing age, and may occur as the result of genetic loss of heterozygosity at the progesterone receptor (PR) gene locus.2, 3 Conversely, elevated progesterone levels experienced during pregnancy transiently and reversibly increase breast cancer risk4, but significantly reduce ovarian cancer risk in Asian, European, and North American populations.5 Consistent with these findings, use of hormonal contraceptives (that include a progestin) is associated with increased risk of breast cancer6–9 but reduced risk of ovarian cancer.9–11 During ovulation the release of a mature follicle into the fallopian tube space requires shedding of ovarian epithelial cell layers followed by local proliferation that is analogous to wound repair. High levels of progesterone experienced during pregnancy or during the luteal phase of the normal menstrual cycle prevent follicle development. Thus, progesterone is believed to exert its protective effect on ovarian cancer in part by reducing the number of times ovulation occurs (i.e. via fewer cycles of ovulation-associated “damage and repair”).
Similar to PR expression in luminal (estrogen receptor (ER)-positive) breast tumors, PR expression in ovarian tumors is a favorable prognostic marker associated with longer progression-free survival.12–20 Roughly 35% of ovarian tumors (all subtypes) express PR; PR expression is highest in the endometrioid (67%) and serous (35%) sub-types.21, 22 Full-length PR-B and N-terminally truncated PR-A (−164 amino acids) isoforms are encoded by the same gene and mRNAs. PR isoforms are ligand-activated transcription factors with distinct transcriptional activities. Following ligand binding, PRs dimerize (A:A, B:B, and A:B) and are retained in the nucleus where they repress or activate transcription of PR-target genes, either directly through binding to progesterone response elements (PREs) on chromatin, or indirectly via tethering interactions with other transcription factors (e.g. AP1, SP1, STATs, FOXO).23–26
Studies of isoform-specific knockout mice determined that PR-B is required for mammary gland development, while PR-A is required for uterine development and reproductive actions.27–30 While PR-A and PR-B share structural and sequence identity downstream of the BUS (B-upstream segment), they are unique transcriptional regulators of distinct gene sets.31 Little is known about how PR isoform-specific transcription is regulated in PR-expressing tissues and tumors; total PR rather than PR isoform expression is measured clinically. Progesterone and progestins, acting through PR-B, are proliferative in the breast.32 However, in the endometrium of the uterus, paracrine signals secreted from PR-A-containing stromal cells antagonize estrogen-induced epithelial hyperplasia.29
Our previous study21 demonstrated that ligand-activated PR-B induces cellular senescence via induction of known cellular senescence mediators, including p21 and p15 via a FOXO1-dependent mechanism in ovarian cancer cells. FOXO1 interacts with steroid hormone receptors (SRs), including the androgen receptor (AR)33, 34, ER alpha (ERα)35, and both PR isoforms, PR-A and PR-B.36, 37 PR-B and FOXO1 were co-recruited to the same PRE-containing region of the p21 upstream promoter. Both proteins were required to activate p21 expression; stable knockdown of FOXO1 or inhibition of FOXO1 activity using the small molecule inhibitor AS1842856 blunted progestin-induced p21 expression in PR-B-expressing cells and blocked PR-dependent cellular senescence.
PR isoform-specific actions have not been studied in ovarian cancer models. In vitro studies primarily performed in breast or uterine cancer models have demonstrated PR-A trans-repression of PR-B, as well as other SRs, including AR and ERα.38 Notably, PR-A expression is markedly reduced relative to PR-B in ovarian tumors.16, 39, 40 To study PR isoform-specific gene regulation and biological consequences, we engineered ovarian cancer (ES-2) cells to stably express either empty vector (control), PR-A-only, or PR-B-only. Our studies indicate that PR-B is the dominant driver of cellular senescence in ovarian cancer cells and reveal a novel mechanism of regulation of hormone sensitivity via PR isoform-specific target gene expression; the presence of activated FOXO1 confers potent PR-B-like transcriptional activity to PR-A. Remarkably, active (de-phosphorylated) FOXO1 is required for phosphorylation of PR-A on Ser294 and transactivation of PR-B at PR-B target genes. A clear understanding of PR isoform-specific actions, interactions, and required co-regulators may reveal novel ways to pharmacologically select for PR-driven inhibitory over proliferative actions in hormone driven cancers.
Materials and Methods
Cell Culture and stable cell line generation
The human PR-B gene was previously cloned into the pEGFP-N3 vector (Clontech Laboratories, Inc.), which also served as the Empty Vector (EV) control vector.21, 41 GFP-tagged EV control, PR-A, and PR-B (with the isoform A start site mutated to Ala) stable clonal cell lines were generated using the parental ES-2 cell line as a model system. Stable cell lines were generated by transfecting cells with 2 µg of their respective plasmids using FuGene HD® transfection reagent (Roche, #04709691001) according to manufacturer’s instructions. Twenty-four hours post-transfection, cells were selected and maintained with McCoy’s 5A Modified medium supplemented with 10% charcoal-stripped fetal bovine serum (i.e. DCC) (Hyclone, #SH30068.03), 100 units/mL penicillin, 100 µg/mL streptomycin, and 0.5 mg/mL of G418 sulfate (Corning, #61-234-RG). Fluorescence-activated cell sorting (FACS) with a FACSAria II cell sorter (BD Biosciences) was used to purify GFP+ EV, PR-A−, and PR-B-containing cells by removing any low and non-GFP-expressing cells. Clones were then established and cultured from the FACS-purified population.
Stable FOXO1 expression cells were generated by infecting ES-2 PR-A-expressing cells (clone #1, #5) with the retroviral pBabe puro L vector (which also served as the EV control) containing the constitutively active FOXO1 (FOXO1-AAA).42 The pBabe puroL HA FKHR AAA plasmid was a gift from William Sellers (Addgene #9025). Cells were selected in and maintained as described earlier with 1 µg/mL of puromycin.21
Ex vivo culture of human ovarian tumors
Ovarian cancer tissues were provided by the University of Minnesota Biological Materials Procurement Network (BioNet). All de-identified tissue samples received in this study were obtained with written informed consent in accordance with the University of Minnesota Institutional Review Board under exemption status.
Dissection, plating, and treatments of ovarian cancer tissue were performed as described previously,43–45 with a few exceptions. After surgical excision and pathological examination, fresh ovarian cancerous tissue was placed in 10 mL of McCoy’s 5A medium supplemented with 5% DCC for transport back to the laboratory. Tissue was dissected into 1-mm3 pieces and cultured in duplicate wells containing presoaked gelatin sponges (Ethicon, Inc., #1969) in 12-well plates containing 1.5 mL explant media (McCoy’s 5A medium supplemented with 10% DCC, hydrocortisone (0.01 mg/mL), and insulin (0.01 mg/mL)) within one hour of receiving tissue from BioNet. Tissue cultures were placed in a 37°C incubator with 5% CO2 for 24 hr. Afterwards, media was gently aspirated from each well. 1.5 mL explant media containing 10 nM R5020 or equal volume vehicle (ethanol) was added to corresponding treatment wells and placed in a 37°C incubator with 5% CO2. To equilibrate the sponges with hormone treatments, media was gently aspirated every 1 hr and replenished with fresh explant media containing 10 nM R5020 or equal volume vehicle (ethanol) for a total three times. Plates were returned to a 37°C incubator with 5% CO2 for 48 hr. Afterwards, tissues were gently removed from the sponges with sterile forceps and processed for RNA or protein isolation as described below.
Reagents
Cells were treated with the following reagents (when applicable): R5020 (Perkin Elmer, #NLP004005MG) and AS1842856 (EMD Millipore, #344355). For experiments with AS1842856, cells were pretreated for 18–24 hr prior to the addition of R5020 in combination treatment studies.
Luciferase and transfection assays
Cells were cotransfected overnight using FuGene HD® transfection reagent (Roche) according to manufacturer’s instructions with either 0.5 µg of a PRE-containing31 (2X-PRE) firefly luciferase reporter construct, 1 µg of a p21 promoter-containing firefly luciferase reporter construct21, or 4 µg (for p21 promoter-luciferase assay) or 0.5 µg (for RT-qPCR analysis of endogenous genes in PR-B cells) of GFP-tagged N3-PRA vector. The constitutively active pRL-TK-Renilla luciferase construct (Promega, #E2241) (10 ng) was cotransfected as a transfection control. Luciferase assays were performed as previously described21 using the dual luciferase reporter assay (Promega, #E1910).
In experiments using HeLa cells, 10 ng of GFP-tagged EV or PR-A vector, 10 ng pcDNA3 empty vector (pc-EV) or pcDNA3 Flag FKHR46 (pc-FOXO1 WT), 0.5 µg of a PRE-containing31 (2X-PRE) firefly luciferase reporter construct, and 10 ng of a constitutively active pRL-TK-Renilla luciferase construct (Promega, #E2241) were transiently cotransfected. pcDNA3 Flag FKHR was a gift from Kunliang Guan (Addgene plasmid # 13507).
One microgram of pcDNA3 empty vector, pcDNA3 Flag FKHR WT, or Flag FKHR AAA46 were transiently transfected in PR-B+ (clone #1) cells. pcDNA3 Flag FKHR WT and FKHR AAA was a gift from Kunliang Guan (Addgene plasmid # 13507, 13508).
Gene expression profiling
Clonal ES-2 cells stably expressing GFP-tagged EV (clone #3), PR-A (clone #7), and PR-B (clone #1) and breast cancer cells T47D Y, YA, and YB originally described by Sartorius et al47 were hormonally starved in modified improved MEM (IMEM) (Gibco, catalog #A10488) plus 5% DCC for 24 hr. Afterwards, cells were treated with R5020 (10 nM) or vehicle control for 24 hour prior to RNA extraction using the RNeasy kit (QIAgen, #74104). DNase I treated (QIAgen, #79254) triplicate RNA samples were prepared for expression analysis using the Illumina HT-12v4 bead chip platform according to the manufacture’s protocols. Data were analyzed within R software48 using the Bioconductor49 package, lumi50 where raw intensities were log2 transformed and quantile normalized. Differentially expressed genes were analyzed using the limma package51, where empirical Bayes was used to better estimate the variance of the genes. Gene expression data presented contain log2 normalized intensities and biological comparisons presented (e.g. R5020/vehicle) contain log2 fold change with the Benjamini and Hochberg (BH) adjusted P value.52 Heat maps were generated by unsupervised hierarchical clustering of genes via the ‘aheatmap’ function in the NMF R package.53 Clustering was performed using Pearson distance and average linkage. Rows were scaled to have mean zero and standard deviation equal to one. All gene expression data is available in the NCBI Gene Expression Omnibus (GEO) database (accession number: GSE69296): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=chkzqyeojjwnrql&acc=GSE69296
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from cells in triplicate wells using TriPure Isolation Reagent (Roche, #11667165001) and isopropanol precipitation. RNA (1.0 µg) was reverse transcribed to cDNA according to manufacturer’s instructions using the qScript cDNA SuperMix (Quanta Biosciences, #95048-100). qPCR was performed using Light Cycler® FastStart DNA Master SYBR Green I (Roche, #12239264001) on a Light Cycler® 480 II Real-Time PCR System (Roche). qPCR cycling conditions with primer sequences (available upon request) and concentrations were performed as described previously.21
Immunoblotting
Western blots were performed as previously described.21 Protein lysates from the ex vivo culture of primary human ovarian tumors were assayed with 4–20% gradient SDS-PAGE precasted gels. Western blots were probed using the following primary antibodies: PR-A/B (H-190) and p21 (C-19) were purchased from Santa Cruz Biotechnology; FOXO1 (#2880), phospho-AKT (Ser473) (#9271), and AKT (#9272) was purchased from Cell Signaling Technologies; and Actin (Sigma-Aldrich, #A4700). Phospho-Ser190 PR anti-sera was purchased from NeoMarkers (#MS-1331-P1). Custom made phospho-Ser294 PR antibodies (clone 8508) were commissioned from Thermo Fisher Scientific designed to recognize the following phospho-specific peptide sequence: C-PMAPGR(pS)PLATTV-amide. HRP-conjugated goat anti-rabbit and goat anti-mouse (Bio-Rad, #170–6515 and #170–6516) secondary antibodies were used to detect their respective primary antibodies, and immunoreactive proteins were visualized on Kodak X-OMAT LS film (Carestream Health, #864–6770) following ECL detection with Super Signal® West Pico Maximum Sensitivity Substrate (Pierce, #34087). All Western blotting experiments were performed at a minimum in triplicate, and representative experiments are shown in each respective figure.
Flow Cytometry
Cells were treated in 5% DCC with either 10 nM R5020 or equal volume vehicle (ethanol) for 96 hrs. Cells were collected, fixed and stained as described previously.21 Propidium iodide staining was detected using a LSRII (BD Biosciences, #H4760). Cells were gated for cell cycle phases using BD Biosciences FACSDiva 8.0 software. Cell cycle profiles were analyzed using FlowJo vX software (Tree Star Inc.).
Chromatin Immunoprecipitation (ChIP)
Cells were treated for 1 hr with either R5020 (10 nM) or equal volume vehicle (ethanol) in 5% DCC and cell samples were fixed, harvested, and lysed according to optimized manufacturer’s instructions using the ChIP-IT™ Express Magnetic Chromatin Immunoprecipitation Kit (Active Motif, #53008). Samples were homogenized using a Bioruptor sonicator (Diagenode, Inc.). ChIP reactions were incubated overnight on an end-to-end rotator using 100 µL of isolated chromatin and either 2 µg of PR-A/B antibody (Ab-8), FKHR (H-128) antibody (Santa Cruz Biotechnology, #sc-11350), or 0.4 µg of normal mouse or rabbit IgG (Santa Cruz Biotechnology, #sc-2025 # sc-2027). Samples were washed, eluted, reverse cross-linked, and treated with Proteinase K according to manufacturer’s instructions (Active Motif). DNA was analyzed by RT-qPCR as described above.
5-bromo-2’-deoxyuridine (BrdU) Assay
Cells were plated in 96-well plates in IMEM plus 5% DCC. Twenty-four hours later, cells were treated with either R5020 (10 nM) or equal volume vehicle (ethanol) in IMEM plus 5% DCC for 96 hr. Three hours prior to the 96 hr timepoint, 10× BrdU antibody from the BrdU Cell Proliferation Assay Kit (Cell Signaling, #6813) was prepared in IMEM and then added to each well for a final concentration of 1×. Plates were returned to a 37°C incubator with 5% CO2 for 3 hr. Cells were fixed, DNA was denatured, and detection of BrdU incorporation into cellular DNA during cell proliferation was conducted according to manufacturer’s protocol.
Senescence Associated-β-galactosidase (SAβGal) Activity Assays
Cells were continuously treated for 96 hr in IMEM plus 5% DCC. Cells were washed, fixed, and stained for SAβGal activity according to manufacturer’s instructions using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, #9860). Staining of cell nuclei was achieved with DAPI containing ProLong® Gold antifade reagent (Invitrogen, #P-36931). All bright-field and fluorescent cell images described herein were acquired with a Leica DM4000B microscope (Leica Microsystems, Inc.) and captured using the Leica Application Suite software (version 4.2). Percentage of positive SAβGal cells (blue staining with enlarged nuclei) was determined from quantifying three fields at 100× magnification using ImageJ software. Values were normalized to total nuclei present per field from DAPI staining.
Statistical Analysis
All reported values represent the mean ± the standard deviation (SD). Data shown are representative of the indicated replicates of each experiment. Statistical analyses were performed using a Student’s t-test where significance was determined with 95% confidence (*p ≤ 0.05, **p ≤ 0.01).
Results
Creation of PR isoform specific ovarian cancer cell models
Cell line models derived from human tumors frequently lose SR expression when propagated in monolayer tissue cultures. We detected low to negligible levels of PR mRNA and protein in a panel of well-characterized ovarian cancer cell lines including the clear cell carcinoma cell line, ES-2.21 PR expression in these models was only modestly induced by estrogen. Thus, to study the impact of progesterone/PR signaling on ovarian cancer cell biology without the added complexity of estrogen addition (PR is an ER target gene), we generated ES-2 cell models stably expressing either PR-A or PR-B. We selected ES-2 cells due to their inherent aggressive nature and rapid growth rate54, low endogenous PR mRNA levels21, and molecular and genomic characteristics that are similar to primary tumors of high-grade serous ovarian carcinomas (the most common cancer of the ovary).55
We initially created pooled populations of ES-2 cells stably transfected with GFP-tagged PR-A, GFP-tagged PR-B (with the PR-A start site mutated), or GFP-only empty vector (EV) control (Figure 1A). GFP-tagged PRs are fully functional relative to untagged PRs in COS and HeLa cell models.41, 56 To verify that GFP-tagged PR isoforms behave as ligand-activated transcription factors in progestin-treated ES-2 cells, PR transcriptional activity in pooled populations of ES-2 cells expressing EV control, PR-A, and PR-B was evaluated using PRE-luciferase reporter assays. Similar to results obtained in breast cancer cell models57, PR-B was more transcriptionally active (~5-fold) relative to PR-A expressed in ES-2 ovarian cancer cells as measured using this minimal (2X-PRE-driven) promoter; the synthetic progestin R5020 (10 nM; for 18hr) significantly increased luciferase expression in ES-2 cells expressing PR-A (1.8 fold) and PR-B (9.5 fold) relative to vehicle or EV (GFP-only) controls (Figure 1A). Both isoforms underwent a slight up-shift in gel mobility upon R5020 treatment (Figure 1A inset), indicating direct PR phosphorylation (further addressed below). Endogenous PR-A (HEF1) and PR-B (BIRC3) target genes were also selectively upregulated in an isoform-specific manner in pooled ES-2 cell populations relative to vehicle and EV controls (Figure 1B). Interestingly, basal BIRC3 mRNA levels were higher in cell pools expressing PR-A only relative to EV control and untreated PR-B expressing cells (Figure 1B), suggestive of potential ligand-independent actions of PR-A in this setting (further addressed below).
Figure 1. Stable expression of PR isoforms in ES-2 cells.
(A) Inset, Western blot analysis showing total PR expression in ES-2 cell pools expressing GFP-tagged empty vector control (EV), GFP-tagged PR-A (PR-A), or GFP-tagged PR-B (PR-B) and treated without or with R5020 (10 nM) for 24 hr. ES-2 cells expressing EV, PR-A, or PR-B were transiently transfected with a progesterone response element (2X-PRE) containing luciferase reporter gene and treated for 18 hr with R5020 (10 nM). Relative luciferase units (RLU) were normalized to the mean result ± standard deviation (SD) for Renilla luciferase expression (n=3, **p≤0.01). (B) RT-qPCR analysis of HEF1 and BIRC3 mRNA expression after 24 hr R5020 (10 nM) treatment in ES-2 cell pools expressing EV, PR-A or PR-B (n=3, *p≤0.05 **p≤0.01). (C) Western blot analysis of total PR expression in ES-2 cells stably expressing GFP-tagged EV control (clone #3), GFP-tagged PR-A (GFP-PR-A clone #1, #5, #4, #7), or GFP-tagged PR-B (GFP-PR-B clone #1, #3) relative to T47D breast cancer cells stably expressing PR-A-only (YA), PR-B-only (YB), and both endogenous PR isoforms (CO). Actin served as a loading control. (D) Western blot analysis of PR-A and PR-B phosphorylation at Ser294 and Ser190, and total PR protein expression in ES-2 PR-expressing cells treated with R5020 (10 nM) for 1 hr. ◆◆denotes a non-specific band present in the phospho-PR Ser294 blot. Actin served as a loading control.
PR expression in pooled populations likely varies between individual cells. To directly compare PR isoform-specific actions in ES-2 ovarian cancer cells to well characterized breast cancer models, multiple clonal PR+ ES-2 cell lines were isolated and expanded after selection for PR protein expression levels comparable to PR expressed in T47D breast cancer cell lines stably expressing either PR-A only (YA), PR-B only (YB), or constitutively expressing both endogenous isoforms in the absence of estrogen (CO)47 (Figure 1C). One GFP-only EV control clone (EV #3), four GFP-PR-A-expressing clones (PR-A #1, #5, #4, #7) and two GFP-PR-B-expressing clones (PR-B #1, #3) were selected for further study. PR phosphorylation status was examined in PR- containing clonal cell lines following R5020 (10 nM for 1 hr) treatment. Similar to pooled populations (Figure 1A inset), R5020 induced a subtle up-shift in PR gel mobility in studies with clonal cell lines as measured by Western blotting (Figure 1D). PR Ser190 is a constitutive phosphorylation site similarly present in each clone. PR Ser294 is a proline-directed (i.e. primarily phosphorylated by CDKs and/or MAPKs)58–60 phosphorylation site robustly phosphorylated in PR-B-expressing cells in the presence of R5020 (a non-specific protein just below PR-B was detected in all lanes). Notably, PR-A-expressing clones exhibited varied ligand-dependent phosphorylation of Ser294, with robust levels detected in PR-A-expressing clone #7 cells relative to clone #4 cells. Phosphorylation of Ser294 occurs on PR-B, but not PR-A, in breast cancer cell models.57, 61 Together, these data confirm that GFP-tagged PR-A and PR-B stably expressed in ES-2 cells can bind ligand, undergo phosphorylation at well-characterized sites, and selectively regulate isoform-specific endogenous target genes.
PR isoforms regulate unique gene sets in ovarian relative to breast cancer cells
To assign isoform-specific transcriptional actions to PR-A and PR-B stably expressed in ovarian cancer cells, we performed genome-wide transcriptional profiling of representative clonal ES-2 cell lines expressing either EV (clone #3), PR-A (clone #7), and PR-B (clone #1) following treatment without or with R5020 (10 nM for 24 hr) and created representative heat maps (Figure 2A) and Venn diagrams (Figure 2B–C) illustrating isoform-specific gene regulation (>2-fold up or downregulated genes are shown). Cells expressing either PR-A-only or PR-B-only regulate distinct gene clusters relative to the same parental cells expressing EV (GFP-only). Simply the expression of PRs in the absence of exogenously added ligand dramatically altered the transcriptome. Strikingly, in the absence of ligand, PR-A altered the expression of more genes (100 genes downregulated and 61 genes upregulated) relative to PR-B (31 genes downregulated and 17 genes upregulated; Figure 2B). Ligand-independent actions of PR-A (as measured by gene array) were also reported using isoform-specific and inducible breast cancer models.62, 63 In contrast to our findings with PR-A-expressing cells, in the presence of ligand, PR-B (132 genes upregulated and 69 genes downregulated) was the more active transcriptional regulator relative to PR-A (53 genes downregulated and 38 genes upregulated). These data suggest that PR-B is the dominant hormone-sensitive PR isoform in ovarian cancer cells, while PR-A may mediate significant ligand-independent actions, but functions predominantly (in the absence or presence of ligand) via repressive actions (further addressed below).
Figure 2. Gene expression profiling of PR-A and PR-B transcriptional activity in ovarian and breast cancer cells.
(A) Heat map highlighting the transcriptional profiles between ES-2 ovarian cancer cells stably expressing EV control (clone #3), PR-A (clone #7), and PR-B (clone #1). Cells were treated with vehicle or R5020 (10 nM) for 24 hr and harvested RNA was subjected to Illumina gene profiling as described in Methods. Genes differentially expressed >2-fold are displayed for each treatment group. The experiment was performed in triplicate. (B) Venn diagrams showing the number of unique genes downregulated or upregulated >2-fold in the absence of R5020 treatment in PR-A− (clone #7) and PR-B-expressing (clone #1) cells. (C) Venn diagrams depicting the number of unique genes downregulated or upregulated >2-fold with R5020 treatment in PR-A− (clone #7) and PR-B-expressing (clone #1) cells. (D) Heat map highlighting the transcriptional profiles between breast cancer cells expressing EV control (T47D Y), PR-A (T47D YA), and PR-B (T47D YB). The cells were treated with vehicle or R5020 (10 nM) for 24 hr and harvested RNA was subjected to Illumina gene profiling as in part A. Genes differentially expressed >2-fold are displayed for each treatment group. The experiment was performed in triplicate. (E) Venn diagrams depicting the number of unique genes downregulated or upregulated >2-fold in the absence of R5020 treatment in T47D YA and YB cells. (F) Venn diagrams depicting the number of unique genes downregulated or upregulated >2-fold with R5020 treatment in T47D YA and YB cells.
Regulation of isoform-specific PR target genes in ES-2 cells suggests important functional differences between PR isoforms (Figure 2B–C). To understand what specific cellular pathways may be isoform-specifically regulated, we performed Gene Ontology (GO) analysis for gene terms significantly regulated by either PR-A or PR-B (Supplementary Figure 1). In the presence of progestin, gene terms significantly upregulated by PR-A (38 genes, 27 of which were also upregulated by PR-B) were enriched in cell adhesion, regulation of cell-substrate adhesion, extracellular matrix organization, and growth factor binding. Gene terms significantly downregulated by PR-B in response to progestin (69 genes, 30 of which were also down-regulated by PR-A) were also associated with regulation of the extracellular matrix, but significantly included genes associated with regulation of the basement membrane. Gene terms associated with pathways regulating the cellular response to oxygen levels were significantly upregulated by ligand-bound PR-B. Other PR-isoform specific regulated gene terms significantly associated with known pathways as defined by GO are listed in Supplementary Figure 1.
In contrast to its protective role in reproductive tissues, progesterone is a potent mitogen in the mammary gland.64 PR-B, but not PR-A, is required for ductal side-branching and alveologenesis that occurs during mammary gland development. Breast cancer cell models expressing PR-B, but not PR-A, grow in soft agar in response to progestin treatment.65–67 Indeed, both overlapping and isoform-specific PR target gene up- and downregulation has been extensively reported in breast cancer models.31, 62 To illustrate potentially distinct transcriptional actions of PR isoforms in ovarian relative to breast cancer models, we repeated whole genome profiling studies in T47D cells stably expressing either PR-A-only (YA) or PR-B-only (YB), using the naturally occurring PR-null variant parental T47D cell line (Y) as a control. As above for ES-2 cells, T47D cells were treated with R5020 (10 nM) for 24 hr, and global gene expression profiles were measured using whole genome gene expression bead (Illumina) arrays. As in our ovarian cancer cell models, transcriptional differences between PR-null T47D cells and parental T47D cells stably expressing either PR-A or PR-B were readily observed (Figure 2D). However, in sharp contrast to hormone-naive ovarian cancer models, in the absence of progestin, breast cancer cells expressing PR-B upregulated more genes (194 genes) relative to cells expressing PR-A (111 genes) (Figure 2E). In the absence of ligand, expression of PR-A (168 genes) or PR-B (157 genes) resulted in downregulation of a similar number of genes from distinct but largely overlapping subsets (103 genes in common) (Figure 2E). In the presence of progestin, PR-A and PR-B both activated and repressed numerous genes from distinct but largely overlapping subsets (Figure 2F). Thus, in contrast to PR expression in ovarian cancer models (where there is modest overlap between PR-A and PR-B regulated gene sets, and PR-B appears to be the dominant hormone-regulated isoform) PR expression in breast cancer models exhibits moderate overlap between PR-A and PR-B regulated gene sets and both isoforms are highly responsive to hormone when these models are assayed using similar conditions (i.e. compare Figure 2 to Supplementary Figure 2). We detected no differences in transcriptional activity between GFP-tagged PRs and untagged PRs in multiple cell types (data not shown).
Subsequently, differential regulation of PR isoform-specific target genes identified using the Illumina platform (Figure 2A–C) was validated by RT-qPCR in multiple ES-2 clones (Figure 3). Progestin-induced expression of selected PR-A and PR-B target genes was evaluated at 24 hr and 96 hr (R5020; 10 nM). PR-A target genes, CRISPLD1 and WISP1, known to be involved in the regulation of the extracellular matrix, were robustly induced following R5020 treatment of PR-A expressing clones (#4 and #7) relative to modest or insignificant regulation in PR-B expressing clones (#1 and #3). CRISPLD1 belongs to a super-family of proteins that have extracellular endocrine or paracrine functions and are also involved in the regulation of extracellular matrix and in cell-cell adhesion during fertilization.68 WISP1 binds to proteoglycans, decorin and biglycan, present in the extracellular matrix of connective tissues.69 PR-B target genes BIRC3 (a mediator of cancer cell pro-survival and a well-characterized PR-B target gene in breast66 and endometrial70 cell models) and GZMA (a novel PR-B target gene identified herein and a mediator of cytotoxic T-cell responses significantly elevated in the serum of women with ovarian cancer71) were robustly induced in PR-B-expressing clones (#1 and #3) relative to weak or insignificant induction in PR-A-expressing clones (#4 and #7) or controls (Figure 3B). Basal expression of BIRC3 noted in PR-A+ ES-2 pools (Figure 1B) validated in clonal PR-A-expressing clones (Figure 3B; #4 and #7 at 24 hr) and increased over time in untreated cells (compare vehicle-treated 24 to 96 hrs for each clone) but was primarily hormone-regulated in PR-B+ clones. Similarly, PDLIM1 (a novel PR target gene identified herein that encodes a cytoskeleton associated protein linked to breast cancer progression as a mediator of cell migration and invasion72) was similarly validated with robust induction at both time points in PR-A as well as PR-B-expressing clones; highest total levels of hormone-induced PDLIM1 expression were noted in PR-B expressing clones.
Figure 3. Validation of PR isoform-specific gene targets in ovarian cancer cells.
RT-qPCR analysis of PR-A selective target gene mRNAs encoding (A) CRISPLD1 and WISP1 or PR-B selective target gene mRNAs encoding (B) BIRC3, GZMA, and PDLIM1 following 24 hr and 96 hr R5020 (10 nM) treatment in ES-2 cells stably expressing EV control (clone #3), PR-A (clone #4, #7), or PR-B (clone #1, #3) (n=3, *p≤0.05, **p≤0.01).
PR-B activates a FOXO1/p21 senescence program repressed by PR-A in ovarian cancer cells
To more fully understand the basis of PR isoform-specific gene regulation in ovarian cancer cells, we considered the largely repressive actions of (primarily unliganded) PR-A relative to the predominantly hormone-sensing actions of (liganded) PR-B (compare Figure 4A, left to right Venn diagram). Only four genes were significantly repressed by PR-A and activated by liganded-PR-B: KYNU, FOXO1, p21, and ZDHHC9 (Figure 4A). KYNU is involved in the biosynthesis of NAD cofactors from tryptophan catabolism through the kynurenine pathway. The expression of KYNU is significantly downregulated in several cancer types, such as in invasive ductal breast carcinomas73, pediatric acute myeloid leukemias harboring IDH mutations74, and highly aggressive osteocarcinoma cell lines.75 ZDHHC9 is a palmitoyltransferase specific to HRAS and NRAS76 and reported to be widely overexpressed in human cancers.77 Notably, we previously identified both FOXO1 and p21 as required factors for progestin-mediated cellular senescence in PR-B+ ovarian cancer cells.21 Our microarray experiments revealed repression of both p21 and FOXO1 mRNA expression in untreated or treated PR-A-expressing (clone #7) ES-2 cells relative to EV (clone #3) controls (Figure 4B). In contrast to PR-A+ cells, R5020 treatment (24 hr) significantly upregulated p21 and FOXO1 mRNA in PR-B+ cells (clone #1). These results (i.e. robust PR-B-selective upregulation of FOXO1, p21, and the FOXO1 target gene, p15) were validated by RT-qPCR in multiple clones of ES-2 cells stably expressing either PR-B-only or PR-A-only and treated with R5020 for 24 and 96 hrs (Figure 4C–E). FOXO1, p21, and p15 mRNA levels were either slightly upregulated or unchanged in PR-A+ cells relative to their pronounced induction in PR-B+ cells (24 hr). These genes were also insensitive to progestin in EV+ (clone #3) controls (Figure 4C–E). Western blot analysis confirmed these results (Figure 4F), demonstrating robust upregulation of FOXO1 and p21 protein with 96 hr R5020 treatment in PR-B+ clones (#1 and #3). Interestingly, R5020 treatment weakly downregulated FOXO1 protein levels in PR-A+ clones relative to PR-B+ clones (Figure 4F), while FOXO1 and p21 levels remained unaltered in EV+ control cells following 96 hr R5020 treatment (data not shown). Taken together, these data indicate that while progestin-treated PR-A+ cells are capable of modestly inducing FOXO1 and p21 at the mRNA level, changes in protein expression do not persistently manifest (i.e. by as late as 96 hrs). In the presence of progestin, PR-B is a stronger transcriptional activator of known senescence mediators relative to PR-A.
Figure 4. Progestin treatment induces isoform-specific differential expression of cellular senescence mediators in ovarian cancer cells.
(A) Venn diagram describing the number of unique genes downregulated in the absence of R5020 treatment in ES-2 cells stably expressing PR-A (clone #7) and upregulated in ES-2 cells stably expressing PR-B (clone #1) with R5020 treatment. Four genes from the shared Venn category are listed. (B) Relative gene expression of FOXO1 and p21 in ES-2 cells stably expressing EV control (clone #3), PR-A (clone #7), or PR-B (clone #1) treated with vehicle or R5020 (10 nM) for 24 hr as determined by Illumina microarray experiments (Figure 2A). RT-qPCR analysis of (C) FOXO1, (D) p21, and (E) p15 mRNA expression following 24 hr and 96 hr R5020 (10 nM) treatment of ES-2 cells stably expressing EV control (clone #3), PR-A (clone #4, #7), PR-B (clone #1, #3) (n=3, *p≤0.05, **p≤0.01). (F) Western blot analysis of PR, FOXO1, and p21 protein expression in ES-2 cells stably expressing PR-A (clone #4, #7) or PR-B (clone #1, #3) after 96 hr treatment of R5020 (10 nM). Actin served as a loading control. (G) ChIP assays showing PR recruitment to a PRE-containing region of the p21 promoter. EV control and PR-A-expressing cells (clone #4, #7) were stimulated with vehicle or R5020 (10 nM) for 1 hr. Fixed lysates were subjected to ChIP assays as described in Methods using specific antibodies targeting PR (or IgG control). Additional data shown are represented as the average fold recruitment (R5020/Vehicle) of PR to a PRE-containing region of the p21 promoter from three separate experiments. (H) ChIP assays demonstrating detection of H3K4me2 at the PRE-containing region of the p21 promoter. EV control, PR-A− (clone #7), and PR-B-expressing (clone #1) cells were stimulated with vehicle or R5020 (10 nM) for 1 hr. Fixed lysates were subjected to ChIP assays as described in Methods using specific antibodies targeting H3K4me2 (or IgG control).
To demonstrate the repressive action of PR-A on the p21 promoter, we performed chromatin immunoprecipitation (ChIP) assays to determine if liganded PR-A binds to PRE-containing regions of the endogenous p21 promoter. Indeed, in the presence of progestin, PR-A was significantly recruited (9- and 21-fold in clones #4 and #7, respectively) to a PRE-containing region downstream of the p21 transcriptional start site previously identified by ChIP-Seq studies conducted in PR+ breast cancer models78 and validated in our previous study21. While some basal level of PR was detected in vehicle-treated EV (PR-low) controls, ligand-dependent PR recruitment was minimal and comparable to background (IgG control) levels (Figure 4G). Previously21, we detected very low PR mRNA levels in ES-2 cells relative to T47D breast cancer cells. While PR protein expression is undetectable in ES-2 (EV) cells as measured by Western blotting and PRE-driven luciferase reporter assays, low levels of PR protein may occupy the p21 promoter as detected by highly sensitive ChIP assays. Additional data (Figure 4G) representing the average fold recruitment (R5020/Vehicle) of three separate experiments illustrates weak PR recruitment in EV control (clone #3) cells relative to PR-A-expressing clones. We previously reported21 similar ligand-dependent recruitment (5-fold relative to controls) of PR-B to the same PRE-containing region of the p21 promoter.
Taken together, our data suggest that PR-B-containing transcriptional complexes activate p21 transcription, while PR-A-containing complexes are repressive. Changes in gene expression are linked to epigenetic histone tail modifications, such as methylation and acetylation. Histone H3 Lys4 dimethylation (H3K4me2) is an epigenetic modification associated with transcriptional activation. To measure the level of H3K4me2 (i.e. as a surrogate marker of p21 gene activation) at the same PRE/PR-containing p21 promoter region, EV control (clone #3), PR-A-expressing (clone #7), and PR-B-expressing (clone #1) cells were treated with vehicle control or R5020 (10 nM for 1 hr) and the degree of histone H3 Lys4 dimethylation was measured by ChIP assay (Figure 4H). H3K4me2 levels were significantly elevated (6-fold) only in progestin-treated ES-2 cells stably expressing PR-B (clone #1) relative to ES-2 cells stably expressing PR-A (clone #7) and EV (clone #3) control. These results suggest that PR-A is recruited to the p21 promoter, but is incapable of mediating robust hormone-dependent p21 gene activation.
PR-B robustly induces cellular senescence relative to PR-A in ovarian cancer cells
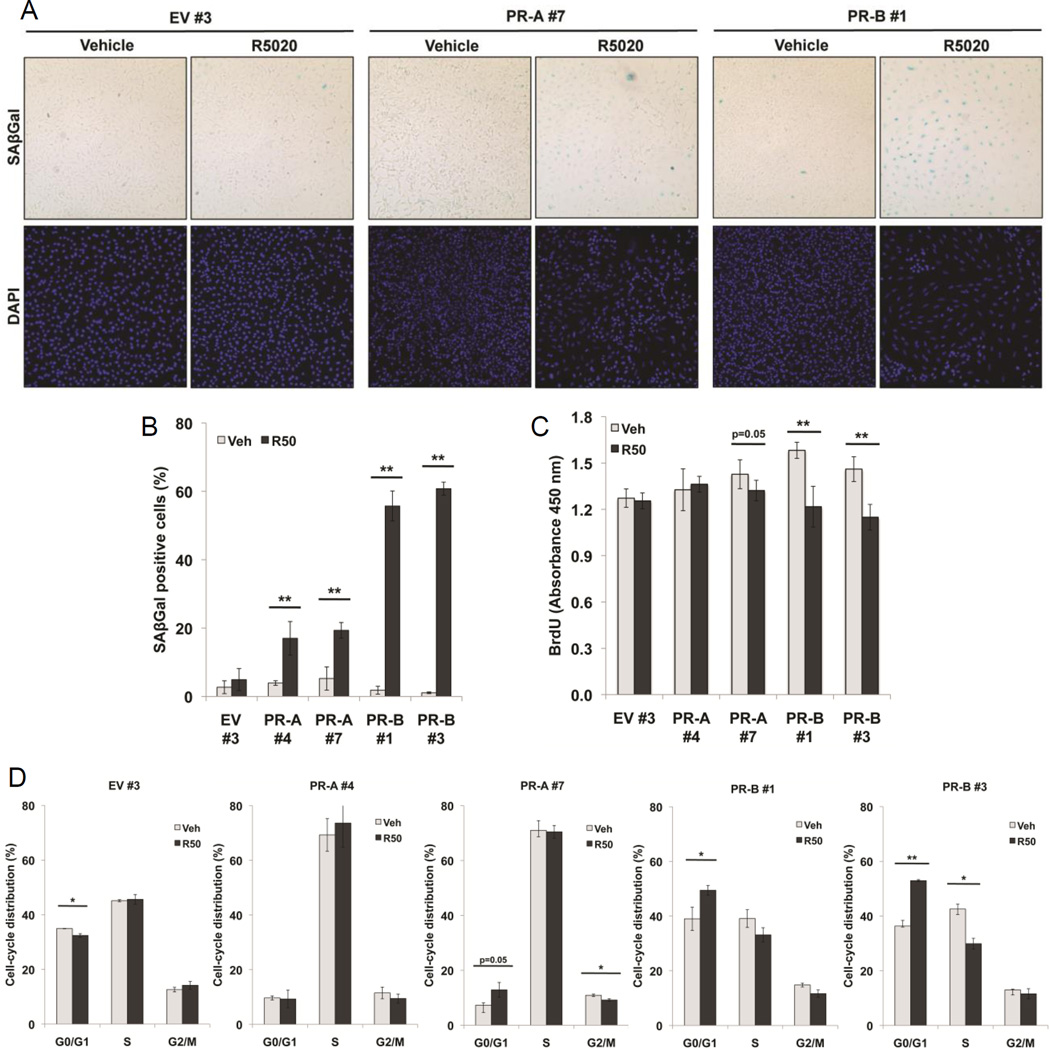
To account for the differences in PR isoform-specific regulation of senescence mediators (Figure 4), we evaluated the senescence phenotype in PR-A and PR-B expressing ES-2 cells. Our previous study demonstrated that in the presence of progestin, PR-B promoted cellular senescence, a cell fate wherein cells remain viable and long-lived, but are non-proliferative.21 A common marker of cellular senescence is the accumulation of endogenous lysosomal β-galactosidase. We estimated the percentage of senescent ES-2 cells via measurement of the activity of senescence-associated β-galactosidase (SAβGal) at pH 679 (i.e. blue stained cells with enlarged nuclei). Representative images showing SAβGal and DAPI staining performed in clonal ES-2 cell lines expressing EV (clone #3), PR-A-only (clone #7), or PR-B-only (clone #1) and treated (96 hr) with either R5020 or vehicle control are depicted (Figure 5A) along with quantitation of SAβGal positive cells in five clonal cell lines (Figure 5B). As expected, R5020 (96 hr) significantly induced senescence as measured by SAβGal in PR-B-expressing ES-2 cells (clones #1 and #3 exhibited 51% and 59% positive cells, respectively) relative to vehicle controls. In contrast, PR-A-expressing ES-2 cells (clones #4 and #7) exhibited significantly less SAβGal (21% and 22%, respectively) relative to PR-B-expressing ES-2 cells. Minimal SAβGal levels were observed in EV #3 (2%) and vehicle-treated cells (Figure 5A–B).
Figure 5. PR isoform expression and activity induces cellular senescence.
(A) Representative staining for SAβGal activity in EV control (clone #3), PR-A− (clone #7) or PR-B-expressing (clone #1) ES-2 cells treated with R5020 (10 nM) for 96 hrs (magnification = 100×). Cell samples were mounted onto glass slides using ProLong® Gold Antifade Reagent with DAPI (Invitrogen) for bright-field and fluorescent microscopy. B) Percentage of positive SAβGal cells in EV control (clone #3) and PR-A− (clone #4, #7) and PR-B-expressing (clone #1, #3) cells treated with R5020 (10 nM) for 96 hr was determined by quantifying three fields at 100× magnification. Values were normalized to total nuclei present in each field as determined by DAPI staining (n=3, **p≤0.01). (C) BrdU incorporation analysis of EV control (clone #3), PR-A− (clone #4, #7) or PR-B-expressing (clone #1, #3) cells continuously treated with R5020 (10 nM) for 96 hrs. BrdU was added to the wells, and cells were incubated for 3 hr prior to fixing the cells and denaturing the DNA according to manufacturer’s protocol (n=2, **p≤0.01). (D) Cell cycle analysis by propidium iodide staining of EV control (clone #3) PR-A− (clone #4, #7) or PR-B-expressing (clone #1, #3) cells treated with R5020 (10 nM) for 96 hr (n=2, *p≤0.05 **p≤0.01).
Cellular senescence is often associated with a decrease in cell proliferation that is accompanied with cell cycle exit (i.e. cells accumulate in G1). Thus, we examined BrdU incorporation to detect altered proliferation following progestin treatment of PR-expressing ovarian cancer cells. After 96 hr R5020 treatment, BrdU incorporation was significantly inhibited in PR-B-expressing cell clones, indicating that R5020 attenuated DNA synthesis and halted proliferation. R5020 treatment did not significantly alter BrdU incorporation in EV (clone #3) and PR-A-expressing (clone #4) cells, and only weakly inhibited PR-A+ clone #7 cells relative to that observed in PR-B-expressing clones (Figure 5C). Finally, cell cycle (FACS) analysis of PR-B-expressing cells, as measured by propidium iodide staining, confirmed a significant increase in the percentage of cells in the G0/G1 phase of the cell cycle accompanied by a decrease in the percentage of cells in S phase following 96 hr of R5020 exposure relative to vehicle controls (Figure 5D). Similar to the above findings using BrdU, progestin treatment elicited minimal or insignificant effects on the cell cycle distribution of ES-2 cells expressing either EV (clone #3) or PR-A (clones #4 and #7; weak but significant effects were again observed in PR-A clone #7). Taken together, our data suggest that in the presence of progestin, PR-B is a stronger promoter of cellular senescence relative to PR-A in ovarian cancer cells.
Active FOXO1 expression confers PR-B-like behavior to PR-A-containing cells
Similar to cell cycle studies performed in breast cancer models,65, 80 relative to PR-B, PR-A often fails to elicit robust induction of cell cycle mediators (p21) and their associated biological responses (i.e. biphasic regulation of cell proliferation in breast models), but may weakly mimic PR-B. Recall that progestin treatment weakly induced FOXO1 mRNA expression (Figure 4C) but weakly downregulated FOXO1 protein levels (Figure 4F) in FOXO1-expressing PR-A+ clones (90023;4 and #7) relative to PR-B+ clones (at 96 hr). We thus evaluated if FOXO1 activity was required for the weak but significant induction of cellular senescence in PR-A-expressing clone #7 using the selective FOXO1 small molecule inhibitor, AS1842856 (AS)21, 81 (Supplementary Figure 3). R5020 treatment of PR-A+ (clone #7) ES-2 cells for 96 hr modestly induced FOXO1 protein and mRNA expression relative to vehicle controls (Supplementary Figure 3A–B). Treatment with AS alone did not affect basal PR-A or FOXO1 protein and mRNA expression, but AS blocked R5020-induced FOXO1 protein and mRNA expression (Supplementary Figure 3A–B). FOXO1 gene expression is known to be auto-regulated (i.e. FOXO1 contributes to regulation of its own gene expression).82 Similar results were observed for R5020-induced p21 and p15 mRNA expression (Supplementary Figure 3C–D). Consistent with these results, progestin-induced senescence, as measured by SAβGal activity, was significantly reduced upon inhibition of FOXO1 by AS (100 nM) in combination with R5020 for 96 hr relative to R5020-only treated controls (Supplementary Figure 3E). Together, these data implicate FOXO1 as a required mediator of senescence-associated gene expression in PR-A+ ES-2 cells treated with progestin.
Progesterone, via PR-dependent rapid signaling actions, transiently activates numerous protein kinases, including the PI3K/AKT pathway in diverse cell types.83, 84 Because AKT signaling is a key input to FOXO1 inactivation (i.e. via direct phosphorylation), we examined the level of AKT activity in our PR+ ES-2 ovarian cancer cell clones. ES-2 cells were plated in media devoid of growth factors (see Methods) and treated with a time course (0–6 hrs) of R5020 (10 nM). Notably, basal AKT activity was undetectable in multiple clones of untreated PR-A+ or PR-B+ ES-2 cells. However, R5020 treatment of either PR-A− or PR-B-expressing ES-2 cells transiently activated AKT as measured by Ser473 phosphorylation at 30 min with peak levels observed at ~1 hr; AKT activity subsided by 4 hr and returned to near basal levels by 6 hr R5020 treatment (Figure 6A). Similar results were observed in additional distinct clones of PR-A+ and PR-B+ cells (data not shown).
Figure 6. Active FOXO1 restores PR-A sensitivity to progestins and induces cellular senescence.
(A) Western blot analysis of total PR, AKT phosphorylation at Ser473, and total AKT protein expression in ES-2 PR-A+ (clone #1) and PR-B+ (clone #1) cells treated with R5020 (10 nM) over a time course of 6 hr. (B) Western blot analysis of PR and FOXO1 protein expression in PR-A-expressing cells (clone #1, #5) stably expressing either EV control or constitutively active FOXO1 (AAA). Actin served as a loading control. (C) RT- qPCR analysis of p21 mRNA expression in cells expressing PR-A (clone #1, #5) and either EV control or constitutively active FOXO1 (AAA) and treated with vehicle or R5020 (10 nM) for 24 hr and 96 hr (n=3, *p≤0.05 **p≤0.01). (D) ChIP assays showing PR and FOXO1 recruitment to the p21 promoter. PR-A-expressing (clone #1) cells stably expressing either EV control or constitutively active FOXO1 (AAA) were stimulated with vehicle or R5020 (10 nM) for 1 hr. Fixed lysates were subjected to ChIP assays as described in Methods using antibodies targeting PR, FOXO1, or IgG control and RT-qPCR was performed on isolated DNA. (E) Representative staining for SAβGal activity in PR-A-expressing cells (clone #1) expressing either EV control or constitutively active FOXO1 (AAA) and treated with vehicle or R5020 (10 nM) for 96 hrs (magnification = 100×). Cell samples were mounted onto glass slides using ProLong® Gold Antifade Reagent with DAPI (Invitrogen) for bright-field and fluorescent microscopy. Percentage of positive SAβGal cells in PR-A-expressing cells (clone #1, #5) expressing either EV control or constitutively active FOXO1 (AAA) and treated with vehicle or R5020 (10 nM, 96 hr) was determined from quantifying three fields at 100× magnification. Values were normalized to total nuclei present in each field from DAPI staining (n=2, **p≤0.01). (F) Western blot analysis of PR phosphorylated on Ser294 or Ser190 and total PR in PR-A+ (clone #1) cells stably expressing either EV control or constitutively active FOXO1 (AAA) treated with either vehicle control, R5020 (R50, 10 nM) or CDB-4124 (CDB, 1 µM) for 1 hr. (G) RT-qPCR analysis of GZMA and IGFBP1 mRNA expression in PR-A-expressing cells (clone #1, #5) co-expressing either EV control or constitutively active FOXO1 (AAA) and treated with vehicle or R5020 (10 nM) for 24 hr and 96 hr (n=3, *p≤0.05 **p≤0.01).
We next speculated that overexpression of FOXO1 in PR-A-expressing cells would confer PR-B-like behavior and thereby “rescue” hormone sensitivity and senescence-associated gene regulation similar to that induced in PR-B+ cells. PR-A-expressing ES-2 clones were screened for low to undetectable FOXO1 protein levels by Western blot (Figure 6B). These clones were fully capable of inducing robust expression of the PR-A target gene WISP1, but failed to upregulate PR-B-selective genes including FOXO1 following 96 hrs R5020 treatment (data not shown). The PI3K/AKT pathway is constitutively activated in a majority of ovarian cancers.85 Phosphorylation of FOXO1 at Thr24, Ser256, and Ser319 by AKT or other kinases negatively regulates its nuclear localization and transcriptional activity.46, 86 We therefore utilized the constitutively active form of FOXO1 (FOXO1-AAA) that cannot be phosphorylated by AKT.42 FOXO1-AAA or empty vector control (EV) were stably expressed by retroviral transduction of PR-A+/FOXO1-low (clones #1 and #5) ES-2 cells. Expression of total FOXO1 protein was confirmed by Western blotting (Figure 6B).
Notably, R5020 only weakly increased p21 mRNA expression after 96 hr in PR-A+ (clone #1) cells expressing EV (FOXO1-null) control. However, forced FOXO1-AAA expression in these cells significantly further increased p21 mRNA induction in the presence of R5020 relative to vehicle controls at both 24 hr and 96 hr (Figure 6C). These results essentially repeated in an additional ES-2 cell line co-expressing PR-A (clone #5) and FOXO1-AAA (Figure 6C). Similar results were observed in HeLa cells transiently expressing PR-A and FOXO1 as measured via 2X-PRE-luciferase reporter assays and RT-qPCR analysis of p21 expression (Supplementary Figure 4). ChIP assays revealed that upon R5020 treatment (10 nM; 1 hr), PR-A was again recruited to the p21 promoter in PR-A+ EV-expressing (clone #1) cells, and significantly further recruited upon exogenous FOXO1-AAA expression (Figure 6D). FOXO1-AAA recruitment to this site also occurred in the absence of ligand but was significantly increased in response to R5020 treatment (Figure 6D). Consistent with these results, overexpression of FOXO1-AAA in PR-A+ cells (clones #1 and #5) conferred increased SAβGal activity upon R5020 treatment relative to vehicle and EV controls (Figure 6E). The level of SAβGal activity detected in FOXO1-AAA overexpressed PR-A+ ES-2 cells was similar to that of PR-B+ cells when treated with R5020 (Figure 5A–B). Induction of SAbGal in R5020-treated PR-A+ cells expressing FOXO1-AAA (but not EV) was accompanied by cellular senescence-associated changes in cell cycle distribution (i.e increased percentage of cells in G0/G1; data not shown).
These data suggest that expression of activated FOXO1-AAA in PR-A+ cells confers PR-B-like behavior (i.e. hormone responsive induction of p21 and senescence). To get at the mechanism for this effect, we assayed ligand-dependent and independent PR phosphorylation as a surrogate marker of heightened progesterone responsiveness.87 Notably, phosphorylation of PR-B Ser294 mediates changes in PR-B promoter selection and hormone sensitivity, primarily assayed in breast cancer models.57, 88–90 We included both R5020 (a PR agonist) and the selective PR antagonist, CDB-4124 (CDB). Surprisingly, while PR-Ser190 phosphorylation remained unaltered across all conditions, addition of either R5020 or CDB similarly induced robust PR-Ser294 phosphorylation in PR-A+ (clone #1) ES-2 cells expressing FOXO1-AAA relative to EV-expressing and vehicle-treated controls (Figure 6F). Similar results were observed in a separate PR-A+ ES-2 clone (#5; data not shown). These data suggest that overexpression of activated FOXO1-AAA enhances PR-A Ser294 phosphorylation and transcriptional activity in a manner similar to PR-B activity on PR-B-selective target genes (e.g. p21). Surprisingly, overexpression of FOXO1-AAA in PR-A+ (clones #1 and 5) cells also significantly increased mRNA expression of the PR-B-selective genes, GZMA and IGFBP1 (Figure 6G). Together, these data suggest that FOXO1 is a key mediator of enhanced hormone sensitivity on selected PR target genes (p21, GZMA, IGFBP1). Ligand-bound PR-A is fully capable of undergoing phosphorylation at Ser294 and regulating PR-B-only target genes when activated FOXO1 is present.
PR-A transactivation of PR-B is dependent on FOXO1 activity
PR-A is capable of inhibiting the transcriptional activity of PR-B, as well as other SRs, including glucocorticoid, mineralocorticoid, androgen, and estrogen receptors.38 Trans-repression of PR-B by PR-A has been primarily measured using reporter-gene assays91, 92 rather then on endogenous genes and requires PR-A SUMOylation.93 Our data suggest that PR-A is primarily repressive on the p21 promoter, but high levels of activated FOXO1 reversed this effect and enhanced both PR-A Ser294 phosphorylation and hormone sensitivity (i.e. of PR-A on PR-B target genes; Figure 6). To address PR-B target gene expression when PR-A and PR-B are co-expressed, we transiently transfected PR-A into PR-B-expressing ES-2 cells. PR-B+ ES-2 cells (clone #1) were transiently transfected with either GFP-tagged EV (N3-EV, control) or GFP-tagged PR-A (N3-PRA) and a p21-promoter-driven luciferase reporter21 (Figure 7A). As previously reported21 R5020 (10 nM for 24 hr) significantly induced p21 promoter activity in PR-B-only expressing cells containing the EV control (~2-fold) as measured using p21-luciferase assays. Surprisingly, transient expression of PR-A further increased ligand-induced p21 promoter activity (5-fold) in PR-B+ ES-2 cells. These results were confirmed at the level of transcription of the endogenous p21 promoter; addition of PR-A to PR-B+ ES-2 cells (clone #1) significantly increased p21 mRNA expression (i.e. from 3-fold in EV-expressing cells to 6-fold upon addition of PR-A; Figure 7B). Furthermore, in the presence of progestin, the PR-B-selective genes, GZMA and IGFBP1, were significantly increased upon expression of PR-A in PR-B+ ES-2 cells (clone #1; Figure 7C). Together, our data suggest that when FOXO1 is present (a PR-B target gene), PR-A is equally hormone responsive (i.e. relative to PR-B) and capable of robust transactivation of selected PR-B target genes (p21, GZMA, IGFBP1).
Figure 7. PR-A transactivation of PR-B in PR-B-expressing ovarian cancer cells is dependent on FOXO1 activity.
(A) GFP-tagged empty vector (N3-EV) or GFP-tagged PR-A (4 µg) were transiently transfected into PR-B-expressing (clone #1) ES-2 cells with a p21 promoter-driven luciferase reporter construct and treated for 24 hr with R5020 (10 nM). Relative luciferase units (RLU) were normalized to the mean result ± standard deviation (SD) for Renilla luciferase expression (n=2, *p≤0.05, **p≤0.01, ◆◆p≤0.01). RT-qPCR analysis of (B) p21, (C) GZMA, and IGFBP1 mRNA expression in PR-B-expressing (clone #1) cells transiently transfected with either GFP-tagged empty vector (N3-EV) or GFP-tagged PR-A (N3-PRA) (0.5 µg). Cells were treated for 24 hr with R5020 (10 nM) (n=2, **p≤0.01, ◆◆p≤0.01). (D) RT-qPCR analysis of GZMA and IGFBP1 mRNA expression in PR-B+ (clone #1) cells transiently transfected with either GFP-tagged empty vector (N3-EV) or GFP-tagged PR-A (N3-PRA) (0.5 µg) and treated with R5020 (10 nM), AS1842856 (AS, 100 nM), or the combination of AS1842856 and R5020 for 24 hr (n=2, *p≤0.05, **p≤0.01, ◆p≤0.05). (E) Western blot analysis of phospho-Ser294 PR-A and PR-B, total PR, and FOXO1 protein expression in PR-expressing ES-2 cells treated with R5020 (10 nM), AS1842856 (AS, 100 nM), or the combination of AS1842856 and R5020 for 1 hr. Actin served as a loading control.
To determine cooperative transcriptional activity in cells expressing both PR-A and PR-B was FOXO1-dependent, we utilized the selective FOXO1 small molecule inhibitor, AS1842856 (AS). Empty Vector (N3-EV, control) or PR-A (N3-PRA) was transfected into PR-B+ ES-2 cells followed by treatment with either vehicle control, AS (100 nM), R5020 (10 nM) or the combination of AS and R50 for 24 hr. Again, the PR-B target genes, GZMA and IGFBP1, were significantly increased upon expression of PR-A in PR-B+ cells following R5020 treatment (Figure 7D). Treatment with AS alone did not alter basal levels of GZMA or IGFBP1, but AS significantly blocked R5020-induced GZMA and IGFBP1 expression when either Empty Vector (EV) or PR-A was expressed in PR-B+ cells (Figure 7D).
The loss of progestin responsiveness when FOXO1 activity was blocked in PR+ cells suggested that PR Ser294 phosphorylation may also be downregulated. We therefore measured PR Ser294 phosphorylation following exposure (1 hr) to either vehicle control, AS (100 nM), R5020 (10 nM), or the combination of AS and R5020 in PR-A+ and PR-B+ cells (Figure 7E). As in Figure 1D, PR Ser294 phosphorylation was detected in PR-A+ (clone #7) and PR-B+ (clone #1) cells following R5020 treatment, and this site was not regulated in Vehicle or AS-treated controls. Notably, AS blocked R5020-induced Ser294 phosphorylation in PR-A− and PR-B expressing cells. Similar results were observed in additional PR+ clonal cell lines (data not shown). Conversely, overexpression of FOXO1 increased Ser294 phosphorylation (Supplementary Figure 5). Flag-tagged FOXO1 wildtype (WT), Flag-tagged FOXO1-AAA, or Empty Vector (EV) control plasmids were transfected into PR-B-expressing cells (clone #1) and PR Ser294 phosphorylation was detected by Western blotting. Forced expression of either WT or AAA-mutant FOXO1 increased PR-B Ser294 phosphorylation (~2-fold) in response to R5020 and relative to EV controls. FOXO1-AAA expression also weakly increased Ser294 phosphorylation relative to similarly transfected WT FOXO1. Overall, these data implicate FOXO1 as a key cofactor required for maximal hormone sensitivity that mediates increased PR Ser294 phosphorylation and robust transactivation of selected PR-B target genes.89, 90, 94
Progestins upregulate p21 and FOXO1 mRNAs in PR-B positive ovarian tumors
To validate our novel in vitro findings in ovarian cancer cell line models defined herein, we assessed the impact of PR signaling in primary human ovarian tumors using an innovative ex vivo culture system.43–45 Notably, tumor tissues cultured using this 3D system retain features of the original tumor, including SR expression. As illustrated in Figure 8A, ex vivo cultures of fresh primary ovarian tissue obtained from cytoreductive surgery were established using dissected tissues mounted on pre-soaked gelatin sponges equilibrated in culture media containing either vehicle control or R5020 (10 nM, 48 hr). A total of seven de-identified ovarian carcinoma tissues were obtained from the University of Minnesota’s tissue procurement facility (BioNet) and cultured as described in Methods (Figure 8B). Tumors included in this study expressed the PR-B isoform only or both PR-A and PR-B (Figure 8B); we did not observe any tumors expressing PR-A only. A high-grade serous subtype tumor (OVC-1) retained expression of the PR-B isoform (two film exposures depicting PR isoform expression in tumor lysates and T47D CO whole cell lysate as a positive control are shown; Figure 8C). In the presence of progestin, FOXO1 and p21 mRNA expression were significantly induced relative to vehicle controls (Figure 8C). A separate PR-B+ primary ovarian tumor (OVC-6) was similarly responsive to progestin treatment (48 hr); p21 mRNA but not FOXO1 mRNA expression was significantly induced upon R5020 treatment (Figure 8D). Similarly, FOXO1 mRNA but not p21 mRNA expression was significantly induced with R5020 treatment in a PR-A+/PR-B+ primary ovarian tumor (OVC-7) (Figure 8E). Additional hormone-responsive tumors are indicated (Figure 8B). Notably, only three out of seven primary ovarian tumors were responsive to progestin treatment, as measured by upregulation of either FOXO1 and/or p21. As noted above, high AKT activity in ovarian tumors may account for loss of progesterone sensitivity via inactivation of FOXO1. We therefore screened duplicate samples of primary ovarian tumors in our study with an antibody against phospho-Ser473 (active) AKT. As predicted, tumors with high AKT activity (OVC-3, OVC-4, OVC-5, OVC-6) either did not respond or significantly downregulated FOXO1 or p21 mRNA levels, whereas tumors with low basal AKT activity (OVC-1, OVC-7) remained sensitive to progestin (Figure 8F). These data confirm that endogenously expressed PR-B induces p21 and FOXO1 expression in unmodified human primary tumors exposed to progestin. However, significant tumor heterogeneity in the response of ovarian tumors to progestins may relate in part to PR isoform expression as well as FOXO1 expression and activity (i.e. as influenced by AKT and other kinases that inactivate FOXO1 via phosphorylation events; see discussion).
Figure 8. Progestin mediates p21 and FOXO1 expression ex vivo in PR+ human primary ovarian tumors.
(A) Workflow schematic of ex vivo ovarian tumor explant assay as described in Methods. (B) Overview of patient age, final diagnosis, PR expression, and significant regulation of FOXO1 or p21 mRNA levels with R5020 (10 nM) treatment in seven ovarian cancer tumors collected in this study. denotes positive expression of PR-A or PR-B as determined by Western blot analysis (as in C–D). ↓, ↑, or NS denote significant upregulation, downregulation, or no significance, respectively, of R5020-induced changes in the expression of p21 and FOXO1 mRNA levels relative to same-tumor vehicle controls. (C-E) PR protein (Western blots; left) and p21 and FOXO1 mRNA (RT-qPCR; right) expression in human ovarian patient tumor OVC-1, OVC-6, and OVC-7 explants treated without (vehicle) or with R5020 (10 nM) for 48 hrs. In Western blots, PR isoforms expressed in explants are shown relative to T47D CO breast cancer cells (positive control); actin served as a loading control. Data reflect the mean of duplicate samples. (F) Western blot analysis of phospho-Ser473 and total AKT expression in duplicate samples of human ovarian patient tumor explants. Data represented is from the same experimental film exposures, but from separate blots.
Herein, our findings are consistent with a model that underscores the importance of activated (i.e. de-phosphorylated) FOXO1 as a mediator of PR hormone responsiveness (Figure 9). In the absence of FOXO1 expression, PR-A primarily represses cellular senescence mediators, p21 and p15, and remains relatively insensitive to added progestin. In sharp contrast, PR-B-expressing cells induce abundant FOXO1, p21, and p15 expression in response to hormone. FOXO1 and PR-B further cooperate to promote a robust cellular senescent phenotype.21 When both isoforms are present, active FOXO1 confers increased hormone responsiveness to PR-A at PR-B target genes, effectively “switching” the genetic programming to induce robust cellular senescence. Overall, we conclude that limiting expression of FOXO1 or AKT-driven FOXO1 phosphorylation and inactivation are important inputs to predicting progesterone/PR-driven responses in ovarian cancers.
Figure 9. Proposed model of PR-A− and PR-B-induced cellular senescence in ovarian cancer cells.
PR-A primarily acts to repress expression of p15 or p21 and weakly induce cellular senescence in PR-A+ cells. In contrast, hormone-stimulated PR-B+ cells upregulate FOXO1, p21, and p15 expression. Elevated levels of FOXO1 (a PR-B target gene) then cooperate with PR-B to further upregulate FOXO1, p15, and p21 expression and robustly induce cellular senescence. Expression of PR-A in PR-B+/FOXO1+ significantly enhances progestin-dependent expression of PR-B target genes, p21, GZMA, and IGFBP1 and promotes cellular senescence. In the absence of FOXO1 expression, PR-A and PR-B regulate proliferative and/or pro-survival genes to promote alternate genetic programs.
Discussion
PR isoform expression in ovarian tumors
Herein we modeled PR isoform expression in ovarian cancer cells to understand mechanisms of progesterone/PR-driven gene expression and cell biology. To date, two studies with large patient cohorts have examined the relative distribution of total PR levels within ovarian carcinomas. We first reported that in a cohort of 504 ovarian tumors, 35% were PR+, with the highest PR expression in endometrioid (67%) and serous (35%; low grade serous, 64%) subtypes.21 In accordance with our study, the international Ovarian Tumor Tissue Analysis (OTTA) consortium examined the association of ER and PR expression and survival in 2,933 invasive epithelial ovarian tumors with similar results.22 Furthermore, the OTTA study confirmed the prognostic significance of PR expression in ovarian tumors; high PR expression (≥50% tumor cell nuclei staining) in high-grade serous ovarian carcinomas was associated with a significant improvement in survival.
PR isoforms are expressed from a single gene via alternate usage of at least two internal translational start sites (thus potentially creating full-length PR-B, truncated PR-A, or further truncated and transcriptionally inactive PR-C isoforms). Although numerous mRNAs capable of encoding both PR-A and PR-B have been characterized95, isoform-specific expression is also regulated via two distinct upstream (i.e. distal and proximal) promoter regions.96 At least three studies16, 39, 40 have reported differential expression of PR isoforms in ovarian tumors. Akahira and colleagues16 investigated PR-A and PR-B by immunohistochemistry of 107 patient tumors. They observed the dominant expression of PR-B across all of the histological subtypes, with PR-B frequently expressed in the serous subtype; PR-A was weakly expressed in mucinous followed by serous.16 In a follow-up study, Akahira et al evaluated PR isoform expression via immunohistochemical staining in normal (n=8), benign serous cystadenomas (n=10), borderline serous adenocarcinomas with low malignant potential (n=8), and malignant serous adenocarcinomas (n=24).39 They reported that PR-A expression was decreased or absent in malignant tissues relative to normal, whereas PR-B expression was present across all tissue cases in that study. The authors concluded that the absence of PR-A expression in malignant ovarian tissues is associated with the development of ovarian tumors. Finally, Lenhard et al40 examined the expression of PR isoforms in 155 ovarian patient cases, and reported PR-B expression in serous and endometrioid subtypes, while PR-A was present in serous followed by modest expression in endometrioid and clear cell.40 Ultimately, these studies observed dominant expression of PR-B in ovarian tumors across all subtypes with reduced or absent expression of PR-A. The mechanism for loss of PR-A relative to PR-B in malignant ovarian tumors is unknown. However, in endometrial cancer cells, enhanced DNA methylation was detected at the PR gene locus when PTEN expression was deleted.97 Approximately 7% of ovarian tumors evaluated in the 2011 TCGA analysis98 harbored PTEN deletions, while 18% and 9% contained activating mutations of either PI3K or AKT, respectively.98 Similar to these findings in ovarian cancer, methylation of the PR-A but not PR-B promoter upstream of the PR gene occurs in advanced breast cancers and is significantly associated with tamoxifen-resistance.99
To our knowledge, we are the first to examine the biological consequences of PR isoform-specific gene regulation in ovarian cancer models, as well as to directly compare PR isoform-selective transcriptomes between ovarian and breast cancer models cultured under similar conditions (Figures 2–3, Supplementary Figure 2). Remarkably, in ovarian cancer models, we found that PR-A predominantly up- or down-regulates genes independently of ligand, while PR-B is the dominant hormone-responsive receptor; we were also surprised to discover relatively modest overlap between PR-A and PR-B target genes. In contrast, we observed much target gene overlap between PR-A and PR-B in similarly designed breast cancer models, where relative to PR-A, PR-B predominantly up-regulated target genes in the absence of progestin. Ligand-independent actions of PR-B have been well-characterized in breast cancer models67, 90, 100 Surprisingly, there was also relatively little overlap between ovarian and breast cancer models with regard to hormone-regulated PR-target genes, with nine genes regulated by PR-A and 16 genes regulated by PR-B in both breast and ovarian cancer models (Supplementary Figure 2). However, while PR-A− and PR-B-regulated genes were distinct in ovarian relative to breast cancer models, both isoforms regulated similar pathways involved in cell growth, proliferation, cell death, and survival (as predicted using Ingenuity Pathway analysis).
Progesterone is proliferative in breast, but inhibitory in reproductive tissues
The paradoxical effects of progesterone observed in ovarian relative to breast cancer models may be attributed to differential co-factor availability101 (discussed below) as well as differential cross-talk between PR and growth factor-mediated signaling pathways (i.e. protein kinases). PR-B, but not PR-A, functions outside the nucleus to rapidly activate protein kinases (c-Src, MAPK, AKT) in part via ligand-induced interaction between a poly-Pro-rich region unique to PR-B and c-Src kinase (wherein c-Src is activated by SH3-domain ligation with PR).102–104 In breast cancer models, rapid progesterone-induced MAPK activation (i.e. downstream of c-Src) or CDK2 robustly phosphorylates PR-B (but not PR-A) at Ser294.65, 89 In this context, PR-B Ser294 phosphorylation is required for regulation of selected target genes in either the absence (ex. IRS-2, STC1) or presence (ex. MSX2, RGS2, MAP1A, PDK4) of hormone.90 Thus, as expected, we observed robust ligand-induced phosphorylation of PR-B Ser294 in intact ES-2 cells (Figure 1D). However, in stark contrast to breast cancer models (i.e. where PR-A is not well phosphorylated on Ser29457, 61), we were surprised to detect phosphorylation of PR-A Ser294 in intact ES-2 cells (Figure 1D). In breast cancer models, PR-B Ser294 phosphorylation confers hypersensitivity to progestin, increases the rate of ubiquitinylation of PR-B (an activation step for several SRs105), delays SUMOylation on K38857, promotes ligand-independent transcriptional activity106, and profoundly alters promoter selectivity.90 Ligand- or growth-factor-induced PR-A Ser294 phosphorylation is generally undetectable.57, 61 Consistent with these findings, we demonstrated that PR-A K388 SUMOylation (a transcriptionally repressive modification) occurs very rapidly (15 mins) and is sustained relative to PR-B in response to progestin or progestin plus growth factor (EGF) treatment.57 Surprisingly, herein, we observed increased PR-A Ser294 phosphorylation when FOXO1-AAA was expressed in ES-2 ovarian cancer models. Similar results were observed in PR-B+ cells (Supplemental Figure 5) and the FOXO1 inhibitor blocked Ser294 phosphorylation of both isoforms (Figure 7E). These data suggest that activated FOXO1 may serve to co-recruit protein kinases capable of phosphorylating Ser294 (MAPKs, CDKs) to PR-containing transcriptional complexes. Taken together, our data suggest that increased PR-A Ser294 phosphorylation in the presence of activated FOXO1 may account for its ability to transactivate PR-B-specific target genes when these isoforms are co-expressed in PR-B+ cells (Figure 7). PR-A and PR-B cross-talk in the context of multiple post-translational modifications, including SUMOylation, is a topic of further study.
FOXO1 as a critical cofactor in ovarian cancer biology
Herein, our data reveal a mechanism whereby PR isoforms may maintain either distinct or overlapping transcriptional gene programs via context-dependent upregulation of key PR cofactors. PR-B but not PR-A induces FOXO1, the same pathway cofactor it requires to induce robust p21-dependent (i.e. via PR-B/FOXO1 complexes) cellular senescence in ovarian cancer cells.21 In contrast, the relative in-availability of FOXO1 severely limits hormone-sensitivity in ovarian cancer cells expressing PR-A. In PR-A+ ES-2 cells, ligand-independent repression of FOXO1 mRNA may account for concomitant minimal regulation of p21 and p15 mRNAs and weak induction of cellular senescence when FOXO1 levels are also relatively low. Surprisingly, exogenous expression of active FOXO1 (FOXO1-AAA) restored hormone sensitivity in PR-A+ cells and conferred PR-B-like transcriptional activity at selected PR-B target genes (p21, GZMA, IGFBP1). In this context (PR-A plus activated FOXO1-AAA), progestin induced a robust cellular senescence phenotype similar to that observed in PR-B+ cells. Ultimately, our study underscores the critical importance of FOXO1 as a required cofactor for PR-dependent actions in the ovary.
Steroid hormone action in ovarian cancer is grossly understudied relative to breast or prostate cancers. There is an urgent need to focus on the early events related to the contribution of hormones in the context of altered signaling events (loss of p53 or PTEN, activation of AKT signaling) that may predispose women to increased risk of ovarian cancer. Deregulation of FOXO1 is associated with tumorigenesis and cancer progression across multiple cancer types. A recent PAthway Representation and Analysis by Direct Inference on Graphical Models (PARADIGM) analysis of mRNA expression and copy number data revealed 80 significant pathways altered in primary ovarian serous cystadenocarcinomas; the FoxO family signaling pathway was identified as significantly altered in this subtype.107 FOXO1 expression and activity is downregulated through alterations in upstream regulators, post-translational deregulation, or by genetic mutations108. Notably, AKT negatively regulates FOXO1 through phosphorylation and prevents FOXO1 nuclear accumulation, thus impairing target gene regulation.108 In other studies, AKT-dependent phosphorylation of FOXO1 was identified as both a mechanism of FOXO1 nuclear export and protein turnover/downregulation.46, 86 Activating mutations of PI3Ks or inactivating mutations (i.e. functional loss) of PTEN are common early events in ovarian cancer. For example, activated AKT (i.e. downstream of these events) may impair PR-induced senescence signaling by nuclear exclusion of FOXO1 (Figure 8E). The early loss or inactivation of FOXO1 may render PR “incompetent” at genes required for the induction of cellular senescence, leading to the loss of protective “sensing” by progesterone in ovarian tumors, and perhaps also in early PR+ breast tumors. Notably, analysis of The Cancer Genome Atlas data revealed significantly decreased FOXO1 mRNA expression in breast tumors relative to normal breast tissue (p<2.2×10−16). Thus, we propose that FOXO1 acts as a “molecular switch” that confers highly hormone-sensitive cell growth inhibition/senescence to PR+ epithelial cells, but may allow progesterone to stimulate proliferation in a highly context-dependent manner. Upon loss of FOXO1, PR interaction with other available co-factors may mediate profound changes in gene expression and associated cell biology (i.e. from senescence induction to activation of proliferative/pro-survival programs). For example, STAT3 and STAT5 appear to be primary cofactors that direct PR-B-selective gene expression and increased breast cancer cell proliferation and survival.109–111 Furthermore, in proliferating (S-phase) breast cancer models, CK2 confers ligand-independent PR-B target gene selection via phosphorylation of Ser81 (not found on PR-A) and recruitment of MKP3/DUSP6.111 These examples illustrate how early genetic events (i.e. mutations that alter kinase signaling) have profound consequences on SR-mediated (i.e. epigenetic) regulation of gene programs and associated changes in cell and cancer biology.
Our data highlight the requirement of FOXO1 for PR transcriptional regulation and specificity. A previous study demonstrated that constitutively active FOXO1 enhanced the ligand-dependent transcriptional activity of PR-A in PRE-luciferase assays.36 One caveat of luciferase assays is that they primarily measure SR transcriptional activity when bound to minimal promoter elements in artificial contexts, limiting interpretations relevant to promoter selectivity during transcriptional regulation of endogenous gene promoters (i.e. in chromatin). Thus, while PR-A may appear to be functional” in PRE-luciferase assays, it may repress selected endogenous genes in the context of chromatin. Herein, we validated that FOXO1 enhanced the ligand-dependent transcriptional activity of PR-A as measured via the levels of endogenous p21, GZMA, and IGFBP1 mRNAs (ES-2 cells; and in HeLa cells for p21) and by readout of PRE-luciferase (HeLa cells). As noted above, regulation of FOXO1 is complex. Notably, progestins transiently activated AKT in our ES-2 cells (Figure 6A). In this context, FOXO1 protein turnover (in response to R5020) is likely more visible in PR-A expressing cells relative to PR-B expressing cells (Figure 4F) since progestins activate AKT in cells expressing either isoform (Figure 6A), but only PR-B (but not PR-A) induces robust FOXO1 mRNA and protein expression (Figure 4). When we stably expressed the constitutively active FOXO1-AAA in PR-A+ ES-2 lacking detectable FOXO1, the sensitivity of PR-A to hormone was greatly enhanced and this formerly repressive isoform transactivated the p21 promoter (Figure 6). We conclude that constitutively active FOXO1 modulates PR-A hormone-sensitivity and influences the specificity of gene regulation, thereby mimicking the actions of PR-B.
Conclusions and Future Directions
In light of our findings herein, PR-B-dominance in ovarian tumors is intriguing. Early loss or inactivation of FOXO1 (i.e. via functional loss of PTEN and/or activation of PI3K/AKT signaling) is predicted to block induction of the senescence pathway, effectively “lifting” progesterone-mediated protection in PR-B+ ovarian cancer cells. It is tempting to speculate that in the absence of active FOXO1, PR-B acts as a driver of a proliferative/pro-survival transcriptional program similar to that observed in breast cancer models67, 90, 100, 111 In support of this concept, flow cytometry experiments revealed a large spike in the percentage of PR-B+ ES-2 cells in S phase upon FOXO1 knock-down relative to shRNA controls.21 The context-dependent actions of PR-B in ovarian cancer models with chronically elevated AKT (i.e. inactivated FOXO1) signaling are a topic of further investigation.
While total PR levels are routinely measured in breast and endometrial cancers for clinical management and disease treatment, very few studies have examined the levels of PR isoforms in ovarian cancers. We collected a total of seven primary ovarian tumors and detected a dominance of PR-B-only in four tumors, while three tumors co-expressed PR-B and PR-A. In the ex vivo culture system, three high-grade serous ovarian tumors recapitulated our in vitro findings of PR-B-dependent induction of FOXO1 and/or p21 upon progestin treatment. As discussed above, aside from considerable tumor heterogeneity, several factors may account for lack of sensitivity to progestin in the other ovarian tumors; unresponsive tumors may harbor mutations that render FOXO1 inactive and unable to direct PR-dependent regulation of FOXO1 and p21 (i.e. required for senescence induction).
In summary, our data reveal distinct tissue-specific actions of PR-A and PR-B in ovarian relative to extensively studied breast cancer models. Our observations have important clinical implications with regard to the development of isoform-specific and/or tissue-selective progestins for endocrine therapies. The ability of PR-A to inhibit hyperplastic proliferation in the uterus, together with reduced proliferative activity when PR-A is activated in ovarian and mammary gland tissue strongly indicates that targeted activation of PR-A with isoform-specific agonists will likely have a protective effect against uterine, breast, and ovarian carcinogenesis. Conversely, it may be desirable to inhibit the actions of PR-B, particularly when FOXO1 is inactivated in the context of heightened kinase signaling that is a hallmark of hormone-driven cancers. The quantification of PR isoforms and their key cofactors and target genes (i.e. FOXO1, p21) rather than total PR levels will be essential to improving the efficacy of disease management by isoform-specific selective PR modulators (SPRMs).
Supplementary Material
Implications.
This study indicates FOXO1 as a critical component for progesterone signaling to promote cellular senescence and reveals a novel mechanism for transcription factor control of hormone sensitivity.
Acknowledgements
We would like to thank members of the University of Minnesota Masonic Cancer Center’s Flow Cytometry Core Facility, the University Imaging Centers, the Minnesota Supercomputing Institute, and the University of Minnesota Genomics Center for their assistance in data acquisition, processing, and analysis. CDB-4124 (Proellex) was a kind gift from Ronald Wiehle (Repros Therapeutics). This study was supported by National Institutes of Health (NIH) grant R01 CA159712 and R01 CA159712-S1 (to C.A.L.), Cancer Biology Training Grant NIH T32 CA009138 (to C.H.D), National Center for Advancing Translational Sciences of the National Institutes of Health Award UL1TR000114 (to C.H.D), and a University of Minnesota Doctoral Dissertation Fellowship (C.H.D.).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
Conception and design: C.H. Diep and C.A. Lange
Development of methodology: C.H. Diep
Acquisition of data: C.H. Diep
Analysis and interpretation of data (e.g. statistical analysis, computational analysis of microarray data): C.H. Diep, T.P. Knutson, and C.A. Lange
Writing, review, and/or revision of manuscript: C.H. Diep and C.A. Lange
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute. 2015 [Google Scholar]
- 2.Edmondson RJ, Monaghan JM. The epidemiology of ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2001;11:423–429. doi: 10.1046/j.1525-1438.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 3.Gabra H, Watson JE, Taylor KJ, Mackay J, Leonard RC, Steel CM, Porteous DJ, Smyth JF. Definition and refinement of a region of loss of heterozygosity at 11q23.3-q24.3 in epithelial ovarian cancer associated with poor prognosis. Cancer Res. 1996;56:950–954. [PubMed] [Google Scholar]
- 4.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiologic reviews. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 5.Banks E, Beral V, Reeves G. The epidemiology of epithelial ovarian cancer: a review. International Journal of Gynecological Cancer. 1997;7:425–438. [Google Scholar]
- 6.Collaborative Group on Hormonal Factors in Breast C. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DJ, Colditz GA, Hankinson SE, Malspeis S, Spiegelman D, Chen W, Stampfer MJ, Willett WC. Oral contraceptive use and breast cancer: a prospective study of young women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:2496–2502. doi: 10.1158/1055-9965.EPI-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Effect of depo-medroxyprogesterone acetate on breast cancer risk among women 20 to 44 years of age. Cancer Res. 2012;72:2028–2035. doi: 10.1158/0008-5472.CAN-11-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstetrics and gynecology. 2014;124:292–299. doi: 10.1097/AOG.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson SE, Colditz GA, Hunter DJ, Spencer TL, Rosner B, Stampfer MJ. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstetrics and gynecology. 1992;80:708–714. [PubMed] [Google Scholar]
- 11.Schildkraut JM, Calingaert B, Marchbanks PA, Moorman PG, Rodriguez GC. Impact of progestin and estrogen potency in oral contraceptives on ovarian cancer risk. Journal of the National Cancer Institute. 2002;94:32–38. doi: 10.1093/jnci/94.1.32. [DOI] [PubMed] [Google Scholar]
- 12.Hempling RE, Piver MS, Eltabbakh GH, Recio FO. Progesterone receptor status is a significant prognostic variable of progression-free survival in advanced epithelial ovarian cancer. American journal of clinical oncology. 1998;21:447–451. doi: 10.1097/00000421-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Munstedt K, Steen J, Knauf AG, Buch T, von Georgi R, Franke FE. Steroid hormone receptors and long term survival in invasive ovarian cancer. Cancer. 2000;89:1783–1791. doi: 10.1002/1097-0142(20001015)89:8<1783::aid-cncr19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Sinn BV, Darb-Esfahani S, Wirtz RM, Budczies J, Sehouli J, Chekerov R, Dietel M, Denkert C. Evaluation of a hormone receptor-positive ovarian carcinoma subtype with a favourable prognosis by determination of progesterone receptor and oestrogen receptor 1 mRNA expression in formalin-fixed paraffin-embedded tissue. Histopathology. 2011;59:918–927. doi: 10.1111/j.1365-2559.2011.04028.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindgren P, Backstrom T, Mahlck CG, Ridderheim M, Cajander S. Steroid receptors and hormones in relation to cell proliferation and apoptosis in poorly differentiated epithelial ovarian tumors. International journal of oncology. 2001;19:31–38. [PubMed] [Google Scholar]
- 16.Akahira J, Inoue T, Suzuki T, Ito K, Konno R, Sato S, Moriya T, Okamura K, Yajima A, Sasano H. Progesterone receptor isoforms A and B in human epithelial ovarian carcinoma: immunohistochemical and RT-PCR studies. Br J Cancer. 2000;83:1488–1494. doi: 10.1054/bjoc.2000.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecologic oncology. 2005;96:671–677. doi: 10.1016/j.ygyno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Hogdall EV, Christensen L, Hogdall CK, Blaakaer J, Gayther S, Jacobs IJ, Christensen IJ, Kjaer SK. Prognostic value of estrogen receptor and progesterone receptor tumor expression in Danish ovarian cancer patients: from the 'MALOVA' ovarian cancer study. Oncology reports. 2007;18:1051–1059. [PubMed] [Google Scholar]
- 19.Tangjitgamol S, Manusirivithaya S, Khunnarong J, Jesadapatarakul S, Tanwanich S. Expressions of estrogen and progesterone receptors in epithelial ovarian cancer: a clinicopathologic study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2009;19:620–627. doi: 10.1111/IGC.0b013e3181a44b62. [DOI] [PubMed] [Google Scholar]
- 20.Yang XY, Xi MR, Yang KX, Yu H. Prognostic value of estrogen receptor and progesterone receptor status in young Chinese ovarian carcinoma patients. Gynecologic oncology. 2009;113:99–104. doi: 10.1016/j.ygyno.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle. 2013;12:1433–1449. doi: 10.4161/cc.24550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieh W, Kobel M, Longacre TA, Bowtell DD, Defazio A, Goodman MT, Hogdall E, Deen S, Wentzensen N, Moysich KB, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. The lancet oncology. 2013 doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. The Journal of biological chemistry. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 24.Stoecklin E, Wissler M, Schaetzle D, Pfitzner E, Groner B. Interactions in the transcriptional regulation exerted by Stat5 and by members of the steroid hormone receptor family. The Journal of steroid biochemistry and molecular biology. 1999;69:195–204. doi: 10.1016/s0960-0760(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 25.Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio C, et al. Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Molecular and cellular biology. 2004;24:7260–7274. doi: 10.1128/MCB.24.16.7260-7274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, Nawaz Z, Yee D, Barr FG, Diab SG, et al. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. The Journal of biological chemistry. 2001;276:27907–27912. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 27.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 28.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 29.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. The Journal of biological chemistry. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 32.McGowan EM, Russell AJ, Boonyaratanakornkit V, Saunders DN, Lehrbach GM, Sergio CM, Musgrove EA, Edwards DP, Sutherland RL. Progestins reinitiate cell cycle progression in antiestrogen-arrested breast cancer cells through the B-isoform of progesterone receptor. Cancer Res. 2007;67:8942–8951. doi: 10.1158/0008-5472.CAN-07-1255. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Molecular and cellular biology. 2003;23:104–118. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. The Journal of biological chemistry. 2007;282:7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 35.Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. The Journal of biological chemistry. 2001;276:33554–33560. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- 36.Rudd MD, Gonzalez-Robayna I, Hernandez-Gonzalez I, Weigel NL, Bingman WE, 3rd, Richards JS. Constitutively active FOXO1a and a DNA-binding domain mutant exhibit distinct co-regulatory functions to enhance progesterone receptor A activity. Journal of molecular endocrinology. 2007;38:673–690. doi: 10.1677/JME-07-0017. [DOI] [PubMed] [Google Scholar]
- 37.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biology of reproduction. 2005;73:833–839. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarpin KM, Graham JD, Mote PA, Clarke CL. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nuclear receptor signaling. 2009;7:e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akahira J, Suzuki T, Ito K, Kaneko C, Darnel AD, Moriya T, Okamura K, Yaegashi N, Sasano H. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Japanese journal of cancer research : Gann. 2002;93:807–815. doi: 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lenhard M, Tereza L, Heublein S, Ditsch N, Himsl I, Mayr D, Friese K, Jeschke U. Steroid hormone receptor expression in ovarian cancer: Progesterone receptor B as prognostic marker for patient survival. BMC cancer. 2012;12:553. doi: 10.1186/1471-2407-12-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Molecular and cellular biology. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centenera MM, Gillis JL, Hanson AR, Jindal S, Taylor RA, Risbridger GP, Sutherland PD, Scher HI, Raj GV, Knudsen KE, et al. Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin Cancer Res. 2012;18:3562–3570. doi: 10.1158/1078-0432.CCR-12-0782. [DOI] [PubMed] [Google Scholar]
- 44.Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, Liu F, Planck JL, Ravindranathan P, Chinnaiyan AM, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer discovery. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, Knudsen ES. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle. 2012;11:2756–2761. doi: 10.4161/cc.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. The Journal of biological chemistry. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 47.Sartorius CA, Groshong SD, Miller LA, Powell RL, Tung L, Takimoto GS, Horwitz KB. New T47D breast cancer cell lines for the independent study of progesterone B-and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54:3868–3877. [PubMed] [Google Scholar]
- 48.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [ http://www.R-project.org]. [Google Scholar]
- 49.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 51.Smyth GK. Bioinformatics and computational biology solutions using R and Bioconductor. Springer; 2005. Limma: linear models for microarray data; pp. 397–420. [Google Scholar]
- 52.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 53.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC bioinformatics. 2010;11:367. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IWF, Heine AA, Smid M, et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9:e103988. doi: 10.1371/journal.pone.0103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nature communications. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim CS, Baumann CT, Htun H, Xian W, Irie M, Smith CL, Hager GL. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol Endocrinol. 1999;13:366–375. doi: 10.1210/mend.13.3.0247. [DOI] [PubMed] [Google Scholar]
- 57.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 58.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Molecular and cellular biology. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 62.Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol Endocrinol. 2005;19:574–587. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- 63.Jacobsen BM, Richer JK, Schittone SA, Horwitz KB. New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. The Journal of biological chemistry. 2002;277:27793–27800. doi: 10.1074/jbc.M202584200. [DOI] [PubMed] [Google Scholar]
- 64.Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13:385–396. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 65.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Molecular and cellular biology. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Molecular and cellular biology. 2011;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daniel AR, Gaviglio AL, Knutson TP, Ostrander JH, D'Assoro AB, Ravindranathan P, Peng Y, Raj GV, Yee D, Lange CA. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2014 doi: 10.1038/onc.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibbs GM, Roelants K, O'Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocrine reviews. 2008;29:865–897. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- 69.Desnoyers L, Arnott D, Pennica D. WISP-1 binds to decorin and biglycan. The Journal of biological chemistry. 2001;276:47599–47607. doi: 10.1074/jbc.M108339200. [DOI] [PubMed] [Google Scholar]
- 70.Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, Hanifi-Moghaddam P, Schink J, Kim JJ. Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis. Hormones & cancer. 2011;2:170–181. doi: 10.1007/s12672-011-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mielczarek-Palacz A, Sikora J, Kondera-Anasz Z, Bednarek I. Cytotoxic reaction mediators: granzymes A and B in women with ovarian cancer. Tissue antigens. 2014;83:409–413. doi: 10.1111/tan.12347. [DOI] [PubMed] [Google Scholar]
- 72.Liu Z, Zhan Y, Tu Y, Chen K, Liu Z, Wu C. PDZ and LIM domain protein 1(PDLIM1)/CLP36 promotes breast cancer cell migration, invasion and metastasis through interaction with alpha-actinin. Oncogene. 2015;34:1300–1311. doi: 10.1038/onc.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 74.Damm F, Thol F, Hollink I, Zimmermann M, Reinhardt K, van den Heuvel-Eibrink MM, Zwaan CM, de Haas V, Creutzig U, Klusmann JH, et al. Prevalence and prognostic value of IDH1 and IDH2 mutations in childhood AML: a study of the AML-BFM and DCOG study groups. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25:1704–1710. doi: 10.1038/leu.2011.142. [DOI] [PubMed] [Google Scholar]
- 75.Lauvrak SU, Munthe E, Kresse SH, Stratford EW, Namlos HM, Meza-Zepeda LA, Myklebost O. Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J Cancer. 2013;109:2228–2236. doi: 10.1038/bjc.2013.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swarthout JT, Lobo S, Farh L, Croke MR, Greentree WK, Deschenes RJ, Linder ME. DHHC9 and GCP16 constitute a human protein fatty acyltransferase with specificity for H- and N-Ras. The Journal of biological chemistry. 2005;280:31141–31148. doi: 10.1074/jbc.M504113200. [DOI] [PubMed] [Google Scholar]
- 77.Young E, Zheng ZY, Wilkins AD, Jeong HT, Li M, Lichtarge O, Chang EC. Regulation of Ras localization and cell transformation by evolutionarily conserved palmitoyltransferases. Molecular and cellular biology. 2014;34:374–385. doi: 10.1128/MCB.01248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, Liu T, Zhang Y, Brown M, Liu XS. A comprehensive view of nuclear receptor cancer cistromes. Cancer Res. 2011;71:6940–6947. doi: 10.1158/0008-5472.CAN-11-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 81.Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, Shibasaki M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Molecular pharmacology. 2010;78:961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 82.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. The Journal of biological chemistry. 2009;284:10334–10342. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sagare-Patil V, Vernekar M, Galvankar M, Modi D. Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol Cell Endocrinol. 2013;374:82–91. doi: 10.1016/j.mce.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Alkhalaf M, El-Mowafy A, Karam S. Growth inhibition of MCF-7 human breast cancer cells by progesterone is associated with cell differentiation and phosphorylation of Akt protein. Eur J Cancer Prev. 2002;11:481–488. doi: 10.1097/00008469-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nature genetics. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 86.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. The Journal of biological chemistry. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 87.Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. Journal of molecular endocrinology. 2015;54:R31–R53. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beck CA, Zhang Y, Weigel NL, Edwards DP. Two types of anti-progestins have distinct effects on site-specific phosphorylation of human progesterone receptor. The Journal of biological chemistry. 1996;271:1209–1217. doi: 10.1074/jbc.271.2.1209. [DOI] [PubMed] [Google Scholar]
- 89.Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- 90.Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, Lange CA. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast cancer research : BCR. 2012;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- 92.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 93.Abdel-Hafiz H, Dudevoir ML, Horwitz KB. Mechanisms underlying the control of progesterone receptor transcriptional activity by SUMOylation. The Journal of biological chemistry. 2009;284:9099–9108. doi: 10.1074/jbc.M805226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei LL, Krett NL, Francis MD, Gordon DF, Wood WM, O'Malley BW, Horwitz KB. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Mol Endocrinol. 1988;2:62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- 96.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. The EMBO journal. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janzen DM, Rosales MA, Paik DY, Lee DS, Smith DA, Witte ON, Iruela-Arispe ML, Memarzadeh S. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013;73:4697–4710. doi: 10.1158/0008-5472.CAN-13-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pathiraja TN, Shetty PB, Jelinek J, He R, Hartmaier R, Margossian AL, Hilsenbeck SG, Issa JP, Oesterreich S. Progesterone receptor isoform-specific promoter methylation: association of PRA promoter methylation with worse outcome in breast cancer patients. Clin Cancer Res. 2011;17:4177–4186. doi: 10.1158/1078-0432.CCR-10-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dressing GE, Knutson TP, Schiewer MJ, Daniel AR, Hagan CR, Diep CH, Knudsen KE, Lange CA. Progesterone receptor-cyclin D1 complexes induce cell cycle-dependent transcriptional programs in breast cancer cells. Mol Endocrinol. 2014;28:442–457. doi: 10.1210/me.2013-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS One. 2012;7:e35859. doi: 10.1371/journal.pone.0035859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. The EMBO journal. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Molecular cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 104.Saitoh M, Ohmichi M, Takahashi K, Kawagoe J, Ohta T, Doshida M, Takahashi T, Igarashi H, Mori-Abe A, Du B, et al. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-kappaB cascade in human breast cancer cells. Endocrinology. 2005;146:4917–4925. doi: 10.1210/en.2004-1535. [DOI] [PubMed] [Google Scholar]
- 105.Salghetti SE, Caudy AA, Chenoweth JG, Tansey WP. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- 106.Daniel AR, Knutson TP, Lange CA. Signaling inputs to progesterone receptor gene regulation and promoter selectivity. Mol Cell Endocrinol. 2009;308:47–52. doi: 10.1016/j.mce.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harvard BIoMa. Broad Institute TCGA Genome Data Analysis Center (2015): PARADIGM pathway analysis of mRNA expression and copy number data. 2015 [Google Scholar]
- 108.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 109.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, et al. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src- dependent mechanism in breast cancer cells. Molecular and cellular biology. 2005;25:4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Obr AE, Grimm SL, Bishop KA, Pike JW, Lydon JP, Edwards DP. Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol Endocrinol. 2013;27:1808–1824. doi: 10.1210/me.2013-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hagan CR, Knutson TP, Lange CA. A Common Docking Domain in Progesterone Receptor-B links DUSP6 and CK2 signaling to proliferative transcriptional programs in breast cancer cells. Nucleic Acids Res. 2013;41:8926–8942. doi: 10.1093/nar/gkt706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.