Abstract
The autoimmune regulator (Aire) was initially identified as the gene causing multiorgan system autoimmunity in humans, and deletion of this gene in mice also resulted in organ-specific autoimmunity. Aire regulates the expression of tissue-specific antigens (TSAs) in medullary thymic epithelial cells (mTECs), which play a critical role in the negative selection of autoreactive T cells and the generation of regulatory T cells. More recently, the role of Aire in the development of mTECs have helped elucidate its ability to present the spectrum of TSAs needed to prevent autoimmunity. Molecular characterization of the functional domains of Aire have revealed multiple binding partners that assist Aire’s function in altering gene transcription and chromatin remodeling. These recent advances have further highlighted the importance of Aire in central tolerance.
Keywords: Aire, mTEC, APS-1, autoimmunity
Introduction
The immune system has evolved a complex set of responses to fight off unwanted infections through the generation of a T cell repertoire that can recognize a broad array of antigens. The unfortunate consequence of such diversity is the potential for the generation of an autoreactive T cell that recognizes a self-antigen. To combat this problem, the immune system has evolved elegant tolerance mechanisms to prevent their generation and to keep them in check when present.
Over the last decade, there have been rapid advances in our understanding of critical pathways that regulate immune tolerance, and many of these recent insights have relied, in part, on genetics and rare Mendelian forms of autoimmunity. Study of immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, a rare monogenic disorder due to Foxp3 mutations, helped establish the critical role of T regulatory (Treg) cells in preventing organ-specific autoimmunity.1 Defects in innate sensing pathways also play a critical role in tolerance, as shown by studies of patients with Aicardi-Goutières syndrome, a disease characterized by neurological dysfunction and lupus-like features.2 Among these findings has been the discovery of the function of the autoimmune regulator (Aire) gene. It has become clear that Aire plays a key role in establishing T cell tolerance to self, particularly in the thymus. Here, we review some of the recent progress in elucidating the function of Aire and its effects on the immune system.
Autoimmune polyglandular syndrome type 1: a Mendelian organ-specific autoimmune syndrome
The role of Aire in immune tolerance was first made evident with its identification as the causative gene for mediating multiorgan system autoimmunity, termed autoimmune polyglandular syndrome type 1 (APS-1) or autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED).3,4 It is primarily considered an autosomal recessive disorder that occurs with variable frequency, depending on ethnic background. Studies have now identified autosomal dominant mutations that cluster in plant homeodomain 1 (PHD1) and one unique mutation (G228W) in the SAND domain.5–7 These patients typically develop the classic constellation of autoimmune adrenocortical failure (i.e., Addison’s disease), autoimmune hypoparathyroidism, and chronic mucocutaneous candidiasis, as well as other autoimmune manifestations.8–11 The disease usually starts in childhood, evolving over time and having heterogeneity in its organ involvement. These patients develop a spectrum of autoantibodies to organ tissue mediating immune destruction.12 More recently, it was found that patients develop autoantibodies to cytokines, such as type I interferons (IFN-ω and IFN-α) and T helper (TH)17-related cytokines (IL-22, IL-17F, and IL-17A).13–15 Almost all patients with Aire deficiency develop type I interferon autoantibody, which serves as an excellent biomarker for this disease. Interestingly, the expected clinical consequences of increased viral susceptibility due to the presence of these autoantibodies do not occur. On the other hand, autoantibodies to the TH17-related cytokines do correlate with candidiasis, supporting the role of the TH17 pathway in providing immunity to candidal infection.14,15
It is now appreciated that, in addition to genetic defects, acquired defects in Aire activity may occur. For example, reduced Aire expression can be present in a type of thymic tumor known as a thymoma, whose presence is often associated with autoimmunity.16 Some of these patients interestingly develop myasthenia gravis with autoantibodies such as anti-acetylcholine receptors. They rarely show the symptoms that are seen in APS-1 patients.17 These two disease processes stemming from a deficiency in Aire both result in autoimmunity, suggesting a clear role of this gene in breaking immune tolerance. To better understand the underlying mechanism, four research groups have generated Aire-deficient mouse models that shared features similar to human APS-1 patients.18–21,22 These mice develop lymphocytic infiltrates similar to those in humans, but in different organs such as the salivary gland, eye, reproductive organs, and liver.19,23 They also develop tissue-specific autoantibodies, as do APS-1 patients.19,23 Taken together, Aire deficiency, whether in humans or mice, leads to autoimmunity, clearly highlighting the importance of this gene in immune tolerance.
Immunological functions of Aire
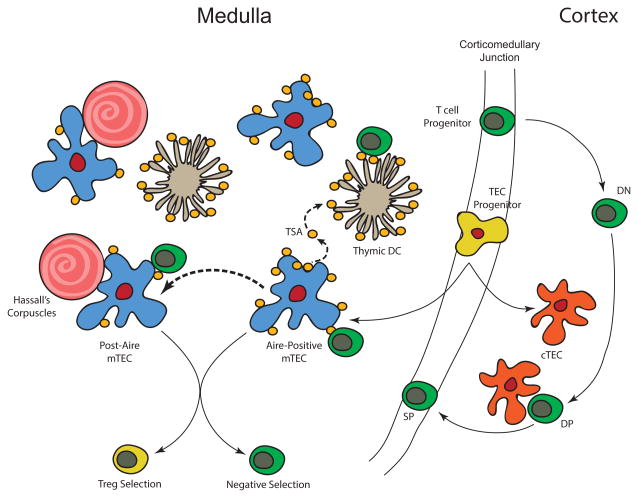
We have now come to appreciate that Aire plays a key role in thymic tolerance (Fig. 1). Aire is primarily expressed in the thymic medulla, suggesting a possible role in T cell tolerance, and was shown to express various tissue-specific genes within the thymus in cells known as medullary thymic epithelial cells (mTECs). Aire knockout mice show reduced expression of a subset of tissue-specific genes, which correlate with the specific organ involvement and associated autoantibodies. Taken together, the functions of Aire clearly affect thymic tolerance.
Figure 1.
The role of Aire in central tolerance. Central tolerance primarily occurs in the medullary compartment of the thymus where T cells develop (T cell progenitor to double-negative (DN) T cells to double-positive (DP) T cells and single-positive (SP) T cells). Aire is expressed in medullary thymic epithelial cells (mTECs) derived from TEC progenitors located in the corticomedullary junction of the thymus. These Aire+ mTECs express tissue-specific antigens (TSAs) and can hand-off these antigens to thymic dendritic cells (DCs). Post-Aire cells also express TSAs but at a lower level and are found near Hassall’s corpuscles. Expression of TSAs in the medulla affect Treg cell generation and negative selection of T cells, which are critical for maintaining central tolerance.
The role of Aire in negative selection
The role of Aire in negative selection (i.e., deletion of autoreactive cells) was first illustrated using a transgenic mouse system bearing a neoantigen and a specific T cell receptor recognizing the neoantigen. The absence of Aire allows these autoreactive T cells to escape into the periphery where, if Aire was present, these autoreactive cells would be deleted,23,24 which is also seen if there is a TCR transgene specific for self-antigens.25,26 In addition, endogenous T cells directed to a specific self-antigen can be detected in the periphery in the absence of Aire, and their presence is correlated with disease.26 Deletion is primarily mediated by Aire expression in mTECs, and recent data suggest that thymic B cells may also express low levels of Aire and participate in T cell selection.27
Aire in Treg cell selection
Initial data did not strongly support a role for Aire in Treg cell generation. Treg cell numbers and frequency are generally similar between Aire-deficient and wild-type mice, and the transfer of Treg cells did not prevent or rescue autoimmunity in Aire-deficient mice.23,28 However, patients with AIRE mutations show decreased Treg cell numbers, and in vitro suppression assays revealed defects suggesting a role of Aire in Treg cells.29,30
To further explore the effects of Aire on Treg cell biology, a transgenic system expressing hemagglutinin (HA) on mTECs using the Aire promoter provided initial insight that Treg cells can be selected by specific Aire-driven antigen on mTECs.31 These data were further strengthened in the context of an endogenous antigen that was Aire dependent; in this model, antigen-specific Treg cells failed to develop in the absence of Aire, clearly illustrating the role of Aire in the selection of specific Treg cells.32
Given how Aire affects specific Treg cell selection, one could speculate that Aire would likely affect the Treg TCR repertoire. To evaluate this possibility, the Treg TCR repertoire was examined in mice with fixed TCRb with limited TCRa arrangements. It was found that the Treg TCR repertoire was similar between Aire-sufficient and Aire-deficient mice;33 however, this limited TCR repertoire may have excluded specific TCRs that are Aire dependent. With new and improved sequencing methodology, a broader and more natural TCR repertoire analysis was performed on mice with a fixed TCRβ chain with the endogenous Tcra locus. In this model, TCR repertoire differences were revealed, and Aire specifically affected those Treg TCRs that were lower in frequency, which may explain why such differences were not initially seen in a limited TCR repertoire model analysis.34
In addition to its effects on Treg cell selection, Aire may also play a role in the generation of unique Treg cell subsets that are generated during the perinatal period.35 These perinatal Treg cells have been shown to be better suppressors in vitro and may operate more efficiently in vivo in the adoptive transfer setting. How Aire influences this unique population of Treg cells remains open to question, as the exact antigen specificities for potential Aire-dependent Treg cells in this setting have not been determined. Previous work by two independent studies have shown that grafting of Aire wild-type and knockout thymic lobes into a single mouse was not sufficient to prevent autoimmunity.20,23 If Treg cell selection was the primary problem in the Aire-deficient model, the result of these latter experiments would be predicted to be protection against autoimmunity. Taken together, it does appear that Aire can influence the Treg cell repertoire; however, the degree to which this contributes to autoimmunity in the Aire-deficient setting remains open to debate.
The role of Aire in peripheral tolerance
Aire also potentially plays a role in peripheral tolerance. In humans, Aire has been found in lymph nodes at both the transcript and protein levels,4,36 and in mice, the detection of peripheral Aire had been more challenging until the generation of an Aire-reporter mouse line. Aire expression was then found to be present in secondary lymphoid organs, such as peripheral lymph nodes and spleen.37 These Aire-expressing cells are bone marrow derived and are similar to dendritic cells (DCs) in cell morphology, expression of Zbtb46, and presence of certain markers such as CD45 and CD11c, although at a lower level than DCs. Expression profiling of these cells revealed a distinct pattern that is different from both plasmacytoid and conventional DCs.37,38 These characteristics suggest a potentially new cell population that has been termed extrathymic Aire-expressing cells (eTACs).
Functionally, eTACs may play a role in maintaining peripheral T cell tolerance. When eTACs were engineered to express a specific antigen, the antigen-specific CD8+ T cells were deleted.37 In contrast, antigen-specific CD4+ T cells were rendered anergic. These antigen-specific CD4+ T cells had elevated PD-1 levels and other markers associated with tolerance, and they were unable to produce IFN-γ. TCR signaling in these CD4+ T cells are impaired in the ability to increase calcium and activate the Ras–MAPK pathway by phosphorylating ERK. The eTACs were found to be unresponsive to inflammatory signals that thus did not upregulate costimulatory markers, such as CD80, supporting their ability to lead to T cell anergy.38 Altogether, the data suggest eTACs as a potential new cell population that may play a role in peripheral T cell tolerance, complementing the important tolerogenic role of Aire in the thymus.
In addition to eTACs, two other cell types have also recently been observed to express Aire. Through the use of an Aire-GFP BAC transgenic reporter, thymic B cells can, to some extent, acquire Aire expression.27 Interestingly, this expression appears to occur through a CD40 signaling pathway and requires cognate help interactions with developing thymocytes. Additionally, these thymic B cells can contribute to thymic selection in transgenic models. It remains unclear how Aire is functioning in these cells, given that these Aire-expressing thymic B cells were not enriched for tissue-specific antigens (TSAs). In addition to thymic B cells, keratinocytes were also shown to express Aire under an inflammatory condition in an oncogenic-driven tumor model.39 In this setting, Aire itself may be a contributor to the transformation process, but precisely how this happens and how widespread this process is in other tumors remains to be determined.
New insights into mTEC development and turnover
Aire plays several roles in the immune system to prevent the development of autoimmunity. On a cellular level, it functions to upregulate expression of TSA, and it plays a role in the differentiation of a specialized subset of the thymus known as mTECs (Fig. 1).There is also some speculation that TSA expression may be linked to maturation of these cells.40
Aire controls TSA expression
It is well established that mTECs express a range of TSAs that play a role in negative selection and Treg cell generation. Previously, it was estimated that roughly 3000 genes were expressed in these cell types and play a role in the selection of T cells.41 However, this covers only a fraction of all known genes, suggesting incomplete self-antigen display for T cell selection, resulting in potential escape of autoreactive T cells. Recently, through the use of singlecell sequencing, a broader expression profile was identified, revealing that roughly 90% of all known genes are indeed expressed in TECs, including Aire+ mTECs.42 Many genes were previously masked because of low levels of expression in the bulk population.42 It was also recently by a comparison of expression profiles between cells that there is coordinated expression of gene clusters, which is speculated to be attributed to chromatin remodeling. While there is this coordinated expression of gene clusters on a single-cell level, the establishment of the gene cluster appears to be stochastic since there is variation on an individual level.43,44 Furthermore, the TECs can also hand-off these antigens to other thymic antigen presenting cells, such as DCs.34,45 Collectively, these cells allow for a wide breath of antigen presentation, resulting in improved T cell selection and self-tolerance.
Aire in medullary thymic epithelial cells
The role of Aire in mTEC development has been illustrated using an in vivo ablation system. Using an Aire-containing BAC transgenic model, diphtheria toxin receptor (DTR) was expressed on Aire+ mTECs to mark them for ablation, and deletion was controlled temporally with diphtheria toxin (DT) administration. Loss of these cells did result in decreased negative selection and Treg cell production as anticipated. Surprisingly, these ablated cells were able to recover after 5 days.46 Bromodeoxyuridine (BrdU) labeling experiments as well as fate mapping studies revealed that these Aire+ cells have a half-life of 7–14 days.46–48 Taken together, the data suggest a progenitor cell population that replenishes the Aire+ mTEC population.
Recent studies have identified this progenitor cell population using different methods (Fig. 1).49–51 One research group identified this progenitor cell line by identifying a rare cell population derived from adult mouse thymic stromal cells that formed thymospheres, a property of progenitor cells. They were Sca1 positive, and negative for CD24, CD45, and EpCam.49 The other group identified this progenitor population by subsetting adult thymic stromal cells using Sca1 and alpha-6 integrin, both of which are markers found in other epithelial progenitor cells50 and are localized to the corticomedullary junction. Both research groups found that these cells had low mitotic activity, self-renewal capacity, and an ability to give rise to cTEC and mTEC lineages, including Aire+ cells. These cells were also found in FoxN1-deficient mice, an athymic mouse line, suggesting that they are not FoxN1 dependent.49,50 Furthermore, it was demonstrated that these progenitor cells, specifically Cld3,4hi, could prevent the development of autoimmunity in the aly/aly mouse model.52 The identification of this progenitor cell identifies the cellular source for replenishing the mTECs that undergo rapid turnover, which helps ensure a near complete presentation of the self-antigen repertoire of mTECs, such that autoimmunity can be prevented through proper negative selection and Treg cell generation.
Preventing autoimmunity can also negatively affect the ability of the immune system to fend off tumors since the effector T cells against these tumor antigens may have been deleted or suppressed. Thus, temporary blockade of mTECs could provide a small window of opportunity for tumor-specific T cells to escape tolerance mechanisms. Studies have previously shown that RANK–RANKL and CD40–CD40L play a role in the development of Aire+ mTECs.53–56 RANK signaling blockade in vivo is able to deplete Aire+ mTECs,46,57 leading to alterations in negative selection by preventing thymic deletion and to loss of Treg cell development in both a restricted and polyclonal T cell repertorie.57 Anti-RANKL treatment in a B16 melanoma model allowed temporary escape of these self-antigen–specific T cells to target the tumor and improve survival.57 Modulation of the mTEC compartment could potentially be part of the immunotherapy regimen for treating cancers.
Post-Aire cells
Initial data suggested that Aire expression reflects the terminal mTEC state;47 however, new data have delineated a post-Aire state, using various fate-mapping mouse models.35,46,48,58–60 The post-Aire cell population is regulated by the lymphotoxin signaling pathway,58 whereas RANK and CD40L signaling may not play key roles as seen in the development of Aire+ mTECs.60 Furthermore, these post-Aire cells express intermediate levels of TSAs;46 are positive for involucin, a terminal maturation marker for keratinocytes; and have been associated with Hassall’s corpuscles structures found in human thymus.61 Hassall’s corpuscles have been suggested to play a role in Treg cell induction on the basis of identification of TSLP+ DCs in these structures and their generation of human Treg cells in an in vitro system. 62 Thus, it is tantalizing to speculate that these cells could be mediating this role in vivo.
Molecular mechanisms of Aire
Aire was initially hypothesized to behave as a transcription factor that turned on TSAs to facilitate negative selection of autoreactive thymocytes. However, several aspects of Aire and its gene expression pattern was not conducive to features of classical transcription factors. First, Aire regulates the expression of thousands of TSAs and thus it is unlikely that there is a consensus DNA sequence that Aire binds.63 Second, the transcriptional start sites were different from the endogenous start site, and Aire uses different transcriptional machinery.64 Third, the pattern of TSA expression varied between cell types.37,65–67 Taken together, the data show that Aire functions differently from classical transcription factors, given the diverse expression pattern.
Structural and functional domains of Aire
Insight into the molecular function of Aire arose from characterization of its structure and functional domains. Aire contains a caspase-recruitment domain (CARD), a SAND domain, two PHD fingers, and LXXLL motifs (Fig. 2). The CARD domain participates in oligomerization of Aire, and the PHD1 domain in Aire binds unmethylated histone H3 at lysine-4 (H3K4m0), which is associated with repressed areas of transcription.68–71 Recognition of this histone mark directs Aire to help in the expression of Aire-dependent TSAs, but it is not the primary mechanism for directing Aire to these target genes.72 The SAND domain binds other proteins, rather than DNA. The LXXLL motifs are thought to recruit Aire-binding partners. Taken together, the properties of Aire transcription and its link to epigenetics through the H3K4Me0 recognition motif suggest that Aire may use a fascinating array of mechanisms to target and promote TSA expression. Gaining insight on these pathways has been challenging because of limited cell numbers in vivo, but inroads have been made using transfection models.
Figure 2.
Aire protein domains. Aire contains multiple functional domains, including caspase-recruitment domain (CARD), SAND (Sp100, AIRE-1, NucP41/75, and DEAF-1) domain, two plant homeodomain (PHD) fingers, proline rich region (PRR), and four LXXLL motifs. These domains bind different Aire-binding partners and epigenetic marks that result in Aire’s unique functions.
Aire in transcription
Several Aire-binding partners play a role in directing transcription by elongation and potentially altering splicing (Fig. 3). The first Aire-binding protein identified was cyclic AMP response element–binding protein (CBP),73 which is a common transcriptional coactivator that colocalizes with Aire in the nucleus and together can drive expression of reporters and endogenous genes.74 The common Finnish mutation, R257X, disrupts the binding of Aire to CBP. CBP was shown to acetylate Aire in the SAND domain, which can alter the expression pattern for Aire-dependent TSAs.75
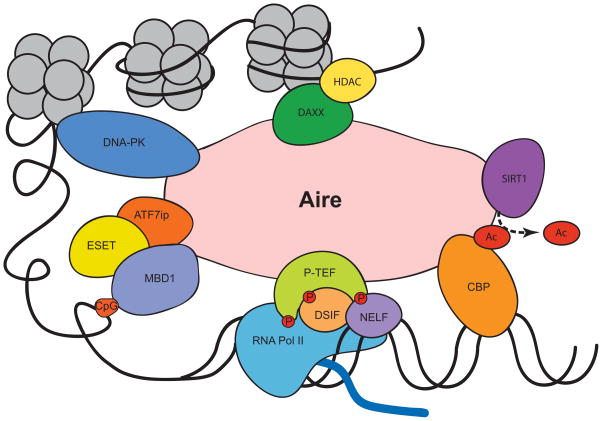
Figure 3.
Aire-binding partners. Aire binds to multiple protein partners, enabling an influence on both transcription and chromatin remodeling.
DNA-dependent protein kinase (DNA–PK) was identified in two different screens searching for Aire-binding partners.76,77 It has been speculated that the associated DNA–PK complex induces double-stranded DNA breaks and leads to removal of nucleosomes, allowing for expression of repressed genes.78 Recently, a patient with mutations in DNA–PK developed autoimmune symptoms; studies suggested an inability to express Aire-dependent TSAs, whereas Aire-independent TSAs were unaffected.79
Positive transcription elongation factor b (P-TEFb) is another protein that interacts with Aire and is positioned in areas of stalled transcription.80 P-TEFb phosphorylates RNA polymerase II, DRB sensitivity-inducing factor (DSIF), and negative elongation factor (NELF). Phosphorylation of these proteins leads to release of stalled RNA polymerase II, allowing for expression of TSA transcripts.81 Patients with a mutation causing a deletion of the C-terminus leads to an inability to bind P-TEFb and a block in TSA expression.82 RNAi screens identified additional factors that further support the role of P-TEFb in mediating Aire-directed TSA expression.83
Aire can also interact with proteins involved in splicing77 and can lead to increased splicing of mRNA.82 More recently, a reanalysis of microarray and RNA-Seq data from murine mTECs showed that the presence of Aire was associated with increased alternative spliced isoforms in TSAs, suggesting that the actions of Aire lead to increased exposure to alternative TSA isoforms.84
Recently, another protein, sirtuin-1 (SIRT1), was shown to affect TSA gene expression in Aire+ mTECs, and its deficiency led to organ-specific autoimmunity.85 Sirt1 is highly expressed in mTECs and in vitro assays suggest association with Aire. It deacetylates proteins such as Aire (acetylated by CBP), which is speculated to affect TSA expression in an Aire-dependent manner.85
Aire in chromatin remodeling
Given the ability of Aire to express a wide spectrum of TSAs, it has been speculated that Aire participates in chromatin remodeling, which opens up areas of repressed transcription, allowing transcription of a broad array of genes (Fig. 3). The genes regulated by Aire are clustered but do not have any specific chromosomal preferences, and the pattern is stochastic, suggesting a role for Aire in epigenetic mechanisms.64,86 Also, as mentioned above, the PHD1 domain recognizes histone mark, which lends further support for a role in chromatin remodeling.
One protein partner suggesting a role in this process is DAXX, which was identified from a yeast two-hybrid system using the HSR/CARD domain as bait. The W78R mutation, a known mutation present in APS-1 patients, disrupts binding of this protein to Aire. DAXX is known to bind histones and recruit histone deacetylases and also binds and recruits other proteins (Pax3, Pax5, p53, glucocorticoid receptor, and HDAC). Together, the actions of DAXX and its protein complex implicates Aire in epigenetics and can alter its accessibility to other transcriptional targets.87
Recently, our research group identified two new Aire-binding partners that provide additional evidence for Aire in chromatin remodeling. The MBD1–ATF7ip complex targets Aire to repressed areas of transcription with methylated CpG dinucleotides. In particular, the SAND domain, which was previously thought to be a potential DNA-binding domain but lacks the canonical sequence, interacts with ATF7ip. The dominant disease-causing G228W mutation is located in the SAND domain and disrupts the interaction. Findings on this mechanism help elucidate how Aire can drive TSA expression in different cell types due to different methylation patterns based on the cellular environment.88 Taken together, it now appears that Aire likely exploits several different types of binding partners and methods to activate transcription of TSAs, and there may be other similar properties that remain to be identified.
Conclusions
Advancing our understanding of the function of Aire has provided insight into self-tolerance mechanisms and the development of autoimmunity, as well as the development cancer. More research is needed to further elucidate the molecular mechanisms by which Aire promotes TSA expression. It also remains to be determined if there are other factors in the thymus that may carry out similar activities to those of Aire, as there remains significant TSA expression in the thymus of Aire-deficient animals. Finally, recent work has demonstrated the potential for selective manipulation of thymic Aire-expressing cells, which could open the door to developing a potential therapy for diseases such as malignancy.
Acknowledgments
We would like to thank Jimmy Chen for assistance with the figures. This work was supported by the U.S. National Institutes of Health Grant R01 AI097457 (M.S.A.) and the Scientist Development Award from the Rheumatology Research Foundation (A.Y.C.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Frontiers in immunology. 2012:3. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crow YJ, Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nature Reviews Immunology. 2015 doi: 10.1038/nri3850. [DOI] [PubMed] [Google Scholar]
- 3.Aaltonen J, Björses P, Perheentupa J, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 4.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 5.Cetani F, Barbesino G, Borsari S, et al. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. The Journal of Clinical Endocrinology & Metabolism. 2001;86:4747–52. doi: 10.1210/jcem.86.10.7884. [DOI] [PubMed] [Google Scholar]
- 6.Ilmarinen T, Eskelin P, Halonen M, et al. Functional analysis of SAND mutations in AIRE supports dominant inheritance of the G228W mutation. Hum Mutat. 2005;26:322. doi: 10.1002/humu.20224. [DOI] [PubMed] [Google Scholar]
- 7.Oftedal BE, Hellesen A, Erichsen MM, et al. Dominant Mutations in the Autoimmune Regulator AIRE Are Associated with Common Organ-Specific Autoimmune Diseases. Immunity. 2015;42:1185–96. doi: 10.1016/j.immuni.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 8.SPINNER MW, BLIZZARD RM, CHILDS B. Clinical and genetic heterogeneity in idiopathic Addison's disease and hypoparathyroidism. The Journal of Clinical Endocrinology & Metabolism. 1968;28:795–804. doi: 10.1210/jcem-28-6-795. [DOI] [PubMed] [Google Scholar]
- 9.Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:154–62. [PubMed] [Google Scholar]
- 10.Ahonen P. Autoimmune polyendocrinopathy–candidosis–ectodermal dystrophy (APECED): autosomal recessive inheritance. Clin Genet. 1985;27:535–42. doi: 10.1111/j.1399-0004.1985.tb02037.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–36. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 12.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann N Y Acad Sci. 2011;1246:77–91. doi: 10.1111/j.1749-6632.2011.06308.x. [DOI] [PubMed] [Google Scholar]
- 13.Meager A, Visvalingam K, Peterson P, et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS medicine. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarpino S, Di Napoli A, Stoppacciaro A, et al. Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas. Clinical & Experimental Immunology. 2007;149:504–12. doi: 10.1111/j.1365-2249.2007.03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ströbel P, Murumägi A, Klein R, et al. Deficiency of the autoimmune regulator AIRE in thymomas is insufficient to elicit autoimmune polyendocrinopathy syndrome type 1 (APS-1) J Pathol. 2007;211:563–71. doi: 10.1002/path.2141. [DOI] [PubMed] [Google Scholar]
- 18.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey C, Winqvist O, Puhakka L, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda N, Mitani T, Takeda N, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–70. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 21.Hubert FX, Kinkel SA, Crewther PE, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182:3902–18. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 23.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 25.Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013;73:2104–16. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi RT, DeVoss JJ, Moon JJ, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A. 2012;109:7847–52. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamano T, Nedjic J, Hinterberger M, et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42:1048–61. doi: 10.1016/j.immuni.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Lei Y, Ripen AM, Ishimaru N, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–94. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kekalainen E, Tuovinen H, Joensuu J, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–15. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 30.Laakso SM, Laurinolli T, Rossi LH, et al. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3< sup> </sup> precursors and impaired activated population. J Autoimmun. 2010;35:351–7. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Aschenbrenner K, D'Cruz LM, Vollmann EH, et al. Selection of Foxp3 regulatory T cells specific for self antigen expressed and presented by Aire medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 32.Malchow S, Leventhal DS, Nishi S, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–24. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 2010;184:6865–73. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry JS, Lio CW, Kau AL, et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–26. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–94. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poliani PL, Kisand K, Marrella V, et al. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. The American journal of pathology. 2010;176:1104–12. doi: 10.2353/ajpath.2010.090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner JM, Devoss JJ, Friedman RS, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–7. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner JM, Metzger TC, McMahon EJ, et al. Extrathymic< i> Aire</i>-Expressing Cells Are a Distinct Bone Marrow-Derived Population that Induce Functional Inactivation of CD4< sup> </sup> T Cells. Immunity. 2013;39:560–72. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobbs RP, Lessard JC, Coulombe PA. Keratin intermediate filament proteins - novel regulators of inflammation and immunity in skin. J Cell Sci. 2012;125:5257–8. doi: 10.1242/jcs.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto M, Nishikawa Y, Nishijima H, Morimoto J, Matsumoto M, Mouri Y. Which model better fits the role of aire in the establishment of self-tolerance: the transcription model or the maturation model? Front Immunol. 2013;4:210. doi: 10.3389/fimmu.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyewski B, Derbinski J. Self-representation in the thymus: an extended view. Nature Reviews Immunology. 2004;4:688–98. doi: 10.1038/nri1436. [DOI] [PubMed] [Google Scholar]
- 42.Sansom SN, Shikama-Dorn N, Zhanybekova S, et al. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24:1918–31. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennecke P, Reyes A, Pinto S, et al. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat Immunol. 2015 doi: 10.1038/ni.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meredith M, Zemmour D, Mathis D, Benoist C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat Immunol. 2015;16:942–9. doi: 10.1038/ni.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metzger TC, Khan IS, Gardner JM, et al. Lineage tracing and cell ablation identify a post-aire-expressing thymic epithelial cell population. Cell reports. 2013;5:166–79. doi: 10.1016/j.celrep.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–8. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishikawa Y, Nishijima H, Matsumoto M, et al. Temporal lineage tracing of Aire-expressing cells reveals a requirement for Aire in their maturation program. J Immunol. 2014;192:2585–92. doi: 10.4049/jimmunol.1302786. [DOI] [PubMed] [Google Scholar]
- 49.Ucar A, Ucar O, Klug P, et al. Adult thymus contains FoxN1(-) epithelial stem cells that are bipotent for medullary and cortical thymic epithelial lineages. Immunity. 2014;41:257–69. doi: 10.1016/j.immuni.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong K, Lister NL, Barsanti M, et al. Multilineage potential and self-renewal define an epithelial progenitor cell population in the adult thymus. Cell Rep. 2014;8:1198–209. doi: 10.1016/j.celrep.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Hamazaki Y, Fujita H, Kobayashi T, et al. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat Immunol. 2007;8:304–11. doi: 10.1038/ni1438. [DOI] [PubMed] [Google Scholar]
- 52.Sekai M, Hamazaki Y, Minato N. Medullary Thymic Epithelial Stem Cells Maintain a Functional Thymus to Ensure Lifelong Central T Cell Tolerance. Immunity. 2014;41:753–61. doi: 10.1016/j.immuni.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Rossi SW, Kim MY, Leibbrandt A, et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–72. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akiyama T, Shimo Y, Yanai H, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–37. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 55.Hikosaka Y, Nitta T, Ohigashi I, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–50. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Roberts NA, White AJ, Jenkinson WE, et al. Rank signaling links the development of invariant γδ T cell progenitors and Aire medullary epithelium. Immunity. 2012;36:427–37. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan IS, Mouchess ML, Zhu ML, et al. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med. 2014;211:761–8. doi: 10.1084/jem.20131889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White AJ, Nakamura K, Jenkinson WE, et al. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185:4769–76. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishikawa Y, Hirota F, Yano M, et al. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J Exp Med. 2010;207:963–71. doi: 10.1084/jem.20092144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Laan M, Bichele R, Kisand K, Scott HS, Peterson P. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Frontiers in immunology. 2012:3. doi: 10.3389/fimmu.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yano M, Kuroda N, Han H, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–38. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe N, Wang Y, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+ CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 63.Derbinski J, Gabler J, Brors B, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villasenor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci U S A. 2008;105:15854–9. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerau-de-Arellano M, Mathis D, Benoist C. Transcriptional impact of Aire varies with cell type. Proc Natl Acad Sci U S A. 2008;105:14011–6. doi: 10.1073/pnas.0806616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci U S A. 2008;105:15860–5. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taubert R, Schwendemann J, Kyewski B. Highly variable expression of tissue-restricted self-antigens in human thymus: Implications for self-tolerance and autoimmunity. Eur J Immunol. 2007;37:838–48. doi: 10.1002/eji.200636962. [DOI] [PubMed] [Google Scholar]
- 68.Chignola F, Hetenyi C, Gaetani M, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–6. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakravarty S, Zeng L, Zhou M. Structure and site-specific recognition of histone H3 by the PHD finger of human autoimmune regulator. Structure. 2009;17:670–9. doi: 10.1016/j.str.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chignola F, Gaetani M, Rebane A, et al. The solution structure of the first PHD finger of autoimmune regulator in complex with non-modified histone H3 tail reveals the antagonistic role of H3R2 methylation. Nucleic Acids Res. 2009;37:2951–61. doi: 10.1093/nar/gkp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh AS, Kuo AJ, Park SY, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proc Natl Acad Sci U S A. 2008;105:15878–83. doi: 10.1073/pnas.0808470105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 2010;107:13016–21. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pitkanen J, Doucas V, Sternsdorf T, et al. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J Biol Chem. 2000;275:16802–9. doi: 10.1074/jbc.M908944199. [DOI] [PubMed] [Google Scholar]
- 74.Pitkänen J, Rebane A, Rowell J, et al. Cooperative activation of transcription by autoimmune regulator AIRE and CBP. Biochem Biophys Res Commun. 2005;333:944–53. doi: 10.1016/j.bbrc.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 75.Saare M, Rebane A, Rajashekar B, Vilo J, Peterson P. Autoimmune regulator is acetylated by transcription coactivator CBP/p300. Exp Cell Res. 2012;318:1767–78. doi: 10.1016/j.yexcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 76.Liiv I, Rebane A, Saare M, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2008;1783:74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–35. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 78.Mahaney B, Meek K, Lees-Miller S. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–50. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathieu AL, Verronese E, Rice GI, et al. PRKDC mutations associated with immunodeficiency, granuloma, and autoimmune regulator-dependent autoimmunity. J Allergy Clin Immunol. 2015;135:1578–1588.e5. doi: 10.1016/j.jaci.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–23. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giraud M, Yoshida H, Abramson J, et al. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proc Natl Acad Sci U S A. 2012;109:535–40. doi: 10.1073/pnas.1119351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zumer K, Plemenitas A, Saksela K, Peterlin BM. Patient mutation in AIRE disrupts P-TEFb binding and target gene transcription. Nucleic Acids Res. 2011;39:7908–19. doi: 10.1093/nar/gkr527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giraud M, Jmari N, Du L, et al. An RNAi screen for Aire cofactors reveals a role for Hnrnpl in polymerase release and Aire-activated ectopic transcription. Proc Natl Acad Sci U S A. 2014;111:1491–6. doi: 10.1073/pnas.1323535111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keane P, Ceredig R, Seoighe C. Promiscuous mRNA splicing under the control of AIRE in medullary thymic epithelial cells. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu785. [DOI] [PubMed] [Google Scholar]
- 85.Chuprin A, Avin A, Goldfarb Y, et al. The deacetylase Sirt1 is an essential regulator of Aire-mediated induction of central immunological tolerance. Nat Immunol. 2015 doi: 10.1038/ni.3194. [DOI] [PubMed] [Google Scholar]
- 86.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–66. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meloni A, Fiorillo E, Corda D, et al. DAXX is a new AIRE-interacting protein. J Biol Chem. 2010;285:13012–21. doi: 10.1074/jbc.M109.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waterfield M, Khan IS, Cortez JT, et al. The transcriptional regulator Aire coopts the repressive ATF7ip-MBD1 complex for the induction of immunotolerance. Nat Immunol. 2014;15:258–65. doi: 10.1038/ni.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]