Summary
The mechanism of colonization of intercellular spaces by the soil‐borne and vascular plant‐pathogenic bacterium Ralstonia solanacearum strain OE1‐1 after invasion into host plants remains unclear. To analyse the behaviour of OE1‐1 cells in intercellular spaces, tomato leaves with the lower epidermis layers excised after infiltration with OE1‐1 were observed under a scanning electron microscope. OE1‐1 cells formed microcolonies on the surfaces of tomato cells adjacent to intercellular spaces, and then aggregated surrounded by an extracellular matrix, forming mature biofilm structures. Furthermore, OE1‐1 cells produced mushroom‐type biofilms when incubated in fluids of apoplasts including intercellular spaces, but not xylem fluids from tomato plants. This is the first report of biofilm formation by R. solanacearum on host plant cells after invasion into intercellular spaces and mushroom‐type biofilms produced by R. solanacearum in vitro. Sugar application led to enhanced biofilm formation by OE1‐1. Mutation of lecM encoding a lectin, RS‐IIL, which reportedly exhibits affinity for these sugars, led to a significant decrease in biofilm formation. Colonization in intercellular spaces was significantly decreased in the lecM mutant, leading to a loss of virulence on tomato plants. Complementation of the lecM mutant with native lecM resulted in the recovery of mushroom‐type biofilms and virulence on tomato plants. Together, our findings indicate that OE1‐1 produces mature biofilms on the surfaces of tomato cells after invasion into intercellular spaces. RS‐IIL may contribute to biofilm formation by OE1‐1, which is required for OE1‐1 virulence.
Keywords: biofilm, intercellular spaces, lectin, Ralstonia solanacearum, soil‐borne vascular plant‐pathogenic bacterium, virulence
Introduction
Bacteria adhere to and grow on almost any surface, forming architecturally complex communities, termed biofilms (Branda et al., 2005; Hall‐Stoodley and Stoodley, 2009). Phytopathogenic bacteria reportedly produce biofilm‐like aggregations in xylem vessels of host plants, as well as on the surfaces of roots and leaves, but not in intercellular spaces (Danhorn and Fuqua, 2007; Mansfield et al., 2012). Biofilm development contributes to the virulence of phytopathogenic bacteria through various mechanisms, including the blockage of xylem vessels, increased resistance to plant antimicrobial compounds and/or enhanced colonization of specific habitats.
Biofilm formation by Gram‐negative proteobacteria is a temporal process involving transitions through distinct stages of multicellular organization. These stages have been operationally identified as planktonic, surface attachment, microcolony formation, biofilm formation and dispersal (Monds and O'Toole, 2009). Microcolony formation can happen by either clonal growth of attached cells or active translocation of cells across the surface. Microcolonies grow in size and coalesce to form biofilms. After the formation of microcolonies, the production of quorum‐sensing signals is required for the formation of mature biofilms (Bogino et al., 2013; Rinaudi and Giordano, 2010). Biofilms can dissolve, releasing cells from the biofilm. Biofilms are assemblages of microorganisms embedded in a matrix of extracellular polymers that adhere to each other and to a surface, thereby enabling adaptation to fluctuating environmental conditions in a social manner (Davey and O'Toole, 2000; Morris and Monier, 2003). Biofilm configurations range in complexity from flat, relatively featureless films to tightly clustered aggregates to complex heterogeneous cellular arrangements, such as towers and streamers (Parsek and Fuqua, 2004; Ramey et al., 2004; Webb et al., 2003).
Bacterial wilt caused by Ralstonia solanacearum (Yabuuchi et al., 1995) is one of the most devastating bacterial plant diseases in the tropics, the subtropics and warm temperature regions worldwide (Hayward, 1991). Ralstonia solanacearum is a soil‐borne bacterium that normally invades plant roots from the soil through wounds or natural openings where secondary roots emerge (Araud‐Razou et al., 1998), colonizes intercellular spaces of the root cortex and vascular parenchyma, and eventually enters xylem vessels and spreads up into the stems and leaves through the xylem (Vasse et al., 1995). Its high level of multiplication leads to wilting symptoms as a result of reduced sap flow caused by the presence of a large number of bacterial cells and exopolysaccharide (EPS) slime produced by the bacterium in some xylem vessels (Genin and Denny, 2012). Thus, we can speculate that R. solanacearum cells produce biofilm‐like aggregations on the surfaces of xylem vessels. It has also been reported that R. solanacearum forms biofilm‐like aggregations on tomato seedling roots (Kang et al., 2002; Yao and Allen, 2006). However, the structures and mechanisms of the biofilm produced by R. solanacearum have not been analysed. Furthermore, the involvement of biofilm formation in its virulence has not been elucidated.
The expression of pathogenicity factors in R. solanacearum is controlled by a complex regulatory network that responds to environmental conditions, the presence of host cells and bacterial density (Schell, 2000). At the centre of this network is a LysR family transcriptional regulator, PhcA (Brumbley et al., 1993), which, directly or through intermediary regulatory genes, coordinates the expression of several virulence factors (Huang et al., 1995). In particular, the function of PhcA is regulated in response to cell density by a quorum‐sensing mechanism (Flavier et al., 1997).
Ralstonia solanacearum possesses hrp (hypersensitive response and pathogenicity) genes encoding structural constituents of the type III secretion system (T3SS), which translocates effector proteins out of the cell (Van Gijsegem et al., 1995). This activation is sensed by the outer membrane receptor PrhA, which transduces signals through the PrhI and PrhR two‐component and complex regulatory cascade integrated by PrhJ, HrpG and HrpB regulators (Aldon et al., 2000; Brito et al., 1999, 2002; Valls et al., 2006). Interestingly, functional PhcA suppresses expression of the prhIR operon, leading to suppression of hrp gene expression (Genin et al., 2005; Yoshimochi et al., 2009).
When R. solanacearum invades intercellular spaces, tobacco plants first induce Sec14P‐mediated phospholipid signalling and produce phosphatidic acid in chloroplast membranes, leading to the induction of jasmonic acid‐ and reactive oxygen‐mediated defence (Kiba et al., 2014, 2012). Tobacco plants invaded by the virulent OE1‐1 strain of R. solanacearum induce the expression of the phosphatidic acid phosphatase gene, dephosphorylating phosphatidic acid into diacylglycerol (Nakano et al., 2013). This leads to the suppression of jasmonic acid‐ and reactive oxygen‐mediated defence. Then, OE1‐1 can colonize intercellular spaces. The expression of the phosphatidic acid phosphatase gene is not induced by infection with T3SS‐deficient mutants. Furthermore, the T3SS‐deficient mutants cannot colonize intercellular spaces and lose their virulence (Kanda et al., 2003a). Therefore, the colonization in intercellular spaces is required for R. solanacearum virulence. However, the mechanism by which R. solanacearum colonizes intercellular spaces remains unknown.
A method for the synchronized invasion of R. solanacearum into the intercellular spaces of roots has not been developed. To investigate the precise kinetics of bacterial gene expression in plants, we used plant leaves infiltrated with bacteria (Kanda et al., 2003a, 2003b, 2008, 2009; Shinohara et al., 2005). Mutants of virulence‐related genes grew similarly in leaves inoculated by leaf infiltration and roots inoculated by root dipping. Furthermore, the mutants showed the same virulence on host plants inoculated by leaf infiltration as those inoculated by root dipping. In this study, to precisely analyse the mechanism by which R. solanacearum strain OE1‐1 colonizes intercellular spaces, the behaviour of OE1‐1 cells was observed in the intercellular spaces of tomato leaves infiltrated with OE1‐1. Observation under a scanning electron microscope (SEM) showed that OE1‐1 cells produce biofilm structures on tomato cells adjacent to intercellular spaces. We also found mushroom‐type biofilms produced by OE1‐1 cells incubated in fluids of apoplasts including intercellular spaces, but not xylem fluids from tomato plants. Interestingly, sugar application led to enhanced biofilm formation by OE1‐1. Using the OE1‐1 biofilm formation system, the involvement of lecM, which encodes a lectin RS‐IIL, in biofilm formation was analysed.
Results
Cells of R. solanacearum strain OE1‐1, after invasion into the intercellular spaces of tomato leaves, produce biofilm‐like structures
We first created a green fluorescent protein (GFP)‐labelled OE1‐1: gOE1‐1. gOE1‐1 was a transformant of OE1‐1 containing the GFP open reading frame from pCmGFP (Srikhanta et al., 2009) fused to a constitutively active promoter of RSc0297 from OE1‐1 integrated into the bacterial chromosome via transposon Tn7 (Y. Mori and Y. Hikichi, unpublished data). Leaves of 5‐week‐old tomato plants were infiltrated with gOE1‐1 suspension in a 20‐μL volume at an optical density at 600 nm (OD600) of 0.1 using a 1‐mL disposable syringe. The gOE1‐1‐derived fluorescence was observed under a fluorescent phase contrast microscope. The gOE1‐1‐derived fluorescence was dispersed throughout the intercellular spaces and aggregated at 18 h post‐infiltration (Fig. 1).
Figure 1.

Colonization of intercellular spaces of tomato leaves by Ralstonia solanacearum strain OE1‐1. Fluorescence from green fluorescent protein (GFP)‐labelled R. solanacearum strain OE1‐1 (gOE1‐1) in intercellular spaces of tomato leaves 18 h after infiltration was observed under a fluorescent phase contrast microscope.
To assess whether the aggregation of OE1‐1 cells in intercellular spaces is involved in biofilm production by OE1‐1 cells, tomato leaves with the lower epidermis layers excised at 18 and 24 h post‐infiltration with OE1‐1 were observed under a SEM. OE1‐1 cells formed microcolonies on the surfaces of tomato cells adjacent to intercellular spaces at 18 h post‐infiltration (Fig. 2a), and then aggregated surrounded by an extracellular matrix and formed biofilm‐like structures at 24 h post‐infiltration (Fig. 2b). Furthermore, planktonic cells, which are thought to be released from dissolving biofilm, were also observed at 24 h post‐infiltration (Fig. 2b). No bacteria were observed in the intercellular spaces of non‐inoculated tomato leaves (Fig. 2c).
Figure 2.

Formation of a microcolony (a) and a biofilm (b) produced by Ralstonia solanacearum strain OE1‐1 on the surfaces of tomato cells adjacent to intercellular spaces. Tomato leaves with the lower epidermis layers excised at 18 h (a) and 24 h (b) post‐infiltration with OE1‐1, and non‐inoculated leaf with the lower epidermis layers excised at 24 h (c), were observed under a scanning electron microscope. Arrows show planktonic OE1‐1 cells released from the biofilm structure. Scale bar, 5 μm.
Cells of R. solanacearum strain OE1‐1 incubated in apoplast fluids from tomato plants produce mushroom‐type biofilms
To assess the biofilm formation of OE1‐1 cells in vitro, we analysed OE1‐1 cells incubated in apoplast fluids (Shinohara et al., 2005; de Wit and Spikman, 1982; Zuluaga et al., 2013) and xylem fluids (Shinohara et al., 2005; Zuluaga et al., 2013) from tomato plants. OE1‐1 was incubated in apoplast fluids and xylem fluids in polyvinylchloride (PVC) microtitre plate wells for 24 h and the bacterial density reached OD600 = 0.8. Under these conditions, OE1‐1 cells incubated in apoplast fluids produced significantly more biofilm than those incubated in xylem fluids (Fig. 3a).
Figure 3.
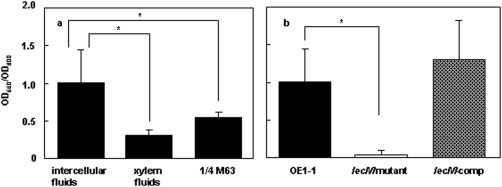
Biofilm formation by Ralstonia solanacearum strains in vitro. (a) Biofilm formation by R. solanacearum strain OE1‐1 incubated in apoplast fluids and xylem fluids from tomato plants, and in ¼ × M63 medium in PVC plate wells stained with crystal violet. (b) Biofilm formation by strains OE1‐1, OE1‐1‐lecM::EZ‐Tn5 (lecM mutant) and the lecM mutant complemented with native lecM (lecM‐comp) incubated in ¼ × M63 medium in PVC plate wells stained with crystal violet. Asterisks indicate significant difference from wild‐type cells growing in apoplast fluids (P < 0.05, t‐test).
Investigations of living bacterial biofilms using advanced microscopy have shown that these sessile communities often consist of mushroom‐shaped complex multicellular structures (Klausen et al., 2003). To analyse the configuration of biofilm structures, OE1‐1 was incubated on nano‐percolators for 24 h, when the bacterial density reached OD600 = 0.6. The configurations of biofilms produced by OE1‐1 were observed under a SEM. We observed tightly clustered aggregates of OE1‐1 cells incubated in apoplast fluids (Fig. 4a) and mature mushroom‐type biofilm structures (Fig. 4b). Furthermore, planktonic cells, which are thought to be released from dissolving mushroom‐type biofilm, were also observed (Fig. 4c). The biofilms of OE1‐1 cells incubated in xylem fluids were significantly less developed than those in apoplast fluids (Fig. 4d). We observed clustered aggregates of OE1‐1 cells, but did not observe any mature mushroom‐type biofilm produced by OE1‐1 (Fig. 4e).
Figure 4.

Observation of biofilm configurations produced by Ralstonia solanacearum strains under a scanning electron microscope. OE1‐1 was incubated in apoplast fluids (a–c) and xylem fluids (d, e) from tomato plants on nano‐percolators. OE1‐1‐lecM::EZ‐Tn5 (lecM mutant) (f, g) and the lecM mutant complemented with native lecM (lecM‐comp) (h) were incubated in apoplast fluids from tomato plants on nano‐percolators. Arrows show planktonic OE1‐1 cells released from biofilm structures. Scale bar, 5 μm.
Sugars influence biofilm formation by cells of R. solanacearum strain OE1‐1
Zuluaga et al. (2013) reported that the tomato apoplast is significantly richer in sugars, such as sucrose, fructose, glucose, galactose and mannose, than the xylem, and R. solanacearum probably catabolizes abundant apoplast sugars. To elucidate why OE1‐1 cells produce significantly more biofilm in apoplast fluids than in xylem fluids, we analysed biofilm formation by OE1‐1 cells in ¼ × M63 medium, including 1.0% arabinose, fructose, fucose, galactose or mannose, in PVC plate wells stained with crystal violet. OE1‐1 incubated in ¼ × M63 medium produced significantly less biofilm than OE1‐1 incubated in apoplast fluids (Fig. 3a). OE1‐1 incubated in ¼ × M63 medium, including sugars in the order arabinose > mannose > fructose, produced significantly more biofilm than OE1‐1 incubated in ¼ × M63 medium (Fig. 5a).
Figure 5.

Biofilm formation by strains OE1‐1 (a) and OE1‐1‐lecM::EZ‐Tn5 (lecM mutant, b) incubated in ¼ × M63 medium containing 1.0% arabinose (Ara), fructose (Fru), fucose (Fuc), galactose (Gal) or mannose (Man) in PVC plate wells stained with crystal violet. Asterisks indicate significant difference from wild‐type cells growing in apoplast fluids (P < 0.05, t‐test).
lecM is involved in the colonization of intercellular spaces by R. solanacearum strain OE1‐1 and its virulence on tomato plants
Ralstonia solanacearum produces a lectin, RS‐IIL, encoded by lecM, with high sequence similarity to Pseudomonas aeruginosa lectin PA‐IIL (Sudakevitz et al., 2002, 2004). The deduced amino acid sequence showed that RS‐IIL has signal peptides in its N‐terminus (Sudakevitz et al., 2002, 2004). Non‐enzymatic proteins in the matrix, such as the cell surface‐associated and extracellular carbohydrate‐binding proteins, lectins, are involved in the formation and stabilization of the polysaccharide matrix network and constitute a link between the bacterial surface and EPSs, forming biofilms (Flemming and Wingender, 2010). RS‐IIL exhibits mannose, fructose, fucose, galactose and arabinose affinity (Sudakevitz et al., 2004).
To assess the involvement of lecM in the colonization of intercellular spaces by OE1‐1, leaves of 5‐week‐old tomato plants were infiltrated with R. solanacearum strains in a 20‐μL volume at OD600 = 0.1. The population of the lecM mutant (OE1‐1‐lecM::EZ‐Tn5) in infiltrated leaves was significantly less than that of OE1‐1 and the lecM mutant transformed with native lecM (Fig. 6a, lecM‐comp). All tomato plants wilted 10 days after inoculation with strains OE1‐1 and lecM‐comp (Fig. 7a). On the contrary, the lecM mutant lost its virulence on tomato plants (Fig. 7a).
Figure 6.

Population dynamics of Ralstonia solanacearum strains OE1‐1, OE1‐1‐lecM::EZ‐Tn5 (lecM mutant) and the lecM mutant complemented with native lecM (lecM‐comp) in leaves (a) and roots (b) of tomato plants inoculated by leaf infiltration and root dipping, respectively. Asterisks indicate significant difference from the wild‐type (P < 0.05, t‐test). CFU, colony‐forming unit.
Figure 7.

Virulence of Ralstonia solanacearum strains on tomato plants. Bacterial wilt on 8‐week‐old tomato plants inoculated by leaf infiltration (a) and root dipping (b) with R. solanacearum strains OE1‐1, OE1‐1‐lecM::EZ‐Tn5 (lecM mutant) and the lecM mutant complemented with native lecM (lecM‐comp). Plants were rated on the following disease index scale: 0, no wilting; 1, 1%–25% wilting; 2, 26%–50% wilting; 3, 51%–75% wilting; 4, 76%–99% wilting; 5, dead.
When inoculated by root dipping, the population of the lecM mutant in roots was significantly lower than that of OE1‐1 and lecM‐comp (Fig. 6b), and the lecM mutant lost its virulence (Fig. 7b). lecM‐comp recovered its virulence on tomato plants, similar to OE1‐1.
The lecM mutation leads to reduced attachment ability
Ralstonia solanacearum produces biofilms on both abiotic and biotic surfaces (Kang et al., 2002; Meng et al., 2011; Yao and Allen, 2006; Zhang et al., 2014). During biofilm formation, cells of strain OE1‐1 first attach to the surface and then produce flat microcolonies, followed by mushroom‐type biofilms. We observed the attachment ability of strains incubated in apoplast fluids under a phase contrast microscope. OE1‐1 cells attached to cellulose membranes (Fig. 8a), similarly to glass slides (Fig. 8b). Attachment of the lecM mutant to glass slides (Fig. 8c) was significantly less than that of OE1‐1 (Fig. 8b) and lecM‐comp (Fig. 8d).
Figure 8.

Attachment ability and microcolony formation of Ralstonia solanacearum strains. Nitrocellulose membranes (2 × 2 cm2) prepared from Visking cellulose tubes (a) and glass slides (b–d) were soaked in suspensions of R. solanacearum strains OE1‐1 (a, b), OE1‐1‐lecM::EZ‐Tn5 (lecM mutant, c) and the lecM mutant complemented with native lecM (lecM‐comp, d).
The lecM mutation leads to reduced biofilm formation
We next analysed the biofilm formation by R. solanacearum strains in apoplast fluids in PVC plate wells stained with crystal violet. The strains were incubated in apoplast fluids in PVC microtitre plate wells for 24 h (bacterial density at OD600 = 0.8). Under these conditions, the lecM mutant produced significantly less biofilm than OE1‐1 and lecM‐comp (Fig. 3b).
To analyse the configurations of biofilms produced by the strains, they were incubated in apoplast fluids on nano‐percolators for 24 h (bacterial density at OD600 = 0.6). The configurations of the biofilms produced by the strains under these conditions were observed under a SEM. The lecM mutant cells aggregated significantly less than did OE1‐1 (Fig. 4f) and produced immature biofilms (Fig. 4g). Mushroom‐type biofilm structures produced by lecM‐comp were observed (Fig. 4h).
RS‐IIL shows affinity for sugars in the order d‐mannose > d‐fructose > l‐fucose > l‐galactose > d‐arabinose (Sudakevitz et al., 2004). Furthermore, the application of d‐arabinose, d‐mannose and d‐fructose enhanced biofilm formation by OE1‐1 cells (Fig. 5a). We analysed the biofilm formation by lecM mutant cells in ¼ × M63 medium, including each of these sugars at 1.0%, in PVC plate wells stained with crystal violet. No sugar application influenced the biofilm formation by lecM mutant cells (Fig. 5b).
Expression of lecM depends on both HrpG and PhcA
The expression of lecM was reported to be regulated positively by HrpG (Valls et al., 2006). However, biofilm formation of R. solanacearum depends on functional PhcA activated by quorum sensing (Genin and Denny, 2012), and functional PhcA represses the expression of the hrp regulon (Genin et al., 2005; Yoshimochi et al., 2009). We speculated that, if lecM is involved in biofilm formation, lecM expression may be dependent not only on HrpG, but also PhcA. We investigated the expression of lecM in strain OE1‐1, a hrpG mutant and a phcA‐deleted mutant grown in ¼ × M63 medium at OD600 = 0.01 and OD600 = 0.3 using real‐time quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) assays. The expression of lecM in OE1‐1 at OD600 = 0.01 was significantly lower than at OD600 = 0.3 (Fig. 9). At both OD600 = 0.01 and OD600 = 0.3, the expression of lecM in the hrpG mutant was significantly lower than in OE1‐1. Furthermore, the expression of lecM in the phcA‐deleted mutant was also significantly lower than in OE1‐1 at both OD600 = 0.01 and OD600 = 0.3. These results demonstrate that OE1‐1 at OD600 = 0.3 expresses lecM more strongly than at OD600 = 0.01, and lecM expression depends on HrpG and PhcA.
Figure 9.
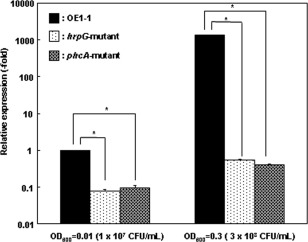
Expression of lecM in Ralstonia solanacearum strain OE1‐1 cultured in apoplast fluids from tomato plants, harvested at an optical density at 600 nm (OD600) of 0.01 and 0.3, and used for RNA extraction as described in Experimental procedures. The rpoD gene was used as an internal control for quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). The RNA levels of the analysed genes are expressed relative to the rpoD expression level. The experiment was performed at least twice using independent batches of samples with similar results. Results from a single representative sample are shown. The means ± standard deviation (SD) (error bars) of three determinations from cDNA from a single representative sample are shown. CFU, colony‐forming units.
The lecM mutation leads to reduced EPS I productivity
Bacterial surface components and extracellular compounds, such as EPSs, as well as primarily flagella and lipopolysaccharides are crucial for auto‐aggregation and biofilm development in most bacterial species (Bogino et al., 2013). Therefore, we quantified the total major EPS, EPS I, produced by comparable numbers (1.0 × 107 colony‐forming units (CFU), OD600 = 0.01) of R. solanacearum cells growing on ¼ × M63 plates using enzyme‐linked immunosorbent assay (ELISA). The lecM mutant produced significantly less EPS I than did OE1‐1 and lecM‐comp (Fig. 10).
Figure 10.

Immunological quantification of exopolysaccharide I (EPS I) in supernatants using enzyme‐linked immunosorbent assay (ELISA) with anti‐R. solanacearum EPS I antibodies [optical density at 650 nm (OD650)]. Asterisks indicate significant differences in OD650 values from wild‐type cells (P < 0.05, t‐test).
Discussion
We have focused on the colonization of intercellular spaces by strain OE1‐1, which is required for its virulence (Hikichi et al., 2007). Microscopic observations in this study showed aggregation of R. solanacearum cells in the intercellular spaces of tomato plants (Figs 1 and 2a) and biofilm formation on the surfaces of tomato cells adjacent to intercellular spaces (Fig. 2b). Furthermore, OE1‐1 cells produced mature mushroom‐type biofilms when incubated in apoplast fluids from tomato plants (Fig. 4a–c). These results demonstrate that OE1‐1 cells attach to tomato cells and produce mature biofilms, resulting in the colonization of intercellular spaces. To our knowledge, this is the first report of biofilm formation by R. solanacearum on host plant cells after invasion into intercellular spaces and of mushroom‐type biofilms produced by R. solanacearum in vitro.
Some proteins in the biofilm matrix have structural functions. Examples of such extracellular proteins include PA‐IL and PA‐IIL in Pseudomonas aeruginosa (Diggle et al., 2006; Tielker et al., 2005). These two lectins have been shown to be closely associated with the virulence of the bacterium, contributing to its biofilm‐producing aggregation (Gilboa‐Garber, 1986), host specificity (Gilboa‐Garber, 1996) and virulence (Gilboa‐Garber and Garber, 1989). A lectin, RS‐IIL, encoded by lecM, shows high sequence similarity to PA‐IIL (Sudakevitz et al., 2004). Mutation of lecM led to a significant decrease in biofilm formation (Fig. 3b). The lecM mutant did not produce any mushroom‐type biofilms when incubated in apoplast fluids from tomato plants (Fig. 4g). Furthermore, the mutant showed significantly less colonization of intercellular spaces than the wild‐type strain OE1‐1 (Fig. 6), losing its virulence on tomato plants inoculated using not only the leaf infiltration method (Fig. 7a), but also the root dipping method (Fig. 7b). Therefore, RS‐IIL encoded by lecM is involved in biofilm formation by the vascular plant‐pathogenic bacterium R. solanacearum strain OE1‐1 on the surfaces of tomato cells adjacent to intercellular spaces. This biofilm formation by OE1‐1 cells after invasion into the intercellular spaces of roots may be required for its virulence.
Proteinaceous appendages, pili and flagella, are bacterial virulence factors that lead to pathogenesis in plant, animal and human hosts and play a key role during colonization steps (Bogino et al., 2013). The initial stage of biofilm formation is dependent on bacterial motility mediated by the polar flagellum and multiple type IV pili, which enable the free‐swimming phenotype to reach a suitable surface and the surface‐motile phenotype to adhere to and move on the surface (Conrad, 2012). These appendages thus have a dual role as motile machines that allow movement among surfaces and adhesins that fix bacteria to surfaces (Petrova and Sauer, 2012). It is thought that a lack of motility increases biofilm formation in R. solanacearum (Meng et al., 2011; Yang et al., 2013; Yao and Allen, 2006; Zhang et al., 2014). The R. solanacearum strain AW1 uses type IV pili for surface adhesion and twitching motility (Kang et al., 2002). Polar adhesion to plant cells is mediated by PilA. The pilA mutant is less virulent and fails to form three‐dimensional aggregates. Interestingly, the attachment ability of the lecM mutant was significantly less than that of OE1‐1 (Fig. 8c). Positive regulation by both HrpG and PhcA resulted in the expression of lecM during biofilm formation by OE1‐1 (Fig. 9). Thus, in the initial stage of biofilm formation, RS‐IIL may be involved in the attachment of OE1‐1 to surfaces in cooperation with flagella and type IV pili.
Sudakevitz et al. (2004) reported that extracts of the R. solanacearum strain GMI1000 had fucose‐ and fructose/mannose‐specific haemagglutinating activities that could be attributed to two lectins, RSL and RS‐IIL, respectively. RS‐IIL exhibits mannose, fructose, fucose, galactose and arabinose affinity. RS‐IIL shows high sequence similarity to PA‐IIL, which is the outer membrane protein of P. aeruginosa. Because mannose, fucose, fructose and arabinose are widely distributed in plant cell walls, Sudakevitz et al. (2004) speculated that RS‐IIL may adhere to these plant cell wall compounds and contribute to bacterial virulence. However, OE1‐1 cells attached to glass slides similarly to cellulose membranes (Fig. 8a,b). Furthermore, lecM mutation resulted in reduced attachment of the bacteria to glass slides (Fig. 8c). These results suggest that adhesion of RS‐IIL to these plant cell wall compounds may not be directly involved in the attachment of OE1‐1 cells to plant cell surfaces.
The production of PA‐IIL has been shown to be regulated by quorum‐sensing signals (Winzer et al., 2000). Transcriptome analysis of R. solanacearum strain GMI1000 and its hrp mutants showed positive regulation of lecM expression by HrpG (Valls et al., 2006). Furthermore, the expression of lecM was dependent not only on HrpG, but also PhcA (Fig. 8). The activation of PhcA is regulated in response to cell density by a quorum‐sensing mechanism (Flavier et al., 1997). At a bacterial cell density of more than 107 CFU/mL, functional PhcA activated through quorum sensing suppresses the expression of the prhIR operon, leading to the suppression of hrp gene expression (Genin et al., 2005; Yoshimochi et al., 2009). Thus, we propose that positive regulation of lecM expression by both HrpG and PhcA may ensure the expression of lecM at any bacterial cell density (Fig. 9). Furthermore, the expression of lecM in OE1‐1 at OD600 = 0.3 was significantly higher than that at OD600 = 0.01. Lectins bind bacterial cells to the polymeric matrix (Diggle et al., 2006; Tielker et al., 2005). The application of arabinose, fructose and mannose, for which RS‐IIL reportedly exhibits affinity, resulted in enhanced biofilm formation by OE1‐1 cells (Fig. 5a). However, the application of these sugars did not enhance biofilm formation of the lecM mutant (Fig. 5b). Furthermore, lecM mutation resulted in reduced EPS I production of the bacteria (Fig. 10) and immature biofilm formed by the bacteria (Fig. 4g). Therefore, the affinity of RS‐IIL for sugars may contribute to certain stages of biofilm formation by OE1‐1, such as the formation and stabilization of the polysaccharide matrix network and linkage between the bacterial surface and EPSs.
Zuluaga et al. (2013) reported that the tomato apoplast is significantly richer in sugars, such as sucrose, fructose, glucose, galactose and mannose, than the xylem, and R. solanacearum probably catabolizes all abundant apoplast sugars. OE1‐1 cells incubated in apoplast fluids produced significantly more biofilm than those incubated in xylem fluids (Fig. 3). Furthermore, we did not observe any mushroom‐type biofilm of OE1‐1 cells incubated in xylem fluids (Fig. 4e). These results suggest that apoplasts, such as intercellular spaces, may provide favourable conditions for biofilm formation by OE1‐1 cells because they are rich in sugars. Because of their poor sugar contents, OE1‐1 cells aggregate in xylem fluids, but show lower biofilm formation.
Our present results showed that apoplast fluids are preferable for biofilm formation by OE1‐1, compared with xylem fluids. Therefore, we postulate that, after invasion into roots, OE1‐1 cells invading intercellular spaces first attach to the surfaces of host cells adjacent to intercellular spaces. RS‐IIL may be involved in the attachment of OE1‐1 cells. The first step of biofilm formation is the attachment phase, in which cells reversibly attach to surfaces and eventually become irreversibly attached (Monds and O'Toole, 2009). The transition from reversible to irreversible attachment is critical for biofilm formation. After attachment to plant cells, OE1‐1 cells express the hrp genes to construct the T3SS and secrete effectors. This leads to the suppression of the innate immunity of host plants (Kiba et al., 2014, 2012; Nakano et al., 2013), allowing the bacteria to grow and establish microcolonies. Substantial growth of the bacteria results in the induction of quorum sensing, leading to biofilm formation. RS‐IIL may also be involved not only in the attachment of OE1‐1 cells on plant cells, but also the formation and stabilization of the polysaccharide matrix network and linkage between the bacterial surface and EPSs, implicated in the biofilm formation of OE1‐1. Thus, biofilm formation by OE1‐1 cells on the surfaces of tomato cells after invasion into intercellular spaces is required for its virulence.
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Ralstonia solanacearum strains were routinely grown in ¼ × M63 medium (Cohen and Rickenberg, 1956) at 30°C and Escherichia coli strains were grown in Luria–Bertani (LB) medium (Hanahan, 1983) at 37°C. The following antibiotics were used in selective media in the amounts indicated (μg/mL): ampicillin, 75; chloramphenicol, 50; gentamycin, 50; kanamycin, 50.
Table 1.
Strains and plasmids used in this study.
| Relevant characteristics | Source | |
|---|---|---|
| Plasmids used for cloning | ||
| pMD20 | pUC19 derivative, Ampr | Takara (Ohtsu, Japan) |
| pMD20gOE1‐1 | pMD20 derivative carrying a 1.6‐kbp fragment containing GFP under the control of the promoter of Rsc0297, Ampr | This study |
| pUC18‐mini‐Tn7T‐Gm | Gmr | Choi et al. (2005) |
| pTNS2 | Helper plasmid carrying T7 transposase gene | Choi et al. (2005) |
| pHSG398 | cmlr | Takara (Ohtsu, Japan) |
| pUCD800 | pSa ori, pBR322 ori, sacB, Kmr | Gay et al. (1985) |
| pHSG398lecM | pHSG398 derivative carrying a 3.8‐kbp fragment containing lecM and sacB from pUCD800, cmlr | This study |
| pMD20lecM | pMD20 derivative carrying a 1.3‐kbp fragment containing lecM | This study |
| Final vectors introduced in Ralstonia solanacearum by natural transformation | ||
| pgOE1‐1 | pUC18‐mini‐Tn7T‐Gm derivative carrying GFP conjugated to Rsc0297 promoter, Gmr | This study |
| pHHSG398lecM::EZ‐Tn5 | pHSG398lecM derivative inserted with Tn EZ::TN <KAN‐2> | This study |
| pUC18‐mini‐Tn7T‐Gm‐lecM | pUC18‐mini‐Tn7T‐Gm derivative carrying a 1.3‐kbp fragment containing lecM | This study |
| Escherichia coli strain | ||
| DH5α | recA1 endA1 gyrA96 thi‐1 hsdR17supE44 Δ(lac)U169(ϕ80lacΔM15) | Takara (Ohtsu, Japan) |
| R. solanacearum strains | ||
| OE1‐1 | Wild‐type strain, phylotype I, race 1, biovar 4 | Liu et al. (2009) |
| gOE1‐1 | Transformant of OE1‐1 with pgOE1‐1, Gmr | This study |
| OE1‐1‐lecM::EZ‐Tn5 | lecM:: Tn EZ::TN <KAN‐2> mutant, Kmr | This study |
| lecM‐comp | Transformant of OE1‐1‐lecM::EZ‐Tn5 with pUC18‐mini‐Tn7T‐Gm‐lecM, Kmr, Gmr | This study |
Ampr, ampicillin resistant; cmlr, chloramphenicol resistant; GFP, green fluorescent protein; Gmr, gentamycin resistant; Kmr, kanamycin resistant.
General DNA manipulations
The isolation of genomic DNA, plasmid DNA manipulations and PCR were performed using standard techniques (Sambrook et al., 1989). Ralstonia solanacearum was transformed by electroporation as described previously (Allen et al., 1991). Double‐stranded DNA sequencing templates were prepared with Fast Gene™ Plasmid miniprep kits (NIPPON Genetics, Tokyo, Japan) and sequences were determined using an Automated DNA Sequencer Model 373 (Applied Biosystems, Foster City, CA, USA). DNA sequence data were analysed using the DNASYS‐Mac software (Hitachi Software Engineering, Yokohama, Japan). Enzymes, including restriction endonucleases (Takara, Ohtsu, Japan), were used according to the manufacturer's instructions.
Creation of GFP‐labelled OE1‐1, gOE1‐1
A 910‐bp fragment was PCR amplified from the genomic DNA of OE1‐1 with the following primers: 5′‐TGCTCGATGCGCTGTCGATC‐3′ (named cPfliC‐1F) and 5′‐TTTACTCATGGTGCACCTCCGGGCGGAAAC‐3′ (297GFP‐1R). A 727‐bp fragment was PCR amplified from pCmGFP with the following primers: 5′‐GAGGTGCACCATGAGTAAAGGAGAAGAAC‐3′ (297GFP‐2F) and 5′‐TTATTTGTATAGTTCATCCATGCC‐3′ (GFP‐2R). A 1.6‐kbp fragment was PCR amplified using these two fragments as templates, and cPfliC‐1F and GFP‐2R as primers. The PCR fragment was inserted into pMD20 (Takara), creating pMD20gOE1‐1. The 1.6‐kbp BamHI‐ and HindIII‐digested fragment of pMD20gOE1‐1 was ligated into the BamHI and HindIII sites of pUC18‐mini‐Tn7T‐Gm (Choi et al., 2005), creating pgOE1‐1. This plasmid was electroporated into OE1‐1 cells with the T7 transposase expression vector pTNS2 (Choi et al., 2005), and the gentamycin‐resistant transformant, gOE1‐1, was selected. DNA sequencing of PCR‐amplified DNA fragments, using cPfliC‐1F and GFP‐2R as primers, was performed to verify that the inserted GFP gene was fused to a constitutively active promoter of RSc0297 in OE1‐1 (data not shown).
Creation of a lecM mutant and a complementation construct
A 3.8‐kbp fragment was PCR amplified from the genomic DNA of OE1‐1 with the following primers (added BamHI sites are shown in italic): 5′‐CGGGATCCATGTCTTCCGGTCTTGCCCAGG‐3′ (Bam‐lecM‐FW) and 5′‐CGGGATCCATGGGACCAGGCTTCCAGGACG‐3′ (Bam‐lecM‐RV). The BamHI‐digested fragment and a 2.6‐kbp BamHI‐ and PstI‐digested DNA fragment containing sacB from pUCD800 (Gay et al., 1985) were ligated into the BamHI and PstI sites of pHSG398 (Takara) to create pHHSG398lecM. The EZ:TN transposome‐mediated insertion system (Epicentre, Madison, WI, USA) was also used. The transposon EZ::TN <KAN‐2> was inserted into pHHSG398lecM following the manufacturer's instructions. Transposition clones were selected by plating on PY medium (Kanda et al., 2003a) containing kanamycin and chloramphenicol, creating pHHSG398lecM::EZ‐Tn5. DNA sequencing of PCR‐amplified DNA fragments using 5′‐CTTTCGGAGATTCACTCGC‐3′ (lecM‐pSQFW) and 5′‐AAGCAGTGGGAACCGATCAG‐3′ (lecM‐pSQRV) as primers was performed to verify the correct insertion of EZ::TN <KAN‐2> into lecM (data not shown), showing that the transposon was inserted between the 40th and 41st nucleotides of lecM. This plasmid was electroporated into OE1‐1 cells and the kanamycin‐resistant, chloramphenicol‐sensitive and sucrose‐resistant recombinant, OE1‐1‐lecM::EZ‐Tn5 (lecM mutant), was selected. DNA sequencing of PCR‐amplified DNA fragments using 5′‐GAAATACGGGATCGACCACC‐3′ (lecM‐SQFW) and 5′‐GCGGTACGGTTCGAGCAC‐3′ (lecM‐SQRV) as primers was performed to verify the correct substitution of the mutated lecM in OE1‐1 (data not shown).
A 1.3‐kbp fragment was PCR amplified from the genomic DNA of OE1‐1 with the following primers: 5′‐ACTACGAGGCCTACCTGTAC‐3′ (lecM‐souho‐FW) and 5′‐AGCGTGGAAAGACAAACGGC‐3′ (lecM‐souho‐RV). The PCR fragment was inserted into pMD20, creating pMD20lecM. The 1.3‐kbp BamHI‐ and HindIII‐digested fragment of pMD20lecM was ligated into the BamHI and HindIII sites of pUC18‐mini‐Tn7T‐Gm, creating pUC18‐mini‐Tn7T‐Gm‐lecM. This plasmid was electroporated into the lecM mutant with the T7 transposase expression vector pTNS2, and the kanamycin‐ and gentamycin‐resistant transformant, lecM‐comp, was selected. DNA sequencing of PCR‐amplified DNA fragments using lecM‐souho‐FW and lecM‐souho‐RV as primers was performed to verify insertion of lecM in the lecM mutant (data not shown).
Quantitative real‐time PCR of lecM
Total RNA was isolated from R. solanacearum strains at OD600 = 0.01 and OD600 = 0.3 using a High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Reverse transcription was carried out with 500 ng of total RNA using a PrimeScript RT Reagent Kit (Takara) according to the manufacturer's instructions. qRT‐PCR was carried out in a 20‐μL reaction mixture containing 1 μL of cDNA stock and 10 pm of respective primers using the SYBR GreenER qPCR Reagent System (Invitrogen, Tokyo, Japan), with an Applied Biosystems 7300 real‐time PCR system. The cycling parameters were the same for all primers: initial 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 31 s. Melting curve runs were performed at the end of each PCR to verify the specificity of the primers by the presence of a single product. DNA fragments of lecM and rpoD were synthesized using 5′‐TCAGCCCAGCGGCCAGTTCAG‐3′ (lectin‐FW) and 5′‐AGATGGGAGTCGTCGTCGTCGTG‐3′ (rpoD‐RV), respectively. PCR was then carried out using 5′‐GGCTCAGCAAGGTGTATTCACGC‐3′ (lectin‐ORF‐FW1) and lectin‐FW to amplify a 343‐bp DNA fragment specific to lecM, and 5′‐ATCGTCGAGCGCAACATCCC‐3′ (rpoD‐FW) and rpoD‐RV to amplify a 331‐bp DNA fragment specific to rpoD. The specificity of the primers under these PCR conditions was initially verified by agarose gel electrophoresis, which yielded a single product of the expected molecular size. We also checked the sequences of the amplified DNA fragments by direct sequencing with the forward primer of each respective gene. Relative quantification of gene expression was carried out according to the instructions for the Applied Biosystems 7300 real‐time PCR system, using the comparative cycle threshold (Ct) method to calculate the quantity value. All values were normalized to the expression value of the rpoD gene as an internal standard in each cDNA stock. Expression analyses were carried out with at least two biological replications to ensure that expression patterns were reproducible. Characteristic data are shown in the figures. Standard deviations and differences between the expression ratios of non‐treated controls and other samples were tested for statistical significance using the t‐test.
Observation of fluorescence derived from gOE1‐1 in the intercellular spaces of tomato leaves under a fluorescent phase contrast microscope
Leaves of 5‐week‐old tomato plants (Solanum lycopersicum cv. Ohgata‐Fukuju) were infiltrated with the GFP‐labelled OE1‐1 strain of R. solanacearum (gOE1‐1) in 50 μL of bacterial suspension at 1.0 × 108 CFU/mL using a disposable 1‐mL syringe (SS‐01T, Terumo, Tokyo, Japan). After incubation for 18 h at 25°C, the fluorescence derived from gOE1‐1 was observed 18 h after infiltration under a fluorescent phase contrast microscope (FSX‐100, Olympus, Tokyo, Japan).
Observation of R. solanacearum cells in intercellular spaces of tomato leaves under a SEM
Leaves of 5‐week‐old tomato plants were infiltrated with strain OE1‐1 in 50 μL of bacterial suspension at 1.0 × 108 CFU/mL using a disposable 1‐mL syringe. After incubation for 18 and 24 h at 25°C, the tomato leaves were fixed with 2.5% glutaraldehyde in 0.1 m phosphate‐buffered saline (PBS, pH 7.4) at room temperature for 2 h under vacuum conditions. After washing the leaves with PBS twice and drying at room temperature, the lower epidermis layers were detached using tweezers. The epidermis‐stripped leaves were vacuum deposited with platinum using an ion sputter coater (NeoCoater MP‐19020NCTR, JEOL, Tokyo, Japan) according to the manufacturer's instructions. The sample on the filter was mounted directly on the specimen holder and examined using a JSM‐6010LV SEM (JEOL).
Observation of R. solanacearum cells incubated on nano‐percolators under a SEM
Overnight cultures of R. solanacearum strains adjusted to OD600 = 0.005 (10 μL) were used to inoculate 190 μL of medium on nano‐percolator filters (JEOL) and incubated without shaking for 24 h at 30°C. After removal of the supernatant, 100 µL of ice‐cold 4% paraformaldehyde in PBS was added to the bacteria on the nano‐percolator filters. The fixation was continued at room temperature for 1 h. The fixed bacteria were washed twice with deionized water and then dried at room temperature. The bacteria on the nano‐percolator filters were vacuum deposited with gold using an ion sputter coater (Hitachi E‐1010, Hitachi High‐Technologies Corporation, Tokyo, Japan) according to the manufacturer's instructions. The sample on the filter was mounted directly on the specimen holder and examined using a Miniscope® TM‐3000 SEM (Hitachi High‐Technologies Corporation) and a JSM‐6010LV SEM.
Biofilm assays
The production of biofilms by R. solanacearum strains was measured in vitro using a minor modification of the PVC microtitre plate assay (O'Toole and Kolter, 1998). Briefly, 5 μL of overnight cultures of R. solanacearum adjusted to OD600 = 0.005 were used to inoculate 95 μL of medium in wells of a PVC microtitre plate (NUNC MICRO WELL PLATE, Thermo Fisher Scientific Inc., Waltham, MA, USA) and incubated without shaking for 24 h at 30°C. For quantification of biofilm development, 25 μL of 1.0% crystal violet solution was added to the wells. After 15 min of incubation, the unbound crystal violet stain was gently removed with a pipette and the wells were washed with distilled water, 70% ethanol and distilled water in turn. The crystal violet in each well was solubilized by adding 100 μL of 100% ethanol and quantified by absorbance at 550 nm. The biofilm formation value was normalized according to the number of cells. This value was termed relative biofilm formation (OD550/OD600).
Attachment ability and microcolony formation
Nitrocellulose membranes (2 ×2 cm2), prepared from Visking cellulose tubing, and glass slides were soaked in suspensions of R. solanacearum strains at 1.0 × 108 CFU/mL in Petri dishes (diameter, 9 cm). After incubation for 24 h at 30°C, the nitrocellulose membranes and glass slides were observed under a phase contrast microscope (FSX‐100, Olympus).
EPS I productivity
Quantitative analyses of EPS I production were conducted using ELISA. The overnight culture of R. solanacearum strains was rinsed, and diluted to a cell density of 1.0 × 103 CFU/mL. A 100‐μL aliquot of this cell suspension was spread on plates of ¼ × M63 agar medium and incubated for 2 days at 30°C. The cells were then resuspended to 1.0 × 105 CFU/mL. Cell density was confirmed through dilution plating. EPS I was quantified using anti‐R. solanacearum EPS I antibodies with ELISA (Agdia Inc., Elkhart, IN, USA) according to the manufacturer's instructions per 100 μL volume (1.0 × 104 CFU) of cell suspension (three technical replicates were assessed). EPS I productivity was quantified by absorbance at 650 nm.
Virulence assays
Tomato plants were grown in pots containing a mixture of vermiculite and peat moss (3 : 1) in a growth room at 25°C under 10 000 lx for 16 h per day; they were watered with fivefold‐diluted Hoagland's solution (Hikichi et al., 1999). Eight‐week‐old tomato plants were inoculated with bacteria using two inoculation methods. For leaf infiltration into tomato leaves (Roberts et al., 1988), 50 μL of bacterial suspension at 1.0 × 108 CFU/mL was infiltrated into tomato leaves using a disposable 1‐mL syringe. For root dip inoculation (Hikichi et al., 1999), the roots of tomato plants were soaked in bacterial suspension at 1.0 × 108 CFU/mL for 30 min and then washed in running water. Plants inoculated using root dipping were grown in water‐culture pots (Yamato Water Culture Pot No. 1, Yamato Plastic Co. Ltd., Yamatotakada, Japan) with fivefold‐diluted Hoagland's solution. For all assays, inoculum concentrations were determined spectrophotometrically and confirmed by dilution plating. All inoculated plants were grown in a growth room at 25°C under 10 000 lx for 16 h per day. Within each trial, 12 plants of each strain were treated, yielding 60 plants per strain. Plants were coded and inspected for wilting symptoms daily after inoculation. Plants were rated on a disease index scale as follows: 0, no wilting; 1, 1%–25% wilting; 2, 26%–50% wilting; 3, 51%–75% wilting; 4, 76%–99%; 5, dead. Each assay was repeated in five successive trials.
Bacterial populations in tomato plants
The bacteria‐infiltrated area (0.5 cm2) in tomato leaves was excised from five plants of each set daily after infiltration. Roots were excised 0, 1, 2 and 3 days after inoculation from five plants of each set that were inoculated with bacteria through the roots. Each sample was ground in 1 mL of distilled water using a mortar and pestle. A 0.1‐mL sample of the original suspension or a 10‐fold serial dilution was spread onto three plates of Hara–Ono medium (Hara and Ono, 1983). For the lecM mutant and lecM‐comp, the medium contained kanamycin at 50 μg/mL and gentamycin at 50 μg/mL, respectively. Colonies were counted after 2 days of incubation at 30°C.
Apoplast and xylem extractions
The apoplast fluid from the leaves was extruded by the modified methods of de Wit and Spikman (1982), Shinohara et al. (2005) and Zuluaga et al. (2013). Briefly, the third leaf from the apex of 5‐week‐old tomato plants was cut, washed with distilled water and dried with a paper towel. Then, one to three leaves were introduced into a 1‐mL syringe with 15 mL of distilled water and pressure–vacuum cycles were applied until the leaves were completely infiltrated. After infiltration, the leaves were carefully removed from the syringe and blotted with a paper towel. Each leaf was then introduced into a 5‐mL syringe placed inside a 50‐mL conical tube containing a 1.5‐mL collection tube. Apoplast extract was collected by centrifugation of the tubes at 4 000 g for 5 min at room temperature. The fraction collected in the 1.5‐mL tube was centrifuged again for 5 min at 4000 g at room temperature. The supernatant was filtered through a membrane filter (pore size, 0.2 μm) (Minisart CE; Sartorius, Gottingen, Germany) and stored at −20°C until use.
The xylem fluid from stems was extruded by the modified methods of Shinohara et al. (2005) and Zuluaga et al. (2013). Briefly, 5‐week‐old tomato plants were cut at the stems approximately 10 cm above the ground with a razor blade. The cut stem was rinsed with 2 mL of distilled water and dried with a paper towel to remove the content from cut cells and the first exuded sap. The stems were cut by a razor blade and were then introduced into a 5‐mL syringe placed inside a 50‐mL conical tube containing a 1.5‐mL collection tube. Xylem extract was collected by centrifugation of the tubes at 4000 g for 10 min at room temperature. The fraction collected in the 1.5‐mL tube was centrifuged again for 5 min at 4000 g at room temperature. The supernatant was filtered through a 0.2‐μm membrane filter and stored at −20°C until use.
Nucleotide sequence accession numbers
The nucleotide sequences of RSc0297 and lecM of strain OE1‐1 were deposited in DDBJ/GenBank/EMBL under accession numbers LC051047 and LC051048, respectively.
After acceptance of this article, Meng et al. (2015) reported involvement of lecM in biofilm formation by R. solanacearum strain UW551 and its virulence on potato. Their data supported our observation indicating that RS‐IIL encoded by lecM is involved in biofilm formation by strain OE1‐1 after invading intercellular spaces contribute its virulence on tomato plants.
Acknowledgements
We thank the members of our laboratory, Dr Ayami Kanda and Ms. Shiho Ishikawa for biofilm analysis. This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Nos. 24580066, 25292029, 26660036), a research grant from the Multidisciplinary Science Cluster, Life and Environmental Medicine Science Unit, Kochi University, and a research grant from Sumitomo Chemical Co. Ltd.
References
- Aldon, D. , Brito, B. , Boucher, C. and Genin, S. (2000) A bacterial sensor of plant cell contact controls the transcriptional induction of Ralstonia solanacearum pathogenicity genes. EMBO J. 19, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, C. , Huang, Y. and Sequeira, L. (1991) Cloning of genes affecting polygalacturonase production in Pseudomonas solanacearum . Mol. Plant–Microbe Interact. 4, 147–154. [Google Scholar]
- Araud‐Razou, I. , Vasse, J. , Montrozier, H. , Etchebar, C. and Trigalet, A. (1998) Detection and visualization of the major acidic exopolysaccharide of Ralstonia solanacearum and its role in tomato root infection and vascular colonization. Eur. J. Plant Pathol. 104, 795–809. [Google Scholar]
- Bogino, P.C. , Oliva, M.M. , Sorroche, F.G. and Giordano, W. (2013) The role of bacterial biofilms and surface components in plant–bacterial associations. Int. J. Mol. Sci. 14, 15 838–15 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda, S.S. , Vik, S. , Friedman, L. and Kolter, R. (2005) Biofilms: the matrix revisited. Trends Microbiol. 13, 20–26. [DOI] [PubMed] [Google Scholar]
- Brito, B. , Marenda, M. , Barberis, P. , Boucher, C. and Genin, S. (1999) prhJ and hrpG, two new components of the plant signal‐dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum . Mol. Microbiol. 31, 237–251. [DOI] [PubMed] [Google Scholar]
- Brito, B. , Aldon, D. , Barberis, P. , Boucher, C. and Genin, S. (2002) A signal transfer system through three compartments transduces the plant cell contact‐dependent signal controlling Ralstonia solanacearum hrp genes. Mol. Plant–Microbe Interact. 15, 109–119. [DOI] [PubMed] [Google Scholar]
- Brumbley, S.M. , Carney, B.F. and Denny, T.P. (1993) Phenotype conversion in Pseudomonas solanacearum due to spontaneous inactivation of PhcA, a putative LysR transcriptional regulator. J. Bacteriol. 175, 5477–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K.H. , Gaynor, J.B. , White, K.G. , Lopez, C. , Bosio, C.M. , Karkhoff‐Schweizer, R.R. and Schweizer, H.P. (2005) A Tn7‐based broad‐range bacterial cloning and expression system. Nat. Methods, 2, 443–448. [DOI] [PubMed] [Google Scholar]
- Cohen, G.N. and Rickenberg, H.V. (1956) La galactoside‐perméase d’Escherichia coli . Ann. Inst. Pasteur (Paris), 91, 693–720. [PubMed] [Google Scholar]
- Conrad, J.C. (2012) Physics of bacterial near‐surface motility using flagella and type IV pili: implications for biofilm formation. Res. Microbiol. 163, 619–629. [DOI] [PubMed] [Google Scholar]
- Danhorn, T. and Fuqua, C. (2007) Biofilm formation by plant‐associated bacteria. Annu. Rev. Microbiol. 61, 401–422. [DOI] [PubMed] [Google Scholar]
- Davey, M.E. and O'Toole, G.A. (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle, S.P. , Stacey, R.E. , Dodd, C. , Cámara, M. , Williams, P. and Winzer, K. (2006) The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa . Environ. Microbiol. 8, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Flavier, A.B. , Clough, S.J. , Schell, M.A. and Denny, T.P. (1997) Identification of 3‐hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum . Mol. Microbiol. 26, 251–259. [DOI] [PubMed] [Google Scholar]
- Flemming, H.C. and Wingender, J. (2010) The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633. [DOI] [PubMed] [Google Scholar]
- Gay, P. , Le Coq, D. , Steinmetz, M. , Berkelman, T. and Kado, C.I. (1985) Positive selection procedure for entrapment of insertion sequence elements in gram‐negative bacteria. J. Bacteriol. 164, 918–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin, S. and Denny, T.P. (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. [DOI] [PubMed] [Google Scholar]
- Genin, S. , Brito, B. , Denny, T.P. and Boucher, C. (2005) Control of the Ralstonia solanacearum type III secretion system (Hrp) genes by the global virulence regulator PhcA. FEBS Lett. 579, 2077–2081. [DOI] [PubMed] [Google Scholar]
- Gilboa‐Garber, N. (1986) Lectins of Pseudomonas aeruginosa: properties, biological effects and applications In: Microbial Lectins and Agglutinins: Properties and Biological Activity (Mirelman D., ed.), pp. 255–269. New York: John Wiley and Sons. [Google Scholar]
- Gilboa‐Garber, N. (1996) Towards anti‐Pseudomonas aeruginosa adhesion therapy. Adv. Exp. Med. Biol. 408, 39–50. [DOI] [PubMed] [Google Scholar]
- Gilboa‐Garber, N. and Garber, N. (1989) Microbial lectin cofunction with lytic activities as a model for a general basic lectin role. FEMS Microbiol. Rev. 5, 211–321. [DOI] [PubMed] [Google Scholar]
- Hall‐Stoodley, L . and Stoodley, P. (2009) Evolving concepts in biofilm infections. Cell Microbiol. 11, 1034–1043. [DOI] [PubMed] [Google Scholar]
- Hanahan, D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Hara, H. and Ono, K. (1983) Ecological studies on the bacterial wilt of tobacco, caused by Pseudomonas solanacearum E. F. Smith. I. A selective medium for isolation and detection of P . solanacearum. Bull. Okayama Tob. Exp. Stn. 42, 127–138. [Google Scholar]
- Hayward, H.C. (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum . Annu. Rev. Phytopathol. 29, 65–87. [DOI] [PubMed] [Google Scholar]
- Hikichi, Y. , Nakazawa‐Nasu, Y. , Kitanosono, S. , Suzuki, K. and Okuno, T. (1999) The behavior of genetically lux‐marked Ralstonia solanacearum in grafted tomato cultivars resistant or susceptible to bacterial wilt. Ann. Phytopathol. Soc. Jpn. 65, 597–603. [Google Scholar]
- Hikichi, Y. , Yoshimochi, T. , Tsujimoto, S. , Shinohara, R. , Nakaho, K. , Kanda, A. , Kiba, A. and Ohnishi, K. (2007) Global regulation of pathogenicity mechanism of Ralstonia solanacearum . Plant Biotechnol. 24, 149–154. [Google Scholar]
- Huang, J. , Carney, B.F. , Denny, T.P. , Weissinger, A.K. and Schell, M.A. (1995) A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum . J. Bacteriol. 177, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, A. , Ohnishi, S. , Tomiyama, H. , Hasegawa, H. , Yasukohchi, M. , Kiba, A. , Ohnishi, K. , Okuno, T. and Hikichi, Y. (2003a) Type III‐secretion machinery deficient mutants of Ralstonia solanacearum lose their ability to colonize, resulting in loss of pathogenicity. J. Gen. Plant Pathol. 69, 250–257. [Google Scholar]
- Kanda, A. , Yasukohchi, M. , Ohnishi, K. , Kiba, A. , Okuno, T. and Hikichi, Y. (2003b) Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant–Microbe Interact. 16, 447–455. [DOI] [PubMed] [Google Scholar]
- Kanda, A. , Tsuneishi, K. , Mori, A. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2008) An amino acid substitution at position 740 in σ70 of Ralstonia solanacearum strain OE1‐1 affects its in planta growth. Appl. Environ. Microbiol. 74, 5841–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, A. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2009) Implication of C‐terminal mutation of PopA of Ralstonia solanacearum strain OE1‐1 in development of bacterial wilt. Plant Pathol. 58, 159–169. [Google Scholar]
- Kang, Y. , Liu, H. , Genin, S. , Schell, M.A. and Denny, T.P. (2002) Ralstonia solanacearum requires type‐4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol. Microbiol. 46, 427–437. [DOI] [PubMed] [Google Scholar]
- Kiba, A. , Galis, I. , Hojo, Y. , Ohnishi, K. , Yoshioka, H. and Hikichi, Y. (2014) SEC14 phospholipid transfer protein is involved in lipid signaling‐mediated plant immune responses in Nicotiana benthamiana . PLoS One, 9, e98150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba, A. , Nakano, M. , Vincent‐Pope, P. , Takahashi, H. , Sawasaki, T. , Endo, Y. , Ohnishi, K. , Yoshioka, H. and Hikichi, Y. (2012) A novel Sec14 phospholipid transfer protein from Nicotiana benthamiana is up‐regulated in response to Ralstonia solanacearum infection, pathogen associated molecular patterns and effector molecules and involved in plant immunity. J. Plant Physiol. 169, 1017–1022. [DOI] [PubMed] [Google Scholar]
- Klausen, M. , Aaes‐Jogenen, A. , Molin, S. and Tolker‐Nielsen, T. (2003) Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50, 61–68. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Kanda, A. , Kiba, A. , Hikichi, Y. , Aino, M. , Kawaguchi, A. , Mizoguchi, S. , Nakaho, K. , Shiomi, H. , Takikawa, Y. and Ohnishi, K. (2009) Molecular typing of Japanese strains of Ralstonia solanacearum and the relationship with the ability to induce a hypersensitive reaction in tobacco. J . Gen. Plant Pathol. 75, 369–380. [Google Scholar]
- Mansfield, J. , Genin, S. , Magori, S. , Citovsky, V. , Sriariyanum, M. , Ronald, P. , Dow, M. , Verdier, V. , Beer, S.V. ; Machado, M.A. , Toth, I. , Salmond, G. and Foster, G.D. (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Babujee, L. , Jacobs, J.M. and Allen, C. (2015) Comparative transcriptome analysis reveals cool virulence factors of Ralstonia solanacearum race 3 biovar 2. PLOS ONE 10, e0139090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Yao, J. and Allen, C. (2011) A MotN mutant of Ralstonia solanacearum is hypermotile and has reduced virulence. J. Bacteriol. 193, 2477–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monds, R.D. and O'Toole, G.A. (2009) The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 17, 73–87. [DOI] [PubMed] [Google Scholar]
- Morris, C.E. and Monier, J.M. (2003) The ecological significance of biofilm formation by plant‐associated bacteria. Annu. Rev. Phytopathol. 41, 429–453. [DOI] [PubMed] [Google Scholar]
- Nakano, M. , Nishihara, M. , Yoshioka, H. , Takahashi, H. , Sawasaki, T. , Ohnishi, K. , Hikichi, Y. and Kiba, A. (2013) Suppression of DS1 phosphatidic acid phosphatase confirms resistance to Ralstonia solanacearum in Nicotiana benthamiana . PLoS One, 8, e75124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, G.A . and Kolter, R. (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295–304. [DOI] [PubMed] [Google Scholar]
- Parsek, M.R. and Fuqua, C. (2004) Biofilms 2003: emerging themes and challenges in studies of surface‐associated microbial life. J. Bacteriol. 186, 4427–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova, O.E. and Sauer, K. (2012) Sticky situations: key components that control bacterial surface attachment. J. Bacteriol. 194, 2413–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey, B.E. , Koutsoudis, M. , von Bodman, S.B. and Fuqua, C. (2004) Biofilm formation in plant–microbe associations. Curr. Opin. Microbiol. 7, 602–609. [DOI] [PubMed] [Google Scholar]
- Rinaudi, L.V. and Giordano, W. (2010) An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 304, 1–11. [DOI] [PubMed] [Google Scholar]
- Roberts, P.D. , Denny, T.P. and Schell, M.A. (1988) Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J. Bacteriol. 170, 1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schell, M.A. (2000) Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 38, 263–292. [DOI] [PubMed] [Google Scholar]
- Shinohara, R. , Kanda, A. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2005) Contribution of folate biosynthesis to Ralstonia solanacearum proliferation in the intercellular spaces. Appl. Environ. Microbiol. 71, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikhanta, Y.N. , Dowideit, S.J. , Edwards, J.L. , Falsetta, M.L. , Wu, H.J. , Harrison, O.B. , Fox, K.L. , Seib, K.L. , Maguire, T.L. , Wang, A.H. , Maiden, M.C. , Grimmond, S.M. , Apicella, M.A. and Jennings, M.P. (2009) Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog. 5, E1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakevitz, D. , Imberty, A. and Gilboa‐Garber, N. (2002) Production, properties and specificity of a new bacterial L‐fucose‐ and D‐arabinose‐binding lectin of the plant aggressive pathogen Ralstonia solanacearum and its comparison to related plant and microbial lectins. J. Biochem. 132, 353–358. [DOI] [PubMed] [Google Scholar]
- Sudakevitz, D. , Kostlánová, N. , Blatman‐Jan, G. , Mitchell, E.P. , Lerrer, B. , Wimmerová, M. , Katcoff, D.J. , Imberty, A. and Gilboa‐Garber, N. (2004) A new Ralstonia solanacearum high‐affinity mannose‐binding lectin RS‐IIL structurally resembling the Pseudomonas aeruginosa fucose‐specific lectin PA‐IIL. Mol. Microbiol. 52, 691–700. [DOI] [PubMed] [Google Scholar]
- Tielker, D. , Hacker, S. , Loris, R. , Strathmann, M. , Wingender, J. , Wilhelm, S. , Rosenau, F. and Jaeger, K.E. (2005) Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology, 151, 1313–1323. [DOI] [PubMed] [Google Scholar]
- Valls, M. , Genin, S. and Boucher, C. (2006) Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum . PLoS Pathog. 2, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijsegem, F. , Gough, C. , Zischek, C. , Niqueux, E. , Arlat, M. , Genin, S. , Barberis, P. , German, S. , Castello, P. and Boucher, C. (1995) The hrp gene cluster of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol. Microbiol. 15, 1095–1114. [DOI] [PubMed] [Google Scholar]
- Vasse, J. , Frey, P. and Trigalet, A. (1995) Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum . Mol. Plant–Microbe Interact. 8, 241–251. [Google Scholar]
- Webb, J.S. , Givskov, M. and Kjellebeg, S. (2003) Bacterial biofilms: prokaryotic adventures in multicellularity. Curr. Opin. Microbiol. 6, 578–585. [DOI] [PubMed] [Google Scholar]
- Winzer, K. , Falconer, C. , Garber, N.C. , Diggle, S.P. , Camara, M. and Williams, P. (2000) The Pseudomonas aeruginosa lectins PA‐IL and PA‐IIL are controlled by quorum sensing and by RpoS. J. Bacteriol. 182, 6401–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, P.J.G. and Spikman, G. (1982) Evidence for the occurrence of race‐ and cultivar‐specific elicitors of necrosis in intercellular fluids of compatible interactions of Cladosoprium fulvum and tomato. Physiol. Plant Pathol. 21, 1–11. [Google Scholar]
- Yabuuchi, E. , Kosako, Y. , Yano, I. , Hotta, H. and Nishiuchi, Y. (1995) Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff, 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. and Ralstonia eutropha (Davis, 1969) comb. nov. Microbiol. Immunol. 39, 897–904. [DOI] [PubMed] [Google Scholar]
- Yang, W.C. , Lin, Y.M. , Cheng, Y.S. and Cheng, C.P. (2013) Ralstonia solanacearum RSc0411 (lptC) is a determinant for full virulence and has a strain‐specific novel function in the T3SS activity. Microbiology 159, 1136–1148. [DOI] [PubMed] [Google Scholar]
- Yao, J. and Allen, C. (2006) Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum . J. Bacteriol. 188, 3697–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimochi, T. , Hikichi, Y. , Kiba, A. and Ohnishi, K. (2009) The global virulence regulator PhcA negatively controls the Ralstonia solanacearum hrp regulatory cascade by repressing expression of the PrhIR signaling proteins. J. Bacteriol. 191, 3424–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Xu, J. , Xu, J. , Zhang, H. , He, L. and Feng, J. (2014) TssB is essential for virulence and required for type VI secretion system in Ralstonia solanacearum . Microb. Pathog. 74, 1–7. [DOI] [PubMed] [Google Scholar]
- Zuluaga, A.P. , Puigvert, M. and Valls, M. (2013) Novel plant inputs influencing Ralstonia solanacearum during infection. Front. Microbiol. 4, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]


