Abstract
The intestine is supported by a complex vascular system that undergoes dynamic and transient daily shifts in blood perfusion, depending on the metabolic state. Moreover, the intestinal villi have a steep oxygen gradient from the hypoxic epithelium adjacent to the anoxic lumen to the relative higher tissue oxygenation at the base of villi. Due to the daily changes in tissue oxygen levels in the intestine, the hypoxic transcription factors hypoxia-inducible factor (HIF)-1α and HIF-2α are essential in maintaining intestinal homeostasis. HIF-2α is essential in maintaining proper micronutrient balance, the inflammatory response, and the regenerative and proliferative capacity of the intestine following an acute injury. However, chronic activation of HIF-2α leads to enhanced proinflammatory response, intestinal injury, and colorectal cancer. In this review, we detail the major mechanisms by which HIF-2α contributes to health and disease of the intestine and the therapeutic implications of targeting HIF-2α in intestinal diseases.
Keywords: hypoxia, inflammatory bowel disease, ulcerative colitis, Crohn’s disease, iron, colon cancer
INTRODUCTION
The small intestine (duodenum, jejunum, and ileum) and large intestine (cecum and colon) are structurally and mechanistically suited to providing an epithelial barrier from the luminal contents and diverse commensal bacteria. The surface area of the intestine is 300 m2 (1), due to fingerlike villi projections and microvilli on epithelial cells, which aids in nutrient absorption and diffusion rate. Intestinal epithelium contains numerous specialized cells that encompass enterocytes that aid in absorption and secretory cells such as goblet, Paneth, and enteroendocrine cells. The intestine is the most regenerative tissue in the body, and the epithelium completely renews every 5 to 6 days. The self-renewal capacity of the intestine is under tight regulation by the cross talk between numerous pathways such as Hedgehog, Notch, and Wnt–β-catenin signaling cascades. The complexity of intestine morphology and function is also regulated by numerous factors such as diet, hormones, microbiota, and the enteric and central nervous systems. Among these factors, recent emphasis focusing on intestinal oxygen homeostasis has determined a central role for oxygen dynamics in maintaining intestinal homeostasis. Dysregulation of oxygen gradients is observed in numerous intestinal diseases such as inflammatory bowel disease (IBD) and colorectal cancer (CRC). The role of hypoxia-inducible factor (HIF)-1α in intestinal homeostasis has been extensively reviewed (2–4). In this article, we describe the role of intestinal HIF-2α in gastrointestinal (GI) homeostasis and disease.
OXYGEN DYNAMICS OF THE INTESTINE
Luminal Oxygen Homeostasis
The intestine is adjacent to a large number of commensal microorganisms termed the microbiota. More than 70% of the total microbiota in human body are colonized in the intestinal tract. The adult intestine contains up to 1013–1014 microorganisms that outnumber human cells by a factor of 10. Between 500 and 1,000 species of gut microbes have been characterized, and most of them are strict anaerobes, composed mainly of two bacterial phyla, namely Bacteroidetes and Firmicutes (5). The gut microbiota have a central role in our physiology, and metagenomic studies have demonstrated a tight correlation between specific bacterial composition and diverse diseases, including irritable bowel syndrome, IBD, CRC, type 2 diabetes, liver disease, obesity, cardiovascular disorders, and neurodegenerative diseases (5a). The diversity and number of microbiota increase along the longitudinal axis of the intestine from the small intestine to the colon. After birth, utilization of the luminal oxygen by the aerobes and facultative anaerobes establishes an anaerobic environment in the intestine (6). Estimates of the oxygen gradient in the intestine vary depending on the methods used for measurements (7). Noninvasive approaches using special electron paramagnetic resonance demonstrated a partial pressure of oxygen (pO2) of 58, 32, 11, and 3 mm Hg in the stomach, duodenum, distal small intestine, and colon, respectively (8). More recent data using phosphorescent probes provide a more precise and dynamic assessment of luminal oxygen. These data demonstrate that oxygen levels at the interface of the epithelium are higher than in the central lumen. Oxygen consumption by aerotolerant bacteria close to the epithelium renders the central lumen anaerobic. Moreover, an increase in host oxygenation leads to an increase in luminal oxygen levels, leading to a more aerotolerant microbiota at the epithelia-lumen interface (7). The changes in luminal oxygen gradient in GI diseases and the reciprocal effects on host response are not presently clear. Indeed, IBD is associated with increased oxidative stress in epithelial cells, which may impact luminal oxygen levels, resulting in the accumulation of aerotolerant pathogens and in dysbiosis.
Epithelial Oxygen Homeostasis
Epithelial oxygen homeostasis has been well studied in normal and diseased intestines. The gradient in the mucosal pO2 between the capillary bed and intestinal epithelial cells allows for efficient diffusion of oxygen to metabolically active intestinal epithelial cells. Intestinal oxygenation is driven by sympathetic stimulations, neurotransmitters, gut hormones, and metabolic activity (9). Intestinal oxygen dynamics rapidly change under various physiological conditions. During fasting, the intestine is relatively hypoxic, and following feeding, active transport of sodium and glucose by the intestinal villi increases local oxygen demand. Postprandial hyperemia delivers oxygen to intestinal mucosa to meet the oxygen demands (10). Sympathetic stimulation decreases vascular tone, whereas parasympathetic tone increases vascular tone. The blood circulation in the muscular layer is under sympathetic regulation, whereas the blood circulation in the mucosal layer is locally regulated; for instance, food intake increases mucosal hyperemia (10). Food composition also influences oxygenation of the intestine. Protein and fat significantly induce oxygen uptake (11). Neurotransmitters such as histamines and bradykinin increase, whereas serotonin and vasopressin decrease, intestinal blood flow. Similarly, hormones such as endothelin-1, glucagon, vasoactive intestinal polypeptide, neurotensin, and cholecystokinin-8 regulate intestinal blood flow and oxygenation (9). Thus, the intestine exists in a daily dynamic flux with respect to intestinal oxygenation. Hypoxic probes such as pimonidazole and 2-nitroimidazole compounds have been used to determine pO2 in tissues in vivo. The pO2 is low at the tip relative to the base of the villi (11a). During inflammatory injury and in colon tumors, an increase in hypoxia is observed throughout the epithelium (12), and the mechanisms involved and significance of epithelial hypoxia in intestinal homeostasis are discussed in more detail below. A dynamic relationship of the microbiota regulating intestinal oxygenation was also recently shown. Microbiota, through production of short-chain fatty acids (SCFA) such as butyrate, can regulate oxygen consumption in intestinal epithelial cells (12).
OXYGEN-SENSITIVE TRANSCRIPTION FACTORS
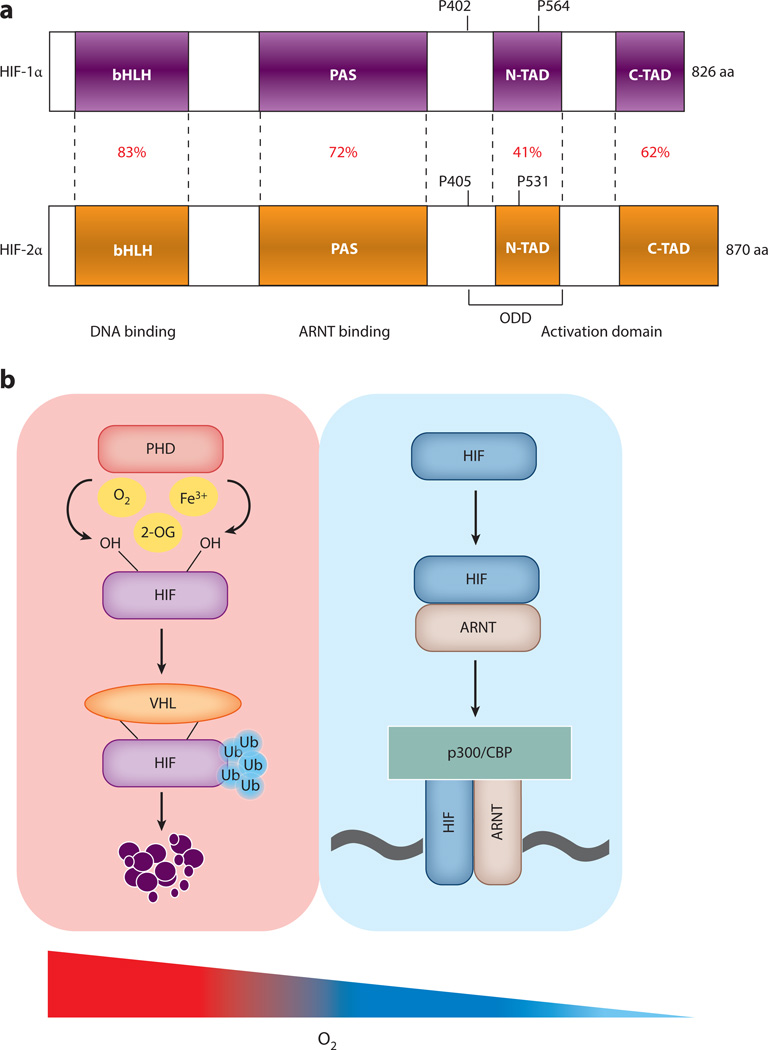
Cellular adaptation to hypoxia is mediated by the HIF family of transcription factors. HIFs belong to the basic helix-hoop-helix Per-Arnt-Sim (bHLH-PAS) family of transcription factors. HIFs and core components are evolutionarily conserved in all metazoans (13). However, only vertebrates acquired additional hypoxic transcription factors through genome duplication. In vertebrates, there are three isoforms: HIF-1α, HIF-2α, and HIF-3α. HIFα heterodimerizes with the constitutively expressed aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIFβ) subunit and binds to HIF response elements (HREs) present in the promoters of HIF target genes (14, 15). Structurally, HIFs contain an N-terminal bHLH domain (a DNA-binding domain), followed by a PAS domain (required for heterodimerization with ARNT), a highly conserved oxygen-dependent degradation domain (ODD), and N-terminal and C-terminal transactivation domains (16, 17) (Figure 1a). HIF expression is regulated mainly at the posttranslational level. In normoxia, two proline residues in the ODD are hydroxylated by prolyl hydroxylase domain (PHD)-containing enzymes (18, 19). Hydroxylated HIFs are bound by E3 ubiquitin ligase, which contains the von Hippel–Lindau (VHL) protein (20, 21). VHL mediates polyubiquitination and subsequent proteasomal degradation of HIF. However, in hypoxic conditions, because HIF is no longer hydroxylated, it is not recognized by VHL, therefore resulting in the stabilization of HIF and in an increase in the expression of HIF target genes (20) (Figure 1b). HIF-1α is ubiquitously expressed. In contrast, in situ hybridization demonstrated selective expression of HIF-2α in endothelial cells, and therefore the gene that encoded HIF-2α was named endothelial PAS protein 1 (Epas1) (22). Further characterization of the HIF-2α protein identified its expression in several additional cell types and tissues, including the intestine. Intestinal epithelial cells express both HIF-1α and HIF-2α (23, 24). However, better analysis is required to understand whether HIF-1α and HIF-2α are expressed in different subsets of intestinal epithelial cells. Such analysis would be challenging to undertake due to low levels of HIF-1α and HIF-2α under basal conditions.
Figure 1.
Regulation of hypoxia-inducible factor (HIF) function. (a) HIF-1α and HIF-1α have highly homologous modular domains: a basic helix-loop-helix (bHLH) domain, a Per-Arnt-Sim (PAS) domain, and a transactivation domain that includes the N-terminal activation domain (N-TAD) and the C-terminal activation domain (C-TAD). The homology of each domain is represented as a percentage. The two proline residues in the oxygen-dependent degradation domain (ODD) are critical for the stability of HIF-1α and HIF-2α. (b) Prolyl hydroxylase domain (PHD) enzymes use molecular oxygen, 2-oxoglutarate (2-OG), and ferric iron (Fe3+) to hydroxylate the two proline residues on HIF. This step is required for the binding of the Von Hippel–Lindau (VHL) tumor suppressor protein. VHL binding recruits E3 ubiquitin (Ub) ligases, which degrade HIFα subunits under normoxic conditions through proteasome-mediated degradation. A decrease in cellular oxygen inhibits PHD activity, leading to HIFα stabilization, binding to ARNT, and increased HIFα transcriptional activity. Other abbreviations: ARNT, aryl hydrocarbon receptor nuclear translocator; CBP, CREB-binding protein.
Regulation of HIF-2α
Analysis of tissues from colon cancer patients demonstrated discrete and overlapping patterns of HIF-1α and HIF-2α expression (25). This distinct function of HIFs suggests that there exist mechanisms that can specifically regulate HIF-1α and/or HIF-2α in the intestine. PHD enzymes may provide a level of specificity for HIF-1α or HIF-2α activation. In the liver, PHD3 is the major isoform that regulates HIF-2α (26). However, in the intestine, PHDs have overlapping functions, and deletion of all three PHDs is required for efficient HIF-1α and HIF-2α expression (27) (Table 1). PHDs are regulated not only by oxygen availability but also by several metabolites (12, 28–30). The specificity of metabolites for HIF-1α or HIF-2α has not been clearly characterized.
Table 1.
The regulation and function of HIF-1α and HIF-2α in intestinal homeostasis
| HIF-1α | HIF-2α | |
|---|---|---|
| Regulation and target specificity | ||
| Regulation by PHD | PHD1/2/3 (27) | PHD1/2/3 (27) |
| Acetylation | Acetylated by p300 (152) | Acetylated by CBP (44) |
| IRP | Not well characterized | Represses HIF-2α translation (31) |
| HAF | Induces degradation (37) | Increases HIF-2α transcriptional activity (47) |
| USF-2 | Dispensable for HIF-1α (46) | Transcriptional activator (46) |
| Yin yang-1 | Stabilizes and increases transcriptional activity (48) |
Acts as corepressor (48) |
| c-myc | Antagonizes c-myc activity (154) |
Potentiates c-myc activity (43) |
| Intestinal homeostasis | ||
| Embryogenesis | Not known | Maintains stemness by regulating Oct-4 expression (60, 60a) |
| Wnt/β-catenin | Competes with TCF-4 and inhibits β-catenin activity (155) |
Enhances β-catenin–driven transcription (61) |
| Notch signaling | Activates (62) | Inhibits (62) |
| Intestinal iron absorption | ||
| Role | Not a major player | Iron transporter genes such as DMT-1, Dcytb, and FPN-1 are direct HIF-2α target genes (38, 49, 75–77) |
| IBD | ||
| Intestinal injury | Decreases intestinal injury (109, 110) |
Acutely decreases intestinal injury by activating mucosal immune response but chronically increases injury (102) |
| Barrier function | Is protective by increasing expression of genes such as those encoding defensin, trefoil factor, and mucin (150, 151) |
Dual role: Acute activation leads to enhanced barrier function by increasing creatine kinase expression (102), whereas chronic activation decreases barrier function by inducing caveolin expression (45) |
| Mucosal inflammation | Results in an anti- inflammatory response (109, 110, 150) |
Promotes inflammation (24, 24,102) |
| Macrophage | Is expressed in M1 macrophages, induces iNOS expression, and increases nitric oxideproduction (95) |
Is expressed in M2 macrophages, induces Arg-1 expression, and decreases nitric oxideproduction (31, 95) |
| Neutrophils | Increases survival (153) | Increases survival and inflammatory response |
| Mucosal fibrosis | Not characterized | Induces fibrosis by regulating MMPs and prolyl hydroxylases (105–108) |
| Colon cancer | ||
| Tumor progression | Does not promote colon cancer (124) |
Promotes progression of colon cancer (24, 108, 122) |
| Tumor angiogenesis | Induces VEGF expression (126) |
Induces expression of VEGF, CD34, VE-cadherin, and CD105 (126–128) |
| Tumor metabolism | Regulates glycolysis (38) | Regulates cellular ATP levels, glycolysis, and amino acid and lipid metabolism (38, 134–138) |
| Tumor elicited inflammation | Anti-inflammatory (109, 110, 150) |
Proinflammatory by inducing macrophage infiltration and neutrophil activation (24, 96–98, 108) |
| Radiation-induced toxicity | ||
| Effect | No effect | Protective (27) |
In macrophages, Th2 cytokines such as IL-4 and IL-13 can selectively increase HIF-2α protein expression, and further investigation is needed to assess whether these cytokines can also activate HIF-2α in intestinal epithelial cells (31) (Figure 2). Poly ADP-ribose and polymerase-1 (PARP-1) specifically regulates HIF-2α mRNA expression and protects against VHL-mediated degradation via direct binding to HIF-2α. Similarly, phosphatase and tensin homolog (PTEN) regulates HIF-2α expression at the transcriptional level and through regulation of PHD2. In addition, Rictor-associated mTORC increases the translation of HIF-2α (32–34) (Figure 2). Mir-155 and hypoxia-associated factor (HAF) specifically inhibit translation and promote degradation of HIF-1α, whereas Kelch-like-20 is essential for HIF-2α stabilization (35–37) (Figure 2). Therefore, these factors promote the HIF-2α-specific response following hypoxia.
Figure 2.
Mechanisms that mediate HIF-2α specificity. HIF-2α specificity is regulated at the levels of mRNA expression, mRNA translation, protein stability, and transcriptional activity. Blue arrows indicate an increase in expression or activity, and red arrows denote a decrease in expression or activity. Abbreviations: Ac, acetylation; ARNT, aryl hydrocarbon receptor nuclear translocator; CBP, CREB-binding protein; EIF, eukaryotic initiation factor; HAF, hypoxia-associated factor; IL, interleukin; IRP1, iron-regulatory protein 1; KLHL-20, Kelch-like-20; MAZ, myc-associated zinc finger protein; PARP-1, poly ADP-ribose and polymerase-1; PTEN, phosphatase and tensin homolog; Sirt1, sirtuin 1; Ub, ubiquitin; USF-2, upstream stimulating factor-2; UTR, untranslated region; YY-1, yin yang-1.
The best-known pathways that specifically regulate HIF-2α in the intestine are iron-driven pathways. PHDs are iron-dependent enzymes, and chelation of iron activates both HIF-1α and HIF-2α in cell lines. However, iron-deficient diets selectively induce HIF-2α expression in mice intestines (38). In addition, HIF-2α mRNA contains a highly conserved iron-responsive element (IRE) in the 5′-untranslated region (UTR). Iron-regulatory proteins (IRP)1 and IRP2 bind with high affinity to IREs on HIF-1α and HIF-2α, as shown in in vitro RNA binding assays. However, IRP binding leads to the inhibition of HIF-2α (but not HIF-1α) translation, with IRP1 the major regulator of the HIF-2α translation (39, 40) (Table 1 and Figure 2). The mechanism of differential regulation of HIF-1α and HIF-2α by IRP is not completely elucidated. Several selective inhibitors of HIF-2α translation that initiate and enhance IRP1 binding to the HIF-2α UTR have been developed as therapeutic targets for renal cell carcinoma (40). Recent work has demonstrated that IRP1 interaction is a physiological regulator of HIF-2α protein expression. Disruption of IRP1 in mouse models led to selective HIF-2α activation and to increased expression of HIF-2α target genes (41, 42).
Target Gene Specificity of HIF-2α
HIF-1α and HIF-2α are regulated in a similar fashion, bind to the same HRE, and share many overlapping genes and function. However, with the generation of knockout mice and tissue-specific conditional mice, it became clear that HIF-1α and HIF-2α have distinct functions and can regulate distinct genes.
The precise mechanisms that determine the target specificity of HIF-1α and HIF-2α are not known. HIF isoforms bind to HREs with similar affinities. Domain-swapping experiments demonstrated that the N-terminal transactivation domain (N-TAD) of HIF-1α and HIF-2α regulates gene specificity (43). These data prompted the search for HIF-2α-selective interacting partners. CREB-binding protein (CBP) directly binds to and increases the acetylation of HIF-2α, which increases the recruitment and transcriptional activity of HIF-2α on its target genes. Conversely, deacetylation of HIF-2α by Sirtuin 1 (Sirt1) decreases its transcriptional activity (44). HIF-2α interaction with C-myc or myc-associated zinc finger protein (MAZ) directs HIF-2α specificity on selective genes and is essential in cell proliferation and the inflammatory response (43, 45). Upstream stimulating factor-2 (USF-2) acts as a coactivator specifically for HIF-2α-dependent genes and is dispensable for the transcriptional induction of HIF-1α target genes (46). More recent work has identified that HIF-2α binding to SUMOlyated hypoxia-associated factor (HAF) increases its transcriptional activity, whereas HAF induces HIF-1α degradation, independently of SUMOlyation (47). In addition to coactivators, yin yang-1 (YY-1), a specific corepressor, directly binds and inhibits the transcriptional activity of HIF-2α (48) (Table 1). Moreover, the importance of ancillary sequences adjacent to the HRE has been determined for HIF-2α-specific targets such as divalent metal transporter-1 (DMT-1, also known as Slc11a2) (38, 49). The Dmt1 promoter maintains HIF-2α specificity on a short, 200-bp proximal promoter region, which contains a canonical HRE and adjacent binding sites for CAAT enhancer binding proteins (38, 49). Indeed, promoter analysis of HIF-2α-specific target genes also revealed the presence of a putative binding site for the ETS family of transcription factors, suggesting a potential interaction between ETS family members and HIF-2α in determining the specificity (50). In addition, genome-wide mapping of HIF-1α and HIF-2α promoter occupancy using ChIP-seq has identified several canonical HRE-binding sites in the promoter selectively bound by either HIF-1α or HIF-2α, providing further evidence that ancillary sequences may have critical roles in maintaining target specificity (51).
ROLE OF HIF-2α IN INTESTINAL DEVELOPMENT
Oxygen gradients are central to development in several tissues. Although oxygen is not classically defined as a morphogen, oxygen levels through HIF signaling perform functions similar to those of well-characterized morphogens in tissue development. The first characterized role of oxygen levels in embryogenesis was demonstrated four decades ago: In vitro neurulation required hypoxic cell culture conditions (52). During early embryogenesis, oxygen and nutrient diffusion is efficient. However, during gastrulation (as the embryo continues to grow and divide), the efficiency of passive diffusion of nutrients and oxygen significantly decreases. Under these conditions, HIF signaling is essential, which is underscored by embryonic lethality in mouse models with disruption of HIF signaling. Hif1α-, Hif2α-, and Vhl-null mice are embryonic lethal. Hif2α-null embryos die at day E16.5 due to dysregulated cardiac output and catecholamine production. An independent group demonstrated that Hif2α-null embryos die at day E11.5–12.5 due to defects in vascular development and remodeling (53, 54). These discrepancies are most likely due to background differences influenced by genetic modifiers. When background influences were closely assessed, no surviving Hif2α-null pups on the 129S6/SvEvTac or C57BL/6J background were found (55). However, the F1 hybrid mating resulted in Hif2α-null mice at significantly reduced Mendelian numbers. Multiple-organ failure such as retinopathy, hepatic steatosis, cardiac hypertrophy, skeletal myopathy, hypocellular bone marrow, and azoospermia in surviving Hif2α-null mice suggests the critical role of HIF-2α in multiple tissues. (55). Hif2α-null mice also exhibited mitochondrial disorders. The heterozygous mice from the 129S6/SvEvTac strain did not demonstrate any overt phenotype. In addition to the severe phenotypes associated with Hif1α-null mice on the C57BL/6J strain background, Hif2α-heterozygous mice demonstrated a decrease in survival, with a mild anemic phenotype (55). Notably, the intestines were not assessed in these studies.
The development and maintenance of intestinal epithelial cells represent a well-studied prototypical model that is widely used to understand stem cell homeostasis. The murine primitive gut tube forms at day E9.5, which is composed of a central lumen encircled by a sheet of epithelium derived from the endoderm (56). Embryonic intestinal epithelium is a pseudostratified epithelium (57), and at E14.5, villus morphogenesis is initiated by remodeling from the flat luminal surface to form fingerlike villi projections (57a). The adult intestinal epithelial lining is a highly regenerative tissue in which stem cell dynamics have been well characterized (58, 58a). Disruption of HIF-2α and ARNT using villin-cre transgenic mice does not result in any overt histological changes in the intestine (24, 59). The finding that there is no overt role of HIF-2α in intestinal stem cell dynamics is surprising because HIF-2α specifically regulates expression of Oct4 and that of its downstream target genes Sox2 and Nanog (60), which are essential for maintaining the stem cell and pluripotent properties of the embryonic stem cells of the inner cell mass. High levels of Oct4 correlates with elevated levels of HIF-2α in the inner mass of the blastocyst compared with the trophectoderm (60a). Moreover, Notch and WNT pathways are critical in intestinal stem cell dynamics, and HIF-2α interacts with and regulates both Notch and Wnt signaling pathways (58, 61–63) (Table 1). Similar to the case for the intestine, Hif2α disruption in hematopoietic stem cells did not alter stem cell pluripotency or maintenance (64, 65). However, Hif2α disruption in the hematopoietic stem cell niche led to a significantly decreased pluripotency of the hematopoietic stem cells (64). This finding suggests that HIF-2α expression in mesenchyme or stromal niche compartments may be critical for stem cell dynamics in the intestine.
HIF-2α AND INTESTINAL IRON ABSORPTION
Recent reviews detail the role of the intestine in systemic iron homeostasis (66, 67). Iron is an essential micronutrient for proper oxygen transport, and there is a well-conserved, bidirectional link between hypoxia and iron signaling. Seventy to eighty percent of iron is used for red blood cell synthesis as an essential prosthetic group in hemoglobin. One to two milligrams of iron are lost on a daily basis, and intestinal iron absorption is the only known mechanism to recover the daily loss. Dietary iron (Fe3+) is reduced by duodenal ferric reductase (DcytB) and is transported into the enterocyte by the apical transporter DMT-1 (68–70). Iron is stored in the enterocyte in ferritin cages or is exported out into the blood through the basolateral iron transporter ferroportin (FPN, also known as Slc40a1) (71–73). Intestinal iron absorption is regulated by both systemic and local mechanisms. Hepcidin is a liver-derived hormone that is regulated by systemic iron levels. High iron levels increase, whereas iron deficiency inhibits, hepcidin expression. Hepcidin is the major systemic regulator that blocks intestinal iron absorption by binding and degrading FPN (74) (Figure 3). In addition, intestinal HIF-2α is a major mechanism by which local intestinal iron absorption from the gut lumen is regulated. HIF-2α is the key transcription factor that promotes intestinal iron absorption by regulating the expression of DcytB, DMT-1, and FPN in the intestine (38, 49, 75–77) (Table 1). The mechanism by which an increase in systemic iron demand can regulate intestinal HIF-2α has not been clearly characterized. During iron deficiency, the iron-dependent PHDs have decreased activity, leading to HIF-2α activation (38). However, a decrease in red blood cell numbers through bleeding, blood disorders such as thalassemia, or treatment with phenylhydrazine increases intestinal iron absorption independently of local iron changes (75, 77). In this case, the decrease in intestinal pO2 is thought to stabilize HIF-2α. However, in both cases there is selective activation of HIF-2α, but not of HIF-1α, suggesting that other mechanisms are required.
Figure 3.
Intestinal iron absorption. (a) Normal systemic iron levels increase circulating hepcidin levels, which degrades ferroportin (FPN). (b) Low systemic iron or anemia decreases hepcidin levels, resulting in stabilization of FPN. Also, HIF-2α stabilization in the intestine leads to transcriptional increases in divalent metal transporter-1 (DMT-1), duodenal ferric reductase (DcytB), and FPN. Other abbreviations: ARNT, aryl hydrocarbon receptor nuclear translocator; HIF-2α, hypoxia-inducible factor-2α.
HIF-2α AND INFLAMMATORY BOWEL DISEASE
IBD, a chronic, persistent, ulcerative disease of the small and/or large intestine, affects approximately 1% of the population in North America and Europe (78). IBD is divided into two major subgroups: ulcerative colitis (UC) and Crohn’s disease (CD). In UC, inflammation is seen primarily in the colon. In CD, inflammatory lesions are observed throughout the GI tract and are characterized by edematous submucosa, ulcerated mucosa, and granulomas, eventually leading to cancerous lesions in all colon segments (79). The precise etiology of IBD is unknown; however, genome-wide association studies identified 99 genetic loci as potential risk factors in the pathogenesis of IBD (80). Several of those genes have been shown to be critical in regulating the mucosal immune response and epithelial barrier integrity. Moreover, recent studies have shown the importance of the microbiota in the onset and progression of IBD (81).
HIF-2α Activation in Inflammatory Bowel Disease
HIF-1α and HIF-2α are highly expressed in the epithelial cells of UC and CD patients and in mouse models of colitis (23, 24). The physiological hypoxia that is normally localized at the villi tips extends down toward the base following inflammatory injury (82). Several mechanisms are important for the increase in hypoxia and in the subsequent stabilization and activation of HIF-1α and HIF-2α in IBD. Patients with chronic intestinal inflammation exhibit microvascular abnormalities, such as vasculitis characterized by decreased intestinal perfusion and limited oxygen supply (83). Moreover, activation of the inflammatory response occurs at a significant expense of energy and oxygen, resulting in hypoxic foci in the inflamed mucosa (23). The inflamed mucosa also undergoes both a metabolic shift due to changes in the inflammatory cells and differential expression of various metabolic genes, leading to altered metabolite levels. For instance, increased expression or mutations in succinate dehydrogenase (commonly seen in high-altitude dwellers) result in induction of HIF expression (84). Increased succinate levels inhibit prolyl hydroxylases and stabilize HIF protein expression in inflamed tissues (29). Like succinate, other TCA cycle metabolites such as α-ketoglutarate and fumarate also regulate HIF expression by modulating PHD enzyme activity (85, 86). Moreover, highly metabolic and highly oxygen-consuming innate immune cells such as monocytes, macrophages, dendritic cells, and neutrophils are recruited to the inflamed foci (4). Transmigrating neutrophils deplete local intestinal oxygen levels due to their ability to undergo respiratory bursts (87).
As introduced above in the section entitled “Oxygen Dynamics of the Intestine,” there is also an intimate bidirectional link between the microbiota and the intestinal mucosal hypoxic response (7, 12). Among the major questions that remain is whether the cross talk between the microbiota and the HIF-2α response is critical in the progression of IBD. Although this question has not been addressed specifically for HIF-2α, the hypoxic response in epithelial cells is dependent on the microbiota metabolites (12). Therefore, it is critical to understand how changes in the gut microbiota during IBD impact HIF-1α or HIF-2α signaling. Lastly, both cytokines and reactive oxygen species activate HIF-2α (31, 88). Thus, HIF-2α activation is a complex and multifactorial pathway, and how these pathways coordinate a HIF-2α response in IBD is unclear.
Mucosal Inflammation and Regeneration
The intestinal epithelial cells act as a functional barrier separating the underlying immune cells from luminal antigens. Studies in the last two decades have demonstrated that epithelial cells express a large number of proinflammatory mediators and chemokines that respond rapidly to injury and infection. Unlike in many other tissues, a basal inflammatory response in intestinal epithelial cells serves as a rheostat in maintaining intestinal homeostasis. This characteristic is best demonstrated in mice whose NFκB response is constitutively activated or disrupted in intestinal epithelial cells. NFκB is a well-characterized inflammatory transcription factor that regulates a large battery of proinflammatory mediators. Disruption of the NFκB response in intestinal epithelial cells led to spontaneous inflammation and cell apoptosis, whereas constitutive activation of NFκB initiated an increased inflammatory response and led to increased susceptibility to acute models of colitis and tumorigenesis (89–92). As does NFκB, HIF-2α directly regulates the expression of several proinflammatory mediators. Chronic activation of HIF-2α signaling in intestinal epithelial cells led to spontaneous colitis, and disruption of intestinal HIF-2α decreased inflammation following treatment with the colitic agent dextran sulfate sodium (DSS) (24) (Table 1). These data suggest an important role of HIF-2α in epithelium-elicited inflammatory response following injury. Although HIF-2α activation led to increased injury in an inflammatory model, HIF-2α activation was protective in a radiation-induced model of injury through vascular endothelial growth factor (VEGF)- and angiogenesis-dependent pathways. Activation of intestinal HIF-2α led to increased crypt regeneration following radiation-induced intestinal injury (27) (Table 1). These data suggest an important role of HIF-2α in both initiation of inflammation and resolution of injury.
Although studies have focused on the intestinal epithelium with respect to HIF-2α in the onset and resolution of intestinal inflammation, HIF-2α is also expressed in immune cells (93). The best-characterized immune cell that expresses HIF-2α is the macrophage. Macrophages accumulate in ischemic and hypoxic sites and are essential in the progression of IBD (94, 95). Macrophages can be polarized, depending on the microenvironmental cues, and are classified as M1 (classically activated) or M2 (alternatively activated). The M1/M2 macrophage subsets are characterized on the basis of the differential metabolism of l-arginine. M1 macrophages express increased levels of inducible nitric oxide (iNOS), which converts l-arginine to l-citrulline and nitric oxide. Alternatively activated M2 macrophages express arginase I (Arg1), which catabolizes l-arginine to l-ornithine. Th1 and Th2 cytokines promote M1 and M2 macrophage polarization, respectively (95). The role and significance of macrophage polarization in IBD are not clear and remain to be determined. M2-stimulating cytokines such as IL-4 and IL-13 highly induce Hif2α mRNA and protein expression, and HIF-2α in turn increases Arg1 expression in macrophages (31) (Table 1). Although M2 macrophages have an anti-inflammatory role and are critical in inflammatory resolution, HIF-2α is essential in activating inflammatory genes following hypoxia or LPS treatment in macrophages (96). Moreover, HIF-2α in macrophages is critical for macrophage recruitment in an acute colitis model by regulating CXR4 and CSFR signaling (96, 97). Recent data also show HIF-2α expression in neutrophils. HIF-2α activation led to neutrophilic inflammation, whereas disruption of Hif2α led to increased apoptosis and decreased inflammation (98) (Table 1). HIF-2α is expressed in several other immune cells, but the role of HIF-2α in these cell types has not been thoroughly explored (93).
HIF-2α and Intestinal Barrier Function
The intestinal barrier is essential in limiting systemic exposure of luminal bacteria and antigens. The intestinal barrier is composed of three components: (a) a mechanical barrier (the intestinal epithelial lining and mucin secreted from goblet cells), (b) an immune barrier, and (c) a microbial barrier (symbiotic bacteria). Intestinal epithelial barrier integrity depends on a molecular network of proteins that form a semipermeable barrier between enterocytes but restricts bacterial and damaging substances. Desmosomes, adherens junctions, and tight junctions form the core networks of the barrier (99). IBD patients exhibit increased intestinal permeability as measured by a lactulose/mannitol test (100). Similarly, animal models of colitis demonstrate increased barrier permeability (101). HIF-2α has a complex role in barrier function. HIF-2α specifically regulates creatine kinases, which are essential for rapid ATP generation in intestinal epithelial cells and are critical for tight junction assembly and epithelial integrity (102). Deletion of HIF-2α in cell lines led to increased expression of proteins involved in apical junction assembly and decreased transepithelial resistance, an accurate measure of barrier integrity (102). However, HIF-2α overexpression in intestinal epithelial cells led to increased caveolin-1 expression. Caveolin-1 is essential for occludin turnover in tight junctions, and increased caveolin-1 expression leads to higher endocytosis and permeability (45) (Table 1).
HIF-2α and Intestinal Fibrosis
Intestinal fibrosis is a common feature present in more than 30% of IBD patients. In UC, fibrosis is seen primarily in the mucosa and submucosa of the large intestine. In CD, fibrotic lesions are observed throughout the GI tract (103). Fibrosis alters the structure and basic functionality of the respective tissue and may lead to deleterious effects such as occlusion, intestinal rupture, and cancer. Current anti-inflammatory treatments do not resolve intestinal fibrosis, possibly due to its long progression and complex mechanisms (103). Therefore, understanding the mechanisms that regulate fibrotic remodeling is vital in effective treatment of intestinal fibrosis and its associated complications. Fibrosis is characterized by excess deposition of extracellular matrix (ECM) that is initiated by an imbalance between matrix metalloproteases (MMPs) and tissue inhibitors of matrix metalloproteases (TIMPs) (104). MMPs released by the epithelial cells at the site of injury and infiltrating inflammatory cells help to digest the ECM, which is required for the reepithelialization and proliferation of adjacent epithelial cells to resolve the wound (104). Key MMPs that are involved in ECM digestion are direct HIF-2α target genes (105). Moreover, collagen prolyl hydroxylases (P4HA1, P4HA2, and P4HA3) that are critical for the maturation of collagen fibrils and fibrosis are HIF target genes. In breast-derived cell lines, these hydroxylases are activated exclusively by HIF-1α (106), but in the liver and intestine, the genes encoding these hydroxylases are activated by HIF-2α (107, 108) (Table 1). Presently, it is not known whether HIF-2α activation in inflamed intestinal tissues is essential for intestinal fibrosis. However, in the liver, HIF-2α activation is sufficient to induce a fibrogenic response (107).
HIF-1α Versus HIF-2α in Inflammatory Bowel Disease
Several labs have shown that HIF-1α activation in epithelial cells results in decreased intestinal injury and inflammation in IBD through regulation of epithelial barrier integrity and the mucosal immune response (2, 3, 23). Physiological hypoxia in intestinal epithelial cells is critical for the maintenance of barrier function. Deletion of intestinal epithelial HIF-1α leads to increased susceptibility to intestinal injury and inflammation in mouse models (23). Currently, HIF-1α is a bona fide therapeutic target for IBD, and several PHD inhibitors are protective in mouse models of colitis (109, 110). Conversely, HIF-2α activation in intestinal epithelial cells promotes inflammation, decreases barrier function, and increases susceptibility in acute models of colitis (24, 45, 102). Disruption of HIF-2α is protective in mouse models of DSS-induced colitis (24). These data suggest opposing functions in colitis. However, recent data demonstrating an important role of HIF-2α in intestinal regeneration paint a more complex view of HIF-2α in intestinal injury and inflammation (27). The temporal regulation of HIF-1α and HIF-2α has not been assessed following intestinal injury, and both HIF-1α and HIF-2α may be essential in an optimal inflammation/resolution response of the intestine. HIF-1α increases barrier integrity, and HIF-2α activates the mucosal immune response to clear the infection or injurious stimuli and to promote regeneration. Indeed, temporal regulation of HIF-1α has been demonstrated, whereby HIF-1α-mediated activation of miR-155 represses HIF-1α expression in prolonged hypoxia (35). Therefore, in chronic colitis, unchecked activation of HIF-2α with diminished expression of HIF-1α may lead to chronic inflammation and injury (Figure 4).
Figure 4.
The role of hypoxia-inducible factor (HIF)-1α and HIF-2α in inflammatory bowel disease. HIF-1α activation in the intestine increases barrier-protective genes such as those encoding multidrug resistance 1a (Mdr1a), 5′-ectonucleotidase (Cd73), mucin, trefoil factor 1 (TFF1), and claudin 1 (Cldn1) and activates a protective innate immune response. HIF-2α activation in the intestine induces proinflammatory mediators such as tumor necrosis factor α (TNFα), cyclooxygenase 2 (COX2), and microsomal prostaglandin synthase 1 (mPGES1). The hypoxic response is initiated and/or maintained by cross talk with the microbiota and transmigrating immune cells (represented by dotted arrowed lines).
HIF-2α AND COLON CANCER
Oxygen concentrations decrease in solid tumors (111). In small tumors, transient changes in oxygen levels are observed, whereas in large tumors chronic hypoxia is observed due to a decrease in blood perfusion (112). Tumor hypoxia is associated with an increase in metastatic potential and with poorer prognosis. There are eight biological properties—hallmarks of cancer—that a cancer cell acquires during the multistep development of cancer: (a) sustaining proliferative signaling, (b) evading growth suppressors, (c) activating invasion and metastasis, (d) enabling replicative immortality, (e) inducing angiogenesis, (f) resisting cell death, (g) deregulating cellular energetics, and (h) avoiding immune destruction. In addition, two enabling factors are instrumental in acquiring and enhancing the hallmarks: (a) genome instability and mutation and (b) tumor-promoting inflammation (113). Tumor hypoxia is involved in each hallmark and enabling characteristic.
CRC is the second-leading cause of cancer deaths in the United States. Eighty percent of sporadic CRCs are associated with a loss-of-function mutation of the APC (adenomatous polyposis coli) tumor suppressor gene. Among the various predisposing factors of CRC, IBD increases the risk of colon cancer, and cancers resulting from IBD are termed colitis-associated colon cancer (CAC) (114, 115). In contrast to the case for sporadic CRC, TP53 mutations occur as an early genetic event and precede APC mutations in the progression of CAC (116–118). In spite of these differences, increased protein expression of HIF-2α is observed in both sporadic CRC and CAC (24). Unlike the case for CRC, more than 50% of cases of clear cell renal carcinoma harbor mutations of Vhl, leading to HIF-2α activation (119). The Cancer Genome Atlas (TCGA) demonstrated that fewer than 4% of colon tumors harbor a mutation in Vhl, Hif2α, or PHD enzymes (PHD1–PHD3) (120). Thus, decreased oxygen homeostasis is a likely mechanism of HIF-2α activation. Altered methylation of the Hif2α gene is also observed in CRC, suggesting an involvement of epigenetic mechanisms in HIF-2α expression (121). Decreased growth in colon cancer–derived cell lines and xenograft models after HIF-2α knockdown demonstrates a central role for HIF-2α in colon cancer (122). However, a contrasting role for HIF-2α as a potential oncogene has been proposed. Loss of HIF-2α in SW480 cells resulted in increased cell proliferation and decreased apoptotic activity, suggesting an antitumor role for HIF-2α (123). These differences may be attributed to the colon cancer–derived cell lines used for the studies. In sporadic CRC and CAC mouse models, HIF-2α expression in intestinal epithelial cells robustly increases tumor multiplicity and progression (24, 108). This effect is specific for HIF-2α, as HIF-1α activation in intestinal epithelial cells does not increase tumorigenesis in sporadic CRC or CAC models (124). Moreover, administration of acriflavine, a dual HIF-1α and HIF-2α inhibitor, significantly decreased CAC (125).
HIF-2α and the Tumor Angiogenic Response
A well-characterized pathway downstream of HIF signaling is angiogenesis. Angiogenesis is a coordinated physiological process to build new blood vessels from existing vessels. When tumors outgrow the minimal limits of oxygen diffusion, angiogenic processes are essential for the growth and progression of these tumors. The gene encoding VEGF, the major growth factor that initiates angiogenesis, is a well-characterized HIF target gene. Although both HIF-1α and HIF-2α can induce expression of the VEGF gene in vitro, there is a higher selectivity for HIF-2α in vivo (126) (Table 1). The tumor angiogenic program constitutes numerous cells: endothelial cell precursors, hematopoietic stem cells, and myeloid cells. Epithelial cells in tumors also display endothelial cell–like features by expressing endothelial and embryonic vasculogenesis-related surface markers such as VE-cadherin, CD34, and CD105 (127). Cancer cells can induce angiogenesis by a phenomenon termed vasculogenic mimicry by increasing the expression of VE-cadherin or CD144 (127). HIF-2α (but not HIF-1) specifically activates VE-cadherin expression (128) (Table 1). The importance of angiogenesis is underscored by drugs that are currently used or in FDA trials to block angiogenesis. In colon cancer, the 5-year survival rate is less than 10%. The most widely used treatment options for patients with CRC are antimetabolite-based drugs such as capecitabine, floxuridine, and fluorouracil, alone or in combination with oxaliplatin or irinotecan (129). Recent data have demonstrated that the addition of the angiogenic inhibitor bevacizumab to the existing treatment regimen for CRC improves overall survival and delays disease progression (130).
HIF-2α and Tumor Cell Metabolism
Cancer cells produce ATP from glucose in glycolysis through the Warburg effect. Glycolysis results in the accumulation of lactate and other glycolytic intermediates that are eventually redirected for the biosynthesis of the amino acids, fatty acids, and nucleotides required by rapidly growing tumor cells. Lactate is also generated from the TCA cycle intermediate malate, which is converted into pyruvate by malic enzyme and subsequently into lactate by lactate dehydrogenase A. Although utilization of glycolysis is a well-characterized metabolic adaptation in tumors, current evidence suggests that tumor cells have far more diverse metabolic adaptation mechanisms than previously realized (131). The HIF response is essential for these adaptive changes in cancer cell metabolism (132). HIF-1α can directly regulate many glycolytic genes, and data from mouse models suggest that HIF-2α is also critical for this glycolytic metabolism in the intestine (38). The increased glycolytic ability of cancer cells has been clinically useful in cancer diagnosis by FDG-PET scans. Hypoxia and the oncogene c-myc are essential in regulating cancer metabolism (133). In specific tumors, coordinated regulation of growth and metabolism is through HIF-2α regulation of c-myc DNA binding and transcriptional activity (43). c-myc increases mitochondrial biogenesis by increasing the expression of mitochondrial transcription factor A and mitochondrial iron levels. Mitochondrial biogenesis helps to generate carbon intermediates from the glucose; such intermediates are essential for de novo synthesis of amino acids and nucleotides. Thus, HIF-2α supports cancer cell growth by providing carbon intermediates. Pyruvate kinase M2 (PKM2) forms dimers or tetramers and favors aerobic glycolysis by irreversible catalysis of phosphoenol pyruvate to pyruvate and ATP (134) (Table 1). PKM2 also acts as a coactivator and increases the transcriptional activity of c-myc, HIF-1α, and HIF-2α by a positive feedback mechanism (135–137). Moreover, the ability of cancer cells to produce lipid precursors was previously unknown because lipogenesis is a highly energetic and oxygen-consuming process. Scavenging mechanisms were thought to be the major source of lipids in cancer. Metabolomics data have demonstrated that hypoxic cells, through HIF-2α, use reductive glutamine metabolism for lipid biosynthesis (138) (Table 1). Most work on colon cancer metabolism has been done in cell lines and xenograft models. However, metabolism in colonocytes and metabolism in CRC-derived cell lines vary significantly. The major energy source of colonocytes in vivo is bacteria-derived SCFA. SCFA help to improve intestine barrier function through activation of the AMPK pathway (139). A recent study reported that utilization of butyrate by colonocytes depletes cellular oxygen levels, resulting in activation of HIF-1α signaling, which plays an important role in maintaining barrier function (12). However, whether SCFA metabolism can also induce HIF-2α expression in colonocytes is unknown. The role of HIF-2α in cancer cell metabolism with respect to CRC has not been adequately defined.
HIF-2α and Tumor-Promoting Inflammation
Chronic inflammation is a critical mechanism that modulates colon cancer. As mentioned above, the risk for developing colon cancer is significantly higher in IBD patients than in the general population. A meta-analysis performed on the role of IBD in colon cancer demonstrated that the risk of colon cancer in IBD patients is directly proportional to the extent and duration of the active disease. In a subsequent study, the severity of IBD was correlated with an increased progression of neoplasias (140). Mice that overexpress HIF-2α in intestinal epithelial cells have increased colonic inflammation and demonstrate a robust increase in tumor number and progression (24). The increase in colon tumors following HIF-2α activation is significantly decreased following treatment with nonsteroidal anti-inflammatory drugs, demonstrating that HIF-2α-dependent inflammation has a critical role in tumorigenesis (43, 108) (Table 1).
The vast majority of CRCs arise without any preceding inflammation but rather are marked by a moderate, low-grade, intrinsic inflammation that is also a major regulator of colon carcinogenesis. As sporadic CRC progresses, the inflammatory gene signatures are similar to those observed in CAC. Furthermore, like most solid tumors, CRC exhibits immune/inflammatory infiltrates referred to as tumor-elicited inflammation (141). The growth-promoting role of inflammation in CAC is well established. In contrast, the current role of tumor-elicited inflammation in CRC patients has not been defined, although in mouse models the tumor-elicited inflammation observed in sporadic CRC is growth promoting (138, 141). Mechanistically, the initiation of tumor-elicited inflammation is attributed to an increase in the permeability of the tumor epithelium, leading to an influx of luminal antigens and activation of the inflammatory response (141). Other mechanisms may also be critical for the initiation of tumor-elicited inflammation because such inflammation is also observed in tumors that arise in sterile sites. However, treating mice with broad-spectrum antibiotics significantly decreased the diversity and number of microbiota and decreased inflammation and tumor numbers (141). HIF-2α activation decreases epithelial barrier function (45, 102) and may be an important mechanism by which the HIF-2α proinflammatory response increases colon tumorigenesis.
Inflammatory lesions observed in CAC or sporadic CRC express several cytokines and angiogenic factors that are critical in proliferative and prosurvival functions. The role of NFκB as a major regulator of several protumorigenic factors in epithelial cells and myeloid cells is well characterized (89). In addition, HIF-2α regulates proinflammatory mediators and angiogenic factors that can contribute to both tumor-elicited inflammation and CAC (142). Lastly, the tumorigenic role of HIF-2α is not limited to its expression in epithelial cells. HIF-2α expressed in tumor-associated macrophages (TAMs) is critical for the infiltration of TAMs and for tumorigenesis. Mice with a disruption of HIF-2α in macrophages exhibit decreased TAMs, tumor number, and tumor burden in CAC mouse models (96) (Table 1).
HIF-2α and Metallomics of Colorectal Cancer
The nutritional and metabolic requirements of cancer cells are an area that holds great therapeutic promise. One area of cancer metabolism that has not been clearly detailed is the requirements of essential micronutrients. The most convincing epidemiological data for the role of micronutrients in cancer cell growth come from studies on patients with altered iron homeostasis. Several meta-analysis studies found that increased iron exposure had a positive correlation with CRC risk (143, 144), whereas low systemic iron decreased risk for CRC (145). Consistent with these data, low-iron diets protect against, and high iron potentiates, tumor growth in mouse models of CRC (108, 146). Colon tumor epithelium has high iron levels that correspond with increases in DMT-1 expression (147). Although other transition metals have not been assessed in such detail, analysis of the TCGA database (https://tcga-data.nci.nih.gov/tcga/) demonstrates an increase in zinc and copper transporters as well. HIF-2α was sufficient to activate DMT-1 in tumor epithelium, and HIF-2α-driven CRC was significantly decreased following low-iron treatment (108). Therefore, a major mechanism by which HIF-2α increases tumor growth in CRC may be through iron.
THERAPEUTIC IMPLICATIONS OF TARGETING HIF-2α
HIF-2α-specific inhibitors have been characterized and may be useful treatments in IBD and CRC (40, 148). Moreover, disruption of HIF-2α signaling is effective in decreasing tissue iron accumulation in secondary hemochromatosis models (77). Thus, pharmacological inhibition of HIF-2α may have therapeutic efficacy in iron overload anemia such as that resulting from sickle cell and β-thalassemia. Although HIF-2α inhibitors are still being characterized, several PHD inhibitors that activate HIF-2α are under preclinical or clinical investigation for potential therapeutic use in the treatment of iron-related diseases such as anemia. FG-4592 is under phase III clinical trials for the treatment of anemia in chronic kidney disease patients (149). Moreover, these agents are useful in decreasing intestinal permeability through a HIF-1α-dependent mechanism and could restore the regenerative capacity of the intestine through HIF-2α. However, caution is needed in the chronic use of such agents because they may induce deleterious HIF-2α-mediated inflammation and may increase the risk of CRC.
SUMMARY POINTS.
Luminal and epithelial oxygen gradients are set up by complex cross talk between the intestinal epithelial cells, the vasculature, and the microbiota.
HIF-1α and HIF-2α have distinct and overlapping functions in the intestine.
HIF-2α is essential in iron absorption and in maintaining the epithelial barrier.
Intestinal disruption of HIF-2α is dispensable for normal tissue proliferation but is essential for regeneration following radiation.
Aberrant and chronic activation of HIF-2α in the intestine leads to inflammation and to decreased epithelial barrier function and promotes CRC.
FUTURE ISSUES.
Mouse models should be developed to accurately study the temporal regulation of HIF-1α and HIF-2α.
The role of HIF-2α in specific immune cell populations during intestinal injury and inflammation needs to be characterized.
Pathways that cross talk with HIF-2α in the intestine should be explored.
Pathways that lead to specific HIF-2α expression or activation need investigation.
Acknowledgments
We apologize to colleagues whose works are not cited due to space limitations. This work was supported by NIH grants CA148828 and DK095201 (to Y.M.S.). S.K.R. was supported by a postdoctoral fellowship from the American Heart Association (15POST22650034).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Helander HF, Fandriks L. Surface area of the digestive tract—revisited. Scand. J. Gastroenterol. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 2.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140:1748–1755. doi: 10.1053/j.gastro.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS. 2007;104:13780–137885. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 6.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He G, Shankar RA, Chzhan M, Samouilov A, Kuppusamy P, Zweier JL. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. PNAS. 1999;96:4586–4591. doi: 10.1073/pnas.96.8.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J. Surg. Res. 2000;93:182–196. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd AP, Riedel GL. Intramural distribution of intestinal blood flow during sympathetic stimulation. Am. J. Physiol. Heart Circ. Physiol. 1988;255:H1091–H1095. doi: 10.1152/ajpheart.1988.255.5.H1091. [DOI] [PubMed] [Google Scholar]
- 11.Crissinger KD, Burney DL. Influence of luminal nutrient composition on hemodynamics and oxygenation in developing intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1992;263:G254–G260. doi: 10.1152/ajpgi.1992.263.2.G254. [DOI] [PubMed] [Google Scholar]
- 11a.Shepherd AP, Kiel JW. A model of countercurrent shunting of oxygen in the intestinal villus. Am. J. Physiol. Heart Cir. Physiol. 1992;262:H1136–H1142. doi: 10.1152/ajpheart.1992.262.4.H1136. [DOI] [PubMed] [Google Scholar]
- 12.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 16.Erbel PJ, Card PB, Karakuzu O, Bruick RK, Gardner KH. Structural basis for PAS domain heterodimerization in the basic helix--loop--helix-PAS transcription factor hypoxia-inducible factor. PNAS. 2003;100:15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinoshita K, Kikuchi Y, Sasakura Y, Suzuki M, Fujii-Kuriyama Y, Sogawa K. Altered DNA binding specificity of Arnt by selection of partner bHLH-PAS proteins. Nucleic Acids Res. 2004;32:3169–3179. doi: 10.1093/nar/gkh637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 19.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 20.Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 21.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 22.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 23.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi H, Pino MS, Zeng M, Shirasawa S, Chung DC. Oncogenic KRAS and BRAF differentially regulate hypoxia-inducible factor-1α and-2α in colon cancer. Cancer Res. 2009;69:8499–8506. doi: 10.1158/0008-5472.CAN-09-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi CM, Finger EC, Krieg AJ, Wu C, Diep AN, et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 2013;19:1325–1330. doi: 10.1038/nm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med. 2014;6:236ra64. doi: 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768. [PubMed] [Google Scholar]
- 29.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Raimundo N, Baysal BE, Shadel GS. Revisiting the TCA cycle: signaling to tumor formation. Trends Mol. Med. 2011;17:641–649. doi: 10.1016/j.molmed.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Flores A, Aguilar-Quesada R, Siles E, Pozo S, Rodriguez-Lara MI, et al. Interaction between PARP-1 and HIF-2α in the hypoxic response. Oncogene. 2014;33:891–898. doi: 10.1038/onc.2013.9. [DOI] [PubMed] [Google Scholar]
- 33.Qi Y, Liu J, Saadat S, Tian X, Han Y, et al. PTEN induces apoptosis and cavitation via HIF-2-dependent Bnip3 upregulation during epithelial lumen formation. Cell Death Differ. 2015;22:875–884. doi: 10.1038/cdd.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1α and 2α on mTORC1 and mTORC2. J. Biol. Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1α activity during prolonged hypoxia. Mol. Cell. Biol. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higashimura Y, Terai T, Yamaji R, Mitani T, Ogawa M, et al. Kelch-like 20 up-regulates the expression of hypoxia-inducible factor-2α through hypoxia- and von Hippel–Lindau tumor suppressor protein–independent regulatory mechanisms. Biochem. Biophys. Res. Commun. 2011;413:201–205. doi: 10.1016/j.bbrc.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1α, leading to its oxygen-independent degradation. Mol. Cell. Biol. 2008;28:7081–7095. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2α expression in iron deficiency. Nat. Struct. Mol. Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer M, Ebert BL, Neil C, Brenner K, Papaioannou I, et al. Small-molecule inhibitors of HIF-2α translation link its 5UTR iron-responsive element to oxygen sensing. Mol. Cell. 2008;32:838–848. doi: 10.1016/j.molcel.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson SA, Nizzi CP, Chang YI, Deck KM, Schmidt PJ, et al. The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 2013;17:282–290. doi: 10.1016/j.cmet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh MC, Zhang DL, Jeong SY, Kovtunovych G, Ollivierre-Wilson H, et al. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α. Cell Metab. 2013;17:271–281. doi: 10.1016/j.cmet.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2α promotes hypoxic cell proliferation by enhancing c-Myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, Xu M, Hogg RT, Li J, Little B, et al. The acetylase/deacetylase couple CREB-binding protein/Sirtuin 1 controls hypoxia-inducible factor 2 signaling. J. Biol. Chem. 2012;287:30800–30811. doi: 10.1074/jbc.M111.244780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie L, Xue X, Taylor M, Ramakrishnan SK, Nagaoka K, et al. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol. Cell. Biol. 2014;34:3013–3023. doi: 10.1128/MCB.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawlus MR, Wang L, Ware K, Hu CJ. Upstream stimulatory factor 2 and hypoxia-inducible factor 2α (HIF2α) cooperatively activate HIF2 target genes during hypoxia. Mol. Cell. Biol. 2012;32:4595–4610. doi: 10.1128/MCB.00724-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh MY, Nguyen V, Lemos R, Jr, Darnay BG, Kiriakova G, et al. Hypoxia-induced SUMOylation of E3 ligase HAF determines specific activation of HIF2 in clear-cell renal cell carcinoma. Cancer Res. 2015;75:316–329. doi: 10.1158/0008-5472.CAN-13-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrella BL, Brinckerhoff CE. PTEN suppression of YY1 induces HIF-2 activity in von-Hippel-Lindau-null renal-cell carcinoma. Cancer Biol. Ther. 2009;8:1389–1401. doi: 10.4161/cbt.8.14.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Investig. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–5647. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- 51.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morriss GM, New DA. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J. Embryol. Exp. Morphol. 1979;54:17–35. [PubMed] [Google Scholar]
- 53.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2α plays an important role in vascular remodeling. PNAS. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat. Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 56.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, et al. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, et al. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 58a.Carulli AJ, Samuelson LC, Schnell S. Unraveling intestinal stem cell behavior with models of crypt dynamics. Integr. Biol. 2014;6:243–257. doi: 10.1039/c3ib40163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator–dependent system in gut. J. Clin. Investig. 2007;117:1940–1950. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, et al. HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60a.Wicklow E, Blij S, Frum T, Hirate Y, Lang RA, et al. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLOS Genet. 2014;10:e1004618. doi: 10.1371/journal.pgen.1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi H, Chun YS, Kim TY, Park JW. HIF-2α enhances β-catenin/TCF-driven transcription by interacting with β-catenin. Cancer Res. 2010;70:10101–10111. doi: 10.1158/0008-5472.CAN-10-0505. [DOI] [PubMed] [Google Scholar]
- 62.Hu YY, Fu LA, Li SZ, Chen Y, Li JC, et al. Hif-1α and Hif-2α differentially regulate Notch signaling through competitive interaction with the intracellular domain of Notch receptors in glioma stem cells. Cancer Lett. 2014;349:67–76. doi: 10.1016/j.canlet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Houshmand G, Mishra S, Fong GH, Gittes GK, Esni F. Impaired pancreatic development in Hif2-α deficient mice. Biochem. Biophys. Res. Commun. 2010;399:440–445. doi: 10.1016/j.bbrc.2010.07.111. [DOI] [PubMed] [Google Scholar]
- 64.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2α is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 65.Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, et al. Hif-2α is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 66.Mastrogiannaki M, Matak P, Peyssonnaux C. The gut in iron homeostasis: role of HIF-2 under normal and pathological conditions. Blood. 2013;122:885–892. doi: 10.1182/blood-2012-11-427765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah YM, Xie L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology. 2014;146:630–642. doi: 10.1053/j.gastro.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 69.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 70.McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291:1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 71.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 72.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 73.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 74.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 75.Anderson ER, Xue X, Shah YM. Intestinal hypoxia-inducible factor-2α (HIF-2α) is critical for efficient erythropoiesis. J. Biol. Chem. 2011;286:19533–19540. doi: 10.1074/jbc.M111.238667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140:2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson ER, Taylor M, Xue X, Ramakrishnan SK, Martin A, et al. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. PNAS. 2013;110:E4922–E4930. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 2011;43:1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 82.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bacaner MB. Quantitative measurement of regional colon blood flow in the normal and pathological human bowel. Gastroenterology. 1966;51:764–777. [PubMed] [Google Scholar]
- 84.Cerecer-Gil NY, Figuera LE, Llamas FJ, Lara M, Escamilla JG, et al. Mutation of SDHB is a cause of hypoxia-related high-altitude paraganglioma. Clin. Cancer Res. 2010;16:4148–4154. doi: 10.1158/1078-0432.CCR-10-0637. [DOI] [PubMed] [Google Scholar]
- 85.Hewitson KS, Lienard BM, McDonough MA, Clifton IJ, Butler D, et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 86.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, et al. NAD(P)H oxidases regulate HIF-2α protein expression. J. Biol. Chem. 2007;282:8019–8026. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 89.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 91.Shaked H, Hofseth LJ, Chumanevich A, Chumanevich AA, Wang J, et al. Chronic epithelial NF-κB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. PNAS. 2012;109:14007–14012. doi: 10.1073/pnas.1211509109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 93.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 95.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Investig. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, et al. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson AA, Elks PM, Marriott HM, Eamsamarng S, Higgins KR, et al. Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood. 2014;123:366–376. doi: 10.1182/blood-2013-05-500207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, et al. Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology. 1989;97:927–931. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 101.Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 102.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. PNAS. 2013;110:19820–19825. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease—current knowledge and future perspectives. J. Crohn’s Colitis. 2008;2:279–290. doi: 10.1016/j.crohns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006;318:933–938. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- 105.Yang S, Kim J, Ryu JH, Oh H, Chun CH, et al. Hypoxia-inducible factor-2α is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 106.Gilkes DM, Bajpai S, Chaturvedi P, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 2013;288:10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Qu A, Taylor M, Xue X, Matsubara T, Metzger D, et al. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472–483. doi: 10.1002/hep.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xue X, Taylor M, Anderson E, Hao C, Qu A, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res. 2012;72:2285–2293. doi: 10.1158/0008-5472.CAN-11-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 112.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 113.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 114.Itzkowitz SH, Yio X. Inflammation and cancer. IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 115.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 117.Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602–1610. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 118.Redston MS, Papadopoulos N, Caldas C, Kinzler KW, Kern SE. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995;108:383–392. doi: 10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 119.Sourbier C, Srivastava G, Ghosh MC, Ghosh S, Yang Y, et al. Targeting HIF2α translation with Tempol in VHL-deficient clear cell renal cell carcinoma. Oncotarget. 2012;3:1472–1482. doi: 10.18632/oncotarget.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rawluszko-Wieczorek AA, Horbacka K, Krokowicz P, Misztal M, Jagodzinski PP. Prognostic potential of DNA methylation and transcript levels of HIF1A and EPAS1 in colorectal cancer. Mol. Cancer Res. 2014;12:1112–1127. doi: 10.1158/1541-7786.MCR-14-0054. [DOI] [PubMed] [Google Scholar]
- 122.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2α oncogenic axis. PNAS. 2009;106:21306–21311. doi: 10.1073/pnas.0906432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Imamura T, Kikuchi H, Herraiz MT, Park DY, Mizukami Y, et al. HIF-1α and HIF-2α have divergent roles in colon cancer. Int. J. Cancer. 2009;124:763–771. doi: 10.1002/ijc.24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xue X, Ramakrishnan SK, Shah YM. Activation of HIF-1α does not increase intestinal tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G187–G195. doi: 10.1152/ajpgi.00112.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shay JE, Imtiyaz HZ, Sivanand S, Durham AC, Skuli N, et al. Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis. 2014;35:1067–1077. doi: 10.1093/carcin/bgu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, et al. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 128.Le Bras A, Lionneton F, Mattot V, Lelievre E, Caetano B, et al. HIF-2α specifically activates the VE-cadherin promoter independently of hypoxia and in synergy with Ets-1 through two essential ETS-binding sites. Oncogene. 2007;26:7480–7489. doi: 10.1038/sj.onc.1210566. [DOI] [PubMed] [Google Scholar]
- 129.Masi G, Allegrini G, Cupini S, Marcucci L, Cerri E, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann. Oncol. 2004;15:1766–1772. doi: 10.1093/annonc/mdh470. [DOI] [PubMed] [Google Scholar]
- 130.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 131.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 132.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J. Clin. Investig. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang W, Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle. 2013;12:3154–3158. doi: 10.4161/cc.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luo W, Hu H, Chang R, Zhong J, Knabel M, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang W, Zheng Y, Xia Y, Ji H, Chen X, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat. Cell Biol. 2012;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013;143:1872–1881. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- 140.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr. Top. Microbiol. Immunol. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ashmore JH, Rogers CJ, Kelleher SL, Lesko SM, Hartman TJ. Dietary iron and colorectal cancer risk: a review of human population studies. Crit. Rev. Food Sci. Nutr. 2015 doi: 10.1080/10408398.2012.749208. In press. [DOI] [PubMed] [Google Scholar]
- 144.Nelson RL. Iron and colorectal cancer risk: human studies. Nutr. Rev. 2001;59:140–148. doi: 10.1111/j.1753-4887.2001.tb07002.x. [DOI] [PubMed] [Google Scholar]
- 145.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J. Natl. Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 146.Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, et al. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep. 2012;2:270–282. doi: 10.1016/j.celrep.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–1460. doi: 10.1136/gut.2006.094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Scheuermann TH, Li Q, Ma HW, Key J, Zhang L, et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 2013;9:271–276. doi: 10.1038/nchembio.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beuck S, Schanzer W, Thevis M. Hypoxia-inducible factor stabilizers and other small-molecule erythropoiesis-stimulating agents in current and preventive doping analysis. Drug Test. Anal. 2012;4:830–845. doi: 10.1002/dta.390. [DOI] [PubMed] [Google Scholar]
- 150.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, et al. Hypoxia-inducible factor 1–dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, et al. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 2013;6:1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geng H, Liu Q, Xue C, David LL, Beer TM, et al. HIF1α protein stability is increased by acetylation at lysine 709. J. Biol. Chem. 2012;287:35496–35505. doi: 10.1074/jbc.M112.400697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. J. Exp. Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 155.Kaidi A, Williams AC, Paraskeva C. Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]