Abstract
Multidetector CT (MDCT) is an imaging technique that provides otherwise unobtainable information in the diagnostic work-up of patients presenting with acute abdominal pain. A correct working diagnosis depends essentially on understanding the individual patient's clinical data and laboratory findings. In haemodynamically stable patients with acute severe and generalized abdominal pain, MDCT is now the preferred imaging test and gives invaluable diagnostic information, also in unstable patients after stabilization. In this descriptive review, we focus our attention on acute, severe and generalized or undifferentiated non-traumatic abdominal pain. The main differential diagnoses are acute pancreatitis, gastrointestinal perforation, ruptured abdominal aneurysm and acute mesenteric ischaemia. We will provide radiologist readers with a technical guide to optimize MDCT imaging protocols and list the major CT signs essential to reach a correct diagnosis and guide the best treatment.
INTRODUCTION
Acute abdominal pain is a common condition accounting for 4–5% of all emergency department admissions,1 involving a sudden onset of severe pain developing over hours associated with abdominal tenderness and rigidity. According to the 0–10 point pain numeric rating scale (NRS-11),2 severe pain is described as 8 or higher. Patients are triaged by the emergency department nurse as Level 2, according to the emergency severity index.3
Rapid and accurate diagnosis is essential. Although clinical data, physical examination and laboratory test results allow the clinician to reach a “working clinical diagnosis”, clinical and laboratory assessment often yields inconclusive results, especially if pain spreads throughout the abdomen rather than involving a specific region or abdominal quadrant.1
Abdominal pain has innumerable possible origins ranging from benign self-limiting to life-threatening diseases. In patients with severe and generalized abdominal pain, the main causes are acute pancreatitis, bowel perforation, ruptured abdominal aneurysm and acute mesenteric ischaemia (AMI).
Ample information including a randomized trial shows that the best diagnostic imaging test in these clinical scenarios is multidetector CT (MDCT).1–4 MDCT imaging improves diagnostic accuracy (from 71% to 93%)4 and increases the clinical diagnostic confidence level. In a cohort study comparing ultrasound and CT in 1021 consecutive patients, CT was significantly more sensitive than ultrasound (89% vs 70%, p < 0.001), although the approach achieving the highest sensitivity was a diagnostic strategy combining an initial ultrasound scan, followed by CT, only when ultrasound examination yielded negative or inconclusive findings.4
In patients with severe and generalized acute abdominal pain, the currently preferred imaging modality is contrast-enhanced MDCT.1–4 Patients presenting with haemodynamic instability should undergo MDCT immediately after stabilization.1
In this descriptive review, we analyse MDCT protocols and the diagnostic role of MDCT in patients presenting to the emergency department with clinically suspected acute pancreatitis, gastrointestinal perforation, ruptured aneurysm and AMI. We especially offer information useful to radiologists wishing to use state-of-the-art MDCT scanners in emergency.
MULTIDETECTOR CT PROTOCOL
The MDCT acquisition protocol should be tailored to the working diagnosis.5 If the working diagnosis is weak, as it often is in patients presenting to the emergency department with severe, generalised abdominal pain, patients should undergo a detailed MDCT study. The MDCT protocol should include a pre-contrast scan followed by dynamic images acquired in the arterial and portal venous phases without oral contrast medium (CM).
If MDCT scans for severe abdominal pain are to reach the correct diagnosis or establish the extent of disease whenever possible, patients should receive intravenous CM. CM is essential for diagnosing vascular disease (aortic or splanchnic vessel bleeding) as well as for bowel ischaemia.5 Contrast-enhanced images are helpful in delineating the bowel wall in patients with poor fat planes and free fluid surrounding the bowel. Bowel wall delineation is essential in many acute abdominal conditions especially in locating the bowel wall perforation site. And in patients with suspected acute pancreatitis, pancreatic contrast enhancement should be assessed for disease staging.
All patients with acute abdomen should undergo an unenhanced scan. An unenhanced scan is useful, for example, to localize intramural haematoma in a rupturing aortic aneurysm.6 Our experience shows that findings from the pre-contrast scan can also be used to optimize the post-contrast scan protocol.
When MDCT is needed to investigate organ arterial enhancement, the scanning protocol should include an arterial phase. Suspected vascular disease requires an early arterial phase (EAP), whereas arterial parenchymal enhancement necessitates a late arterial phase (LAP).6,7 Both acquisitions necessitate a CM bolus monitoring technique, but they differ in delay from the trigger (usually 100 HU) measured in the arterial district of reference. An EAP should start 8 s after the trigger, whereas the delay for a LAP is 18 s. For EAP, the CM injected concentrates in the arterial vessels, whereas in the LAP, it enhances arterial vessels and parenchyma.6,7
Consensus recommends portal venous phase using a best guess standardized scan delay of 70 s after CM injection.1–5,7–9 Acute pancreatitis requires a pancreatic arterial phase scan (acquired 45 s after the injection starts). A delayed phase is rarely recommended except for patients with acute pyelonephritis.5
Once the radiologist has chosen the acquisition protocol, CM injection should be optimized by setting CM volume, or iodine dose, and flow rate, or iodine delivery rate (IDR).8 For a vascular study, CM volume can be calculated according to the scan time. The injection time should equal the scan time (for the arterial phase) plus the scan delay (8 s). For EAP, choosing a proper IDR is crucial because this setting specifically influences vascular attenuation. Most investigators suggest an IDR of 2 g of iodine (gI) per second.8 This rate means that for a CM with an iodine concentration of 400 mgI dl−1, CM should be injected at 5 ml s−1.8
For non-vascular studies or multiphasic acquisitions (EAP and portal phase), CM volume should be calculated according to patient size using two approaches. The first depends on total body weight (TBW) and the second depends on lean body weight (LBW). The first method requires injecting 0.625 gI per kilogram of TBW, and the second method requires injecting 0.750 mgI per kilogram of LBW.9 In the emergency setting, the method, based on TBW, should take precedence because it takes less time to calculate. LBW can, nevertheless, be quickly calculated using dedicated nomograms based on the patient's sex, height and weight.8,9
Intravenous CM raises problems in patients with impending renal insufficiency; in these cases, the risk of acute renal failure should be carefully weighed against the added value for diagnosis and patient management.
Oral or rectal CM has little use in patients with severe abdominal pain. Giving an oral contrast agent delays CT acquisition and the lack of contrast agent within the bowel loops seems not to hamper CT reading.10
Scan settings include a collimation ranging between 0.5 and 2.5 mm that modern scanners provide from the diaphragm dome to the pubic symphysis within seconds.5 Automatic tube current modulation or iterative reconstruction, available on last-generation scanners, can reduce ionizing radiation exposure particularly in young patients.11
Thanks to progress in MCDT technology and software exceedingly helpful diagnostic information on severe abdominal pain comes from advances in image post-processing techniques.1,12 Multiplanar reformation (MPR) increases the radiologist's confidence level for diagnosis12 and may help in delineating the bowel wall contour, whereas MPR, maximum intensity projection and three-dimensional volume rendering may help in vascular diseases.
CLINICAL SETTINGS
Acute pancreatitis
Acute pancreatitis is diagnosed on clinical and laboratory data alone without imaging. Imaging, nevertheless, has a pivotal role in assessing the severity of disease, evaluating underlying causes and identifying complications, and it is, therefore, essential for patient management.13
The revised Atlanta classification13 classifies acute pancreatitis into interstitial oedematous pancreatitis (IEP) and acute necrotizing pancreatitis (ANP) and distinguishes two stages: an early stage taking place within the first week after disease onset and a late stage after the first week.14–17
In the early stage, severity is assessed on clinical variables (haematocrit; APACHE II and Ranson scores, serum C-reactive protein levels, pulmonary complications), because the need for treatment is determined primarily by the presence or absence of organ failure caused by the systemic inflammatory response syndrome and much less by morphological findings involving the pancreas and peripancreatic region.
The late stage begins after the first week and may extend for weeks to months; it is characterized by increasing necrosis, infection and persistent multiorgan failure.18 Treatment is determined from clinical variables and morphological criteria as defined by MDCT.19,20
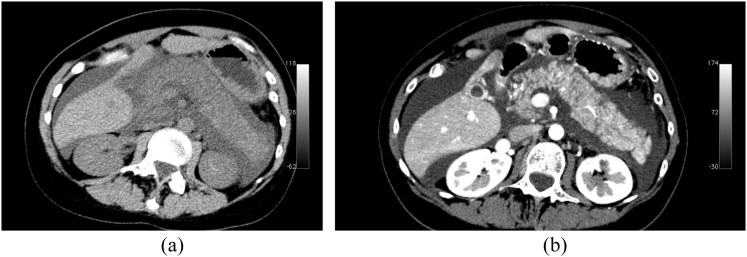
In patients with IEP, contrast-enhanced CT demonstrates a localized or diffusely enlarged pancreas, with normal homogeneous or slightly heterogeneous pancreatic parenchyma enhancement related to oedema (Figure 1).14 In early-stage mild disease, peripancreatic and retroperitoneal tissues may appear normal or may show inflammatory changes including “mistiness” or fat stranding with peripancreatic fluid in variable amounts.
Figure 1.
Multidetector CT study acquired in a patient affected by interstial acute pancreatitis. (a) Unenhanced CT image. (b) Contrast-enhanced CT image. Pancreas is diffusely enlarged and shows low density on unenhanced scan. Pancreatic gland is surrounded by fluid. On contrast-enhanced image, pancreatic gland shows homogeneous enhancement.
The revised Atlanta classification system distinguishes three forms of ANP: parenchymal necrosis alone, peripancreatic necrosis alone and pancreatic and peripancreatic necrosis.13,14 Parenchymal necrosis alone is seen in <5% of patients and appears on contrast-enhanced MDCT images as a lack of parenchymal enhancement.21
Peripancreatic necrosis alone, observed in approximately 20% of patients,22 is diagnosed when MDCT images show heterogeneous unenhanced areas containing non-liquefied components. Peripancreatic necrosis commonly involves the retroperitoneum and lesser sac. The clinical importance of peripancreatic necrosis alone lies in the fact that patients with this condition have a better prognosis than patients with pancreatic parenchymal necrosis.13,14
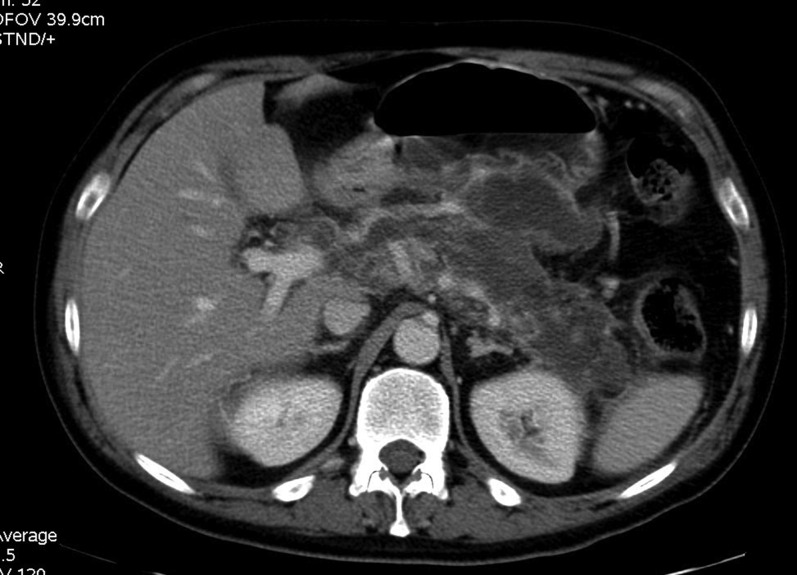
Acute pancreatic parenchymal necrosis with peripancreatic necrosis is the most common type and is seen in 75–80% of patients with ANP19 (Figure 2). On CT scans, ANP has imaging appearances similar to those for pancreatic parenchymal necrosis alone and peripancreatic necrosis alone combined.22
Figure 2.
Multidetector CT study acquired in a patient affected by necrotizing pancreatitis: axial contrast-enhanced CT scan showing diffuse lack of the pancreatic gland enhancement with fluidification of pancreatic tissue as well as peripancreatic fat.
Acute pancreatitis can be accompanied by parenchymal or peripancreatic collections. The revised Atlanta classification makes an important distinction between fluid and no liquefied collections.14 Acute collections are referred to as either acute peripancreatic fluid collections or as acute necrotic collections, depending on the absence or presence of necrosis. IEP can be associated with acute peripancreatic fluid collection and, over time, with pancreatic pseudocysts. Necrotizing pancreatitis in its three forms can be associated with acute necrotic collection and, over time, with walled-off necrosis, a thickened non-epithelialized wall between the necrosis and the adjacent tissue, representing a maturing collection (Figure 3).
Figure 3.
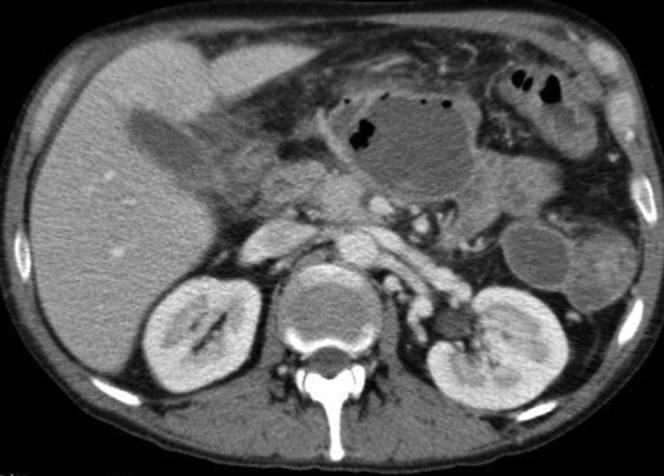
A contrast-enhanced CT image acquired in a patient affected by necrotizing pancreatitis and infected walled-off necrosis: CT image shows a capsulated fluid collection in the pancreatic area with internal gas bubbles.
All four types of pancreatic fluid collections can be sterile or infected. Contrast-enhanced MDCT images showing gas bubbles within the collection suggest infection.23,24
Gastrointestinal perforation
Another possible cause of severe abdominal pain is gastrointestinal perforation. Gastroduodenal perforation causes particularly severe pain owing to the dramatic effect of peptic acid on the peritoneum.25
Non-traumatic breach of the gastrointestinal tract wall can arise from peptic ulcer disease, inflammatory disease, diverticulitis, iatrogenic factors, foreign body and neoplasm. Bowel perforation may complicate other bowel diseases such as small-bowel obstruction and mesenteric ischaemia. Correctly identifying the presence, location and cause of the perforation is essential for appropriate management and surgical planning. MDCT is extremely accurate in depicting extraluminal gas, and it has 86% accuracy in predicting the site of the perforation.25 The diagnosis of GI tract perforation is based on direct CT findings, such as bowel wall discontinuity, and on indirect signs, such as bowel wall thickening, abnormal bowel wall enhancement, perigastroduodenal fluid, stranding and gas bubbles close to the affected wall. Direct visualization of bowel wall discontinuity can specify the presence and site of GI tract perforation, which is marked by a low-attenuating cleft that usually runs perpendicular to the bowel wall on CT. MDCT visualizes this cleft less frequently than free air. A cleft is usually seen in <50% of the patients with GI tract perforation.26 When axial CT images are inconclusive, bowel wall discontinuity appears more clearly on MPR.
Another MDCT approach to identify the site of perforation entails investigating free-air distribution. Upper GI tract perforations (stomach or duodenal bulb) cause gas to accumulate in the supramesocolic compartment, whereas perforations in the distal small and large bowel loops distend either the inframesocolic cavity alone or the inframesocolic and supramesocolic compartments.
If a perforation involves retroperitoneal structures, the second and third duodenal segments, ascending and descending colon or the middle third of the rectum gas, may be present in the retroperitoneal space, usually the anterior pararenal space.27 In patients with gastro-oesophageal perforation, gas can spread into the mediastinum as well as the peritoneal cavity.
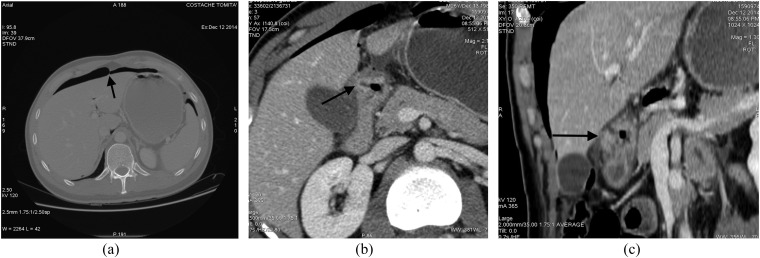
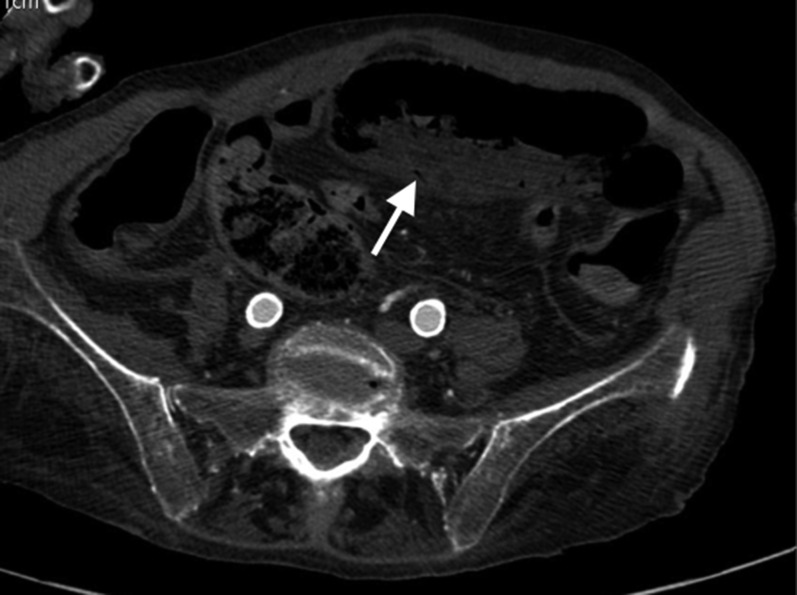
When free gas is seen outlining the intrahepatic fissure and ligamentum teres (known as ligamentum teres sign) (Figure 4), this MDCT appearance often reflects a perforation in the duodenal bulb or stomach.28 Also the “periportal free gas sign” (air around the portal vein), strongly suggests upper GI tract perforation.
Figure 4.
Images from a multidetector CT study acquired in a patient affected by perforated duodenal peptic ulcer: (a) axial CT image with wide window setting; (b) contrast-enhanced axial image; (c) an oblique multiplanar reformation. (a) Intraperitoneal free air is clearly visible in the anterior perihepatic space, crossing the midline and accentuating the falciform ligament (arrow). Tiny air bubbles are also visible at the hepatic hilum, close to the caudate lobe and in the sperisplenic space. (b) Thickening of bulbar duodenal wall with deep penetrating ulcer (arrow) is visible. (c) A duodenal wall cleft is better visualized (arrow).
Another sign, “falciform ligament sign” (free gas or a gas–fluid level crossing the abdominal midline and delineating the falciform ligament), is seen more frequently in perforations involving the proximal GI tract (stomach, duodenum, jejunum).25–28
Ruptured aneurysm
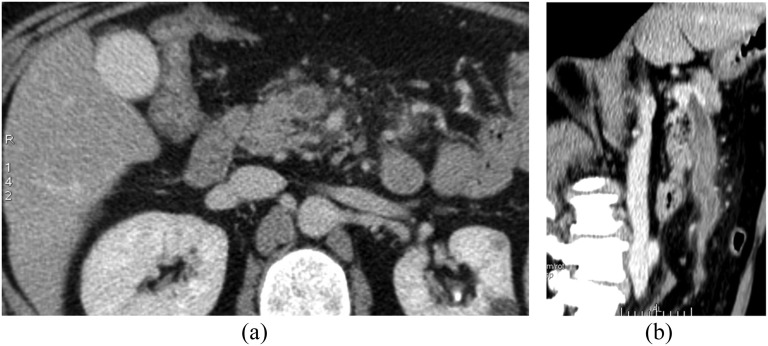
A dramatic emergency event requiring prompt diagnosis is rupture or impending rupture of abdominal aortic aneurysm. Most abdominal vascular ruptures manifest as a retroperitoneal haematoma. Periaortic blood may extend into the perirenal space, the pararenal space or both.29 Intraperitoneal extravasation may be an immediate or a delayed finding (Figure 5).
Figure 5.
Contrast-enhanced multidetector CT images acquired in a patient presenting with ruptured right iliac aneurysm with hemoperitoneum. (a) The site of aneurysm rupture is well depicted (arrow); (b) massive pelvic hemoperitoneum with active extravasation of iodinate contrast medium is shown.
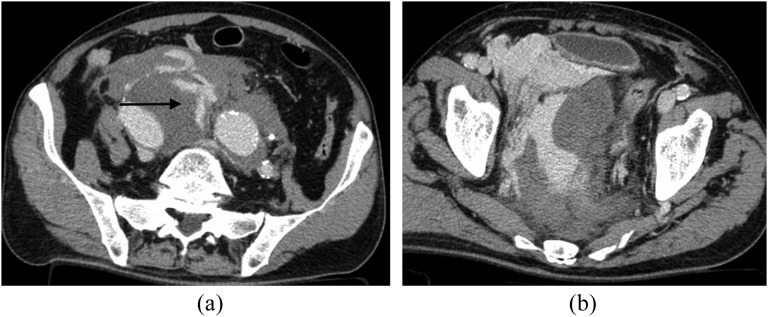
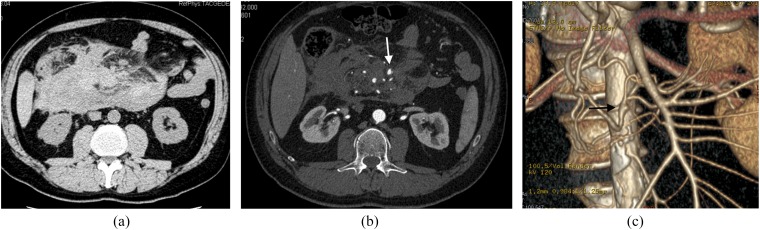
In patients presenting with a retroperitoneal haematoma and normal abdominal aorta, the radiologist should carefully seek a splanchnic vessel aneurysm (Figure 6). A retroperitoneal haematoma adjacent to an abdominal aortic aneurysm is the most common imaging finding in abdominal aortic aneurysm rupture.30
Figure 6.
Images from multidetector CT (MDCT) study acquired in a patient with rupturing inferior pancreaticoduodenal artery (IPDA) aneurysm. (a) Unenhanced CT image: huge retroperitoneal haematoma is clearly visible in the mesenteric root. (b) Axial contrast-enhanced MDCT image acquired in the early arterial phase; in this image, it is very difficult to identify a tiny aneurysm of a splanchnic arterial vessel (arrow). (c) Three-dimensional volume rendering reconstruction; in this image, a small aneurysm of the IPDA with tiny aneurysm wall bleb is clearly visible (arrow).
An important MDCT imaging feature that may be seen in a contained ruptured abdominal aortic aneurysm is the draped aorta sign.31 This sign is considered present either when the posterior wall of the aorta is not identifiable as distinct from adjacent structures or when it closely follows the contour of adjacent vertebral bodies. A well-defined peripheral crescent of increased attenuation within the thrombus of a large abdominal aortic aneurysm is a CT sign of acute or impending rupture.32 This finding is best appreciated on unenhanced CT images.
Hyper-attenuating crescents have been attributed histopathologically to haemorrhage into the mural thrombus or into the aneurysm wall, with clefts of blood seeping from the lumen into the thrombus. The haemorrhage later penetrates and weakens the aneurysm wall, thus placing the aneurysm at risk for frank rupture.32
Acute mesenteric ischaemia
The aetiology of AMI is secondary to reduced mesenteric blood flow to the bowel owing to several conditions.
These conditions can be categorized into two major aetiological groups: occlusive mesenteric ischaemia and non-occlusive mesenteric ischaemia (NOMI). Occlusive mesenteric ischaemia refers to a vascular obstruction, either arterial or venous, underlying the ischaemic damage to the bowel wall.
NOMI occurs in condition of systemic hypoperfusion without the evidence of vascular obstruction (hypovolaemia, cardiac failure, intraoperative hypotension).33
MDCT signs of AMI can be categorized into vascular and bowel wall signs. Considering vascular signs of AMI, splanchnic vessel emboli appear on EAP MDCT as filling defects in the vascular lumen of mesenteric arteries. A mesenteric vein thrombosis appears slightly hyperdense in the unenhanced scan and is usually associated with an enlarged vein and stranding of the surrounding fat and appears as a filling defect of the venous lumen during the portal venous phase.34
Considering bowel wall signs of AMI, we should consider abnormalities of bowel wall thickness, bowel lumen and bowel wall enhancement. During AMI and mesenteric infarction, bowel wall appearances vary widely at MDCT scan. Variability depends on the pathogenesis of bowel ischaemia as well as on the acuteness, duration, site and extent of the ischaemic attack and the state of the collateral circulation and reperfusion damage. Bowel wall thickening is an aspecific imaging finding, but it is the most frequently observed MDCT finding in mesenteric ischaemia and is caused by mural oedema, haemorrhage or superinfection in the ischaemic bowel wall. The degree of thickening is usually <1.5 cm, typically 8–9 mm,33–35 and is often observed in mesenteric venous occlusion and mesenteric arterial occlusion after reperfusion. Conversely, in exclusively arterial occlusive mesenteric ischaemia or infarction, the bowel wall becomes thinner rather than thicker owing to lack of arterial flow, mural oedema or haemorrhage. Bowel wall thinning referred to as “paper-thin wall” is caused by “tissue volume loss” and vessels in the bowel wall and by loss of intestinal muscular tone with bowel lumen dilatation (Figure 7).34–40
Figure 7.
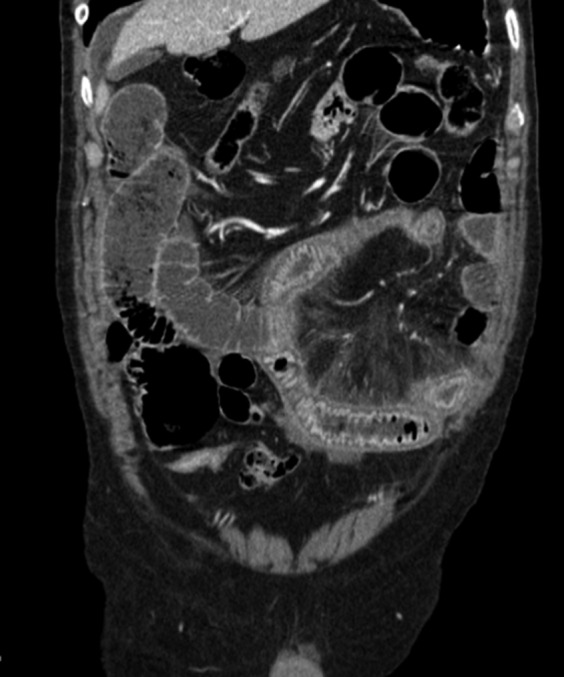
A coronal multiplanar reformation contrast-enhanced CT images acquired in a patient affected by strangulated small-bowel obstruction and acute mesenteric ischaemia. Ischaemic bowel loops show wall thickening and increased bowel wall enhancement with target appearance.
On contrast-enhanced MDCT, a highly specific, but not sensitive, finding for AMI is the absence of bowel wall contrast enhancement or diminished bowel wall contrast enhancement.34–40
The halo sign or target appearance also suggests mesenteric ischaemia, representing hyperaemia and hyperperfusion associated with surrounding mural oedema, and can be seen in arterial occlusion after reperfusion, non-occlusive and veno-occlusive bowel ischaemia and strangulation (Figure 7).34–40
Pneumatosis intestinalis (air in the bowel wall) indicates transmural infarction; this event implies a poor patient prognosis with high mortality (Figure 8).33–40
Figure 8.
Axial (a) and oblique multiplanar reformation (b) contrast-enhanced CT images acquired in a patient with acute superior mesenteric vein thrombosis.
In patients with AMI, MDCT is also able to depict other signs concerning mesentery, peritoneal cavity and solid organs, such as alteration of mesenteric fat streakiness, air into mesenteric venous vessels and portal venous system, free peritoneal fluid and solid organ ischaemia in cases of NOMI (Figures 9 and 10).
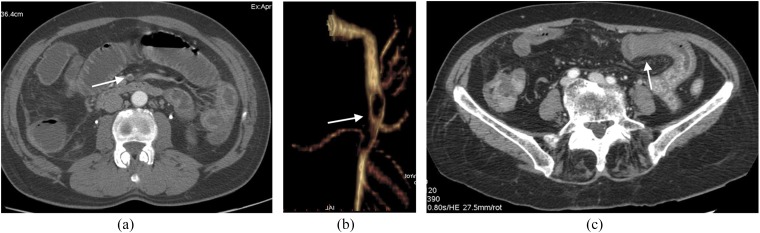
Figure 9.
A 55-year-old male patient with acute mesenteric ischaemia due to superior mesenteric artery (SMA) embolization. (a) Arterial embolus as a filling defect (arrow) of SMA on axial contrast-enhanced early arterial phase CT axial image. (b) The extension of SMA embolization is visible on three-dimensional volume rendering selective reconstruction of SMA (arrow). (c) Necrotic changes of bowel wall (arrow) with lack of contrast enhancement.
Figure 10.
An axial contrast-enhanced CT image acquired in a patient affected by small bowel infarction. Necrotic bowel loop shows dilated lumen, “paper-thin” bowel wall with lack of enhancement. Some vasa recta are gas-filled (arrow).
Other entities
Severe and generalized abdominal pain can also have numerous non-surgical causes, such as diabetic ketoacidosis, herpes zoster, sickle cell crisis, acute porphyria, abdominal organ embolization from endocarditis and adrenal crisis.1 In these conditions, the role of MDCT is limited by the lack of specific findings, although it can be useful in the differential diagnosis with surgery-related abdominal pain and in patient follow-up after diagnosis. In sickle cell crisis, MDCT may show splenomegaly, splenic infarction and free peritoneal fluid,41 whereas in acute porphyria and abdominal organ embolization from endocarditis, MDCT can show multiple infarctions in solid organs and peritoneal fluid. Only in adrenal crisis (Addison's crisis), MDCT shows rather specific findings, including bilateral adrenal gland enlargement with high density on pre-contrast scan owing to adrenal haemorrhage. These patients usually already have a diagnosis of Addison disease and present with hyponatraemia, abdominal pain and hypotensive shock.
CONCLUSION
In this descriptive review, we analysed MDCT protocols and the diagnostic role of MDCT in patients presenting to the emergency department with clinically suspected acute pancreatitis, gastrointestinal perforation, ruptured aneurysm and AMI. We especially offer information useful to radiologists wishing to use state of the art MDCT scanners in emergencies.
MDCT gives invaluable diagnostic information in the management of patients with severe non-traumatic abdominal pain. To ensure that patients undergo the most appropriate MDCT diagnostic protocol, the radiologist should be present in the MDCT suite with the radiographer right from the patient arrival. Attention to proper technique and protocol and knowledge of the clinical data and laboratory results is essential for optimizing the CT examination and maximizing diagnostic accuracy. Correctly interpreting MDCT findings enables a rapid diagnosis, thus saving precious time in patient management. Further research directions in emergency imaging include making CT scanning easier for uncooperative patients and reducing ionizing radiation exposure.
Contributor Information
Pasquale Paolantonio, Email: paolantoniopasquale@hotmail.com.
Marco Rengo, Email: marco.rengo@uniroma1.it.
Riccardo Ferrari, Email: ferraririccardo@gmail.com.
Andrea Laghi, Email: andrea.laghi@uniroma1.it.
REFERENCES
- 1.Stoker J, van Randen A, Laméris W, Boermeester MA. Imaging patients with acute abdominal pain. Radiology 2009; 253: 31–46. doi: 10.1148/radiol.2531090302 [DOI] [PubMed] [Google Scholar]
- 2.Bijur P, Latimer C, Gallagher J. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med 2003; 10: 390–2. [DOI] [PubMed] [Google Scholar]
- 3.American College of Emergency Physicians (2010). ACEP policy statements: triage scale standardization. Dallas, TX: American College of Emergency Physicians. Cited 1 June 2011. Available from: http://www.acep.org/content.aspx?id=29828&terms=triage%scale [Google Scholar]
- 4.Sala E, Watson CJ, Beadsmoore C, Groot-Wassink T, Fanshawe TR, Smith JC, et al. A randomized, controlled trial of routine early abdominal computed tomography in patients presenting with non-specific acute abdominal pain. Clin Radiol 2007; 62: 961–9. doi: 10.1016/j.crad.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 5.Urban BA, Fishman EK. Tailored helical CT evaluation of acute abdomen. Radiographics 2000; 20: 725–49. [DOI] [PubMed] [Google Scholar]
- 6.Abbas A, Brown IW, Peebles CR, Harden SP, Shambrook JS. The role of multidetector-row CT in the diagnosis, classification and management of acute aortic syndrome. Br J Radiol 2014; 87: 20140354. doi: 10.1259/bjr.20140354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsushima Y, Yamada S, Aoki J, Motojima T, Endo K. Effect of contrast-enhanced computed tomography on diagnosis and management of acute abdomen in adults. Clin Radiol 2002; 57: 507–13. doi: 10.1053/crad.2001.0925 [DOI] [PubMed] [Google Scholar]
- 8.Rengo M, Bellini D, de Cecco CN, Osimani M, Vecchietti F, Caruso D, et al. The optimal contrast media policy in CT of the liver. Part I: technical note. Acta Radiol 2011; 52: 467–72. [DOI] [PubMed] [Google Scholar]
- 9.Rengo M, Bellini D, de Cecco CN, Osimani M, Vecchietti F, Caruso D, et al. The optimal contrast media policy in CT of the liver. Part II: clinical protocols. Acta Radiol 2011; 52: 473–80. [DOI] [PubMed] [Google Scholar]
- 10.Huynh LN, Coughlin BF, Wolfe J, Blank F, Lee SY, Smithline HA. Patient encounter time intervals in the evaluation of emergency department patients requiring abdominopelvic CT: oral contrast versus no contrast. Emerg Radiol 2004; 10: 310–13. [DOI] [PubMed] [Google Scholar]
- 11.Silva AC, Lawder HJ, Hara A, Kujak J, Pavlicek W. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol 2010; 194: 191–9. doi: 10.2214/AJR.09.2953 [DOI] [PubMed] [Google Scholar]
- 12.Paulson EK, Jaffe TA, Thoma J, Harris JP, Nelson RC. MDCT of patient with acute abdominal pain; a new perspective using coronal reformations from submillimiter isotropic voxels. AJR Am J Roentgenol 2004; 183: 899–906. doi: 10.2214/ajr.183.4.1830899 [DOI] [PubMed] [Google Scholar]
- 13.Sarr MG. 2012 revision of the Atlanta classification of acute pancreatitis. Pol Arch Med Wewn 2013; 123: 118–24. [DOI] [PubMed] [Google Scholar]
- 14.Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for radiologist and its effect on treatment. Radiology 2012; 262: 751–64. doi: 10.1148/radiol.11110947 [DOI] [PubMed] [Google Scholar]
- 15.Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg 2002; 89: 298–302. doi: 10.1046/j.0007-1323.2001.02025.x [DOI] [PubMed] [Google Scholar]
- 16.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut 2004; 53: 1340–4. doi: 10.1136/gut.2004.039883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besselink MG, van Santvoort HC, Witteman BJ, Gooszen HG; Dutch Acute Pancreatitis Study Group. Management of severe acute pancreatitis: it's all about timing. Curr Opin Crit Care 2007; 13: 200–6. doi: 10.1097/MCC.0b013e328015b8af [DOI] [PubMed] [Google Scholar]
- 18.Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg 2000; 87: 1019–23. doi: 10.1046/j.1365-2168.2000.01464.x [DOI] [PubMed] [Google Scholar]
- 19.Balthazar EJ. Staging of acute pancreatitis. Radiol Clin North Am 2002; 40: 1199–209. doi: 10.1016/S0033-8389(02)00047-7 [DOI] [PubMed] [Google Scholar]
- 20.Mortele KJ, Ip IK, Wu BU, Conwell DL, Banks PA, Khorasani R. Acute pancreatitis: imaging utilization practices in an urban teaching hospital—analysis of trends with assessment of independent predictors in correlation with patient outcomes. Radiology 2011; 258: 174–81. doi: 10.1148/radiol.10100320 [DOI] [PubMed] [Google Scholar]
- 21.Sakorafas GH, Tsiotos GG, Sarr MG. Extrapancreatic necrotizing pancreatitis with viable pancreas: a previously under-appreciated entity. J Am Coll Surg 1999; 188: 643–8. doi: 10.1016/S1072-7515(99)00045-9 [DOI] [PubMed] [Google Scholar]
- 22.Tann M, Maglinte D, Howard TJ, Sherman S, Fogel E, Madura JA, et al. Disconnected pancreatic duct syndrome: imaging findings and therapeutic implications in 26 surgically corrected patients. J Comput Assist Tomogr 2003; 27: 577–82. doi: 10.1097/00004728-200307000-00023 [DOI] [PubMed] [Google Scholar]
- 23.Vege SS, Fletcher JG, Talukdar R, Sarr MG. Peripancreatic collections in acute pancreatitis: correlation between computerized tomography and operative findings. World J Gastroenterol 2010; 16: 4291–6. doi: 10.3748/wjg.v16.i34.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashley SW, Perez A, Pierce EA, Brooks DC, Moore FD, Jr, Whang EE, et al. Necrotizing pancreatitis: contemporary analysis of 99 consecutive cases. Ann Surg 2001; 234: 572–9; discussion 579–580. doi: 10.1097/00000658-200110000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SH, Shim SS, Jeong YY, Heo SH, Kim JW, Kang HK. Gastrointestinal tract perforation: MDCT findings according to perforation sites. Korean J Radiol 2009; 10: 63–70. doi: 10.3348/kjr.2009.10.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh JP, Steward MJ, Booth TC, Mukhtar H, Murray D. Evolution of imaging for abdominal perforation. Ann R Coll Surg Engl 2010; 92: 182–8. doi: 10.1308/003588410X12664192075251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grassi R, Romano S, Pinto A, Romano L. Gastro-duodenal perforations: conventional plain film, US and CT findings in 166 consecutive patients. Eur J Radiol 2004; 50: 30–6. doi: 10.1016/j.ejrad.2003.11.012 [DOI] [PubMed] [Google Scholar]
- 28.Ghahremani GG. Radiologic evaluation of suspected gastrointestinal perforations. Radiol Clin North Am 1993; 31: 1219–34. [PubMed] [Google Scholar]
- 29.Rakita D, Newatia A, Hines J, Siegel D, Friedman B. Spectrum of findings of rupture and impending rupture of abdominal aortic aneurysms. Radiographics 2007; 27: 497–507. doi: 10.1148/rg.272065026 [DOI] [PubMed] [Google Scholar]
- 30.Siegel CL, Cohan RH, Korobkin M, Alpern MB, Courneya DL, Leder RA. Abdominal aortic aneurysm morphology: CT features in patients with ruptured and non-ruptured aneurysm. AJR Am J Roentgenol 1994; 163: 1123–9. [DOI] [PubMed] [Google Scholar]
- 31.Halliday KE, Al-Kutoubi A. Draped aorta: CT sign of contained leak of aortic aneurysms. Radiology 1996; 199: 41–3. doi: 10.1148/radiology.199.1.8633170 [DOI] [PubMed] [Google Scholar]
- 32.Gonsalves CF. The hyperattenuating crescent sign. Radiology 1999; 211: 37–8. doi: 10.1148/radiology.211.1.r99ap1137 [DOI] [PubMed] [Google Scholar]
- 33.Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL. Harrison's principles of internal medicine. 16th edn. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 34.Menke J. Diagnostic accuracy of multi detector CT in acute mesenteric ischaemia: systematic review and meta-analysis. Radiology 2010; 256: 93–101. [DOI] [PubMed] [Google Scholar]
- 35.Reginelli A, Lacobellis F, Berritto D, Gagliardi G, Di Grezia G, Rossi M, et al. Mesenteric ischaemia: the importance of differential diagnosis for the surgeon. BMC Surg 2013; 13: S51. doi: 10.1186/1471-2482-13-S2-S51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischaemia: a clinical review. Arch Intern Med 2004; 164: 1054–62. doi: 10.1001/archinte.164.10.1054 [DOI] [PubMed] [Google Scholar]
- 37.Mazzei MA, Mazzei FG, Marrelli D, Imbriaco G, Guerrini S, Vindigni C, et al. Computed tomographic evaluation of mesentery: diagnostic value in acute mesenteric ischaemia. J Comput Assist Tomogr 2012; 36: 1–7. doi: 10.1097/RCT.0b013e31823b4465 [DOI] [PubMed] [Google Scholar]
- 38.Mazzei MA, Volterrani L. Nonocclusive mesenteric ischaemia: think about it. Radiol Med 2015; 120: 85–95. doi: 10.1007/s11547-014-0460-6 [DOI] [PubMed] [Google Scholar]
- 39.Romano S, Lassandro F, Scaglione M, Romano L, Rotondo A, Grassi R. Ischaemia and infarction of the small bowel and colon: spectrum of imaging findings. Abdom Imaging 2006; 31: 277–92. doi: 10.1007/s00261-005-0376-7 [DOI] [PubMed] [Google Scholar]
- 40.Horton KM, Fishman EK. Multidetector CT angiography in the diagnosis of mesenteric ischaemia. Radiol Clin North Am 2007; 45: 275–88. doi: 10.1016/j.rcl.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 41.Magid D, Fishman E, Charache S, Siegelman SS. Abdominal pain in sickle cell disease: the role of CT. Radiology 1987; 163: 325–8. doi: 10.1148/radiology.163.2.3562812 [DOI] [PubMed] [Google Scholar]