Abstract
Previous studies have identified an association between the gene glyoxalase 1 (Glo1) and anxiety-like behavior in mice and have shown that the substrate of GLO1, methylglyoxal, is a competitive partial agonist at GABAA receptors. Given the well-established role of GABAA receptors in the behavioral effect of ethanol, we investigated the role of Glo1 in regulating voluntary ethanol consumption in mice using the drinking in the dark (DID) paradigm. Transgenic mice overexpressing Glo1 on both FVB/NJ (FVB) or C57BL/6J (B6) backgrounds showed increased voluntary ethanol consumption compared to their wild-type littermates in DID. Furthermore, transgenic Glo1 knockdown mice on a B6 background showed decreased voluntary ethanol consumption in DID. These genetic manipulations of Glo1 had no effect on sucrose, saccharin or water consumption. Finally, we found that a small molecule GLO1 inhibitor (S-bromobenzylglutathione cyclopentyl diester (pBBG; 6.25, 12.5 mg/kg) reduced ethanol consumption compared to vehicle treated B6 mice without altering saccharin or water consumption. Sucrose consumption was only reduced by the higher (12.5 mg/kg) dose of pBBG. We did not observe differences in the loss of righting reflex or ethanol-induced foot slips on the balance beam in response to acute ethanol administration (LORR: 4g/kg, Balance Beam: 1.25g/kg) in B6 or FVB mice overexpressing Glo1, nor in B6 mice treated with pBBG. These data are the first to implicate Glo1 in ethanol-related behaviors and suggest that GLO1 inhibitors may have therapeutic potential for the treatment of alcohol use disorders.
Keywords: Alcohol use disorder, drinking in the dark, ethanol consumption, GLO1, methylglyoxal
Introduction
Alcohol use disorders (AUD) are characterized by ‘a problematic pattern of alcohol use leading to clinically significant impairment or distress’ (DSM V). There are a dearth of pharmacological treatments for AUDs and those that exist are only modestly effective and may even be ineffective in certain individuals (Dawson, Goldstein, & Grant, 2007; Maisel, Blodgett, Wilbourne, Humphreys, & Finney, 2012). Further, AUDs share high comorbidity with several psychiatric disorders including generalized anxiety disorder (GAD) (Boschloo et al., 2011; Grant et al., 2004; Smith & Randall, 2012) and these comorbid disorders are associated with worse treatment outcomes (Bruce et al., 2005; Driessen et al., 2001; Smith & Book, 2010). Thus, identifying novel treatments for AUD, especially ones that might also address psychiatric co-morbidities is of critical importance.
While it is impossible to fully recapitulate AUDs in model organisms, key aspects of AUD can be modeled and may be used to evaluate the potential effectiveness of novel therapeutic targets. Binge drinking is defined as drinking enough to obtain a blood alcohol concentration (BAC) of 0.08g/dL or above (National Institute on Alcohol Abuse and Alcoholism, 2004). Binge drinking is a risk factor for the development of AUDs (Viner & Taylor, 2007) and accounts for a large portion of harm that is associated with AUDs (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011). The drinking in the dark (DID) paradigm was developed to model binge drinking in rodents (Rhodes, Best, Belknap, Finn, & Crabbe, 2005). DID takes advantage of the tendency of mice to voluntarily consume large amounts of ethanol when it is presented for a limited period of time during the dark phase of the light cycle. Under these conditions several inbred mouse strains will freely consume enough ethanol to achieve BAC greater than 0.08 g/dL, and will thus demonstrate overt signs of behavioral intoxication (Rhodes et al., 2005)). Importantly, current treatments for AUDs such as naltrexone and acamprosate, reduce ethanol consumption in this model without altering water or sucrose consumption, illustrating the strong predictive validity of DID (Gupta et al., 2008; Kamdar et al., 2007).
Several previous studies have identified an association between expression of the gene, glyoxalase 1 (Glo1) and anxiety-like behavior in mice (Distler et al., 2012b; Hovatta et al., 2005; Williams et al., 2009). Glo1's transcript, GLO1, is a ubiquitous cytosolic enzyme that mediates the detoxification of methylglyoxal (MG), which is a non-enzymatic by-product of glycolysis (Thornalley, 1996). We previously showed that transgenic overexpression of Glo1 increased anxiety-like behavior and that direct administration of MG had the opposite effect and decreased anxiety-like behavior in mice. Further, a pharmacological inhibitor of GLO1, S-bromobenzylglutathione cyclopentyl diester (pBBG), increased MG concentrations in brain and reduced anxiety-like behavior. We subsequently determined that MG is a competitive partial agonist at GABAA receptors, likely explaining the effect of GLO1 on anxiety-like behavior (Distler et al., 2012b).
Many of the behavioral effects associated with ethanol use are mediated through the actions of ethanol at GABAA receptors and modulation of GABAA receptor activation alters both the effects of ethanol and voluntary ethanol consumption (Grobin, Matthews, Devaud, & Morrow, 1998; Kumar et al., 2009; Liang & Olsen, 2014; Moore et al., 2007). As GLO1 regulates MG concentrations and MG is a competitive partial agonist at GABA-A receptors, we speculated that increased Glo1 expression and corresponding decreases in MG would increase ethanol consumption, while reduced Glo1 expression and corresponding increases in MG would decrease ethanol consumption.
In the current study we examined the effect of overexpression and knockdown of Glo1 on DID. In addition, we evaluated the effect of a small molecule GLO1 inhibitor (pBBG) on DID.
Materials and Methods
Mice
Transgenic (TG) mice overexpressing Glo1 on either a FVB/NJ (FVB) or C57BL/6J (B6) background were generated by insertion of a BAC transgene, as previously described (Distler et al., 2012b). FVB TGs used in this paper had approximately 35 copies of the transgene while B6 TGs had approximately 8 copies; previously published estimates of brain mRNA suggest that these transgenes induced 17-fold (FVB) and 5-fold (B6) increases in Glo1 mRNA relative to wild-type (WT) littermates (Distler et al., 2012b). Glo1 knock-down (KD) mice were generated on a C57BL/6 background in the lab of Dr. Michael Brownlee (Albert Einstein College of Medicine, Bronx, NY) and show an approximately 45-65% reduction in GLO1 enzymatic activity as previously described (El-Osta et al., 2008). KD mice have been maintained in our lab by continuing to backcross to B6 for more than 5 generations. In all studies, TG, KD and their WT littermates were tested at ages 10-16 weeks and both males and females were used. For studies using the GLO1 inhibitor (pBBG), male B6 mice were purchased from The Jackson Laboratory (JAX) and tested when they were 8-12 weeks old. All mice were group housed on a reverse light cycle (12/12 hour light/dark, lights on at 22:30) for at least 2 weeks prior to testing. All mice were singly housed beginning exactly 5 days before the start of DID testing. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago and performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Drinking in the dark (DID)
Our studies were performed using the two day DID model of binge drinking as described in Rhodes et al. (2007). Briefly, three hours into the dark cycle, mice were given access to 20% ethanol (vol/vol) for 2 hours on day 1 and for 4 hours on day 2. Using the same 2 day DID paradigm, we replaced ethanol with either 10% sucrose (wt/vol), 0.2% Saccharin (wt/vol) or water as controls for general consummatory behavior. Mice had at least 1 day of rest between studies. For example, in one week, sucrose testing took place on a Monday and Tuesday, the mice were undisturbed on Wednesday, then water testing took place Thursday and Friday. For studies using the GLO1 inhibitor pBBG, mice followed a similar testing schedule, but received injections of the inhibitor 2 hours before testing only on day 2 for each DID study. The 2 hour time-point was used to allow time for accumulation of MG in the brain through a reduction in MG clearance. At this time-point, we previously reported increases of MG in the brain and differences in other behaviors (Distler et al., 2012a, 2013). TG and KD mice underwent testing in the following order: sucrose, water, saccharin and ethanol. Mice receiving the GLO1 inhibitor pBBG underwent testing in a different order: sucrose, saccharin, water and ethanol. There was no methodological reason for this difference in order. Blood samples were taken for BEC analysis immediately following the 4 hour exposure to ethanol on day 2 of DID.
Loss of Righting Reflex (LORR)
Novel sets of ethanol naïve mice (FVB TG, B6 TG, and their WT littermates or WT B6 from JAX for pBBG studies) were used for the LORR study. A 4 g/kg dose of ethanol was administered IP using a 20% ethanol solution that was prepared by diluting a 95% ethanol stock solution with 0.9% saline. LORR was defined as the time at which a mouse could no longer right itself twice within 30 seconds. Mice taking longer than 3.5 minutes to lose their righting reflex were deemed to have received a misplaced injection and were excluded from analysis. Of 112 mice tested, 8 were excluded for either failing to lose their righting reflex (5 of 8) or for taking longer than 3.5 minutes to lose their righting reflex (3 of 8). Of the 8 that were excluded, 4 were FVB (1 TG, 3 WT) and 4 were B6 that were purchased from JAX for the pharmacological studies. No mice were excluded from the B6 Glo1 overexpressing line. Duration of LORR was defined as the time at which a mouse regained the ability to right itself 3 times in 60 seconds minus the time it achieved LORR. For mice that received the GLO1 inhibitor pBBG, pBBG injections occurred 2 hours before mice received ethanol injections.
Balance Beam
Novel sets of ethanol naïve mice (FVB TG, B6 TG and their WT littermates or WT B6 from JAX for pBBG studies) were used for the balance beam study. The day before testing, mice were trained to traverse a balance beam (97cm length, 16 mm wide, suspended 56 cm above the floor) by placing mice at one end of the balance beam and encouraging them, if necessary, to walk to the other side of the balance beam by a light nudge at the base of the tail using the eraser end of a pencil. Previous studies (Linsenbardt, Moore, Griffin, Gigante, & Boehm nd, 2011; J. S. Rhodes et al., 2007) have shown that this training is sufficient to have mice traverse the beam during testing without encouragement. FVB and B6 TG mice were tested on the balance beam over 2 days. On day 1 they received no injections and baseline foot slips were assessed. On day 2, all mice received 1.25g/kg ethanol 10 minutes before being placed on the balance beam. In a separate study, we used a 3×2 experimental design to assess interactions between drug (VEH, MG or pBBG) and ethanol (saline or ethanol) on ataxia (foot slips). WT B6 JAX mice received injections of either VEH or 6.25mg/kg pBBG 2 hours before testing and then received another injection of either saline, 50mg/kg MG, 1.25g/kg ethanol or 50mg/kg MG + 1.25g/kg ethanol 10 minutes before testing. Mice were then placed on one end of the balance beam and allowed to traverse to the other end while hind foot slips were recorded by an observer blind to treatment conditions.
Ethanol Metabolism
Novel sets of ethanol naïve mice (FVB TG, B6 TG, B6 KD and their WT littermates) were used to determine ethanol metabolism. A 2 g/kg dose of ethanol was administered IP using a 20% ethanol solution that was prepared by diluting a 95% stock solution with 0.9% saline. Blood (20 μl) was taken from the tail at 15, 30, 60 and 120 minutes post injection and blood ethanol concentrations (BECs) were determined as described below.
BEC
Blood samples were processed and analyzed as in Barkley-Levenson and Crabbe (2012). Briefly, 20 μl blood samples taken immediately after the 4 hour ethanol exposure on day 2 of DID, during the ethanol metabolism study or immediately upon regain of LORR were immediately placed into a microcentrifuge tube containing 50 μl zinc sulfate and placed on ice. Following collection of all blood samples, 50 μl of 0.3N barium hydroxide and 300 ul distilled water were added and samples were centrifuged at 12,000 RPM for 5 minutes. The supernatant was then removed, placed in a sealed, air-tight container, and frozen until analyzed by gas chromatography. Samples were compared to a standard ethanol concentration curve.
Drugs
S-bromobenzylglutathione cyclopentyl diester (pBBG) was synthesized as followed: In a dry glass vial, L-Glutathione (307 mg, 1 mmol) was dissolved in water (2 mL) at room temperature for 5 minutes. 2 mL of 6N NaOH was added slowly followed by the dropwise addition of solution of 4-bromobenzyl bromide (1.1 mmol) in methanol (2 mL). The reaction mixture was stirred at room temperature for 3 hours. The product was precipitated by adding 6N HCL (2 mL). The precipitate was washed with water and dried. The crude product was dissolved in cyclopentanol (10 ml). To the solution, few drops of concentrated sulfuric acid were added and stirred for 48 hours. The completion of reaction is monitored by LC/MS until the disappearance of starting material. The product was precipitated by adding hexanes. The precipitation step was repeated thrice giving 122 mg (20% yield). 1H NMR (DMSO-d6) δ 8.52 (t, 1H, J = 5.9 Hz, NH), 8.27 (d, 1H, J = 5.9 Hz, NH), 7.49 (d, 2H, J = 8.6 Hz), 7.29 (d, 2H, J = 8.6 Hz), 7.09 (s (broad), 2H, NH2) 5.18 (m, 1H), 5.05 (m, 1H), 4.56 (m, 1H), 3.95, (m, 1H), 3.79 (d, J = 5.6 Hz, 2H), 3.73 (s, 2H), 2.75 (m, 1H), 2.53 (m, 1H), 2.42 (m, 2H), 2.09 (m, 2H), 1.6-1.9 (m, 16H); ESI MS (+ve) 613.16 m/z; found 614.36 (M+H). pBBG was dissolved in vehicle (8% DMSO/18% Tween80/74% PBS) and administered IP. Methylglyoxal (Sigma-Aldrich, M0252) was dissolved in 0.9% saline or 20% ethanol in 0.9% saline and administered IP.
Statistical Analysis
Data were analyzed using t-Test or ANOVA. Holm-Sidak multiple comparisons procedures were used to determine which doses yielded significantly different responses. p-values less than 0.05 were considered significant.
Results
TG mice on both FVB and B6 backgrounds showed significantly increased ethanol consumption over the 4 hours of drinking on day 2 compared to their WT littermates in DID (Fig. 1a-b). Mice on an FVB background showed significant main effects of genotype and sex (Fig 1a; F(1,30) = 4.643, F(1,30) = 6.913; p<0.05 for both) with TGs drinking more ethanol than WTs and females drinking more than males. There was no significant interaction. Mice on a B6 background also showed a significant main effect of genotype (Fig 1b; F(1,38) = 4.251; p<0.05), but showed no significant effect of sex or interaction. Conversely, KD mice showed significantly reduced ethanol consumption (Fig. 1c). There was a significant main effect of both genotype and sex (Fig 1c; F(1,45) = 4.633, p<0.05; F(1,45) = 8.951, p<0.01), with KDs drinking less ethanol than WTs and females drinking more than males. There was no significant interaction. BECs were positively correlated with ethanol consumption in all strains (FVB: R2 = 0.3, p<0.01; B6: R2 = 0.2, p<0.05; KD: R2 = 0.2, p<0.01). Importantly, genotype had no effect on water, sucrose or saccharin consumption in B6 or FVB TG mice or in B6 KD mice, though there was a trending effect of reduced saccharin consumption in KD mice (Fig. S1a-I; p>0.05 for all consummatory behaviors; F(1,16)=4.097, p=0.064 for saccharin in KD). There was no difference in ethanol metabolism in B6 or FVB TG mice or in B6 KD mice (data not shown).
Figure 1. Glo1 expression regulates EtOH consumption.
Mice overexpressing Glo1 (TG) on a (A) FVB background (n=20 WT, 16 TG) and (B) B6 background showed increased EtOH consumption over a 4 hr period in DID (n=21 per genotype). (C) Glo1 knockdown (KD; B6 background) mice show reduced EtOH consumption over a 4 hr period in DID (n=21 WT, 26 KD). *p<0.05 by Two-Way ANOVA.
As Glo1 knockdown mice showed reduced ethanol consumption, we next investigated the therapeutic potential of GLO1 inhibition by using a pharmacological inhibitor of GLO1, pBBG (Distler et al., 2012b; Thornalley et al., 1996). Male B6 WT mice received an IP injection of pBBG (0, 6.25, or 12.5 mg/kg) 2 hours before testing on day 2 of the DID paradigm. There was a significant overall effect of treatment on ethanol consumption (Fig. 2a; F(2,43) = 4.712; p<0.05). Post hoc tests revealed that both doses significantly reduced ethanol consumption compared to vehicle treatment (p<0.05). BECs were positively correlated with ethanol consumption (R2 = 0.3, p<0.001). There was no effect of pBBG on water consumption or saccharin consumption, but sucrose consumption was reduced following the 12.5 mg/kg dose of pBBG (Fig. 2b-d; F(2, 41) = 8.354; p<0.001; post hoc for 0 vs 12.5 mg/kg p<0.002). However, the 6.25 mg/kg dose of pBBG did not change sucrose consumption.
Figure 2. The GLO1 inhibitor pBBG reduces EtOH consumption.
Acute IP injection (2hrs before testing) with the indicated doses of pBBG reduces (A) EtOH consumption at multiple doses, but has no effect on (B) water or (C) 0.2% saccharin consumption. (D) Sucrose consumption was reduced only at the 12.5 mg/kg dose. n=14-15 per group for each test *p<0.05, ** p<0.01 by Holm-Sidak (comparisons to VEH).
In a separate set of studies we found that there was a significant effect of treatment on ethanol consumption when using higher doses of pBBG (25mg/kg and 50mg/kg; Fig. S2a; F(2,42) = 9.113; p<0.001). However, these doses also changed consumption of water and sucrose consumption, which confounds the interpretation of DID. Specifically, there was a significant main effect of treatment on water consumption [F(2,41) = 3.522; p<0.05]; post hoc tests were suggestive for both doses (Fig. S2b; 0 vs 25 mg/kg: p=0.079; 0 vs 50 mg/kg: p=0.053). There was no effect of treatment on saccharin consumption (Fig. S2c), but there was a significant effect of treatment on sucrose consumption (Fig S2d; F(2,42) = 6.201; p<0.01); post hoc tests were suggestive for the 25 mg/kg dose (p=0.064) and were significant for the 50 mg/kg dose (p<0.05).
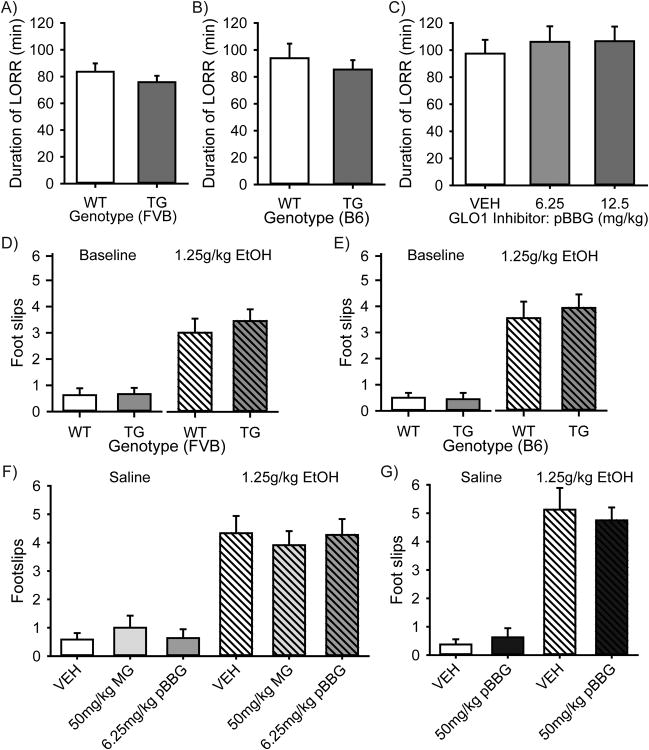
Finally, we performed the LORR and balance beam tests to evaluate whether manipulations of GLO1 altered sensitivity to the sedative or ataxic effects of ethanol. In LORR, we observed no significant differences on duration of LORR between TG Glo1 overexpressing and WT mice on either a FVB or B6 background (Fig. 3a-b; p>0.05 by Two-Way ANOVA). Similarly, we did not observe any significant effects of treatment of either 6.25 or 12.5mg/kg pBBG on duration of LORRin male B6 WT mice (Fig. 3c; p>0.05 by One-Way ANOVA). No differences in BECs were seen upon the regain of righting reflex between genotypes within either strain (FVB or B6) nor in pBBG treatment groups in B6 WT mice (data not shown). On the balance beam, mice overexpressing Glo1 (TG) showed no differences in foot slips at baseline or following ethanol injections on either an FVB background (Fig 3d, p>0.05 by Two-Way ANOVA), or B6 background (Fig 3e, p>0.05 by Two-Way ANOVA). In WT B6 mice, ethanol treatment significantly increased foot slips (Fig.3f; F(1,68)=84.479, p<0.001), but there was no effect of drug treatment (VEH, 50mg/kg MG or 6.25mg/kg pBBG) nor was there an interaction (p>0.05 for drug treatment or interaction by Two-way ANOVA). In a separate study in WT B6 mice using a higher dose of pBBG (50mg/kg), ethanol treatment significantly increased foot slips (Fig 3g; F(1,31)=84.621, p<0.001), but again, there was no effect of drug treatment nor was there an interaction (p>0.05 by Two-way ANOVA).
Figure 3. EtOH induced LORR and balance beam foot slips are not altered in Glo1 TG overexpressing mice or in mice treated with MG or pBBG.
Mice overexpressing Glo1 (TG) on an (A) FVB background (n=9 WT, 13 TG), or (B) B6 background (n=15 WT, 11 TG) show no differences in duration of LORR following a 4g/kg EtOH injection. (C) WT male B6 mice treated with 0, 6.25 or 12.5 mg/kg pBBG 2 hours before EtOH injections showed no differences in duration of LORR (n=19-20 per group). On the balance beam, mice overexpressing Glo1 (TG) showed no differences in foot slips at baseline or following 1.25g/kg EtOH injections on either an (D) FVB background (n=8 WT, 9 TG), or (E) B6 background (n=8 WT, 9 TG). (F) In WT B6 mice (n=11-12 per group) EtOH treatment significantly increased foot slips (p<0.001 Two-way ANOVA), but there was no interaction between drug (VEH, 50mg/kg MG or 6.25mg/kg pBBG) and EtOH treatment (G) Using a higher dose of pBBG (50mg/kg) in WT B6 mice (n=8 per group), EtOH treatment significantly increased foot slips (p<0.001 by Two-way ANOVA), but again, there was no interaction between drug and EtOH treatment.
Discussion
Our data demonstrate a novel role for GLO1 in the regulation of ethanol consumption. We observed increased ethanol consumption in FVB and B6 TG mice overexpressing Glo1. Conversely, we observed decreased ethanol consumption following both genetic knockdown of Glo1 (KD) and pharmacological inhibition of GLO1 by pBBG. To the best of our knowledge, these are the first studies to demonstrate that manipulations of Glo1 expression and enzymatic inhibition can alter voluntary ethanol consumption. These data suggest that pharmacological inhibition of GLO1 could be used to reduce voluntary ethanol consumption.
Importantly, neither overexpressing nor knocking down Glo1 affected general consummatory behavior as there was no effect on 10% sucrose or water consumption. Additionally, genotype did not alter ethanol metabolism. While all doses of the GLO1 inhibitor (pBBG) reduced ethanol consumption, the higher doses altered consumption of sucrose (12.5, 25 and 50 mg/kg) and water (25, 50 mg/kg) consumption. No doses of pBBG altered saccharin consumption nor did genotype have an effect on saccharin consumption in TG mice. While there was a non-significant trending effect of genotype on 0.2% saccharin consumption in KD mice, the consistent effects of Glo1 manipulations on ethanol consumption and lack of general effect of Glo1 manipulation on other consummatory behaviors suggests that Glo1 manipulations are not leading to decreased ethanol drinking through changes in their tastant sensitivity. Additionally, the DID studies may be limited by effects from either repeated testing or order of testing. However, this again seems unlikely given the complimentary and inverse effects of Glo1 overexpression versus Glo1 knockdown or GLO1 inhibition.
Correlations between ethanol drinking and BEC were somewhat modest, though they were not dissimilar to those seen by others (Wilcox et al., 2013) and may be a reflection of the extended access (4 hrs) wherein mice will show different patterns of drinking. For example, Wilcox et al (2013) showed that in DID mice may “front-load” or drink ethanol at the highest rate during the first 15 minutes of ethanol access which could lead to high overall drinking, but lower than expected BECs at the end of the session.
In studies using Glo1 overexpressing mice or Glo1 knockdown, both males and females were used. We saw no interactions between sex and genotype in any of our measures. A limitation of the GLO1 inhibitor studies is that we did not use females. While the lack of female subjects in the inhibitor study makes it unclear whether the GLO1 inhibitor would reduce ethanol consumption in females, the effects seen in the transgenic animals suggest ethanol consumption in females would respond to GLO1 inhibition.
We have previously shown that MG, which is metabolized by GLO1, is a competitive partial agonist at GABAA receptors (Distler & Palmer, 2012; Distler et al., 2012b; McMurray et al., 2014). We suspect that the changes in MG concentrations, which are caused by manipulations of Glo1 expression or inhibition (Distler et al., 2012b), modulate ethanol consumption via the action of MG at GABAA receptors. There is a well-established role of the GABAA receptor system in regulating ethanol consumption (Kumar et al., 2009). Indeed, GABAergic drugs such as muscimol and THIP reduce ethanol consumption as measured using DID, though they also reduce other consummatory behavior such as sucrose and water consumption (Moore et al., 2007). Additionally, a recent mouse study found that GABAA receptor-mediated signaling was depressed in the striatum following repeated ethanol consumption through 6 weeks of DID (Wilcox et al., 2013). Our data are consistent with those supporting a role for GABAA receptors in the regulation of ethanol consumption in DID and show that these effects can be obtained via manipulation of Glo1.
It is possible that MG and GLO1 are involved in the normal regulation of alcohol consumption through the activity of MG at GABAA receptors. MG is an endogenously produced byproduct of glycolysis (Thornalley, 1996). However, MG is also found in almost all foods and in many alcoholic beverages (Angeloni, Zambonin, & Hrelia, 2014; Nemet, Varga-Defterdarović, & Turk, 2006; Ojeda, Wrobel, Escobosa, Garay-Sevilla, & Wrobel, 2014). Whether concentrations of MG reach pharmacologically meaningful levels is unknown, but it raises the possibility that direct ingestion of MG may be an important component of the pharmacological properties of fermented beverages. MG may provide negative feedback on alcohol consumption whereby alcohol increases MG levels both through endogenous production and exogenous ingestion. High levels of MG may occupy GABAA receptors and lead to a reduction in ethanol consumption. This reduction may be the result of antagonistic-like properties of MG by reducing the maximal amplitude of GABAergic currents because of its actions as a partial agonist. This may be similar to decreased ethanol consumption seen after systemic administration GABAA antagonists (Chester & Cunningham, 2002; Koob, Sanna, & Bloom, 1998; Koob, 2006). Alternatively, increased activation from baseline could increase sensitivity to the hypnotic or ataxic effects of alcohol use and lead to early termination of drinking similar to the reduction in consumption others have seen using GABAA receptor agonists such as muscimol (Moore et al., 2007). However, this is unlikely as we see no differences in LORR or footslips in either TG mice or mice treated with the GLO1 inhibitor and suggests the effect of GLO1 regulation on ethanol consumption is not mediate through alterations in the hypnotic or ataxic effects of ethanol. The ability of GLO1 inhibitors to reduce ethanol consumption in DID suggest GLO1 inhibitors may be a viable for the treatment of alcohol use disorders. We previously showed that GLO1 inhibitors reduce anxiety-like behavior in mice and have suggested that GLO1 inhibitors could be used for the treatment of anxiety disorders (Distler & Palmer, 2012; Distler et al., 2012b; McMurray et al., 2014), which are highly comorbid with AUDs (Smith & Randall, 2012). Current pharmacological treatments for anxiety disorders include selective serotonin reuptake inhibitors (e.g. fluoxetine), serotonin-norepinephrine reuptake inhibitors or benzodiazepines (e.g. diazepam) that are positive allosteric modulators that do not directly activate GABAA receptor (e.g. diazepam, a benzodiazepine) (Smith & Randall, 2012). Those most commonly used for the treatment of AUDs act either as a mu-opioid antagonist or an NMDA receptor modulator (e.g. naltrexone, acamprosate respectively) (Yahn, Watterson, and Olive 2013). Based on our pre-clinical models, GLO1 inhibition is suspected to reduce anxiety-like behavior and ethanol consumption by a mechanism that may be distinct from those currently in use. We have not yet explored the abuse potential for GLO1 inhibitors; were GLO1 inhibition to show low or no abuse potential this would be an additional advantage relative to many currently used GABAA acting anxiolytics. Prior studies have not identified sedative effects of pBBG at the doses used within these studies (Distler et al., 2012b). Additionally, the LORR studies suggest that GLO1 inhibition may not potentiate the sedative effects of ethanol, if correct this would be an additional advantage to the use of GLO1 inhibition to treat AUDs.
The studies presented here suggest that manipulation of Glo1 can influence ethanol consumption, thus offering a novel target for the treatment of AUDs. Our previous studies have established a therapeutic potential for GLO1 inhibition in the treatment of anxiety disorders, which in conjunction with the data presented here, suggest GLO1 inhibition may be of particular interest for treatment of comorbid AUD and anxiety disorder.
Supplementary Material
Acknowledgments
The authors would like to thank John Crabbe, Stephanie Spence, Pamela Metten, Igor Ponomarev, Charissa Newkirk and Stephanie Dulawa for technical advice and assistance related to DID procedures and BEC analysis. This work was supported by MH079103 (AAP), AA13519 (JC), AA020245 (JC) and DA031090 (LAA).
Footnotes
Author contributions: KMJM and AAP were responsible for study concept and design. KMJM was responsible for data collection. KMJM drafted the manuscript. LAA, JAC and PS synthesized the pBBG used in this study. All authors provided critical revision of the manuscript and approved the final version for publication.
References
- Angeloni C, Zambonin L, Hrelia S. Role of Methylglyoxal in Alzheimer's Disease. Biomed Res Int. 2014:1–12. doi: 10.1155/2014/238485. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Crabbe JC. Ethanol Drinking Microstructure of a High Drinking in the Dark Selected Mouse Line. Alcohol Clin Exp Res. 2012;36:1330–1339. doi: 10.1111/j.1530-0277.2012.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, Smit JH, Van Den Brink W, Veltman DJ, Beekman ATF, Penninx BWJH. Comorbidity and risk indicators for alcohol use disorders among persons with anxiety and/or depressive disorders: Findings from the netherlands study of depression and anxiety (NESDA) J Affect Disord. 2011;131:233–242. doi: 10.1016/j.jad.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers Ka, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am J Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester Ja, Cunningham CL. GABAA receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/S0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Dawson Da, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: A 3-year follow-up. Alcohol Clin Exp Res. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Distler MG, Gorfinkle N, Papale La, Wuenschell GE, Termini J, Escayg A, Winawer MR, Palmer Aa. Glyoxalase 1 and its substrate methylglyoxal are novel regulators of seizure susceptibility. Epilepsia. 2013;54:649–57. doi: 10.1111/epi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler MG, Palmer Aa. Role of Glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet. 2012;3:250. doi: 10.3389/fgene.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest. 2012;122:2306–15. doi: 10.1172/JCI61319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill a, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. doi: http://dx.doi.org.proxy1.lib.umanitoba.ca/10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–2417. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grobin aC, Matthews DB, Devaud LL, Morrow aL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis Aa, Miller Sa, Martinez M, Cohn Ka, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–8. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hovatta I, Tennant RS, Helton R, Marr Ra, Singer O, Redwine JM, Ellison Ja, Schadt EE, Verma IM, Lockhart DJ, Barlow C. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller Sa, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/S0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for GABA in alcohol dependence. Adv Pharmacol. 2006;54:205–229. doi: 10.1016/S1054-3589(06)54009-8. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow aL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Olsen RW. Alcohol use disorders and current pharmacological therapies: the role of GABAA receptors. Acta Pharmacol Sin. 2014;35:981–93. doi: 10.1038/aps.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, Boehm SL. Tolerance to Ethanol's Ataxic Effects and Alterations in Ethanol-Induced Locomotion Following Repeated Binge-Like Ethanol Intake Using the DID Model. Alcohol Clin Exp Res. 2011;35:1246–1255. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray KMJ, Distler MG, Sidhu PS, Cook JM, Arnold La, Palmer Aa, Plant LD. Glo1 inhibitors for neuropsychiatric and anti-epileptic drug development. Biochem Soc Trans. 2014;42:461–7. doi: 10.1042/BST20140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsl. 2004;3:3. [Google Scholar]
- Nemet I, Varga-Defterdarović L, Turk Z. Methylglyoxal in food and living organisms. Mol Nutr Food Res. 2006;50:1105–1117. doi: 10.1002/mnfr.200600065. [DOI] [PubMed] [Google Scholar]
- Ojeda AG, Wrobel K, Escobosa ARC, Garay-Sevilla ME, Wrobel K. High-performance liquid chromatography determination of glyoxal, methylglyoxal, and diacetyl in urine using 4-methoxy-o-phenylenediamine as derivatizing reagent. Anal Biochem. 2014;449:52–58. doi: 10.1016/j.ab.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn Da, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn Da, Garland T, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Smith JP, Book SW. Comorbidity of generalized anxiety disorder and alcohol use disorders among individuals seeking outpatient substance abuse treatment. Addict Behav. 2010;35:42–45. doi: 10.1016/j.addbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Randall CL. Anxiety and Alcohol Use Disorders. Alcohol Res. 2012;34:414–431. [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, Ladan MJ, Double J. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol. 1996;51:1365–72. doi: 10.1016/0006-2952(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–73. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Viner RM, Taylor B. Adult outcomes of binge drinking in adolescence: findings from a UK national birth cohort. J Epidemiol Community Health. 2007;61:902–907. doi: 10.1136/jech.2005.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Carlson VCC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez Va. Repeated Binge-Like Ethanol Drinking Alters Ethanol Drinking Patterns and Depresses Striatal GABAergic Transmission. Neuropsychopharmacology. 2013;39:579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Wiltshire T, Su AI, et al. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One. 2009;4:e4649. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahn SL, Watterson LR, Olive MF. Safety and efficacy of acamprosate for the treatment of alcohol dependence. Subst Abuse. 2013;6:1–12. doi: 10.4137/SART.S9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.