Abstract
Oxygen levels and mechanical properties provide vital cues to regulate myriad cellular functions and stem cell fate decisions. Here, we present a hybrid hydrogel system in which we can control independently oxygen levels and mechanical properties. We designed, synthesized and analyzed a hybrid hydrogel system comprised of two polymer backbones, gelatin and dextran. Both polymers were crosslinked via a laccase-mediated, oxygen consuming reaction. By specifically controlling the concentration of phenolic molecules available to react in our hydrogel, we could precisely control the time in which the hydrogel remained hypoxic (TH). We were able to achieve a range of TH from the order of minutes to greater than 10 hours. Additionally, by incorporating a secondary crosslinker, transglutaminase, mechanical properties could be adjusted in a user-defined fashion, with dynamic elastic modulus (G′) values ranging from <20 Pa to >1 kPa. Importantly, oxygen levels and substrate mechanical properties could be individually tuned and decoupled in our hybrid hydrogels, while retaining the potential to study possible synergistic effects between the two parameters. By precisely controlling oxygen tension and mechanical properties, we expect that research utilizing the new hybrid hydrogels will enhance our understanding of the complex 3D cellular processes mediated by each parameter individually and may also hold clinical interest as acellular therapies.
Introduction
The ever increasing complexity and precision control of hydrogel biomimetic properties have enabled great strides to be taken in a broad range of interdisciplinary fields, perhaps most importantly at the intersection of materials engineering and biomedical sciences. Applications for hydrogel technologies have expanded to include controlled cell [1–5] and drug/growth factor delivery [6–14], engineered tissue [15–17], acellular therapeutics [18], and more recently, as scaffolds to study the complex interplay between cells and their microenvironment. Many important instructive interactions between cells and their microenvironment have been studied and defined utilizing hydrogel systems, which have sought to isolate specific variables. Important parameters of the cellular microenvironment that have been incorporated into hydrogel design and subsequently shown to affect cell function, phenotype, and fate include the effects of mechanical properties [19–21], degradation [22–24], protein tethering [25], cell adhesion [26, 27], geometry [28–30], topography [31, 32], oxygen tension [33, 34], and the presence of signaling molecules including growth factors and cytokines [35]. Gaining a fundamental understanding of the factors which control the most downstream effects and elicit the most robust responses will likely lead to important steps towards the development and translation of a wide range of novel therapies.
Substrate stiffness and oxygen tension have emerged as two vital parameters that have influenced tissue engineering and regenerative medicine, as well as for numerous basic science studies. Indeed, these two factors elicit numerous downstream effects and have proven to be two of the most potent regulators of cellular function. Of particular interest to our group, oxygen tension and substrate stiffness play vital roles in vascular morphogenesis (including angiogenesis and vasculogenesis)[21, 23, 31, 33] and cancer progression and metastasis, specifically tumor angiogenesis [36–39].
Extremely low levels of oxygen have proven detrimental to the success of tissue engineered constructs, owing to the diffusion limit of oxygen and nutrients and ultimately the necrosis imparted by the lack of these two key regulators of cell survival. However, low levels of oxygen, defined as hypoxia (<5% O2) have been shown to be a potent promoter of the angiogenic program both for the body’s natural healing of injured tissue and for tumor angiogenesis [40, 41]. Hypoxia inducible factors (HIFs) are transcription factors that regulate numerous downstream effects including the production of important pro-angiogenic signaling molecules that aid in the development of neovasculature [40]. In addition to its effect on more mature endothelial and endothelial progenitor cells, hypoxia also plays an important role in determining stem cell fate. In the developing embryo, dissolved oxygen levels range form 2–9% [42]. As the vasculature develops under hypoxic conditions, exact mimicry of the embryonic differentiation conditions necessitates a hypoxic microenvironment. Indeed, priming pluripotent stem cells in a hypoxic microenvironment enhances their differentiation along a vascular lineage and improves their ability to form vascular networks [33].
The importance of substrate stiffness on cell function and stem cell fate decisions has been made abundantly clear over the last ten years. Studies pertaining to angiogenesis and vasculogenesis are largely limited to mature and progenitor cells indicating that that soft substrates (< 1 kPa) are generally more conducive to the formation of functional vascular networks. Stiffer substrates often limit cell migration and the eventual coalescing of endothelial cells to form perfusable, lumenal structures [21]. Further, the elasticity of the ECM has proven a vital signaling cue for metastatic cancer [37].
Others and us have utilized hydrogels as an important tool to study the interactions between cells and their microenvironment. Recently, we have developed both gelatin (Gtn) and dextran (Dex) based oxygen-controlling hydrogels, which we have termed hypoxia-inducible (HI) hydrogels [34, 43]. Ferulic acid (FA), a phenol containing molecule that also contains a carboxyl end group, was conjugated to the amine groups readily present on Gtn via a carboiimide-mediated reaction. Dextran was first aminated (DexE), then phenol containing tyramine (TA) was conjugated through amine reactive polyethylene glycol (PEG). The hydrophilic PEG linker was used to enhance the crosslinking reactivity. The crosslinking groups are FA and NH-PEG-TA for the Gtn and Dex-based hydrogels, respectively. Hydrogels were formed individually using either Gtn or Dex polymers through an enzymatic laccase-mediated crosslinking reaction. Gtn and Dex hydrogels were formed by formation of di-ferulic acid and di-tyramine bonds, respectively.
In both of these hydrogel systems, the time which hypoxia is maintained and the hydrogel mechanical properties are directly related to the composition of polymer and the concentration of laccase used. Specifically, as the concentration of polymer is increased, both stiffness and time hypoxic (TH) increase accordingly in a highly predictable fashion. As the importance of both of these parameters in cellular responses have been described individually, we propose a hydrogel system which can precisely control each of these parameters individually, while also retaining the ability to study potential synergistic effects between the two variables.
Here we show that by incorporating non-conjugated phenol containing molecules prior to hydrogel crosslinking, TH is significantly increased with only mild effects on hydrogel stiffness. Further, by incorporating a secondary crosslinker, effects on mechanical properties can be induced with negligible effects on TH. Transglutaminase provides an ideal secondary crosslinker, as it is able to enzymatically crosslink the amine groups readily present on the gelatin backbone, as well as the unconjugated amine groups on the dextran backbone [44, 45]. By controlling these two variables independently, we can gain an increased understanding into the effects of two parameters vital to vascular morphogenesis and cancer progression.
Results and Discussion
Hybrid Gtn-Dex hydrogel formation and characterization
Gelatin-based and dextran-based polymers were synthesized as previously described [34, 43]. The chemical structures of the conjugated crosslinking groups (FA and NH-PEG-TA) are provided in Figure 1 and were confirmed with 1H-NMR (Supplementary Figure 1). In order to determine the degree of substitution (DS) of phenol containing moieties, UV/vis spectroscopy was performed. The DS values obtained here were similar to those obtained in previous studies, indicating the reproducibility of these synthesis protocols. The DS of FA for gelatin-based polymers (Gtn-FA) was 55.31±5.67 μmol/g of polymer (DS 55), while the DS of TA for aminated, PEG-linked dextran-based polymers (DexE-PT) was 174.81±3.27 μmol/g of polymer (DS 175). Additional small batch-to-batch variability was accounted for when separate batches of polymer were used. These results are summarized in Table 1.
Figure 1. Crosslinking chemistry for hybrid Gtn-FA and DexE-PT hydrogels.
(A) Hypoxia inducible hydrogels are formed via laccase-mediated dimerization of phenolic moieties. Laccase catalyzes the reduction of atmospheric O2 to water, resulting in the oxidation of phenol-containing ferulic acid (FA,
 ) and tyramine (TA,
) and tyramine (TA,
 ) conjugated to Gtn and Dex, respectively, forming crosslinked polymer networks. (B) Additional non-conjugated phenol-containing molecules (e.g. TA,
) conjugated to Gtn and Dex, respectively, forming crosslinked polymer networks. (B) Additional non-conjugated phenol-containing molecules (e.g. TA,
 ) are added prior to the reaction, resulting in decreased cross-linking density, and thus slightly decreased stiffness. The same reaction kinetics are employed, resulting in nearly identical oxygen consumption and a decoupling of oxygen concentration and mechanical properties. (C) Secondary crosslinking via transglutaminase-mediated dimerization of primary amines (
) are added prior to the reaction, resulting in decreased cross-linking density, and thus slightly decreased stiffness. The same reaction kinetics are employed, resulting in nearly identical oxygen consumption and a decoupling of oxygen concentration and mechanical properties. (C) Secondary crosslinking via transglutaminase-mediated dimerization of primary amines (
 ) present on both polymer backbones leads to increased stiffness, while not affecting oxygen consumption. By controlling the number of phenolic moieties (both conjugated and unconjugated), as well as controlling the crosslinking density by secondary crosslinking, precise control over both oxygen concentration and mechanical stiffness may be acheived.
) present on both polymer backbones leads to increased stiffness, while not affecting oxygen consumption. By controlling the number of phenolic moieties (both conjugated and unconjugated), as well as controlling the crosslinking density by secondary crosslinking, precise control over both oxygen concentration and mechanical stiffness may be acheived.
Table 1.
Concentrations of Crosslinking Moieties
| Polymer backbone | DS of Phenol Moiety (μmol/g polymer) | Available –NH2 (μmol/g polymer) | Total Available Crosslinking Molecules (μmol/g polymer) |
|---|---|---|---|
| Gtn-FA | 55.31±5.67 | 394.69±6.73 | ~450.00 |
| DexE-PT | 174.81±3.27 | 0.64±0.31 | ~175.45 |
In order to form hybrid Gtn-Dex hydrogels, a laccase-mediated enzymatic crosslinking reaction was employed, as shown in Figure 1. A constant concentration of laccase (25 U/ml) was used in all hydrogels, as this concentration exhibited high crosslinking efficiency and good biocompatibility. Hydrogels were formed at 37°C in order to enhance the enzymatic activity, as well as to recapitulate the in vivo setting and further display the potential for the formation of these hydrogels in situ. Further, reaction conditions, including pH and ion strength were chosen to mimic the in vivo microenvironment to enable study of these hydrogels in a highly biomimetic fashion. By combining the two polymer backbones, homo-crosslinking (Gtn-FA to Gtn-FA; DexE-PT to DexE-PT) and hetero-crosslinking (Gtn-FA to DexE-PT) occurs yielding hybrid hydrogels (Figure 1A). As the oxygen consumption is highly dependent on the phenol concentration of the constituents of the hydrogel, and laccase crosslinking is inherently linked to phenol concentration, it follows that with increased crosslinking, increases in TH and mechanical properties occur in concert. In order to account for this, we utilized non-conjugated, phenol-containing molecules, TA, to increase TH without increasing the mechanical properties of the hydrogel. As shown in Figure 1B, non-conjugated molecules either bind to other non-conjugated molecules, or interrupt some of the crosslinking by binding to conjugated phenolic moieties. As such, the TH can be controlled precisely by the addition of given concentrations of TA in a hydrogel and the mechanical properties will show a slight decrease to account for the decrease in crosslinking. Oppositely, increases in mechanical properties may be achieved by employing a secondary crosslinking reaction. Both polymers used here (Gtn-FA and DexE-PT) contain unconjugated primary amines. These primary amines can be crosslinked enzymatically using microbial transglutaminase (mTG). Transglutaminase, like laccase, has been used extensively in the food industry and has recently found a niche in biomedical applications through crosslinking of primary amine containing polymers, such as collagen and gelatin [44, 46]. This secondary crosslinking has theoretically insignificant effects on hydrogel oxygen levels, and can act to increase crosslinking density and thus increase the mechanical properties of the hydrogel, as seen in Figure 1C. To determine the number of available crosslinking primary amines on each polymer, primary amines were tagged with fluorescamine and subsequently quantified using a fluorescence microplate reader. Predictably, there were a large number of excess primary amines on Gtn-FA polymers (394.69±6.73 μmol/g of polymer), indicating potential for a more significant increase in mechanical properties of these hydrogels compared to the relatively low unconjugated primary amine concentration on DexE-PT polymer (0.64±0.31 μmol/g of polymer). With only trace concentrations of available primary amines to crosslink through mTG on DexE-PT polymers, the secondary crosslinking will likely be dominated by the Gtn-FA portion of hybrid hydrogels. The results presented here are summarized in Table 1.
Hybrid Gtn-Dex hydrogels for control of oxygen tension and mechanical properties
In order to examine the hypothesis that both oxygen tension and elastic modulus will increase as the concentration of phenolic moieties increases (Figure 1), a range of hybrid Gtn-Dex polymers were synthesized and analyzed. The gelatin backbone of Gtn-FA provides many important components often present in natural polymers. The biodegradability and presence of cell adhesion motifs are both of the utmost importance when studying cells in vitro, and are critical to vascular morphogenesis [47]. One major drawback of the use of a Gtn based backbone in our system is the limited conjugation efficiency of phenolic moieties and the subsequent limitation of TH. Prolonged hypoxia could only be maintained in this system by way of cell oxygen consumption in vitro [34], or by suspected oxygen consumption of surrounding tissue upon injection in vivo. To account for these limitations, a dextran based hydrogel (DexE-PT) was developed. Conjugation efficiency of DexE-PT was 3–4 times higher than for Gtn-FA (see Table 1), leading to prolonged hypoxia controlled solely by the crosslinking reaction [43]. The major limitation of dextran based hydrogels is the lack of cell adhesion binding sites. By combining these two hydrogels, a highly bioactive hydrogel with prolonged hypoxia is achieved.
Hybrid hydrogels were formed by ranging Gtn-FA and DexE-PT concentrations. Bounds of our region of study were defined by previous results reported in [34, 43]. Gtn-FA 3 wt% was shown to be an appropriate hydrogel composition for studies both in vitro and in vivo, while DexE-PT 10 wt% yielded the highest TH of any hydrogel tested. Combinations of hydrogels with total concentrations of phenolic moieties falling between these two hydrogels (Gtn-FA 3 wt% and DexE-PT 10 wt%) were tested. As shown in Figure 2A, increasing concentrations of phenol containing molecules led to predictable increases in TH. Further, we determined the dynamic elastic modulus (G′) and found that as phenol concentrations increased, G′ increased accordingly (Figure 2B). When normalizing G′ to relative total number of phenolic molecules, we found that increasing phenol concentration resulted in both increased TH and G′ (Figure 2C). This type of predictability can enable a user-defined TH and G′ to develop hydrogels for highly specific applications.
Figure 2. Dissolved oxygen (DO) and rheological characterization for hybrid hydrogels.
(A) DO levels were measured at the bottom of hydrogels (3.13 mm thick, 25 U/ml laccase) as a function of time. (B) Rheological characterization of hydrogels as measured by elastic modulus (G′) using dynamic time sweep. (C) DO and elastic modulus are coupled: as phenol concentration increases, both stiffness and time hypoxic increase.
Decoupling TH and mechanical properties through addition of non-conjugated phenol-containing molecules
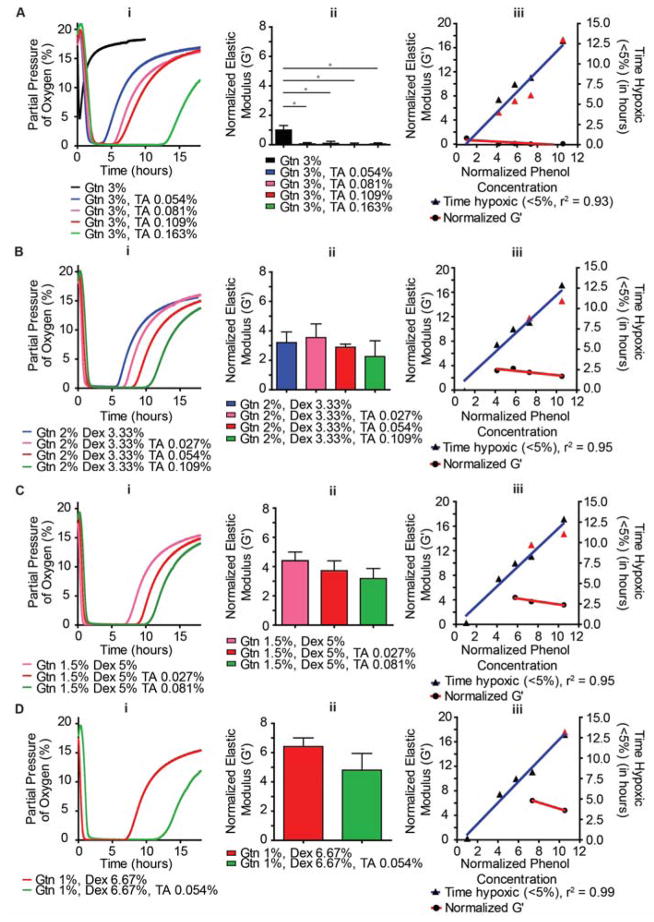
In order to decouple TH and G′, addition of non-conjugated TA to a range of hybrid polymers was performed. As a demonstration of the precision of this approach, concentrations of TA were determined as to match each of the oxygen graphs (with higher TH) in Figure 2A. In other words, specific concentrations of TA were added to Gtn-FA 3 wt% to match TH for each of the hybrid hydrogels. The concentrations of TA added to the Gtn-FA 3 wt% polymer enabled precision control over TH (Figure 3A(i)). By matching the phenol concentrations with the addition of TA, TH was matched for a variety of hydrogel compositions (Figure 3B(i);D(i)). We next examined the effect of this non-conjugated TA on G′, and found that as predicted, decreases in G′ result from interruption of crosslinks by non-conjugated TA (Figure 3A(ii);D(ii)). The data here are presented as a normalized elastic modulus (G′) (normalized to G′ of Gtn-FA 3 wt%), in order to make this data easier to compare between rheological tests. With the addition of TA to Gtn-FA, the already low crosslinking density was decreased further, resulting in a highly viscous fluid, rather than a hydrogel. The importance of a secondary crosslinker for these types of hydrogels is discussed below. Notably, highly predictable TH may be achieved by simply adding non-conjugated phenol containing molecules. These molecules have minimal effect on mechanical properties of the hydrogels, enabling the potential study of a range of TH, while restricting mechanical properties to a small range of values which are not strictly dependent on phenol concentration (Figure 3A(iii);D(iii)).
Figure 3. Addition of non-conjugated TA enables control over TH with minimal effects on G′.
(A(i) –D(i)) DO levels were measured at the bottom of hydrogels for hydrogels with addition of TA: A (Gtn-FA 3%), B (Gtn-FA 2%, DexE-PT 3.33%), C (Gtn-FA 1.5%, DexE-PT 5%), D (Gtn-FA 1%, DexE-PT 6.67%). (A(ii) – D(ii)) Rheological characterization of the same hydrogel formulations. (A(iii) – D (iii)). DO and G′ are no longer coupled and precision control over each variable is shown. Black triangles represent hybrid hydrogel TH (no TA); red triangles represent hybrid hydrogel TH with additional non-conjugated TA. * Indicates P<0.05
Decoupling Mechanical Properties and TH Through Secondary Crosslinking
In order to further decouple oxygen tension and mechanical properties, the addition of a secondary crosslinker, transglutaminase (TG), was examined. By maintaining a consistent oxygen tension and providing additional crosslinks between polymer chains, the opposite effect of addition of non-conjugated TA may be achieved. In other words, stiffer hydrogels with shorter TH can theoretically be developed. TG is a naturally occurring crosslinker that catalyzes the formation of N ε-(γ-glutamyl) lysine amide bonds between gelatin or collagen polymer chains [44, 46]. TG has been used in a number of collagen or gelatin containing hydrogels due to its improved cytocompatibility over other crosslinkers and potential as an in situ crosslinker, owing to its relatively short gelation time at body temperature and pH [44]. Its crosslinking potential can be expanded beyond just gelatin and collagen to include crosslinking of primary amine containing polymer chains, such as DexE-PT. Mammalian TG inherently relies on the presence of proenzymes and calcium ions to reach its maximum potency. However, innocuous microbial TG (mTG) has seen widespread use in the food industry, is U.S. Food and Drug Administration approved, and functions independently of proenzymes and calcium ions [44], thus further indicating its vast potential in biomedical applications.
The effects of a mTG on oxygen tension are shown in Figure 4A. Hydrogels were formed with the same concentration of laccase as described above (25 U/ml), but with the addition of mTG at 0.3 U/ml, as described in [45]. As expected, the addition of mTG had minimal effect on TH. However, the addition of mTG only had insignificant or neglible effects on the mechanical properties of the hybrid hydrogels (Figure 4B, C). Predicted increases in G′ were not observed, particularly in those hydrogels with high concentration of the Gtn-FA component. These hydrogels have a significant increase in the number of potential crosslinking sites upon the addition of secondary crosslinker, mTG (Table 1). Hydrogels with less or no gelatin component only have a slight increase in potential crosslinking sites. This predicted difference was not evident in the rheological characterization of these hydrogels (Figure 4B). It is possible that the faster reaction kinetics of the laccase enzyme led to decreased availability of crosslinking sites for mTG due to steric hindrance and decreased polymer chain mobility. In order to combat these minor effects in future studies, increases in mTG concentration will be investigated, along with their accompanying cytotoxicity tests, as well as a two step crosslinking in which mTG is added first, then laccase is added so as both enzymes complete their respective catalytic crosslinking reactions at the same time and are both minimally hindered in their reactions.
Figure 4. Dissolved oxygen (DO) and rheological characterization for hybrid hydrogels with secondary crosslinking microbial transglutaminase (mTG).
(A) DO levels were measured at the bottom of hydrogels (3.13 mm thick, 25 U/ml laccase, 0.3 U/ml mTG) as a function of time. (B) Rheological characterization of hydrogels as measured by viscoelastic modulus (G′) using dynamic time sweep and normalized to Gtn-FA 3% in order to provide a direct comparison between hydrogels. (C) DO and viscoelastic modulus remain coupled, but less strictly. * indicates P<0.05.
Another important function of mTG component becomes clear when Gtn-FA hydrogels with high TA concentration are studied. These combinations were essentially viscous fluids rather than hydrogels (data not shown). By adding mTG along with non-conjugated TA, hydrogel formation will likely occur, leading to an enhanced breadth of possible studies for these hybrid hdyrogels, as well as enhanced therapeutic potential as a stand-alone treatment option.
Varying Oxygen Tension and Mechanical Stiffness Alters Vasculogenesis In Vitro
In order to establish the viability of these hydrogels for biological experimentation, in particular the effects of varying oxygen tension and mechanical stiffness on vascular morphogenesis, we utilized endothelial colony forming cells (ECFCs). The cytocompatibility of both polymers (Gtn and Dex), which contain free phenol groups in the same concentrations as the non-conjugated phenol groups tested here, and both enzymes (laccase and mTG) have been established previously [34, 43, 44]).
Encapsulation of ECFCs within hydrogels with a range of properties was tested. As described previously [34], soft hydrogels with moderate hypoxic conditions (Gtn-FA 3%) are amenable to vascular network formation (Figure 5). Upon investigation of other hybrid hydrogels, several interesting conclusions can begin to be drawn. As a proof of concept, hybrid hydrogels (Gtn-FA 2%, DexE-PT 3.33%) were tested (Figure 5). Interestingly, following the same encapsulation protocol, the ECFCs in the hybrid hydrogels seemed to aggregate much more readily than in the Gtn-FA 3% hydrogels. This increased aggregation is likely a result of the decreased availability of cell adhesion sites in the hybrid hydrogels, likely leading to more cell-cell interactions in the initial encapsulation. The well documented steps of vascular morphogenesis [24] were not seen in the Gtn-FA 2%, DexE-PT 3.33% hybrid hydrogels. Vacuole formation was noticeably absent, and sprouting cells appeared to bridge from one ‘island’ of cell aggregates to another ‘island’.
Figure 5. Vascular morphogenesis in an array of microenvironmental conditions.
Gtn-FA 3% hydrogels (soft, moderate hypoxia) were amenable to network formation, which followed the well understood vascular morphogenetic process of vacuole formation, followed by lumen formation and ultimately branching and sprouting to form complex networks. Hybrid hydrogels (Gtn-FA 2%, DexE-PT 3.33%) (stiff, severe hypoxia) permitted sprouting, but not extensive network formation. Interestingly, cells seemed to aggregate and form sprouts between groups of cell aggregates without the initial stage of vascular morphogenesis (vacuole/lumen formation). Gtn-FA 3%, TA 0.054% hydrogels (soft, severe hypoxia) matched the oxygen tension in Gtn-FA 2%, DexE-PT 3.33%, but were soft. Again, cell aggregates led to sprouting, but in these softer hydrogels, more robust networks formed. Gtn-FA 3%, 0.3 U/ml mTG (stiff, moderate hypoxia) permitted the initial stages of vascular morphogenesis, but completely inhibited branching and sprouting. Vacuoles/lumen indicated by arrowheads (▶), sprouting indicated by arrows (
 ). Scale bars 100 μm.
). Scale bars 100 μm.
To further prove the novelty and efficacy of the hybrid hydrogels described, Gtn-FA 3% hydrogels with additional TA (0.054%) to match the oxygen tension of the Gtn-FA 2%, DexE-PT 3.33% hybrid hydrogels were examined (Figure 5). Robust structures were formed even in these extreme hypoxic conditions (~0% O2) indicating the ability for ECFCs to survive and even thrive in low hypoxic conditions. Further indications for an interplay between oxygen tension and mechanical properties are evidenced here, as few vascular morphogenetic structures were formed in the severely hypoxic ‘stiff’ hybrid gels (Gtn-FA 2%, DexE-PT 3.33%), while extensive network formation was present in the softer hydrogels (Gtn-FA 3%, TA 0.054%).
Finally, hybrid hydrogels with the same TH as Gtn-FA 3%, but approximately two times the elastic modulus, were tested (see Figure 4). By adding mTG, stiffer hydrogels were formed (Gtn-FA 3%, mTG; Figure 5). As previously observed [21], ECFCs in these hydrogels formed much less robust networks. They followed the initial stages of vascologenesis (vacuole formation), but were unable to progress to form sprouts and vascular networks. This may reveal an additional specification about the importance of the mechanical stiffness on vascular network formation and display the precision with which ECFCs sense their surrounding microenvironment. The small, but clearly significant, change in elastic modulus prevented completion of vascular network formation. These results may also indicate that mechanical stiffness plays an important role in the later stages of vascular morphogenesis, as the increase in stiffness did not impede the initial stages of the process. In stiff gels with longer TH, sprouts were able to form, while in stiff gels with briefer TH, vasculogenic potential was limited.
Taken together, these results indicate the sensitivity of ECFCs to form vascular networks, highlighting the need for a system such as the one presented here to tease out the exact mechanisms of vasculogenesis that permit the most robust and ultimately functional vascular networks.
Conclusions
By varying the composition of hydrogel backbone components, predictable changes in TH and mechanical properties were achieved. Choosing a specific composition of polymers allows for user-defined control of both of these parameters, indicating the potential for such hybrid hydrogel systems in a range of cellular studies in vitro and as potential treatment options in vivo. However, these studies are restricted by the short TH and low elastic modulus achievable by Gtn-FA hydrogels and the limited bioactivity of the DexE-PT hydrogels. By decoupling both TH and elastic modulus, significant enhancement of control of these properties is achieved. Addition of non-conjugated TA allows for significant increases in TH, while minimally affecting the mechanical properties of hydrogels. Utilizing a secondary crosslinker (mTG), which has negligible effects on TH, while increasing the elastic modulus will also aid in controlling these hydrogels. Perhaps most importantly, this secondary crosslinker will help when designing hybrid hydrogels with high concentration of TA and low inherent crosslinking density (e.g. Gtn-FA). Secondary crosslinking will also allow prolonged hypoxia beyond the 10–12 hour range exhibited in this study.
Choosing specific polymer compositions also enables control over the concentration of cell adhesion motifs, a vital regulator of cellular function. Because DexE-PT does not contain cell binding sites, the cell adhesion is strictly regulated by the Gtn-FA component. Varying this component will allow investigation of the potential synergistic effects of TH and elastic modulus as they pertain to cell adhesion. Additionally, because two degradation mechanisms are necessary to degrade hybrid hydrogels (collagenase for Gtn, dextranase for Dex), control over degradation of these hydrogels can be incorporated into the design. The range of elastic moduli presented in this study, from <20 Pa to 1 kPa, will allow study of the range of mechanical properties experienced by the cells of the vasculature, from development to maturity [21, 48]. Such potential studies may help elucidate the mechanisms controlling myriad downstream effects on angiogenesis and vasculogenesis, towards the development of potential novel therapeutics.
Another important aspect of these hybrid hydrogels is the ability to control the oxygen gradient. Oxygen gradients exist throughout the body. As such, cellular function is highly dependent on specific oxygen tension, indicating the importance of studying oxygen gradients in both tissue engineering and tumor biology. While not measured here, there are likely a range of oxygen gradients that may be developed utilizing these hydrogels. For example, while most of the hybrid hydrogels shown here reach 0% oxygen tension, there are likely significant differences in the steepness of the oxygen gradient and how long this oxygen gradient is maintained. Altering the crosslinking density will change the pore size and ultimately the oxygen diffusion through these hydrogels, which may enable precision control of oxygen gradients.
Varying TH and the mechanical properties of these hydrogels without changing any aspects of polymer synthesis enables a process that is highly repeatable and has potential for scale up in the future, as these hydrogels are optimized for high throughput studies and as disease treatment therapies.
Experimental
Materials
Gelatin (Gtn, type A from porcine skin, <300 bloom), 3-methoxy-4-hydroxycinnamic acid (ferulic acid, FA), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), dimethyl sulfoxide (DMSO), Dextran (Dex, MW 70,000), 2–bromoethylamine hydrobromide (BEAHB), triethylamine (TEA), 4–dimethylaminopyridine (DMAP), p–nitro–phenylchloroformate (PNC), poly(ethylene glycol) (PEG, MW 4000), tyramine (TA), laccase (lyophilized powder from mushroom, ≥4.0 units/mg), and deuterium oxide (D2O) were purchased from Sigma-Aldrich (Saint Louis, MO) and used as obtained without purification. Microbial transglutaminase (mTG) (Activa-TI) was purchased from Ajinomoto, Inc. Dulbecco’s Phosphate-Buffered Saline (DPBS), Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and trypsin were all purchased from Gibco, Invitrogen (Life Technologies, CA). Dialysis membranes (molecular cutoff = 3500 Da; molecular cutoff = 6000–8000 Da) were purchased from Spectrum Laboratories (Rancho Dominguez, CA). Endothelial growth media-2 (EGM2) was purchased from Lonza (Walkersville, MD) and used to culture endothelial colony forming cells (ECFCs) (Lonza) on collagen I coated cell culture plates (BD Biosciences, Franklin Lakes, NJ).
Synthesis of gelatin-g-ferulic acid (Gtn-FA)
Gelatin-g-ferulic acid (Gtn-FA) was synthesized using EDC and NHS as coupling reagents. A mixture of DMSO and distilled (DI) water (1:1 volume ratio) was prepared as a solvent. Gtn (1.0 g) was dissolved in 50 ml of solvent at 40°C. FA (0.78 g, 4.0 mmol) was dissolved in 20 ml of the solvent and reacted with EDC (0.92 g, 4.8 mmol) and NHS (0.64 g, 5.6 mmol) (carobxyl:EDC:NHS = 1:1.2:1.4) at room temperature for 15 min to activate the terminal carboxyl groups of FA. The activated solution was then added to the Gtn solution, and a conjugative reaction was conducted at 40°C for 24 hours. Following completion of the reaction, the solution was dialyzed against DI water for 5 days (molecular weight cutoff = 3500 Da) and then lyophilized.
Synthesis of aminated dextran–g–poly(ethylene glycol)–tyramine (DexE–PT)
Aminated–dextran (DexE) was synthesized by coupling of BEAHB and Dex using TEA as a catalyst. Briefly, Dex (1.5 g, 0.02 mmol) was dissolved in 60 mL of anhydrous DMSO at 50°C and reacted with TEA (6.2 mL, 44.5 mmol) for 30 min. BEAHB (4.6 g, 22.5 mmol) was dissolved in 50 mL of DMSO then added to the Dex solution and the reaction was conducted at 50°C for 24 hours. Following completion of the reaction, the solution was dialyzed against water for 5 days (molecular weight cutoff = 6000–8000 Da) and then lyophilized. For the synthesis of amine–reactive PEG, the terminal hydroxyl groups of PEG were activated with excess PNC. PEG (10 g, 2.5 mmol) was dissolved in 100 mL of anhydrous dichloromethane (DCM) at room temperature under a nitrogen atmosphere. DMAP (0.916 g, 7.5 mmol) and TEA (0,759 g, 7.5 mmol) were dissolved in 20 mL of DCM and then added to the PEG solution. The mixture was reacted at room temperature for 15 min to activate the terminal hydroxyl groups. The PNC solution (1.511 g, 7.5 mmol) dissolved in DCM was added dropwise to the activated PEG solution, and the reaction was conducted under a nitrogen atmosphere at room temperature for 24 hours. The molar ratio of PEG:DMAP:TEA:PNC was 1:3:3:3. Following completion of the reaction, the solvent was evaporated using a rotary evaporator at 30°C. The concentrated solution was precipitated in cooled diethyl ether. The precipitate was filtered and dried overnight under vacuum to give a white powder of amine–reactive PEG (PEG–(PNC)2). Finally, DexE–PEG–TA (DexE–PT) was synthetized by coupling DexE and TA using PEG–(PNC)2. For this synthesis, PEG–(PNC)2 (3.2 g, 0.8 mmol) was dissolved in 30 mL of anhydrous DMSO at room temperature under a nitrogen atmosphere. The TA (0.11 g, 0.8 mmol) solution dissolved in 30 mL of anhydrous DMSO and was added dropwise to the PEG–(PNC)2 solution. The reaction was performed at room temperature under a nitrogen atmosphere for 6 hours to give mono-TA conjugated PEG (PNC–PEG–TA). The molar ratio of PEG–(PNC)2:TA was 1: 1. The PNC–PEG–TA solution was applied to DexE (0.25 g, 3.6 μmol) solution and dissolved in 50 mL of anhydrous DMSO. The reaction was conducted at room temperature under a nitrogen atmosphere for 24 hours. Following completion of the reaction, the solution was dialized against a saltwater solution (NaCl 0.01 nM) to remove PNC salt and unconjugated molecules for 5 days (molecular cutoff = 6000–8000 Da), then further purified by dialysis against distilled water (molecular cutoff = 6000–8000 Da) for 1 day to remove NaCl molecules. The purified solution was lyophilized to give the DexE PT–polymer.
Characterization of hydrogel precursor solutions
The degrees of substitution (DS) of FA and TA were measured using a UV/Vis spectrometer (SpectraMax; Molecular Devices, Sunnyvale, CA). Gtn-FA polymer (10 mg) was dissolved in 1ml of a DMSO:DI water (1:1) solution, and the absorbance was measured at 320 nm. DexE-PT polymer (10 mg) was dissolved in 1 ml of DMSO, and the absorbance was measured at 275 nm. The concentrations of the conjugated FA and TA molecules were calculated from a calibration curve given by monitoring the absorbance of known concentrations of FA or TA. The availability of primary amines for mTG crosslinking was analyzed using fluorescamine. Following manufacturer instructions, polymer solutions of 10 mg/ml PBS (for Gtn-FA and DexE-PT) were measured following reaction (15 min) with fluorescamine with a fluorescence microplate reader (SpectraMax Gemini XPS; Molecular Devices, Sunnyvale, CA) at excitation/emission 380/470 nm. Polymers were compared to a calibration curve of glycine with known concentrations. The chemical structures of Gtn-FA and DexE-PT were characterized using a 1H NMR spectrometer (Bruker AMX-300 NMR spectrometer, Billerica, MA). Gtn-FA and DexE-PT polymer solutions (10 mg/ml of D2O) were prepared and analyzed.
Preparation of hypoxia-inducible hydrogels
Hydrogel precursor solutions (Gtn-FA, DexE-PT, laccase, mTG) were prepared in DBPS. Enzymes were maintained at final concentrations of 25 U/ml (laccase)[34, 43] and 0.3 U/ml (mTG)[45]. Hypoxia-inducible (HI) hydrogels were prepared by mixing aqueous Gtn-FA and/or DexE-PT polymers and laccase and/or mTG solutions. Hydrogels were prepared in 1.5 ml vials in a 3:1 polymer:enzyme ratio. Polymer solutions (Gtn-FA 4.0 wt%, DexE-PT 13.3 wt%) and enzyme solutions (100 U/ml laccase and/or 1.2 U/ml mTG) were mixed by pipetting to form hydrogels. All gels were formed at 37°C.
O2 measurement
We measured dissolved O2 (DO) levels noninvasively at the bottom of hydrogels using commercially available sensor patches (Presens, Regensburg, Germany). To measure O2 levels at the bottom of hydrogels, the polymer solutions were added on top of the sensors, which were immobilized in each well of a 96–well plate (BD Bioscience), and then mixed with laccase solutions to form hydrogels. All experiments were conducted in a controlled environment at 37°C and 5% CO2 in a standard incubator.
Measurement of viscoelastic properties
Rheological analysis of the HI hydrogels was performed using a rheometric fluid spectrometer (RFS3, TA Instruments, New Castle, DE) with a 25mm parallel plate geometry. For analysis of viscoelastic properties of preformed hydrogels in their equilibrium swelling state, hydrogel discs were prepared and swelled in DPBS for >12 hours. For dynamic time sweep, we monitored the elastic modulus (G′) and viscous modulus (G″) at 1% strain and a frequency of 10 Hz at 37°C with a constant force of ~10 gm.
ECFC encapsulation
Endothelial colony forming cells (ECFCs) (Lonza, Walkersville, MD) were cultured in endothelial growth media-2 (EGM2; Lonza) prepared according to manufacturer instructions with an additional 10% FBS, on standard tissue culture plates coated with type I collagen (BD Biosciences, Franklin Lakes, NJ). For combination hydrogels (without additional nonconjugated TA or mTG), polymer solutions were dissolved in 1X DPBS (pH 7.4) and mixed with ECFC pellets to provide cell suspension (4 million cells/ml), and then laccase solution (100 U/ml) was added at a volume ratio of 3:1 (polymer solution:laccase solution) and gently mixed at 37°C. The final concentration of laccase was 25 U/ml in all hydrogels and the final concentration of each polymer was varied to achieve a range of TH and mechanical properties. TA was added to polymer solutions to prolong TH. To increase mechanical properties, mTG was added to laccase stock solutions to a concentration of 1.2 U/ml, as described previously [45], to achieve a final concentration of 0.3 U/ml. Each mixture (cells, polymer, crosslinker(s)) was placed in the 96-well plate and allowed ample time to completely form a hydrogel at 37°C. Following complete hydrogel curing, 200 μl of endothelial growth media (Lonza) was added and cells were cultured under standard cell culture conditions (37°C, 5% CO2) for up to 6 days. The culture medium was replaced every day. Bright field images were taken daily to monitor network formation. After 6 days in culture, hydrogels were fixed with 3.7% paraformaldehyde for 30 minutes at room temperature, then washed three times with 1X DPBS. Encapsulated ECFCs were then permeabilized with 0.1% Triton X-100 for 1 hour and then incubated in 1% BSA blocking solution for 1 hour at room temperature. Hydrogels were then stained with phalloidin overnight and DAPI for 15 minutes. Hydrogels were analyzed using confocal microscopy (LSM 780, Carl Zeiss)
Supplementary Material
Acknowledgments
This research was partially funded by National Institute of Health grants R01HL107938 and AHA-Established Investigator Award (to SG).
References
- 1.Park KM, et al. Thermosensitive chitosan-Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomaterialia. 2009;5(6):1956–1965. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Park JS, et al. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. Journal of Controlled Release. 2007;124(1–2):51–59. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Park H, et al. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford MC, et al. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraehenbuehl TP, et al. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30(26):4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zisch AH, et al. Covalently conjugated VEGF-fibrin matrices for endothelialization. Journal of Controlled Release. 2001;72(1–3):101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 7.Peattie RA, et al. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25(14):2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 8.Jeon O, et al. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. Journal of Controlled Release. 2005;105(3):249–259. doi: 10.1016/j.jconrel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. Journal of Thrombosis and Haemostasis. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 10.Phelps EA, et al. Bioartificial matrices for therapeutic vascularization. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zieris A, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. Journal of Controlled Release. 2011;156(1):28–36. doi: 10.1016/j.jconrel.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Saik JE, et al. Covalently immobilized platelet-derived growth factor-BB promotes antiogenesis in biomirnetic poly(ethylene glycol) hydrogels. Acta Biomaterialia. 2011;7(1):133–143. doi: 10.1016/j.actbio.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacchi V, et al. Long-lasting fibrin matrices ensure stable and functional angiogenesis by highly tunable, sustained delivery of recombinant VEGF(164) Proceedings of the National Academy of Sciences of the United States of America. 2014;111(19):6952–6957. doi: 10.1073/pnas.1404605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chwalek K, et al. Glycosaminoglycan-based hydrogels to modulate heterocellular communication in in vitro angiogenesis models. Scientific Reports. 2014:4. doi: 10.1038/srep04414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chemical Reviews. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 17.Seliktar D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science. 2012;336(6085):1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 18.Sun GM, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):20976–20981. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Wen JH, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature Materials. 2014;13(10):979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanjaya-Putra D, et al. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. Journal of Cellular and Molecular Medicine. 2010;14(10):2436–2447. doi: 10.1111/j.1582-4934.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials. 2013;12(5):458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanjaya-Putra D, et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood. 2011;118(3):804–815. doi: 10.1182/blood-2010-12-327338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanjaya-Putra D, et al. Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials. 2012;33(26):6123–6131. doi: 10.1016/j.biomaterials.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Materials. 2012;11(7):642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 26.Moon JJ, et al. Micropatterning of Poly(Ethylene Glycol) Diacrylate Hydrogels with Biomolecules to Regulate and Guide Endothelial Morphogenesis. Tissue Engineering Part A. 2009;15(3):579–585. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, et al. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials. 2013;34(12):2865–2874. doi: 10.1016/j.biomaterials.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson LE, Moura ME, Gerecht S. Guiding endothelial progenitor cell tube formation using patterned fibronectin surfaces. Soft Matter. 2010;6(20):5109–5119. [Google Scholar]
- 29.Raghavan S, et al. Geometrically Controlled Endothelial Tubulogenesis in Micropatterned Gels. Tissue Engineering Part A. 2010;16(7):2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusuma S, et al. Micropattern size-dependent endothelial differentiation from a human induced pluripotent stem cell line. Journal of Tissue Engineering and Regenerative Medicine. 2015:n/a–n/a. doi: 10.1002/term.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickinson LE, et al. Endothelial cell responses to micropillar substrates of varying dimensions and stiffness. Journal of Biomedical Materials Research Part A. 2012;100A(6):1457–1466. doi: 10.1002/jbm.a.34059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipski AM, et al. The effect of silica nanoparticle-modified surfaces on cell morphology, cytoskeletal organization and function. Biomaterials. 2008;29(28):3836–3846. doi: 10.1016/j.biomaterials.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusuma S, et al. Low Oxygen Tension Enhances Endothelial Fate of Human Pluripotent Stem Cells. Arteriosclerosis Thrombosis and Vascular Biology. 2014;34(4):913–920. doi: 10.1161/ATVBAHA.114.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nature Communications. 2014:5. doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blatchley MR, Gerecht S. Acellular implantable and injectable hydrogels for vascular regeneration. Biomedical materials (Bristol, England) 2015;10(3):034001. doi: 10.1088/1748-6041/10/3/034001. [DOI] [PubMed] [Google Scholar]
- 36.Shen YI, et al. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomaterials Science. 2014;2(5):655–665. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levental KR, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature Reviews Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 39.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129(3):465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. Hypoxia-Inducible Factors in Physiology and Medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilkes DM, et al. Hypoxia-inducible Factor 1 (HIF-1) Promotes Extracellular Matrix Remodeling under Hypoxic Conditions by Inducing P4HA1, P4HA2, and PLOD2 Expression in Fibroblasts. Journal of Biological Chemistry. 2013;288(15):10819–10829. doi: 10.1074/jbc.M112.442939. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nature Reviews Molecular Cell Biology. 2008;9(4):285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park KM, Blatchley MR, Gerecht S. The Design of Dextran-Based Hypoxia-Inducible Hydrogels via In Situ Oxygen-Consuming Reaction. Macromolecular Rapid Communications. 2014;35(22):1968–1975. doi: 10.1002/marc.201400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yung CW, et al. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. Journal of Biomedical Materials Research Part A. 2007;83A(4):1039–1046. doi: 10.1002/jbm.a.31431. [DOI] [PubMed] [Google Scholar]
- 45.Paguirigan AL, Beebe DJ. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nature Protocols. 2007;2(7):1782–1788. doi: 10.1038/nprot.2007.256. [DOI] [PubMed] [Google Scholar]
- 46.McDermott MK, et al. Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules. 2004;5(4):1270–1279. doi: 10.1021/bm034529a. [DOI] [PubMed] [Google Scholar]
- 47.Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. American Journal of Pathology. 2000;156(5):1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nature Reviews Molecular Cell Biology. 2009;10(1):34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.