Abstract
This review highlights a selection of recently published literature in the area of osteoarthritis biology. Major themes transpiring from a PubMed search covering the year between the 2014 and the 2015 OARSI World Congress are explored. Inflammation emerged as a significant theme, revealing complex pathways that drive dramatic changes in cartilage homeostasis and in the synovium. Highlights include a homeostatic role for CXC chemokines in cartilage, identification of the zinc-ZIP8-MTF1 axis as an essential regulator of cartilage catabolism, and the discovery that a small aggrecan fragment can have catabolic and pro-inflammatory effects through Toll-like receptor 2. Synovitis can promote joint damage, partly through alarmins such as S100A8. Synovitis and synovial expression of the pro-algesic neurotrophin, Nerve Growth Factor, are associated with pain. Increasingly, researchers are considering specific pathogenic pathways that may operate in distinct subsets of osteoarthritis associated with distinct risk factors, including obesity, age, and joint injury. In obesity, the contribution of metabolic factors and diet is under intense investigation. The role of autophagy and oxidative stress in age-related osteoarthritis has been further explored. This approach may open avenues for targeted treatment of distinct phenotypes of osteoarthritis. Finally, a small selection of novel analgesic targets in the periphery is briefly discussed, including calcitonin gene-related peptide and the neuronal sodium voltage-gated channels, Nav1.7 and Nav1.8.
Keywords: Ageing, Animal models, Autophagy, Cartilage, Chondrocytes, Extracellular matrix, Inflammation, Joint, Obesity, Osteoarthritis, Pain, Post-traumatic Osteoarthritis, Synovium
Search criteria and selection process
Papers published online between February 2014 and April 2015 were identified by performing a PubMed search using the following terms: “osteoarthritis”, “animal models”, “joint”, “cartilage”, “chondrocytes”, “synovium”, “subchondral bone”, “risk factors”, “ageing”, “obesity”, “post-traumatic”, and “pain”. From this search, papers were selected in order to illustrate active research topics. The papers included below are a personal selection and by no means capture the wealth of meritorious studies published in the past year. Rather, they were chosen because they exemplify some overarching themes that are emerging in the field, and it is hoped that this selection will entice the reader to conduct a more in-depth exploration of these themes. In addition, reference is made to several recent review articles that provide background information to these main themes.
Mechanisms of inflammation in cartilage and synovium that drive joint organ failure
All articular tissues – including cartilage, subchondral bone, synovium, intra-articular fat, meniscus, ligaments, and peri-articular muscles - can be affected by osteoarthritis (OA) pathology. Individual tissues and the crosstalk between them likely contribute to disease progression and pain (reviewed in [1, 2]. Emphasis in the past year was on complex pathways, especially in articular cartilage and synovium, that drive catabolism and low-grade inflammation and how these processes affect global joint metabolism (for review, see [3]).
Altered cartilage homeostasis
In the course of OA, articular chondrocytes are exposed to a range of insults, including aberrant biomechanical stresses, pro-inflammatory cytokines and chemokines and extensive changes in the extracellular matrix (ECM). This provokes a phenotypic shift in chondrocytes and disturbs cartilage homeostasis [4]. Several novel pathways that contribute to and perpetuate these dramatic phenotypic changes were uncovered in the past year.
mRNA decay in healthy versus OA chondrocytes
mRNA levels are not just controlled by the rate of synthesis but also by the rate of degradation (i.e. “post-transcriptional regulation”). Tew and colleagues examined mRNA decay in the transcriptome of healthy and OA human chondrocytes, by microarray analysis following an actinomycin D chase [5]. They reported that the majority of chondrocyte-expressed transcripts were stable, but a subset exhibited rapid decay with, interestingly, a clear bias toward shortening of mRNA half-life in OA chondrocytes. Short-lived transcripts included genes involved in transcriptional regulation, localized to the nucleus, or involved in the regulation of programmed cell death. Additionally, the study identified genes related to ECM turnover, such as ADAMTS-1, ADAMTS-5, ADAMTS-9, the hyaluronic acid synthase, HAS2, the heparan sulfate sulfotransferase, HS3ST3A1, and also the NFκB complex component, RELA. These short-lived transcripts were overall more highly expressed in OA chondrocytes, which may mean that they are associated with processes that rely on rapid and flexible gene responses in OA [5]. This first-of-its-kind study adds a new dimension to the regulation of chondrocyte phenotypic stability.
A homeostatic role for CXCR2 signaling in articular cartilage
CXC chemokines with an ELR motif are heparin-binding chemokines that signal through CXCR1 and CXCR2 and have been targeted in inflammatory arthritis because of their chemotactic properties. However, an unexpected homeostatic role for CXCR2 signaling in articular cartilage was revealed by Sherwood and co-workers [6], who found that the CXCR1/2 ligand, CXCL6, was present in the territorial matrix of healthy human cartilage, bound to heparan sulfate proteoglycans. In contrast, in early human or murine OA, CXCL6 was no longer detected. CXCR2-deficient mice showed more severe cartilage damage 8 weeks after destabilization of the medical meniscus (DMM) than wild types, while in vitro disruption of CXCR2 signaling resulted in loss of mRNA for the transcription factor, SOX9, COL2A1 mRNA and aggrecan mRNA in primary human chondrocytes. The authors concluded that CXCL6 may support chondrocyte phenotypic stability through SOX9 and that the loss of CXCL6 from degrading cartilage may contribute to the characteristic changes in the phenotype of the OA chondrocyte.
The zinc-ZIP8-MTF1 axis is an essential regulator of the catabolic cascade in cartilage
Kim et al. reported that the zinc (Zn2+) importer, ZIP8, was increased in human and murine OA cartilage, raising intracellular Zn2+ in chondrocytes [7]. ZIP8-mediated Zn2+ influx upregulated chondrocyte expression of catabolic enzymes, including MMP3, MMP9, MMP12, MMP13, and ADAMTS-5. Chondrocyte-specific ZIP8 overexpression in mice (using a Col2a1 promoter) resulted in cartilage damage and subchondral bone (SCB) sclerosis without overt synovitis. After DMM surgery, these mice developed accelerated cartilage destruction and SCB changes, while there was no effect on synovitis and osteophytes. Conversely, chondrocyte-specific conditional Zip8 knockout mice showed less cartilage damage and SCB sclerosis 8 weeks after DMM, without protection from synovitis and osteophyte growth. Further, the transcription factor, MTF1, was identified as an essential mediator of Zn2+/ZIP8-induced catabolism. This study establishes the zinc-ZIP8-MTF1 axis as a novel therapeutic target in OA. It also substantiates the notion that cartilage damage can drive changes in other joint tissues, in this case the SCB – but remarkably, there was no effect on synovium or osteophytes.
Catabolic and pro-inflammatory effects of an aggrecan fragment mediated through TLR2
It has long been recognized that matrix molecules and fragments thereof, including fibronectin, tenascin C, and hyaluronan fragments, can act as Damage Associated Molecular Patterns (DAMPs). These DAMPs activate Pattern Recognition Receptors (PRR) such as Toll-like receptors (TLR) and Receptor for Advanced Glycation Endproducts (RAGE) that are locally expressed in the joint, initiating a cascade of inflammatory cytokine production [3]. Lees and co-workers investigated bio-activity of an aggrecan fragment that is generated when ADAMTS-4/5 cleave the interglobular domain of the aggrecan core protein at the 374ARGS cleavage site and the remaining G1-EGE373 stub is subsequently cleaved by MMPs at DIPEN341, resulting in a 32-amino acid fragment [8]. A synthetic 32-mer peptide caused a pro-catabolic, anti-anabolic, and pro-inflammatory response in vitro in murine and human chondrocytes, increasing mRNA expression for several proteases, including MMP-13 and ADAMTS-5, and decreasing mRNA for matrix molecules, including Col2A1 and aggrecan. These effects are mediated through TLR2 and are NFκB-dependent. It was confirmed that the native, glycosylated 32-mer also has biological activity. This is the first demonstration that a TLR ligand can be derived from one of the major cartilage macromolecules. This aggrecan fragment adds to the pool of DAMPs that can originate from degrading cartilage and amplify the pro-inflammatory and catabolic network in the OA joint. The specific role of the 32-mer aggrecan fragment within the innate immune network in vivo needs to be determined.
The pathogenic role of synovium
Clinical and imaging studies provide substantial evidence that low-grade synovitis is associated with accelerated cartilage loss as well as with symptoms (reviewed in [9]). The recent studies discussed below shed light on synovial pathways that may contribute to OA disease.
The role of the alarmins, S100A8 and S100A9, in OA with pronounced synovitis
S100A8 and S100A9 are abundantly present in OA joints. A few years ago, it was reported that these alarmins have pro-catabolic effects on chondrocytes via TLR4, and that they contribute to OA pathogenesis in collagenase-induced OA (CIOA), a model with pronounced synovial inflammation, but not in the DMM model, which exhibits low-grade synovitis [10]. The same authors now reported that intra-articularly (IA) deposited adipose-derived stem cells were efficacious in reducing cartilage damage and osteophytes in CIOA but not after DMM - indicating that synovial activation drives the protective effects of locally administered adipose-derived stem cells [11]. Efficacy in CIOA was related to rapid suppression of synovial activation, suppression of S100A8/A9 and IL-1 in the joint and of S100A8/A9 serum levels [11]. These findings further reinforce the concept that these alarmins may contribute to OA progression in subsets with a high degree of synovitis, and this could be therapeutically exploited. OA joints may, of course, contain a variety of DAMPs in addition to S100 proteins, including ECM degradation products that signal through various PRRs. Hence, it is of interest that female TLR1, TLR2, TLR4, TLR6, or MyD88 deficient mice were not protected from cartilage damage or synovial inflammation 8 weeks after partial removal of the medial meniscus [12]. These paradoxical findings further illustrate the need for careful assessment of the role of the innate immune network in different subsets of OA and at different stages of disease.
PAR2 ablation modulates synovial macrophage activation one week after DMM and results in cartilage protection at 8 weeks
The pro-inflammatory G-Protein Coupled Receptor (GPCR), proteinase-activated receptor-2 (PAR2), is elevated in human OA cartilage and synovium. Jackson et al. compared the progression of OA in wildtype versus PAR2-/- mice up to 8 weeks after DMM and demonstrated chondroprotection in PAR2-/- mice, confirming older studies [13]. In vitro studies, however, failed to reveal a direct chondroprotective effect of PAR2 ablation, suggesting that the primary protective mechanisms of PAR2 ablation may occur through extra-cartilagenous pathways. One week after DMM, wildtype mice showed clear synovitis with increased synovial gene expression of pro-inflammatory cytokines and metalloproteases and increased numbers of CD4+ T-lymphocytes and activated macrophages (assessed by FACS analysis), while PAR2-deficient mice contained fewer macrophages. This suggests that synovitis early on after DMM surgery, although mild in comparison to more inflammatory models, may be instrumental for disease progression.
Synovitis and pain
Stoppiello and co-workers aimed to identify histopathological features associated with symptomatic knee OA [14]. They harvested medial tibial plateaux and synovia from 29 asymptomatic donors (postmortem) and 29 symptomatic donors (total knee replacement) who were matched for macroscopic tibiofemoral chondropathy. They analyzed similarities and differences in the histological features between those two groups and found that the symptomatic chondropathy group displayed greater loss of cartilage integrity and alterations in chondrocyte morphology than did the asymptomatic chondropathy group. However, the features most strongly associated with pain were synovitis (8 out of 29 symptomatic knees showed severe inflammation) and the synovial area that stained positive for the pro-algesic neurotrophin, nerve growth factor (NGF). NGF staining mostly co-localized with synovial fibroblasts but some CD68+ macrophages in the sublining of the synovium also stained for NGF. These results underscore the relationship between pain and synovitis and strongly suggest a pro-algesic role for synovial NGF. Thus, defining a role for NGF in synovitis may provide a better rationale to pursue anti-NGF therapies in subsets of patients.
Distinct risk factors drive distinct phenotypes of osteoarthritis
Symptomatic OA is a highly heterogeneous disease, comprising overlapping but distinct phenotypes that present with common clinical and pathological features [15]. A clinically relevant stratification can be based on the risk factors that underlie the disease, such as obesity, age, or joint injury. It is likely that these phenotypes may warrant different therapeutic approaches, and researchers are increasingly studying specific pathogenic pathways associated with these risk factors.
Metabolic factors that link obesity and OA
Obesity is one of the strongest predictors of OA development [16]. Weight increase alters joint loading and damages the joint, but inflammatory and metabolic characteristics of obesity affect joint health as well [17]. Unravelling the contributions of metabolic factors to OA pathogenesis independently from weight increase is a daunting task that requires innovative approaches.
Polyunsaturated fatty acids and diet
Since diet is a modifiable factor that can be studied in a clinical setting, it is of great potential interest to model its effect in animals. McNeill et al. fed C57BL/NIA mice either a calorie-restricted (CR) or an ad libitum (AL) diet for two years. They found that Mankin score, synovitis, and bone mineral density were similar between the heavier AL mice and the lighter CR mice, and concluded that dietary composition may matter more than caloric content for OA development [18]. The authors went on to test this hypothesis by feeding mice diets with different ratios of ω-6 to ω-3 polyunsaturated fatty acids (PUFAs), since this ratio is considered one of the most important dietary mediators of inflammation [19]. Four diets were compared: (1) a high-fat diet (HFD) rich in Saturated Fatty Acids (SFA); (2) an ω-6 PUFA HFD; (3) an anti-inflammatory ω-3 PUFA-HFD; and (4) a control diet. Weight increase was higher in all three HFD groups than in the controls, but development of OA after DMM surgery was very different: while ω-3 PUFAs attenuated OA and synovitis in obese mice, SFA and ω-6 PUFA independently acted as a detrimental factor. Interestingly, 12 weeks after DMM, ω-6 mice had a higher synovial macrophage score than the other groups. The study concluded that injury-induced OA was associated with dietary content but not with body weight. In contrast, Cai and co-workers reported that a life-long reduction in the ω-6:ω-3 ratio did not alter the development of idiopathic moderate knee OA in middle-aged mice [20]. They studied development of spontaneous OA in Fat-1 transgenic mice, which convert dietary ω-6 to ω-3 PUFAs endogenously. When fed an ω-6 PUFA diet for approximately a year, Fat-1 mice showed a reduction in systemic TNF-α and IL-6, but they were not protected from OA.
Collectively, these findings reinforce that distinct mechanisms may drive distinct OA phenotypes – and this must be taken into account when modeling the disease in small animals (reviewed in [21]).
Proteins related to lipid metabolism
Peroxisome proliferator-activated receptors (PPARs), PPARα, PPARγ, and PPARδ, a family of nuclear receptors activated by lipid ligands, are expressed in OA chondrocytes. PPARδ functions in lipid metabolism, apoptosis, and immune regulation. Ratneswaran and co-workers reported that PPARδ activation promotes fatty acid oxidation in chondrocytes, as well as catabolic processes [22]. Chondrocyte-specific PPARδ knock-out mice were protected from cartilage damage 8 weeks after DMM. This study was the first to interrogate the role of PPARδ in OA and offers a glimpse into the link between lipid metabolism and cartilage health.
Ageing and Osteoarthritis
Ageing is a key risk factor for OA [16] and an active area of research aims to resolve mechanisms that contribute to age-associated OA. While all tissues in the joint change with age, the majority of studies aiming to solve the link between OA and ageing focus on cartilage ageing and chondrocyte senescence (reviewed in [23, 24]).
The role of autophagy in cartilage homeostasis and ageing
Autophagy is a cytoplasmic program for the removal of damaged cellular components in conditions of oxidative stress [25]. Cellular accumulation of damaged proteins and organelles, due to defective autophagy, is one of the hallmarks of ageing [26]. Caramés and co-workers reported the first in vivo analysis of basal autophagy activation in cartilage, using GFP-LC3 reporter mice [27]. They found a reduction in number of autophagic vesicles in chondrocytes with age, and a decrease in the autophagy proteins, Atg5 and LC3. Cartilage damage, monitored up to 28 months, progressed with age and was subsequent to autophagy. This suggests that age-related decreases in autophagy in chondrocytes contribute to joint damage. The importance of autophagy for cartilage homeostasis was also demonstrated in a study by Vasheghani et al., who subjected cartilage-specific PPARγ knockout mice to DMM [28]. They found accelerated cartilage degradation, overproduction of catabolic mediators, chondrocyte apoptosis, increased expression of mammalian target for rapamycin (mTOR, a negative regulator of authophagy) and suppression of autophagy markers. Importantly, PPARγ-mTOR double knock-out rescued this phenotype.
Since autophagy is a druggable pathway [25], these findings suggest that it could be targeted in age-associated OA. Indeed, an in vivo study investigated the effect of IA administration of the mTOR inhibitor, rapamycin (twice a week for 8 weeks), on experimental OA induced by DMM [29]. Treatment markedly attenuated cartilage damage 8 and 12 weeks after surgery. This study was performed in young mice, and it will be most interesting to see if the beneficial effect can be reproduced in models of age-associated OA.
Oxidative stress is major contributor to age-associated chronic diseases, including OA
Mitochondrial dysfunction is one of the hallmarks of ageing, and impairment in the serine/threonine kinase AMPK, a master regulator of cellular energy management, contributes to this [26]. AMPK inhibits pro-catabolic responses of chondrocytes to biomechanical stress and inflammation, and its signaling decreases with age [3]. Zhao and collaborators reported that the chondroprotective effect of AMPK is at least partly mediated by 2 major downstream targets, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1) and FoxO3A [30]. PGC-1 and FoxO3A expression levels decreased in cartilage from ageing mice and mice with OA. The role of FoxO transcription factors in ageing cartilage was further addressed in two studies by Akasaki et al. [31, 32], who reported that in ageing human joints, expression of FOXO1 and FOXO3 was reduced in the superficial zone of cartilage regions exposed to maximal weight bearing. They also found that reduced expression of FoxO transcription in chondrocytes increased susceptibility to cell death by oxidative stress.
Using human cartilage from donors 24-81 years, Loeser and collaborators reported an age-related decline in proteoglycan synthesis stimulated by insulin-growth factor (IGF)-1 and IGF-1 plus osteogenic protein-1 (OP-1) [33]. Induction of oxidative stress inhibited both IGF-1- and OP-1-stimulated proteoglycan synthesis. Signaling studies showed that oxidative stress inhibited IGF-1-stimulated Akt phosphorylation while increasing phosphorylation of ERK, and that these effects were greater in cells from older donors.
Posttraumatic Osteoarthritis
Posttraumatic OA (PTOA) develops after joint injury and therefore offers the opportunity for early therapeutic intervention to prevent progressive disease. Clinical and animal studies indicate that pro-inflammatory cytokines are elevated after joint injury. Hence the role of local and systemic inflammation in the development of PTOA was tested in a model where closed intra-articular fracture in the lateral tibial plateau of the mouse knee results in OA [34]. Two treatment protocols were compared: either a single IA injection of IL-1RA (anakinra) or sTNFRII (etanercept) or systemic administration of IL-1RA or sTNFRII for 4 weeks after fracture. Interestingly, only IA inhibition of IL-1 significantly prevented cartilage degeneration and synovial inflammation. These data suggest that IL-1, rather than TNF-α, may be critical in the acute phase following joint injury and that acute treatment with local IL1RA may be able to prevent cartilage degeneration and synovitis in PTOA.
New targets for osteoarthritis pain
Clinical OA is defined by the presence of symptoms that drive the sufferer to seek medical help. Pain is the major symptom of OA, and current evidence supports the idea that it is generated through continuous nociceptive peripheral input from the OA joint (reviewed in [35]). Therefore, researchers are exploring novel analgesic targets in the joint and in the peripheral nervous system.
Nerve growth factor
Since initial clinical trial results with antibodies against NGF were very encouraging, in spite of the occurrence of poorly understood side effects (reviewed in [36]), several companies are targeting NGF for OA pain (either through antibodies or through blockade of its receptor, TrkA), thereby encouraging basic research in this area. As discussed above, synoviocytes are a source of NGF and synovial expression of this neurotrophin was found to be associated with pain in human knee OA [14]. Cartilage, however, can also be a source of NGF and two groups reported on stimuli that can induce NGF in chondrocytes, supporting the concept that cartilage, while aneural, may indirectly generate pain: IL-1β and visfatin induced NGF in murine and human chondrocytes [37] and TGF-β induced NGF mRNA in bovine and human chondrocytes in an ALK5/Smad2/3-dependent manner [38]. The latter study adds a new layer to the complex pathogenic role of TGF-β in OA pathogenesis. A first study investigating the effect of NGF blockade in experimental OA was reported [39]. A single dose of anti-NGF antibody exerted a long-lasting beneficial effect on pain during motion, as assessed by monitoring gait, in the rat mono-iodoacetate (MIA) model, while there was no effect on macroscopic joint lesions. Histology of the joints was not performed in this study.
Novel targets under investigation in preclinical OA models
Several interesting studies explored putative novel targets for OA pain in relevant models. One study uncovered a role for the neuropeptide, calcitonin gene-related peptide (CGRP), in peripheral neuronal sensitization (a hallmark of pain) in both the rat MIA and the rat medial meniscal tear (MMT) model [40]. Researchers at Eli Lilly tested the analgesic effect of a newly developed antibody against CGRP in the same two models and found that CGRP blockade led to a dose-dependent and prolonged pain reduction, as measured by weight-bearing asymmetry [41].
Experiments using in vivo electrophysiology to assess the effects of selective antagonists of neuronal sodium voltage-gated channels, Nav1.7- and Nav1.8, in the rat MIA model suggest that these channels play a pivotal role in nociceptive pathways in this model [42]. These channels merit further exploration as potential targets for OA pain [43].
Since the translational utility of different experimental OA models for mimicking OA pain is currently poorly understood [21], it can be expected that more in-depth analysis of these novel targets in different model systems will enhance our understanding of their pathogenic role in OA-related pain.
Conclusion
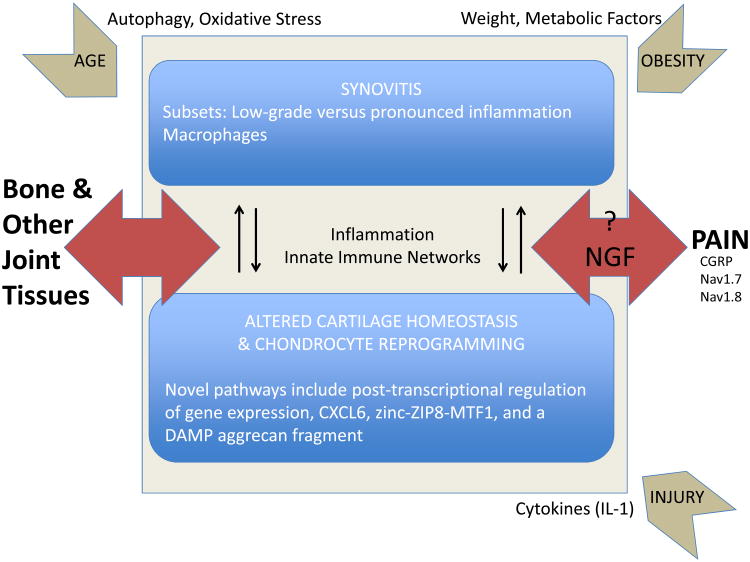
Within the general concept that OA represents a failure of the joint as an organ [1], research in the past year has focused on a multitude of pro-inflammatory and pro-catabolic pathways that initiate and maintain dramatic changes in chondrocyte phenotype, cartilage homeostasis, and in the synovium (Fig. 1). The integration of the different pathways results in complex networks that drive disease progression, affect other articular tissues such as the subchondral bone, and promote pain. These networks may be modulated by factors such as obesity, diet, age, and joint injury. This general picture provides great complexity, but also offers a tremendous opportunity for targeted intervention in distinct but overlapping subsets of OA.
Fig. 1.
Research in the past year has focused on a multitude of pro-inflammatory and pro-catabolic pathways that initiate and maintain dramatic changes in chondrocyte phenotype, cartilage homeostasis, and in the synovium. The integration of the different pathways results in complex networks that drive disease progression, affect other articular tissues such as the subchondral bone, and promote pain. These networks may be modulated by factors such as obesity, diet, age, and joint injury.
Acknowledgments
The author acknowledges funding by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR064251 and R01AR060364).
Footnotes
Author contribution: Anne-Marie Malfait searched the literature, summarized the findings, and wrote the manuscript.
Conflict of interests: The author has no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol. 2013;9(8):485–497. doi: 10.1038/nrrheum.2013.72. [DOI] [PubMed] [Google Scholar]
- 3.Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tew SR, McDermott BT, Fentem RB, Peffers MJ, Clegg PD. Transcriptome-wide analysis of messenger RNA decay in normal and osteoarthritic human articular chondrocytes. Arthritis Rheumatol. 2014;66(11):3052–3061. doi: 10.1002/art.38849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherwood J, Bertrand J, Nalesso G, Poulet B, Pitsillides A, Brandolini L, et al. A homeostatic function of CXCR2 signalling in articular cartilage. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Lees S, Golub SB, Last K, Zeng W, Jackson DC, Sutton P, Fosang AJ. Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol. 2015;67(5):1240–1249. doi: 10.1002/art.39063. [DOI] [PubMed] [Google Scholar]
- 9.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64(5):1466–1476. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
- 11.Schelbergen RF, van Dalen S, ter Huurne M, Roth J, Vogl T, Noel D, et al. Treatment efficacy of adipose-derived stem cells in experimental osteoarthritis is driven by high synovial activation and reflected by S100A8/A9 serum levels. Osteoarthritis Cartilage. 2014;22(8):1158–1166. doi: 10.1016/j.joca.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Nasi S, Ea HK, Chobaz V, van Lent P, Liote F, So A, Busso N. Dispensable role of myeloid differentiation primary response gene 88 (MyD88) and MyD88-dependent toll-like receptors (TLRs) in a murine model of osteoarthritis. Joint Bone Spine. 2014;81(4):320–324. doi: 10.1016/j.jbspin.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Jackson MT, Moradi B, Zaki S, Smith MM, McCracken S, Smith SM, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis Rheumatol. 2014;66(12):3337–3348. doi: 10.1002/art.38876. [DOI] [PubMed] [Google Scholar]
- 14.Stoppiello LA, Mapp PI, Wilson D, Hill R, Scammell BE, Walsh DA. Structural associations of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2014;66(11):3018–3027. doi: 10.1002/art.38778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, Hochberg MC. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19(5):478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54(4):588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 18.McNeill JN, Wu CL, Rabey KN, Schmitt D, Guilak F. Life-long caloric restriction does not alter the severity of age-related osteoarthritis. Age (Dordr) 2014;36(4):9669. doi: 10.1007/s11357-014-9669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CL, Jain D, McNeill JN, Little D, Anderson JA, Huebner JL, et al. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai A, Hutchison E, Hudson J, Kawashima Y, Komori N, Singh A, et al. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthritis Cartilage. 2014;22(9):1301–1309. doi: 10.1016/j.joca.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malfait AM, Little CB. On the Predicitve Utility of Animal Models of Osteoarthritis. Arthritis Research Therapy. 2015 doi: 10.1186/s13075-015-0747-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratneswaran A, LeBlanc EA, Walser E, Welch I, Mort JS, Borradaile N, Beier F. Peroxisome proliferator-activated receptor delta promotes the progression of posttraumatic osteoarthritis in a mouse model. Arthritis Rheumatol. 2015;67(2):454–464. doi: 10.1002/art.38915. [DOI] [PubMed] [Google Scholar]
- 23.Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108–113. doi: 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mobasheri A, Matta C, Zakany R, Musumeci G. Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas. 2015;80(3):237–244. doi: 10.1016/j.maturitas.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125(1):1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carames B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67(6):1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasheghani F, Zhang Y, Li YH, Blati M, Fahmi H, Lussier B, et al. PPARgamma deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann Rheum Dis. 2015;74(3):569–578. doi: 10.1136/annrheumdis-2014-205743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama K, Kawakami Y, Kobayashi M, Greco N, Cummins JH, Matsushita T, et al. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res Ther. 2014;16(6):482. doi: 10.1186/s13075-014-0482-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Petursson F, Viollet B, Lotz M, Terkeltaub R, Liu-Bryan R. Peroxisome proliferator-activated receptor gamma coactivator 1alpha and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014;66(11):3073–3082. doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK. FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 2014;66(12):3349–3358. doi: 10.1002/art.38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz MK. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage. 2014;22(1):162–170. doi: 10.1016/j.joca.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S. Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol. 2014;66(8):2201–2209. doi: 10.1002/art.38641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16(3):R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malfait AM, Schnitzer T. Toward a mechanism-based approach of pain management in osteoarthritis. Nature Reviews Rheumatology. 2013;9:654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage. 2015;23 Suppl 1:S8–17. doi: 10.1016/j.joca.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Pecchi E, Priam S, Gosset M, Pigenet A, Sudre L, Laiguillon MC, et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res Ther. 2014;16(1):R16. doi: 10.1186/ar4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaney Davidson EN, van Caam AP, Vitters EL, Bennink MB, Thijssen E, van den Berg WB, et al. TGF-beta is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis Cartilage. 2015;23(3):478–486. doi: 10.1016/j.joca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa G, Koya Y, Tanaka H, Nagakura Y. Long-term analgesic effect of a single dose of anti-NGF antibody on pain during motion without notable suppression of joint edema and lesion in a rat model of osteoarthritis. Osteoarthritis Cartilage. 2015;23(6):925–932. doi: 10.1016/j.joca.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014;66(8):2188–2200. doi: 10.1002/art.38656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22(4):578–585. doi: 10.1016/j.joca.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Rahman W, Dickenson AH. Osteoarthritis-dependent changes in antinociceptive action of Nav1.7 and Nav1.8 sodium channel blockers: An in vivo electrophysiological study in the rat. Neuroscience. 2015;295:103–116. doi: 10.1016/j.neuroscience.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman W, Dickenson AH. Emerging targets and therapeutic approaches for the treatment of osteoarthritis pain. Curr Opin Support Palliat Care. 2015;9(2):124–130. doi: 10.1097/SPC.0000000000000125. [DOI] [PubMed] [Google Scholar]