Abstract
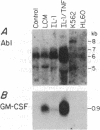
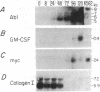
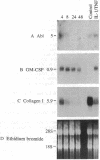
Analysis of protooncogene RNA expression in marrow stromal cells from long-term marrow culture demonstrated high levels of c-abl 5-, 6-, and 7-kilobase (kb) RNA transcripts. In experiments on three independently derived simian virus 40-transformed marrow stromal cell lines, the expression of these c-abl transcripts was further increased in response to recombinant tumor necrosis factor alpha (1000 units/ml) and interleukin 1 alpha (10 units/ml). Although lymphocyte-conditioned medium predominantly up-regulated the 5-kb transcript, interleukin 1 alpha primarily affected the 6-kb transcript. The up-regulation of the 5-kb c-abl message correlated with up-regulation of the granulocyte/macrophage colony-stimulating factor transcript and down-regulation of procollagen I transcripts in transformed cells. These data suggest that c-abl plays roles in the regulation of extracellular matrix expression and in the regulation of hematopoietic growth factors by stromal cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. F., 3rd, Collins S. J. Heterogeneity in expression of the bcr-abl fusion transcript in CML blast crisis. Leukemia. 1987 Oct;1(10):718–724. [PubMed] [Google Scholar]
- Andrews D. F., 3rd, Nemunaitis J., Tompkins C., Singer J. W. Effect of 5-azacytidine on gene expression in marrow stromal cells. Mol Cell Biol. 1989 Jun;9(6):2748–2751. doi: 10.1128/mcb.9.6.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anklesaria P., Kase K., Glowacki J., Holland C. A., Sakakeeny M. A., Wright J. A., FitzGerald T. J., Lee C. Y., Greenberger J. S. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Rubin C. M., Westbrook C. A., Paskind M., Baltimore D. The first intron in the human c-abl gene is at least 200 kilobases long and is a target for translocations in chronic myelogenous leukemia. Mol Cell Biol. 1987 Sep;7(9):3231–3236. doi: 10.1128/mcb.7.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell M. A., Anderson D., Cerretti D. P., Price V., McKereghan K., Tushinski R. J., Mochizuki D. Y., Larsen A., Grabstein K., Gillis S. Cloning, sequence, and expression of a human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6250–6254. doi: 10.1073/pnas.82.18.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Slamon D. J., Nimer S. D., Golde D. W., Gasson J. C. Regulation of expression of human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8669–8673. doi: 10.1073/pnas.83.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Coleman H., Groudine M. Expression of bcr and bcr-abl fusion transcripts in normal and leukemic cells. Mol Cell Biol. 1987 Aug;7(8):2870–2876. doi: 10.1128/mcb.7.8.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Konopka J. B., Witte O. N. Activation of the c-abl oncogene by viral transduction or chromosomal translocation generates altered c-abl proteins with similar in vitro kinase properties. Mol Cell Biol. 1985 Jan;5(1):204–213. doi: 10.1128/mcb.5.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. G., Sieff C. A., Gross R. G., Rozans M. K., Miller R. A., Rappeport J. M., Nathan D. G. Decreased hematopoietic accessory cell function following bone marrow transplantation. Exp Hematol. 1987 Nov;15(10):1013–1021. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fibbe W. E., van Damme J., Billiau A., Goselink H. M., Voogt P. J., van Eeden G., Ralph P., Altrock B. W., Falkenburg J. H. Interleukin 1 induces human marrow stromal cells in long-term culture to produce granulocyte colony-stimulating factor and macrophage colony-stimulating factor. Blood. 1988 Feb;71(2):430–435. [PubMed] [Google Scholar]
- Gillery P., Coustry F., Pujol J. P., Borel J. P. Inhibition of collagen synthesis by interleukin-1 in three-dimensional collagen lattice cultures of fibroblasts. Experientia. 1989 Jan 15;45(1):98–101. doi: 10.1007/BF01990461. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. The human v-abl cellular homologue. J Mol Appl Genet. 1983;2(1):57–68. [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. M., Mardon G., Bishop J. M., Varmus H. E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988 Jun;8(6):2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Lin N., Adamson J. W. Interleukin 1 stimulates fibroblasts to synthesize granulocyte-macrophage and granulocyte colony-stimulating factors. Mechanism for the hematopoietic response to inflammation. J Clin Invest. 1988 Jan;81(1):92–97. doi: 10.1172/JCI113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Activation of the abl oncogene in murine and human leukemias. Biochim Biophys Acta. 1985 Nov 12;823(1):1–17. doi: 10.1016/0304-419x(85)90012-5. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lin J. X., Vilcek J. Tumor necrosis factor and interleukin-1 cause a rapid and transient stimulation of c-fos and c-myc mRNA levels in human fibroblasts. J Biol Chem. 1987 Sep 5;262(25):11908–11911. [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Recombinants within the tyrosine kinase region of v-abl and v-src identify a v-abl segment that confers lymphoid specificity. Mol Cell Biol. 1988 Jan;8(1):234–240. doi: 10.1128/mcb.8.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D., Hermans A., von Lindern M., van Agthoven T., de Klein A., Mackenbach P., Grootegoed A., Talarico D., Della Valle G., Grosveld G. Molecular characterization of the testis specific c-abl mRNA in mouse. EMBO J. 1987 Dec 20;6(13):4041–4048. doi: 10.1002/j.1460-2075.1987.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munker R., Gasson J., Ogawa M., Koeffler H. P. Recombinant human TNF induces production of granulocyte-monocyte colony-stimulating factor. Nature. 1986 Sep 4;323(6083):79–82. doi: 10.1038/323079a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J., Andrews D. F., Crittenden C., Kaushansky K., Singer J. W. Response of simian virus 40 (SV40)-transformed, cultured human marrow stromal cells to hematopoietic growth factors. J Clin Invest. 1989 Feb;83(2):593–601. doi: 10.1172/JCI113922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppi C., Shore S. K., Reddy E. P. Nucleotide sequence of testis-derived c-abl cDNAs: implications for testis-specific transcription and abl oncogene activation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8200–8204. doi: 10.1073/pnas.84.23.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C., Wolgemuth D. J. Haploid expression of a unique c-abl transcript in the mouse male germ line. Mol Cell Biol. 1985 Jul;5(7):1791–1794. doi: 10.1128/mcb.5.7.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M. W., Capozza M. A., Wang J. Y. Differential expression of type-specific c-abl mRNAs in mouse tissues and cell lines. Mol Cell Biol. 1988 Oct;8(10):4547–4551. doi: 10.1128/mcb.8.10.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rovera G., Valtieri M., Mavilio F., Reddy E. P. Effect of Abelson murine leukemia virus on granulocytic differentiation and interleukin-3 dependence of a murine progenitor cell line. Oncogene. 1987 Mar;1(1):29–35. [PubMed] [Google Scholar]
- Sandmeyer S., Gallis B., Bornstein P. Coordinate transcriptional regulation of type I procollagen genes by Rous sarcoma virus. J Biol Chem. 1981 May 25;256(10):5022–5028. [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Savin K. W., Adams F. C., Devereux L. M., Jose D. G., de Kretser T. A. Two families of abl-related transcripts in human haematopoietic cells differing in their homology to v-abl. Mol Biol Med. 1984 Dec;2(6):397–409. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Roe B. A., Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986 Oct 24;47(2):277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- Sieff C. A., Niemeyer C. M., Mentzer S. J., Faller D. V. Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells. Blood. 1988 Oct;72(4):1316–1323. [PubMed] [Google Scholar]
- Singer J. W., Charbord P., Keating A., Nemunaitis J., Raugi G., Wight T. N., Lopez J. A., Roth G. J., Dow L. W., Fialkow P. J. Simian virus 40-transformed adherent cells from human long-term marrow cultures: cloned cell lines produce cells with stromal and hematopoietic characteristics. Blood. 1987 Aug;70(2):464–474. [PubMed] [Google Scholar]
- Song Z. X., Shadduck R. K., Innes D. J., Jr, Waheed A., Quesenberry P. J. Hematopoietic factor production by a cell line (TC-1) derived from adherent murine marrow cells. Blood. 1985 Aug;66(2):273–281. [PubMed] [Google Scholar]
- Takahashi M., Yamada H., Bean M. A., Singer J. W. Helper (OKT4) T cells from human T cell colonies produce potent colony-stimulating activity. Int J Cell Cloning. 1986 Nov;4(6):406–414. doi: 10.1002/stem.5530040602. [DOI] [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Tobler A., Johnston D., Koeffler H. P. Recombinant human tumor necrosis factor alpha regulates c-myc expression in HL-60 cells at the level of transcription. Blood. 1987 Jul;70(1):200–205. [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Cellular RNA homologous to the Abelson murine leukemia virus transforming gene: expression and relationship to the viral sequence. Mol Cell Biol. 1983 May;3(5):773–779. doi: 10.1128/mcb.3.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Wagner K., Griffin J. D. Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in acute myeloblastic leukemia. J Clin Invest. 1987 Jan;79(1):100–106. doi: 10.1172/JCI112769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]