Abstract
Thrombotic events while receiving anti-platelet agents (APA) are more common in individuals with vs. without chronic kidney disease (CKD). Data on anti-platelet effects of APA in CKD are scarce and limited by lack of baseline platelet function before APA treatment. We hypothesized individuals with stages 4–5 CKD vs. no CKD have higher baseline platelet aggregability and respond poorly to aspirin and clopidogrel. In a prospective controlled study, we measured whole blood platelet aggregation (WBPA) in 28 CKD and 16 non-CKD asymptomatic stable outpatients not on dual APA, frequency-matched for age, gender, obesity and diabetes mellitus. WBPA was re-measured after 2 weeks of each aspirin and aspirin plus clopidogrel. The primary outcome was percent inhibition of platelet aggregation (IPA) from baseline. The secondary outcome was residual platelet aggregability (RPA) (proportion with <50% IPA). Baseline platelet aggregability was similar between groups except adenosine diphosphate (ADP)-induced WBPA, which was higher in CKD vs. non-CKD; median (IQR) =13.5 (9.5, 16.0) vs. 9.0 (6.0, 12.0) Ω, p=0.007. CKD vs. non-CKD participants had lower clopidogrel-induced IPA, 38% vs. 72%, p=0.04. A higher proportion of CKD vs. non-CKD participants had RPA after clopidogrel treatment (56% vs. 8.3%, p=0.01). There were no significant interactions between CKD and presence of CYP2C19 polymorphisms for platelet aggregability in clopidogrel-treated participants. In conclusion, CKD vs. non-CKD individuals exhibited similar platelet aggregation at baseline, similar aspirin effects and higher residual platelet aggregability on clopidogrel, which was independent of CYP2C19 polymorphisms.
Keywords: platelets, aspirin, kidney, thrombosis
Chronic kidney disease (CKD) patients are at higher risk for thrombotic cardiovascular events as compared to those without CKD.1, 2 Although there are several factors explaining increased thrombotic events in CKD patients, poor efficacy of antiplatelet agents (APA) is a well-known risk factor for increased thrombotic events.2, 3 However, previous studies reporting presence of residual platelet aggregability (RPA) were limited by the use of less physiological techniques, such as optical aggregometry, to measure platelet function,4–7 and the inclusion of primarily End Stage Renal Disease (ESRD) and not non-dialysis CKD patients,8, 9 where earlier intervention for secondary cardiovascular prevention may be more efficacious. These studies also suffered from failure to measure before and after-treatment effects of APA on platelet aggregability and lacked non-CKD controls.4, 6 Surprisingly, there are little data on how baseline platelet function may differ in stable CKD vs. non-CKD individuals.10 It is also unclear whether there are differences in platelet aggregation in response to APA based on the presence or absence of CKD11 or as related to the presence of cytochrome P450 polymorphisms such as cytochrome P450 2C19 (CYP2C19), a major drug metabolic enzyme involved in the conversion of ingested clopidogrel pro-drug into its active metabolite.12
METHODS
We conducted the Whole Blood Platelet Aggregation in Chronic Kidney Disease on Aspirin (WICKD onASA) study, an open-label prospective clinical trial, to compare the degree of platelet inhibition from baseline by aspirin (81 mg/d) and by a combination of aspirin (81 mg/d) and clopidorgrel (75 mg/d) in a frequency-matched cohort of asymptomatic non-dialysis CKD patients and controls without CKD. We hypothesized that as compared to those without kidney disease, those with CKD: 1) have higher platelet aggregability at baseline as measured by ex vivo whole blood impedance platelet aggregation; and 2) have a reduced response to aspirin and clopidogrel, as measured by lower inhibition of platelet aggregation (IPA) from baseline and higher RPA. Finally, we explored whether there was an interaction between CKD and presence of CYP2C19 polymorphisms for platelet aggregability in clopidogrel-treated patients. Institutional Review Board approval was obtained. Adults ≥ 21 years old were recruited from outpatient clinics at Parkland, Dallas Veterans Affairs, and the University of Texas Southwestern (UTSW) Medical Centers in Dallas, Texas from December 10, 2012 to January 31, 2014. Non-CKD participants were included if there was no evidence of kidney disease as defined by estimated glomerular filtration rate (eGFR) ≥90 mL/min/1.73 m2, urine albumin-to-creatinine ratio (UACR) <30 mg/g, and no detectable kidney disease by other methods defined by National Kidney Foundation guidelines.13 CKD patients were those with non-dialysis CKD stages 4–5, with an eGFR of <30 mL/min/1.73 m2 for at least 3 months, calculated.13 Exclusion criteria were similar to those used in previous platelet studies:4 presence of ESRD, kidney transplantation, hospitalization or surgery within 3 months, cardiovascular event within 12 months, surgery within 3 months, history of blood dyscrasias, active bleeding or bleeding diathesis, gastrointestinal bleeding in the last 6 months, active malignancy or liver disease, ongoing use of APA or anti-thrombotic drugs, <30 days treatment with a glycoprotein IIb/IIIa antagonist, hematocrit <25%, white blood cell count >20,000/μL, or platelet count <50,000/μL. Patients who were taking aspirin for primary cardiovascular prevention were included but asked to discontinue use for at least 2 weeks prior to baseline measurements. The 2 groups were frequency-matched for decade of age,14 gender,15 presence of diabetes4 and each 5 kg/m2 of body mass index (BMI),16 as these characteristics were reported to influence platelet function.
On visit 1, demographic, clinical, and medication use data were collected. Data on the following variables were obtained: 1) diabetes mellitus (defined as glycosylated hemoglobin level ≥6.3%, fasting blood glucose level ≥126 mg/dL, or use of hypoglycemic agents); 2) hyperlipidemia (use of anti-lipid medications, total cholesterol ≥240 mg/dL or serum low density lipoprotein (LDL) ≥160 mg/dL), current smoking (any cigarette smoking in the past 30 days); and 3) hypertension (an average of 4 office BP readings ≥140/90 mm Hg and/or use of antihypertensive medication). Dietary supplements were discontinued for 2 weeks prior to the study.4, 17 Patients were also asked to not smoke18 or eat foods known to affect platelet function (coffee, chocolate, grapes, and alcohol) 48 hours prior to every sample collection.17 Diet and physical activity were assessed at every visit by the administration of the Modified 24-hour Dietary Recall and the Stanford 7-day Physical Activity Recall interviewer-administered questionnaires.16, 17 Venipuncture was also performed at each visit for complete blood count, WBPA measurements and adenosine triphosphate (ATP) secretions. Patients were dispensed chewable aspirin 81 mg/d. After 2 weeks, patients returned for Visit 2. WBPA and ATP secretion measurements were repeated. Two buccal swabs were collected for genotype analysis. Aspirin 81 mg/d and clopidogrel 75 mg/d were dispensed. Patients returned in 2 weeks for Visit 3. WBPA induced by ADP was measured to analyze clopidogrel effect. Patients were asked to take APA on the morning of venipuncture so that everyone received phlebotomy within 3–4 hours of pill intake. Pill counts were performed for adherence.
The primary outcome measure was the inhibition of platelet aggregation (IPA), defined as follows:5, 7 %IPA = (baseline aggregation) - (post-treatment aggregation)/(baseline aggregation). The secondary outcome measure was RPA, defined as the proportion with <50% inhibition of aggregation from baseline.5, 10 WBPA was measured by ex vivo whole blood impedance platelet aggregometry (Chrono-log Corporation, Havertown, PA 19083, USA).19 ATP secretion was measured using the firefly luciferin-luciferase reaction (Chrono-log, Havertown, PA 19083, USA). Genotyping of all CYP2C19 alleles was performed from buccal swabs using real-time polymerase chain reaction (Harmonyx Diagnostics Inc. Cordova, TN, USA).12 Major alleles, CYP2C19*2 and CYP2C19*17 genotypes were classified based on the standardized metabolic phenotypes.12 For the purposes of testing 2-way interactions with CKD, intermediate (CYP2C19*1/*2 and CYP2C19*2/*17) and poor (CYP2C19*2/*2) metabolic groups were collapsed into one category as poor metabolic phenotype.12
For sample size calculations, we used published data on healthy volunteers as historical controls17 and unpublished pilot data from the Chronic Kidney Disease Antidepressant Sertraline Trial for CKD patients. 20 From log-transformed pilot data (non-Gaussian), a coefficient of variation of 0.84 (SD/Mean= 1.35/1.6 in log units) was calculated. With a sample of 30 patients (20 CKD and 10 non-CKD) and a two-sided α of 0.05, the study would have 84% power to detect at least a 4 Ω between-group difference in the geometric means of WBPA induced by 0.5 mM AA at baseline or on-treatment with APA. Student’s t-test or Wilcoxon rank sum test was used to compare continuous and Fisher’s Exact to compare categorical variables among CKD vs. non-CKD groups. Mixed model repeated measures (MMRM) analyses were used to compare WBPA induced by 0.5 mM AA, 2 μg/mL collagen and ADP responses (change) to aspirin and dual therapy between groups.21 The model had group factor, treatment factor (repeated, pre- vs. post-), and interaction between group and treatment as covariates; the participant was modeled as a random effect. RPA was compared between-groups using the Fisher’s Exact test. Proportions of metabolic phenotypes of CYP2C19 polymorphisms were analyzed between groups based on the presence of CKD and compared using Fisher’s Exact test. ANOVA was used to test any 2-way interactions of poor (vs. normal) metabolic phenotype X CKD and rapid (vs. normal) metabolic phenotype X CKD on IPA after clopidogrel treatment. All available data were included in the analyses; the mixed model can accommodate cases with incomplete data. Analyses were performed with SAS version 9.3 (SAS Institute, NC).
RESULTS
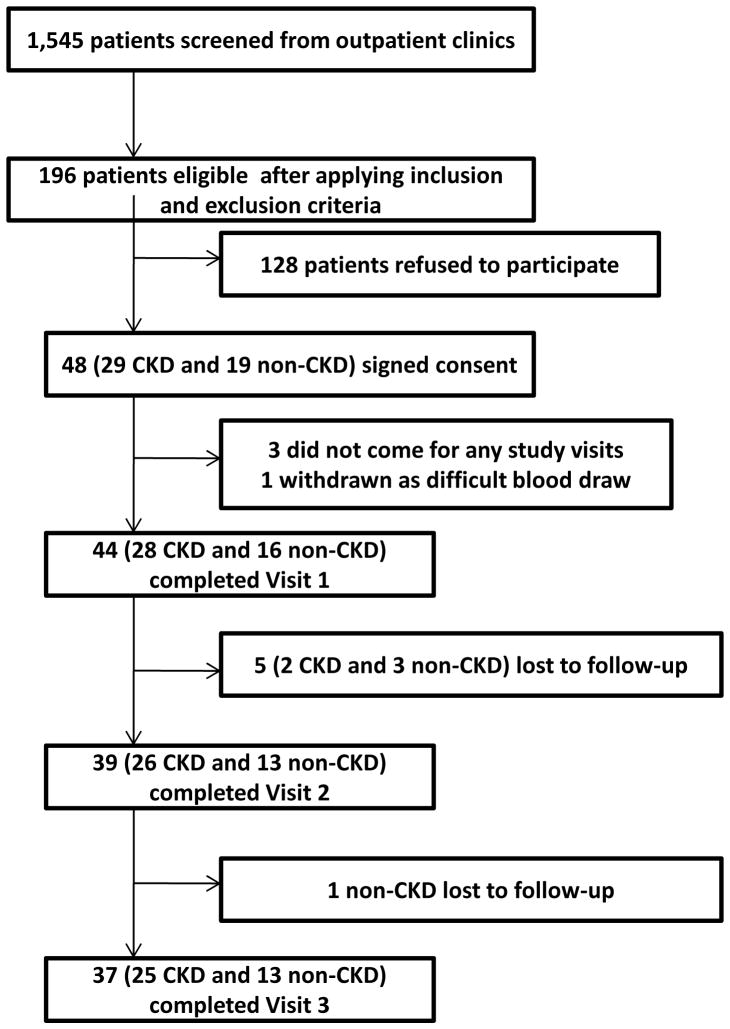
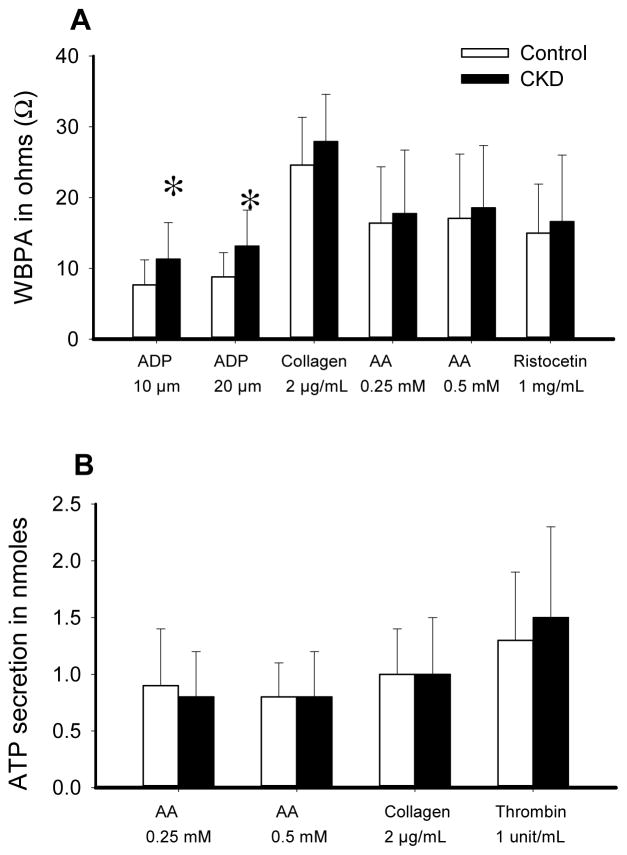
Figure 1 shows the exclusionary cascade for derivation of the cohorts. Baseline characteristics, based on presence or absence of CKD, are listed in Table 1. A higher proportion of participants with CKD vs. no CKD reported use of beta-blockers and statins (Table 1). Those with CKD compared to no CKD had a lower mean hemoglobin concentration and higher glycosylated hemoglobin, serum uric acid, phosphorus, and intact parathyroid hormone levels (Table 1). There were no between-group differences in the baseline values for ATP secretions and WBPA except when induced by ADP (Table 2 and Figure 2).
Figure 1.
Exclusionary cascade for derivation of sample. CKD, Chronic Kidney Disease.
Table 1.
Baseline Characteristics of the Cohort
| Chronic Kidney Disease | |||
|---|---|---|---|
| Variable | No (n =16) | Yes (n =28) | p-value |
| Age (years) | 49 ± 11 | 52 ± 10 | 0.48* |
|
| |||
| BMI (kg/m2) | 29.8 ± 4.9 | 32.0 ± 5.5 | 0.19* |
|
| |||
| Woman | 8 (50%) | 12 (43%) | 0.65 |
|
| |||
| Black | 4 (25%) | 15 (54%) | 0.06 |
|
| |||
| Diabetes mellitus | 7 (44%) | 13 (46%) | 0.86 |
|
| |||
| Proton pump inhibitor use | 1 (6%) | 1 (4%) | 1.00 |
|
| |||
| Beta blocker use | 0 | 21 (75%) | <0.0001 |
|
| |||
| Statin use | 5 (31%) | 19 (68%) | 0.02 |
|
| |||
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker use | 5 (31%) | 12 (43%) | 0.45 |
|
| |||
| Allopurinol use | 0 | 6 (21%) | 0.07 |
|
| |||
| Baseline use of aspirin | 4 (25%) | 12 (43%) | 0.24 |
|
| |||
| eGFR (mL/min/1.73 m2) | 101 ± 15 | 17 ± 7 | <0.0001* |
|
| |||
| Urine albumin-to-creatinine ratio (mg/g) | 5 (2.5–8.8) | 1,282 (143–1,848) | <0.0001** |
|
| |||
| Hemoglobin (g/dL) | 14.4 ± 1.7 | 11.2 ± 1.7 | <0.0001* |
|
| |||
| Hematocrit (%) | 42.6 ± 4.2 | 33.9 ± 5.1 | <0.0001* |
|
| |||
| White blood cell count (K per μL) | 6 ± 2 | 8 ± 2 | 0.06* |
|
| |||
| Platelet count (K per μL) | 217 ±55 | 232 ± 55 | 0.43* |
|
| |||
| Serum total cholesterol (mg/dL) | 180 ± 33 | 170 ± 42 | 0.46* |
|
| |||
| Serum triglycerides (mg/dL) | 112 (55–211) | 124 (104–161) | 0.44** |
|
| |||
| Serum low density lipoprotein (mg/dL) | 106 (70–124) | 94 (61–118) | 0.38** |
|
| |||
| Serum total bilirubin (mg/dL) | 0.6 (0.4–0.7) | 0.3 (0.2–0.5) | 0.02** |
|
| |||
| Glycosylated hemoglobin (%) | 6.4 ± 0.4 | 6.8 ± 0.4 | <0.0001* |
|
| |||
| Serum albumin (g/dL) | 4.2 ± 0.4 | 4.0 ± 0.5 | 0.13* |
|
| |||
| Serum uric acid (mg/dL) | 5.1 ± 1.8 | 8.9 ± 2.0 | <0.0001* |
|
| |||
| Serum calcium (mg/dL) | 9.3 ± 0.3 | 9.1 ± 0.8 | 0.31* |
|
| |||
| Serum phosphorus (mg/dL) | 3.3 ± 0.3 | 4.6 ± 1.1 | <0.0001** |
|
| |||
| Serum parathyroid hormone (pg/mL) | 42 (34–46) | 197 (143–358) | <0.0001** |
|
| |||
| Serum 25-hydroxyvitamin D (ng/mL) | 29 ± 15 | 28 ± 14 | 0.80* |
Student’s t-test used for comparison
Wilcoxon rank sum test used for comparison
Table 2.
Whole Blood Platelet Aggregation and Adenosine Triphosphate Secretion at Baseline, on Aspirin and on Aspirin/Clopidogrel
| Chronic kidney disease | |||
|---|---|---|---|
| Profile | No | Yes | p-value |
|
|
|||
| Visit 1: Baseline | |||
|
| |||
| Aggregation to 0.5 mM arachidonic acid (Ω) | 18.0 (10.0, 20.0) | 21.0 (10.5, 24.5) | 0.27 |
|
| |||
| Aggregation to 0.25 mM arachidonic acid (Ω) | 17.0 (9.0, 21.0) | 19.5 (12.0, 22.0) | 0.43 |
|
| |||
| Aggregation to 20 μM adenosine diphosphate (Ω) | 9.0 (6.0, 12.0) | 13.5 (9.5, 16.0) | 0.007* |
|
| |||
| Aggregation to 10 μM adenosine diphosphate (Ω) | 8.0 (5.0, 11.0) | 12.5 (8.0, 14.5) | 0.02* |
|
| |||
| Aggregation to 2 μg/mL collagen (Ω) | 25.0 (20.0, 30.0) | 28.5 (23.5, 31.5) | 0.21 |
|
| |||
| Aggregation to 1 mg/mL ristocetin (Ω) | 14.0 (9.0, 22.0) | 14.0 (9.5, 20.0) | 0.92 |
|
| |||
| Secretion to 0.5 mM arachidonic acid (nmoles) | 0.81 (0.65, 0.98) | 0.67 (0.52, 1.05) | 0.79 |
|
| |||
| Secretion to 0.25 mM arachidonic acid (nmoles) | 0.92 (0.60, 1.33) | 0.75 (0.5, 0.94) | 0.24 |
|
| |||
| Secretion to 2 μg/mL collagen (nmoles) | 1.05 (0.79, 1.28) | 0.92 (0.62, 1.32) | 0.72 |
|
| |||
| Secretion to 1 unit/mL thrombin (nmoles) | 1.28 (0.82, 1.64) | 1.32 (0.93, 1.84) | 0.54 |
|
| |||
| Visit 2: On Aspirin | |||
|
| |||
| Aggregation to 0.5 mM arachidonic acid (Ω) | 0 | 0 | 0.67 |
|
| |||
| Aggregation to 0.25 mM arachidonic acid (Ω) | 0 | 0 | 0.50 |
|
| |||
| Aggregation to 20 μM adenosine diphosphate (Ω) | 10.0 (8.0, 11.0) | 11.0 (8.0, 16.0) | 0.19 |
|
| |||
| Aggregation to 10 μM adenosine diphosphate (Ω) | 9.0 (7.0, 11.0) | 10.5 (8.0, 14.0) | 0.14 |
|
| |||
| Aggregation to 2 μg/mL collagen (Ω) | 19.0 (16.0, 22.0) | 19.5 (16.0, 26.0) | 0.72 |
|
| |||
| Aggregation to 1 mg/mL ristocetin (Ω) | 6.0 (4.0, 6.0) | 7.5 (6.0, 12.0) | 0.04 |
|
| |||
| Secretion to 0.5 mM arachidonic acid (nmoles) | 0 | 0 (0, 0.15) | 0.03* |
|
| |||
| Secretion to 0.25 mM arachidonic acid (nmoles) | 0 (0, 0.1) | 0 (0, 0.11) | 1.0 |
|
| |||
| Secretion to 2 μg/mL collagen (nmoles) | 0.48 (0.4, 0.61) | 0.59 (0.4, 0.83) | 0.20 |
|
| |||
| Secretion to 1 unit/mL thrombin (nmoles) | 0.82 (0.77, 1.55) | 1.32 (1.09, 1.47) | 0.21 |
|
| |||
| Visit 3: On Aspirin/Clopidogrel | |||
|
| |||
| Aggregation to 20 μM adenosine diphosphate (Ω) | 3.0 (0, 4.0) | 8.0 (1.0, 12.0) | 0.04* |
|
| |||
| Aggregation to 10 μM adenosine diphosphate (Ω) | 1.0 (0, 3.0) | 7.0 (1.0, 12.0) | 0.02* |
Denotes statistically significant p-values for Wilcoxon rank sum test comparing CKD yes vs. no
Figure 2.
A. Whole blood platelet aggregation (WBPA) induced by various agonists at baseline in chronic kidney disease (CKD) participants (closed bars) vs. non-CKD controls (open bars), expressed in ohms: 10 μM adenosine diphosphate (ADP10), 20 μM adenosine diphosphate (ADP20), 2 μg/mL collagen (collA2), 0.25 mM arachidonic acid (AAA0.25), 0.5 mM arachidonic acid (AAA00.5) and 1 mg/mL ristocetin (R_1mg_mL). B. Adenosine triphosphate (ATP) secretion response to various agonists at baseline in the CKD vs. non-CKD groups, expressed in nanomoles (nmoles): ATP secretion to 0.25 mM arachidonic acid (AAS0_25), to 0.5 mM arachidonic acid (AAS0_5), to 2 μg/mL collagen (CollS2), and to 1 unit/mL thrombin (ThrombinS). Results are presented as means ±standard deviation. Between-group significant differences are shown with asterisk (CKD vs. non-CKD), p=0.02 for ADP10 and p=0.007 for ADP20.
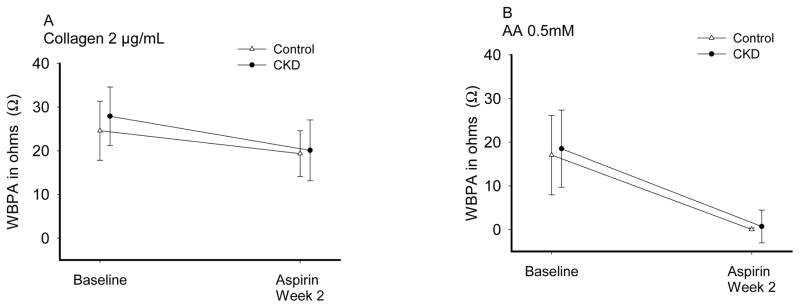
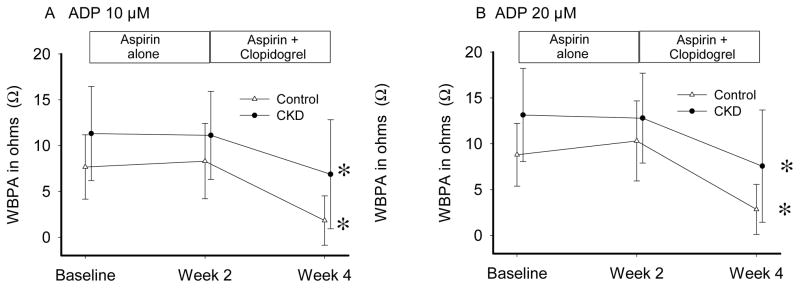
Aspirin effect17, 21 (Table 2; Figure 3) was evaluated at Visit 2 by the change from baseline (Visit 1) in platelet aggregation induced by AA and collagen after 2 weeks of aspirin 81 mg/d. Both groups had near complete IPA to AA on aspirin (Table 3), group x time interaction p=0.78. Response to collagen was incomplete in both groups, group x time interaction p=0.29. Finally, proportion of patients with RPA on-aspirin was not different between-groups (Table 4). Clopidogrel effect19 was evaluated at Visit 3 by ADP response after 2 weeks of aspirin/clopidogrel (Table 2; Figure 4). IPA in response to clopidogrel was significantly lower in CKD vs. non-CKD individuals (Table 3). Additionally, a higher proportion of CKD vs. non-CKD participants had RPA after clopidogrel treatment (Table 4). Group x time interaction was not significant (p =0.46 for 10 μM ADP and p=0.42 for 20 μM ADP). There were no differences in the metabolizer phenotypes between groups (Table 5). Figure 5 shows platelet-inhibitory responses to clopidogrel in the two groups based on the four metabolic phenotypes (CKD x metabolic phenotype interaction p >0.1). There were no hospitalizations or bleeding episodes requiring APA discontinuation or blood transfusion. Three CKD and 2 non-CKD participants experienced self-limited spontaneous bruises or nose bleeds on aspirin alone. Two CKD and 7 non-CKD patients experienced similar episodes on aspirin/clopidogrel.
Figure 3.
Aspirin effect in the chronic kidney disease (CKD) and the non-CKD groups as measured by the change in whole blood platelet aggregation (WBPA) induced by A) 0.5 mM arachidonic acid (AAA0.5) and B) 2 μg/mL collagen (CollA2) after 2 weeks of 81 mg of aspirin daily. Results are presented as mean and standard deviation. CKD is represented with closed circles and non-CKD with open triangles. There was a significant decrease in WBPA in both groups with CollA2 (p <0.0001) and with AAA0.5 (p <0.0001). The response at 2 weeks was not statistically different between CKD and non-CKD groups: group x time interaction p =0.29 for CollA2 and p =0.78 for AAA0.5.
Table 3.
Inhibition of Platelet Aggregation from Baseline in Response to Aspirin and Aspirin/Clopidogrel
| Inhibition of platelet aggregation measured by | Chronic kidney disease | |||
|---|---|---|---|---|
| Visits | No | Yes | p-value | |
| Aspirin effect | ||||
|
| ||||
| 0.25 mM arachidonic acid (%) | Visit 2 vs. Visit 1 | 95 ± 17 | 95 ± 21 | 0.58 |
|
| ||||
| 0.5 mM arachidonic acid (%) | Visit 2 vs. Visit 1 | 99 ± 3 | 97 ± 17 | 0.67 |
|
| ||||
| 2 μg/mL collagen (%) | Visit 2 vs. Visit 1 | 28 (16, 37) | 30 (7, 42) | 0.56 |
|
| ||||
| Clopidogrel effect | ||||
|
| ||||
| 10 μM adenosine diphosphate (%) | Visit 3 vs. Visit 1 | 88 (65, 100) | 50 (10, 85) | 0.046 |
|
| ||||
| 20 μM adenosine diphosphate (%) | Visit 3 vs. Visit 1 | 72 (59, 100) | 38 (20, 88) | 0.04 |
p-value for Wilcoxon rank sum test comparing CKD yes vs. no
Table 4.
Residual Platelet Aggregation on Aspirin and on Aspirin/Clopidogrel
| Chronic kidney disease | ||||
|---|---|---|---|---|
| Residual platelet aggregation measured by | Visits | No | Yes | p-value |
| On aspirin | ||||
|
| ||||
| 0.25 mM arachidonic acid (%) | Visit 2 vs. Visit 1 | 7.7 | 4.2 | 1.0 |
|
| ||||
| 0.5 mM arachidonic acid (%) | Visit 2 vs. Visit 1 | 0.0 | 3.9 | 1.0 |
|
| ||||
| 2 μg/mL collagen (%) | Visit 2 vs. Visit 1 | 92.3 | 84.6 | 0.65 |
|
| ||||
| On aspirin/clopidogrel | ||||
|
| ||||
| 10 μM adenosine diphosphate (%) | Visit 3 vs. Visit 1 | 16.7 | 48.0 | 0.08 |
|
| ||||
| 20 μM adenosine diphosphate (%) | Visit 3 vs. Visit 1 | 8.3 | 56.0 | 0.01 |
p-value for Fisher’s Exact test comparing proportion of participants with CKD yes vs. no
Figure 4.
Clopidogrel effect in the chronic kidney disease (CKD) (closed circles) vs. the non-CKD (open triangles) groups in whole blood platelet aggregation (WBPA) induced by A. 10 μM adenosine diphosphate (ADP10) and B. 20 μM adenosine diphosphate (ADP20) on 2 weeks of 81 mg of aspirin and 75 mg of clopidogrel daily. Results are presented as means ± standard deviation. Asterisk (*) shows that at Week 4 vs. Week 2, there was a significant decrease in WBPA in both groups induced by 10 μM ADP (p<0.0001) and 20 μM ADP (p<0.0001). The responses over time, i.e. at 2 weeks (aspirin 81 mg) and 4 weeks (aspirin 81 mg + clopidogrel 75 mg) of treatment, were not statistically different between CKD and non-CKD groups.
Table 5.
Frequencies for CYP2C19 According to Allele, Genotype, and Predicted Metabolic Phenotype
| Allele | NCBI SNP ID | Genotype | Chronic kidney disease | Metabolic phenotype | Chronic kidney disease | ||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| (n=26) | (n=13) | (n=26) | (n=13) | ||||
| *17 | rs12248560 | *1/17 | 6 (23%) | 2 (15%) | Rapid | 7 (27%) | 2 (15%) |
| *17/*17 | 1 (4%) | 0 | |||||
| *1/*1 | 9 (35%) | 5 (38%) | Normal | 9 (35%) | 5 (38%) | ||
| *2 | rs4244285 | *2/*17 | 2 (8%) | 3 (23%) | Intermediate | 9 (35%) | 6 (47%) |
| *1/*2 | 7 (27%) | 3 (23%) | |||||
| *2/*2 | 1 (4%) | 0 | Poor | 1 (3%) | 0 | ||
p-value = 0.84 for Fisher’s Exact test comparing proportions of metabolic phenotypes between CKD yes vs. no
CYP2C19, cytochrome P450 2C19; CYP2C19*1, wild type; NCBI, National Center for Biotechnology Information; SNP ID, single nucleotide polymorphism identification number
Figure 5.
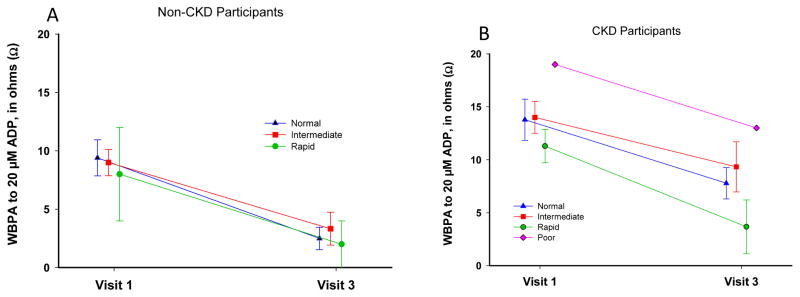
Clopidogrel effect in the chronic kidney disease (CKD) (right-side) vs. the non-CKD (left-side) groups in whole blood platelet aggregation (WBPA) induced by 20 μM adenosine diphosphate (ADP 20) based on metabolic phenotypes of CYP2C19 polymorphisms.
DISCUSSION
Our study reports 3 key findings in CKD patients when compared to those without kidney disease: 1) similar platelet function profiles at baseline, except higher ADP-induced platelet aggregation in CKD; 2) similar aspirin effect; and, 3) poor response to the platelet-inhibitory effects of clopidogrel among CKD patients, independent of CYP2C19*2 and CYP2C19*17 polymorphism effects.
We report baseline similarity in platelet aggregation of non-dialysis patients with CKD stages 4–5 compared to non-CKD, except an abnormality in the ADP pathway. These findings are particularly important as scarce data exist on platelet aggregation and secretion profiles of non-dialysis CKD patients,4, 6 and there are no published controlled studies reporting baseline platelet aggregation profiles of patients with non-dialysis CKD before treatment with APA.2 Early published case series used bleeding time (BT), capillary fragility time (CFT) or other methods to measure platelet dysfunction in patients with severe uremia,22–24 and are of limited clinical utility in modern-day practice given that it is uncommon to find such severe degree of untreated uremia. Studies in uremic dogs reported no between-group differences in platelet aggregation profiles when compared to controls.25 Later uncontrolled reports from ESRD cohorts that used older methods of platelet function testing were conflicting, reporting either normal or impaired ADP-induced platelet aggregation.8, 9 These studies employed assays including optical aggregometry, multi-plate analyzers or platelet function analyzer (PFA)-100 which are sub-optimal for testing platelet function and the results may not be reproducible. 19, 21, 26 The WBPA method is superior to the these methods as it is more sensitive, evaluates platelets in a physiological milieu in the presence of red and white blood cells known to affect platelet function, and does not require centrifugation which can injure platelets.26 As a result, knowledge gaps existed in our understanding of uremic platelet dysfunction from studies that were limited by lack of controls, or performed in uremic dogs25 or ESRD.6, 22–24 Findings of our study address these gaps in knowledge by including a control group, studying CKD stages 4–5 patients where previous data on platelet aggregation was lacking and using a validated method of platelet aggregation measurement in whole blood.19
We report no differences in platelet response to 81 mg of aspirin between two groups. These are similar to findings in healthy volunteers where WBPA induced by AA and collagen were inhibited completely and incompletely, respectively, by 2 weeks of aspirin 81 mg/d.17 Although, we observed reduction in WBPA by collagen from 29 to 20 Ω in CKD and from 25 to 19 Ω in non-CKD individuals on-aspirin (p=0.56), a cross sectional study of aspirin-treated diabetic patients observed higher proportion of CKD vs. non-CKD patients had RPA measured by collagen (49% vs. 41%, p=0.004).4 This study did not define CKD based on guidelines and failed to assess before- and after- aspirin effects. Therefore, we addressed these limitations and found that AA and collagen pathways of platelet aggregation may be inhibited to a similar extent in patients with stages 4–5 CKD as in those without kidney disease.
We extend findings of previous studies by reporting poor response to clopidogrel in CKD stages 4–5 vs. non-CKD patients, independent of CYP2C19 polymorphism effects.11 Previous cross-sectional studies reported presence of RPA in nearly 40–60% of patients with CKD on-clopidogrel.4, 6 However, these studies had various limitations: a) Angiolillo et al. did not measure before- and after-treatment effects on platelets and over-fitted the model;4 b) Woo et al. showed lower ADP-induced platelet aggregation in ESRD patients vs. controls but used the optical method of platelet assay;5 c) Htun et al. reported poor response to clopidogrel in an uncontrolled analysis of CKD and ESRD patients without measuring before- and after-treatment effects;6 and, d) Park et al. reported similar findings of high RPA on-clopidogrel in Korean men with CKD but failed to adjust for DM.7 Our study addresses these limitations and extends these findings by using a validated contemporary method to measure aggregation; including a control; measuring before- and after-treatment effects of clopidogrel on platelet aggregation; and analyzing CYP2C19 polymorphisms.
Although our study may be limited by residual confounding due to small sample size and was not randomized, the two groups were frequency-matched for characteristics previously reported to influence platelet function. Another limitation is that given hemostatic mechanisms are complex, platelet function measured ex vivo by WBPA may be different from platelet function in vivo, and we investigated only one pathway of hemostasis that would be potentially different among patients with and without CKD. In addition, platelet function assays may have suboptimal reproducibility.27 As such, previously reported variability in antiplatelet effects of clopidogrel is partly explained by CYP2C19 polymorphisms.28,29 Therefore, we measured CYP2C19 polymorphisms that may affect clopigogrel-induced platelet inhibition.30 Future studies are needed to confirm these findings, determine if such differences may explain the increased incidence of thrombotic events in APA-treated patients with vs. without CKD, and investigate whether RPA on-clopidogrel translates into a modifiable clinical risk.
Acknowledgments
We thank Robert F. Reilly (M.D.) for advice about study conception and design. We thank Steven Creacy, PhD, at Harmonyx Diagnostics for analyzing and interpreting genetic polymorphisms. We also thank Kyle West (M.S.), Mieshia Beamon (M.S.), and Staci Schwartz (B.A.) for assistance with recruitment and study visits.
Grant support: Supported by an American Heart Association grant (12CRP11830004) awarded to N.J.; VA MERIT grant (CX000217-01) and grant R01DK085512, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) awarded to S.S.H; and by UTSW O'Brien Kidney Research Core Center (NIDDK, P30DK079328) and Center for Translational Medicine (NIH, UL1TR001105).
Footnotes
Clinical Trial Registration: NCT01768637
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Machecourt J, Danchin N, Lablanche JM, Fauvel JM, Bonnet JL, Marliere S, Foote A, Quesada JL, Eltchaninoff H, Vanzetto G, Investigators E. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2002;39:1113–1119. doi: 10.1016/j.jacc.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 2.Jain N, Hedayati SS, Sarode R, Banerjee S, Reilly RF. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol. 2013;8:665–674. doi: 10.2215/CJN.06790712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aradi D, Komocsi A, Vorobcsuk A, Rideg O, Tokes-Fuzesi M, Magyarlaki T, Horvath IG, Serebruany VL. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J. 2010;160:543–551. doi: 10.1016/j.ahj.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Woo JS, Kim W, Lee SR, Jung KH, Kim WS, Lew JH, Lee TW, Lim CK. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. Am Heart J. 2011;162:1018–1025. doi: 10.1016/j.ahj.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Muller K, Bigalke B, Stellos K, May AE, Flather M, Gawaz M, Geisler T. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol. 2011;22:627–633. doi: 10.1681/ASN.2010020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Kim W, Park C, Kang W, Hwang S, Kim W. A Comparison of Clopidogrel Responsiveness in Patients with versus without Chronic Renal Failure. The American journal of cardiology. 2009;104:1292–5. doi: 10.1016/j.amjcard.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Cetin O, Bekpinar S, Unlucerci Y, Turkmen A, Bayram C, Ulutin T. Hyperhomocysteinemia in chronic renal failure patients: relation to tissue factor and platelet aggregation. Clin Nephrol. 2006;65:97–102. doi: 10.5414/cnp65097. [DOI] [PubMed] [Google Scholar]
- 9.Ho SJ, Gemmell R, Brighton TA. Platelet function testing in uraemic patients. Hematology. 2008;13:49–58. doi: 10.1179/102453308X315834. [DOI] [PubMed] [Google Scholar]
- 10.Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny JL, Trenk D, Sibbing D, Gurbel PA Working Group on On-Treatment Platelet R. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 11.Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL, Jr, Xu K, Parise H, Brodie BR, Stuckey TD, Stone GW. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents registry. Circ Cardiovasc Interv. 2015;8:e001683. doi: 10.1161/CIRCINTERVENTIONS.115.001683. [DOI] [PubMed] [Google Scholar]
- 12.Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR Clinical Pharmacogenetics Implementation C. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease. evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 14.Leng WX, Ren JW, Cao J, Cong YL, Cui H, Hu GL, Hu QQ, Niu H, Fan L. Chronic kidney disease--is it a true risk factor of reduced clopidogrel efficacy in elderly patients with stable coronary artery disease? Thromb Res. 2013;131:218–224. doi: 10.1016/j.thromres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, Herzog W, Drolet M, Pakyz R, Newcomer S, Sack P, Karon H, Ryan KA, Zhao Y, Shi X, Mitchell BD, Shuldiner AR. Aspirin Resistance in healthy drug-naive men versus women (from the Heredity and Phenotype Intervention Heart Study) Am J Cardiol. 2009;104:606–612. doi: 10.1016/j.amjcard.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordeaux BC, Qayyum R, Yanek LR, Vaidya D, Becker LC, Faraday N, Becker DM. Effect of obesity on platelet reactivity and response to low-dose aspirin. Prev Cardiol. 2010;13:56–62. doi: 10.1111/j.1751-7141.2009.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qayyum R, Becker DM, Yanek LR, Moy TF, Becker LC, Faraday N, Vaidya D. Platelet inhibition by aspirin 81 and 325 mg/day in men versus women without clinically apparent cardiovascular disease. Am J Cardiol. 2008;101:1359–1363. doi: 10.1016/j.amjcard.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurbel PA, Bliden KP, Logan DK, Kereiakes DJ, Lasseter KC, White A, Angiolillo DJ, Nolin TD, Maa JF, Bailey WL, Jakubowski JA, Ojeh CK, Jeong YH, Tantry US, Baker BA. The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: the PARADOX study. J Am Coll Cardiol. 2013;62:505–512. doi: 10.1016/j.jacc.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Dyszkiewicz-Korpanty A, Olteanu H, Frenkel EP, Sarode R. Clopidogrel anti-platelet effect: an evaluation by optical aggregometry, impedance aggregometry, and the platelet function analyzer (PFA-100) Platelets. 2007;18:491–496. doi: 10.1080/09537100701280654. [DOI] [PubMed] [Google Scholar]
- 20.Jain N, Trivedi MH, Rush AJ, Carmody T, Kurian B, Toto RD, Sarode R, Hedayati SS. Rationale and design of the Chronic Kidney Disease Antidepressant Sertraline Trial (CAST) Contemp Clin Trials. 2013;34:136–144. doi: 10.1016/j.cct.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyszkiewicz-Korpanty AM, Kim A, Burner JD, Frenkel EP, Sarode R. Comparison of a rapid platelet function assay--Verify Now Aspirin--with whole blood impedance aggregometry for the detection of aspirin resistance. Thromb Res. 2007;120:485–488. doi: 10.1016/j.thromres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Salzman EW, Neri LL. Adhesiveness of blood platelets in uremia. Thromb Diath Hemorrh. 1966;15:84. [PubMed] [Google Scholar]
- 23.Horowitz HI, Cohen BD, Martinez P, Papayonau MF. Defective ADP-induced platelet factor-3 activation in uremia. Blood. 1967;30:331–340. [PubMed] [Google Scholar]
- 24.Eschbach JW, Harker LA, Dale DC. The hematological consequences of renal failure. In: Brenner BM, Rector FC, editors. The Kidney. 1. Philadelphia: WB Saunders; 1976. pp. 1531–33. [Google Scholar]
- 25.Sloand J, Sloand E. Studies on platelet membrane glycoproteins and platelet function during hemodialysis. J Am Soc Nephrol. 1997;8:799–803. doi: 10.1681/ASN.V85799. [DOI] [PubMed] [Google Scholar]
- 26.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin Appl Thromb Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 27.Grove EL, Hvas AM, Johnsen HL, Hedegaard SS, Pedersen SB, Mortensen J, Kristensen SD. A comparison of platelet function tests and thromboxane metabolites to evaluate aspirin response in healthy individuals and patients with coronary artery disease. Thromb Haemost. 2010;103:1245–1253. doi: 10.1160/TH09-08-0527. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: A review of the evidence. J Am Coll Cardiol. 2005;45:1157–1164. doi: 10.1016/j.jacc.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Frelinger AL, 3rd, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, Michelson AD. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: a 700-patient study of aspirin resistance. Circulation. 2006;113:2888–2896. doi: 10.1161/CIRCULATIONAHA.105.596627. [DOI] [PubMed] [Google Scholar]
- 30.Gurbel PA, Shuldiner AR, Bliden KP, Ryan K, Pakyz RE, Tantry US. The relation between CYP2C19 genotype and phenotype in stented patients on maintenance dual antiplatelet therapy. Am Heart J. 2011;161:598–604. doi: 10.1016/j.ahj.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]