Abstract
Chronic aflatoxin exposure has long been related to hepatocellular carcinoma (HCC). Recently, its association with gallbladder cancer (GBC) was postulated. Here we present the data supporting this hypothesis in Chile, the country with the highest GBC mortality worldwide with age-standardized mortality rates (ASMR) of 10.3 in women and 5.04 in men.
The highest GBC rates occur in Southern Chile (ASMR= 18), characterized by: high Amerindian ancestry, associated with high bile acid synthesis and gallstones; high poverty and high cereal agriculture, both associated with aflatoxin exposure. Aflatoxins have been detected in imported and locally grown foods items. We estimated population dietary exposure ranging from 0.25 to 35.0 ng/kg-body weight/day. The only report on human exposure in Chile found significantly more aflatoxin biomarkers in GBC than in controls (Odds Ratio= 13.0).
The hypothesis of aflatoxin-GBC causal link in the Chilean population is supported by: genetically-determined rapid cholesterol excretion and high gallstones prevalence (49.4%); low prevalence of HCC (ASMR=4.99) and low HBV infection (0.15%) the main co-factor of aflatoxins in HCC risk.
If the association between aflatoxins and GBC were confirmed, public health interventions based on food regulation could have a substantial public health impact.
Keywords: aflatoxin, gallbladder cancer, gallstones
Introduction
A previous report hypothesized that excretion of excess of cholesterol from the liver into the gallbladder can lead to the simultaneous pumping of a food carcinogen into the bile. This carcinogen would then be stored and concentrated in the gallbladder, increasing the risk of gallbladder cancer (GBC) (1). One xenobiotic of particular interest is aflatoxin. Here we explore the data that supports the hypothesis that chronic exposure to aflatoxin may be a causal factor for GBC. Chile is the ideal place to study this hypothesis given its high burden of GBC in geographic defined areas.
Aflatoxins
Aflatoxins (B1, B2, G1 and G2) are secondary metabolites of Aspergillus flavus and Aspergillus parasiticus fungi that naturally contaminate food, including a wide range of products like cereals, oilseeds, spices, tree nuts, milk, meat and dried fruit (2). Agricultural products grown between latitudes 40° North and 40° South are at highest risk of becoming contaminated with aflatoxins and risk of consumption of contaminated products is particularly high in developing countries, associated with poverty, shortage and no strict food regulations (3,4,5). However, because of globalization no Region of the world is free from these toxins (6).
Aflatoxins are potent hepatotoxins and carcinogens, being aflatoxin B1 (AFB1) the most prevalent and toxic (7). The International Agency for Research on Cancer (IARC) classifies aflatoxins as carcinogenic to humans (Group 1) based on cohort studies which found increased risk for hepatocellular carcinoma (HCC) in individuals exposed to aflatoxins (relative risk=1.6–16.1), as well as several case-series and case–control studies. Further, carcinogenicity of aflatoxins has been shown in several experimental animal and mechanistic studies (6). The mutagenesis process is the result of the metabolism of aflatoxin by cytochrome P450s (CYPs) to the reactive 8,9-epoxide form, a genotoxic metabolite that can bind to essential proteins and react with DNA in the N7 position of guanines, forming DNA adducts. AFB1 adducts can result in GC to TA transversions of the tumor suppressor TP53 gene, which can lead to cancer (8). According to the IARC TP53 database, 39% of the HCC is associated to this transvertion followed by transitions GC to AT (9). In areas where aflatoxin exposure is high, up to 50% of HCC tumors have a specific point mutation in codon 249 of this gene (6).
Susceptibility to aflatoxin hepatotoxicity is associated with the expression level of CYP enzymes in the liver (10). Cofactors for aflatoxin-related carcinogenesis include liver infection and inflammation (11), nutritional factors, younger age and testosterone concentration (3); but aflatoxin’s most potent cofactor is hepatitis B virus (HBV) (6, 12, 13). HBV may increase aflatoxin metabolism (6) through induction of the CYPs, increasing the 8,9-epoxide in the liver. Also, chronic HBV infection leads to necrosis and regeneration of hepatocytes, which may predispose to TP53 mutation when exposed to aflatoxin (13).
Given the genotoxic properties of the metabolized aflatoxins, we hypothesize that these toxins are a causal factor for other digestive cancers, if specific co-factors were present. In particular, we aim to review the basis and evidence that chronic exposure to aflatoxins is a causal factor for GBC in the presence of high gallstone prevalence.
Gallbladder cancer
GBC is the most common malignancy of the biliary tract, with striking geographic variation worldwide, suggesting a strong role of environmental factors. The highest rates of GBC are found in Latin America and Asia, with intermediate rates in Eastern and Central Europe, and very low rates in the United States and most Western and Mediterranean European countries (14).
Known risk factors for GBC include gallstones (15), obesity, parity (16), increased endogenous and exogenous estrogen levels (17–19) and poverty (20). GBC risk has also been positively associated with total carbohydrate and calorie intake (21) and red chili pepper consumption (22,23). The association with red chili pepper consumption is in apparent contradiction with the anti-carcinogenic effect of capsaicin, its active compound. This paradox could potentially be explained by contamination of chili pepper with carcinogens (24).
GBC is more frequent in populations with a high prevalence of gallstones, such as the Amerindians of North, Central and South America (Table 1) (28, 32), and the risk is particularly high among Amerindian women of low socioeconomic status (20, 33). In Chile, it has been shown that Amerindians are especially susceptible to GBC due to the presence of lithogenic cholesterol genes (26) with an associated increase in bile acid synthesis (34).
Table 1.
Prevalence of gallstones in selected populations.
| Selected populations (Reference) | Gallstones (%) | |
|---|---|---|
| Women | Men | |
| North- Amerindians (25) | 64.1 | 29.5 |
| South- Amerindians (26) | 49.4 | 12.6 |
| Chileans (26) | 37.4 | 14.5 |
| Peruvians (27) | 16.1 | 10.7 |
| Bolivians (28) | 15.7 | 7.5 |
| White- American (25) | 16.6 | 8.6 |
| Black- American (25) | 13.9 | 5.3 |
| Brazilian (28) | 6 | 2 |
| Chinese (29) | 3.7 | 6.9 |
| Sub-Saharan Black Africans (25,30,31) | 2.9–5.1 | 5.1 |
GBC incidence and mortality rates in Chile are among the highest in the world with age-standardized mortality rates (ASMR) of 10.3 in women and 5.04 in men (35). In 2006, a GBC prevention program was implemented in the Country which was based in preventive cholecystectomy for people aged 35 to 49 years carrying gallstones, resulting in thousands of patients being cholecystectomized annually (36). But health care resources have been insufficient to cover the target population.
GBC is more frequent in the Southern Regions of Chile (VII to X) (20, 33, 37), where the proportion of Amerindian population (38) and of poverty (39) are the highest (Figure 1). Since aflatoxins and GBC are both associated with poverty and the latter with Amerindian origin (4, 5, 20), aflatoxins may be the environmental factor in the gene-environment causal chain to GBC.
Figure 1.
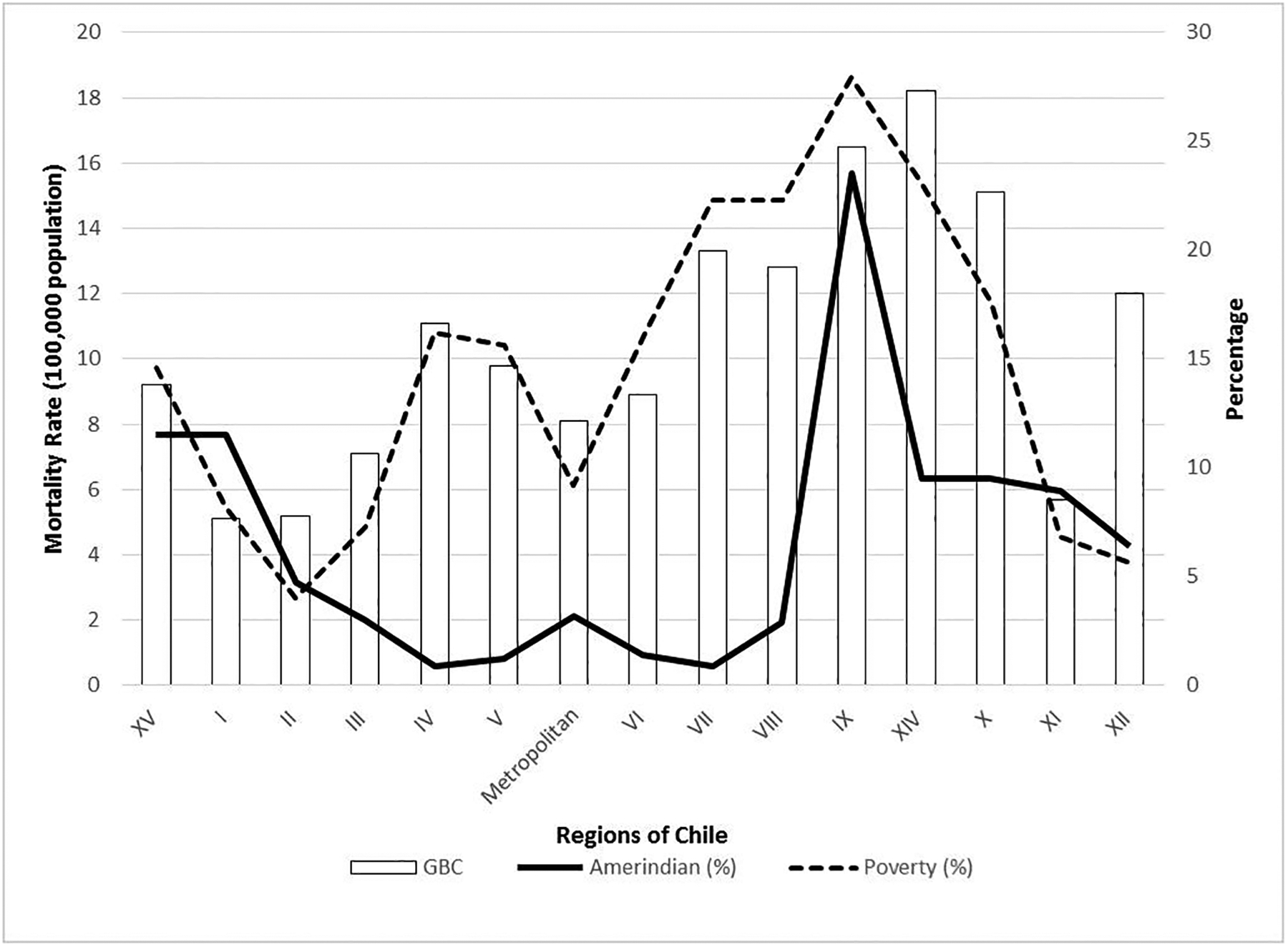
GBC mortality, poverty and Amerindian in the Chilean Regions from North to South.
Hypothesis
Chronic exposure to aflatoxins will preferentially increase risk of GBC rather than HCC in populations with 1) genetic predisposition toward rapid cholesterol excretion into the gallbladder, 2) high prevalence of gallstones, and 3) low prevalence of HBV infection.
Evaluation of the hypothesis
Experimental and epidemiological data
Biliary excretion is the major excretory route of metabolized aflatoxin, which can reach high concentrations in the bile (40,41); thus, the gallbladder is a repository of these toxins. In experimental animals (pigs and dogs), a single oral dose of aflatoxin causes inflammation, edema and hemorrhage of the gallbladder; subsequent daily doses cause biliary proliferation (42). Sieber et al. (43) reported gallbladder and bile duct tumors developing in nonhuman primates 10 to 12 years after receiving AFB1 orally over a course of 117–136 weeks (515.49 to 1354.24 mg total AFB1).
The first and only report on human exposure, a case control study by Nogueira et al. (23) in Chile, found significantly more circulating aflatoxin-albumin adducts in GBC patients compared to gallstone patients (OR= 6.8; 95% CI, 1.9 to 29.0) or population controls (OR= 13.0; 95% CI, 3.0 to 52.5). In this study, 35% of participants have detectable levels of aflatoxin-albumin adducts, ranging from 0.3 to 238.45 pg/mg (23).
As in HCC, somatic TP53 mutations associated with GBC are common (44–47). According to the IARC TP53 database, most of mutation types associated to GBC are GC to AT transitions, having GC to TA transvertions in 8.18% of the cases (9). Mutations in TP53 related to GBC have shown geographic and ethnic variation suggesting both exogenous and endogenous causation (47). In Chile, TP53 mutations associated to GBC showed GC to TA transvertions in 6% of the cases (44); similar of what is shown in the IARC database.
Sources of exposure to aflatoxins
Food
Information of aflatoxin in food in Chile is scarce. In 2009, the Chilean government established aflatoxin regulation and in 2015 established limits of 10 ppb for total aflatoxin and 0.5 ppb for aflatoxin M1, a metabolite of AFB1 excreted through milk (48). Aflatoxin regulation control has consisted in a non-systematic sampling of approximately 40 food items per year, covering a few Regions, resulting in aflatoxin detection in some food items tested, mainly imported peanuts and spices but also in locally grown foods like corn and powdered chili (Table 2).
Table 2.
Aflatoxins in food and estimated mean dietary exposure of the Chilean population, based on the Global Environment Monitoring System consumption Cluster diets (56).
| Food commodity (Reference) | Positive samples | Aflatoxins levels (ppb) | Year of sampling | Origin | C05 consumption g/day/person | Aflatoxins mean level (ppb) | Mean exposure (ng/kg-bw/day)c |
|---|---|---|---|---|---|---|---|
| Corn (49) | 1/9 (11) | 5 | 1990 | National | Cereals: 358.4 | 5 | 29.87 |
| Peanut (49) | 1/9 (11) | 60 | 1989 | Imported | Treenuts: 15.9 | 7.32 | 1.94 |
| Japanesse peanut (50) | 1/3 (33) | 33.3 | 2009 | Imported | |||
| Almondsa | 5/5 (100) | 2.1 −3.1 | 2013 | Imported | |||
| Peanuta | 1/11 (9) | 0.2 | 2013 | National | |||
| Nutmeg (51) | 5/5 (100) | 14.5 – 35.8 | 2010 | Imported | Spices: 4.4 | 3.38 | 0.25 |
| Red chili (51) | 3/3 (100) | 9.9 – 10.9 | 2010 | Imported | |||
| Nutmeg (52) | 5/5 (100) | 173.3 | 2011 | Imported | |||
| Curry (52) | 1/5 (20) | 23 | 2011 | Imported | |||
| Red chili (53) | 1/1 (100) | 4.9 | 2011 | National | |||
| White pepper (54) | 3/3 (100) | 2.5– 4.7 | 2012 | Imported | |||
| Black pepper(54) | 2/4 (50) | 2.6– 3.3 | 2012 | Imported | |||
| Paprika (54) | 3/4 (75) | 1– 1.3 | 2012 | Imported | |||
| Merquena | 5/15 (33) | 0.2–0.9 | 2013 | National | |||
| Milk | N.Sb | N.Sb | Milk: 174.9 | 1 | 2.92 | ||
| Total | 34.97 |
Public Health Institute of Chile 2013, unpublished data.
No sampled in Chile. It was assumed a mean concentration of 1 ppb.
Dietary exposure (mean concentration level in food x daily amount consumed/body weight) measured in ng per kg of body weight per day, calculated with an average of 60 kg of body weight.
We estimated the Chilean dietary exposure to aflatoxins (according to 55) based on: i) Chilean (and other Latin-American countries) typical diet according to the WHO Global Environment Monitoring System (GEMS) (56) that estimates a daily consumption per person of 15.9 g of nuts, 4.4 g of spices, 358.4 g of cereals and 175 g of milk and; ii) aflatoxin mean levels of the food items measured in Chile (Table 2). We estimated that Chileans have an intermediate level of exposure ranging from 0.25 to 34.97 ng/kg-body weight (bw)/day, with cereal as the main contributor (Table 2). Based on these estimates, Chileans would consume higher amounts of aflatoxins than Europeans (0.93–2.45 ng/kg-bw/day) or residents of the United States (2.7 ng/kg-bw/day) but lower than residents in Africa (0.3–180 ng/kg-bw/day) or Asia (0.3–53 ng/kg-bw/day) (57).
Occupational
While the main source of aflatoxin exposure is the ingestion of contaminated food, inhalation could be an important route of exposure for agricultural workers during crop harvest and storage (58–60) and cereal handlers like bakers and mill workers (61). Occupational exposure to aflatoxin has been associated with HCC (61, 62) and other digestive cancers, such as esophageal cancer in Iran (63) and gallbladder and extrahepatic bile duct cancers in Denmark (64). In Chile, cereal production (mainly corn, wheat and rice) is concentrated in the Central and Southern Regions (between Regions IV and X) (65), coinciding with the high risk areas of GBC (Figure 1). Thus, occupational exposure to aflatoxin may also contribute to GBC risk among the agricultural workers of Southern Chile.
Plausibility of a causal role of aflatoxins in GBC
If aflatoxin exposure were common in Chile, however, it begs the question why Chile has relatively low rates of HCC (Table 3). One reason is the very low rate of HBV infection (0.15%) (70), the most potent co-factor for aflatoxin in HCC. Additionally, some have proposed that the metabolic disorder behind gallstones somehow protects the liver from xenobiotics (1). Individuals who synthetize and rapidly excrete excess of cholesterol into bile are prone to develop gallstones (34), and since xenobiotics are excreted together with cholesterol (71), the time that xenobiotics remain in the liver is reduced in rapid cholesterol excretors. By the same token, xenobiotics accumulate in the gallbladder. In addition, gallstones cause local mucosal irritation and chronic inflammation (72) increasing aflatoxin toxicity in the gallbladder. Another co-factor acting in Chile is gallbladder chronic infection, in particular by Salmonella typhi. Bacterial chronic infection may contribute to malignant transformation through the degradation of bile constituents, chronic inflammation or alteration of tumor suppressor genes (28, 73). Chile suffered a high outbreak of typhoid fever from 1976 to 1986 (73), leaving behind a large reservoir of chronic carriers (74) who are thought to be at increased risk of GBC (Koshiol J personal communication).
Table 3.
Hepatocellular carcinoma (HCC) and gallbladder cancer (GBC) incidence, percent of population infected with hepatitis B (HBV) infection and aflatoxin mean exposure in selected countries.
According to our hypothesis, the rapid excretion of xenobiotics protects the liver from the hepatocarcinogenic toxin. The fact that HCC mortality has been historically low in Chile is consistent with this hypothesis. Intriguingly, an upward trend in HCC mortality has been reported over the last 15 years, concurrent with the stabilization of GBC mortality (37) (Figure 2). The increase in HCC could be the result of the obesity epidemic (75) while the drop of GBC could be the result of the systematic increase in the cholecystectomy rate observed over the last 20 years. Another explanation of the reverse trend of these two cancers is that cholecystectomy increased risk of HCC, which has been reported in several cohort studies (76–78).
Figure 2.
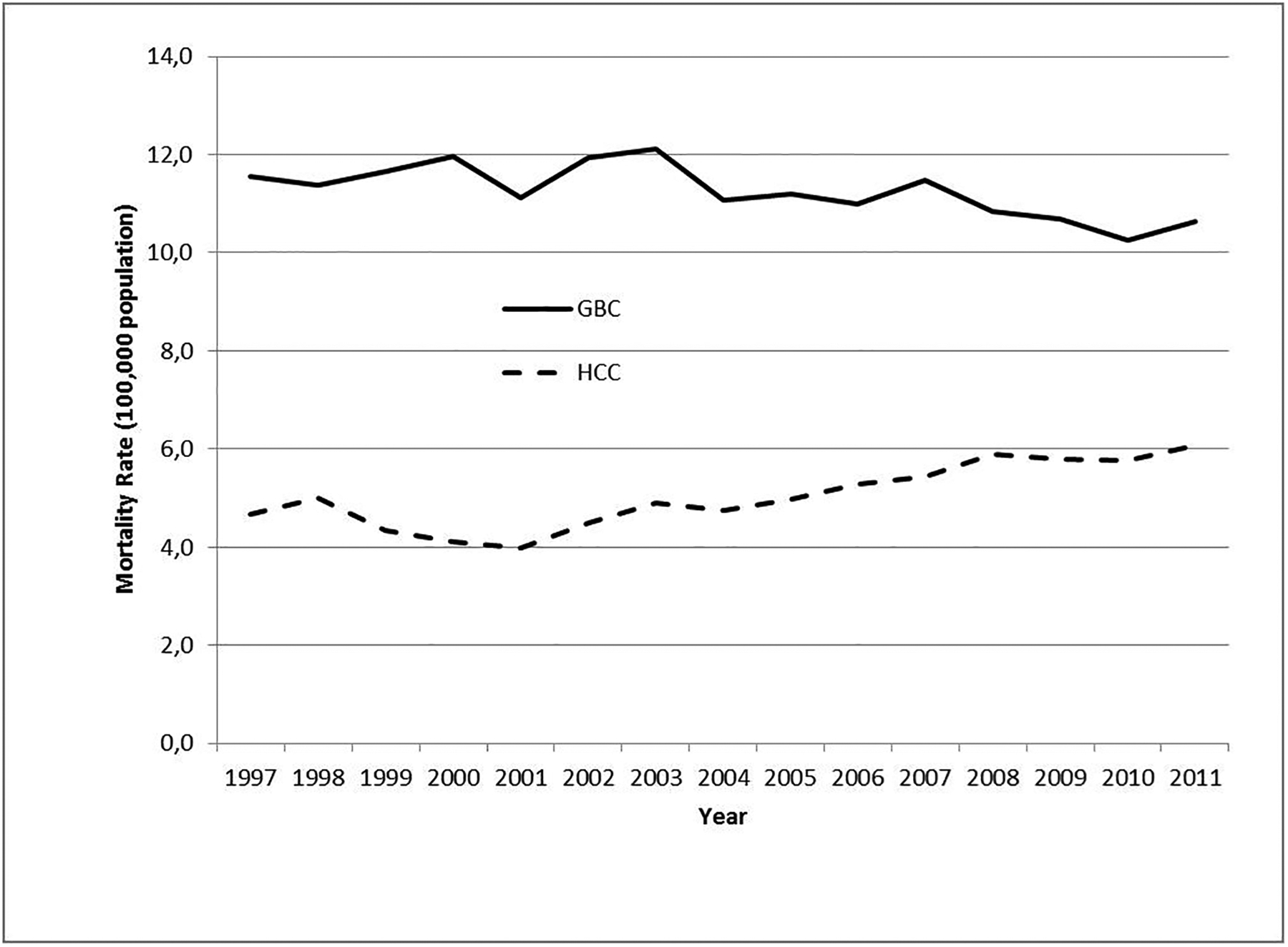
GBC and HCC mortality in Chile 1997– 2011.
Thus, the lack of HBV for liver carcinogenesis and the potential for genetic susceptibility through dominant lithogenic genes, widely distributed in populations with South Amerindian ancestors (26), may explain how aflatoxins could contribute to high rates of GBC without causing high rates of HCC.
Discussion
Chronic diseases like cancer are multifactorial and depend on the interaction of genetics with environmental and other cofactors (e.g., chronic infections). Levels of exposure to the carcinogen are also important. High levels of aflatoxin biomarkers (> 250 pg/mg aflatoxin-albumin adduct) have been found in regions with high risk of HCC such as The Gambia (79) and China (80), which also have high rates of HBV infection (Table 3). Other cofactors for aflatoxin may include Hepatitis C virus in Taiwan (81) and malaria, kwashiorkor and HIV/AIDS in Africa (5); all of which are very low in Chile. High HBV rates and low genetic predisposition to gallstones (Table 1) could explain the low GBC incidence rates in Africa (Table 3).
In countries with intermediate exposure to aflatoxins like Chile, Malaysia (82, 83) and Brazil (84), HCC incidence will vary according to the HBV rate (Table 3). The highest GBC incidences in the world are in Chile and Bolivia (Table 3), which share the same GEMS diet with possibly similar aflatoxin exposure, Amerindian miscegenation prone to lithogenic genes and gallstones (Table 1) and low HBV rates (Table 3). HBV is low to intermediate in most Latin American countries, but is highly endemic in the Amazon Basin (85), shared by Peru, Bolivia and Brazil; up to 14% of some Amazon rural groups are hepatitis B virus surface antigen (HBsAg) seropositive (67). This may explain why Peru, with the same GEMS diet and Amerindian miscegenation as Chile, presents higher HCC rates and intermediate GBC rates (Table 3).
Conclusion
Epidemiological, experimental and occupational data supports the hypothesis that aflatoxins are carcinogens for the gallbladder. Low population rates of HBV infection, genetic predisposition to rapid cholesterol synthesis and excretion, high prevalence of gallstones and chronic bacterial infection may help explain the aflatoxin-GBC association in Chile. The characterization of aflatoxin exposure in Chile through biomarkers studies in the population and measurement of aflatoxin levels in incriminated food-items is urgently needed.
If aflatoxins were a causal factor for GBC, public health intervention should move from its current emphasis on secondary prevention through cholecystectomy to invest in primary prevention through food safety regulation, which would likely have a broader and more cost-effective impact.
Acknowledgments:
Authors would like to Vanessa Van de Wyngard for the revision of the final draft.
Financial support:
ACCDiS/FONDAP #15130011
Footnotes
Competing financial interests: The authors have no competing financial interests to declare.
References
- 1.Venniyoor A Cholesterol gallstones and cancer of gallbladder (CAGB): molecular links. Med Hypotheses 2008; 70(3):646–53. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Public Health Strategies for Preventing Aflatoxin Exposure, <http://www.who.int/ipcs/events/2005/workshop_report.pdf>; 2005. [accessed 4 January 2015].
- 3.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 2004; 80(5):1106–22. [DOI] [PubMed] [Google Scholar]
- 4.Hawkes C, Ruel M. The links between agriculture and health: an intersectoral opportunity to improve the health and livelihoods of the poor. Bull World Health Organ 2006; 84(12):984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagacha JM, Muthomi JW. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol 2008; 10:124(1):1–12. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer. Chemical agents and related occupations. IARC Monogr Eval Carcinog Risk Hum 2012; 100F:227–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett JW, Klich M. Mycotoxins. ClinMicrobiol Rev 2003; 16(3):497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton DI, Ramsdell HS, Gordon EN. Biotransformation of aflatoxins. In: Eaton DL, Groopman JD (ed) The toxicology of aflatoxins: human health, veterinary, and agricultural significance. London, Academic Press, 45–65; 1993. [Google Scholar]
- 9.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 2007; 28(6):622–9. Version November 2013 [DOI] [PubMed] [Google Scholar]
- 10.Wild CP, Turner PC. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002; 17:471–81. [DOI] [PubMed] [Google Scholar]
- 11.Barton CC, Hill DA, Yee SB, Barton EX, Ganey PE, Roth RA. Bacterial lipopolysaccharide exposure augments aflatoxin B(1)-induced liver injury. Toxicol Sci 2000; 55(2):444–52. [DOI] [PubMed] [Google Scholar]
- 12.Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health 2008; 29:187–203. [DOI] [PubMed] [Google Scholar]
- 13.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int 2003; 23(6):405–9. [DOI] [PubMed] [Google Scholar]
- 14.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014; 6:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep 2005; 7:132–40. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Wang B, Qiao L. Association between obesity and gallbladder cancer. Front Biosci 2012; 17:2550–8. [DOI] [PubMed] [Google Scholar]
- 17.Andreotti G, Hou L, Gao YT, Brinton LA, Rashid A, Chen J, et al. Reproductive factors and risks of biliary tract cancers and stones: a population-based study in Shanghai, China. Br J Cancer 2010; 102(7):1185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, la Vecchia C. Hormone replacement therapy and cancer risk: a systematic analysis from a network of case-control studies. Int J Cancer 2003; 105(3):408–12. [DOI] [PubMed] [Google Scholar]
- 19.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The relationship between reproductive history and cholecystectomy in older women. J Clin Epidemiol 1990; 43(7):687–92. [DOI] [PubMed] [Google Scholar]
- 20.Andia ME, Hsing AW, Andreotti G, Ferreccio C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer 2008; 123(6):1411–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, et al. Epidemiologic aspects of gallbladder cancer: A case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997; 89:1132–8. [DOI] [PubMed] [Google Scholar]
- 22.Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K, et al. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer 2002; 102:407–11. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira L, Foerster C, Groopman J, Egner P, Koshiol J, Ferreccio C et al. Association of Aflatoxin With Gallbladder Cancer in Chile. JAMA 2015; 313(20):2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bley K, Boorman G., Mohammad B, Mckenzie D, Babbara S. Comprehensive Review of the Carcinogenic and Anticarcinogenic Potential of Capsaicin. Toxicol Pathol 2012; 40(6):847–73. [DOI] [PubMed] [Google Scholar]
- 25.Stinton LM, Shaffer EA. Epidemiology of Gallbladder Disease: Cholelithiasis and Cancer. Gut and Liver 2012; 6(2):172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miquel JF, Covarrubias C, Villaroel L, Mingrone G, Greco AV, Puglielli L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology 1998; 115(4):937–46. [DOI] [PubMed] [Google Scholar]
- 27.Moro PL, Checkley W, Gilman RH, Cabrera L, Lescano AG, Bonilla JJ, Silva B. Gallstone disease in Peruvian coastal natives and highland migrants. Gut 2000; 46(4):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. Cancer J Clin 2001; 51(6):349–64. [DOI] [PubMed] [Google Scholar]
- 29.Chen CY, Lu CL, Huang YS, Tam TN, Chao Y, Chang FY, Lee SD. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing 1998; 27(4):437–41. [DOI] [PubMed] [Google Scholar]
- 30.Ibitoye BO, Adisa AO, Makinde ON, Ijarotimi AO. Prevalence and complications of gallstone disease among pregnant women in a Nigerian hospital. Int J Gynaecol Obstet 2014; 125(1):41–3. [DOI] [PubMed] [Google Scholar]
- 31.Obajimi MO, Atalabi MO, Ogbole GI, Adeniji-Sofoluwe AT, Agunloye AM, Adekanmi AJ, et al. Abdominal ultrasonography in HIV/AIDS patients in southwestern Nigeria. BMC Med Imaging 2008; 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemrow SM, Perdue DG, Stewart SL, Richardson LC, Jim MA, French HT, et al. Gallbladder cancer incidence among American Indians and Alaska Natives, US, 1999–2004. Cancer 2008; 113(5 Suppl):1266–73. [DOI] [PubMed] [Google Scholar]
- 33.Bertran E, Heise K, Andia ME, Ferreccio C. Gallbladder cancer: incidence and survival in a high-risk area of Chile. Int J Cancer 2010; 127(10):2446-54. [DOI] [PubMed] [Google Scholar]
- 34.Gälman C, Miquel JF, Pérez RM, Einarsson C, Ståhle L, Marshall G, et al. Bile acid synthesis is increased in Chilean Hispanics with gallstones and in gallstone high-risk Mapuche Indians. Gastroenterology 2004; 126(3):741–8. [DOI] [PubMed] [Google Scholar]
- 35.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, < http://globocan.iarc.fr >; 2013. [accessed 2 June 2015]. [Google Scholar]
- 36.Heath Ministry of Chile. Guía Clínica Colecistectomía preventiva en adultos de 35 a 49 años, http://web.minsal.cl/portal/url/item/72205a1420599f92e04001011f016d02.pdf; 2010. [accessed 20 June 2014].
- 37.Health statistics of Heath Ministry of Chile. 2011. Mortalidad por algunas causas según regiones 2000 a 2011, <http://www.deis.cl/?p=2541>; 2011. [accessed 20 February 2015].
- 38.Social Development Ministry of Chile. Resultados de Encuesta Casen 2013, <http://www.ministeriodesarrollosocial.gob.cl/resultados-encuesta-casen-2013/>; 2013. [accessed 16 April 2015].
- 39.National Institute of Estatistics of Chile. Censo Nacional 2002, <http://www.ine.cl/cd2002/>; 2002. [accessed 6 March 2015].
- 40.Emerole GO. Excretion of aflatoxin B1 as a glutathione conjugate. Eur J Drug Metab Pharmacokinet 1981; 6(4):265–8. [DOI] [PubMed] [Google Scholar]
- 41.Harland EC, Cardeilhac PT. Excretion of carbon-14-labeled aflatoxin B1 via bile, urine, and intestinal contents of the chicken. Am J Vet Res 1975; 36(7):909–12. [PubMed] [Google Scholar]
- 42.Newberne PM, Butler WH. Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals: a review. Cancer Res 1969; 29(1):236–50. [PubMed] [Google Scholar]
- 43.Sieber SM, Correa P, Dalgard DW, Adamson RH. Induction of osteogenic sarcomas and tumors of the hepatobiliary system in nonhuman primates with aflatoxin B1. Cancer Res 1979; 39: 4545–54. [PubMed] [Google Scholar]
- 44.Moreno M, Pimentel F, Gazdar AF, Wistuba II, Miquel JF. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder carcinoma. Ann Hepatol 2005; 4(3):192–9. [PubMed] [Google Scholar]
- 45.Javle M, Rashid A, Churi C, Kar S, Zuo M, Eterovic AK, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol 2014; 5(4):701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013; 45(12):1470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asai T, Loza E, Roig GV, Ajioka Y, Tsuchiya Y, Yamamoto M, et al. High frequency of TP53 but not K-ras gene mutations in Bolivian patients with gallbladder cancer. Asian Pac J Cancer Prev 2014; 15(13):5449–54. [DOI] [PubMed] [Google Scholar]
- 48.Food Sanitary Regulation of Chile. DTO. N° 977/96; D.OF.13.05.97, amendment Art. 1°, N° 2 del Dto. N° 63/09, <http://web.minsal.cl/reglamento_san_alimentos>; 2015. [accessed 25 May 2015].
- 49.Cornejo J, Villarroel O. Antecedentes generales sobre las aflatoxinas y otras micotoxinas y elementos a tener en cuenta para el diseño de prácticas correctas de cultivo y elaboración de nueces, <http://web.minsal.cl/portal/url/item/72fd6274dad8792ee04001011f0109e4.pdf>; 2012. [accessed 4 June 2015].
- 50.Public Health Institute of Chile. Micotoxinas en alimentos de consumo directo en Chile, Monitoreo de micotoxinas realizado por el Instituto de Salud Pública de Chile Años 2008–2009, <http://www.ispch.cl/sites/default/files/documento/2013/05/MICOTOXINAS%20EN%20ALIMENTOS%20DE%20CONSUMO%20DIRECTO%20EN%20CHILE%202008-2009.pdf >; 2009. [accessed 8 June 2015].
- 51.Public Health Institute of Chile. Informe Monitoreo de micotoxinas en alimentos, <http://www.ispch.cl/centrodedocumentacion>; 2010. [accessed 8 June 2015].
- 52.Public Health Institute of Chile. Informe Monitoreo de micotoxinas en alimentos Año 2011, <http://www.ispch.cl/sites/default/files/documento_tecnico/2012/06/informe_micotoxinas_2011.pdf>; 2011. [accessed 8 June 2015].
- 53.Tsuchiya Y, Terao M, Okano K, Nakamura K, Oyama M, Ikegami K, et al. Mutagenicity and mutagens of the red chili pepper as gallbladder cancer risk factor in Chilean women. Asian Pac J Cancer Prev 2011; 12(2):471–6. [PubMed] [Google Scholar]
- 54.Public Health Institute of Chile. Informe de resultados de vigilancia de laboratorios. Micotoxinas en alimentos, <http://www.ispch.cl/centrodedocumentacion>; 2012. [accessed 8 January 2015]. [Google Scholar]
- 55.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellularcarcinoma: a risk assessment. Environ Health Perspect 2010; 118: 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sy MM, Feinberg M, Verger P, Barré T, Clémençon S, Crépet A. New approach for the assessment of cluster diets. Food Chem Toxicol 2013; 52: 180–187. [DOI] [PubMed] [Google Scholar]
- 57.Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. WHO Tech Rep Ser 2008; 947: 163. [Google Scholar]
- 58.Autrup JL, Schmidt J, Autrup H. Exposure to aflatoxin B1 in animal-feed production plant workers. Environ Health Perspect 1993; 99:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh SK, Desai MR, Pandya GL, Venkaiah K. Airborne aflatoxin in the grain processing industries in India. Am Ind Hyg Assoc 1997; J 58:583–6. [DOI] [PubMed] [Google Scholar]
- 60.Viegas S, Faisca VM, Dias H, Clerigo A, Carolino E, Viegas C. Occupational exposure to poultry dust and effects on the respiratory system in workers. J Toxicol Environ Health A 2013; 76(4–5):230–9. [DOI] [PubMed] [Google Scholar]
- 61.Saad-Hussein A, Taha MM, Beshir S, Shahy EM, Shaheen W, Elhamshary M. Carcinogenic effects of aflatoxin B1 among wheat handlers. Int J Occup Environ Health 2014; 20(3):215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai H, Mo X, Yang Y, He K, Xiao J, Liu C, et al. Association between aflatoxin B1 occupational airway exposure and risk of hepatocellular carcinoma: a case-control study. Tumour Biol 2014; 35(10):9577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghasemi-Kebria F, Joshaghani H, Taheri NS, Semnani S, Aarabi M, Salamat F, et al. Aflatoxin contamination of wheat flour and the risk of esophageal cancer in a high risk area in Iran. Cancer Epidemiol 2013; 37(3):290–3. [DOI] [PubMed] [Google Scholar]
- 64.Olsen JH, Dragsted L, Autrup H. Cancer risk and occupational exposure to aflatoxins in Denmark. Br J Cancer 1988; 58:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Statistical Institute of Chile. Agropecuaria: Informe Anual 2012, <http://www.ine.cl/canales/menu/publicaciones/calendario_de_publicaciones/pdf/agropecuarias_informe_anual_2012.pdf>; 2012. [accessed 10 June 2014].
- 66.Pkids on line. Hepatitis B Global Infection Rates, <http://www.pkids.org/files/pdf/phr/02-09globalhbv.pdf> ; 2006. [accessed 16 June 2015].
- 67.León P, Venegas E, Bengoechea L, Rojas E, López JA, Elola C, Echevarría JM. Prevalence of infections by hepatitis B, C, D and E viruses in Bolivia. Rev Panam Salud Publica 1999; 5(3):144–51. [DOI] [PubMed] [Google Scholar]
- 68.Segovia M, Galván K; García V, Huamaní L, Gotuzzo E Prevalencia de marcadores serológicos para hepatitis B y delta e infección intrafamiliar en el valle del río Pampas, Perú. Rev Perú Med Exp Salud Publica 2002; 19 (2): 57–62. [Google Scholar]
- 69.Cabezas C Situación y control de la hepatitis B y Delta en el Perú. Acta Med Peruana 2008; 25 (2): 96–112. [Google Scholar]
- 70.Heath Ministry of Chile. Informe anual, Hepatitis B, año 2011 Dpto. de Epidemiología, DIPLAS-MINSAL, <http://epi.minsal.cl/epi/html/bolets/reportes/HepatitisB/HepB_2011.pdf>; 2011. [accessed 15 June 2015].
- 71.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol Rev 2010; 62(1):1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaffer EA. Gallbladder cancer: the basics. Gastroenterol Hepatol 2008; 4(10):737–41. [PMC free article] [PubMed] [Google Scholar]
- 73.Ferreccio C Salmonella typhi and Gallbladder Cancer. In: Khan AA (ed) Bacteria and Cancer, Chapter 5. New York, Springer, pp 117–38; 2012. [Google Scholar]
- 74.Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis 1982; 146(6): 724–726. [DOI] [PubMed] [Google Scholar]
- 75.Albala C, Vio F, Kain J, Uauy R.Nutrition transition in Chile: determinants and consequences. Public Health Nutr 2002;5(1A):123–8. [DOI] [PubMed] [Google Scholar]
- 76.Lagergren J, Mattsson F, El-Serag H, Nordenstedt H. Increased risk of hepatocellular carcinoma after cholecystectomy. Br J Cancer 2011; 105(1):154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kao WY, Hwang CY, Su CW, Chang YT, Luo JC, Hou MC, et al. Risk of hepato-biliary cancer after cholecystectomy: a nationwide cohort study. J Gastrointest Surg 2013; 17(2):345–51. [DOI] [PubMed] [Google Scholar]
- 78.Vogtmann E, Shu XO, Li HL, Chow WH, Yang G, Ji BT, et al. Cholelithiasis and the risk of liver cancer: results from cohort studies of 134,546 Chinese men and women. J Epidemiol Community Health 2014; 68(6):565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turner PC, Mendy M, Whittle H, Fortuin M, Hall AJ, Wild CP.Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Trop Med Int Health 2000;5(12):837–41. [DOI] [PubMed] [Google Scholar]
- 80.Gan LS, Skipper PL, Peng XC, Groopman JD, Chen JS, Wogan GN, Tannenbaum SR. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis 1988; 9(7):1323–5. [DOI] [PubMed] [Google Scholar]
- 81.Chen CH, Wang MH, Wang JH, Hung CH, Hu TH, Lee SC, et al. Aflatoxin exposure and hepatitis C virus in advanced liver disease in a hepatitis C virus endemic area in Taiwan. Am J Trop Med Hyg 2007; 77(4):747–52. [PubMed] [Google Scholar]
- 82.Leong YH, Rosma A, Latiff AA, Ahmad NI. Exposure assessment and risk characterization of aflatoxin B1 in Malaysia. Mycotoxin Res 2001; 27(3):207–14. [DOI] [PubMed] [Google Scholar]
- 83.Mohd-Redzwan S, Jamaluddin R, Abd-Mutalib MS, Ahmad Z. A mini review on aflatoxin exposure in Malaysia: past, present and future. Front Microbiol 2013; 4:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jager AV, Tedesco MP, Souto PCMC, Oliveira CAF. Assessment of aflatoxin intake in São Paulo, Brazil. Food Control 2013; 33 (1):87–92. [Google Scholar]
- 85.Viana S, Paraná R, Moreira RC, Compri AP, Macedo V. High prevalence of hepatitis B virus and hepatitis D virus in the western Brazilian Amazon. Am J Trop Med Hyg 2005; 73(4):808–14. [PubMed] [Google Scholar]


