Abstract
Zinc finger protein, X-linked (ZFX) gene locus on the human X chromosome is structurally similar to the zinc finger protein, Y-linked gene. Its role in human laryngeal squamous cell carcinoma (LSCC) is still not clearly defined. This study was focused on investigating the role of zinc-finger protein X-linked (ZFX) in human LSCC. Expression levels of ZFX were examined in LSCC tissues, corresponding adjacent non-tumoral tissues and vocal leukoplakia tissues by immunohistochemistry (IHC). The association with the expression level of ZFX and LSCC clincopathological parameters was analyzed. The prognostic value of ZFX expression was also analyzed. Lentivirus-mediated RNA interference was applied to silence ZFX expression and the effects of ZFX knockdown on the growth of human LSCC primary cells was investigated. Overexpression of ZFX was found in LSCC tissues. The expression of ZFX was associated with the clinical stage of LSCC. Patients with higher level of ZFX experienced a poorer prognosis compared to those with lower level of ZFX. Knockdown of ZFX inhibited cell proliferation, colony formation and migration of LSCC primary cells. Moreover, ZFX silencing induced cell apoptosis. These results provide the convincing evidence for the first time that ZFX plays an important role in LSCC development and could be a potential therapeutic target or prognostic predictor for LSCC.
Keywords: Laryngeal squamous cell carcinoma (LSCC), zinc finger protein x-linked (ZFX), prognosis, proliferation, apoptosis
Introduction
Laryngeal squamous cell carcinoma (LSCC) is the second most common squamous cell carcinoma of the head and neck and is the eleventh most common form of malignancy among worldwide [1]. About 2.4% of the world new malignancy cases are laryngeal cancer [2]. With the process of industrialization, both incidence and mortality from LSCC is rapidly increasing in the whole world [3]. In recent years, although the survival rate of several kinds of cancer has undergone improvement with the development of new medical technology these years, the survival rate of laryngeal cancer was in decline [4].
Zinc finger protein X-linked (ZFX) is one of the zinc finger protein super family. It is closely related to the regulation of cell proliferation, apoptosis and cell cycle. Recently, emerging studies have indicated that ZFX can play a key role in the initiation and development of several human malignancies [5,6]. Other studies suggested ZFX involved in the metastasis of human gallbladder cancer and non-small cell lung cancer [7,8].
In this study, we aimed to investigate the expression of ZFX in LSCC tissues, corresponding adjacent non-tumoral tissues and vocal leukoplakia tissues. We investigate the association between ZFX level and clinicopathological parameters of LSCC. We evaluate the prognosis value of ZFX in patients with LSCC. What more, we employ lent virus-mediated short hairpin RNA (shRNA) to silence ZFX expression in human LSCC primary cells and investigate the effects in cell proliferation and apoptosis after knockdown of ZFX.
Materials and methods
Patients and tissues
A total of 88 males and 9 females (between 32 and 82 years old with an average 59.96 years old) that underwent surgery for LSCC, 25 males and 3 females (between 32 and 75 years old with an average 51.14 years old) who underwent surgery for vocal leukoplakia were recruited for the study. None of the LSCC patients were treated with radiotherapy, chemotherapy or other therapies before surgery. Respectively, patients with LSCC were classified according to different clinicopathlogical parameters, including tumor T-level, local cervical lymph node metastasis, clinical stage and histopathological differentiation. Among these clinicopathlogical parameters, different clinical stages were divided into two groups: early-stage group (stage I and II) and middle-advanced stage group (stage III and IV) (Table 1). Totally, 50 LSCC tissue samples and 47 paired tissue samples (40 males and 7 females, between 32 and 75 years old with an average 61.21 years old), of which 47 were tumor samples of LSCC and 47 were adjacent non-tumoral samples from the same patients, were collected and examined for ZFX protein expression. 28 vocal leukoplakia tissue samples were also collected and examined for ZFX protein expression.
Table 1.
Expression of ZFX protein in LSCC grouped by different clinicopathlogical parameters
| Clinicopathlogical parameters | n | Optical density (Mean ± SD) | P value |
|---|---|---|---|
| T-level | 0.358 | ||
| T1 | 19 | 0.464±0.026 | |
| T2 | 28 | 0.467±0.024 | |
| T3 | 29 | 0.471±0.032 | |
| T4 | 21 | 0.480±0.024 | |
| Cervical lymph node metastasis | 0.601 | ||
| Without cervical lymph node metastasis | 77 | 0.470±0.028 | |
| With cervical lymph node metastasis | 20 | 0.475±0.024 | |
| Clinical stage | 0.004 | ||
| Early (StageI + II) | 44 (19+25) | 0.462±0.028 | |
| Middle-advanced (Stage III + IV) | 53 (32+21) | 0.478±0.025 | |
| Differentiated degree | 0.350 | ||
| Poorly differentiated | 13 | 0.462±0.019 | |
| Moderately differentiated | 48 | 0.470±0.029 | |
| Well-differentiated | 36 | 0.475±0.027 |
All patients we selected were treated in Department of Head and Neck Surgery, Beijing Tongren Hospital from Jan 2010 to Jan 2012 and have complete follow-up records. All patients were provided informed consent before their participation. This study was approved by the ethics committee of Capital Medical University, Beijing, China.
Reagents and apparatus
Rabbit anti-human ZFX antibody was purchased from Biosynthesis Biotechnology Co. Ltd (Beijing, China). PowerVisionTM immunohistochemistry kit was purchased from ZSGB-Bio (Beijing, China). AgeI, EcoRI were purchased from NEB (Ipswich, MA, USA). SYBR Green Master Mix Kit was purchased from TaKaRa (Dalian, China). pHelper 1.0, pHelper 2.0, and pGCSL-GFP plasmids were purchased from Genechem Co. Ltd (Shanghai, China). RNeasy Midi Kit was from Qiagen (Valencia, CA, USA). Trizol and Super ScriptII reverse transcriptase were purchased from Invitrogen (Carlsbad, CA, USA). Roswell Park Memorial Institute 1640 (RPMI 1640), Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Cambex, MD, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Bromodeoxyuridine (Brdu) ELISA kit was purchased from Roche (Basel, Switzerland). Methyl thiazolyl tetrazolium (MTT) was purchased from Ding Guo Co. Ltd (Beijing, China). Dimethyl sulfoxide (DMSO) was purchased from Sinopharm Group Co. Ltd (Shanghai, China). Transwell® was purchased from Corning Life Science (Tewksbury, MA, USA). All other chemicals were obtained from Sigma (St. Louis, MO, USA). Microplate reader (Elx800) was purchased from Biotek (Winooski, VT, USA). Optical microsope and image acquisition software (Dp2-BSW) was purchased from Olympus (Tokyo, Japan).
Immunohistochemistry (IHC) and staining image analysis
Paraffin-embedded serial sections were 4 μm, with conventional dewaxing and hydration. After that, 3% hydrogen peroxide was incubated for 5 min at room temperature (RT), and then PBS washed. The sections were locked with skimmed milk powder for 20 min at RT and probed with 1:75 rabbit anti-ZFX antibody at 4°C over night. After PBS washing, the sections were incubated with biotinylated goat anti-rabbit immunoglobulins at RT for 40 min, and then PBS washed. At last, the sections were developed with DAB, stained with hematoxylin, dehydrated, and mounted. We set up PBS instead of primary antibody as negative control. ZFX protein is located in the nucleus and cytoplasm with brown or yellow masculine performance.
To detect the protein expression of ZFX, we adopted image analysis to analyze the immunohistochemistry staining. Five fields in each slices were selected randomly under high magnification (400×). Leica IM50 picture collecting system and Leica Qwin software was used to count the mean optical density (OD) of ZFX positive cells in each field, and then, OD value of five fields was averaged for ZFX expression in each slice. The analysis was performed by two independent researchers, who were blind to the clinicopathlogicalal parameters of patients.
Cell culture
The LSCC primary cells originated from one primary LSCC patient and derived from the surgical specimen. Human embryonic kidney (HEK) 293T cell line was obtained from American Type Culture Collection (ATCC). LSCC primary cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS and penicillin/streptomycin. HEK 293T was maintained in DMEMsupplemented with 10% heat-inactivated FBS and penicillin/streptomycin. All cell cultures were incubated at 37°C in humidified atmosphere of 5% CO2.
Construction of ZFX shRNA lentivirus
The following oligonucleotide was synthesized. ZFX small interfering RNA (siRNA) was 5’-GTCGGAAATTGATCCTTGTAA-3’. The negative control siRNA was 5’-TTCTCCGAACGTGTCACGT-3’. The stem-loop-stem oligos (shRNAs) were synthesized, annealed and ligated into the AgeI/EcoRI-linearized pGCSIL-GFP vector. The lentiviaral-based shRNA expressing vectors were confirmed by DNA sequencing. The generated plasmids were named as pGCSIL-GFP-shZFX or -shNC.
HEK 293T cells (1.2×107) were seeded in 10-cm dishes and cultured for 24 hours to reach 70-80% confluence. The medium was replaced with serum-free DMEM two hours before transfection. Three plasmids including 20 μg pGCSIL-GFP-shZFX or -shNC, 15 μg packaging vector pHelper 1.0 and 10 μg VSVG expression plasmid vector pHelper 2.0 were added to Opti-MEM. The volume of the mixture was adjusted to 2.5 ml. 100 μl of Lipofectamine were added to 2.4 ml Opti-MEM. The two kinds of mixtures were mixed and added to the cells. The cells were incubated for 5 hours before replacing the medium with 10 ml of complete DMEM medium (with 10% FBS). Lentiviral particles were harvested at 48 hours after transfection, followed by purification by ultra centrifugation. As the lentivirus carries green fluorescence protein (GFP), the viral titer was determined by end-point dilution assay through counting GFP-expressing cells under fluorescence microscopy 96 hours after transfection.
RNA isolation and qRT-PCR
According to manufacturer’s instruction, total RNA was extracted using RNeasy Midi Kit. cDNA was synthesized using Super ScriptII reverse transcriptase. Briefly, a mixture containing 1 μg of total RNA, 0.5 μg oligo-dT primer (Genechem Co. Ltd, Shanghai, China) and nuclease-free water in a total volume of 15 μl was heated at 70°C for 5 min and then cooled on ice for another 5 min. The mixture was supplemented with 2 μl 10× buffer and 200U Super ScriptII reverse transcriptase up to a final volume of 20 μl, followed by incubation at 42°C for 60 min. qRT-PCR analyses for target mRNAs were performed using SYBR Green Master Mix Kit. The primer sequences for PCR amplification of ZFX gene were 5’-GGCAGTCCACAGCAAGAAAC-3’ and 5’-TTGGTATCCGAGAAAGTCAGAAAG-3’. GAPDH was applied as an internal control. The Primer sequences of GAPDH were 5’-TGACTTCAACAGCGACACCCA-3’ and 5’-CACCCTGTTGCTGTAGCCAAA-3’. 2-ΔΔCT was calculated and used as an indication of the relative expression levels.
MTT assay
After 24, 48, 72, 96 and 120 h of transfection, the viability of LSCC primary cells were evaluated by using the MTT assay according to manufacturer’s instruction. Cells were digested and resuspended thoroughly. Cell number was then counted using a hemocytometer, and 3000 cells per well were added in 96-well plates and cultured at 37°C in a 5% CO2 incubator. 10 μl of MTT solution was added to the culture medium and incubated for another 4 h. The culture medium was discarded and 150 μl DMSO was added to dissolve formazan. The absorbance was determined at 490 nm wavelength.
BrdU-proliferation assay
Brdu-proliferation assay was used to evaluate the proliferation of LSCC primary cells after transfection. LSCC primary cells after lentivirus infection were seeded in 96-well plates. After 24 and 96 h of transfection, respectively, cell were incubated with 10 μM BrdU for 2 h. Quantitative sandwich enzyme immunoassay technique was used to evaluate the level of BrdU incorporation as indicated by the manufacturer. Cell fixation was performed using FixDenat (200 μl/well) for 30 min and blocked with 5-10% BSA for another 30 min at room temperature. Then, cells were treated with diluted anti-BrdU-POD for 90 min at room temperature. The absorbance of samples was measured at 450 nm to determine BrdU incorporation ratio.
Cell colony formation assay
A total of 600 cells were plated in complete growth media at 37°C in a 5% CO2 incubator for 14 days and allowed to grow until visible colonies formed. Cell colonies were fixed with paraformaldehyde, stained with GIEMSA, washed and air dried. Colonies with more than 50 cells were counted under fluorescence microscopy.
Cell migration assay
The 8 μm pore-sized transwell inserts were coated with matrigel on the inner layer. pGCSIL-GFP-shZFX or -shNC infected LSCC primary cells were resuspended in serum-free medium and adjusted the density to 1×105/ml. The cells were allowed to migrate for 24 h at 37°C in a humidified incubator with 5% CO2. Non-invading cells were gently removed from the interior of the inserts with a cotton-tipped swab. Invasive cells were stained with GIEMSA staining. Then light absorbance was detected at 570 nm wavelength (OD570).
5000 cells were also seeded in 96-well plates. Then MTT assay were carried out by using CellTiter 96 Aqueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA). The absorbance was determined at 490 nm wavelength (OD490). The average cell migration rate was calculated and represented as OD570/OD490.
Cell apoptosis measurement
The apoptotic cells were detected with the Annexin V-APC Apoptosis Ddetection Kit (BioVision, Mountain View, CA, USA). In brief, 5×105 LSCC primary cells were infected with lentivirus expressing pGCSIL-GFP-shZFX or -shNC and incubated at 37°C for 4 days. After collection and washing with PBS buffer, cells were resuspended in 500 μl of 1x binding buffer. A total of 5 μl Annexin V-APC and 5 μl of PI were added and incubated at RT for 5 min in dark. Finally, cells were analyzed by FacScan (BD FACS Calibur, BD Bioscience, San Jose, CA, USA).
Statistical analysis
For Immunohistochemistry data, all data were expressed as mean ± SD. The quantitative data were approximate normal distribution and had constant variances. The independent-samples t test was used to evaluate the ZFX protein expression differences between the vocal leukoplakia and LSCC samples. The paired t test was used to evaluate the ZFX protein expression differences between the LSCC samples and adjacent non-tumoral samples. Sing factor analysis was used to evaluate the ZFX protein expression differences with different clinicopathlogical parameters.
The survival rate of LSCC patient was estimated using the Kaplan-Meier curves. The comparisons of Kaplan-Meier curves by different clinicopathlogical parameters were performed by the Log-rank test. Cox proportional hazard model was performed to evaluate the independent risk factors of survival time and prognosis.
All data of cell function study in LSCC primary cells, including MTT assay, BrdU-proliferation assay, cell colony formation assay, migration assay and flow cytometry analysis, were expressed as mean ± SD. The MTT assay was performed in quintuplicate at every timing point; the other assays mentioned above were performed in triplicate. The Student t test was used to evaluate the differences between the pGCSIL-GFP-shZFX group and -shNC group.
All data analysis was performed by using SPSS 20.0 software. P<0.05 was considered as statistically significant.
Results
ZFX was expressed both in the nucleus and cytoplasm of LSCC tissue, adjacent non-tumoral tissue and vocal leukoplakia
Initially, LSCC tissues, vocal leukoplakia tissues and adjacent non-tumoral tissues were examined and confirmed using hematoxylin and eosin (H&E) staining. Then, ZFX expression status was examined using immunohistochemistry (IHC) staining. All of the tissues mentioned above showed positive ZFX staining on different levels. Immunohistochemistry (IHC) staining revealed that ZFX protein was mainly expressed in the nucleus in LSCC tissue, adjacent non-tumoral tissue and vocal leukoplakia tissue. Its expression was also observed in the cytoplasm among all kinds of tissues mentioned above (Figure 1).
Figure 1.

Expression of ZFX proteins in LSCC tissue, adjacent non-tumoral tissue and vocal leukoplakia tissue using immunohistochemistry (IHC) and H&E staining (left 100×, right 400×). T tumor tissue, A adjacent non-tumoral tissue, V vocal leukoplakia tissue.
Expression levels of ZFX protein in LSCC tissues, adjacent non-tumoral tissues and vocal leukoplakia tissues
To detect the protein expression of ZFX in LSCC tissues, adjacent non-tumoral tissues and vocal leukoplakia tissues, immunohistochemistry and computer image analysis was applied. ZFX expression was quantified depending on the optical density of IHC signals. According to the result, the expression level of ZFX protein in LSCC was significantly higher than that in vocal leukoplakia (P<0.01) (Figure 2A). Furthermore, we found the expression of ZFX protein in early-stage LSCC was also statistically higher than that in vocal leukoplakia (P<0.01) (Figure 2B).
Figure 2.
Expression quantification of ZFX proteins in tissue samples. Quantification of ZFX expression depending on optical density of ZFX positive signals in IHC slices. A. LSCC tissue vs. vocal leukoplakia tissues. B. Early-stage LSCC tissue vs. vocal leukoplakia tissues. C. Corresponding LSCC tissues vs. adjacent non-tumoral tissues. D. Non-tumoral tissues vs. vocal leukoplakia tissues. The asterisk above the bar denotes statistically significant differences from the control group (P<0.05).
Among the 97 samples of LSCC, 47 of which had corresponding adjacent non-tumoral tissues. So we compared the expression level of ZFX protein in these LSCC tissues and corresponding adjacent non-tumoral tissues. The result revealed a markedly higher of ZFX protein level in LSCC than that in adjacent non-tumoral tissues (P<0.01) (Figure 2C).
A comparison with ZFX protein level between adjacent non-tumoral tissues and vocal leukoplakia tissues was performed in order to investigate if there were differences of ZFX expression between normal tissues and precancerous lesion tissues. The result showed no statistically difference of ZFX in both tissues (P>0.05) (Figure 2D).
Expression differences of ZFX protein in LSCC grouped by different clinicopathlogical parameters
The expression differences of ZFX protein in LSCC with different clinicopathlogicalal parameters were summarized in Table 1. According to the result, the level of ZFX was demonstrated to have a statistical difference between early-stage LSCC and middle-advanced LSCC (P<0.01). However, there was no difference between ZFX protein level and other clinicopathlogicalal parameters, including pathologic differentiation degree (P>0.05), T-level (P>0.05) and cervical lymph node metastasis (P>0.05). Taken together, the expression level of ZFX protein showed a significantly association with the clinical stage of LSCC.
Prognostic significance of ZFX protein expression in LSCC
To determine if ZFX could be used as an independent risk factor for poor prognosis of postoperative LSCC patients, six conventional clinicopathological factors were picked, including age, pathologic differentiation degree, T-level, cervical lymph node metastasis, clinical stage and the expression level of ZFX protein. The level of ZFX protein were split at the median of optical density (0.477) into two groups: low expression group (≤0.477) and high expression group (>0.477).
The Kaplan-Meier method was performed to investigate the influence of ZFX protein levels on the prognosis of LSCC patients. As shown in Figure 3, patients with high expression of ZFX was shown to have a lower overall survival rate than patients with low expression of ZFX (P=0.000). Then ZFX protein levels and other clinicopathological factors mentioned above were analyzed by Log-rank test. Univariate analysis suggested that ZFX expression, together with T-level, cervical lymph node metastasis, were significantly correlated with overall survival of LSCC patients (P=0.000, P=0.02, P=0.000). Moreover, multivariate analysis indicated that ZFX protein level (RR=3.298, P=0.008) and cervical lymph node metastasis (RR=2.900, P=0.007) were independent prognostic factors for overall survival of LSCC patients (Table 2).
Figure 3.

Kaplan-Meier graph showing the overall survival for patients with LSCC. Patients with high expression of ZFX had a significant lower overall survival rate than those with low expression of ZFX (P<0.01).
Table 2.
Prognosis analysis for classified factors influencing the overall survival rate of LSCC patients
| Clinicopathlogical parameters | n | Survival rate | Log-rank | P value | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n | Percent (%) | P value | RR (95% CI) | ||||
| Age | 0.386 | 0.520 | |||||
| ≤60 years old | 48 | 35 | 73.0 | ||||
| >60 years old | 49 | 33 | 67.4 | ||||
| Differentiated degree | 1.054 | 0.590 | |||||
| Poorly differentiated | 13 | 10 | 76.9 | ||||
| Moderately differentiated | 48 | 35 | 72.9 | ||||
| Well-differentiated | 36 | 23 | 63.9 | ||||
| T-level | 9.878 | 0.020 | |||||
| T1 | 19 | 15 | 78.9 | ||||
| T2 | 28 | 22 | 78.6 | ||||
| T3 | 29 | 21 | 72.4 | ||||
| T4 | 21 | 10 | 47.6 | ||||
| Cervical lymph node metastasis | 16.205 | 0.000 | 0.007 | 2.900 (1.347-6.245) | |||
| Without cervical lymph node metastasis | 77 | 60 | 77.9 | ||||
| With cervical lymph node metastasis | 20 | 8 | 40 | ||||
| Clinical stage | 0.339 | 0.560 | |||||
| Early | 44 | 32 | 72.7 | ||||
| Middle-advanced | 53 | 36 | 67.9 | ||||
| Expression level of ZFX | 13.150 | 0.000 | 0.008 | 3.298 (1.368-7.949) | |||
| Low expression group | 51 | 44 | 86.3 | ||||
| High expression group | 46 | 24 | 52.2 | ||||
Expression of ZFX was suppressed by pGCSIL-GFP-shZFX in LSCC primary cells
To further explorer the biological role of ZFX in LSCC, we generated lentiviruses that expressed the ZFX-specific siRNA or control siRNA, respectively. The lentiviral vector system (pGCSIL-GFP-shZFX and -shNC) down-regulated the expression of ZFX in LSCC primary cells. As expected, a high percentage (>80%) of LSCC primary cells expressed GFP after lentivirus transfection (Figure 4A), indicating the high efficiency and stability of transfection. ZFX mRNA level in pGCSIL-GFP-shZFX-infected cells was reduced in comparison with control (Figure 4B). It suggested that the siRNA targeting ZFX is effective to silence ZFX gene in LSCC primary cells.
Figure 4.

The expression of ZFX was dramatically knocked down by lentivirus mediated shZFX transfection. A. Microscope pictures of GFP-expressing cells after 96 h after pGCSIL-GFP-shZFX or -shNC transfection, including fluorescence microscope images and light microscope images (100×). B. Quantitative analysis of ZFX by qRT-PCR. The asterisk above the bar denotes statistically significant differences from the control group, P<0.05.
Reduction of ZFX affects cell proliferation of LSCC primary cells
MTT and colony formation assays were performed to investigate the effect of ZFX reduction in LSCC primary cells. Both assays revealed reduction of ZFX dramatically inhibited proliferation of LSCC primary cells compared with that of control cells (Figure 5A, 5B). Furthermore, BrdU assay showed that ZFX-inhibited cells displayed lower DNA synthesis levels than that in control cells after 96 h of transfection (Figure 5C). Taken together, these data suggested that ZFX may function as an oncogenic regulator of LSCC.
Figure 5.

The effect of ZFX knockdown on the proliferation of human LSCC primary cells. A. MTT assay of cell proliferation in LSCC primary cells infected with pGCSIL-GFP-shZFX or -shNC. The asterisk under the line denotes statistically significant differences from the control group (P<0.05). B. Knockdown of ZFX decreased colony number of LSCC primary cells. pGCSIL-GFP-shZFX or -shNC infected LSCC primary cells were cultured for 2 weeks and stained with GIEMSA. Quantitative analysis of colony formation was shown. C. A BrdU ELISA was performed to determine proliferation of LSCC primary cells after knockdown of ZFX. The asterisk above the bar denotes statistically significant differences from the control group (P<0.05).
Reduction of ZFX inhibited the migration of LSCC primary cells
The migration capacity of LSCC primary cells was measured via Transwell migration assays. Compared with control cells, the average migration rate displayed a consistent reduction when cells were infected with the pGCSIL-GFP-shZFX (0.816±0.091 vs 2.048±0.082, P<0.05) (Figure 6). The result illustrated that ZFX may be involved in cell migration of LSCC primary cells.
Figure 6.
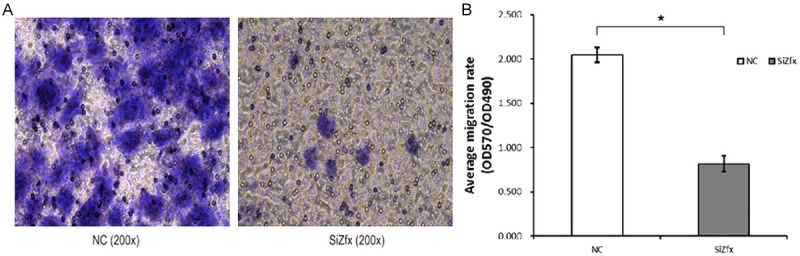
The effect of ZFX-knockdown on cell migration in human LSCC primary cells. A. Transwell assay in LSCC primary cells infected with pGCSIL-GFP-shZFX and -shNC. Migrated cells on the lower surface of the transwell filter were stained by GIEMSA, photographs were taken after 24 h post-migration (200×). B. The average migration rate was measured in the Transwell assay. The average cell migration rate was calculated and represented as OD570/OD490. The asterisk above the bar denotes statistically significant differences from the control group (P<0.05).
Apoptosis induced by the reduction of ZFX gene
In order to examine whether knockdown of ZFX affect cell survival, cell apoptosis was detected by flow cytometry assay. After 6 days of infection with pGCSIL-GFP-shZFX, the apoptotic cells were determined by Annexin V-APC staining (Figure 7). The results showed apoptosis rate reached approximately 30% treated with pGCSIL-GFP-shZFX, compared with less than 5% in control cells. Taken together, ZFX has been shown to be a regulator in apoptosis and cell growth.
Figure 7.
Apoptosis induction in LSCC primary cells infected with lentivirus expressing ZFX-specific siRNA. A. Cell apoptosis by annexin-V staining was analyzed with flow cytometry. B. FACS analysis of Annexin V-APC stained control cells or ZFX-knockdown cells 5 days after transient infection. Data shown here is the mean ± SD of cell percentage in apoptosis from three independent experiments. The asterisk above the bar denotes statistically significant differences from the control group (P<0.05).
Discussion
Zinc finger protein X-linked (ZFX) belongs to the zinc finger protein super family. Located at the X chromosome, ZFX is highly conservative in vertebrate and has a similar structure with Zfy which located at the Y chromosome. ZFX’s sequence contains 13 C2H2 zinc finger structures with an acidic transcriptional activation domain (AD), a nuclear localization sequence (NLS) and a DNA binding domain (DBD) [9]. Research showed ZFX played an important role in various biological processes, such as gene expression, cell differentiation and embryonic development [10]. As a regulation factor, ZFX participates in cell cycle control, self-renewal in several sorts of stem cell, cell proliferation and the regulation of cancer related gene or pathway [11]. Previous studies have indicated that ZFX can function as an oncoprotein in various types of cancer such as gastric cancer [12], gallbladder cancer [7] and hepatocellular cancer [13]. However, limited data are available about the prognostic significance of ZFX in cancer, and less known about its role in LSCC.
In this study, firstly, we performed immunohistochemistry and image analysis to detect the expression of ZFX protein in LSCC tissues, vocal leukoplakia tissues and corresponding adjacent non-tumoral tissues. We found that the ZFX protein level in tumor tissues was significantly higher than that in corresponding adjacent non-tumoral tissues, implying a possible involvement of ZFX in LSCC development. We compared the difference of ZFX protein level between LSCC and vocal leukoplakia, and found the ZFX protein level in LSCC was markedly higher than that in vocal leukoplakia. Even in early-stage LSCC, the level of ZFX protein still showed an overexpression compared with its level in vocal leukoplakia. Vocal leukoplakia is precancerous lesion of laryngeal. Its incidence of malignant change is nearly 8%. The malignant change of vocal leukoplakia is strongly related to the level of dysplastic vocal fold keratinized epithelium [14]. Present result revealed an up-regulated expression of ZFX protein during the malignant transformation from vocal leukoplakia to LSCC, which suggested a potential role of ZFX in the early diagnosis of LSCC.
Further exploration to examine the expression differences of ZFX in LSCC grouped by clinicopathlogical parameters was performed and showed that the expression of ZFX was associated with the clinical stage of LSCC. ZFX protein level was significantly higher in middle-advanced LSCC than that in early-stage LSCC. The result shown here have been supported by previous studies in other tumors, which have also suggested that over-expression of ZFX is essential for the progress and metastasis of malignancies [5-7]. Although no statistically association was found with the expression of ZFX protein and other clinicopathlogical parameters (pathologic differentiation degree, T-level and cervical lymph node metastasis), we still need to treat LSCC as a whole. The early lesion of LSCC has limited size, limited involvement, and low probability of cervical lymph node metastasis or distant metastasis. Conversely, the advanced lesion is always characterized by extensive local aggression, cervical lymph node metastasis and distant metastasis. We speculate the overexpression of ZFX during the advancement of LSCC might be a result of multiple factor influences.
In the clinical management, prognostic biomarkers have been regarded as necessary tools for predicting the progression of disease. Yan et al. has reported that ZFX could be a prognostic factor for cancer patients [15]. In order to investigate whether ZFX could be used as a clinical predictor for LSCC patients, we performed a survival analysis by using Kaplan-Meier method as well as Cox proportional hazard model. Interestingly, we found that patients with high expression of ZFX had a lower survival rate, and ZFX protein level was an independent prognostic factor for LSCC patients. Higher level of ZFX protein in LSCC might be a sign of a tumor that is able to grow faster and more aggressive. As a result, the tumor is more likely to have extensive local involvement, early metastasis and recurrence. Studies have highlighted the presence of cancer stem cells (CSCs) in head and neck squamous cell carcinoma. Research has proven that CD44+ cells in LSCC cell lines contain CSCs. These cells have two characteristics similar to normal stem cells-self-renewal and differentiation potential [16]. These characteristics may contribute to the recurrence and metastasis of cancer. It has been identified in hepatocellular carcinoma cells that ZFX was able to regulate the expression of CSC markers, such as Nanog and SOX-2 [13]. Another study also reported that ZFX controls c-Myc expression to maintain the self-renewal and tumorigenic potential of glioma stem cells [17]. Thus, it is possible that ZFX could promote the survival of LSCC cells partly by inducing cell stemness, which may lead to the recurrence of tumor. It is widely accepted that tumor cells must alter shapes and lose cell-cell junction before invasion and metastasis, which commonly appears to involve epithelial-mesenchymal transition (EMT). Several studies implied the association between ZFX and EMT [8,18]. Moreover, both CSC characteristics and signal pathways ZFX regulate are closely linked to EMT [19,20], also indicating an involvement of ZFX in EMT. In summarize, it is very likely that overexpression of ZFX participated the recurrence and metastasis of LSCC. To the best of our knowledge, this is the first report demonstrating the prognostic significance of ZFX in head and neck cancer patients, and also the first study exploring its role in LSCC.
According to previous studies and our findings, we speculate that ZFX involved in development of LSCC by regulating the function of tumor cells. To further investigate its role in development of LSCC, we designed lentivirus-delivery strategy to suppress the expression of ZFX. As a result, the expression of ZFX in LSCC primary cells was obviously suppressed by lentivirus-mediated shRNA, which indicates the efficiency of shRNA treatment and the delivery system. This lentivirus-mediated delivery system is considered more effective as it allows gene silencing in non-dividing cells; thereby the efficiency and effectiveness of gene silencing system in enhanced [21]. As the expression of ZFX was suppressed, the proliferation and colony formation ability was significantly reduced in LSCC primary cells, and the DNA synthesis level was also markedly impaired. It was also found that the migration of LSCC primary cells was suppressed. Knockdown of ZFX resulted in the inhibition of cell growth and migration, which could contribute to LSCC treatment. Further analysis showed depletion of ZFX induced significantly apoptosis of LSCC primary cells.
In consistent with our previous studies on Hep-2 human LSCC cell line, current study indicated the key role of ZFX in regulation of cell proliferation, and apoptosis. Our prior studied pointed out that knockdown of ZFX with its siRNA attenuated expression of AKT, Survivin and Ki-67 [22]. These three genes belong to the inhibitor of apoptosis (IAP) gene family and their products can prevent apoptotic cell death [23]. Recent studies also reported that ZFX participated cell apoptosis and proliferation in some kind of cancer via the regulation of AKT and Survivin levels, or their targets’ levels. Moreover, ZFX has been confirmed as a transcriptional target of cyclin D1, which act as an important role on cell cycle and apoptosis. And the level of cyclin D1 was found markedly changed after ZFX reduction in these cancer cell lines [24-26]. These changes after ZFX knockdown may also happened in LSCC primary cells and needed to be proved. Yet we may audaciously hypothesized that ZFX binds to the promoter regions of these genes to directly regulate their expression or function with other regulators as inhibitors or promoters in LSCC cells. Further investigation should be considered when extended the usage of ZFX.
In conclusion, the research of ZFX gene and its function in human malignant tumor is still at explorative phase. Strong evidences are needed to further explorer its molecular mechanisms. As the researches for ZFX in head and neck cancer have only just caught on, we hope this study would supply a research basis for understanding the role of ZFX in head and neck squamous carcinoma, especially in LSCC.
Acknowledgements
The authors are thankful for the financial support from National Natural Science Foundation of China (NSFC) (81072204), Beijing Municipal Science & Technology Commission (Z141107002514003) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (XMLX201311).
Disclosure of conflict of interest
None.
References
- 1.Chu EA, Kim YJ. Laryngeal cancer: diagnosis and preoperative work-up. Otolaryngol Clin North Am. 2008;41:673–95. doi: 10.1016/j.otc.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Lin HW, Bhattacharyya N. Staging and survival analysis for nonsquamous cell carcinomas of the larynx. Laryngoscope. 2008;118:1003–13. doi: 10.1097/MLG.0b013e3181671b3d. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman HT, Porter K, Karnell LH, Cooper JS, Weber RS, Langer CJ, Ang KK, Gay G, Stewart A, Robinson RA. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(Suppl 111):1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 5.Wu S, Lao XY, Sun TT, Ren LL, Kong X, Wang JL, Wang YC, Du W, Yu YN, Weng YR, Hong J, Fang JY. Knockdown of ZFX inhibits gastric cancer cell growth in vitro and in vivo via downregulating the ERK-MAPK pathway. Cancer Lett. 2013;337:293–300. doi: 10.1016/j.canlet.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Li K, Xu D, Liu Y, Tang H, Xie Q, Xie L, Liu J, Wang H, Gong Y, Hu Z, Zheng J. ZFX regulates glioma cell proliferation and survival in vitro and in vivo. J Neurooncol. 2013;112:17–25. doi: 10.1007/s11060-012-1032-z. [DOI] [PubMed] [Google Scholar]
- 7.Tan Z, Zhang S, Li M, Wu X, Weng H, Ding Q, Cao Y, Bao R, Shu Y, Mu J, Ding Q, Wu W, Yang J, Zhang L, Liu Y. Regulation of cell proliferation and migration in gallbladder cancer byzinc figer X-chromosomal protein. Gene. 2013;528:261–266. doi: 10.1016/j.gene.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 8.Li K, Zhu ZC, Liu YJ, Liu JW, Wang HT, Xiong ZQ, Shen X, Hu ZL, Zheng J. ZFX knockdown inhibits growth and migration of non-small cell lung carcinoma cell line H1299. Int J Clin Exp Pathol. 2013;6:2460–2467. [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider-Gädicke A, Beer-Romero P, Brown LG, Mardon G, Luoh SW, Page DC. Putative transcription activator with alternative isoforms encoded by human ZFX gene. Nature. 1989;342:708–11. doi: 10.1038/342708a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Su Z, Huang Y, Sun T, Chen S, Wu T, Chen G, Xie X, Li B, Du Z. The ZFX gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J Exp Clin Cancer Res. 2011;30:114. doi: 10.1186/1756-9966-30-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu G, Kim J, Xu Q, Leng Y, Orkin SH, Elledge SJ. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev. 2009;23:837–48. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikpour P, Emadi-Baygi M, Mohammad-Hashem F, Maracy MR, Haghjooy-Javanmard S. Differential expression of ZFX gene in gastric cancer. J Biosci. 2012;37:85–90. doi: 10.1007/s12038-011-9174-2. [DOI] [PubMed] [Google Scholar]
- 13.Lai KP, Chen J, He M, Ching AK, Lau C, Lai PB, To KF, Wong N. Overexpression of ZFX confers self-renewal and chemoresistance properties in hepatocellular carcinoma. Int J Cancer. 2014;135:1790–9. doi: 10.1002/ijc.28819. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett RS, Heckman WW, Isenberg J, Thibeault SL, Dailey SH. Genetic characterization of vocal fold lesions: leukoplakia and carcinoma. Laryngoscope. 2012;122:336–42. doi: 10.1002/lary.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan X, Yan L, Su Z, Zhu Q, Liu S, Jin Z, Wang Y. Zinc-finger protein X-linked is a novel predictor of prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2014;7:3150–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang X, Huang Z, Zhou W, Wu Q, Sloan AE, Ouyang G, McLendon RE, Yu JS, Bao S. The Zinc Finger Transcription Factor ZFX is Required for Maintaining the Tumorigenic Potential of Glioblastoma Stem Cells. Stem Cells. 2014;32:2033–2047. doi: 10.1002/stem.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim J, Cha HJ. Loss of E-cadherin activates EGFRMEK/ERK signaling, which promotes invasion via the ZEB1/MMP2 axis in non-small cell lung cancer. Oncotarget. 2013;4:2512–2522. doi: 10.18632/oncotarget.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Qiu XS. Cancer stem cells and tumor metastasis. J Cancer Res Ther. 2013;9(Suppl):S150–152. doi: 10.4103/0973-1482.122510. [DOI] [PubMed] [Google Scholar]
- 20.Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-tomesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Stewart SA, Dykxhoorn DM, Palliser D, Mizyno H, Yu EY, An DS, Sabatini DM, Chen ISY, Hahn WC, Sharp PA, Weinberg RA, Novina CD. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang JG, Yu Z, Lian M, Ma H, Tai J, Zhang L, Han D. Knockdown of zinc finger protein, Xlinked (ZFX) inhibits cell proliferation and induces apoptosis in human laryngeal squamous cell carcinoma. Mol Cell Biochem. 2012;360:301–7. doi: 10.1007/s11010-011-1069-x. [DOI] [PubMed] [Google Scholar]
- 23.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 24.Fang Q, Fu WH, Yang J, Li X, Zhou ZS, Chen ZW, Pan JH. Knockdown of ZFX suppresses renal carcinoma cell growth and induces apoptosis. Cancer Genet. 2014;207:461–6. doi: 10.1016/j.cancergen.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Lu Y, Zheng Y, Yu X, Xia X, He X, Feng W, Xing L, Ling Z. shRNAmediated silencing of ZFX attenuated the proliferation of breast cancer cells. Cancer Chemother Pharmacol. 2014;73:569–76. doi: 10.1007/s00280-014-2379-y. [DOI] [PubMed] [Google Scholar]
- 26.Casimiro MC, Crosariol M, Loro E, Ertel A, Yu Z, Dampier W, Saria EA, Papanikolaou A, Stanek TJ, Li Z, Wang C, Fortina P, Addya S, Tozeren A, Knudsen ES, Arnold A, Pestell RG. ChIP sequencing of cyclin D1 reveals a transcriptional role in chromosomal instability in mice. J Clin Invest. 2012;122:833–843. doi: 10.1172/JCI60256. [DOI] [PMC free article] [PubMed] [Google Scholar]




