Abstract
Pulmonary hypertension and related pulmonary vascular diseases cause significant morbidities and high mortality and present many unique challenges toward improving outcomes in neonates, infants, and children. Differences between pediatric and adult disease are reflected in controversies regarding etiologies, classification, epidemiology, diagnostic evaluations, and therapeutic interventions. This brief review highlights several key topics reflecting recent advances in the field and identifies persistent gaps in our understanding of clinical pediatric pulmonary hypertension.
Key Words: pediatric cardiology, pediatric pulmonary, pulmonary hypertension
Abbreviations: BPD, bronchopulmonary dysplasia; CDH, congenital diaphragmatic hernia; FDA, US Food and Drug Administration; NT-proBNP, N-terminal pro-brain-type natriuretic peptide; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PPHN, persistent pulmonary hypertension of the newborn; PVR, pulmonary vascular resistance; PVRI, Pulmonary Vascular Research Institute; TAPSE, tricuspid annular plane systolic excursion; WHO, World Health Organization
Pulmonary hypertension (PH), defined as an abnormal elevation of pulmonary arterial pressure, causes significant morbidity and mortality in the pediatric population. Although there are similarities in etiology and disease pathogenesis between pediatric and adult forms of PH, many cardiopulmonary and systemic diseases associated with PH are unique to neonates, infants, and children. There has been growing recognition of the important impact of PH after premature birth and in the settings of developmental lung diseases, genetic syndromes, and diverse factors that reflect interactions between prenatal and postnatal influences (Fig 1). PH-related hospitalizations of children are increasing, which likely reflect improved recognition and awareness of the role of PH in diverse settings, or perhaps, an actual increase in the incidence of disease.1, 2 In this review, we briefly discuss important challenges in the definition, classification, diagnosis, evaluation, and treatment of children with PH, highlighting differences from adult PH.
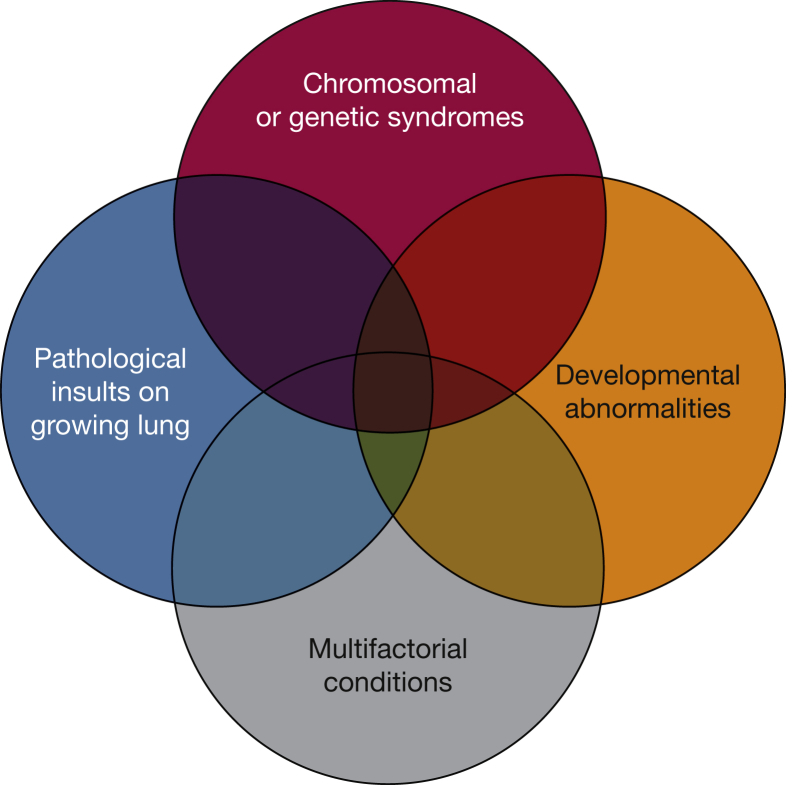
Figure 1.
Venn diagram illustrating the heterogeneity and multifactorial elements in pediatric pulmonary hypertensive vascular disease. Insults on the growing lung may be due to ventilator-induced lug injury. Developmental abnormalities may include conditions such as bronchopulmonary dysplasia. Examples of multifactorial conditions may include aspiration or congenital heart disease.
(From del Cerro et al.6)
Definition
PH in adults refers to an increased mean pulmonary artery pressure (PAP) > 25 mm Hg. Pulmonary arterial hypertension (PAH) refers specifically to precapillary pathology and is defined by mean PAP > 25 mm Hg with a normal pulmonary artery wedge pressure of 15 mm Hg or less. However, PAP must progressively decline during the first few months after birth from values that match systemic arterial levels in utero to values of mean PAP that are similar to adults. Thus, the definition of PH in children is the same as in adults beyond 3 months of age at sea level.3 Failure of the normal transition of the pulmonary circulation at birth leads to sustained elevation of PAP to near systemic levels and causes the syndrome of persistent pulmonary hypertension of the newborn (PPHN) (Table 1). PPHN is associated with diverse disorders, such as meconium aspiration, congenital diaphragmatic hernia, and others, and is characterized by extrapulmonary right-to-left shunting of blood across the patent foramen ovale and/or ductus arteriosus, causing severe hypoxemia.4 Although pulmonary vascular resistance (PVR) is not included in the Nice classification system per se,5 it is included in the pediatric Panama classification because of the greater prevalence of congenital heart diseases, which have vastly different outcomes depending on PVR but with a similar elevation of mean PAP.6 Although diagnostic criteria for PAH are the same in adults and children, some question the use of a mean PAP > 25 mm Hg in infants or young children, whose systemic BP is significantly lower than that of an adult. Many pediatric practitioners use indexed PVR > 3 units × m2 as well as a ratio of PVR to systemic vascular resistance > 0.5 in determining the presence of pediatric PAH.3, 7
Table 1.
Nice Classification of PH
| 1. Pulmonary arterial hypertension |
| 1.1 Idiopathic |
| 1.2 Heritable (1.2.1 BMPR2, 1.2.2 ALK-1, ENG, SMAD9, CAV1, KCNK3, 1.2.3 unknown) |
| 1.3 Drug- and toxin-induced |
| 1.4 Associated (1.4.1 Connective tissue disease, 1.4.2 HIV infection, 1.4.3 Portal hypertension, 1.4.4 Congenital heart diseases, 1.4.5 Schistosomiasis) |
| 1′ Pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis |
| 1″ Persistent PH of the newborn |
| 2. PH from left heart disease |
| 2.1 Left ventricular systolic dysfunction |
| 2.2 Left ventricular diastolic dysfunction |
| 2.3 Valvular disease |
| 2.4 Congenital/acquired left heart inflow/outflow tract obstruction, congenital cardiomyopathies |
| 3. PH from lung disease and/or hypoxia |
| 3.1 COPD |
| 3.2 Interstitial lung disease |
| 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4 Sleep-disordered breathing |
| 3.5 Alveolar hypoventilation disorders |
| 3.6 Chronic exposure to high altitude |
| 3.7 Developmental lung diseases |
| 4. Chronic thromboembolic PH |
| 5. PH from unclear multifactorial mechanisms |
| 5.1 Hematologic disorders: chronic hemolytic anemia, myeloproliferative disorders, splenectomy |
| 5.2 Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis |
| 5.3 Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4 Other: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH |
Pediatric-related revisions to the WHO classification system are highlighted in boldface type. PH = pulmonary hypertension. (From Simonneau et al.5)
Classification
Classification of pediatric PH is challenging because of the many diseases associated with PH across the life span, ranging from the fetus to the onset of adulthood. The World Health Organization (WHO)/World Symposium and Panama classifications have been applied to children with PH; however, controversies persist regarding the relative utility of each system.6, 7 The WHO Symposium in 1998 at Evian originally established a classification schema that described five subgroups of PH disorders, which have been revised in subsequent symposia in Venice (2003), Dana Point (2008), and Nice (2013). Organization of these subgroups was based on a common pathobiology and treatment strategy, which include: PAH (group 1); PH from left heart disease (group 2); PH from chronic lung disease and/or hypoxia (group 3); chronic thromboembolic pulmonary hypertension (group 4); and PH from unclear multifactorial mechanisms (group 5) (Table 1).5, 8, 9 The initial meetings focused primarily on PH in the adult patient, but one of the goals of the most recent meeting in Nice (the 5th World Symposium, in 2013) updated the current classification and included changes that reflect recommendations from the pediatric PH community5, 7 (Table 1). Among several changes, the emphasis on the unique aspects of PPHN is highlighted (group 1″), as well as changes regarding complex congenital heart disease (group 2.4) and the growing importance of developmental lung disorders (group 3.7). Because of the increased recognition of PH associated with complex congenital heart disease, congenital or acquired left-heart inflow/outflow obstructive lesions and congenital cardiomyopathies were added to group 2. Examples of these obstructive lesions include PH resulting from congenital cor triatriatum, supravalvar mitral stenosis, aortic valve stenosis, or pulmonary vein stenosis, which is increasingly recognized as associated with prematurity.10 Segmental PH from systemic blood supply to regions of the lung or discrete stenosis in the pulmonary arterial bed, as seen in patients with pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries,11 was added to group 5. Developmental lung diseases, such as congenital diaphragmatic hernia (CDH) or bronchopulmonary dysplasia (BPD), were emphasized because of the growing recognition of the role of abnormal lung vascular growth in the pathogenesis of PH and impaired lung structure in these disorders.7
A striking difference between the adult and childhood forms of pulmonary hypertensive vascular disease is that during fetal, neonatal, and early postnatal life, the developing lung circulation is exposed to pathological and environmental insults during critical periods of growth and maturation that cause maladaptation at birth, impaired structural and functional maturation, or growth arrest. Although generally helpful, the Nice classification does not specifically include PH caused by prenatal or perinatal diseases associated with PH.
To address the needs for a classification system that incorporates more of the pediatric-specific aspects of pathobiology and related clinical issues, a comprehensive classification of pediatric pulmonary hypertensive vascular disease was proposed at the Panama Pulmonary Vascular Research Institute (PVRI) symposium in 2011, highlighting 10 major categories of pediatric pulmonary vascular disease (Table 2). There is a specific focus on the multifactorial nature of pediatric disease because children may carry multiple diagnoses contributing to pulmonary vascular disease; for example, a single patient may have PH associated with prematurity, congenital heart disease, and postnatal insults on the developing lung (Fig 1). In addition, the Panama classification suggests that congenital heart disease palliated to single-ventricle physiology may not meet traditional criteria for PAH because of their unique circulation. The Panama definition of pulmonary hypertensive vascular disease following a cavopulmonary anastomosis is defined as a PVRI > 3.0 Wood units × m2 or a transpulmonary gradient > 6 mm Hg (as measured by mean PAP – mean left atrial pressure), even if the mean PAP is < 25 mm Hg and does not meet the traditional diagnosis of PH per se.6 These examples illustrate the potential role for a pediatric-specific classification system, but whether such a system will prove practical and useful requires further study.
Table 2.
Panama Classification System of Pediatric Pulmonary Vascular Disease
| Category | Description |
|---|---|
| 1 | Prenatal or developmental pulmonary hypertensive vascular disease |
| 2 | Perinatal pulmonary vascular maladaptation |
| 3 | Pediatric cardiovascular disease |
| 4 | Bronchopulmonary dysplasia |
| 5 | Isolated pediatric pulmonary hypertensive vascular disease (isolated pediatric PAH) |
| 6 | Multifactorial pulmonary hypertensive vascular disease in congenital malformation syndromes |
| 7 | Pediatric lung disease |
| 8 | Pediatric thromboembolic disease |
| 9 | Pediatric hypobaric hypoxic exposure |
| 10 | Pediatric pulmonary vascular disease associated with other system disorders |
PAH = pulmonary arterial hypertension. (From del Cerro et al.6)
Two diseases that best highlight critical aspects of the role of lung vascular development in the pathogenesis and clinical course of pediatric PH are BPD and CDH. Impaired lung development contributes to PH in extremely premature infants, combined with adverse postnatal effects from prolonged ventilator requirement, hypoxia, hyperoxia, and inflammation, which inhibit alveolar and vascular development after birth. BPD, the chronic lung disease associated with prematurity, is diagnosed in up to 68% of infants born before 28 weeks’ gestation.12 PH is reported in 17% to 25% of infants with severe BPD.13, 14 Importantly, early echocardiographic signs of pulmonary vascular disease as early as 7 days of life may be associated with higher risk of late PH in preterm infants at risk of BPD.15 Infants with more severe BPD tend to be at higher risk for development of PH, but there is not an absolute correlation with severity of lung disease.16, 17 Risk factors for PH include lower gestational age, small-for-gestational age birth weight, oligohydramnios, preeclampsia, prolonged duration of mechanical ventilation, and oxygen therapy, which may suggest genetic, epigenetic, or environmental factors.13, 14, 17, 18, 19 Recent data from animal models and humans suggest risk factors distinct from BPD severity may be related to placental hypoperfusion resulting from an imbalance of circulating angiogenic factors such as endostatin, angiopoietin-1, and altered expression of vascular endothelial growth factor receptors and nitric oxide synthase.20, 21, 22, 23, 24, 25 Although PH can resolve with therapy in some premature infants, the persistence of severe PH is associated with high mortality, which has been reported as high as 47%.16 The high mortality likely reflects, at least in part, the ineffectiveness of current therapies at promoting normal lung growth and development. Novel therapies to promote lung growth, such as stem cell therapy, may prove promising in treatment of PH in premature infants.26
Underdevelopment of the distal lung and its vasculature contribute to high risk for severe PH in other developmental diseases, such as CDH. CDH is a defect in diaphragm development present in ∼1:3000 live births, in which abdominal viscera herniate into the chest in fetal life.27, 28 PH has a significant impact on mortality in CDH, with extremely poor survival of CDH infants with severe PH that persists at 6 weeks of age.29, 30 PH in CDH is thought to be due to marked reductions of lung vascular surface area and increased muscularization of distal pulmonary arteries, resulting in lesions often as aggressive as those in older patients with other forms of severe PAH (Fig 2).31 Molecular mechanisms contributing to PH in CDH are uncertain, but experimental studies suggest roles for endothelial dysfunction,32 altered BMPR2 signaling,33, 34 increased endothelin-1,35 and inflammatory cytokines.36, 37
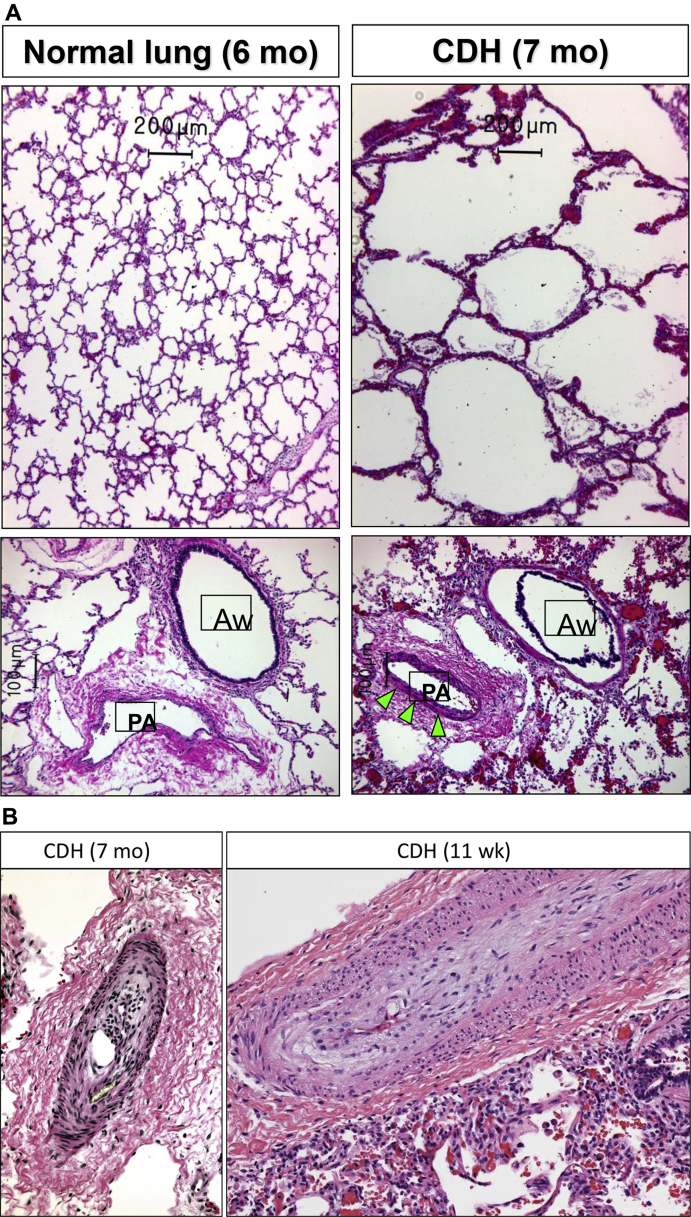
Figure 2.
A, Postmortem lung pathology from a normal lung at 6 months of age and lung from a patient with CDH at 7 months of age. CDH lung shows alveolar simplification (top) and muscularization of the small pulmonary arteries (arrows, bottom). Images courtesy Marcus Davey, PhD. B, Muscular hypertrophy and intimal hyperplasia in pulmonary arteries from lung biopsy specimens of two pediatric patients with CDH and PH. Aw, airway; PA, pulmonary artery.
Left image courtesy Marcus Davey, PhD. Right image courtesy Kudakwashe Chikwava, MBBCh.
Epidemiology
Although the true incidence and prevalence of PH in the pediatric population remain uncertain, recent epidemiologic data from national or international registries have begun to shed some light. The Netherlands PH service registry, which includes 3263 pediatric patients over a 15-year period, estimated an annual incidence of 63.7 cases per million children. PPHN and transient PH following repair of congenital heart disease composed the majority of cases (30.1 and 21.9 cases per million, respectively), whereas idiopathic PAH was 0.7 cases per million and persistent PAH associated with congenital heart disease was 2.2 cases per million. Chromosomal abnormalities, most commonly trisomy 21, were reported in 13% of patients. Excluding cases of transient PH, the majority of cases of pediatric PH were included in Nice group 1 (ie, idiopathic PAH, heritable PAH, or PAH associated with congenital heart disease). Developmental lung disease accounted for just under 6% of cases, which raises some concerns regarding bias in patient referral and inclusion in the registry.38 The Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension (TOPP) registry, which included 362 patients with catheterization-confirmed PH, 317 (88%) of those were classified as PAH (57% idiopathic PAH and 36% associated PAH).39 Efforts are currently underway to create a comprehensive registry of patients with pediatric PH in the United States and Canada by centers in the Pediatric Pulmonary Hypertension Network.40
Survival in pediatric PH has improved dramatically since the advent of targeted PH therapies.41 The Registry to Evaluate Early and Long-Term PAH (REVEAL) included 216 pediatric patients and reported 1-, 3-, and 5-year estimated survival rates of 96 ± 4%, 84 ± 5%, and 74 ± 6%, respectively, with no significant difference between idiopathic PAH and congenital heart disease–associated PAH.42 The UK PH service reported idiopathic PAH survival rates in 2001-2006 of 85.6%, 79.9%, and 71.9% at 1, 3, and 5 years, respectively, and survival of subjects with congenital heart disease with associated PAH of 92.3%, 83.8%, and 56.9% at 1, 3, and 5 years.43 The Netherlands registry reported 1-, 3-, and 5-year survival for patients with progressive PAH as 73%, 63%, and 60%, respectively. Survival was markedly worse in patients with congenital heart disease without shunts or those with PH that persisted after surgical repair.38 PH-related hospitalizations appear to have increased over the past decade; however, because of uncertain factors, such as earlier recognition of disease, diagnosis of PH in more diverse settings, or improved care, hospital mortality has decreased during this period.1, 2 Additional data are needed from prospective registries or informatics studies to better understand the true prevalence, natural history, and outcomes of diverse forms of pediatric PH.
Diagnosis
Delays in making the diagnosis 1 to 2 years after the onset of disease are not uncommon in pediatric PH, which is likely from the nonspecific nature of early symptoms. In the TOPP registry, the most common presenting symptoms were dyspnea on exertion, fatigue, and syncope.39 Children with PAH are often misdiagnosed with more common childhood conditions such as asthma, vasovagal syncope, or seizures before diagnosis with PAH. A standardized approach to diagnostic testing has been recommended in the recently published American Heart Association and American Thoracic Society Joint Guidelines for Pediatric PH Guidelines. Although less common in children than adults, left heart disease is important to exclude before the initiation of targeted PH therapy.3
Echocardiography is widely used in pediatrics as a noninvasive screen for PH and evaluation of structural congenital heart disease. As in adults, multiple parameters are used to estimate the right ventricular/PAP, including tricuspid and pulmonary valve regurgitation jet velocities and interventricular septal position. Moderate or greater septal flattening occurs when right ventricular systolic pressure is at least half systemic.44 Although these parameters show good correlation in adults with PH, one evaluation of children with PH related to BPD showed an echo estimate of PH severity only correlated with hemodynamic assessment 47% of the time.45 Validation of right ventricular systolic and diastolic functional assessment tools including right atrial volume, tricuspid annular plane systolic excursion (TAPSE), fractional area of change, and eccentricity index is ongoing in pediatrics. Age-dependent normal values have been reported for TAPSE.46 TAPSE is reduced in infants with PH.47 Echocardiographic indices of right ventricular function, including TAPSE, have been shown to correlate with outcome in children with IPAH.48
Brain-type natriuretic peptide (BNP), or its cleavage product N-terminal (NT) pro-BNP, has been shown to correlate with severity of PAH in children.49 The REVEAL study found low BNP (< 50 pg/mL) or NT-proBNP (< 300 pg/mL) predict lower mortality in children with PAH.42 BNP may also be a prognostic marker in PH associated with BPD and CDH.50, 51
Advanced imaging is increasingly used in the assessment of pediatric PH. MRI has been used to assess abnormalities of the right ventricular function and volume longitudinally.52 Novel assessment of additional MRI parameters, including wall stress, may also prove useful for assessment of disease severity over time.53 MRI may also play a role in in real-time assessment of PAH because septal curvature was shown to correlate with hemodynamic measurement of PAH severity.54
Although noninvasive tests are clinically useful, cardiac catheterization remains the gold standard for diagnosis and ongoing assessment of PH in children.3 Acute vasodilator testing may be performed for evaluation of potential therapies, notably calcium channel blockers. The criteria for prognosis and use of calcium channel blockers for idiopathic PAH are generally defined as a decrease in mean PAP of at least 20% with no increase or change in cardiac index and decrease or no change in the PVR:systemic vascular resistance ratio.7 A recent study suggests the Sitbon criteria (decrease of mean PAP ≥ 10 mm Hg to a value ≤ 40 mm Hg with increased or unchanged cardiac output)55 may also be useful in predicting calcium channel blocker response or prognosis in children.56 Risks associated with cardiac catheterization in children have been reported to be higher than in adults, up to 4% to 6% risk of complication,57, 58 but can be ameliorated with a standardized approach and careful anesthetic management.59, 60
Predictors of PH severity in pediatrics include clinical evidence of right ventricular failure, growth failure, elevated BNP, and echocardiographic and hemodynamic indicators of severely elevated PAP, PVR, and depressed cardiac index (Table 3).7 Functional assessment is also useful and a pediatric-specific functional classification was recently developed to incorporate developmentally appropriate parameters for ongoing assessment throughout childhood.61
Table 3.
Determinants of Low and High Risk in Pediatric Patients With PAH
| Lower Risk | Determinants of Risk | Higher Risk |
|---|---|---|
| No | Clinical evidence of RV failure | Yes |
| No | Progression of symptoms | Yes |
| No | Syncope | Yes |
| Growth | Failure to thrive | |
| I, II | WHO functional class | III, IV |
| Minimally elevated | BNP/NT-proBNP | Significantly elevated, rising levels |
| Echocardiography | Severe RV enlargement/dysfunction, pericardial effusion | |
| Cardiac index > 3.0 L/min/m2 mPAP/mSAP < 0.75 Acute vasoreactivity |
Hemodynamics | Cardiac index < 2.5 L/min/m2 mPAP/mSAP > 0.75, rising RAP > 10 mm Hg PVRI > 20 WU × m2 |
BNP = brain-type natriuretic peptide; mPAP = mean pulmonary arterial pressure; mSAP = mean systemic arterial pressure; NT-proBNP = N-terminal pro-brain-type natriuretic peptide; PAP = pulmonary artery pressure; PVRI = indexed pulmonary vascular resistance; RAP = right atrial pressure; RV = right ventricular; SAP = systemic arterial pressure; WHO = World Health Organization; WU = wood unit. (From Ivy et al.7)
Treatment
Because of the relative lack of multicenter, randomized clinical trials of PH-specific drug therapies in children, the approach to PH treatment in pediatrics is largely inferred from adult data and related algorithms (Table 4). Other than inhaled nitric oxide therapy for PPHN, there are no pulmonary vasodilator drugs that have been approved for use in children by the Food and Drug Administration (FDA). Small patient numbers preclude traditional large, randomized clinical trials in pediatric PH. Additional challenges exist in assessing response to treatment in children because age-appropriate testing and study end points and goals remain poorly defined. For example, the 6-min walk distance is commonly used in adult trials but has age- and size-related limitations in the pediatric population. TAPSE, WHO functional class, and NTpro-BNP levels at baseline are somewhat predictive of the subsequent outcomes in children, and changes in these parameters often track clinical deterioration.62
Table 4.
Pediatric PH: Guidelines From the American Heart Association and American Thoracic Society
Diagnosis, Assessments, Monitoring
|
Bronchopulmonary Dysplasia
|
Pharmacotherapy
|
Outpatient Care of the Child with PH
|
The Pediatric Task Force Report from the World Symposium Meeting in Nice meeting and the American Heart Association and American Thoracic Society Joint Guidelines recently outlined therapeutic approaches for the use of pulmonary vasodilator therapies in children, but these recommendations are most applicable for children with idiopathic PAH (Fig 3, Table 4).3, 7 Oral agents, including phosphodiesterase type 5 inhibitors and endothelin receptor antagonists, are commonly used in children. Sildenafil is commonly used as a first-line agent and is generally well-tolerated in children, but up to 30% report side effects such as erections, headache, or visual disturbances.63 However, questions regarding the safety of sildenafil therapy for children have been raised.64 The STARTS-1 (Sildenafil in Treatment-Naive Children, Aged 1-17 years, with Pulmonary Arterial Hypertension) trial was a randomized, double-blind placebo controlled trial of sildenafil in treatment-naive children, comparing multiple doses of sildenafil over a 16-week period. Peak oxygen consumption, functional capacity, mean PAP and PVR variably improved in the medium- and high-dose groups compared with placebo with no significant difference in the low-dose group.64 However, the open-label extension study (Long-Term Survival With Oral Sildenafil Monotherapy in Treatment-Naive Pediatric Pulmonary Arterial Hypertension [STARTS-2]) reported increased mortality in the high-dose group, although the authors acknowledged uncertainty about the relationship between dose and survival.65 The FDA warned against using sildenafil in children, but clarified the language after experts raised concerns regarding study design and lack of correlation to clinical practice. Notwithstanding the FDA warning, sildenafil therapy has become a mainstay of treatment of pediatric PAH. There are few studies in non-group 1 PAH, although a recent retrospective study showed sildenafil to be associated with improvement in premature infants with BPD and PH, without drug-related deaths or significant adverse events.66 Multiple small studies have demonstrated safety and efficacy of the endothelin receptor antagonists, bosentan and ambrisentan, in children with PAH, but large-scale studies are lacking.67, 68
Figure 3.
Adapted treatment algorithm for idiopathic or heritable pediatric pulmonary arterial hypertension. CCB = calcium channel blocker.
(From Ivy et al.7)
Prostacyclins have significantly improved survival in pediatric PAH and are recommended as first-line therapy in children with higher risk criteria at diagnosis or those who deteriorate on oral therapies (Fig 3, Table 3).7 Intravenous, subcutaneous, and inhaled prostacyclins are widely used in children with PAH with a trend toward increased use of treprostinil because of the safety profile and subcutaneous delivery. Safe transition from epoprostenol to treprostinil has been reported (Fig 4).69 Treprostinil has been used successfully in infants with PH resulting from BPD70 and CDH.71 Oral treprostinil is being studied in children with PAH. Patients who fail conventional therapy should be considered for lung transplantation. Recently, some children with IPAH or repaired congenital heart disease and suprasystemic PAP have been palliated with a Potts shunt connecting the left pulmonary artery to the descending aorta to allow for a decompressing right-to-left systolic shunt.72
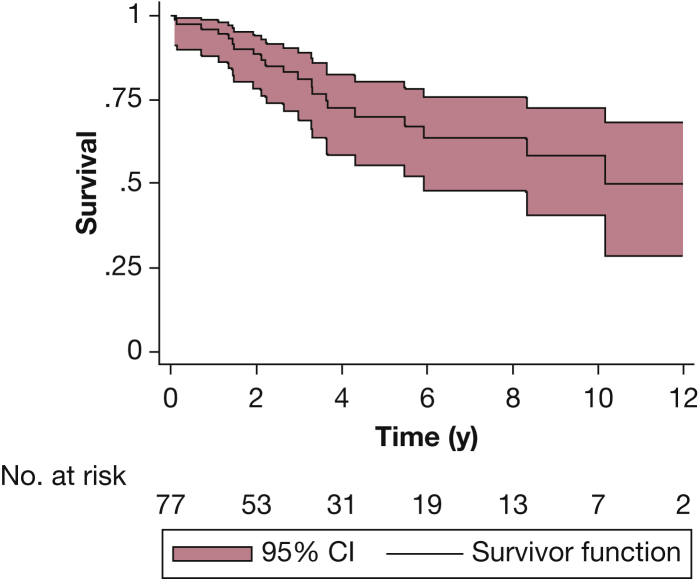
Figure 4.
Kaplan-Meier survival curve in 77 children with PH who were treated with chronic intravenous prostacyclin analogues (including epoprostenol or treprostinil, and those who transitioned from epoprostenol to treprostinil). The shaded areas depict the 95% CI. Transplant-free 5-year survival was 70% (95% CI, 56%-80%).
(From Siehr et al.69)
Conclusions
Despite improvements in the recognition of PH in children and increased survival of patients, PH remains a significant source of morbidity and mortality in many childhood diseases. Although sharing common features with adult PH, pediatric PH remains distinct from adult disease, largely because of issues regarding different disorders associated with PH, especially related to the timing of lung vascular injury and disruption of growth and maturation of the pulmonary circulation. Much needs to be learned about the incidence and prevalence, natural history, and outcomes of distinct pediatric diseases associated with PH. In addition, biomarkers and surrogate end points that are strongly predictive of outcomes are desperately needed for enhancing clinical studies and the care of children with PH, ranging from infancy through adolescence. Despite robust evidence, the recently published American Heart Association and American Thoracic Society Joint Guidelines for Pediatric PH provides recommendations for the diagnosis and treatment of PH in children that highlights key gaps in our knowledge and will serve as a source of ideas for promoting new research. Finally, there is a clear need to encourage the development of interdisciplinary PH programs for children for improving care and outcomes of pediatric PH, which will also enhance multicenter collaborations in research that are needed because of the relatively small numbers of patients at each site, multifactorial disease etiologies, and variations in current practice.
Acknowledgments
Financial/nonfinancial interests: The authors have reported to CHEST the following: S. H. A. is a recipient of grant support from Shire Pharmaceuticals for laboratory research. D. D. I. reports that the University of Colorado contracts with Actelion, Bayer, Lilly, and United Therapeutics for him to be a consultant and steering committee member. None declared (R. K. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was supported by the Jayden DeLuca Foundation; the Leah Bult Foundation; the Frederick and Margaret L Weyerhaeuser Foundation; and National Institutes of Health (NIH)/National Center for Advancing Translations Sciences Colorado CTSA [Grants UL1 TR001082 and NIH U01 HL081335].
References
- 1.Maxwell B.G., Nies M.K., Ajuba-Iwuji C.C., Coulson J.D., Romer L.H. Trends in hospitalization for pediatric pulmonary hypertension. Pediatrics. 2015;136(2):241–250. doi: 10.1542/peds.2014-3834. [DOI] [PubMed] [Google Scholar]
- 2.Frank D.B., Crystal M.A., Morales D.L.S. Trends in pediatric pulmonary hypertension-related hospitalizations in the United States from 2000-2009. Pulm Circ. 2015;5(2):339–348. doi: 10.1086/681226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abman S.H., Hansmann G., Archer S.L. Pediatric pulmonary hypertension guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 4.Jain A., McNamara P.J. Persistent pulmonary hypertension of the newborn: advances in diagnosis and treatment. Semin Fetal Neonatal Med. 2015;20(4):262–271. doi: 10.1016/j.siny.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 6.del Cerro M., Diaz G., Freudenthal F. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1(2):286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivy D.D., Abman S.H., Barst R.J. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D117–D126. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G., Robbins I.M., Beghetti M. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Simonneau G., Galiè N., Rubin L.J. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43(12):S5–S12. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Drossner D.M., Kim D.W., Maher K.O., Mahle W.T. Pulmonary vein stenosis: prematurity and associated conditions. Pediatrics. 2008;122(3):e656–e661. doi: 10.1542/peds.2008-0075. [DOI] [PubMed] [Google Scholar]
- 11.Schuuring M.J., Bouma B.J., Cordina R. Treatment of segmental pulmonary artery hypertension in adults with congenital heart disease. Int J Cardiol. 2013;164(1):106–110. doi: 10.1016/j.ijcard.2011.06.084. [DOI] [PubMed] [Google Scholar]
- 12.Stoll B.J., Hansen N.I., Bell E.F. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An H.S., Bae E.J., Kim G.B. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40(3):131. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat R., Salas A.A., Foster C., Carlo W.A., Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129(3):e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mourani P.M., Sontag M.K., Younoszai A. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2014;191(1):87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khemani E., McElhinney D.B., Rhein L. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 17.Kim D.-H., Kim H.-S., Choi C.W., Kim E.-K., Kim B.I., Choi J.-H. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 18.Jayet P.-Y., Rimoldi S.F., Stuber T. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122(5):488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 19.Check J., Gotteiner N., Liu X. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33(7):553–557. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang J.-R., Karumanchi S.A., Seedorf G., Markham N., Abman S.H. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. AJP Lung Cell Mol Physiol. 2012;302(1):L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mestan K.K., Check J., Minturn L. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35(8):570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D.-H., Kim H.-S. Serial changes of serum endostatin and angiopoietin-1 levels in preterm infants with severe bronchopulmonary dysplasia and subsequent pulmonary artery hypertension. Neonatology. 2014;106(1):55–61. doi: 10.1159/000358374. [DOI] [PubMed] [Google Scholar]
- 23.Rozance P.J., Seedorf G.J., Brown A. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. AJP Lung Cell Mol Physiol. 2011;301(6):L860–L871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacRitchie A.N., Albertine K.H., Sun J. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol - Lung Cell Mol Physiol. 2001;281(4):L1011–L1020. doi: 10.1152/ajplung.2001.281.4.L1011. [DOI] [PubMed] [Google Scholar]
- 25.Afshar S., Gibson L.L., Yuhanna I.S. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol - Lung Cell Mol Physiol. 2003;284(5):L749–L758. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly M., Thébaud B. Stem cells for the prevention of neonatal lung disease. Neonatology. 2015;107(4):360–364. doi: 10.1159/000381135. [DOI] [PubMed] [Google Scholar]
- 27.Stege G., Fenton A., Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112(3 Pt 1):532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- 28.Colvin J., Bower C., Dickinson J.E., Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116(3):e356–e363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- 29.Dillon P.W., Cilley R.E., Mauger D., Zachary C., Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg. 2004;39(3):307–312. doi: 10.1016/j.jpedsurg.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Wynn J., Krishnan U., Aspelund G. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. 2013;163(1):114–119. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geggel R.L., Murphy J.D., Langleben D., Crone R.K., Vacanti J.P., Reid L.M. Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr. 1985;107(3):457–464. doi: 10.1016/s0022-3476(85)80534-5. [DOI] [PubMed] [Google Scholar]
- 32.Acker S.N., Seedorf G.J., Abman S.H., Nozik-Grayck E., Partrick D.A., Gien J. Pulmonary artery endothelial cell dysfunction and decreased populations of highly proliferative endothelial cells in experimental congenital diaphragmatic hernia. AJP Lung Cell Mol Physiol. 2013;305(12):L943–L952. doi: 10.1152/ajplung.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makanga M., Dewachter C., Maruyama H. Downregulated bone morphogenetic protein signaling in nitrofen-induced congenital diaphragmatic hernia. Pediatr Surg Int. 2013;29(8):823–834. doi: 10.1007/s00383-013-3340-6. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann A.D., Friedmacher F., Takahashi H., Hunziker M., Gosemann J.-H., Puri P. Decreased apelin and apelin-receptor expression in the pulmonary vasculature of nitrofen-induced congenital diaphragmatic hernia. Pediatr Surg Int. 2014;30(2):197–203. doi: 10.1007/s00383-013-3450-1. [DOI] [PubMed] [Google Scholar]
- 35.Keller R.L., Tacy T.A., Hendricks-Munoz K. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med. 2010;182(4):555–561. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleck S., Bautista G., Keating S.M. Fetal production of growth factors and inflammatory mediators predicts pulmonary hypertension in congenital diaphragmatic hernia. Pediatr Res. 2013;74(3):290–298. doi: 10.1038/pr.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaible T., Veit M., Tautz J. Serum cytokine levels in neonates with congenital diaphragmatic hernia. Klin Pädiatr. 2011;223(7):414–418. doi: 10.1055/s-0031-1295436. [DOI] [PubMed] [Google Scholar]
- 38.van Loon R.L.E., Roofthooft M.T.R., Hillege H.L. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124(16):1755–1764. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 39.Berger R.M., Beghetti M., Humpl T. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379(9815):537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abman S.H., Raj U. Towards improving the care of children with pulmonary hypertension: the rationale for developing a Pediatric Pulmonary Hypertension Network. Prog Pediatr Cardiol. 2009;27(1-2):3–6. doi: 10.1016/j.ppedcard.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barst R.J., Maislin G., Fishman A.P. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99(9):1197–1208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 42.Barst R.J., McGoon M.D., Elliott C.G., Foreman A.J., Miller D.P., Ivy D.D. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012;125(1):113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 43.Haworth S.G., Hislop A.A. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart. 2008;95(4):312–317. doi: 10.1136/hrt.2008.150086. [DOI] [PubMed] [Google Scholar]
- 44.King M.E., Braun H., Goldblatt A., Liberthson R., Weyman A.E. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation. 1983;68(1):68–75. doi: 10.1161/01.cir.68.1.68. [DOI] [PubMed] [Google Scholar]
- 45.Mourani P.M., Sontag M.K., Younoszai A., Ivy D.D., Abman S.H. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121(2):317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koestenberger M., Ravekes W., Everett A.D. Right ventricular function in infants, children and adolescents: reference values of the Tricuspid Annular Plane Systolic Excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22(6):715–719. doi: 10.1016/j.echo.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Zakaria D., Sachdeva R., Gossett J.M., Tang X., O’Connor M.J. Tricuspid annular plane systolic excursion is reduced in infants with pulmonary hypertension. Echocardiography. 2015;32(5):834–838. doi: 10.1111/echo.12797. [DOI] [PubMed] [Google Scholar]
- 48.Kassem E., Humpl T., Friedberg M.K. Prognostic significance of 2-dimensional, M-mode, and Doppler echo indices of right ventricular function in children with pulmonary arterial hypertension. Am Heart J. 2013;165(6):1024–1031. doi: 10.1016/j.ahj.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 49.Bernus A. Brain natriuretic peptide levels in managing pediatric patients with pulmonary arterial hypertension. CHEST J. 2009;135(3):745. doi: 10.1378/chest.08-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steurer M.A., Moon-Grady A.J., Fineman J.R. B-type natriuretic peptide: prognostic marker in congenital diaphragmatic hernia. Pediatr Res. 2014;76(6):549–554. doi: 10.1038/pr.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuna A., Kandasamy J., Sims B. B-type natriuretic peptide and mortality in extremely low birth weight infants with pulmonary hypertension: a retrospective cohort analysis. BMC Pediatr. 2014;14(1):68. doi: 10.1186/1471-2431-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blalock S., Chan F., Rosenthal D., Ogawa M., Maxey D., Feinstein J. Magnetic resonance imaging of the right ventricle in pediatric pulmonary arterial hypertension. Pulm Circ. 2013;3(2):350. doi: 10.4103/2045-8932.114763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Truong U., Fonseca B., Dunning J. Wall shear stress measured by phase contrast cardiovascular magnetic resonance in children and adolescents with pulmonary arterial hypertension. J Cardiovasc Magn Reson. 2013;15(1):81. doi: 10.1186/1532-429X-15-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandya B., Quail M.A., Steeden J.A. Real-time magnetic resonance assessment of septal curvature accurately tracks acute hemodynamic changes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2014;7(4):706–713. doi: 10.1161/CIRCIMAGING.113.001156. [DOI] [PubMed] [Google Scholar]
- 55.Sitbon O., Humbert M., Jaïs X. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 56.Douwes J.M., van Loon R.L.E., Hoendermis E.S. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: occurrence and prognostic value when comparing three response criteria. Eur Heart J. 2011;32(24):3137–3146. doi: 10.1093/eurheartj/ehr282. [DOI] [PubMed] [Google Scholar]
- 57.Taylor C.J., Derrick G., McEwan A., Haworth S.G., Sury M.R.J. Risk of cardiac catheterization under anaesthesia in children with pulmonary hypertension. Br J Anaesth. 2007;98(5):657–661. doi: 10.1093/bja/aem059. [DOI] [PubMed] [Google Scholar]
- 58.Carmosino M.J., Friesen R.H., Doran A., Ivy D.D. Perioperative complications in children with pulmonary hypertension undergoing noncardiac surgery or cardiac catheterization. Anesth Analg. 2007;104(3):521–527. doi: 10.1213/01.ane.0000255732.16057.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bobhate P., Guo L., Jain S. Cardiac catheterization in children with pulmonary hypertensive vascular disease. Pediatr Cardiol. 2015;36(4):873–879. doi: 10.1007/s00246-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 60.Williams G.D., Maan H., Ramamoorthy C. Perioperative complications in children with pulmonary hypertension undergoing general anesthesia with ketamine. Pediatr Anesth. 2010;20(1):28–37. doi: 10.1111/j.1460-9592.2009.03166.x. [DOI] [PubMed] [Google Scholar]
- 61.Lammers A., del Cerro M., Freudenthal A. Functional classification of pulmonary hypertension in children: report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ. 2011;1(2):280. doi: 10.4103/2045-8932.83445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ploegstra M.-J., Douwes J.M., Roofthooft M.T.R., Zijlstra W.M.H., Hillege H.L., Berger R.M.F. Identification of treatment goals in paediatric pulmonary arterial hypertension. Eur Respir J. 2014;44(6):1616–1626. doi: 10.1183/09031936.00030414. [DOI] [PubMed] [Google Scholar]
- 63.Siehr S.L., McCarthy E.K., Ogawa M.T., Feinstein J.A. Reported sildenafil side effects in pediatric pulmonary hypertension patients. Front Pediatr. 2015;3:12. doi: 10.3389/fped.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barst R.J., Ivy D., Dingemanse J. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73(4):372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 65.Barst R.J., Beghetti M., Pulido T. STARTS-2: Long-Term Survival With Oral Sildenafil Monotherapy in Treatment-Naive Pediatric Pulmonary Arterial Hypertension. Circulation. 2014;129(19):1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 66.Trottier-Boucher M.N., Lapointe A., Malo J. Sildenafil for the treatment of pulmonary arterial hypertension in infants with bronchopulmonary dysplasia. Pediatr Cardiol. 2015;36(6):1255–1260. doi: 10.1007/s00246-015-1154-0. [DOI] [PubMed] [Google Scholar]
- 67.Takatsuki S., Rosenzweig E.B., Zuckerman W., Brady D., Calderbank M., Ivy D.D. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol. 2013;48(1):27–34. doi: 10.1002/ppul.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivy D.D., Rosenzweig E.B., Lemarié J.-C., Brand M., Rosenberg D., Barst R.J. Long-term outcomes in children with pulmonary arterial hypertension treated with bosentan in real-world clinical settings. Am J Cardiol. 2010;106(9):1332–1338. doi: 10.1016/j.amjcard.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siehr S.L., Dunbar Ivy D., Miller-Reed K., Ogawa M., Rosenthal D.N., Feinstein J.A. Children with pulmonary arterial hypertension and prostanoid therapy: long-term hemodynamics. J Heart Lung Transplant. 2013;32(5):546–552. doi: 10.1016/j.healun.2013.01.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferdman D.J., Rosenzweig E.B., Zuckerman W.A., Krishnan U. Subcutaneous treprostinil for pulmonary hypertension in chronic lung disease of infancy. Pediatrics. 2014;134(1):e274–e278. doi: 10.1542/peds.2013-2330. [DOI] [PubMed] [Google Scholar]
- 71.Olson E., Lusk L.A., Fineman J.R., Robertson L., Keller R.L. Short-term treprostinil use in infants with congenital diaphragmatic hernia following repair. J Pediatr. 2015;167(3):762–764. doi: 10.1016/j.jpeds.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baruteau A.E., Belli E., Boudjemline Y. Palliative Potts shunt for the treatment of children with drug-refractory pulmonary arterial hypertension: updated data from the first 24 patients. Eur J Cardiothorac Surg. 2015;47(3):e105–e110. doi: 10.1093/ejcts/ezu445. [DOI] [PubMed] [Google Scholar]