Abstract
Background
The contractility of colonic smooth muscle is dysregulated due to immune/inflammatory responses in inflammatory bowel diseases. Inflammation in vitro induces up-regulation of regulator of G-protein signaling 4 (RGS4) expression in colonic smooth muscle cells.
Aims
To characterize the immune/inflammatory responses and RGS4 expression pattern in colonic smooth muscle after induction of colitis.
Methods
Colitis was induced in rabbits by intrarectal instillation of 2,4,6-trinitrobenzene sulfonic acid (TNBS). Innate/adaptive immune response RT-qPCR array was performed using colonic circular muscle strips. At 1–9 weeks after colonic intramuscular microinjection of lentivirus, the distal and proximal colons were collected, and muscle strips and dispersed muscle cells were prepared from circular muscle layer. Expression levels of RGS4 and NFκB signaling components were determined by Western blot analysis. The biological consequences of RGS4 knockdown were assessed by measurement of muscle contraction and phospholipase C (PLC)-β activity in response to acetylcholine (ACh).
Results
Contraction in response to ACh was significantly inhibited in the inflamed colonic circular smooth muscle cells. RGS4, IL-1, IL-6, IL-8, CCL3, CD1D, and ITGB2 were significantly up-regulated, while IL-18, CXCR4, CD86, and C3 were significantly down-regulated in the inflamed muscle strips. RGS4 protein expression in the inflamed smooth muscles was dramatically increased. RGS4 stable knockdown in vivo augmented ACh-stimulated PLC-β activity and contraction in colonic smooth muscle cells.
Conclusion
Inflamed smooth muscle exhibits up-regulation of IL-1-related signaling components, Th1 cytokines and RGS4, and inhibition of contraction. Stable knockdown of endogenous RGS4 in colonic smooth muscle increases PLC-β activity and contractile responses.
Keywords: Smooth muscle cells, Rabbit, Regulators of G-protein signaling, Colitis, shRNA
Background
Inflammatory bowel disease (IBD) is a cluster of chronic and inflammatory/immune conditions in the gastrointestinal tract, mainly including Crohn’s disease and ulcerative colitis [1]. Understanding the molecular and cellular mechanisms for IBD is fundamental for developing new effective treatments. The inflammatory and immunological mediators have been proposed to contribute significantly to the development of tissue damage and related symptoms in IBD [2]. These inflammatory mediators derive from multiple cell types in the gut including epithelial cells, smooth muscle cells (SMCs), glial cells, interstitial cells of Cajal, and various immune cells. SMCs act as both source and target of these mediators and maintain an intricate and dynamic inflammatory response [2–4]. Different patterns of inflammatory cytokines including T helper 1 (Th1), Th2, and Th17 cytokines have been identified to regulate the contractility of the gastrointestinal smooth muscle [2–5].
The pro-inflammatory cytokines, interleukin (IL-1β) and tumor necrosis factor (TNF-α), are known to inhibit the contraction of gut SMCs [6, 7]. Inhibition of contraction is correlated with changes in neural input and expression of signaling molecules in SMCs. The indirect mechanisms involve the modulation of the neurotransmitter release such as acetylcholine (ACh), norepinephrine, and substance P [8]. The direct mechanisms underlying smooth muscle contractile inhibition involve the cellular regulation or modification in SMCs [8–10]. For example, IL-1β reduced the tone of lower esophageal sphincter through stimulating COX-2-mediated prostaglandin E2 (PGE2) [11]. TNF-α inhibited colonic muscle contraction by up-regulating intercellular adhesion molecule (ICAM)-1 expression [6]. IL-1β-stimulated hydrogen peroxide (H2O2) in the colonic and esophageal sphincter smooth muscles [12] inhibits neurokinin A-induced contractility by decreasing Ca2+ mobilization [11].
G-protein signaling plays key roles in the regulation of smooth muscle function. The strength and duration of G-protein signaling are negatively regulated by a family of GTPase-activating proteins, called regulators of G-protein signaling (RGS). All RGS proteins share a conserved signature domain that binds directly to the activated Gα subunits [13]. Among over 30 mammalian members of RGS family [14], RGS4 is one of the most extensively studied at the structural, biochemical, and cellular/molecular levels [13–15]. RGS4 regulates the strength and duration of the Gαi/o and Gαq/11 family members [16, 17]. RGS4 expression and function have been studied in cardiomyocyte [16], nerve tissue [18], and cancers [19], but little is known in SMCs. RGS4 expression in cardiomyocyte induced by endotoxin and IL-1β [20] may contribute to the deficiency in Gαq-mediated activation of phospholipase C (PLC) by endothelin-1 [21]. RGS4 abnormal function in the brain is linked to schizophrenia [15], Alzheimer’s disease [22], and Huntington’s disease [23]. In human aortic SMCs, high level of RGS4 expression inhibits S1P3 receptor-mediated signaling [24]. We demonstrated that exposures of circular colonic muscle strips and freshly isolated or cultured colonic muscle cells to IL-1β in vitro cause RGS4 up-regulation leading to inhibition of ACh-induced contraction [7]. IL-1β-induced RGS4 up-regulation is mediated by NFκB signaling [25] and modulated by MAP kinases and a PI3-kinase/Akt/GSK3β pathway [26].
The aim of the present study was to characterize the immune/inflammatory response and the in vivo expression pattern of RGS4 in the inflamed colonic smooth muscle using rabbit colitis model induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). To validate the in vivo function of RGS4 in colonic smooth muscle, we also determined the efficacy of lentivirus-mediated RGS4 small hairpin RNA (shRNA) knockdown to regulate smooth muscle contractility [27].
Materials and Methods
TNBS-Induced Colitis in Rabbit
The TNBS-induced colitis in rabbit was prepared through a modification of previously reported protocols [28–30]. Briefly, New Zealand white rabbit (2–2.5 kg, male) with an overnight fast was placed in an animal carrier and was calmed down by gentle touching without any anesthesia. An infant feeding tube (5 Fr, Guardian Catheter™) lubricated with mineral oil was inserted via anus for ~15 cm into the colon and then slowly injected with 75 mg/kg TNBS in 1.0 ml of 50 % ethanol (to break the intestinal epithelial barrier). This procedure took about 2–3 min, and there was no distress or pain to the rabbits. As a control, the same volume of 50 % ethanol was administered. Rabbits were then allowed to recover and have free access to food and water. Anti-inflammatory drugs and other analgesics were not used because they could interfere with the pathophysiological mechanisms being investigated. The clinical index including weight change, food consumption, stool consistency, vital signs, and attitude responses was determined daily. Body weight in all animals was measured daily at 9 a.m. The daily food intake was measured by the 24-h difference between the amount of remaining food and the pre-weighed food. The score of the weight loss was 1–4 (<5, 5–10, 10–15, and >15 %). Blood in the stool was scored as 0, normal; 2, slight bleeding; and 4, gross bleeding. The score for the diarrhea was 0, normal; 2, loose stools; and 4, watery diarrhea. The total score for these three parameters was defined as the clinical index.
Sample Collection and Macroscopic Examinations
Animals were euthanized with an overdose of Euthasol (pentobarbital sodium and phenytoin sodium) solution at day 5 (the time for maximal inflammation) and day 7 (recovery start) after the induction of colitis [28, 29]. The colon was quickly removed, and the colorectum length was measured. The colon was longitudinally opened, and the stool was gently cleared. The extent of gross macroscopic damage was determined and scored (Table 1) according to the criteria modified from previous reports [28].
Table 1.
Criteria for macroscopic scoring of colonic damage from rabbit colitis
| Score | Appearance |
|---|---|
| Whole colon opened longitudinally along the anti-mesenteric border | |
| Primary criteria | |
| 0 | Normal |
| 1 | Localized hyperemia, no ulcers |
| 2 | Ulceration without hyperemia or bowel wall thickening |
| 3 | Ulceration with inflammation at one site |
| 4 | Two or more sites of ulceration and inflammation |
| 5 | Major sites of damage extending > 1 cm along length of colon |
| 6–10 | Major sites of damage extending > 2 cm along length of colon, with score increasing by 1 for each additional 1 cm |
| Other considerations | |
| +x | Thickness of the colon (in mm) |
| +1 | If minor adhesions are present (colon can be easily separated from other tissues) |
| +2 | If major adhesions are present |
| +1 | If animal has diarrhea |
| Total | |
Segments of 5–15th cm from the anus were prepared as distal colon, and 30–40th cm was taken and designated as proximal colon. For histopathological and immunohistochemical studies, a segment (1 cm in length) of entire colon tissue from distal colon (10–11th cm) and proximal colon (35–36th cm) was placed in freshly prepared 4 % paraformaldehyde/0.1 M PBS for overnight and 30 % sucrose for 2–3 days and stored at −70 °C. For the remaining colon, the mucosa was scraped off and the circular muscular strips were cut into 3 pieces. Two pieces were snap-frozen in liquid nitrogen and stored at −70 °C until further processing for mRNA and protein expression. Another piece was used for isolation of SMCs.
Histopathological Examination
The colon tissues from three animals of each group (ethanol control and TNBS) were embedded in one block using OCT compound (Tissue Tek, Elkhart, Ind., USA) and cut in cryostat at −18 °C. All the tissue sections were coded and evaluated in a blind manner by two investigators. The sections (10 μm thickness) were collected on 1 % gelatinized slides and stored at −35 °C before use. The standard hematoxylin and eosin staining was performed for histopathological examination.
Isolation of Colonic SMCs Reverse Transcription (RT) Quantitative PCR (RT-qPCR) and Western Blot Analysis, Assay for Phospholipase C-β Activity, and Measurement of SMC Contraction
Rabbit colonic SMC preparation, and RT-qPCR and Western blot analysis were prepared as we previously described [7, 26]. RT-qPCR analyses were carried out in a LightCycler480 (Roche) using an SYBR® Green PCR Master Mix Kit (Applied Biosystems) as described previously. PLC-β activity was determined in freshly isolated SMCs by measuring the formation of inositol phosphates from the phosphoinositide using ion-exchange chromatography as described previously [31]. Contraction in freshly dispersed muscle cells from colonic circular muscle strips after TNBS colitis or lentivirus infection was measured by scanning microscopy as we described previously [32].
The Rabbit Innate and Adaptive Immune Responses RT2 Profiler PCR Array
To analyze the differential expressions of the multiple genes related to innate/adaptive immune responses, we employed an RT2 Profiler PCR Array (catalog no. panza-052A; SABioscience Corp, Frederick, MA, USA), which used SYBR green-based real-time PCR to simultaneously assay 84 specific genes. The detail description of this PCR array can be found on the Web site (http://www.sabiosciences.com/rt_pcr_product/HTML/PANZ-052Z.html). This array profiles the mRNA expression of 84 genes related to the innate and adaptive immune responses. For the adaptive immunity, Th1, Th2, Th17, and Treg markers/responses can be classified. All the genes are listed in Table 2. After adding cDNA to each well of an RT2 Profiler PCR Array, qPCR analyses were carried out in a LightCycler480 (Roche) using an SYBR® Green PCR Master Mix Kit (Applied Biosystems). The PCR product specificity was validated via the melt curves. Five housekeeping genes in the array, including β-actin (Actb), β2-microglobulin (B2m), hypoxanthine phosphoribosyl transferase 1 (Hprt1), lactate dehydrogenase A (LDHA), and non-POU domain-containing octamer-binding-like protein (NONO), were used for normalization. The Ct value for each gene was normalized to the average Ct of 5 housekeeping genes (ΔCt). A list of differentially expressed genes was identified using unpaired Student’s t test. Fold changes in gene expression between experimental group and control group were calculated. Genes that met statistical significance (p < 0.05) and ≥3-fold changes were considered to be increased or decreased.
Table 2.
Up- or down-regulated genes in ethanol treatment (n = 3) over the normal control animals (n = 3)
| RefSeq | Symbol | Description | Gene name | Folds |
|---|---|---|---|---|
| NM_001171347 | IRF1 | Interferon regulatory factor 1 | IRF1 | 5.45 |
| XM_002708055 | LOC100353137 | Interferon type I-like | IFNa1 | 4.44 |
| XM_002722842 | LOC100344622 | Tyrosine kinase 2-like | TYK2 | 3.53* |
| XM_002714498 | LOC100339322 | Interleukin 17A-like | IL17A | 3.17* |
| NM_001171140 | RAG1 | Recombination-activating gene 1 | RAG1 | 3.04 |
| XM_002708417 | LOC100101588 | Caspase-1 | Caspase-1 | 2.89 |
| NM_001163177 | IL4 | Interleukin 4 | IL4 | 2.73 |
| NM_001163180 | IL2 | Interleukin 2 | IL2 | 2.66 |
| XM_002723759 | LOC100359014 | Myxovirus resistance protein 1 | MX1 | 2.63 |
| NM_001122940 | IL18 | Interleukin 18 (interferon-gamma-inducing factor) | IL18 | 2.25 |
| NM_001089311 | CD1A | CD1a molecule | CD1A | 2.19 |
| XM_002722638 | LOC100354743 | Interleukin 1 receptor-associated kinase 1-like | IRAK1 | 2.10 |
| NM_001195719 | LBP | Lipopolysaccharide-binding protein | LBP | 2.06 |
| NM_001082787 | LY96 | Lymphocyte antigen 96 | LY96 | 2.05 |
| XM_002713735 | NOD1 | Nucleotide-binding oligomerization domain containing 1 | NOD1 | 2.03 |
| NM_001082219 | TLR3 | Toll-like receptor 3 | TLR3 | 1.90 |
| XM_002723869 | LOC100340811 | Myeloid differentiation primary response gene 88-like | Myd88 | 1.85 |
| XM_002710201 | LOC100358075 | Interleukin-5-like | IL5 | 1.80 |
| NM_001204346 | TICAM2 | Toll-like receptor adaptor molecule 2 | TICAM2 | 1.79 |
| XM_002711079 | LOC100352065 | Interleukin 23, alpha subunit p19-like | IL23a | 1.76 |
| XM_002723157 | NLRP3 | NLR family, pyrin domain containing 3 | NLRP3 | 1.68 |
| XM_002722790 | MEFV | Mediterranean fever | MEFV | 1.67 |
| XM_002721749 | ITGAM | Integrin, alpha M (complement component 3 receptor 3 subunit) | ITGAM | 1.66 |
| XM_002719094 | MPO | Myeloperoxidase | MPO | 1.65 |
| XM_002713106 | LOC100345500 | Chemokine (C-C motif) receptor 8 | CCR8 | 1.61 |
| XM_002718142 | LOC100357341 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | IkBa | 1.59 |
| XM_002708040 | DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | DDX58 | 1.56 |
| XM_002710092 | LOC100358676 | Interleukin 13 (predicted)-like | IL13 | 1.56 |
| XM_002719910 | LOC100348270 | Forkhead box P3 | FoxP3 | 1.55 |
| XM_002722022 | LOC100338696 | Putative CD209 protein-like | CD209 | 1.51 |
| XM_002708002 | LOC100358134 | Janus kinase 2 | JAK2 | 1.47 |
| XM_002722561 | LOC100338701 | Macrophage migration inhibitory factor | MIF | 1.43 |
| XM_002712346 | STAT1 | Signal transducer and activator of transcription 1, 91 kDa | STAT1 | 1.42 |
| XM_002709270 | LOC100351814 | Toll-like receptor 1-like | TLR1 | 1.41 |
| XM_002719395 | STAT3 | Signal transducer and activator of transcription 3 (acute-phase response factor) | STAT3 | 1.39 |
| XM_002718628 | LOC100339928 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 | NFkB2 | 1.39 |
| XM_002711889 | LOC100348561 | Mitogen-activated protein kinase 3-like | MAPK3 | 1.38 |
| NM_001082195 | CD14 | CD14 molecule | CD14 | 1.38 |
| XM_002719246 | LOC100348776 | Chemokine (C–C motif) ligand 3-like | CCL3 | 1.36 |
| XM_002709388 | TLR6 | Toll-like receptor 6 | TLR6 | 1.34 |
| NM_001082286 | C3 | Complement component 3 | C3 | 1.34 |
| NM_001082663 | CD80 | CD80 molecule | CD80 | 1.33 |
| NM_001160282 | CD1D | CD1d molecule | CD1D | 1.30 |
| XM_002712124 | CXCR4 | Chemokine (C-X-C motif) receptor 4 | CXCR4 | 1.30 |
| XM_002712347 | STAT4 | Signal transducer and activator of transcription 4 | STAT4 | 1.29 |
| XM_002717290 | LOC100356058 | Lysozyme-like 1-like | LYZ | 1.29 |
| XM_002720937 | STAT6 | Signal transducer and activator of transcription 6, interleukin-4 induced | STAT6 | 1.25 |
| NM_001082732 | TLR4 | Toll-like receptor 4 | TLR4 | 1.24 |
| NM_001081991 | IFNG | Interferon, gamma | IFNG | 1.18 |
| NM_001101682 | ACTA2 | Actin, alpha 2, smooth muscle, aorta | ACTA2 | 1.18 |
| XM_002714645 | MAPK14 | Mitogen-activated protein kinase 14 | MAPK14 | 1.14 |
| XM_002717361 | GATA3 | GATA-binding protein 3 | GATA3 | 1.13 |
| NM_001101683 | ACTB | Actin, beta | ACTB | 1.13 |
| NM_001171125 | CCR5 | Chemokine (C-C motif) receptor 5 (gene/pseudogene) | CCR5 | 1.13 |
| NM_001082781 | TLR2 | Toll-like receptor 2 | TLR2 | 1.12 |
| NM_001082770 | IL1R1 | Interleukin 1 receptor, type I | IL1R1 | 1.11 |
| XM_002721345 | NOD2 | Nucleotide-binding oligomerization domain containing 2 | NOD2 | 1.11 |
| XM_002712221 | LOC100343560 | Interferon induced with helicase C domain 1 | IFIH1 | 1.10 |
| XM_002717421 | IRF6 | Interferon regulatory factor 6 | IRF6 | 1.10 |
| XM_002718365 | LOC100339019 | Soluble mannose-binding lectin-like | MBL2 | 1.08 |
| XM_002723383 | LOC100346936 | Non-POU domain-containing octamer-binding-like | NONO | 1.04 |
| NM_001082064 | IL6 | Interleukin 6 (interferon, beta 2) | IL6 | 1.03 |
| XM_002720089 | LOC100349863 | Chemokine (C-X-C motif) receptor 3 | CXCR3 | 1.01 |
| XM_002715354 | RORC | RAR-related orphan receptor C | RORC | −1.01 |
| XM_002721199 | LOC100349106 | CD40 antigen | CD40 | −1.03 |
| NM_001101684 | IL1A | Interleukin 1, alpha | IL1A | −1.04 |
| NM_001082277 | LDHA | Lactate dehydrogenase A | LDHA | −1.06 |
| XM_002722670 | MAPK8 | Mitogen-activated protein kinase 8 | MAPK8 | −1.12 |
| NM_001082293 | IL8 | Interleukin 8 | IL8 | −1.16 |
| NM_001082216 | IL15 | Interleukin 15 | IL15 | −1.18 |
| NM_001256781 | LOC100358388 | CD40 ligand | CD40L | −1.19 |
| XM_002724155 | LOC100339049 | Integrin beta-2-like | ITGB2 | −1.19 |
| XM_002719731 | LOC100347345 | Mitogen-activated protein kinase 1-like | MAPK1 | −1.24 |
| NM_001082201 | IL1B | Interleukin 1, beta | IL1B | −1.30 |
| NM_001082253 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | −1.31 |
| XM_002709054 | TRAF6 | TNF receptor-associated factor 6, E3 ubiquitin protein ligase | TRAF6 | −1.34 |
| NM_001082263 | TNF | Tumor necrosis factor | TNF | −1.37 |
| NM_001082208 | CD86 | CD86 molecule | CD86 | −1.37 |
| NM_001198947 | C-JUN | C-jun transcription factor | C-JUN | −1.43 |
| XM_002716251 | LOC100347328 | Chemokine (C-C motif) receptor 4 | CCR4 | −1.51 |
| NM_001082207 | CD28 | CD28 molecule | CD28 | −1.64 |
| NM_001082313 | CD4 | CD4 molecule | CD4 | −1.66 |
| XM_002723866 | LOC100339537 | Chemokine (C-C motif) receptor 6-like | CCR6 | −1.76 |
| NM_001082045 | IL10 | Interleukin-10 | IL-10 | −1.88 |
| NM_001082388 | PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | Cox2 | −1.97 |
| NM_001082265 | CRP | C-reactive protein, pentraxin-related | CRP | −2.03 |
| XM_002720515 | LOC100342334 | Fas ligand (TNF superfamily, member 6)-like | FasL | −2.04 |
| NM_001081995 | FAS | Fas (TNF receptor superfamily, member 6) | FAS | −2.08 |
| NM_001082294 | CCL2 | Chemokine (C-C motif) ligand 2 | CCL2 | −3.43 |
Bold values indicate >3-fold changes
p < 0.05 indicates a statistically significant change by the Student’s t test compared to the normal control group
Preparation and Injection of Lentivirus Carrying RGS4 Small Hairpin RNA (shRNA)
Lentiviral vectors encoding shRNA for RGS4 targeting nucleotide 5′-gaggaagtcaagaaatgggc-3′ (pLL-RGS4A) were generated as we previously described [7]. Silencing efficiency and specificity of RGS4A shRNA were validated by Western blot and RT-PCR analysis in cultured colonic SMCs [7]. The recombinant lentivirus carrying pLL-RGS4A-shRNA or empty control lentiviral vector pLL3.7 was produced by calcium phosphate-mediated transient transfection of HEK293T cells according to standard protocols. The viruses were concentrated and purified with ViraBind™ Lentivirus Concentration and Purification Kit (Cell Biolabs Inc.) following the manufacture’s protocol. The purified lentivirus was resuspended in phosphate-buffered saline (PBS). The functional titers were determined by counting EGFP-expressing HEK293T cells at 48 h after infection with serial dilutions of lentiviruses. A typical preparation generated a titer of approximately 108−9 transduction units per ml.
For intramuscular colonic microinjection, a midline laparotomy was performed on animal under anesthesia with i.m. ketamine (40 mg/kg)/xylazine (5 mg/kg)/butorphanol (0.1 mg/kg). Sterile technique was used for the procedure. The left abdominal sidewall was exposed, and a short piece of colon around 15 cm from the anus was microinjected longitudinally into the smooth muscle layers with 5 μl of concentrated lentivirus per point at 10 points about 2 mm separately from each other. Then the muscle incision was closed with a 3-0 absorbable suture through a horizontal mattress stitch, and the skin incision was closed with skin staples. After recovery from anesthesia, animal was returned to normal maintenance cage. At 1, 2, 4, 9 weeks after microinjection, animals were euthanized, and the whole colons were collected for further experiments. The efficiency of lentivirus infection was examined by checking EGFP under confocal fluorescent microscopy.
Statistical Analysis
The nonparametric values for the clinical index, macroscopic change, and histopathological examination were expressed as median for the number of rabbits indicated and analyzed by the Mann–Whitney U test. Quantitative data were presented as mean ± SE of n experiments with a statistical significance (p < 0.05 or 0.01) by the unpaired Student’s t test.
Ethics Statement
The protocols for rabbit TNBS colitis and lentivirus gene delivery were approved by the IACUC committee at Virginia Commonwealth University (# 0510-3402) and Temple University (# 3164).
Results
Rabbit TNBS Colitis Model
TNBS-induced colitis has been well established in rat and mouse [33, 34]. In rabbit, intrarectal instillation of TNBS also induced a typical colitis [28, 30, 35, 36]. After a single injection of TNBS (75 mg/kg), all animals reproducibly developed colitis with diarrhea and rectal bleeding to various extent as well as weight loss by 18.3 ± 4.7 % (n = 8). The clinical index for TNBS-treated animals was 9.4 ± 1.3 (n = 8). On laparotomy at day 5 after TNBS treatment [28, 29], the gross appearance of peritoneal cavity and the distal colon varied with the severity of colitis. In some cases, severe peritoneal inflammatory exudate, tissue swelling, major adhesions, and inflammatory stricture with unformed stool were observed. The gross appearance of the mucosa was normal in the 50 % ethanol-treated animals (Fig. 1a, c). However, mucosal damages including hyperemia, ulceration, wall thickness (Fig. 1b, d) were observed in TNBS-treated animals with a macroscopic score of 13.2 ± 1.8 (n = 6). Histopathological examination of these inflamed tissues showed intensive epithelial damage, crypt abscess, inflammatory leukocyte infiltration, and smooth muscle thickening (Fig. 1e, f).
Fig. 1.
Representative macroscopic and histopathological changes in rabbit colon at 5 days after intrarectal instillation of ethanol (a, c, e) or TNBS (b, d, f). a–d Macroscopic examination showing mucosal ulceration, hyperemia, bleeding, inflammation, and wall thickening (white arrow) in distal colon after TNBS. A piece of tissue was missing due to quick snap frozen for RNA and protein extraction. The inflamed region was shown in the entire colon. e, f Hematoxylin/eosin staining showing extensive mucosal damage, crypt loss, smooth muscle thickening, and intensive inflammatory infiltration in distal colon after TNBS (f) in contrast to ethanol control (e). cm and lm circular and longitudinal muscles, mu mucosal layers, smm submucosal muscles. Scale bars 50 μm
Characteristic Inflammatory/Immune Response in Inflamed Colonic Smooth Muscle
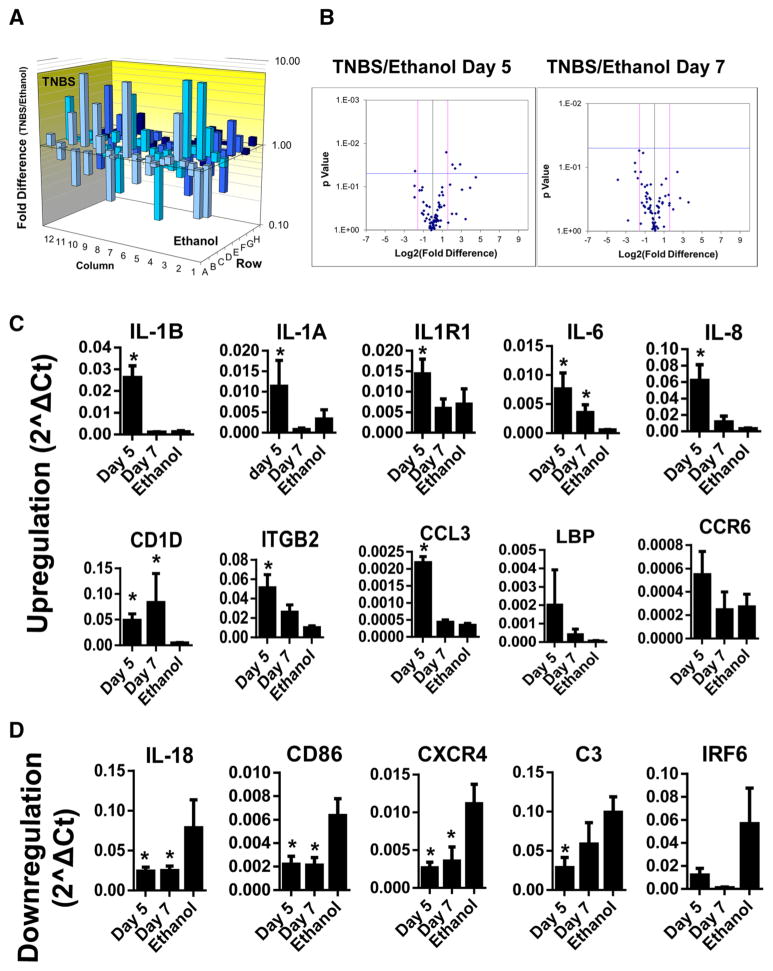
Inflammatory/immune responses have been known to contribute to the pathogenesis of intestinal diseases. Recently, the innate and adaptive immune responses have been shown to play key roles in modulating the intestinal motility [37]. Most studies have focused on the mucosal inflammatory/immune responses from epithelial cells and various immune cells [1]. Increasing experimental evidence showed that SMCs also release inflammatory/immunological mediators that play important role in regulating inflammatory dysregulation of intestinal motility [2–4]. To elucidate the role of smooth muscle-derived immune/inflammatory response in intestinal disorders, we performed RT-qPCR array using the freshly isolated colonic circular smooth muscle strips. The ethanol treatment has been used as a vehicle control for TNBS-induced colitis as it does not induce dramatic histopathological changes in the colon [28, 30, 35, 36]. Most genes in this PCR array exhibited no significant changes between ethanol vehicle and normal control. However, a few genes showed various degrees (>2- to 3-folds) of up- or down-regulation, mainly related to interferon response, Th2 and Th17 cytokine response, despite only IL-17A and TYK2 showing statistical significance (Table 2). Thus, data interpretation should be cautious for the biochemical/molecular and functional evaluation in colitis model, and the ethanol vehicle group should be used for the comparison against TNBS-induced colitis. At day 5 after TNBS treatment, the maximal time for the inflammation, 24 genes from 84 innate/adaptive immune responsive genes exhibited ≥2-fold increase in the mRNA expression, whereas 5 genes displayed ≥2-fold decrease (Table 3; Fig. 2). Student’s t test showed a statistically significant increase in IL-1β, IL-1α, IL-1R1, IL-6, IL-8, CD1D, ITGB2, and CCL3 (MIP1α) (Fig. 2c), but a significant decrease in IL-18, CXCR4, C3, and CD86 (Fig. 2d). At day 7 after TNBS treatment, only CD1D and IL-6 remained significantly higher than the ethanol control, implying that the expression of the inflammation-induced up-regulated genes restored when the colonic inflammation started to recover. However, the expression of genes that are down-regulated continued to decrease significantly at day 7. Additional genes such as IRF6, caspase-1, TLR3 that are not affected at day 5 became further decreased at day 7 despite no statistical significance (Table 3). TNF-α and most Th2/Th17 cytokines did not change significantly in the inflamed smooth muscle (Table 3).
Table 3.
Up- or down-regulated genes in day 5 (n = 5) and 7 (n = 3) after TNBS treatment over ethanol (n = 3)
| Day 5
|
Day 7
|
||
|---|---|---|---|
| Gene | Folds | Gene | Folds |
| IL1B | 36.65* | CD1D | 14.28* |
| LBP | 29.33 | LBP | 6.86 |
| IL8 | 23.64* | FAS | 6.47 |
| IL1A | 18.37* | IL6 | 6.31* |
| IL6 | 11.94* | IL8 | 4.97 |
| RAG1 | 10.28 | Cox2 | 3.52 |
| CD1D | 8.40* | IRAK1 | 3.43 |
| ITGB2 | 5.88* | NOD2 | 2.69 |
| CCL3 | 4.90* | NOD1 | 2.56 |
| IL1R1 | 4.45* | CRP | 2.25 |
| CCR6 | 4.43 | IL1B | 2.10 |
| IFNa1 | 4.25 | ITGB2 | 1.94 |
| Cox2 | 3.57 | STAT3 | 1.85 |
| FAS | 3.36 | NFkB2 | 1.79 |
| CD4 | 3.33 | CD209 | 1.71 |
| TLR4 | 2.87* | RAG1 | 1.64 |
| MBL2 | 2.82 | TICAM2 | 1.56 |
| CD1A | 2.61 | ITGAM | 1.55 |
| CCR4 | 2.47 | CD40 | 1.53 |
| ITGAM | 2.33 | MAPK1 | 1.53 |
| CCL2 | 2.19 | C-JUN | 1.52 |
| CD40 | 2.16 | LDHA | 1.43 |
| IL13 | 2.16 | FoxP3 | 1.42 |
| CCR8 | 2.02 | TYK2 | 1.41 |
| NOD1 | 1.96 | GAPDH | 1.33 |
| CD28 | 1.93 | MBL2 | 1.27 |
| NOD2 | 1.92 | CCL2 | 1.24 |
| TICAM2 | 1.91 | CD1A | 1.23 |
| CD40L | 1.85 | MIF | 1.22 |
| IL4 | 1.79 | ACTA2 | 1.22 |
| CCR5 | 1.74 | MAPK3 | 1.21 |
| IL2 | 1.74 | STAT6 | 1.12 |
| CRP | 1.72 | CCR8 | 1.10 |
| MPO | 1.66 | MAPK8 | 1.08 |
| FoxP3 | 1.60 | CCL3 | 1.08 |
| STAT3 | 1.54 | TLR4 | 1.08 |
| LDHA | 1.53 | IL1R1 | 1.07 |
| FasL | 1.51 | CCR5 | 1.06 |
| ACTA2 | 1.50 | IL13 | 1.06 |
| TNF | 1.50 | MAPK14 | −1.01 |
| RORC | 1.47 | IkBa | −1.01 |
| IkBa | 1.44 | IL4 | −1.01 |
| IL15 | 1.44 | IL23a | −1.01 |
| TYK2 | 1.43 | MPO | −1.04 |
| Myd88 | 1.40 | IFNa1 | −1.04 |
| IRAK1 | 1.37 | CCR6 | −1.06 |
| NFkB2 | 1.34 | CD4 | −1.08 |
| CD14 | 1.32 | IL1A | −1.15 |
| CXCR3 | 1.30 | CD40L | −1.17 |
| IL17A | 1.29 | CCR4 | −1.18 |
| MAPK1 | 1.25 | Myd88 | −1.21 |
| NLRP3 | 1.24 | TRAF6 | −1.24 |
| C-JUN | 1.17 | GATA3 | −1.24 |
| IL23a | 1.17 | MEFV | −1.27 |
| GATA3 | 1.17 | STAT1 | −1.28 |
| MIF | 1.13 | CD14 | −1.30 |
| STAT6 | 1.12 | CD28 | −1.31 |
| MEFV | 1.07 | IL2 | −1.32 |
| TLR2 | 1.07 | TNF | −1.32 |
| ACTB | 1.04 | NLRP3 | −1.35 |
| MAPK3 | 1.01 | NONO | −1.44 |
| IFIH1 | −1.02 | LYZ | −1.53 |
| IRF1 | −1.03 | ACTB | −1.53 |
| CD209 | −1.06 | TLR6 | −1.61 |
| DDX58 | −1.08 | IFNG | −1.62 |
| STAT4 | −1.15 | DDX58 | −1.65 |
| STAT1 | −1.15 | CD80 | −1.67 |
| TRAF6 | −1.20 | IFIH1 | −1.73 |
| LYZ | −1.21 | IL17A | −1.78 |
| MAPK8 | −1.21 | IL15 | −1.78 |
| GAPDH | −1.23 | TLR2 | −1.80 |
| MAPK14 | −1.24 | RORC | −2.02 |
| TLR6 | −1.35 | IRF1 | −2.04 |
| IL10 | −1.37 | MX1 | −2.04 |
| IFNG | −1.41 | IL5 | −2.09 |
| TLR1 | −1.41 | CXCR3 | −2.44 |
| NONO | −1.47 | IL10 | −2.50 |
| CD80 | −1.60 | LY96 | −2.75 |
| LY96 | −1.68 | STAT4 | −2.76 |
| IRF6 | −1.70 | TLR1 | −2.85 |
| CD86 | −1.85* | FasL | −2.92 |
| IL5 | −1.86 | JAK2 | −2.99 |
| Caspase-1 | −1.93 | C3 | −2.99* |
| TLR3 | −2.00 | TLR3 | −3.17 |
| JAK2 | −2.19 | CXCR4 | −3.31* |
| C3 | −2.27* | CD86 | −3.78* |
| MX1 | −2.69 | IL18 | −4.92* |
| CXCR4 | −2.98* | Caspase-1 | −6.01 |
| IL18 | −3.36* | IRF6 | −20.97 |
Bold values indicate >3-fold changes
p < 0.05 indicates a statistically significant change by the Student’s t test compared to the ethanol group
Fig. 2.
Statistically significant changes in mRNA expression of IL-1 signaling, Th1 cytokines, and inflammatory mediators in colonic smooth muscle after rabbit TNBS-induced colitis. a The 3D profile graphs showing the fold difference in expression of each gene between the TNBP and ethanol groups in the 96-well format of the PCR array. Columns pointing up (with z-axis values >1) indicate an up-regulation of gene expression, and columns pointing down (with z-axis values <1) indicate a down-regulation of gene expression in the TNBS group relative to the ethanol group. b The Volcano plot graphs the log2 of the fold change in each gene’s expression between the samples versus its p value from the Student’s t test. The black vertical line indicates fold changes of 1. The pink vertical lines the 3-fold changes in gene expression. The blue horizontal line indicates p < 0.05 by unpaired two-tailed Student’s t test. c, d Diagram illustration of representative genes using relative gene expression (2ΛCt) for statistical analysis by the Student’s t test. *p < 0.05 indicates significant changes of each gene at day 5 (n = 5) and day 7 (n = 3) after TNBS treatment as compared with the corresponding ethanol control
Inflammation-Induced Contractile Inhibition of Isolated Smooth Muscle Cells
Several studies showed a decrease in colonic muscle contraction in TNBS colitis models. Decrease in muscle contraction could be due to decrease in neural input and/or changes in signaling pathways that regulate muscle contraction [7–10]. To address the contribution of the SMCs to the inflammation-induced colonic dysmotility, the single SMCs freshly isolated from the inflamed colon circular muscle strips were used for measurement of contractile responses. Contraction in response to muscarinic M3 receptor activation was measured by treatment of cells with ACh in the presence of muscarinic M2 receptor antagonist as described previously [7]. As shown in Fig. 3a, contraction of colonic SMCs was significantly reduced after TNBS-induced colitis as compared with ethanol control. These data suggest that the inflamed SMCs could contribute to altered motility in TNBS-induced colitis.
Fig. 3.

Inhibition of colonic smooth muscle contraction (a) and up-regulation of RGS4 mRNA (b) and protein (c) 5 days after rabbit TNBS-induced colitis. a Contraction of freshly dispersed colonic circular smooth muscle cells (SMCs) after treatment (30 s) with acetylcholine (0.1 μM) plus M2 receptor antagonist methoctramine (0.1 μM) was measured by scanning microscopy. Contraction was expressed as percent decrease in cell length from before acetylcholine treatment. b, c The levels of RGS4 mRNA and protein in colonic circular muscle strips were determined by RT-qPCR and Western blot, respectively. GAPDH was used as internal normalization control. Values are mean ± SE of 3–4 experiments. **p < 0.01 indicates significant difference from corresponding vehicle control. The number in c indicates individual animal. The ratio indicates the relative density over corresponding GAPDH. The fold change represents the average expression levels in TNBS group over the average levels in ethanol group
Up-Regulation of RGS4 in Inflamed Muscle Contributing to the Inhibition of Muscle Contraction
Immune response/inflammatory mediators induce different pattern of muscle contraction in gastrointestinal tract. Th1 cytokines such as IL-1β, TNF-α, IL-6, IL-8 induce an inhibitory effect, while Th2 cytokines such as IL-4, IL-5, IL-12 induce a stimulatory effect on the contractile responses of smooth muscle to excitatory neurotransmitters [3, 5, 6, 9, 10, 38]. Th17 cytokines may involve both inhibitory and stimulatory effects [39]. As shown above, IL-1 signaling and Th1 cytokines in the colonic smooth muscles were most affected by TNBS-induced colonic inflammation. This is consistent with previous reports showing that IL-1 is a predominant cytokine in mediating inhibition of contraction [3, 6, 9, 10]. IL-1β stimulation significantly up-regulates RGS4 expression in cultured and freshly isolated colonic SMCs from healthy rabbit [7, 25, 26]. In vitro studies showed that up-regulated RGS4 contributes significantly to the inhibitory effect of IL-1β on ACh-stimulated contraction in rabbit colonic SMCs [7, 25, 26]. To address whether up-regulation of RGS4 is involved in inflammation-induced inhibition of muscle contraction in vivo, we examined the expression pattern of RGS4 by RT-qPCR and Western blot analysis in colonic circular muscle strips after TNBS-induced colitis. As shown in Fig. 3b, c, RGS4 expression was significantly increased at both mRNA and protein levels after experimental colitis.
RGS4 Silencing In Vivo Augments ACh-Induced PLC-β Activation and Muscle Contraction
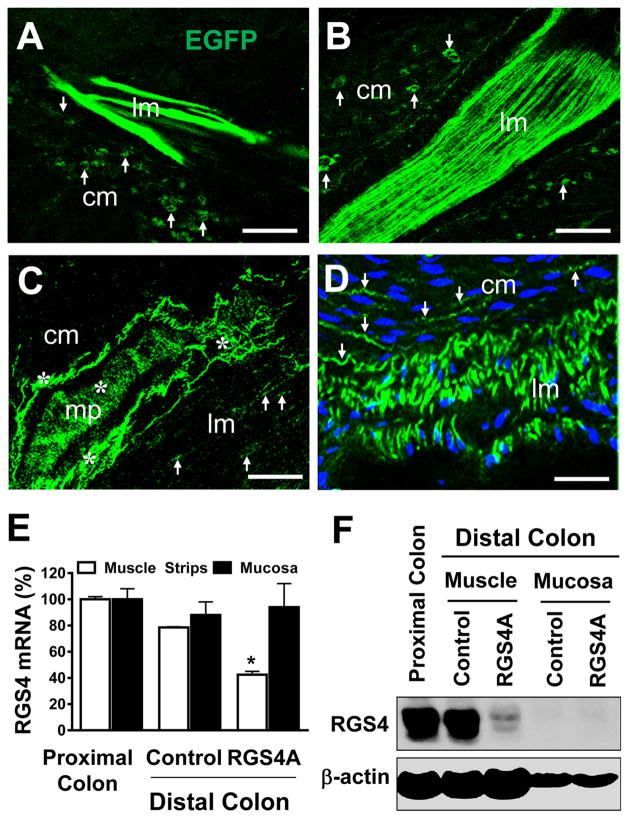
Our previous in vitro studies showed that knockdown of endogenous RGS4 in cultured SMCs and freshly isolated muscle strips by RGS4A shRNA blocked IL-1β-induced inhibition of smooth muscle contraction [7]. To further determine the role of endogenous RGS4 in mediating IL-1β-induced inhibitory effect on colonic SMCs in animal model, we first examined the feasibility and efficiency of lentivirus-mediated RGS4A shRNA in adult rabbit colon and then determined the effect of in vivo RGS4 knockdown on colonic smooth muscle motility. Lentivirus was used because of its high efficiency for gene delivery in non-dividing cells and long-term stable expression of target gene or shRNA. Intramuscular microinjection of lentivirus carrying efficient RGS4A shRNA into the distal colon through abdominal surgery was performed to suppress RGS4 expression in vivo. The feasibility and efficiency of lentiviral transduction were confirmed by the expression of the internal EGFP marker (Fig. 4a–d). As compared with the empty lentiviral vector pLL3.7, the lentiviral RGS4A shRNA efficiently inhibited RGS4 expression at both mRNA and protein levels in muscle strips of distal but not proximal colon at 2 weeks after microinjection into the muscle layers of distal colon (Fig. 4e, f). In the mucosal layer of distal colon, the level of RGS4 mRNA showed no significant difference between the empty vector and RGS4A shRNA (Fig. 4e), and the level of RGS4 protein was undetectable by Western blot (Fig. 4f). These data suggest that lentivirus-mediated RGS4A shRNA efficiently and stably silences the expression of endogenous RGS4 in vivo.
Fig. 4.
Effective knockdown of endogenous RGS4 in rabbit colonic smooth muscles after colonic intramuscular microinjection of RGS4A shRNA-expressing lentivirus. a–d Direct microscopic observation of EGFP (green) showing efficient transduction of lentivirus in circular SMCs (arrows) and myenteric plexus (mp, stars) at 2 (a, c) and 4 weeks (b, d) after infection. Scale bars 40 μm. The cm and lm indicate circular and longitudinal smooth muscle layers, respectively. The nucleus is stained with DAI (blue). e, f RT-qPCR and Western blot analysis at 2 weeks after intramuscular infection showing dramatic reduction of RGS4 mRNA and protein by RGS4 shRNA in circular muscle strips of distal colon. Values are mean ± SE of 3 experiments. *p < 0.05 indicates significant difference from corresponding empty vector control
RGS4 is a negative regulator of Gαq-dependent PLC-β activation and contraction of colonic SMCs [7, 17]. To examine the functional outcome of stable RGS4 knockdown in colonic SMCs in vivo, we measured PLC-β activity and contraction of colonic SMCs freshly isolated from colonic circular muscle strips at 9 weeks after infection of lentivirus carrying efficient RGS4A shRNA. In vivo knockdown of RGS4 expression in the SMCs of the distal colon significantly increased PLC-β activation in response to Gq-coupled m3 receptor agonist, ACh. PLC-β activation in response to Gi-coupled δ opioid receptor agonist, DPDPE, however, was not affected (Fig. 5a). Previous studies have shown that PLC-β activity in response to DPDPE is regulated by Gαi2-associated RGS12 [40]. ACh-stimulated PLC-β activation in the proximal colon was also not affected (Fig. 5a), and this was consistent with no change in the RGS4 expression in the proximal colon after microinjection at the distal colon (Fig. 4e, f). Contraction of colonic circular SMCs in response to stimulation with different concentration of ACh was significantly augmented by in vivo knockdown of RGS4 expression in the distal colon but not proximal colon (Fig. 5b). Taken together, these data suggest that RGS4 silencing in vivo specifically promotes ACh-induced PLC-β activation and smooth muscle contraction. The results also confirm that RGS4 is a negative regulator of Gαq-dependent PLC-β activation and muscle contraction in vivo.
Fig. 5.
Significant increase in acetylcholine-stimulated PLC-β activation (a) and initial contraction (b) in the colonic SMCs of distal colon after colonic intramuscular microinjection of RGS4 shRNA-expressing lentivirus. SMCs were isolated from circular colonic muscle strips of distal and proximal colon, labeled with myo-[3H]inositol in serum-free medium for 1 day. The hydrolysis of the phosphoinositide (PI) was stimulated with ACh (0.1 μM) plus M2 receptor antagonist methoctramine (0.1 μM) or DPDPE (1 μM, a specific δ-opioid receptor agonist) for 0.5 min. The level of [3H]inositol phosphate was determined with liquid scintillation and expressed as count per minute (cpm). The dose-dependent contractile responses at 30 s after stimulation with ACh in the presence of M2 receptor antagonist methoctramine (0.1 μM) was measured by scanning microscopy. Contraction was expressed as the percent decrease in cell length from before ACh treatment. Values are mean ± SE of 3 experiments. #p < 0.05 indicates a significant increase in ACh-induced initial contraction compared with the corresponding empty shRNA vector control. Values are mean ± SE of 3 experiments. *p < 0.05 indicates a significant increase in agonist-stimulated PLC-β activity compared with vehicle control. #p < 0.05 indicates a significant increase in ACh-induced PLC-β activity compared with the corresponding empty shRNA vector control
Up-Regulation of IKK2/P65 NFκB Signaling in Inflamed Smooth Muscles
NFκB signaling plays a key role in various types of inflammation. The action of NFκB signaling after colitis in mouse, rat, and human depends upon the induction of various immune/inflammatory responses [41]. In rabbit TNBS colitis model, IL-1β is significantly up-regulated [28, 29, 35]. IL-1β is known to regulate downstream target gene expression through classical and non-classical pathway of NFκB activation. To determine the correlation of in vivo NFκB signaling to RGS4 up-regulation in colonic smooth muscles after colitis, we examined the expression of IKK2/p65 (classical) and IKK1/p52 (non-classical) by performing Western blot analysis. In coincidence with the up-regulation of RGS4, dramatic increase in the protein levels of IKK2 and p65 was observed in colonic smooth muscles after TNBS-induced colitis (Fig. 6). The expression levels of IKK1 and p52 varied with individual animal but did not exhibit any dramatic difference between the inflamed and non-inflamed smooth muscles (Fig. 6). These data suggest that the classical IKK2/p65 pathway is up-regulated in inflamed colonic smooth muscles. This supports our previous studies demonstrating that IL-1β up-regulates RGS4 expression in rabbit colonic SMCs via activation of the classical IKK2/IκBα/p65 signaling in vitro [25].
Fig. 6.
Up-regulation of the classical IKK2/p65 pathway of NFκB activation in inflamed colonic circular smooth muscles. a Western blot analysis was performed with antibodies against indicated component of classical and non-classical NFκB signaling. The number indicates individual animal. The ratio indicates the relative density over corresponding GAPDH. b The fold changes represent the average expression levels in TNBS group over the average levels in control–ethanol group. *p <0.05 indicates a statistical significance by one-tailed Student’s t test
Discussion
In gut smooth muscle cells, RGS4 suppresses the Gαq signaling stimulated by muscarinic M3 or motilin receptors [42], resulting in the inhibition of agonist-induced muscle contraction [7, 17, 25]. During intestinal inflammation, IL-1β-induced up-regulation of RGS4 leads to rapid inactivation of Gαq signaling and decrease in ACh-induced PLC-β activity, MLC20 phosphorylation, and muscle contraction [7, 25, 26]. These in vitro studies using cultured or freshly isolated gut SMCs have demonstrated the important function of RGS4 in regulating colonic muscle contraction. In the present study, we demonstrate for the first time that colonic inflammation induced up-regulation of RGS4 expression in vivo in colonic SMCs, which contributes to the inhibition of colonic muscle contraction. Lentivirus-mediated delivery of RGS4 shRNA through intramuscular colonic microinjection effectively knocked down the expression of endogenous RGS4, leading to the acceleration of ACh-stimulated PLC-β-dependent contraction in colonic SMCs.
Among several colitis animal models, TNBS-induced colitis is a classic model that is used to mimic various clinical and histopathological features of colitis, including neutrophil infiltration, mucosal ulceration, and increased production of inflammatory mediators [33]. TNBS-induced colitis model in rabbits was used in the present study because (1) our previous studies have the signaling pathways involved in the regulation of smooth muscle function in response to both excitatory and inhibitory transmitters [7, 25, 26]; (2) a closer gene homology and functional correlation between rabbit and human have been observed in several disease models [29, 36]; and (3) TNBS-induced colitis in rabbits has been established [28–30, 36]. In this model, TNBS can be administered without any anesthesia. A single dose of TNBS induces a reliable and reproducible model for colitis, showing similar macroscopic damage and histopathological changes in the colon as described previously [28–30, 35, 36].
Changes in the levels of inflammatory mediators and innate/adaptive immune responses have been widely investigated in the mucosa or muscle layers after colitis induced by TNBS and others [43]. In this study, the major genes with statistically significant up-regulation in the inflamed smooth muscles of rabbit colon are the IL-1 signaling (IL-1β, IL-1α, IL-1R1) and its related Th1 cytokines (IL-6, IL-8), which is consistent with previous reports in rodent TNBS colitis models [34]. We also observed that CD1D, ITGB2, and CCL3 (MIP1α) were significantly up-regulated in the inflamed colonic smooth muscles in rabbit colitis model. All these three genes are preferentially expressed in leukocytes. Up-regulation of these genes in the inflamed colonic circular muscles may predominantly reflect the inflammatory extravasation of leukocytes. CD1D is expressed in antigen-presenting cells and presents lipid antigens to natural killer T cells that directly contribute to the intestinal inflammation [44]. ITGB2 gene encodes the common integrin β2 (CD18) that dimerizes with CD11a, b, c, and d to generate LFA-1, Mac-1, CR4, and integrin αDβ2, respectively, and participates in leukocyte adhesion and activation. ITGB2 is dramatically up-regulated in mucosa, submucosa, smooth muscles of colon in IBD patients [45], and colitis animals [46]. The chemokine CCL3 (MIP1α) is well known to be up-regulated in IBD [47] and recruits neutrophils to the inflamed gut tissues [48]. The effects of these up-regulated genes in the inflamed muscles on the intestinal contractility remain to be determined. When the colonic inflammation started to recover at day 7 after TNBS challenge, the expression of the inflammation-induced up-regulated genes restored. Interestingly, several genes such as IL-18, CXCR4, C3, CD86 were down-regulated in the inflamed smooth muscle and continued to be down-regulated when the recovery started, suggesting that these genes may protect against colonic inflammation. However, TNF-α and most Th2/Th17 cytokines did not change significantly in the inflamed smooth muscle, in contrast to their significant changes in mucosal/immune cells as evidenced by previous reports [3, 5, 6, 9, 10, 37–39]. IL-18 (also called interferon-γ-inducing factor) is a member of IL-1 superfamily, activated by caspase-1, and regulates target gene expression through NFκB signaling [49]. In contrast to IL-1, IL-18 (and its related caspase-1 and interferon-γ) is down-regulated in the inflamed smooth muscles, implying that down-regulation may be a protective mechanism against TNBS-induced colitis in rabbit. IL-18 is expressed mainly in the lamina propria mononuclear cells and intestinal epithelial cells. IL-18 exerts a dual role in intestinal inflammation, depending on the cell types and inflammation stages [50]. Further investigation is warranted to elucidate the cellular expression pattern of IL-18 and its signaling after colitis and their functional regulation in intestinal motility.
The pro-inflammatory cytokine IL-1β modulates intestinal motility through regulating contractile proteins and G-protein signaling, which is controlled by the family of RGS proteins. Little is known about the expression and function of RGS proteins in IBD. A series of in vitro studies demonstrated the critical role of RGS4 in mediating IL-1β-induced inhibitory effect on agonist-stimulated contraction of smooth muscle [7, 25, 26]. The present in vivo study provided a convincing evidence that RGS4 is significantly up-regulated in colonic SMCs after colitis. Up-regulated RGS4 expression contributes to the inhibition of SMC contraction in the inflamed colon. To investigate the role of RGS4 in vivo in regulating smooth muscle contraction, we examined the feasibility and efficiency of in vivo silencing of endogenous RGS4 expression via intramuscular microinjection of lentivirus carrying effective RGS4A shRNA. Our previous studies demonstrated the efficiency of lentivirus to infect cultured colonic SMCs [51]. In this study, we demonstrated that intramuscular administration of lentivirus induced a sufficient infection in colonic SMCs in vivo, leading to an effective and long-term knockdown of RGS4 mRNA and protein expression in colonic muscle strips. Such knockdown promotes PLC-β activation and muscle contraction after ACh stimulation in the freshly isolated SMCs. Whether lentivirus-mediated RGS4 shRNA knockdown, particularly in a SMC-specific manner, could reverse inflammation-induced smooth muscle dysfunction needs further investigation using colitis model.
In conclusion, TNBS-induced colitis in rabbit induces up-regulation of IL-1 signaling and RGS4 expression in colonic smooth muscles. Up-regulation of RGS4 contributes to the inflammatory inhibition of muscle contractile responses to agonist stimulation. Down-regulation of RGS4 could restore the contractility of colonic muscle cells. Intervention of RGS4 signaling in colonic muscle cells may provide a potential therapeutic approach for IBD.
Acknowledgments
This work was supported by Grant DK075964 (WH) and DK015564 (KSM) from the National Institutes of Diabetes, and Kidney and Digestive Diseases.
Footnotes
Author’s contribution: YZ carried out the lentivirus injection and Western blot study, performed the statistical analysis, and drafted the manuscript. FL carried out the colitis modeling and RT-qPCR study. CY carried out Western blot and quantification. JH carried out the colitis modeling, histological dissections, and tissue preparation. SM carried out smooth muscle contraction and PLC-β assay. HW carried out PCR array. KSM participated in the experimental design and help to draft the manuscript. WH designed and coordinated the study and drafted the manuscript. The final manuscript was read and approved by all authors.
Compliance with ethical standards
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Yonggang Zhang, Email: zygote@temple.edu.
Fang Li, Email: flihu@temple.edu.
Hong Wang, Email: whmdzju@hotmail.com.
Chaoran Yin, Email: tug21151@temple.edu.
JieAn Huang, Email: jhuangnews@gmail.com.
Sunila Mahavadi, Email: sunila@vcu.edu.
Karnam S. Murthy, Email: skarnam@vcu.edu.
Wenhui Hu, Email: whu@temple.edu.
References
- 1.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2:72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salinthone S, Singer CA, Gerthoffer WT. Inflammatory gene expression by human colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G627–G637. doi: 10.1152/ajpgi.00462.2003. [DOI] [PubMed] [Google Scholar]
- 4.Singer CA, Salinthone S, Baker KJ, Gerthoffer WT. Synthesis of immune modulators by smooth muscles. BioEssays. 2004;26:646–655. doi: 10.1002/bies.20041. [DOI] [PubMed] [Google Scholar]
- 5.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol. 2006;143:389–397. doi: 10.1111/j.1365-2249.2005.02979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazdrak K, Shi XZ, Sarna SK. TNFalpha suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-kappaB-mediated induction of ICAM-1. Gastroenterology. 2004;127:1096–1109. doi: 10.1053/j.gastro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol. 2007;293:C1991–C2000. doi: 10.1152/ajpcell.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1131–G1139. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- 9.Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G274–G284. doi: 10.1152/ajpgi.00512.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 11.Cao W, Cheng L, Behar J, Biancani P, Harnett KM. IL-1beta signaling in cat lower esophageal sphincter circular muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G672–G680. doi: 10.1152/ajpgi.00110.2006. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, Vrees MD, Potenti FM, Harnett KM, Fiocchi C, Pricolo VE. Interleukin 1beta-induced production of H2O2 contributes to reduced sigmoid colonic circular smooth muscle contractility in ulcerative colitis. J Pharmacol Exp Ther. 2004;311:60–70. doi: 10.1124/jpet.104.068023. [DOI] [PubMed] [Google Scholar]
- 13.Riddle EL, Schwartzman RA, Bond M, Insel PA. Multi-tasking RGS proteins in the heart: The next therapeutic target? Circ Res. 2005;96:401–411. doi: 10.1161/01.RES.0000158287.49872.4e. [DOI] [PubMed] [Google Scholar]
- 14.Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol. 2006;17:363–376. doi: 10.1016/j.semcdb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: convergent evidence for regulator of G-protein signaling 4 (RGS4) Biol Psychiatry. 2006;60:534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Hao J, Michalek C, Zhang W, Zhu M, Xu X, Mende U. Regulation of cardiomyocyte signaling by RGS proteins: differential selectivity towards G proteins and susceptibility to regulation. J Mol Cell Cardiol. 2006;41:51–61. doi: 10.1016/j.yjmcc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi-and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA. 1997;94:6159–6163. doi: 10.1073/pnas.94.12.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumins AM, Barker SA, Huang C, et al. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem. 2004;279:2593–2599. doi: 10.1074/jbc.M311600200. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Wolff DW, Wei T, et al. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res. 2009;69:5743–5751. doi: 10.1158/0008-5472.CAN-08-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patten M, Stube S, Thoma B, Wieland T. Interleukin-1beta mediates endotoxin- and tumor necrosis factor alpha-induced RGS16 protein expression in cultured cardiac myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:360–365. doi: 10.1007/s00210-003-0798-0. [DOI] [PubMed] [Google Scholar]
- 21.Mittmann C, Schuler C, Chung CH, et al. Evidence for a short form of RGS3 preferentially expressed in the human heart. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:456–463. doi: 10.1007/s002100000376. [DOI] [PubMed] [Google Scholar]
- 22.Emilsson L, Saetre P, Jazin E. Low mRNA levels of RGS4 splice variants in Alzheimer’s disease: association between a rare haplo-type and decreased mRNA expression. Synapse. 2006;59:173–176. doi: 10.1002/syn.20226. [DOI] [PubMed] [Google Scholar]
- 23.Runne H, Regulier E, Kuhn A, et al. Dysregulation of gene expression in primary neuron models of Huntington’s disease shows that polyglutamine-related effects on the striatal transcriptome may not be dependent on brain circuitry. J Neurosci. 2008;28:9723–9731. doi: 10.1523/JNEUROSCI.3044-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho H, Harrison K, Schwartz O, Kehrl JH. The aorta and heart differentially express RGS (regulators of G-protein signalling) proteins that selectively regulate sphingosine 1-phosphate, angiotensin II and endothelin-1 signalling. Biochem J. 2003;371:973–980. doi: 10.1042/BJ20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu W, Li F, Mahavadi S, Murthy KS. Interleukin-1beta up-regulates RGS4 through the canonical IKK2/IkappaBalpha/NF-kappaB pathway in rabbit colonic smooth muscle. Biochem J. 2008;412:35–43. doi: 10.1042/BJ20080042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3-beta pathway. Am J Physiol Cell Physiol. 2009;296:C1310–C1320. doi: 10.1152/ajpcell.00573.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto H, Kimura T, Haga K, Kasahara N, Anton P, McGowan I. Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors. BMC Gastroenterol. 2010;10:44. doi: 10.1186/1471-230X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony D, Savage F, Boulos P, Hembry R, Sams V, Trevethick M. Effect of methylprednisolone on the ulceration, matrix met-alloproteinase distribution and eicosanoid production in a model of colitis in the rabbit. Int J Exp Pathol. 1997;78:411–419. doi: 10.1046/j.1365-2613.1997.440373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Depoortere I, Van Assche G, Peeters TL. Motilin receptor density in inflamed and noninflamed tissue in rabbit TNBS-induced colitis. Neurogastroenterol Motil. 2001;13:55–63. doi: 10.1046/j.1365-2982.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 30.Hathaway CA, Appleyard CB, Percy WH, Williams JL. Experimental colitis increases blood–brain barrier permeability in rabbits. Am J Physiol. 1999;276:G1174–G1180. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 31.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 32.Misra S, Murthy KS, Zhou H, Grider JR. Coexpression of Y1, Y2, and Y4 receptors in smooth muscle coupled to distinct signaling pathways. J Pharmacol Exp Ther. 2004;311:1154–1162. doi: 10.1124/jpet.104.071415. [DOI] [PubMed] [Google Scholar]
- 33.Qin HY, Wu JC, Tong XD, Sung JJ, Xu HX, Bian ZX. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol. 2011;46:164–174. doi: 10.1007/s00535-010-0321-6. [DOI] [PubMed] [Google Scholar]
- 34.Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneda K, Saitoh F, Shibusawa H, et al. Correlation of neutrophil and monocyte derived interleukin-1 receptor antagonist and interleukin-8 with colitis severity in the rabbit. Cytokine. 2011;56:508–514. doi: 10.1016/j.cyto.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Percy WH, Burton MB, Fallick F, Burakoff R. A comparison in vitro of human and rabbit distal colonic muscle responses to inflammatory mediators. Gastroenterology. 1990;99:1324–1332. doi: 10.1016/0016-5085(90)91157-2. [DOI] [PubMed] [Google Scholar]
- 37.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology. 2005;129:131–141. doi: 10.1053/j.gastro.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390–400. doi: 10.3748/wjg.14.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimple RJ, De Vries L, Tronchere H, et al. RGS12 and RGS14 GoLoco motifs are G alpha(i) interaction sites with guanine nucleotide dissociation inhibitor Activity. J Biol Chem. 2001;276:29275–29281. doi: 10.1074/jbc.M103208200. [DOI] [PubMed] [Google Scholar]
- 41.Nalli AD, Kumar DP, Mahavadi S, et al. Hypercontractility of intestinal longitudinal smooth muscle induced by cytokines is mediated by the nuclear factor-kappaB/AMP-activated kinase/myosin light chain kinase pathway. J Pharmacol Exp Ther. 2014;350:89–98. doi: 10.1124/jpet.113.212522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol. 2007;292:C200–C208. doi: 10.1152/ajpcell.00103.2006. [DOI] [PubMed] [Google Scholar]
- 43.Hommes DW, Meenan J, Dijkhuizen S, Ten Kate FJ, Tytgat GN, Van Deventer SJ. Efficacy of recombinant granulocyte colony-stimulating factor (rhG-CSF) in experimental colitis. Clin Exp Immunol. 1996;106:529–533. doi: 10.1046/j.1365-2249.1996.d01-863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao CM, Zimmer MI, Shanmuganad S, Yu HT, Cardell SL, Wang CR. dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142:326–334. e321–e322. doi: 10.1053/j.gastro.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelbaqi M, Chidlow JH, Matthews KM, et al. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Lab Invest. 2006;86:380–390. doi: 10.1038/labinvest.3700398. [DOI] [PubMed] [Google Scholar]
- 46.Hajj-Hussein IA, Jurjus R, Saliba J, et al. Modulation of Beta2 and Beta3 integrins in experimental colitis induced by iodoacetamide and enteropathogenic E. coli. J Biol Regul Homeost Agents. 2013;27:351–363. [PubMed] [Google Scholar]
- 47.Ajuebor MN, Kunkel SL, Hogaboam CM. The role of CCL3/macrophage inflammatory protein-1alpha in experimental colitis. Eur J Pharmacol. 2004;497:343–349. doi: 10.1016/j.ejphar.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 49.Sollberger G, Strittmatter GE, Garstkiewicz M, Sand J, Beer HD. Caspase-1: the inflammasome and beyond. Innate Immun. 2014;20:115–125. doi: 10.1177/1753425913484374. [DOI] [PubMed] [Google Scholar]
- 50.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 2011;32:171–179. doi: 10.1016/j.it.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu W, Huang J, Mahavadi S, Li F, Murthy KS. Lentiviral siRNA silencing of sphingosine-1-phosphate receptors S1P1 and S1P2 in smooth muscle. Biochem Biophys Res Commun. 2006;343:1038–1044. doi: 10.1016/j.bbrc.2006.03.079. [DOI] [PubMed] [Google Scholar]