Abstract
In all organisms, heat-shock proteins (HSPs) provide an ancient defense system. These proteins act as molecular chaperones by assisting proper folding and refolding of misfolded proteins and aid in the elimination of old and damaged cells. HSPs include Hsp100, Hsp90, Hsp70, Hsp40, and small HSPs. Through its substrate-binding domains, Hsp70 interacts with wide spectrum of molecules, ranging from unfolded to natively folded and aggregated proteins, and provides cytoprotective role against various cellular stresses. Under pathophysiological conditions, the high expression of Hsp70 allows cells to survive with lethal injuries. Increased Hsp70, by interacting at several points on apoptotic signaling pathways, leads to inhibition of apoptosis. Elevated expression of Hsp70 in cancer cells may be responsible for tumorigenesis and for tumor progression by providing resistance to chemotherapy. In contrast, inhibition or knockdown of Hsp70 reduces the size of tumors and can cause their complete regression. Moreover, extracellular Hsp70 acts as an immunogen that participates in cross presentation of MHC-I molecules. The goals of this review are to examine the roles of Hsp70 in cancer and to present strategies targeting Hsp70 in the development of cancer therapeutics.
Keywords: Hsp70, Apoptosis, Cancer therapeutics, Immunogenicity
Introduction
In all organisms, the heat-shock response, induced by a wide range of stimuli, increases expression of a family of proteins called heat-shock proteins (HSPs) [1,2] that act as molecular chaperones and, for cells under stress, exhibit cytoprotective properties [3,4]. Stress beyond a certain threshold induces misfolding and aggregation of proteins and disruption of regulatory complexes [5]. In mammalian cells, the chaperone functions of HSPs maintain and restore cellular homeostasis. Principal heat-shock proteins that have chaperone activity belong to five conserved classes: Hsp33, Hsp60, Hsp70, Hsp90, Hsp100, and the class of small heat-shock proteins (sHSPs). Members of the HSP family, either expressed constitutively or regulated inductively, are transported to various cellular compartments. HSPs of large molecular weight are ATP-dependent molecular chaperones; small HSPs act in an ATP-independent manner. Under physiological conditions, the chaperones also assist in signaling and protein trafficking. However, harmful assaults on cells increase the demand for HSPs (Hsp70 in particular). Among the HSPs, Hsp70 has strong cytoprotective properties. Stress-induced expression of Hsp70 protects cells from lethal injuries and therefore provides a barometer of how pro-apoptotic stimuli elicit a protective response [6–9].
Hsp70 is also involved in cell growth, cell proliferation, and erythroid differentiation during formation of erythrocytes. Accumulation of this protein in erythroblast nuclei protects GATA-1, a transcription factor necessary for erythropoiesis [10]. Malignant cells express higher levels of Hsp70 than normal cells, and high Hsp70 expression is indicative of a tumorigenic phenotype that typically resists chemotherapy and programmed cell death [11–13]. Moreover, Hsp70 interferes at various points on the apoptotic pathways, preventing stress from inappropriately inducing cell death. In addition to its anti-apoptotic function, Hsp70 has an immunomodulatory effect and shows cross presentation with the MHC-I molecule [14]. Hsp70 activates both arms of the immune system (innate and adaptive) and acts as a potent immunomodulator. The goals of the review are to focus on the tumorigenic effects of Hsp70 and potential strategies for treatment of cancers and to assess the immunological role of Hsp70 in cross presentation.
Hsp70: cellular lifeguard
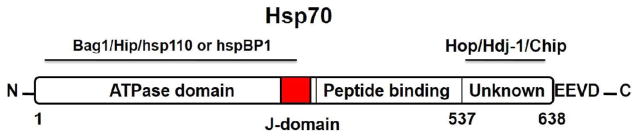
During periods of stress, human cells produce high levels of Hsp70, constitutively expressed as Hsc70, mitochondrial Hsp75, and GRP78, which are found in the endoplasmic reticulum. Hsp70 functions as an ATP-dependent molecular chaperone that assists proper folding/refolding of newly synthesized polypeptide chains, assembly of complex structures, and the transport of proteins through the cell membrane [15–20]. Structurally, Hsp70 possesses a peptide binding domain (PBD) and an N-terminal ATPase domain (ABD) (Fig. 1). The EEVD motif in the carboxyl terminal of the PBD is responsible for substrate binding/refolding (Fig. 1). The ABD induces release of client proteins and accomplishes ATP hydrolysis. Upon ATP binding, a proline residue in the ATPase domain induces a conformational change and causes hydrolysis [24]. Several associated co-chaperones also bind to Hsp70 and regulate its chaperone function. The Hsp70-associated co-chaperones are of three types: J-domain co-chaperones (Hsp40 binds to ABD in Hsp70 and stimulates low ATPase activity for this chaperone); the nucleotide exchange factors Bag-1, Hsp110, or HspBP1 catalyze the release of ADP and complete the Hsp70 ATPase cycle; the TPR domain (Hop, CHIP) chaperones bind to the C-terminal EEVD motif of both Hsp70 and Hsp90 [21–23]. These are essential for the assembly of Hsp70 and Hsp90 complexes (Fig. 1) [23].
Fig. 1.
Structure of Hsp70 with sites of action [21–23]. N-terminal shows ATPase domain with associated co-chaperones (Bag1/Hip/hsp110 or hspBP1). However, C-terminal represents EEVD domain with its co-chaperone (Hop/Hdj-1/Chip) and peptide binding domain. J-domain localizes in the central approximately.
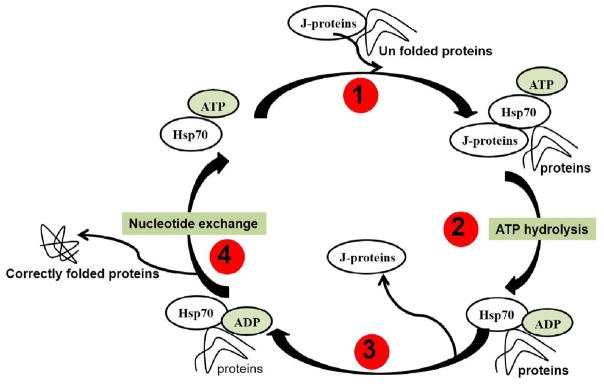
Stress-induced expression of Hsp70 allows cells to cope with large amounts of unfolded and/or denatured proteins. Further, the Hsp70 housekeeping functions involve transportation of precursor proteins into cellular comportments; protein folding (cytosol, endoplasmic reticulum, and mitochondria); degradation of unstable proteins and protein complexes; control of regulatory proteins; and protein refolding (Fig. 2) [25].
Fig. 2.
Diagrammatic presentation of the chaperone functions of Hsp70 [21–23,25]. Hsp70 binds to unfolded protein in the presence of J-proteins using ATP. ATP hydrolysis stimulates J-protein release that further led to release of correctly folded protein(s) and Hsp70 become available for next cycle.
Hsp70: an anti-apoptotic protein
Apoptosis is essential for embryogenesis, development, and maintenance of cellular homeostasis [26,27]. Anti-cancer drugs induce it through a variety of mechanisms [28]. Either an intrinsic (mitochondria-dependent) or extrinsic (death receptor) pathway facilitates the process. Caspase-3, the effector caspase, is involved in both pathways and is responsible for apoptosis in target cells [29].
In response to a death signal, activation of the intrinsic apoptotic pathway results in altered expression of the Bcl2 family of proteins [30]. These include anti-apoptotic (Bcl2 and Bcl-xL); pro-apoptotic (Bax, Bcl-Xs, and Bak); and BH3-only proteins, which function upstream to Bax, Bak, and Bcl-Xs [30–32]. Downstream to mitochondria, released cytochrome c interacts with cytosolic apoptotic protease activation factor-1 (Apaf-1) to form the apoptosome with caspase-9, which leads to activation of caspases [33,34]. In addition to Bcl2 proteins, Smac/Diablo and Htra2/Omi induce apoptosis by blocking inhibitory apoptotic proteins (Fig. 3) [39–41].
Fig. 3.
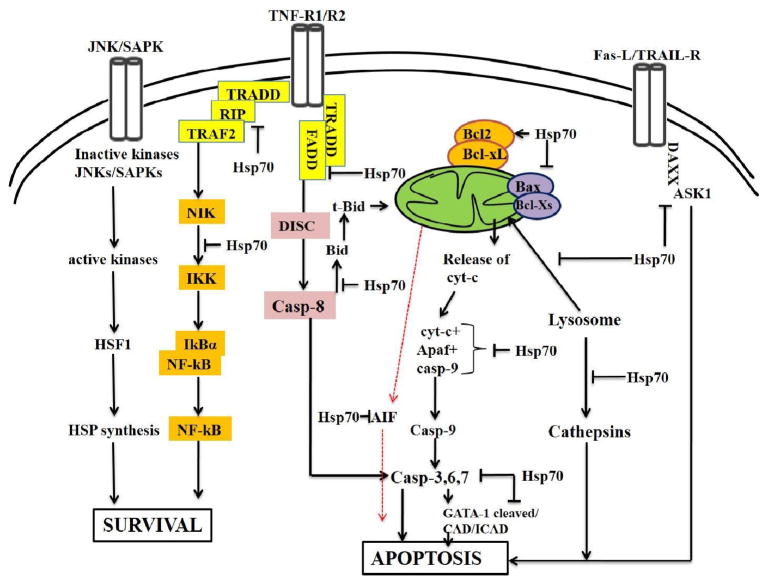
Schematic representation of the function of Hsp70 in the apoptotic signaling and survival pathways [35–38]. SAPKs stimulate synthesis of Hsp70 that leads to cell survival. High Hsp70 interferes at several levels in the apoptotic signaling such as apoptosome, caspases, and cathepsins. Hsp70 also contributes in heterodimerization of bcl2 and Bcl-xL that block Bax heterodimerization.
Extrinsic apoptosis (the death receptor pathway) is triggered by plasma membrane-associated proteins of the TNF-family of receptors, which lead to activation of caspase-8/10 in the death-inducing signaling complex (DISC) [42]. Caspase-8 either directly activates executioner caspases (caspase-3/6/7) or cleaves Bid into t-Bid, which connects the extrinsic and intrinsic apoptotic pathways [43–45]. In apoptotic signaling, highly expressed Hsp70 interferes at several points, including the release of cytochrome c, activation of caspases, accumulation of misfolded proteins, generation of reactive oxygen species, and DNA fragmentation [13,46]. Further, inhibition/knockdown of Hsp70 increases sensitivity of cells to apoptosis [47–49]. Hsp70 inhibits caspase activity directly or indirectly, thereby blocking the intrinsic and extrinsic apoptotic pathways through interaction with key apoptotic proteins at three levels: up-stream to mitochondria, at mitochondria, and post-mitochondria (Fig. 3). Thus, Hsp70 directly or indirectly modulates the intrinsic and extrinsic apoptotic pathways.
Hsp70 and the intrinsic apoptotic pathway
Expression of Hsp70, an evolutionary-conserved protein involved in apoptotic signaling, increases the survivability of cells under stress. Cells with Hsp70 knockdown are sensitive to apoptosis [50]; over-expression of Hsp70 inhibits apoptosis, acting either downstream or upstream to mitochondria.
Furthermore, Hsp70 interacts with nerve growth factor and platelet-derived growth factor and enhances the survival of cells through activation of the PI3K signaling pathway. Activated PI3K leads to activation of ser/thr kinases (Akt/PKB) to generate a growth factor-mediated survival signal. Akt kinase targets Bad and caspase-9 in the apoptotic cascade [51–54]. In K562 cells, Hsp70 stabilizes the Akt/PKB complex [55]. In zebra fish, HspA12B, a member of the Hsp70 family, is required for vasculature development, and, by sustaining Akt activity, is involved in endothelial cell migration and tube formation [56]. Thus, members of the Hsp70 family are implicated in the regulation of cell survival and differentiation. Hsp70 is also involved in the re-phosphorylation and stabilization of proteins through the priming of non-phosphorylated protein kinases [57]. In NIH3T3 cells, Hsp70 inhibits a stress-activated kinase (apoptosis signal regulating kinase-1), and its down-regulation facilitates production of H2O2, ASK-1 activation, and apoptosis [58]. In addition, Hsp70 binds to C-Jun N-terminal kinase (JNK) and blocks its ATP-dependent activation [59]. Mouse embryo fibroblast (MEF) Hsp70.1−/− cells are resistant to JNK-mediated apoptosis, and AEG 3482, an anti-apoptotic compound, inhibits JNK activity by inducing expression of Hsp70 [60]. In primary cultures of IMR90 human fibroblasts, Hsp70 inhibits P38 kinase [61], which participates in a signaling cascade controlling cellular responses to cytokines and stress.
Hsp70 affects the expression of transcription factors associated with proteins of the Bcl2 family [62]. Bcl2 and Bax are the targets of the tumor suppressor protein, p53. In response to DNA damage, transcription of Bcl2 is repressed, and Bax is induced [63]. Tumor cells often have a mutated p53, which forms a stable complex with Hsp70/Hsc70 [64]. Stress-mediated expression of Hsp70 inhibits the nuclear import of p53 [64,65]. However, how Hsp70 regulates NF-kB-function is still poorly understood. Cytosolic Hsp70 could inhibit NF-kB expression, and membrane-bound Hsp70 could induce this transcription factor [66]. In response to similar stimuli, cytosolic and membrane-bound Hsp70 are stimulated [67]. In endothelial cells, stress-induced expression of Hsp70 facilitates TNF-α-mediated apoptosis by blocking the NF-kB survival pathway [68]. Furthermore, Hsp70 blocks NF-kB-activation through inhibition of I-kB-α kinase (IKK) and degradation of I-kB-α [69,70]. Hsp70 may also facilitate the elimination of DNA-damaged cells [71]. Inhibitor of growth (ING) proteins acts as tumor suppressors; their expression is down-regulated in human cancers that transmit a death signal and bind to histones and therefore control chromatin remodeling and p53 activity [72,73]. These proteins boost the function of Hsp70, which in turn induces TNF-α receptor-mediated apoptosis by preventing IKK activity and blocking of NF-kB survival pathways [72]. In addition, Hsp70 coupled with Hsp40 inhibits Bax translocation and thereby prevents permeabilization of mitochondrial membranes and the subsequent release of cytochrome c and apoptosis-inducing factor (AIF) [74]. This function of Hsp70 depends on a chaperone and ATP hydrolysis activity [75].
Hsp70 also acts at the post-mitochondrial level, blocking apoptosis downstream to cytochrome c and upstream to caspase-3 [76]. It inhibits formation of the apoptosome by interaction with its ATPase domain [77,78]. Furthermore, in TNF-α-mediated apoptosis, Hsp70 prevents the characteristic morphological changes of dying cells but does not preclude caspase-3 activation [79]. Activated caspase-3 leads to activation of caspase-activated DNase (CAD), which is responsible for DNA degradation during apoptosis. Hsp70 with its co-chaperones, Hsp40 and inhibitor of CAD (ICAD), regulate enzymatic function and proper folding of CAD. ICAD, with the Hsp70-Hsp40 complex, recognizes and binds to the transit state of CAD and increases its activity in T-cell receptor-mediated T-cells [80]. An early target of caspase-3, poly (ADP-ribose) polymerase (PARP), is necessary to prevent necrosis and inflammation during apoptosis [81,82]. In the nuclei of cells undergoing single-strand DNA breaks after heat treatment, Hsp70 interacts with PARP1, XRCC1, and other DNA repair proteins [81,82]. These findings suggest that Hsp70 restores DNA integrity through formation of the protein repair complex. Caspase-3 targets a transcription factor, GATA-1; however, Hsp70 accumulation in nucleus protects GATA-1 from caspase-3 cleavage and thereby increases erythroid cell differentiation and survival [10].
Hsp70 and the extrinsic/death receptor pathway
Hsp70 blocks TNF-α-mediated apoptosis; however, it fails to protect Bid-homozygous knockdown in MEF cells [83]. In primary human fibroblast cells, Hsp70 inhibits Bid cleavage through activation of caspase-8/10 [83]. TNF-α mediated stimulation of hematopoietic cells induces activation of pro-apoptotic, double-stranded RNA-dependent protein kinase (PKR) [84]. In TNF-α-induced apoptosis, Hsp70 interacts with the FANCC protein (Fanconianemia complementation group C, an inhibitor of PKR) via its ATPase domain and forms a ternary complex with FANCC and PKR [84,85]. It also resists TRAIL-induced apoptosis and formation of a death-inducing signaling complex with death receptors DR4 and DR5 [42]. The function of Hsp70 in Fas-induced apoptosis is poorly understood; however, adverse effects depend on the cell context [86,87].
Hsp70 and the caspase-independent apoptotic pathway
Activation of the intrinsic pathway, triggered by the release of cytochrome c, induces AIF and translocation of endonuclease G (Endo-G) to the nucleus, where it induces caspase-independent nuclear changes [88]. In cells with Apaf-1 and caspase-9 knockdown, Hsp70 inhibits the caspase-independent apoptotic pathway, suggesting that it also prevents cell death in Apaf-1- or caspase-9-knockdown cells incubated with or without a caspase activator/inhibitor. These findings indicate that the cytochrome c/Apaf-1/caspase pathway is not the sole pathway for Hsp70 interactions [89,90]. In cell-free systems, Hsp70 binds to AIF and inhibits AIF-mediated chromatin condensation, a mechanism by which it could protect cells from AIF-induced apoptosis. Thus, endogenous Hsp70 controls AIF-mediated apoptosis; in Jurkat T cells, down-regulation of Hsp70 sensitizes cells to serum withdrawal and AIF release [90]. Further, in Apaf-1−/− cells, Hsp70 inhibits erythroblast apoptosis by blocking nuclear import of AIF [91]. Hsp70 with Endo-G protects against DNA fragmentation; this association could use AIF as a molecular bridge [92]. Hsp70 resides in the endolysosomal membranes of tumor cells and stressed cells; in HeLa cells, it prevents the release of lysosomal cathepsin into the cytosol (Fig. 3) [93,94]. Hsp70-positive lysosomes show increased size and resistance to chemical and physical membrane destabilization [95]. In murine fibroblasts, Hsp70 also protects cells from UV-A-and UV-B-induced apoptosis. This protection is mediated through inhibition of IL-6 release, which is induced by UV light (Fig. 3) [95].
Hsp70: a protein associated with tumorigenicity
Under non-stressed conditions, cells express Hsp70 at basal levels. Enhanced expression, a characteristic of cancerous or stressed cells, increases survival of these cells. Further, anti-cancer therapy elicits Hsp70 expression, which has a cytoprotective effect. Clinical studies indicate that Hsp70 predicts for a poor prognosis because malignant cells express more Hsp70 during tumor progression (endometrial cancers, osteosarcomas, and renal cell tumors) as compared to normal cells [96]. Hsp70 and prostate-specific antigen are markers used to identify patients in early stages of prostate cancer [97]. Further, Hsp70 is abundantly expressed during the progression of chronic myeloid leukemia [98]. In HL-60/BCR-ABL and K562 cells, increased expression of Hsp70 helps cells resist imatinib-mediated cell death (imatinib, a chemotherapeutic agent used to block Bcr-Abl tyrosine kinase activity) [99]. In gastric epithelial cells, the expression of Hsp70 is elevated after infection with Helicobacter pylori [99]. In addition, Hsp70.2, a member of the Hsp70 family, is highly expressed during spermatogenesis and breast cancer progression, thereby delaying senescence [99,100]. Enhanced expression of Hsp70 is associated with tumorigenesis for breast cancer, endometrial cancer, gastric cancer, and acute leukemia; with poor prognoses; and with resistance to chemo- and radiation therapy [13,101–104]. Nuclear accumulation of Hsp70 is a diagnostic marker for epithelial dysplasia, and antibodies against Hsp70are present in sera of patients with hepatocellular carcinoma [105,106] (Table 1).
Table 1.
Demonstrates specific cancers and their association with Hsp70.
| Cancer type | Effects of Hsp70 expression | Ref. | |
|---|---|---|---|
| 1. | Leukemia, MCF-7 breast cancer | High Hsp70 expression increase cancer growth and survival | [107,108] |
| 2. | Gastric, endometrial cancer | Hsp70 silencing with RNAi inhibits human gastric cancer growth and induces apoptosis | [109,110] |
| 3. | Gastric cancer | High Hsp70expression induced cancer survival | [111,112] |
| 4. | Colon and lung cancer | High Hsp70 expression was associated with overall survival High Hsp70 plays cytoprotective role in cancer |
[113–116] |
| 5. | Breast and gastric cancer | Hsp70 showed anticancer effect | [117–119] |
| 6. | Prostate cancer | High Hsp70 contributes in prostate cancer development | [113,120] |
Hsp70, which inhibits apoptosis upstream and downstream to mitochondria, is a promising therapeutic target for lowering drug resistance in cancer cells [42,98]. Stress-mediated expression of Hsp70 promotes tumorigenesis in cancer cells (colon cancer, melanoma, and pancreatic adenocarcinoma); its down-regulation is associated with decreased tumorigenicity [48,49,121–123]. In accord with this, a Hsp70 antisense construct was used to kill cancer cells in the absence of additional stimuli [124]. Down-regulation of Hsp70 is cytotoxic to transformed cells; however, it is undetectable in non-transformed cells [122,125]. Therefore, Hsp70 knockdown sensitizes or kills cancer cells preferentially to normal cells. The constitutively stressed phenotype of cancer cells depends on the cytoprotective function of Hsp70 (Fig. 3).
Hsp70: cancer treatment
Hsp70 is a druggable target in comparison to other HSPs because they are regulated by nucleotides [126,127]. Hsp70/Hsp90 is the only ATPase that is regulated by the inhibition of its ATPase activity [126,127]. Therefore, targeting of Hsp70 is an attractive strategy for cancer treatment. Although several inhibitors have been designed for Hsp90, and some of these are in clinical trials, few are known for Hsp70. Gene transcription of Hsp70 is regulated by the transcription factor, HSF1, which becomes activated in response to stress stimuli [128]. Since activated HSF1 induces Hsp70 expression [128], inhibition of HSF1 could be an effective approach to block the expression of Hsp70. Inhibition of HSF1 activation can be achieved by the flavonoid, quercetin; by diterpenetriperoxide; and by triptolide. These compounds, along with benzopyrene, inhibit the expression of Hsp70; other HSPs may remain unaffected [47,129–133]. In K562 cells, resveratrol, a non-specific inhibitor of Hsp70, inhibits Hsp70 expression by blocking Akt-kinase activity and up-regulating ERK1/2 kinase activity [134].
AIF-derived peptides (150–228aa) targeting Hsp70 sensitize cancer cells to apoptosis [135]. These peptides carry the AIF regions (150aa–228aa) required for Hsp70 binding in its PBD but lack the pro-apoptotic function of AIF. One of these inhibitors, ADD70 (AIF-derived decoy for Hsp70) decreases tumor sizes in rat colon cancers and melanomas (B16F10) and sensitizes these cancers to cisplatin. ADD70 shows anti-tumor effects in syngeneic animals but not in immuno-deficient mice; it also increases tumor-infiltrating cytotoxic CD8+ T-cells [125].
In lymphoma cells, interaction of a small molecule (HS-72) with Hsp70 leads to aggregation of misfolded proteins and destabilization of lysosome membranes, thus inducing autophagic cell death [136]. 2-Phenylacetylene sulfonamide interacts with the C-terminal of Hsp70 and inhibits its expression [137]. In B-CCL cells, however, PES induces the caspase-dependent apoptotic pathway [138]. Hsp90 inhibitors that displace ATP from Hsp70 may target Hsp70. Although further investigations are needed to explore the underlying mechanisms, some results are encouraging [139]. The adenosine-derived compound, VER-155008, targets the ATPase domain of Hsp70/hsc70 and blocks its chaperone activity; it also induces death of colon HCT116 carcinoma cells [140]. To date, however, no evidence derived with intact animals is available. Although azure C, methylene blue, and myricetin are potent inhibitors of human Hsp70, their specificity for tumor-derived Hsp70 remains to be addressed [141] (Table 2).
Table 2.
Demonstrates various Hsp70 inhibitors including their sites of action and applications in pre-clinical clinical and trials. Dotted lines show that the given compound may or may not be beneficial in clinical trials.
| Hsp70 inhibitors | Site of interaction | Tested in clinical trials | Ref. |
|---|---|---|---|
| 1. MKT-077 | N-terminal ATP binding domain | Yes | [126,127] |
| 2. Dihydropyrimidines | N-terminal ATP binding domain | ……. | [126,127,141] [128,129] |
| (i) SW02 | N-terminal ATP binding domain | Yes | |
| (ii) MAL2-IIB | N-terminal ATP binding domain | Yes | |
| (iii) MAL3-101 | N-terminal ATP binding domain | Yes | |
| (iv) NSC630668-R/I | N-terminal ATP binding domain | ……. | |
| 3. Sulfoglycolipids | N-terminal ATP binding domain | ……. | [126,127,142,143] |
| (i) Sulfogalactoglycerolipid | N-terminal ATP binding domain | ……. | |
| (ii) Sulfogalactosylceramide | N-terminal ATP binding domain | ……. | |
| (iii) Adamantyl SGC | N-terminal ATP binding domain | ……. | |
| 4. Flavonoids | N-terminal ATP binding domain | ……. | [126,127,141,144] |
| (i) Epigallocatechin | N-terminal ATP binding domain | Yes | |
| (ii) Myricetin | N-terminal ATP binding domain | ……. | |
| 5. Apoptozole | N-terminal ATP binding domain | ……. | [126,127,145] |
| 6. VER-155008 | N-terminal ATP binding domain | ……. | [126] |
| 7. Aptamer A17 | N-terminal ATP binding domain | ……. | [126,127] |
| 8. Dibenzyl-8-aminoadenosine analog | N-terminal ATP binding domain | ……. | [126,127] |
| 9. cmHsp70.1mAb | Interact with Hsp70 epitope | Yes | [126,127,146] |
| 10. PES | C-terminal/peptide binding domain | Yes | [126,127] |
| 11. Pyrrhocoricin | C-terminal/peptide binding domain | ……. | [126,127,147] |
| 12. Geranylgeranylacetone | C-terminal/peptide binding domain | Yes | [126,127,148] |
| 13. Fatty acid acyl benzamides | C-terminal/peptide binding domain | ……. | [126,127,149] |
| 14. Pifichrin-μ | C-terminal/peptide binding domain | ……. | [126,127,136] |
| 15. Aptamer A8 | C-terminal/peptide binding domain | ……. | [126,127] |
MKT-077, a cationic rhodacyanine dye, acts upon ABD-Hsp70 in cancer cells. This compound, which migrates to mitochondria and inhibits mitochondrial Hsp70, is being tested in Phase-I clinical trials as an anticancer agent [150]. Although MKT-077 does not interact with Hsp70, it deserves further investigation due to its drug-like nature [151]. NSC 630668, a dihydropyrimidine, and a second-generation compound, MAL3-101, inhibit the ATPase activity of Hsp70 and the proliferation of SK-BK-3 cancer cells [152]. MAL2-11B, an inhibitor of polyomavirus, blocks the activity of the viral J-domain protein and T-antigen; further investigations are needed to dissect this process [153]. The combination of peptide aptamers consisting of an E. coli thioredoxin scaffold shows (8aa or 13aa) peptide loops that block Hsp70 expression by interacting with the ATP-binding domain of Hsp70, as determined in yeast 2-hybrid systems. Among these, A17 induces apoptosis in tumors in response to anti-cancer drugs. It inhibits the chaperone activity of Hsp70 but has no effect on Hsp70/Hsp90 [135] (Table 2).
Several synthetic compounds disrupt the interaction between Hsp70 and its co-chaperones. Active in this regard are pyrimidotriazinediones, a new class of drugs that interact with Hop/Hsp70 and are toxic to WST-1 cells [154]. Drugs targeting huntingtin-interacting protein 1 block Hsp70-chaperone activity and stimulate neurodegeneration [155].
Targeting Hsp70 is a new therapeutic approach; most compounds active in this regard are Hsp90/Hsp70 inhibitors that induce apoptosis in cancer cells [50,125]. Various HSP inhibitors are being evaluated in clinical trials. In treated patients, however, there is enhanced expression of Hsp70 in cancer cells, which is not a positive sign, for Hsp70 accumulation reduces the possibility of cell death, thus decreasing the anti-tumor efficacy of Hsp70 inhibitors. Treatment with chemotherapeutic drugs increases the expression of Hsp70 and induces TGF-β-signaling [156]. Hsp70 knockdown by siRNA increases sensitivity of cancer cells to tanespimycin (17-N-allylamino-17-demethoxygeldanamycin, 17-AAG) [157]. However, in HCT116 cells, knockdown of both Hsp70 and Hsc70 induces proteosomal-dependent degradation of Hsp90 client proteins and apoptosis [158]. In addition, a combination of an adenosine-derived inhibitor of Hsp70, VER-155008, and 17-AAG induces apoptosis in HCT116 colon carcinoma cells [140]. Furthermore, the anti-cancer activity of 17-AAG is elevated in colon cancer cells with blocked Hsp70 [125].
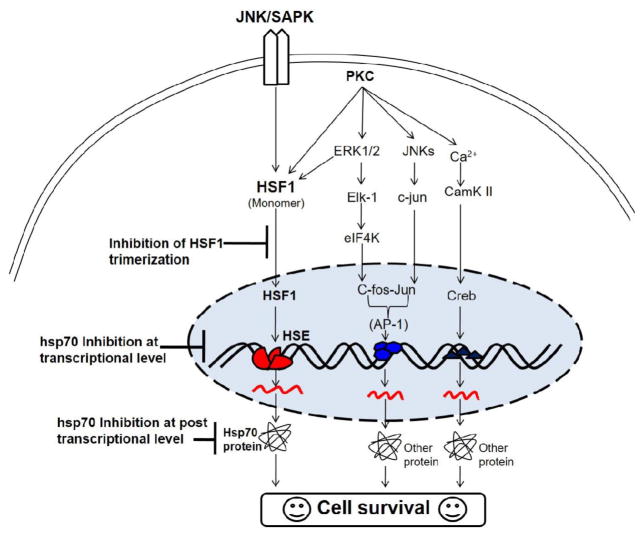
Inhibitors of Hsp70 and Hsp90 combined with histone deacetylase inhibitors increase the cell surface expression of Hsp70 on hematopoietic cancer cells [159]. An early response of extracellular Hsp70 is to activate suppressive myeloid immune cells [160]. Therefore, Hsp70 and Hsp90 inhibitors should be evaluated for a synergistic effect in an experimental model (Fig. 4). Chelerythrine, a benzophenenthridine alkaloid and a specific inhibitor of protein kinase C (PKC), downregulates the expression of Hsp70 in Dalton’s lymphoma cells [164,165]. Staurosporine, a non-specific inhibitor of PKC, also shows a similar effect [165].
Fig. 4.
Schematic representation of the inhibition of Hsp70 in cell survival pathways [161–163]. Hsp70 can be inhibited at transcription and post-transcription levels as indicated in the figure. Inhibition of Hsp70 may results in induction of cell death.
Hsp70: immunological function
In addition, to cytoprotection and chaperone activity, tumor-derived Hsp70 has immunomodulatory function(s) that activate the immune system. The immunogenic function of tumor derived/exogenous Hsp70 is exerted through its antigenic peptides [161].
In humans, the tumor burden enhances the expression of serum Hsp70, and tumor cells are the natural reservoir of Hsp70. Hsp70 is abundantly expressed on tumor cell surfaces during tumor progression; however, treatment with interferon-γ induces the release of Hsc70 from tumor cells [166,167]. Furthermore, members of the Hsp70 family often infiltrate antigen-presenting cells (APCs) and are present in neuroblastomas and lung and colon adenocarcinomas [140,168].
Cytosolic Hsp70 is transported to the plasma membrane in association with other proteins that possess a transmembrane domain. However, lack of a leader peptide does not allow Hsp70 to localize on the membrane in that manner. A possibility is that Hsp70 interacts with lipids in/on the membrane. In PC12 tumor cells, Hsp70 interacts with phosphatidylserine (PS) and shows co-localization with PS in the outer membrane leaflet, which is facilitated by a flipping mechanism [169]. There are two possible mechanisms by which Hsp70 is released from cells: a passive mechanism that results from necrosis, trauma, or surgery, and/or an active mechanism by which non-classical proteins are released from immunologically potent exosomes [170,171]. In HepG2 cells, Hsp70 is released from the extracellular environment after heat shock. Membrane-bound Hsp70acts as an activator of macrophages, free recombinant Hsp70 does not [172].
Hsp70 shows immunogenicity that activates both arms of the immune system (innate and adaptive immune responses) [173,174]. In a receptor-mediated manner, endocytosis of Hsp70 stimulates the MHC-I presentation pathway of professional APCs and cytotoxic T-lymphocytes [76]. Thus, tumor-derived Hsp70 can be used as a tumor-specific vaccine [175]. Hsp70 also induces the release of pro-inflammatory cytokines from innate immune cells, thereby increasing the expression of co-stimulatory molecules [168,176]. Furthermore, Hsp70 activates the lytic machinery of natural killer (NK) cells against tumors expressing Hsp70 on the cell surface [177]. These characteristics of Hsp70 have led to the view that Hsp70 acts as an endogenous adjuvant and immunological danger signal [178].
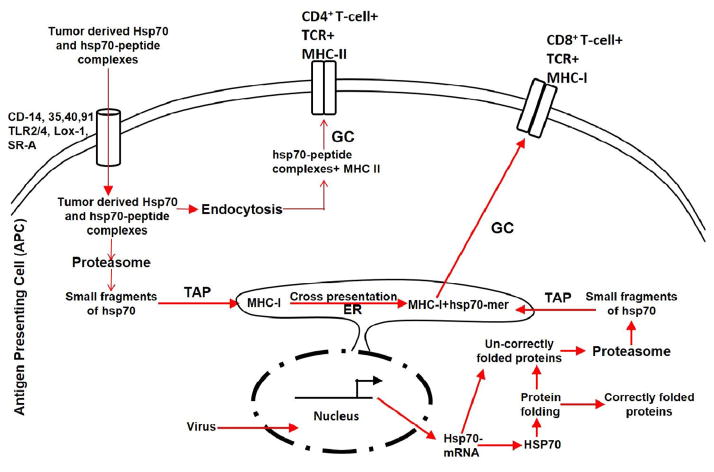
Hsp70 and Hsp90 are regulators of the immune system. Hsp70 elicits anti-cancer immune responses, but Hsc70 does not [179]. Therefore, Hsp70 is the foundation of immunogenicity; following cross presentation of tumor-derived Hsp70 on MHC-I molecules, a T-cell response is generated [162,180–186]. Hsp70 enters into the endogenous antigen-processing pathway, primes CD8+ T cells for antigen production, and becomes involved in cross presentation [162,187–189]. Tumor-derived Hsp70 is endocytosed by APCs with the help of HSP receptors (CD91, CD40, TLR2/4 + CD14, CD35, Lox-1, and SR-A) and presented on the MHC-I molecule, thereby producing a CD8+ T-cell response against cancerous stimuli. Recombinant Hsp70 induces cross presentation via the formation of various complexes and the uptake of antigens. However, recombinant Hsp70 does not stimulate innate immune responses in dendritic or B cells [190]. However, tumor-derived Hsp70 enhances MHC-II restricted peptide presentation and CD4+ T-cell activation [191]. In the absence of immunogenic antigens, Hsp70 gives danger signals for the immune system [192]. Therefore, Hsp70 apparently inhibits tumor growth via two pathways: one antigen-dependent and the other antigen-independent. The C-terminal domain of Hsp70 typically produces an antigen-independent response, which includes stimulation of NK cells against tumor challenges [67]. Treatment with Hsp70 results in a stronger anti-tumor response [193]. Hsp70 also affects cytokines. In APCs, Hsp70 induces the release of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α [67]. In established melanomas, Hsp70 acts as an immune adjuvant that induces TNF-α production [194]. The role of Hsp70 in cytokine production has been studied (Fig. 5) [57,161,195].
Fig. 5.
Diagram depicting involvement of Hsp 70 in cross presentation [161–163]. Receptor mediated endocytosis of Hsp70 was presented by MHC-II in association with CD4+ T cells. However, small fragments of Hsp70 was presented by MHC-I molecule in association with CD8+ T cells, called “cross presentation”.
Hsp70: an immunotherapeutic drug
On the basis of its immunological function, Hsp70 can be used as an immunotherapeutic drug because its potent adjuvant nature induces cancer autoimmunity [196]. Hsp70 derived from A-20 leukemic cells acts as an antigen and induces the production of an A-20-specific antibody, resulting in complement-dependent cytotoxicity against these cells [197]. Further, dendritic cells pulsed with Hsp70 induce immunity against B-16 melanoma, which increases the therapeutic value of a Cox-2 inhibitor [198]. The C-terminus of Hsp70 interacts with CD8+ T and CD4+ T cell epitopes and enhances tumor immunity beyond the effect of the CD8+ T cell epitope alone in eliminating tumor cells [161,199]. In experimental models, a similar interaction with HPV-16 E7 induces an antigen-specific cytotoxic T-cell response [200]. C-terminal interaction of Hsp70 with a recombinant N-domain calreticulin/E7 generates an antitumor immune response [200]. Although some HSP-based vaccines are currently being used in clinical practice, an improved formulation of these vaccines may derived by extraction and use of the Hsp70 complex from dendritic tumor-fused cells [145]. Hsp70-based vaccines derived from the fusions of dendritic and tumor cells reverse immunotolerance of cancers more efficiently than vaccines derived from tumor cells alone [201]. Autologous anti-tumor vaccines prepared with hydroxyapatite particles and Hsp70/27/Grp96 can be used safely [202,203]. NK cells, which are effectors of innate immune responses, are involved in anti-tumor immunity produced by vaccinations with chaperone-rich cell lysates [204]. Hsp70 induces activation of NK cells [205]. A14-mer peptide (TKD), derived from the C-terminal of Hsp70 (PBD) 450aa–463aa, shows similar immunostimulatory capabilities on NK-cells as full-length Hsp70 [206,207]. NK cells incubated with a cytokine plus soluble Hsp70 or the TKD peptide enhance expression of the activating receptor (CD14) and increase migration capacity [205]. Hsp70, present on tumor cell surfaces but not on surfaces of normal cells, is considered as a tumor-selective target. In a mouse model, peripheral blood lymphocytes incubated with TKD-peptide and IL-2 induce migration of NK cells towards Hsp70 membrane-positive tumor cells [208]. An Hsp70-based vaccine is in phase-I clinical trials; an advantage of this approach is the excellent safety and the bioavailability of the synthetic Hsp70 peptide that stimulates NK-cells [209].
Conclusions
Hsp70 is the most ancient anti-stress defensive system that promotes tumor cell survival by interacting at several points in apoptotic signaling pathway(s) [113–116]. Researchers are attempting to improve modern cancer treatment therapies by implementing Hsp70 inhibitors for the development of novel drugs in the near future [117–119]. Hsp70 demonstrates various functions, including acting as a cellular lifeguard and exerting anti-apoptotic effects. It is also involved in modulation of intrinsic and extrinsic apoptotic signaling and, in cancer cells, modulates caspase-independent apoptosis. Since Hsp70 has anti-cancer and immunogenic properties, there is a search for Hsp70 inhibitors. However, preclinical and clinical evaluations have not yet been accomplished, unlike Hsp90 inhibitors, which are already in phase II/III clinical trials. Nevertheless, clinical trial results obtained to date are not favorable. Such results may be due to enhanced expression of Hsp70 in tumors. Extracellular Hsp70 is believed to be immunogenic because it acts as an adjuvant and, combined with chaperone(s), it may prove useful for vaccine production and cancer treatment. Increased expression of extracellular Hsp70 is a sign of a poor prognosis for cancer. In syngeneic mice, inhibition or depletion of Hsp70 causes tumor regression, and Hsp70 inhibition results in an anti-tumor immune response and apoptosis of the target cells. Therefore, targeting Hsp70 is a promising therapy for cancer patients.
Acknowledgments
We thank Dr. Donald Hill for his critical review of the manuscript. The authors have been partially supported by National Institutes of Health grants P20CA192976 (MKM) and P20CA192973 (UM); U.S. Department of Defense grants W911NF-12-1-0073 (MKM) and W911NF-14-1-0064 (MKM); and National Science Foundation grant 1154214 (MKM).
Abbreviations
- HSPs
Heat shock proteins
- Hsp70
Heat shock protein 70
- Hsp75
Heat shock protein 75
- Hsp100
Heat shock protein100
- Hsp90
Heat shock protein 90
- Hsp40
Heat shock protein 40
- Hsp33
Heat shock protein 33
- sHSPs
Small Heat shock proteins
- HspBP1
(Hsp70) binding protein 1
- MHC
Major histocompatibility complex
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- GRP78
Glucose regulated protein 78
- PBD
Peptide binding domain
- ABD
ATP binding domain
- PS
Phosphatidylserine
- PKB
Protein kinase B
- BH
Bcl2 homology
- Bak
Bcl2 homologous antagonist/killer
- Bax
Bcl2 associated X protein
- Bcl-Xs
B cell lymphoma X small
- BclxL
B cell lymphoma x Large
- Bcl2
B cell lymphoma 2
- Bid
BH3 interacting-domain death agonist
- CAD
Caspase-activated DNase
- Caspase
Cysteinyl aspartate protease
- ING
Inhibitor of growth
- IKK
Inhibition of I-kB-α kinase
- ER
Endoplasmic reticulum
- MPT
Membrane permeability transition
- BH3-OFM
BH3-only family member
- OMM
Outer mitochondrial membrane
- PTP
Permeability transition pores
- TM
Transmembrane domains
- CYT-C
Cytochrome-c
- CARD
Caspase activation and recruitment domain
- Apaf-1
Apoptotic protease activating factor-1
- ICE
Interleukin-1β converting enzyme
- DED
Death effector domain
- FADD
Fas-associating death domain
- DISC
Death inducing signaling complex
- TRAIL
TNF-related apoptosis inducing ligand
- TRAIL-R
TNF-related apoptosis inducing ligand-receptor
- Smac
Second mitochondrial activator of caspase
- DIBALO
Direct IAP binding protein with low pI
- Endo-G
Endonuclease G
- DR
Death receptor
- NGF
Nerve growth factor
- NGFR
Nerve growth factor receptor
- siRNA
Short interfering RNA
- RNAi
RNA interference
- TNF-α
Tumor necrosis factor alpha
- ASK-1
Stress activated kinase-1
- FasL
Fas-ligand
- DD
Death domain
- NF-kB
Nuclear factor-kappa-B
- t-Bid
Truncated-Bid
- XIAP
X-linked IAP
- HSFs
Heat shock factors
- HSF1
Heat shock factor1
- DBD
DNA binding domain
- AIF
Apoptosis inducing factor
- SAPK
Stress activated kinase
- JNK
c-Jun N-terminal kinase
- DAG
Diacylglycerol
- PARP
Poly (ADP-ribose) polymerase
- ADD70
AIF-derived decoy for Hsp70
- APCs
Antigen presenting cells
- CD
Cluster of differentiation
- NK cells
Natural killer cells
Footnotes
Conflict of interest
There is no conflict of interest among the authors. The authors alone are responsible for the content and writing of this review.
References
- 1.Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 2.Kroeger H. The induction of new puffing patterns by transplantation of salivary gland nuclei into egg cytoplasma of Drosophila. Chromosoma. 1960;11:129–145. doi: 10.1007/BF00328649. [DOI] [PubMed] [Google Scholar]
- 3.Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 5.Yahara I. Stress-inducible cellular responses. Introduction. EXS. 1996;77:XI–XII. [PubMed] [Google Scholar]
- 6.Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun. 2003;304:505–512. doi: 10.1016/s0006-291x(03)00623-5. [DOI] [PubMed] [Google Scholar]
- 7.Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polla BS, Kantengwa S, Francois D, Salvioli S, Franceschi C, Marsac C, et al. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc Natl Acad Sci USA. 1996;93:6458–6463. doi: 10.1073/pnas.93.13.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HS, Cho SG, Kim CK, Hwang HS, Noh KT, Kim MS, et al. Heat shock protein hsp72 is a negative regulator of apoptosis signal-regulating kinase 1. Mol Cell Biol. 2002;22:7721–7730. doi: 10.1128/MCB.22.22.7721-7730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- 11.Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–271. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 12.Rerole AL, Jego G, Garrido C. Hsp70: anti-apoptotic and tumorigenic protein. Methods Mol Biol. 2011;787:205–230. doi: 10.1007/978-1-61779-295-3_16. [DOI] [PubMed] [Google Scholar]
- 13.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 14.Elsner L, Flugge PF, Lozano J, Muppala V, Eiz-Vesper B, Demiroglu SY, et al. The endogenous danger signals HSP70 and MICA cooperate in the activation of cytotoxic effector functions of NK cells. J Cell Mol Med. 2010;14:992–1002. doi: 10.1111/j.1582-4934.2008.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 16.Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- 19.Murakami H, Pain D, Blobel G. 70-kD heat shock-related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J Cell Biol. 1988;107:2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol. 1992;12:2186–2192. doi: 10.1128/mcb.12.5.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all J domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions. Protein Sci. 2005;14:1697–1709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 23.Ramos C. Molecular chaperones and protein quality control. Protein Pept Lett. 2011;18:100. doi: 10.2174/092986611794474995. [DOI] [PubMed] [Google Scholar]
- 24.Vogel M, Bukau B, Mayer MP. Allosteric regulation of Hsp70 chaperones by a proline switch. Mol Cell. 2006;21:359–367. doi: 10.1016/j.molcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Voos W. A new connection: chaperones meet a mitochondrial receptor. Mol Cell. 2003;11:1–3. doi: 10.1016/s1097-2765(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol. 2002;3:779–787. doi: 10.1038/nrm931. [DOI] [PubMed] [Google Scholar]
- 28.Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, Garrido C. Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia. 2000;14:1833–1849. doi: 10.1038/sj.leu.2401902. [DOI] [PubMed] [Google Scholar]
- 29.Hess CJ, Berkhof J, Denkers F, Ossenkoppele GJ, Schouten JP, Oudejans JJ, et al. Activated intrinsic apoptosis pathway is a key related prognostic parameter in acute myeloid leukemia. J Clin Oncol. 2007;25:1209–1215. doi: 10.1200/JCO.2006.08.4061. [DOI] [PubMed] [Google Scholar]
- 30.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breckenridge DG, Xue D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 2004;16:647–652. doi: 10.1016/j.ceb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhivotovsky B, Orrenius S, Brustugun OT, Doskeland SO. Injected cytochrome c induces apoptosis. Nature. 1998;391:449–450. doi: 10.1038/35060. [DOI] [PubMed] [Google Scholar]
- 35.Gutcher I, Webb PR, Anderson NG. The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol Life Sci. 2003;60:1061–1070. doi: 10.1007/s00018-003-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 40.Verhagen AM, Vaux DL. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2002;7:163–166. doi: 10.1023/a:1014318615955. [DOI] [PubMed] [Google Scholar]
- 41.Creagh EM, Murphy BM, Duriez PJ, Duckett CS, Martin SJ. Smac/Diablo antagonizes ubiquitin ligase activity of inhibitor of apoptosis proteins. J Biol Chem. 2004;279:26906–26914. doi: 10.1074/jbc.M313859200. [DOI] [PubMed] [Google Scholar]
- 42.Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, et al. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105:1246–1255. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- 43.Muzio M. Signalling by proteolysis: death receptors induce apoptosis. Int J Clin Lab Res. 1998;28:141–147. doi: 10.1007/s005990050035. [DOI] [PubMed] [Google Scholar]
- 44.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 45.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 46.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 48.Gurbuxani S, Bruey JM, Fromentin A, Larmonier N, Parcellier A, Jaattela M, et al. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene. 2001;20:7478–7485. doi: 10.1038/sj.onc.1204948. [DOI] [PubMed] [Google Scholar]
- 49.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA. 2000;97:7871–7876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt E, Parcellier A, Gurbuxani S, Cande C, Hammann A, Morales MC, et al. Chemosensitization by a non-apoptogenic heat shock protein 70-binding apoptosis-inducing factor mutant. Cancer Res. 2003;63:8233–8240. [PubMed] [Google Scholar]
- 51.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 53.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 54.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 55.Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem. 2002;277:31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- 56.Hu G, Tang J, Zhang B, Lin Y, Hanai J, Galloway J, et al. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J Cell Sci. 2006;119:4117–4126. doi: 10.1242/jcs.03179. [DOI] [PubMed] [Google Scholar]
- 57.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–22529. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 58.Gabai VL, Yaglom JA, Volloch V, Meriin AB, Force T, Koutroumanis M, et al. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol Cell Biol. 2000;20:6826–6836. doi: 10.1128/mcb.20.18.6826-6836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 60.Lee JS, Lee JJ, Seo JS. HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem. 2005;280:6634–6641. doi: 10.1074/jbc.M412393200. [DOI] [PubMed] [Google Scholar]
- 61.Gabai VL, Mabuchi K, Mosser DD, Sherman MY. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2002;22:3415–3424. doi: 10.1128/MCB.22.10.3415-3424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang B, Liang P, Deng G, Tu Z, Liu M, Xiao X. Increased stability of Bcl-2 in HSP70-mediated protection against apoptosis induced by oxidative stress. Cell Stress Chaperones. 2011;16:143–152. doi: 10.1007/s12192-010-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crowe DL, Sinha UK. p53 apoptotic response to DNA damage dependent on bcl2 but not bax in head and neck squamous cell carcinoma lines. Head Neck. 2006;28:15–23. doi: 10.1002/hed.20319. [DOI] [PubMed] [Google Scholar]
- 64.Zylicz M, King FW, Wawrzynow A. Hsp70 interactions with the p53 tumour suppressor protein. EMBO J. 2001;20:4634–4638. doi: 10.1093/emboj/20.17.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akakura S, Yoshida M, Yoneda Y, Horinouchi S. A role for Hsc70 in regulating nucleocytoplasmic transport of a temperature-sensitive p53 (p53Val-135) J Biol Chem. 2001;276:14649–14657. doi: 10.1074/jbc.M100200200. [DOI] [PubMed] [Google Scholar]
- 66.Chen H, Wu Y, Zhang Y, Jin L, Luo L, Xue B, et al. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006;580:3145–3152. doi: 10.1016/j.febslet.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 67.Mosoian A. Intracellular and extracellular cytokine-like functions of prothymosin alpha: implications for the development of immunotherapies. Future Med Chem. 2011;3:1199–1208. doi: 10.4155/fmc.11.72. [DOI] [PubMed] [Google Scholar]
- 68.Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shanley TP, Ryan MA, Eaves-Pyles T, Wong HR. Heat shock inhibits phosphorylation of I-kappaBalpha. Shock. 2000;14:447–450. doi: 10.1097/00024382-200014040-00005. [DOI] [PubMed] [Google Scholar]
- 70.Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- 71.Zorzi E, Bonvini P. Inducible hsp70 in the regulation of cancer cell survival: analysis of chaperone induction, expression and activity. Cancers (Basel) 2011;3:3921–3956. doi: 10.3390/cancers3043921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng X, Bonni S, Riabowol K. HSP70 induction by ING proteins sensitizes cells to tumor necrosis factor alpha receptor-mediated apoptosis. Mol Cell Biol. 2006;26:9244–9255. doi: 10.1128/MCB.01538-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coles AH, Jones SN. The ING gene family in the regulation of cell growth and tumorigenesis. J Cell Physiol. 2009;218:45–57. doi: 10.1002/jcp.21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 75.Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, et al. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem. 2006;281:7873–7880. doi: 10.1074/jbc.M513728200. [DOI] [PubMed] [Google Scholar]
- 76.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, et al. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 78.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duncan EJ, Cheetham ME, Chapple JP, van der Spuy J. The role of HSP70 and its co-chaperones in protein misfolding, aggregation and disease. Subcell Biochem. 2015;78:243–273. doi: 10.1007/978-3-319-11731-7_12. [DOI] [PubMed] [Google Scholar]
- 81.Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- 82.Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, et al. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones. 2009;14:391–406. doi: 10.1007/s12192-008-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, et al. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37:507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- 84.Pang Q, Keeble W, Christianson TA, Faulkner GR, Bagby GC. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-gamma/TNF-alpha-mediated cytotoxicity. EMBO J. 2001;20:4478–4489. doi: 10.1093/emboj/20.16.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC. The anti-apoptotic function of Hsp70 in the interferon-inducible double-stranded RNA-dependent protein kinase-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J Biol Chem. 2002;277:49638–49643. doi: 10.1074/jbc.M209386200. [DOI] [PubMed] [Google Scholar]
- 86.Schett G, Steiner CW, Groger M, Winkler S, Graninger W, Smolen J, et al. Activation of Fas inhibits heat-induced activation of HSF1 and up-regulation of hsp70. FASEB J. 1999;13:833–842. doi: 10.1096/fasebj.13.8.833. [DOI] [PubMed] [Google Scholar]
- 87.Liossis SN, Ding XZ, Kiang JG, Tsokos GC. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol. 1997;158:5668–5675. [PubMed] [Google Scholar]
- 88.Cande C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ. 2004;11:591–595. doi: 10.1038/sj.cdd.4401400. [DOI] [PubMed] [Google Scholar]
- 89.Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res. 2000;257:58–66. doi: 10.1006/excr.2000.4856. [DOI] [PubMed] [Google Scholar]
- 90.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 91.Lui JC, Kong SK. Heat shock protein 70 inhibits the nuclear import of apoptosis-inducing factor to avoid DNA fragmentation in TF-1 cells during erythropoiesis. FEBS Lett. 2007;581:109–117. doi: 10.1016/j.febslet.2006.11.082. [DOI] [PubMed] [Google Scholar]
- 92.Kalinowska M, Garncarz W, Pietrowska M, Garrard WT, Widlak P. Regulation of the human apoptotic DNase/RNase endonuclease G: involvement of Hsp70 and ATP. Apoptosis. 2005;10:821–830. doi: 10.1007/s10495-005-0410-9. [DOI] [PubMed] [Google Scholar]
- 93.Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–435. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bivik C, Rosdahl I, Ollinger K. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis. 2007;28:537–544. doi: 10.1093/carcin/bgl152. [DOI] [PubMed] [Google Scholar]
- 95.Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jaattela M, et al. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, et al. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3:49–53. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 98.Ray S, Lu Y, Kaufmann SH, Gustafson WC, Karp JE, Boldogh I, et al. Genomic mechanisms of p210BCR-ABL signaling: induction of heat shock protein 70 through the GATA response element confers resistance to paclitaxel-induced apoptosis. J Biol Chem. 2004;279:35604–35615. doi: 10.1074/jbc.M401851200. [DOI] [PubMed] [Google Scholar]
- 99.Pocaly M, Lagarde V, Etienne G, Ribeil JA, Claverol S, Bonneu M, et al. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia. 2007;21:93–101. doi: 10.1038/sj.leu.2404463. [DOI] [PubMed] [Google Scholar]
- 100.Eddy EM. Role of heat shock protein HSP70-2 in spermatogenesis. Rev Reprod. 1999;4:23–30. doi: 10.1530/ror.0.0040023. [DOI] [PubMed] [Google Scholar]
- 101.Targosz A, Pierzchalski P, Krawiec A, Szczyrk U, Brzozowski T, Konturek SJ, et al. Helicobacter pylori inhibits expression of heat shock protein 70 (HSP70) in human epithelial cell line. Importance of Cag A protein. J Physiol Pharmacol. 2006;57:265–278. [PubMed] [Google Scholar]
- 102.Morii T, Ohtsuka K, Ohnishi H, Mochizuki K, Satomi K. Inhibition of heat-shock protein 27 expression eliminates drug resistance of osteosarcoma to zoledronic acid. Anticancer Res. 2010;30:3565–3571. [PubMed] [Google Scholar]
- 103.Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 104.Brondani Da Rocha A, Regner A, Grivicich I, Pretto Schunemann D, Diel C, Kovaleski G, et al. Radioresistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol. 2004;25:777–785. [PubMed] [Google Scholar]
- 105.Seoane JM, Varela-Centelles PI, Ramirez JR, Cameselle-Teijeiro J, Romero MA, Aguirre JM. Heat shock proteins (HSP70 and HSP27) as markers of epithelial dysplasia in oral leukoplakia. Am J Dermatopathol. 2006;28:417–422. doi: 10.1097/01.dad.0000211509.44865.bb. [DOI] [PubMed] [Google Scholar]
- 106.Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Harada T, Fujimoto M, et al. Proteomic analysis of autoantibodies in patients with hepatocellular carcinoma. Proteomics. 2006;6:3894–3900. doi: 10.1002/pmic.200500346. [DOI] [PubMed] [Google Scholar]
- 107.Hatfield MP, Lovas S. Role of Hsp70 in cancer growth and survival. Protein Pept Lett. 2012;19:616–624. doi: 10.2174/092986612800493968. [DOI] [PubMed] [Google Scholar]
- 108.Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones. 2001;6:316–325. doi: 10.1379/1466-1268(2001)006<0316:eoihet>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiang TX, Li Y, Jiang Z, Huang AL, Luo C, Zhan B, et al. RNA interference-mediated silencing of the Hsp70 gene inhibits human gastric cancer cell growth and induces apoptosis in vitro and in vivo. Tumori. 2008;94:539–550. doi: 10.1177/030089160809400416. [DOI] [PubMed] [Google Scholar]
- 110.Du XL, Jiang T, Wen ZQ, Gao R, Cui M, Wang F. Silencing of heat shock protein 70 expression enhances radiotherapy efficacy and inhibits cell invasion in endometrial cancer cell line. Croat Med J. 2009;50:143–150. doi: 10.3325/cmj.2009.50.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153–4161. doi: 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maehara Y, Oki E, Abe T, Tokunaga E, Shibahara K, Kakeji Y, et al. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144–151. doi: 10.1159/000012091. [DOI] [PubMed] [Google Scholar]
- 113.Brusa D, Migliore E, Garetto S, Simone M, Matera L. Immunogenicity of 56 degrees C and UVC-treated prostate cancer is associated with release of HSP70 and HMGB1 from necrotic cells. Prostate. 2009;69:1343–1352. doi: 10.1002/pros.20981. [DOI] [PubMed] [Google Scholar]
- 114.Gunther S, Ostheimer C, Stangl S, Specht HM, Mozes P, Jesinghaus M, et al. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non-small cell lung cancer. Front Immunol. 2015;6:556. doi: 10.3389/fimmu.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, McAlpine SR. Regulating the cytoprotective response in cancer cells using simultaneous inhibition of Hsp90 and Hsp70. Org Biomol Chem. 2015;13:2108–2116. doi: 10.1039/c4ob02531h. [DOI] [PubMed] [Google Scholar]
- 116.Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–125. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 117.Nimmervoll B, Chtcheglova LA, Juhasz K, Cremades N, Aprile FA, Sonnleitner A, et al. Cell surface localised Hsp70 is a cancer specific regulator of clathrin-independent endocytosis. FEBS Lett. 2015;589:2747–2753. doi: 10.1016/j.febslet.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 118.Horibe T, Torisawa A, Kohno M, Kawakami K. Synergetic cytotoxic activity toward breast cancer cells enhanced by the combination of Antp-TPR hybrid peptide targeting Hsp90 and Hsp70-targeted peptide. BMC Cancer. 2014;14:615. doi: 10.1186/1471-2407-14-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang W, Ji W, Hu H, Ma J, Li X, Mei W, et al. Survivin promoter-regulated oncolytic adenovirus with Hsp70 gene exerts effective antitumor efficacy in gastric cancer immunotherapy. Oncotarget. 2014;5:150–160. doi: 10.18632/oncotarget.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardile V, Scifo C, Russo A, Falsaperla M, Morgia G, Motta M, et al. Involvement of HSP70 in resveratrol-induced apoptosis of human prostate cancer. Anticancer Res. 2003;23:4921–4926. [PubMed] [Google Scholar]
- 121.Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, et al. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 2003;22:6669–6678. doi: 10.1038/sj.onc.1206794. [DOI] [PubMed] [Google Scholar]
- 122.Jaattela M. Over-expression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int J Cancer. 1995;60:689–693. doi: 10.1002/ijc.2910600520. [DOI] [PubMed] [Google Scholar]
- 123.Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2:579–584. [PubMed] [Google Scholar]
- 124.Lee JH, Schoffl F. An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet. 1996;252:11–19. doi: 10.1007/s004389670002. [DOI] [PubMed] [Google Scholar]
- 125.Schmitt E, Maingret L, Puig PE, Rerole AL, Ghiringhelli F, Hammann A, et al. Heat shock protein 70 neutralization exerts potent antitumor effects in animal models of colon cancer and melanoma. Cancer Res. 2006;66:4191–4197. doi: 10.1158/0008-5472.CAN-05-3778. [DOI] [PubMed] [Google Scholar]
- 126.Powers MV, Jones K, Barillari C, Westwood I, van Montfort RL, Workman P. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9:1542–1550. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 127.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 128.Kumar S, Tomar MS, Acharya A. HSF1-mediated regulation of tumor cell apoptosis: a novel target for cancer therapeutics. Future Oncol. 2013;9:1573–1586. doi: 10.2217/fon.13.106. [DOI] [PubMed] [Google Scholar]
- 129.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 130.Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–290. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 132.Li M, Wang J, Jing J, Hua H, Luo T, Xu L, et al. Synergistic promotion of breast cancer cells death by targeting molecular chaperone GRP78 and heat shock protein 70. J Cell Mol Med. 2009;13:4540–4550. doi: 10.1111/j.1582-4934.2008.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gong Z, Yang J, Yang M, Wang F, Wei Q, Tanguay RM, et al. Benzo(a)pyrene inhibits expression of inducible heat shock protein 70 in vascular endothelial cells. Toxicol Lett. 2006;166:229–236. doi: 10.1016/j.toxlet.2006.07.307. [DOI] [PubMed] [Google Scholar]
- 134.Banerjee Mustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS ONE. 2010;5:e8719. doi: 10.1371/journal.pone.0008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Correction: peptides and aptamers targeting HSP70: a novel approach for anticancer chemotherapy. Cancer Res. 2015;75:902. doi: 10.1158/0008-5472.CAN-15-0042. [DOI] [PubMed] [Google Scholar]
- 136.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang C, Wang J, Chen Z, Wang Y, Zhang W. 2-Phenylethynesulfonamide prevents induction of pro-inflammatory factors and attenuates LPS-induced liver injury by targeting NHE1-Hsp70 complex in mice. PLoS ONE. 2013;8:e67582. doi: 10.1371/journal.pone.0067582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Steele AJ, Prentice AG, Hoffbrand AV, Yogashangary BC, Hart SM, Lowdell MW, et al. 2-Phenylacetylenesulfonamide (PAS) induces p53-independent apoptotic killing of B-chronic lymphocytic leukemia (CLL) cells. Blood. 2009;114:1217–1225. doi: 10.1182/blood-2008-11-190587. [DOI] [PubMed] [Google Scholar]
- 139.Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- 140.Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol. 2010;66:535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 141.Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, et al. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mamelak D, Lingwood C. The ATPase domain of hsp70 possesses a unique binding specificity for 3′-sulfogalactolipids. J Biol Chem. 2001;276:449–456. doi: 10.1074/jbc.M006732200. [DOI] [PubMed] [Google Scholar]
- 143.Boulanger J, Faulds D, Eddy EM, Lingwood CA. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamete recognition and mycoplasma-related infertility. J Cell Physiol. 1995;165:7–17. doi: 10.1002/jcp.1041650103. [DOI] [PubMed] [Google Scholar]
- 144.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 145.Williams DR, Ko SK, Park S, Lee MR, Shin I. An apoptosis-inducing small molecule that binds to heat shock protein 70. Angew Chem Int Ed Engl. 2008;47:7466–7469. doi: 10.1002/anie.200802801. [DOI] [PubMed] [Google Scholar]
- 146.Balaburski GM, Leu JI, Beeharry N, Hayik S, Andrake MD, Zhang G, et al. A modified HSP70 inhibitor shows broad activity as an anticancer agent. Mol Cancer Res. 2013;11:219–229. doi: 10.1158/1541-7786.MCR-12-0547-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kragol G, Lovas S, Varadi G, Condie BA, Hoffmann R, Otvos L., Jr The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry. 2001;40:3016–3026. doi: 10.1021/bi002656a. [DOI] [PubMed] [Google Scholar]
- 148.Otaka M, Yamamoto S, Ogasawara K, Takaoka Y, Noguchi S, Miyazaki T, et al. The induction mechanism of the molecular chaperone HSP70 in the gastric mucosa by Geranylgeranylacetone (HSP-inducer) Biochem Biophys Res Commun. 2007;353:399–404. doi: 10.1016/j.bbrc.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 149.Liebscher M, Jahreis G, Lucke C, Grabley S, Raina S, Schiene-Fischer C. Fatty acyl benzamido antibacterials based on inhibition of DnaK-catalyzed protein folding. J Biol Chem. 2007;282:4437–4446. doi: 10.1074/jbc.M607667200. [DOI] [PubMed] [Google Scholar]
- 150.Koya K, Li Y, Wang H, Ukai T, Tatsuta N, Kawakami M, et al. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996;56:538–543. [PubMed] [Google Scholar]
- 151.Deocaris CC, Widodo N, Shrestha BG, Kaur K, Ohtaka M, Yamasaki K, et al. Mortalin sensitizes human cancer cells to MKT-077-induced senescence. Cancer Lett. 2007;252:259–269. doi: 10.1016/j.canlet.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 152.Evans CG, Wisen S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 153.Wright CM, Seguin SP, Fewell SW, Zhang H, Ishwad C, Vats A, et al. Inhibition of Simian Virus 40 replication by targeting the molecular chaperone function and ATPase activity of T antigen. Virus Res. 2009;141:71–80. doi: 10.1016/j.virusres.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yi F, Regan L. A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roodveldt C, Bertoncini CW, Andersson A, van der Goot AT, Hsu ST, Fernandez-Montesinos R, et al. Chaperone proteostasis in Parkinson’s disease: stabilization of the Hsp70/alpha-synuclein complex by Hip. EMBO J. 2009;28:3758–3770. doi: 10.1038/emboj.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yun CH, Yoon SY, Nguyen TT, Cho HY, Kim TH, Kim ST, et al. Geldanamycin inhibits TGF-beta signaling through induction of Hsp70. Arch Biochem Biophys. 2010;495:8–13. doi: 10.1016/j.abb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 157.Powers MV, Clarke PA, Workman P. Death by chaperone: HSP90, HSP70 or both? Cell Cycle. 2009;8:518–526. doi: 10.4161/cc.8.4.7583. [DOI] [PubMed] [Google Scholar]
- 158.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 159.Jensen H, Andresen L, Hansen KA, Skov S. Cell-surface expression of Hsp70 on hematopoietic cancer cells after inhibition of HDAC activity. J Leukoc Biol. 2009;86:923–932. doi: 10.1189/jlb.0209056. [DOI] [PubMed] [Google Scholar]
- 160.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kumar S, Deepak P, Kumar S, Jr, Kishore D, Acharya A. Autologous Hsp70 induces antigen specific Th1 immune responses in a murine T-cell lymphoma. Immunol Invest. 2009;38:449–465. doi: 10.1080/08820130902802673. [DOI] [PubMed] [Google Scholar]
- 162.Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 164.Kumar S, Acharya A. Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma. Tumour Biol. 2014;35:129–140. doi: 10.1007/s13277-013-1016-4. [DOI] [PubMed] [Google Scholar]
- 165.Kumar S, Deepak P, Kumar S, Gautam PK, Acharya A. A benzophenanthridine alkaloid, chelerythrine induces apoptosis in vitro in a Dalton’s lymphoma. J Cancer Res Ther. 2013;9:693–700. doi: 10.4103/0973-1482.126485. [DOI] [PubMed] [Google Scholar]
- 166.Bausero MA, Page DT, Osinaga E, Asea A. Surface expression of Hsp25 and Hsp72 differentially regulates tumor growth and metastasis. Tumour Biol. 2004;25:243–251. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, et al. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602–1609. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 169.Schilling D, Gehrmann M, Steinem C, De Maio A, Pockley AG, Abend M, et al. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23:2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 171.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 172.Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 173.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 174.Nicchitta CV. Re-evaluating the role of heat-shock protein-peptide interactions in tumour immunity. Nat Rev Immunol. 2003;3:427–432. doi: 10.1038/nri1089. [DOI] [PubMed] [Google Scholar]
- 175.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 177.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 178.Todryk SM, Melcher AA, Dalgleish AG, Vile RG. Heat shock proteins refine the danger theory. Immunology. 2000;99:334–337. doi: 10.1046/j.1365-2567.2000.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Castelli C, Rivoltini L, Rini F, Belli F, Testori A, Maio M, et al. Heat shock proteins: biological functions and clinical application as personalized vaccines for human cancer. Cancer Immunol Immunother. 2004;53:227–233. doi: 10.1007/s00262-003-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- 181.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 182.Singh-Jasuja H, Hilf N, Scherer HU, Arnold-Schild D, Rammensee HG, Toes RE, et al. The heat shock protein gp96: a receptor-targeted cross-priming carrier and activator of dendritic cells. Cell Stress Chaperones. 2000;5:462–470. doi: 10.1379/1466-1268(2000)005<0462:thspga>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sondermann H, Becker T, Mayhew M, Wieland F, Hartl FU. Characterization of a receptor for heat shock protein 70 on macrophages and monocytes. Biol Chem. 2000;381:1165–1174. doi: 10.1515/BC.2000.144. [DOI] [PubMed] [Google Scholar]
- 184.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163–17171. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 185.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 187.Flechtner JB, Cohane KP, Mehta S, Slusarewicz P, Leonard AK, Barber BH, et al. High-affinity interactions between peptides and heat shock protein 70 augment CD8+ T lymphocyte immune responses. J Immunol. 2006;177:1017–1027. doi: 10.4049/jimmunol.177.2.1017. [DOI] [PubMed] [Google Scholar]
- 188.Habich C, Baumgart K, Kolb H, Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. J Immunol. 2002;168:569–576. doi: 10.4049/jimmunol.168.2.569. [DOI] [PubMed] [Google Scholar]
- 189.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 190.Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, Brauchle C, et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem. 2007;282:31688–31702. doi: 10.1074/jbc.M704129200. [DOI] [PubMed] [Google Scholar]
- 191.Haug M, Schepp CP, Kalbacher H, Dannecker GE, Holzer U. 70-kDa heat shock proteins: specific interactions with HLA-DR molecules and their peptide fragments. Eur J Immunol. 2007;37:1053–1063. doi: 10.1002/eji.200636811. [DOI] [PubMed] [Google Scholar]
- 192.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 193.Zhang H, Huang W. Fusion proteins of Hsp70 with tumor-associated antigen acting as a potent tumor vaccine and the C-terminal peptide-binding domain of Hsp70 being essential in inducing antigen-independent anti-tumor response in vivo. Cell Stress Chaperones. 2006;11:216–226. doi: 10.1379/CSC-191R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sanchez-Perez L, Kottke T, Daniels GA, Diaz RM, Thompson J, Pulido J, et al. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- 195.Reed RC, Berwin B, Baker JP, Nicchitta CV. GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-kappa B activation and nitric oxide production. J Biol Chem. 2003;278:31853–31860. doi: 10.1074/jbc.M305480200. [DOI] [PubMed] [Google Scholar]
- 196.Kottke T, Sanchez-Perez L, Diaz RM, Thompson J, Chong H, Harrington K, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 197.Jimbo J, Sato K, Hosoki T, Shindo M, Ikuta K, Torimoto Y, et al. Induction of leukemia-specific antibodies by immunotherapy with leukemia-cell-derived heat shock protein 70. Cancer Sci. 2008;99:1427–1434. doi: 10.1111/j.1349-7006.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Toomey D, Conroy H, Jarnicki AG, Higgins SC, Sutton C, Mills KH. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine. 2008;26:3540–3549. doi: 10.1016/j.vaccine.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 199.Faure O, Graff-Dubois S, Alves PM, Cornet S, Duffour MT, Scardino A, et al. Induction of multiple CD8+ T cell responses against the inducible Hsp70 employing an Hsp70 oligoepitope peptide. Oncol Rep. 2007;17:679–685. [PubMed] [Google Scholar]
- 200.Liu B, Ye D, Song X, Zhao X, Yi L, Song J, et al. A novel therapeutic fusion protein vaccine by two different families of heat shock proteins linked with HPV16 E7 generates potent antitumor immunity and antiangiogenesis. Vaccine. 2008;26:1387–1396. doi: 10.1016/j.vaccine.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 201.Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J Immunol. 2006;177:5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 202.Ciocca DR, Frayssinet P, Cuello-Carrion FD. A pilot study with a therapeutic vaccine based on hydroxyapatite ceramic particles and self-antigens in cancer patients. Cell Stress Chaperones. 2007;12:33–43. doi: 10.1379/CSC-218R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wooldridge L, Lissina A, Vernazza J, Gostick E, Laugel B, Hutchinson SL, et al. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur J Immunol. 2007;37:1323–1333. doi: 10.1002/eji.200636765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zeng Y, Chen X, Larmonier N, Larmonier C, Li G, Sepassi M, et al. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int J Cancer. 2006;119:2624–2631. doi: 10.1002/ijc.22150. [DOI] [PubMed] [Google Scholar]
- 205.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 206.Botzler C, Li G, Issels RD, Multhoff G. Definition of extracellular localized epitopes of Hsp70 involved in an NK immune response. Cell Stress Chaperones. 1998;3:6–11. doi: 10.1379/1466-1268(1998)003<0006:doeleo>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Multhoff G, Mizzen L, Winchester CC, Milner CM, Wenk S, Eissner G, et al. eat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–1636. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 208.Stangl S, Wortmann A, Guertler U, Multhoff G. Control of metastasized pancreatic carcinomas in SCID/beige mice with human IL-2/TKD-activated NK cells. J Immunol. 2006;176:6270–6276. doi: 10.4049/jimmunol.176.10.6270. [DOI] [PubMed] [Google Scholar]
- 209.Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, et al. Heat Shock Protein 70 (Hsp70) Peptide Activated Natural Killer (NK) Cells for the Treatment of Patients with Non-Small Cell Lung Cancer (NSCLC) after Radiochemotherapy (RCTx) – From Preclinical Studies to a Clinical Phase II Trial. Front Immunol. 2015;6:162. doi: 10.3389/fimmu.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]