Abstract
Background
Nursing home residents often suffer from a multiplicity of medical conditions and take many different drugs. Many drugs are eliminated via the kidneys and thus require dose adjustment in patients with renal insufficiency. This is the first study to address the prevalence of renal insufficiency among nursing home residents in Germany, and the extent to which such persons take drugs that are contraindicated or incorrectly dosed because of renal insufficiency.
Method
We carried out a cross-sectional study in nursing homes in the German regions of Bremen and Lower Saxony. Data were collected by nursing staff and given to us anonymously. Whenever the nursing home data did not include a current creatinine value, the patient’s general practitioner was asked to supply this value. The estimated creatinine clearance (eCCr) was calculated with the Cockcroft-Gault formula.
Results
852 residents of 21 nursing homes were included in the study; eCCr values were obtainable for 685 (80.4%) of them (average age, 83.3 years; 75.2% female). 48.2% of these patients (95% confidence interval [CI] 41.8–54.5) had moderate renal insufficiency (eCCr 59–30 mL/min), and 15.5% (95% CI 12.4–18.6) had severe renal insufficiency (eCCr 30 mL/min). 19.7% were regularly taking at least one medication that was contraindicated or incorrectly dosed in the light of renal insufficiency. Predictors for such inappropriate drug use were advanced age, female sex, arterial hypertension, and polypharmacy. The drugs that were most often inappropriately used were metformin, ramipril, and potassium chloride.
Conclusion
Nursing home residents often suffer from renal insufficiency and should therefore have their creatinine levels measured regularly. A knowledge of the creatinine level is a prerequisite for the proper adjustment of drug doses (if necessary). A practical and compact summary of dose-adjustment recommendations for patients with renal insufficiency would be desirable but is not yet available.
About 800 000 people in Germany live in nursing homes (1). This is a population characterized by a high prevalence of chronic diseases and by physical and cognitive impairment (2– 6). According to the international literature, the percentage of nursing home residents with renal insufficiency, defined as a glomerular filtration rate (GFR) of 60 mL/min, is very high, ranging between 22% and 78% (7– 12). However, at present no reliable figures are available for Germany. In addition, nursing home residents often receive multiple drugs (6, 13). Since about 50% of all drugs or their metabolites are renally eliminated, this particular patient group is at considerably increased risk of inappropriate drug therapy (14). The most frequent causes of inappropriate drug therapy in older persons and nursing home residents relate to prescription (dosage too high, dose intervals too short), compliance, and/or lack of treatment monitoring (15– 17). The consequences can in some cases include severe adverse drug events (AE) leading to hospital admission or even death. However, most of these AE in nursing home residents are classified as potentially avoidable or reducible (16, 17). In patients with renal insufficiency, lack of dose adjustment or the use of drugs that are contraindicated for the patient’s level of renal function are of particular significance. The few international studies that have been carried out in the nursing home setting show that 12% to 29% of residents receive at least one drug at an inappropriate dosage (18– 20). The range of drugs included in the various studies varies widely. How often nursing home residents in Germany receive drugs that are not adjusted for their renal function, or are contraindicated, is unknown.
The aims of this study were therefore to investigate what percentage of nursing home residents have renal insufficiency and how often drugs are inappropriately dosed or are contraindicated for the patient’s renal function.
Methods
Data and study design
The “Inappropriate Medication in Patients with Renal Insufficiency in Nursing Homes” (IMREN) project is a multicenter cross-sectional study carried out between October 2014 and April 2015 in nursing homes in Bremen and the parts of Lower Saxony surrounding Bremen. A convenience sample was taken of nursing homes that were heterogeneous in terms of supporting organization, size, and location. Within this sample, data were recorded for all residents of the participating care units of the homes. There were no exclusion criteria.
Data collection was anonymized; data were recorded exclusively by the nursing staff in the nursing homes, using a piloted data collection form to which the patient’s current medication regimen was attached. Active participation of the residents was not required for this study, which relied exclusively on existing data. Data collected included sociodemographic information such as age and sex, height and weight, diseases present, and care level.
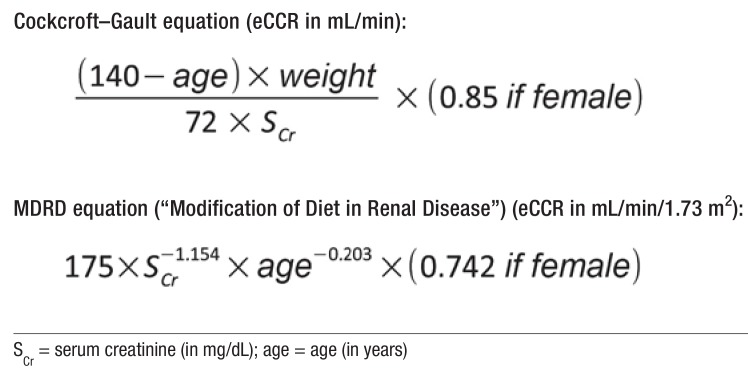
Up-to-date plasma creatinine concentrations were taken from care notes or hospital discharge letters; if no data were present, the care personnel requested them from the patient’s general practitioners. The glomerular filtration rate (GFR) was calculated as estimated creatinine clearance (eCCr) using the Cockcroft–Gault equation, because this is used in published studies (18– 20) and as the reference in the summary of product characteristics (SPC). The Cockcroft–Gault equation includes the following variables: age, sex, body weight, and creatinine concentration (eFigure) (21).
eFigure.
Equations used to estimate creatinine clearance (eCCr) (4)
All data collection forms were computerized by two people independently of each other. The electronic data collection regarding medication regimens was done exclusively by pharmacists. For each drug prescribed, the summary of product characteristics was used as a basis to determine whether the drug was contraindicated or required dosage adjustment in patients with impaired renal function (eTable). Each prescription was individually assessed for whether the dosage was appropriate or the drug contraindicated for the resident’s current level of renal function. If the SPC merely mentioned dose adjustment without giving further details, we made the conservative assumption that the drugs had been correctly adjusted. All dosages were regarded as maintenance dosages. If starting dosages adjusted to the renal function had been required, this could not be taken into account because of the lack of information about treatment duration. All analyses are confined exclusively to scheduled medication. Drugs taken “as needed” and extemporaneous products were not included in the analysis.
eTable. Drugs that are contraindicated or require dose adjustment in patients with renal insufficiency, together with the relevant recommendations from the drug prescribing information.
| Contraindicated in patients with creatinine clearance 90 ml/min | |
| Clemastine | Patients with hepatic and renal insufficiency should not use clemastine, as insufficient data are available for its use in this patient group |
| Methionine | CI: Renal insufficiency |
| Nitrofurantoin | CI: Any degree of renal insufficiency |
| Potassium chloride | CI: Impaired renal excretory function |
| Contraindicated in patients with creatinine clearance 60 ml/min | |
| Leflunomide | CI: Patients with moderate to severe renal insufficiency, as there is insufficient clinical experience of treatment in this patient group |
| Metformin | CI: Renal failure or impaired renal function (creatinine clearance 60 ml/min, reduced to 45 ml/min after the study was carried out) |
| Metformin + vildagliptin | CI: Renal insufficiency or renal impairment, defined as creatinine clearance 60 ml/min |
| Contraindicated in patients with creatinine clearance 50 ml/min | |
| Desmopressin | CI: Moderate or severe renal insufficiency (creatinine clearance 50 ml/min) |
| Contraindicated in patients with creatinine clearance 30 ml/min | |
| Acetylsalicylic acid + paracetamol + caffeine | CI: Severe renal impairment |
| Aluminum oxide + magnesium hydroxide | Should not be used in patients with impaired renal function (creatinine clearance 30 ml/min) |
| Amiloride + HCT | CI: Severe renal impairment (renal insufficiency with pronounced oliguria; creatinine clearance 30 ml/min and/or serum creatinine >1.8 mg/100 mL) |
| Benazepril + HCT | CI: Severe renal impairment (serum creatinine >1.8 mg/dL or creatinine clearance 30 ml/min) |
| Bendroflumethiazide + amiloride | CI: Severe renal impairment (acute renal failure or renal insufficiency with oliguria or anuria; creatinine clearance 30 ml/min and/or serum creatinine >1.8 mg/100 mL) |
| Betamethasone + gentamicin (topical) | Should not be used in patients with advanced renal insufficiency (creatinine clearance 30 ml/min) |
| Brinzolamide | CI: Severe renal impairment. The use of brinzolamide in patients with severely reduced renal function (creatinine clearance 30 ml/min) or patients with hyperchloremic acidosis has not been investigated. since brinzolamide and its main metabolite are excreted through the kidneys, its use in such patients is contraindicated. |
| Calcipotriol | CI: Should not be used in patients with known disorders of calcium metabolism. Should not be used in patients with severe liver and/or kidney disease. |
| Calcium(incl. in combination with vitamin D) | CI: Severe renal insufficiency (creatinine clearance 30 ml/min) |
| Candesartan + HTC | CI: Severely reduced renal function (creatinine clearance 30 ml/min) |
| Captopril + HCT | The combination of captopril and hydrochlorothiazide is contraindicated in patients with severe renal insufficiency (creatinine clearance 30 ml/ min) |
| Chloral hydrate | CI: Severe renal impairment |
| Chlortalidone | CI: Creatinine clearance 30 ml/min |
| Dabigatran | Treatment of patients with severe impairment of renal function (creatinine clearance 30 ml/min) is contraindicated |
| Diclofenac (oral) | CI: Severe renal impairment |
| Dorzolamide | CI: Dorzolamide has not been tested in patients with severe renal impairment (creatinine clearance 30 ml/min) or hyperchloremic acidosis. since dorzolamide and its metabolites are excreted primarily via the kidneys, the use of dorzolamide is contraindicated in these patients. |
| Duloxetine | CI: Severely reduced renal function (creatinine clearance 30 ml/min) |
| Enalapril + HCT | CI: Severely impaired renal function (creatinine clearance 30 ml/min) |
| Enalapril + lercanidipine | CI: In patients with severely renal impairment (creatinine clearance 30 ml/min) or patients receiving hemodialysis |
| Estriol(ovules, vaginal cream, tablets) | CI: Severe renal insufficiency |
| Etoricoxib | No dose adjustment is required in patients with creatinine clearance ≥30 mL/min. The use of etoricoxib in patients with creatinine clearance 30 ml/min is contraindicated |
| Gentamicin (topical) | CI: Should not be used concurrently with systemic aminoglycoside antibiotics or in patients with advanced renal insufficiency (creatinine clearance 30 ml/min), because of the risk of toxic serum concentrations |
| Glibenclamide (USAN: glyburide) | CI: Severely impaired renal function |
| Glimepiride | CI: Severely renal impairment. In patients with severe renal impairment, a change over to insulin is required |
| Hydrochlorothiazide | CI: Creatinine clearance 30 ml/min |
| Hydrotalcite | CI: Patients with severe renal impairment should not use hydrotalcite |
| Ibuprofen (oral) | CI: Severe renal impairment (creatinine clearance 30 ml/min) |
| Indapamide | Treatment is contraindicated in patients with severe renal insufficiency (creatinine clearance 30 ml/min) |
| Irbesartan + HCT | Reduced renal function: Because of the hydrochlorothiazide component, this drug is not recommended for patients with Severe renal impairment (creatinine clearance <30 ml/min). no dose adjustment is required in patients with reduced renal function and creatinine clearance ≥30 ml/min |
| Iron(II) sulfate | CI: Severe renal disease. In some patients with renal anemia, oral substitution with iron(II) sulfate under close monitoring can be beneficial. |
| Lercanidipine | Should not be used in patients with severe renal impairment (creatinine clearance 30 ml/min) |
| Lisinopril + HCT | CI: Creatinine clearance 30 ml/min |
| Losartan + HCT | CI: Creatinine clearance 30 ml/min |
| Mesalazine | Suppositories should not be used in patients with severe renal impairment |
| Metoprolol + HCT | CI: Severe renal impairment (renal insufficiency with oliguria or anuria; creatinine clearance 30 ml/min and/or serum creatinine concentration >1.8 mg/100 mL) |
| Naproxen | CI: Severe renal impairment |
| Nitroxoline | Should not be taken by patients with severe renal impairment |
| Olmesartan + amlodipine + HCT | CI: Severely reduced renal function (creatinine clearance 30 ml/min) |
| Phenprocoumon | CI: Creatinine clearance 30 ml/min (“manifest renal insufficiency” defined as “severe renal insufficiency”) |
| Primidone (INN: desoxyphenobarbital) | CI: Should not be used in patients with severe renal impairment |
| Ramipril + HCT | CI: Creatinine clearance 30 ml/min |
| Reviparin | The use of reviparin is contraindicated in patients with severely reduced renal function (creatinine clearance 30 ml/min) |
| Spironolactone | CI: Creatinine clearance 30 ml/min |
| Spironolactone + furosemide | CI: Creatinine clearance 30 ml/min |
| Sulfasalazine | CI: Severe renal insufficiency |
| Tacalcitol | CI: Severe renal disease, as no clinical evidence is available |
| Telmisartan + HCT | CI: Severe renal damage (creatinine clearance 30 ml/min) |
| Timolol + dorzolamide | CI: Severe renal impairment (creatinine clearance 30 ml/min) |
| Timolol + travoprost | CI: Creatinine clearance 30 ml/min |
| Triamterene + HCT | CI: Creatinine clearance 30 ml/min |
| Valsartan + HCT | Because of the hydrochlorothiazide component, this preparation is contraindicated in patients with severe renal impairment |
| Contraindicated in patients with creatinine clearance 20 ml/min | |
| Methotrexate | CI: Creatinine clearance 20 ml/min |
| Contraindicated in patients with creatinine clearance 15 ml/min | |
| Cotrimoxazole (trimethoprim, sulfamethoxazole) | CI: Creatinine clearance 15 ml/min |
| Contraindicated in patients with creatinine clearance 10 ml/min | |
| Fluoxetine | CI: Severe renal impairment (creatinine clearance 10 ml/min) |
| Contraindicated in patients with creatinine clearance 9 ml/min | |
| Galantamine | Since no data are available on the use of galantamine in patients with severe renal impairment (creatinine clearance 9 ml/min), galantamine should not be used in these patients. |
| Contraindicated in patients with/without concurrent disease/treatment | |
| Fesoterodine | CI: Concurrent use of strong CYP3A4-inhibitors in patients with moderately to severely impaired hepatic or renal function (creatinine clearance 90 ml/min) |
| Fluprednidene + gentamicin (topical) | CI: Should not be used concurrently with systemic aminoglycoside antibiotics or in patients with advanced renal insufficiency (creatinine clearance 30 ml/min), because of the risk of toxic serum concentrations |
| Furosemide | CI: Renal insufficiency with anuria |
| Gentamicin (topical) | CI: Should not be used concurrently with systemic aminoglycoside antibiotics or in patients with advanced renal insufficiency (creatinine clearance 30 ml/min), because of the risk of toxic serum concentrations |
| Metoclopramide | No contraindication exists for metoclopramide given at a low dosage. High-dose metoclopramide therapy is contraindicated in patients with cytostatic-induced nausea and vomiting who have reduced renal function |
| Ropinirole | CI: Severe renal insufficiency (creatinine clearance 30 ml/min) without regular hemodialysis therapy |
| Telmisartan | CI: Concurrent use of telmisartan and aliskiren is contraindicated in patients with reduced renal function (creatinine clearance 60 ml/min). |
| Dose adjustment required in patients with concurrent disease/treatment | |
| Oxybutynin | Caution is advised when using oxybutynin in patients with impaired renal function, especially those with severe renal insufficiency, as no pharmacokinetic data are available for this patient group. Dose reduction may be necessary. |
| Dose adjustment required | |
| Acemetacin | Special care is required in patients with pre-existing renal damage; long-term use of acemetacin mandates regular monitoring of renal function and hemogram. |
| β -Acetyldigoxin | In patients with impaired renal function the dosage of β -acetyldigoxin must be adjusted to renal clearance. |
| Acetylsalicylic acid 500 mg (oral) | In patients with renal impairment the dose must be reduced or the dosing interval extended. |
| Aciclovir (oral) | For patients with renal insufficiency: In patients with impaired renal function a lower dose may suffice for treatment […]. |
| Agomelatine | No significant change in pharmacokinetic parameters was observed in patients with severe renal impairment. However, limited data are available about the use of agomelatine in patients with severe or moderate renal insufficiency. Caution is therefore advised when using it in these patients. |
| Alendronic acid | In patients with creatinine clearance >35 mL/min, dose adjustment is not required. As very little clinical evidence is available, alendronic acid is not recommended for use in patients with impaired renal function with creatinine clearance <35 ml/min. |
| Alendronic acid + cholecalciferol | Is not recommended for patients with impaired renal function with creatinine clearance 35 ml/min, due to lack of evidence. in patients with creatinine clearance >35 mL/min, dose adjustment is not required. |
| Allopurinol | Without dose adjustment, patients with impaired renal function are at risk of overdose, as allopurinol and its metabolites are excreted via the kidneys. To reduce any possible risk, therefore, reduction of the recommended dosage is indicated. In patients with severe renal impairment, a maximum of 100 mg allopurinol/ per day or single doses of 100 mg at intervals longer than 1 day should be given. |
| Amantadine | In patients with impaired renal function the dosage should always be adjusted for the reduction in renal clearance (determined as creatinine clearance). |
| Ambroxol | In patients with impaired renal function, ambroxol drops should only be used with particular caution (i.e., at longer dosing intervals or in reduced doses). |
| Amitriptyline | Dose reduction is indicated in patients with impaired renal or hepatic function. |
| Amoxicillin | In patients with severely impaired renal function with a creatinine clearance 30 ml/min, a reduction in subsequent doses is recommended, since accumulation of amoxicillin is to be expected. patients with a creatinine clearance of 20–30 ml/min should receive two thirds of the normal dose, and those with a creatinine clearance 20 ml/min one third of the normal dose. increasing the dosing interval with monitoring of the effective serum concentration is a possible option.. |
| Amoxicillin + clavulanic acid | In patients with impaired renal function, the dosage should be reduced in accordance with the severity of the impairment, and should be adjusted for body weight. |
| Apixaban | When two of the following three criteria are met, the dosage should be reduced to 2.5 mg every 12 hours: serum creatinine ≥1.5 mg/dL, age ≥80 years, body weight ≤60 kg. In patients with a creatinine clearance of 15–29 mL/min, the dosage should be reduced to 2.5 mg every 12 hours; in those with a creatinine clearance 15 ml/min, apixaban should not be used. |
| Atenolol | Since atenolol is excreted via the kidneys, in patients with impaired renal function the dosage should be adjusted to renal clearance: where creatinine clearance values are reduced to 10–30 mL/min (serum creatinine >1.2 5 mg/dl), the dosage should be reduced to half the standard dosage; for values below 10 ml/min (serum creatinine >5 mg/dL) it should be reduced to a quarter of the standard dosage. |
| Azathioprine | In patients with renal insufficiency, the dosage of azathioprine should be towards the lower end of the normal range. |
| Baclofen | Especially slow dose increments are indicated in older, frailer patients suffering from impaired hepatic and renal function. In patients with impaired renal function the dosage should be reduced. |
| Betaxolol | In the presence of impaired renal function (up to a creatinine clearance of 30 mL/min), dose adjustment is usually unnecessary. Clinical monitoring of the patient is however recommended at the beginning of treatment. In patients with severely impaired renal function (creatinine clearance 30 ml/min) and in patients receiving dialysis, a dose of half a film-coated tablet should not be exceeded. |
| Bimatoprost | Bimatoprost has not been investigated in patients with impaired renal function and should therefore be used only with caution in these patients. |
| Bisoprolol | In patients with severely impaired renal function (creatinine clearance 20 ml/min), it is recommended that a daily dose of 10 mg is not exceeded. |
| Brimonidine | Use in patients with renal impairment: Brimonidine has not been investigated in patients with impaired hepatic or renal function. Caution is advised in the treatment of these patients. |
| Bromazepam | Patients with impaired renal function usually receive half the normal daily dose, i.e., a quarter of a tablet at night (corresponding to 1.5 mg bromazepam) up to a maximum of one tablet (corresponding to a maximum of 6 mg bromazepam). |
| Bromhexine | In patients with impaired renal function, bromhexine drops should only be used with particular caution (i.e., at longer dosing intervals or in reduced doses). |
| Buprenorphine | Excretion via the kidney can be delayed, as about 30% of the administered dose is excreted via the kidneys. In patients with renal insufficiency, accumulation of buprenorphine metabolites is seen. Particular care is indicated in the treatment of patients with impaired renal function or severe renal insufficiency (creatinine clearance 30 ml/min). |
| Bupropion | Patients with renal impairment: The recommended dose in these patients is 150 mg once a day, as bupriopion and its active metabolites can accumulate in these patients to a greater extent than normal. |
| Butylscopolamine + paracetamol | Should be used with particular care in patients with severe renal insufficiency (creatinine clearance 10 ml/min) |
| Calcitriol | Calcitriol increases the serum concentration of inorganic phosphate. In patients with hypophosphatemia this is a positive effect, but for patients with chronic renal insufficiency caution is advised, as here there is a risk of ectopic calcification. |
| Captopril | As captopril is excreted primarily via the kidneys, in patients with impaired renal function the dosage should be reduced or the dosing interval extended. |
| Carbamazepine | In patients with kidney disease a lower dosage is indicated. |
| Cefixime | In patients with markedly impaired renal function the dosage should be reduced. In patients with renal insufficiency (creatinine clearance 20 ml/min) a dose adjustment to one-time administration of 200 mg cefixime per day is recommended. particular care is advised when using cefixime in patients with severe renal impairment (creatinine clearance 10ml/min). |
| Cefuroxime | The safety and efficacy of cefuroxime axetil in patients with renal insufficiency have not been shown. Cefuroxime is mainly excreted via the kidneys. When using cefuroxime In patients with markedly impaired renal function, it is recommended that the dose should be reduced in accordance with the delayed renal elimination. |
| Certoparin | Certoparin sodium should only be used with increased caution in patients with severely impaired renal function (creatinine clearance 30 ml/min). |
| Cetirizine | There are no data to document the efficacy/safety ratio of cetirizine in patients with renal insufficiency. Since cetirizine is mainly excreted via the renal route, in cases where no alternative treatment can be used, the dosing intervals must be individualized according to renal function. |
| Chlordiazepoxide | Patients with impaired renal function usually receive half the recommended dosage. |
| Chlorprothixene | Should only be used with special caution in patients with renal insufficiency. |
| Ciprofloxacin | As ciprofloxacin is largely excreted unchanged via the kidneys, in patients with impaired renal function dose adjustment is required to avoid increased adverse effects due to accumulation of ciprofloxacin. |
| Clodronate | Clodronate is mainly excreted via the kidneys and should therefore be used with particular caution in patients with renal insufficiency. The daily dose should not exceed 1600 mg disodium clodronate continuously. No pharmacokinetic data are available regarding patients with renal insufficiency with a creatinine clearance 10 ml/min receiving treatment with oral clodronate. in such patients treatment should be avoided except in the case of short-term treatment of patients with purely functional renal insufficiency caused by a raised serum calcium concentration. |
| Clomipramine | This drug should only be used after careful weighing of risks against benefits and with appropriate caution in patients with severe hepatic or renal insufficiency. |
| Clonazepam | Should only be used with caution in patients with impaired renal function. The dosage should always be reduced in these patients. |
| Clonidine | Dosage in patients with renal insufficiency: The regulation and treatment of hypertension in patients with renal insufficiency through the use of clonidine hydrochloride requires particular care and frequent blood pressure monitoring. For predialysis patients a dosage of 0.3 mg clonidine hydrochloride orally per day is usually sufficient. Especially careful medical monitoring is necessary in patients with advanced renal insufficiency (creatinine clearance 30 ml/min). |
| Clopidogrel | To date only limited treatment data are available for the use of clopidogrel in patients with renal impairment. Clopidogrel should therefore be used with care in these patients. |
| Cloprednol | Restrictions on use: Renal impairment |
| Codeine | In patients with renal insufficiency and patients on dialysis the elimination of codeine is delayed, and therefore the dosing interval needs to be extended. Codeine should be avoided in patients with advanced renal insufficiency. |
| Cholecalciferol | During long-term treatment serum and urine calcium concentrations should be monitored and renal function tested by serum creatinine determination. This monitoring is especially important in older patients and those being concurrently treated with cardiac glycosides or diuretics. In patients with hypercalcemia or signs of impaired renal function, the dosage should be reduced or treatment stopped. |
| Dalteparin | Should only be used with increased caution in patients at increased potential risk of bleeding, e.g., those with severe renal insufficiency (creatinine clearance 15–29 mL/min). |
| Dantrolene | Caution is advised in patients with pre-existing hyperkalemia (renal insufficiency), as dantrolene has been shown to cause a rise in serum potassium in animal experiments. |
| Diazepam | Patients with impaired renal function usually receive half the recommended daily dose. |
| Digitoxin | Patients with severe hepatic and renal insufficiency may have a reduced digitoxin requirement. |
| Digoxin | In patients with impaired renal function the dosage should be adapted to the patient’s renal clearance. |
| Dihydralazine | Restriction on use: advanced renal insufficiency (creatinine clearance 30 ml/min) |
| Clorazepate | In patients with impaired hepatic and renal function, the dose should be reduced on an individual basis, usually by 50%. |
| Diphenhydramine | Patients with impaired renal function should receive a reduced dose. To be used with caution in patients with impaired renal function. |
| Domperidone | In patients with severe renal insufficiency the elimination half-life increases from 7.4 to 20.8 hours, although the plasma concentrations were below those of normal probands. As only very small amounts of the drug are excreted unchanged via the kidneys, it does not appear necessary to adjust a one-time dose in patients with renal insufficiency. For repeated administration, the dosing frequency should be reduced to once or twice daily, depending on the severity of the impairment, and the dose may need to be reduced. |
| Enalapril | The dosing intervals for enalapril should always be extended and/or the dose reduced. |
| Enoxaparin | Impaired renal function: In patients with mildly or moderately impaired renal function (creatinine clearance 50–80 mL/min or 30–50 mL/min, respectively), dose adjustment is not usually required. However, patients should be carefully monitored. In patients with severely impaired renal function (creatinine clearance 30 ml/min), it is recommended to use the following dosages […] |
| Eprosartan | In patients with mildly to moderately severely impaired renal function (creatinine clearance ≥30 mL/min) no dose adjustment is required. Caution is advised in patients with a creatinine clearance 30 ml/min and in dialysis patients. |
| Escitalopram | In patients with mild to moderately severe renal insufficiency no dose adjustment is required. Caution is advised in patients with severely impaired renal function (creatinine clearance 30 ml/min). |
| Etilefrine | Caution: As etilefrine and its conjugates are mainly excreted renally, accumulation of conjugates may potentially occur in patients with renal insufficiency. |
| Felodipine | The pharmacokinetics are largely unchanged in patients with mildly to moderately severely impaired renal function. In patients with severely impaired renal function, caution is required. |
| Fenoterol | To be used with caution in patients with renal insufficiency |
| Fenoterol and ipratropium bromide | Like other anticholinergics, should only be used with caution in patients with renal insufficiency. |
| Fentanyl | Patients with renal impairment should be carefully monitored and the dosage reduced if necessary. Patients with impaired renal function should, if treated with transdermal fentanyl patches, be carefully monitored for signs of fentanyl intoxication and the dosage should be reduced if necessary. |
| Flecainide | Patients with impaired renal function (creatinine clearance 50 ml/min): in these patients the maximum initial dose should be 100 mg flecainide acetate per day, corresponding to half a tablet twice daily. |
| Fluspirilene | Particular caution is required when using fluspirilene in patients with renal insufficiency. |
| Gabapentin | Dose adjustment may be required in older patients because of age-related reduction in renal function. |
| Haloperidol | Patients with renal insufficiency more frequently show hypotensive reactions to haloperidol and should therefore be closely monitored. |
| Hydromorphone | Patients with renal insufficiency: These patients may require lower doses than other patient groups to achieve adequate analgesia. In patients with creatinine clearance 60 ml/min the initial dose should be reduced, followed by especially careful dose titration. in patients with creatinine clearance 30 ml/min, extending the dosing interval should also be considered. |
| Hydroxyzine | Dose adjustment: Within the recommended dosage range, the dose should be adjusted to the patient’s reaction to the treatment so as to allow any overdose to be recognized as quickly as possible. This applies particularly in the case of patients with renal insufficiency; hydroxyzine is only to be used with particular caution in patients with moderate to severe renal insufficiency. |
| Ibandronate | The use of ibandronate in patients with a serum creatinine concentration >200 μmol/L (2.3 mg/dL) or a creatinine clearance (measured or estimated) 30 ml/min is not recommended because only limited clinical data are available from studies in such patients. in patients with mild or moderate renal insufficiency with a serum creatinine concentration ≤200 μmol/l (2.3 mg/dl) or a creatinine clearance (measured or estimated) ≥30 ml/min no dose adjustment is required. |
| Imipramine | Should only be used after careful weighing of risks versus benefits and with appropriate caution and monitoring in patients with severe renal impairment. |
| Insulin glargine | Patients with impaired renal function may have a reduced insulin requirement due to reduced insulin metabolism. |
| Insulin, human | In patients with hepatic or renal impairment, blood glucose monitoring should be intensified and the insulin dose adjusted on an individual basis. |
| Insulin lispro | Patients with renal damage may have a reduced insulin requirement. |
| Interferon beta-1b | Interferon beta should be used with caution in patients with severe renal impairment, and close monitoring should be considered. |
| Lamotrigine | Caution should be used in administering lamotrigine to patients with impaired renal function. In patients with terminal renal insufficiency the initial doses of lamotrigine should be based on the concurrently administered drugs. A reduced maintenance dose may be effective for patients with significantly impaired renal function. In single-dose studies in subjects with end-stage renal failure, plasma concentrations of lamotrigine were not significantly altered. However, caution should nevertheless be exercised in treating patients with renal insufficiency as accumulation of the glucuronide metabolite is to be expected. |
| Levetiracetam | In older patients with impaired renal function, dose adjustment is recommended […]. |
| Levocetirizine | There are no data to document the efficacy/safety ratio in patients with renal insufficiency. Since levocetirizine is mainly excreted via the kidneys, in cases where no alternative treatment can be used the dosing intervals must be adjusted according to the renal function of the individual. Dose adjustment should be in accordance with the following table […]. |
| Levofloxacin | As levofloxacin is mainly excreted via the kidneys, the dose should be adjusted in patients with impaired renal function. |
| Levomepromazine | In patients with renal insufficiency the dose should be adjusted with particular care, as an increase in adverse events is to be expected in these patients. |
| Levomethadone | Patients with renal insufficiency: Dose reduction is recommended in patients with renal disease. |
| Lisinopril | In patients with impaired renal function, the dose should be adjusted on the basis of the creatinine clearance as shown in the following table […]. |
| Lithium | Should not be taken by patients with acute renal failure; furthermore, in older patients serum lithium concentrations can reach mildly toxic levels because of decreasing renal function, resulting in reduced lithium elimination. Serum lithium concentrations should therefore be monitored more frequently in older patients than in standard cases. |
| Magnesium | There are no absolute, general contraindications that apply in every case to patients receiving electrolytes via the gastrointestinal tract (enteral administration). However, it must always be tested whether any particular cation or anion is contraindicated on the basis of the individual’s electrolyte, fluid, and acid–base balance, especially in patients with excretory disorders (renal insufficiency, anuria) and severe, disease-related fluid loss (exsiccosis).Patients with renal impairment should only take this drug under medical supervision. Long-term high-dose use should be avoided by such patients. |
| Maprotiline | Warnings and precautions: Severe renal impairment |
| Meloxicam | In dialysis patients with severe renal insufficiency, the daily dose should not exceed 7.5 mg meloxicam. In patients with mildly to moderately impaired renal function (e.g., patients with a creatinine clearance of >25 mL/min), no dose reduction is required. |
| Memantine | In patients with mildly impaired renal function (creatinine clearance 50–80 mL/min), no dose adjustment is required. In patients with moderate renal impairment (creatinine clearance 30–49 mL/min) the dose should be 10 mg/day. If this is well tolerated for at least 7 days, the dose may be increased to 20 mg/day in accordance with the standard titration scheme. In patients with severe renal impairment (creatinine clearance 5–29 mL/min), the dose should be 10 mg/day. |
| Metamizole | As excretion rates are reduced in patients with hepatic or renal impairment, repeated high doses should be avoided. For short-term use only, no dose reduction is necessary. No evidence is available regarding long-term use. The dose should be reduced in patients of advanced age, those in a reduced general state of health, or those with reduced creatinine clearance, as excretion of the metabolites of metamizole sodium may be delayed. |
| Metildigoxin | In patients with impaired renal function the dose of metildigoxin should be adjusted according to renal clearance. |
| Mianserin | In patients with impaired renal function, regular monitoring is required in case dose adjustment should become necessary; particular caution is required in patients with severe renal damage. |
| Mirtazapine | Renal insufficiency: Clearance of mirtazapine can be reduced in patients with moderate to severe renal insufficiency (creatinine clearance 40 ml/min). this should be taken into account when prescribing mirtazapine film-coated tablets for patients in this group. |
| Morphine | Titrate dose cautiously in patients with hepatic or renal impairment and those in whom slowed gastrointestinal transit is suspected. |
| Moxonidine | As moxonidine is mainly excreted via the kidneys, caution is advised in patients with impaired renal function. In these patients, careful dose titration is recommended, especially at the start of treatment. The initial dosage should be 0.2 mg/day. If indicated on clinical grounds, and if well tolerated, the daily dose can be increased to a maximum of 0.4 mg in patients with moderately impaired renal function (creatinine clearance >30 mL/min and 60 ml/min) and a maximum of 0.3 mg in patients with severely impaired renal function (creatinine clearance 30 ml/min). |
| Nebivolol | In patients with renal insufficiency the recommended initial daily dose is 2.5 mg nebivolol. If required, the daily dose can be increased to 5 mg. |
| Nortriptyline | In patients with impaired renal function a dose reduction is indicated. |
| Opipramol | In patients with impaired renal function it may be necessary to reduce the dosage, as elimination becomes slower as impairment increases. |
| Oxcarbazepine | In patients with impaired renal function (creatinine clearance 30 ml/min), treatment should start at half the normal initial dosage (300 mg/day) and be increased by increments at least 1 week apart until the desired level of effect is reached. dose increases in patients with impaired renal function require careful monitoring. |
| Oxybutynine | Caution is advised in patients with impaired renal function, especially those with severe renal impairment, as no pharmacokinetic data are available for these patient groups. Dose reduction might be necessary. |
| Oxycodone | Caution is advised in patients with severely impaired renal function. |
| Oxycodone + naloxone | Caution is advised in use in patients with renal impairment. |
| Paracetamol | In patients with renal impairment the dose should be reduced or the dosing interval extended. In patients with severe renal insufficiency (creatinine clearance 10 ml/min), the dosing interval must be at least 8 hours. |
| Paracetamol + codeine | Dose reduction or dosing interval extension are required in patients with severe renal insufficiency (creatinine clearance 10 ml/min) and in dialysis patients. |
| Pentoxyverine | In patients with renal insufficiency caution is advised, as inadequate data are available regarding the use of pentoxyverine in this patient group. |
| Perphenazine | Restrictions on use: Renal impairment |
| Phenoxymethylpenicillin | In newborns and in patients with impaired renal function excretion is delayed. Dosage in impaired renal function: Up to a creatinine clearance rate of 30–15 mL/min and with a dosing interval of 8 hours, dose reduction is generally unnecessary. In patients with anuria, the dosing interval should be extended to 12 hours. |
| Phenytoin | Phenytoin should be used with particular caution in patients with renal impairment. Regular monitoring should be carried out. |
| Piracetam | As piracetam is excreted exclusively via the kidneys, reduced renal function can result in increased plasma concentrations. The daily dose should therefore be determined individually on the basis of renal function. Dose adjustment should be in accordance with the following table […]. |
| Pramipexole | Excretion of pramipexole is dependent on renal function. The following dose scheme is recommended for the start of treatment: In patients with a creatinine clearance rate >50 mL/min no reduction of daily dose or dosing interval is required. In patients with creatinine clearance between 20 and 50 mL/min the initial daily dose of pramipexole should be divided into two separate doses, each consisting of 0.088 mg of the base (0.125 mg of the salt) (0.176 mg of the base or 0.25 mg of the salt per day). A maximum daily dose of 1.57 mg pramipexole base (2.25 mg salt) should not be exceeded. In patients with a creatinine clearance 20 ml/min the daily dose of pramipexole should be given in a single dose, starting with 0.088 mg of the base (0.125 mg salt). a maximum daily dose of 1.1 mg pramipexole base (1.5 mg salt) should not be exceeded. |
| Pravastatin | An initial dose of 10 mg/day is recommended for patients with moderately or severely impaired renal function. The dosage should be adjusted according to lipid response with the patient under medical monitoring. |
| Pregabalin | Pregabalin is mainly eliminated unchanged via the kidneys. As the rate of pregabalin clearance is directly proportional to the rate of creatinine clearance, dose reduction in patients with impaired renal function needs to be individually adjusted to the creatinine clearance. |
| Promethazine | Patients with impaired renal function usually receive half the recommended daily dose (normal maximum dose 50–100 mg given in divided doses). |
| Propiverine | In patients with mildly or moderately impaired renal function no dose adjustment is needed, but these patients should be treated with caution. In patients with severely impaired renal function (creatinine clearance 30 ml/min) the maximum daily dose is 30 mg. |
| Propranolol | In patients with severely impaired renal function the elimination of propranolol is reduced, so dose reduction may be required. |
| Ramipril | In patients with a creatinine clearance of ≥60 mL/min the initial dosage (2.5 mg/day) does not need to be adjusted; the maximum daily dose is 10 mg. In those with a creatinine clearance of 30–60 mL/min the initial dosage (2.5 mg/day) does not need to be adjusted; the maximum daily dose 5 is mg. In those with a creatinine clearance of 10–30 mL/min the initial dose is 1.25 mg/day, and the maximum daily dose is 5 mg. |
| Ramipril + amlodipine | To calculate optimal initial and maintenance dosages in patients with impaired renal function, individualized dose adjustment should be carried out by separate titration of the ramipril and amlodipine components. In patients with impaired renal function no adjustment of the dose of amlodipine is required. In patients with impaired renal function the daily dose of ramipril should be determined on the basis of creatinine clearance: If the creatinine clearance is ≥60 mL/min, no adjustment of the initial dose is necessary; the maximum daily dose is 10 mg […]. |
| Ranitidine | Patients with impaired renal function (creatinine clearance 50 ml/min) should generally take a daily dose of 150 mg. |
| Ringer’s lactate solution | Restrictions on use: Patients with renal insufficiency with a tendency to hyperkalemia |
| Risperidone | Patients with impaired renal function are less able to eliminate the active antipsychotic fraction than adults with normal renal function. Patients with impaired hepatic function have elevated plasma concentrations of the free fraction of risperidone. Irrespective of indication, initial and subsequent dosages should be halved, and dosages should be increased more slowly in patients with impaired renal function. Risperidone should be used with caution in these patient groups. |
| Rivaroxaban | Limited clinical data in patients with severe renal impairment (creatinine clearance 15–29 mL/min) indicate significantly elevated plasma concentrations of rivaroxaban. Rivaroxaban should therefore be used with caution in these patients. The use of rivaroxaban in patients with a creatinine clearance 15 ml/min is not recommended. in patients with mild renal impairment (creatinine clearance 50–80 ml/min) or moderate renal impairment (creatinine clearance 30–49 ml/min), no dose adjustment is required. |
| Roxithromycin | According to available pharmacokinetic studies, dose adjustment is normally not required in patients with renal insufficiency. However, in patients with severe renal insufficiency, monitoring of serum concentrations of roxithromycin is advisable and dose reduction or extension of the dosing interval may become necessary. In patients with simultaneous severe renal and hepatic impairment, serum concentrations of roxithromycin should be regularly monitored dose adjustment may become necessary. |
| Salicylic acid (topical) | Restrictions on use: Renal impairment: should be used only in rare cases and then only on a small area of skin (max. 10 cm2) and for not longer than 3 days. |
| Saxagliptin | Renal insufficiency: For patients with mild renal insufficiency no dose adjustment is recommended. In patients with moderate or severe renal insufficiency the dose should be reduced to 2.5 mg once daily. Saxagliptin is not recommended for use in patients in need of dialysis with end-stage renal disease. |
| Simvastatin | For patients with moderate renal impairment, usually no dose adjustment is required. In patients with severe renal impairment (creatinine clearance <30 ml/min), dosages above 10 mg/day should be considered with care and, if needed, be prescribed with caution. |
| Sitagliptin | If administration of sitagliptin in combination with another antidiabetic drug is being considered, its conditions for use in patients with renal impairment should be checked. For patients with mild renal impairment (creatinine clearance ≥50 mL/min), no dose adjustment is required. For patients with moderate renal impairment (≥30 to 50 ml/min), the dose is 50 mg once daily. for patients with severe renal impairment (30 ml/min) or with end-stage renal disease (esrd) requiring hemodialysis or peritoneal dialysis, the dose is 25 mg once daily. |
| Sodium chloride (i. v.) | Particular caution should be exercised when administering sodium chloride solution to patients with impaired renal function or other conditions or therapies associated with sodium retention. |
| Solifenacin | No dose adjustment is required for patients with mildly to moderately impaired renal function (creatinine clearance >30 mL/min). Patients with severely impaired renal function (creatinine clearance ≤30 mL/min) should be treated with particular caution and should receive no more than 5 mg once daily. |
| Sotalol | Since repeated administration of sotalol to patients with impaired renal function incurs a risk of accumulation, the dosage should be adjusted to renal clearance taking into account heart rate (no lower than 50 bpm) and clinical efficacy. It is recommended that patients with severe renal insufficiency should be given sotalol hydrochloride only with frequent ECG monitoring and monitoring of serum concentrations. It is recommended that in patients whose creatinine clearance is reduced to values of 10–30 mL/min (serum creatinine 2–5 mg/dL) the dosage should be reduced to half, and at values <10 ml/min (serum creatinine >5 mg/dL) it should be reduced to a quarter. |
| Sterofundin (i.v.) | The solution should be used with caution in patients with hypercalcemia and those with impaired renal function or conditions associated with elevated serum vitamin D concentrations. |
| Strontium ranelate | Strontium ranelate is not recommended for use in patients with severe renal impairment (creatinine clearance 30 ml/min). in patients with mild to moderate renal insufficiency (creatinine clearance 30–70 ml/min) no dose adjustment is necessary. |
| Sulpiride | Patients with impaired renal function should be given lower daily doses depending on the severity of impairment. The following guide values should be observed: creatinine clearance 30–60 mL/min, give 50% of the daily dose; creatinine clearance 10–30 mL/min, give 30% of the daily dose; creatinine clearance 10 ml/min, give 20% of the daily dose. |
| Sultamicillin | In patients with severely impaired renal function the excretion of sulbactam and ampicillin is similarly delayed. The dosing intervals for sultamicillin should be extended in accordance with the usual practice for ampicillin. |
| Sumatriptan | Sumatriptan should be used with caution in patients with conditions that can significantly affect the absorption, metabolism, or excretion of the drug, such as impaired hepatic or renal function. |
| Tafluprost | Tafluprost has not been studied in patients with impaired hepatic/renal function, and therefore this drug should be used with caution in such patients. |
| Tamsulosin | Particular care is advised in the treatment of patients with severe renal impairment (creatinine clearance 10 ml/min), as the use of this drug has not yet been studied in this patient group. |
| Temazepam | In patients with markedly impaired renal function caution is advised; it may be advisable to reduce the dosage. |
| Tetrabenazine | In patients with impaired hepatic or renal function the dose should be titrated slowly; it is possible that lower daily doses will be required. |
| Theophylline | Renal impairment: In patients with advanced renal impairment, theophylline metabolites can accumulate. Such patients therefore require smaller doses and dose increments must be carried out particularly carefully. |
| Tiapride | In patients with impaired renal function the dosage should be reduced; if the impairment is severe, tiapride should be discontinued on medical grounds. |
| Timolol and brimonidine | […] has not been investigated in patients with impaired hepatic or renal function. For this reason, caution is advised when treating such patients. |
| Tizanidine | In patients with renal insufficiency (creatinine clearance 25 ml/min) treatment should start with 2 mg daily, followed by slow titration to achieve the effective dose. dose increases should be in increments of no more than 2 mg, depending on tolerability and effectiveness. it is advisable to slowly increase the once-daily dose before increasing the frequency of administration. renal function should be monitored as appropriate in these patients. patients with impaired renal function may need a lower dosage, and for this reason caution is advised when using tizanidine in patients with impaired renal function. |
| Tolperisone | Experience in patients with renal impairment is limited and a higher frequency of adverse events has been observed in this patient group. Therefore, individual titration with close monitoring of the patient’s condition and renal function is recommended in patients with moderate renal impairment. Use of tolperisone is not recommended in patients with severe renal impairment.. |
| Tolterodine | Caution should be exercised when using tolterodine in patients with impaired renal function. In patients with severely impaired renal function (creatinine clearance ≤30 mL/min) the recommended dose is 2 mg once daily. |
| Topiramate | In patients with renal impairment (≤70 mL/min) tolpiramate should be used with caution, as the plasma and renal clearance of topiramate are reduced in these patients. Persons with known renal impairment may require a longer time to reach steady state at each dose. Half of the usual starting and maintenance dose is recommended |
| Torasemide | Because loop diuretics must be actively secreted in the tubules, the dosage must be increased in patients with renal insufficiency. Only limited information is available on dose adjustment in patients with hepatic or renal impairment. |
| Tramadol + paracetamol | In patients with moderate renal impairment (creatinine clearance 10–30 mL/min) the dosing interval should be increased to 12 hours. |
| Trazodone | Restrictions on use: patients with impaired renal or hepatic function (follow dosage instructions) |
| Trimipramine | In older patients with impaired renal function, appropriate investigation and monitoring before and during treatment are required and the dosage should be adjusted. In particular, the dosage should start low, be increased slowly, and a low maintenance dose should be chosen. |
| Trospium | In patients with severely impaired renal function (creatinine clearance 10–30 mL/min), a daily dose of 20 mg should not be exceeded. |
| Tutofusin (i.v.) | Tutofusin should be used only with caution in patients with renal insufficiency with a tendency to hyperkalemia. |
| Urea (topical) | Contraindications: Should not be used on large areas of skin in patients with impaired renal function. |
| Valproate | In patients with renal insufficiency and hypoproteinemia the increase in serum free valproic acid must be taken into account and the dosage reduced accordingly. |
| Vildagliptin | In patients with mild renal impairment (creatinine clearance ≥50 mL/min) dose adjustment is not required. For patients with moderate to severe renal impairment or end-stage renal disease a dose of 50 mg once daily is recommended. |
| Xipamide | Thiazide diuretics and related drugs are fully effective only in patients with normal or at most mildly impaired renal function (serum creatinine concentration 25 mg/l or 220 μmol/l in an adult). in older patients, the dosage of xipamide should be adjusted in relation to age, weight, and sex and according to serum creatinine concentration. when given to treat edema, dosages of up to 40 mg xipamide may be required. in patients with more severe impaired renal function the dosage can be increased to up to 80 mg xipamide daily. increasing the dosage to >80 mg xipamide per day is not recommended. |
| Zuclopenthixol | Particular caution should be exercised in the use of zuclopenthixol in patients with hepatic or renal impairment. |
CI: contraindication; HCT: hydrochlorothiazide; incl.: including
●“Manifest” and “advanced renal insufficiency” are defined as “severe renal insufficiency” (creatinine clearance 30 mL/min)
●Where there are differences in the medical drug prescribing information for drugs containing the same amount of the same active ingredient, the lowest creatinine clearance threshold has been used for all preparations using this active ingredient.
●Drugs for which restrictions for use or warnings such as “should only be used with great caution” are given in relation to impaired renal function have been included in the group of drugs for which dose adjustment is potentially required in patients with impaired renal function.
Sample size calculation
Assuming a prevalence of renal insufficiency of 45% and an intracluster correlation coefficient of 0.01 (22), the intention was to enroll a total of 856 residents in 19 homes (n = 45 per home), in order to calculate a 95% confidence interval (95% CI) with a precision of ± 4% (41% to 49%).
Statistical analysis
All residents for whom at least one eCCr value could be computed were included. The prevalence of moderate (eCCr 59 to 30 mL/min, corresponding to stage 3 in ICD-10) and severe renal insufficiency (eCCr 30 mL/min, corresponding to stages 4 and 5) was calculated on the basis of the most recent creatinine values available. Descriptive statistics were used to analyze the baseline data. Logistic regression was used to determine which variables were associated with inappropriately dosed or contraindicated drugs for the patient;s current renal function. Predictors included in the model were age, sex, care level, polypharmacy (≥5 scheduled medications), and the most frequent co-morbidities. All analyses were cluster-adjusted; for regression analysis, mixed models with random effects were used. SAS 9.4 (SAS Institute, Cary, USA) was used for all analyses.
The study was approved by the ethics committee of the University of Bremen.
Results
Baseline data of the study population
A total of 852 residents (10 to 69 per home) of 21 homes were enrolled (11 in Bremen, 10 in the parts of Lower Saxony surrounding Bremen). At least one eCCr value could be calculated for 685 of these 852 residents (80.4%; 30% to 100% per home). The two groups (those with and those without at least one eCCr value) did not differ significantly in terms of baseline characteristics (Table 1). The analysis reported here is confined to residents with eCCr values. They had an average age of 83.3 years, three quarters of them were female, and they had been in the nursing home for an average of 3.2 years. More than a quarter of the residents had been assessed at care level III, and more than half had dementia.
Table 1. Baseline characteristics of all nursing home residents, those for whom eCCr values were available, and those for whom eCCr values were not available.
| Characteristic | All residents (n=852*1) | eCCr available (n=685*2) | eCCr not available (n=167*3) |
|---|---|---|---|
| Female sex | 76.5% | 75.2% | 82.0% |
| Age in years, mean ± SD (interquartile range; IQR) | 83.5 ± 10.5(79–91) | 83.3 ± 10.6 (79–91) | 84.1 ± 10.1 (80–91) |
| Age group in years | |||
| <70 | 9.5% | 10.2% | 6.6% |
| 70–79 | 18.0% | 18.0% | 18.0% |
| 80–89 | 43.3% | 42.9% | 44.9% |
| ≥90 | 29.2% | 28.9% | 30.5% |
| Time in nursing home in years, mean ± SD (interquartile range; IQR) | 3.2 ± 3.4 (0.9–4.1) | 3.2 ± 3.3 (1.0–4.1) | 2.9 ± 3.4 (0.8–3.7) |
| Legal guardian | 46.2% | 47.7% | 40.1% |
| Care level | |||
| None | 1.7% | 1.6% | 1.8% |
| Level I | 38.6% | 37.1% | 44.8% |
| Level II | 34.3% | 35.3% | 30.3% |
| Level III | 25.4% | 26.0% | 23.0% |
| Body weight in kg, mean ± SD (interquartile range; IQR) | 67.2 ± 16.2 (56.0–75.4) | 67.7 ± 16.6 (56.1–76.0) | 65.2 ± 13.9 (55.5–73.7) |
| Indwelling catheter | 13.4% | 15.1% | 6.2% |
| Feeding tube | 4.1% | 4.4% | 3.1% |
| Chronic diseases | |||
| Arterial hypertension | 59.3% | 60.5% | 54.0% |
| Dementia | 57.7% | 58.6% | 54.1% |
| Diabetes mellitus | 21.9% | 22.8% | 18.1% |
| Stroke | 21.3% | 22.1% | 18.2% |
| Heart failure | 22.5% | 21.4% | 26.9% |
| Coronary heart disease | 13.3% | 13.6% | 11.9% |
| Number of scheduled medications, mean ± SD (interquartile range; IQR) | 6.3 ± 3.4 (4–9) | 6.3 ± 3.3 (4–9) | 6.4 ± 3.6 (4–9) |
| Number of scheduled medications | |||
| <5 (no polypharmacy) | 30.3% | 30.4% | 29.9% |
| ≥5 (polypharmacy) | 69.7% | 69.6% | 70.1% |
*1 Discrepancies are possible depending on the number of missing values; for this group, the number of missing values ranged from 0 (number of scheduled medications) to 34 (legal guardian)
*2Discrepancies are possible depending on the number of missing values; for this group, the number of missing values ranged from 0 (age, sex, and number of scheduled medications) to 24 (legal guardian)
*3Discrepancies are possible depending on the number of missing values; for this group, the number of missing values ranged from 0 (number of scheduled medications) to 10 (legal guardian)
Renal insufficiency
A total of 63.6% (95% CI: [55.8; 71.5]) had renal insufficiency (eCCr <60 mL/min), 48.2% of which with an eCCr of 59–30 and 15.5% with an eCCr of <30 mL/min (Table 2). A constant decline in renal function with age was shown. Thus, severe renal insufficiency (eCCr 30 mL/min) was found in 1.4% of 70-year-olds, but in 29.8% of residents aged at least 90 years. Female nursing home residents had poorer renal function than male residents, although the average age of female residents was also higher (85.0 vs. 78.3 years). The only significant difference between the sexes was in the 70 to 79 year-old age group (eCCr 60 mL/min was 12.5% [4.9; 20.1] in men and 44.0% [26.8; 61.2]) in women; however, case numbers were low.
Table 2. Prevalence of moderate and severe renal impairment (eCCr, Cockcroft–Gault equation) in nursing home residents by age and sex with a 95% confidence interval (95% CI).
| Characteristics | ≥60 mL/min (n=249) | 59–30 mL/min (n=330) | <30 ml/min (n=106) |
|---|---|---|---|
| Sex | |||
| Men (n=170) | 57.1% (49.2–65.0) | 36.5% (30.0–42.9) | 6.5% (3.2–9.7) |
| Women (n=515) | 29.5% (21.8–37.2) | 52.0% (45.6–58.4) | 18.4% (14.7–22.2) |
| Age in years | |||
| <70 (n=70) | 87.1% (77.4–96.9) | 11.4% (2.2–20.6) | 1.4% (0.0–4.6) |
| 70–79 (n=123) | 68.3% (55.9–80.7) | 29.3% (17.6–41.0) | 2.4% (0.0–5.2) |
| 80–89 (n=294) | 30.6% (22.9–38.3) | 54.8% (46.9–62.6) | 14.6% (9.5–19.7) |
| ≥90 (n=198) | 7.1% (3.5–10.7) | 63.1% (55.8–70.5) | 29.8% (22.2–37.4) |
| Total (n=685) | 36.4% (28.5–44.2) | 48.2% (41.8–54.5) | 15.5% (12.4–18.6) |
The most recent creatinine value was a mean of 185 days old (interquartile range [IQR] 67 to 373). In a third of cases (31.6%) it was taken from the hospital discharge letter; in the remaining cases it was requested from the patient’s general practitioner or was recorded in the care notes. The values from both sources were of similar recency (median 185 and 188 days old, respectively). If only the 507 residents (74.1%) whose most recent creatinine values were from the previous 365 days are included, the results are the same as when all residents with recorded values are included (48.9% with an eCCr of 59 to 30 mL/min and 15.8% with an eCCr <30 mL/ min).
Medication adjustment
The 685 residents were receiving a total of 4316 drugs as scheduled medication, of which 2184 (50.6%) were potentially contraindicated or required dosage adjustment in patients with impaired renal function. The most frequently used of these were ramipril, simvastatin, and torasemide (Table 3). However, in only a small number of cases (n = 169; 7.7%) were these actually being incorrectly used in relation to the given patient’s renal function, because the drug was inappropriately dosed (n = 54) or was contraindicated (n = 115) for the patient’s eCCr value. This was most often the case for metformin, ramipril, and potassium chloride (Table 3).
Table 3. Drug prescribing*1.
| Drugs that potentially require dose adjustment or are contraindicated in renal impairment (irrespective of actual renal function in any given patient) | ||
| Rank order | Active ingredient (ATC code) | Number (%) |
| 1. | Ramipril (C09AA05) | 156 (7.1%) |
| 2. | Simvastatin (C10AA01) | 123 (5.6%) |
| 3. | Torasemide (C03CA04) | 120 (5.5%) |
| 4. | Metamizole (N02BB02) | 118 (5.4%) |
| 5. | Furosemide (C03CA01) | 106 (4.9%) |
| Total | 2184 (100%) | |
| Drugs that were inappropriately dosed for the given patient’s level of renal function | ||
| Rank order | Active ingredient (ATC code) | Number (%) |
| 1. | Ramipril (C09AA05) | 22 (40.7%) |
| 2. | Gabapentin (N03AX12) | 7 (13.0%) |
| 3. | Allopurinol (M04AA01) | 6 (11.1%) |
| 4. | Acetyl digoxin (C01AA02)*2 | 2 (3.7%) |
| 5. | Levetiracetam (N03AX14)*2 | 2 (3.7%) |
| Total | 54 (100%) | |
| Drugs that were contraindicated for the given patient’s level of renal function | ||
| Rank order | Active ingredient (ATC code) | Number (%) |
| 1. | Metformin (A10BA02) | 24 (20.9%) |
| 2. | Potassium chloride (A12BA01) | 14 (12.2%) |
| 3. | Hydrochlorothiazide (C03AA03) | 11 (9.6%) |
| 4. | Calcium carbonate and colecalciferol (A12AX01) | 8 (7.0%) |
| 5. | Phenprocoumon (B01AA04)*3 | 5 (4.3%) |
| Total | 115 (100%) | |
*1i.e., drugs that potentially were contraindicated or required dose
adjustment for use in patients with renal impairment, and how often this was not correctly followed in relation to patient’s actual level of renal function. For the underlying recommendations in the relevant SPC for each active ingredient, see eTable
*2There were also two prescriptions each for cetirizine and levocetirizine
*3There were also five prescriptions each for spironolactone and ibuprofen
In total, 135 residents (19.7%) [15.5; 23.9] were receiving at least one drug that was inappropriately dosed or was contraindicated for their renal level of function. This was associated with higher age, female sex, arterial hypertension, and polypharmacy. Care level had no effect (Table 4).
Table 4. Prevalences and variables associated with drugs administered to nursing home residents that are inappropriately dosed or are contraindicated for the patient’s actual renal function*1.
| Characteristic | Prevalence | OR [95%-CI] | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 12.4% | 1 | 0.0468 |
| Female | 22.1% | 1.76 [1.01–3.06] | |
| Age in years | |||
| <70 | 2.9% | 1 | 0.0009 |
| 70–79 | 12.2% | 4.13 [0.87–19.70] | |
| 80–89 | 22.1% | 8.35 [1.89–36.95] | |
| ≥90 | 26.8% | 11.85 [2.63–53.38] | |
| Care level | |||
| None/level I | 21.5% | 1 | 0.94 |
| Level II | 21.0% | 1.09 [0.67–1.76] | |
| Level III | 16.0% | 1.05 [0.59–1.89] | |
| Polypharmacy | |||
| <5 scheduled medications | 6.7% | 1 | <0.0001 |
| ≥5 Scheduled medications | 25.4% | 4.28 [2.32–7.90] | |
| Arterial hypertension | |||
| No | 12.4% | 1 | 0.03 |
| Yes | 24.0% | 1.69 [1.05–2.70] | |
| Diabetes mellitus | |||
| No | 17.7% | 1 | 0.13 |
| Yes | 25.8% | 1.45 [0.90–2.35] | |
| Dementia | |||
| No | 21.4% | 1 | 0.29 |
| Yes | 18.4% | 0.78 [0.49–1.23] | |
*1(Odds ratio [OR] with 95% confidence interval [95% CI]). The final multivariate model was based on n=659 residents, for whom values were available for all of the included variables.
Discussion
Results in the context of the existing literature
This cross-sectional study showed that 63.6% of residents had renal insufficiency. To the best of our knowledge, this is the first published study of renal function in nursing home residents in Germany. Most international studies are from North America and show a very wide range of variation, with values between 22% and 78% (7– 12), although some of this variation is due to known differences between the equations used to estimate creatinine clearance (23, 24). The largest study published so far enrolled 9931 residents in 87 homes in Ontario, Canada, and found an eGFR or eCCr 60 mL/min in 35.7% (“modification of diet in renal disease” [MDRD equation]) and 77.5% respectively (Cockcroft–Gault equation, which we used in our study) (11). Similar differences between the estimating equations can also be identified in our study (60 and 30 mL/min in 63.6% and 15.5% respectively according to Cockcroft–Gault versus 41.5% and 5.5% according to MDRD). This makes clear what a large effect the choice of estimating equation has on the requirement for dose adjustment (25). Where reported, however, all studies, including our own—irrespective of which equation was used—showed renal function reducing with age, and women with lower values than men (8, 10– 12).
How often nursing home residents receive drugs at inappropriate dosages or that are contraindicated for their renal function, has so far as we know only been investigated in three US studies, none of which included as large a number of different drugs as the present one (18– 20). In one study of 1304 nursing home residents, in 11.9% of residents at least one of 21 drugs had not been dose-adjusted (18). In another study, 197 out of 721 residents (27.3%) received at least one of 18 drugs included in the study at an inappropriate dosage (20). The third study included all drugs that were prescribed (55 in total), but only in 90 residents. In that study, 56 residents (62%) received drugs that required dose adjustment, and in 26 (29%) at least one drug was not dose-adjusted (19). Our finding of around 20% appears comparatively low when one remembers that the larger part of our patient group (90.1%) had drugs prescribed that required adjustment or were contraindicated in patients with renal insufficiency. Regarding the frequently used drugs, there were some accordances with previous studies (e.g., allopurinol, hydrochlorothiazide, spironolactone, or gabapentin) (18, 20); other drugs, such as ranitidine, barely appeared in our study (prescribed only three times as scheduled medication). Age and polypharmacy or co-morbidities were associated with non-adjusted medication in both the larger studies (18, 20) and in our own. In the study by Hanlon et al. (18), the odds ratio for female sex was also numerically increased.
Strengths and limitations of the present study
The present study is based on a dataset of almost 700 nursing home residents prescribed about 4300 drugs as scheduled medication. Nevertheless, it is still a convenience sample of nursing homes willing to take part in a study of this kind. This means that selection bias cannot be ruled out. The same is true of the reported creatinine values, as some general practitioners, who measure serum creatinine only sporadically, may not have given information on this point. For 19.6% of residents, eCCr could not be calculated because no blood samples were taken for the purpose of the study. To do this would have required consent from the residents, which would have led to another selection bias. On the other hand, we were able to include all of the residents in the participating care units—an important point for the validity of the study. In one American study that also relied on existing data, 24% or 32% of creatinine values (depending on the calculation method used) were missing (10). The creatinine values available to us were a median of 185 days old. This does not, however, allow us to conclude with certainty that these values are measured too infrequently. A third of the values were taken from hospital discharge letters: in these cases, more recent values may exist that are unknown to the nursing homes. However, restricting the analysis to residents with values from the preceding year did not alter the prevalence of renal insufficiency.
Various equations exist for calculating the GFR, requiring different variables (e.g., cystatin C) and producing different results (8, 21, 23– 26). We decided to use the Cockcroft–Gault equation, because this is commonly in routine use in clinical practice (27, 28) and in studies similar to ours (7, 11, 18– 20, 23). In addition, it overestimates GFR in older persons to a much lesser extent than do the CKD-EPI and MDRD equations—an important consideration in the question of dose adjustment (28). However, restrictions in drug use in persons with impaired renal function are sometimes linked to certain other laboratory values which were not available to us and therefore could not be taken into account in this study.
To date, there is no generally accepted, uniform compilation of drugs that are contraindicated or require dose adjustment in patients with renal insufficiency (18– 20, 25, 29). For this reason, we decided to rely on the SPC, since this is what is used by the personnel prescribing and supplying drugs, and is approved by regulatory authorities and regularly revised. It does, however, sometimes contain recommendations that do not reflect current evidence or routine practice; an example would be the contraindication for metformin in patients with an eGFR 60 mL/min (29– 31), which after the study was concluded was lowered to 45 mL/min. Taking renal artery stenosis into consideration, and with monitoring of creatinine and potassium concentrations, even ACE inhibitors and sartans are recommended for nephroprotection (32, 33). Similarly, calcium channel blockers are used in patients with renal insufficiency to reduce blood pressure, and thiazides and related diuretics are used together with loop diuretics (33). Ultimately, it is up to the treating physician to weigh up risks against benefits, and so it can occasionally happen that even a drug that is contraindicated in patients with kidney disease is beneficial. An example is oral substitution with iron(II) sulfate, which, despite being contraindicated in patients with severe kidney disease, is, with regular monitoring, a mainstay of treatment for renal anemia (34). Other limitations of the study are discussed in the eBox.
eBox. Other limitations of the study.
Another potential source of error is the data input by the nursing staff. Because of data anonymization, it was not possible to investigate the extent to which transcription errors might have occurred, but we carried out numerous plausibility tests. In addition, no information was available about how frequently drugs were titrated, so we regarded all prescriptions as maintenance doses – and it is likely that in most cases this was in fact the case. It should also be mentioned that due to missing renal values, the sample actually studied was smaller than originally planned. Moreover, all that was tested was whether the drugs prescribed were contraindicated or required dose adjustment in relation to renal function. Other reasons for contraindication may exist in addition to this one but were not included in the present study. The same applies to the question of whether a given drug was, or was still, indicated at all, or whether it would have been lower-risk—irrespective of renal function—to reduce the dosage or discontinue altogether (“deprescribe”) certain drugs in very old residents in nursing homes (1). This topic is under debate especially in relation to the primary and secondary medical prevention of cardiovascular disease (2). For example, it was recently shown that discontinuing statins in patients with limited life expectancy can be a safe option and was associated with better quality of life and with discontinuation of other drugs (3).
Conclusions
Renal insufficiency is common in residents of nursing homes. In this patient group, therefore, creatinine values should be determined at least once a year and, ideally, recorded in the care notes so as to be available to all personnel involved in the patient’s care. This would allow renal function to be taken into account even by, for example, emergency or out-of-hours physicians when prescribing. Clearly, this is not regular practice at present.
Overall, with about half of the drugs used for scheduled medication in nursing home residents, caution is needed in patients with impaired renal function. In most of the prescriptions in the present study, this had in fact been taken into account. However, in some cases it was difficult to ascertain whether and which dose adjustments were needed (e.g., for insulins, opioids, or other analgesics). Sometimes the SPC given by different manufacturers is even contradictory. This problem of divergent recommendations has also been described in the literature (25, 29). The call for a thorough review and standardization of prescribing information regarding the use of drugs in patients with renal insufficiency—as formulated in the 2008/2009 action plan for improving drug safety in Germany (35)—therefore needs repeating, with emphasis. Even in the www.dosing.de database set up by Heidelberg University Hospital, dosage recommendations are missing for some active ingredients.
Thus, there exists no unified, practical reference source for everyday clinical use where dose adjustments to be made in patients with renal insufficiency can be looked up. Given the increasing proportion of older people with multiple morbidities, this is a major problem for drug safety. A variety of approaches to a possible solution exist, which could include the following:
Rapid harmonization of summary of product characteristics
Integration with electronic prescribing aids or apps
Optimization of www.dosing.de
Producing a guideline specifically for this purpose, as was done in the Netherlands (36).
Key Messages.
Just under half the nursing home residents in Germany suffer from moderate renal insufficiency (estimated creatinine clearance [eCCr] 59–30 mL/min) and around 15% from severe renal insufficiency (eCCr <30 mL/min).
About half of all drugs used for scheduled medication in nursing home residents require dose adjustment or are even contraindicated in patients with renal insufficiency.
In this study, 25% of nursing home residents were receiving at least nine drugs as scheduled medication.
Almost one in five residents was receiving at least one drug which according to the SPC was inappropriately dosed or was contraindicated for the patient’s current renal function.
When five or more drugs were being administered, the risk of an inappropriately dosed or contraindicated drug was higher than when fewer drugs were being given (OR = 4.28, 95% CI: [2.32; 7.90]).
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
The authors are grateful to Mandy Köhrmann and Katharina Allers for inputting the survey form data and to Birgitt Wiese for statistical support. They thank all the participating homes for their collaboration, and Christian Scholz for technical support.
Footnotes
Conflict of interest statement
This study was supported financially by KfH-Stiftung Präventivmedizin. KfH-Stiftung Präventivmedizin was not involved in the data analysis and had no influence on the preparation of the manuscript or the decision to submit. The authors declare that no other conflicts of interest exist.
References
- 1.Pflegestatistik 2011. Pflege im Rahmen der Pflegeversicherung. Deutschlandergebnisse [ www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Pflege/PflegeDeutschlandergebnisse5224001119004.pdf] (Last accessed on 28 September 2015)
- 2.Reuther S, van Nie N, Meijers J, Halfens R, Bartholomeyczik S. [Malnutrition and dementia in the elderly in German nursing homes. Results of a prevalence survey from the years 2008 and 2009] Z Gerontol Geriatr. 2013;46:260–267. doi: 10.1007/s00391-012-0346-y. [DOI] [PubMed] [Google Scholar]
- 3.Meyer G, Köpke S, Haastert B, Mühlhauser I. Restraint use among nursing home residents: cross-sectional study and prospective cohort study. J Clin Nurs. 2009;18:981–990. doi: 10.1111/j.1365-2702.2008.02460.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann F, Kaduszkiewicz H, Glaeske G, van den Bussche H, Koller D. Prevalence of dementia in nursing home and community-dwelling older adults in Germany. Aging Clin Exp Res. 2014;26:555–559. doi: 10.1007/s40520-014-0210-6. [DOI] [PubMed] [Google Scholar]
- 5.Richter T, Mann E, Meyer G, Haastert B, Köpke S. Prevalence of psychotropic medication use among German and Austrian nursing home residents: a comparison of 3 cohorts. J Am Med Dir Assoc. 2012;13:187.e7–187e13. doi: 10.1016/j.jamda.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JRF. Health status of UK care home residents: a cohort study. Age Ageing. 2014;43:97–103. doi: 10.1093/ageing/aft077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar EA, Ashraf H, Frontini M, Ruiz M, Reske TM, Cefalu C. An analysis of chronic kidney disease risk factors in a Louisiana nursing home population: a cross-sectional study. J La State Med Soc. 2013;165(260 3):265–267. [PubMed] [Google Scholar]
- 8.Chan TC, Yap DYH, Shea YF, Luk KH, Chan HW, Chu LW. Prevalence and associated comorbidities of moderate to severe chronic renal impairment in Chinese nursing home older adults. J Am Med Dir Assoc. 2012;13:630–633. doi: 10.1016/j.jamda.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Joseph J, Koka M, Aronow WS. Prevalence of moderate and severe renal insufficiency in older persons with hypertension, diabetes mellitus, coronary artery disease, peripheral arterial disease, ischemic stroke, or congestive heart failure in an academic nursing home. J Am Med Dir Assoc. 2008;9:257–259. doi: 10.1016/j.jamda.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Schnelle J, Osterweil D, Globe D, Sciarra A, Audhya P, Barlev A. Chronic kidney disease, anemia, and the association between chronic kidney disease-related anemia and activities of daily living in older nursing home residents. J Am Med Dir Assoc. 2009;10:120–126. doi: 10.1016/j.jamda.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int. 2004;65:649–653. doi: 10.1111/j.1523-1755.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson B, Artz AS, Culleton B, Critchlow C, Sciarra A, Audhya P. Prevalence of anemia in the nursing home: contribution of chronic kidney disease. J Am Geriatr Soc. 2007;55:1566–1570. doi: 10.1111/j.1532-5415.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 13.Beloosesky Y, Nenaydenko O, Gross Nevo RF, Adunsky A, Weiss A. Rates, variability, and associated factors of polypharmacy in nursing home patients. Clin Interv Aging. 2013;8:1585–1590. doi: 10.2147/CIA.S52698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann B, Czock D, Keller F. Drug therapy in patients with chronic renal failure. Dtsch Arztebl Int. 2010;107:647–55. doi: 10.3238/arztebl.2010.0647. quiz 655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 16.Jaehde U, Thürmann PA. [Medication safety in nursing homes] Z Evid Fortbild Qual Gesundhwes. 2012;106:712–716. doi: 10.1016/j.zefq.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109:87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older veterans affairs nursing home patients. J Am Med Dir Assoc. 2011;12:377–383. doi: 10.1016/j.jamda.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimi AR, Kennedy K, Thomason M, Crumley J, Bugg A, Peacock E. Improper renal dosing in long-term care facilities. South Med J. 2008;101:802–805. doi: 10.1097/SMJ.0b013e31817f1f71. [DOI] [PubMed] [Google Scholar]
- 20.Papaioannou A, Clarke JA, Campbell G, Bédard M. Assessment of adherence to renal dosing guidelines in long-term care facilities. J Am Geriatr Soc. 2000;48:1470–1473. doi: 10.1111/j.1532-5415.2000.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 22.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57:785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Modig S, Lannering C, Östgren C, Mölstad S, Midlöv P. The assessment of renal function in relation to the use of drugs in elderly in nursing homes; a cohort study. BMC Geriatr. 2011;11 doi: 10.1186/1471-2318-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmarajan TS, Yoo J, Russell RO, Norkus EP. Chronic kidney disease staging in nursing home and community older adults: does the choice of cockcroft-gault, modification of diet in renal disease study, or the chronic kidney disease epidemiology collaboration initiative equations matter? J Am Med Dir Assoc. 2012;13:151–155. doi: 10.1016/j.jamda.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Karsch-Völk M, Schmid E, Wagenpfeil S, Linde K, Heemann U, Schneider A. Kidney function and clinical recommendations of drug dose adjustment in geriatric patients. BMC Geriatr. 2013;13 doi: 10.1186/1471-2318-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drenth-van Maanen AC, Jansen PA, Proost JH, Egberts TC, van Zuilen AD, van der Stap D, van Marum RJ. Renal function assessment in older adults. Br J Clin Pharmacol. 2013;76:616–623. doi: 10.1111/bcp.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nygaard HA, Naik M, Ruths S, Krüger K. Clinically important renal impairment in various groups of old persons. Scand J Prim Health Care. 2004;22:152–156. doi: 10.1080/02813430410006468. [DOI] [PubMed] [Google Scholar]
- 28.Dowling TC, Wang ES, Ferrucci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy. 2013;33:912–921. doi: 10.1002/phar.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanal A, Castelino RL, Peterson GM, Jose MD. Dose adjustment guidelines for medications in patients with renal impairment: how consistent are drug information sources? Intern Med J. 2014;44:77–85. doi: 10.1111/imj.12291. [DOI] [PubMed] [Google Scholar]
- 30.Hanlon JT, Aspinall SL, Semla TP, et al. Consensus guidelines for oral dosing of primarily renally cleared medications in older adults. J Am Geriatr Soc. 2009;57:335–340. doi: 10.1111/j.1532-5415.2008.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkowski B, Tylicki L. Nephroprotective action of renin-angiotensin-aldosterone system blockade in chronic kidney disease patients: the landscape after ALTITUDE and VA NEPHRON-D trails. J Ren Nutr. 2015;25:194–200. doi: 10.1053/j.jrn.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Verbeke F, Lindley E, van Bortel L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the management of blood pressure in non-dialysis-dependent chronic kidney disease: an endorsement with some caveats for real-life application. Nephrol Dial Transplant. 2014;29:490–496. doi: 10.1093/ndt/gft321. [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. [ www.nice.org.uk/guidance/ng8] London: National Clinical Guideline Centre; 2015. Anaemia management in people with chronic kidney disease. (last accessed on 28 September 2015) [Google Scholar]
- 35.Bundesministerium für Gesundheit. Aktionsplan 2008/2009 Zur Verbesserung Der Arzneimitteltherapiesicherheit (AMTS) in Deutschland. [ www.akdae.de/AMTS/Aktionsplan/Aktionsplan-AMTS-2008-2009.pdf] 2007. (last accessed on 28 September 2015)
- 36.Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie (KNMP) Verminderde Nierfunctie; Doseringsadviezen Voor Geneesmiddelen. Den Haag: Geneesmiddel Informatie Centrum. www.knmp.nl/downloads/TabelbijVerminderdenierfunctie.Doseringsadviezenvoorgeneesmiddelen.pdf/view. (last accessed on 28 September 2015)
- e1.Todd A, Holmes HM. Recommendations to support deprescribing medications late in life. Int J Clin Pharm. 2015;37:678–681. doi: 10.1007/s11096-015-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Schwartz JB. Primary prevention: do the very elderly require a different approach? Trends Cardiovasc Med. 2015;25:228–239. doi: 10.1016/j.tcm.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness. JAMA Intern Med. 2015;175:691–700. doi: 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]