ABSTRACT
Understanding the structure of PrPSc and its strain variation has been one of the major challenges in prion disease biology. To study the strain-dependent conformations of PrPSc, we purified proteinase-resistant PrPSc (PrPRES) from mouse brains with three different murine-adapted scrapie strains (Chandler, 22L, and Me7) and systematically tested the accessibility of epitopes of a wide range of anti-PrP and anti-PrPSc specific antibodies by indirect enzyme-linked immunosorbent assay (ELISA). We found that epitopes of most anti-PrP antibodies were hidden in the folded structure of PrPRES, even though these epitopes are revealed with guanidine denaturation. However, reactivities to a PrPSc-specific conformational C-terminal antibody showed significant differences among the three different prion strains. Our results provide evidence for strain-dependent conformational variation near the C termini of molecules within PrPSc multimers.
IMPORTANCE It has long been apparent that prion strains can have different conformations near the N terminus of the PrPSc protease-resistant core. Here, we show that a C-terminal conformational PrPSc-specific antibody reacts differently to three murine-adapted scrapie strains. These results suggest, in turn, that conformational differences in the C terminus of PrPSc also contribute to the phenotypic distinction between prion strains.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are fatal neurodegenerative diseases resulting in the accumulation of the misfolded form of prion protein (PrP) in the brain (1). Prions are disease-causing infectious agents that lack agent-coding nucleic acids (1). The normal cellular glycoprotein PrP (PrPC), which is typically bound to a carboxyl-terminal glycosylphosphatidylinositol (GPI) anchor, can undergo major conformational changes into pathogenic disease-causing forms of PrP (PrPSc). This conversion is induced by the binding and templating effects of preexisting PrPSc (2). Relative to PrPC, PrPSc tends to be rich in β-sheets, detergent insoluble, oligomeric or fibrillar, and partially resistant to proteinase K (PK) digestion. PK treatment of PrPSc typically produces a PK-resistant carboxyl-terminal core referred to as PrPRES or PrP(27-30) (3, 4). Although there is increasing evidence that protease-sensitive forms of disease-associated PrP can also exist in the brains of humans and animals affected with prion diseases (5–8), the presence of PrPRES is a major diagnostic indicator of prion diseases. However, the detailed three-dimensional structures of PrPSc and its variants remain a mystery.
One approach to probing prion structures and studying prion pathogenesis has been the development of PrPSc- and/or PrPRES-selective antibodies (9–21). Despite some successes, the development of PrPSc-specific antibodies with diverse epitopes has been limited by the fact that PrPSc has the same primary sequence as PrPC. This requires PrPSc-specific epitopes to be conformational. However, such potentially distinctive epitopes are often hidden by PrPSc's tightly packed multimeric nature, as well as its heavy glycosylation and GPI anchoring (22, 23).
The existence of prion strains, classically defined by incubation times and neuropathologic profiles of vacuolation in a given host, is another prominent feature of prion diseases (24, 25). Strains have also been discriminated by variable characteristics of PrPSc, such as the glycoform ratio, fibril morphology (26, 27), β-sheet secondary structure (28–30), conformational stability (6, 31), and hydrogen-deuterium (H/D) exchange characteristics (32, 33). Further, in vitro conversion or amplification reactions have shown that the rate of PrPSc-seeded conversion of PrPC can vary from strain to strain (34–39). The existence of multiple prion strains within hosts of a given genotype implies that the phenotypic diversity of prions and PrPSc can be maintained without variations in the primary structure of the constituent PrP molecules. Conversely, it is also true that a single prion strain replicating in hosts of different genotypes can have different biological properties (40, 41). These observations provide evidence that PrPSc conformational diversity is important in defining prion strains.
It has long been evident that prion strains can have different conformations at the N terminus of the PrPSc protease-resistant core. Pioneering studies of hamster-adapted transmissible mink encephalopathy described distinct Hyper and Drowsy strains with fragments of different molecular weights after PK digestion of the TSE agents (42, 43). Types 1 and 2 human Creutzfeldt-Jacob disease (CJD) also have different proteinase-resistant PrPSc fragments, implying a difference in conformation (44, 45). In addition, these strain-specific conformations can be maintained during passages in transgenic mice (34, 44).
In this study, to gain further insight into PrPSc structure and strain-associated diversity, we systematically compared the exposure of epitopes on PrPRES from different murine prion strains using multiple PrP antibodies and indirect enzyme-linked immunosorbent assay (ELISA). Here, we show that a C-terminal conformation-dependent PrPSc antibody can bind differently to three different murine prion strains.
MATERIALS AND METHODS
Animals and rodent-adapted prion strains.
The mouse-adapted scrapie Chandler (also called RML) strain was derived from a “drowsy” goat with experimental scrapie SSBP/1 by subsequent passage in wild-type Rocky Mountain Laboratories (RML) mice (46). The 22L strain was derived from a pool of Cheviot sheep brains affected by SSBP/1 by passage in wild-type C57BL/10 mice (47). The Me7 strain came from a natural case of sheep scrapie in Suffolk sheep passaged in wild-type RIII mice (48). These prion strains were inoculated intracerebrally into wild-type RML mice or anchorless PrP-transgenic (GPIneg) mice (49) as indicated. The health of these rodents was monitored daily. Mice exhibiting neurological signs of TSE disease, as described in previous studies (49, 50), were euthanized according to protocols approved by the Institutional Animal Care and Use Committee (IACUC), and their brains were removed for use in PrPRES purifications. All animal experiments were conducted under an NIH IACUC-approved animal study protocol (numbers 2013-030 and 2010-45).
Purification of PrPRES.
Purification of wild-type PrPRES was previously described (51).
GPIneg PrPRES was purified as previously described (29). Briefly, about 3 g of scrapie-infected GPIneg mouse brains was homogenized in phosphate-buffered saline (PBS) in the presence of protease inhibitors (Roche complete protease inhibitor cocktail tablets). The brain homogenate was adjusted to contain 50 mM Tris, 137 mM NaCl, and 2% Sarkosyl (pH 8.0) and incubated for 20 min at room temperature. Nucleic acids in brain homogenates were digested with Benzonase (25 units/ml) for 30 min at 37°C. Samples were centrifuged at 3,000 × g for 25 min (Beckman 50.2 Ti rotor) to pellet PrPRES and other detergent-insoluble materials. The pellet was gently rinsed with Tris-buffered saline (TBS) (50 mM Tris, pH 8.0, at room temperature, 137 mM NaCl) with 0.2% Sarkosyl. The pellet (omitting the brownish portion) was resuspended in TBS with 0.2% Sarkosyl. The resuspended pellet was digested with PK at 10 μg/ml for 1 h at 37°C, and PK digestion was terminated by adding 0.1 M Pefabloc and incubating on ice for 10 min. PK was removed by the following steps: addition of EDTA (final concentration, 30 mM); incubation for 5 min at room temperature, followed by addition of NaCl (final concentration, 1.7 M); layering onto a sucrose cushion (1 M sucrose, 100 mM NaCl, 0.5% sulfobetaine 3-14, 10 mM Tris-HCl, pH 7.4); and centrifugation as described above through the sucrose cushion. The pellet was resuspended in 500 μl of 0.5% sulfobetaine 3-14 in PBS and centrifuged to wash the pellet. The final pellet was resuspended by sonicating in 500 μl of 0.5% sulfobetaine 3-14 in PBS.
Antibodies.
PrP-specific monoclonal antibodies (MAbs) 6C2 (final concentration in ELISA, 0.5 μg/ml) and 94B4 (0.5 μg/ml) were obtained from Central Veterinary Institute, Netherlands. Anti-PrP R1 (recombinant human Fab fragment antibody; 0.1 μg/ml) was a gift from Stanley Prusiner, University of California, San Francisco (52, 53). PrPSc-specific MAb 6H10 (0.8 μg/ml) (13) and PrP-specific MAb 132 (0.4 μg/ml for wild-type PrPRES; 1 μg/ml for GPIneg PrPRES) (54) were provided by Motohiro Horiuchi, Hokkaido University, Sapporo, Japan. Additional PrP MAbs 6D11 (0.02 μg/ml; Santa Cruz), 8G8 (0.5 μg/ml; Cayman), SAF-70 (0.2 μg/ml for wild-type PrPRES; 0.5 μg/ml for GPIneg PrPRES; SPIbio), SAF-84 (0.04 μg/ml; Cayman), and 8H4 (1.7 μg/ml; Abcam) were purchased. Supernatants of anti-PrP D13 or D18 (recombinant human Fab fragment antibody; 1/200 dilution) hybridoma cells were acquired from Bruce Chesebro, RML, NIH (52).
Indirect ELISA.
Ninety-six-well plates (MaxiSorp C bottom; Nunc) were coated with purified wild-type PrPRES in 20 mM phosphate buffer, pH 7.0, and left to dry overnight (∼16 h) at 37°C. The 96-well plate was coated with GPIneg PrPRES in bicarbonate-carbonate buffer (50 mM; pH 9.6). The next day, the plates were rinsed with TBS (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) five times and treated with 3 M or 8 M guanidine-hydrochloride (GdnHCl) in TN (10 mM Tris-HCl, 150 mM NaCl) buffer, pH 8.0, at room temperature or TN buffer alone (folded) for 1 h at 37°C (100 μl/well). After rinsing in TBS five times, the plates were blocked with 5% fetal bovine serum (FBS) or 5% nonfat milk in TBS with 0.05% Tween (TBST) (300 μl/well) for 2 h at room temperature. Folded (untreated) or GdnHCl-denatured PrPRES was incubated with anti-PrP antibodies (each anti-PrP antibody concentration is indicated in “Antibodies” above; 100 μl/well) for 1 h at room temperature, followed by incubation with goat anti-mouse IgG horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch; 0.2 μg/ml; 100 μl/well) for 1 h at room temperature. For MAbs D13, D18, and R1, goat anti-human IgG F(ab′)2 HRP-conjugated secondary antibody (0.2 μg/ml; Jackson ImmunoResearch) was used. The antigen-antibody complex was visualized with 3,3′,5,5′-tetramethylbenzidine (TMB) (KPL; 100 μl/well). TMB stop solution (KPL; 100 μl/well) was applied to stop the TMB reaction. The plates were read at 450 nm. The mean optical density measurements at 450 nm (OD450) of negative controls (in which primary antibody was omitted) were subtracted from each MAb OD450 value. The subtracted background value (OD450) for each antibody was as follows: ≤0.1 for D13, 6D11, 8G8, 6C2, and MAb 132 (see Fig. 2A to E); ≤0.17 for D18 (see Fig. 2F); ≤0.15 for SAF-70 (see Fig. 3A); ≤0.08 for SAF-84 (see Fig. 3B); ≤0.12 for 8H4 (see Fig. 3C); and ≤0.16 for 94B4 (see Fig. 3D). The results of ELISAs are expressed as averages of triplicate wells with standard deviations (SD) and graphed using GraphPad Prism 6.
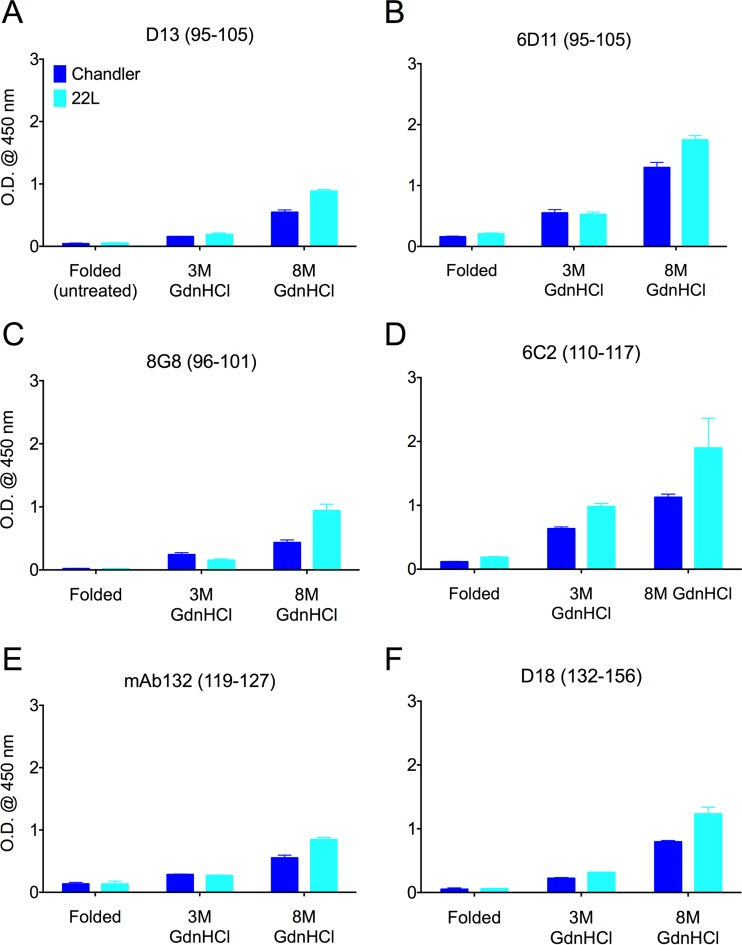
FIG 2.
Epitopes of PrP antibodies with more N-terminal linear epitopes were largely hidden in the folded conformation of PrPRES. Purified PrPRES from wild-type mice inoculated with the Chandler strain and with the 22L strain were used to examine the epitope accessibilities of the indicated more N-terminal PrP antibodies using indirect ELISA. The linear epitopes are shown in parentheses. Each of the epitopes was much less accessible in the folded conformation than after treatments with chaotropic 3 or 8 M GdnHCl solutions. OD450 readings with background subtracted are indicated as the means and SD from triplicate wells. Similar results were obtained in at least three independent experiments.
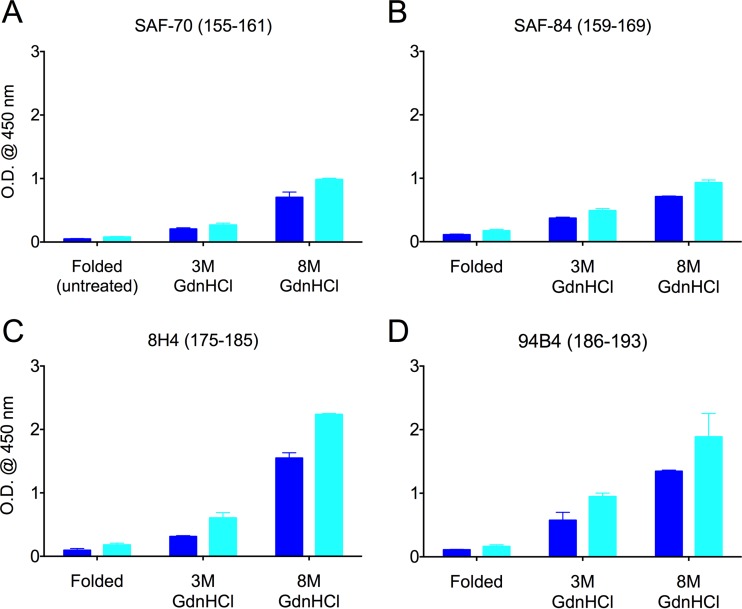
FIG 3.
Epitopes of PrP antibodies with linear epitopes near glycosylation sites were largely hidden in the folded conformation of PrPRES. Preparations of wild-type murine Chandler (dark blue) and 22L (light blue) PrPRES were either folded (untreated) or treated with 3 M or 8 M GdnHCl prior to indirect-ELISA measurements using the indicated primary anti-PrP antibodies, with epitopes shown in parentheses. OD450 readings with background subtracted are indicated as the means and SD from triplicate wells. Similar results were obtained in at least three independent experiments.
To load equivalent amounts of each strain of PrPRES into the ELISA wells and establish linearly responsive conditions for the ELISAs with the different antibodies, the procedure was as follows. Relative concentrations of PrPRES from each preparation were first estimated using semiquantitative immunoblotting to allow initial loading of a nominal 100 ng of PrPRES per well. The immunoblotting analysis was done by following a protocol described previously (29). Prior to making further dilutions of purified PrPRES, all PrPRES strains were sonicated for 10 to 15 s (repeated up to three times), ensuring the pellet was finely dispersed. Finer tuning of the relative amounts of each PrPRES strain bound to the wells was done to give approximately equivalent ELISA OD450 values using several different antibodies with linear PrP epitopes (MAb 132, 6C2, SAF-70, 8H4, and/or 94B4) after a denaturing treatment with 8 M GdnHCl. Then, with equivalent amounts of each strain of PrPRES loaded into each well, the final concentration of each anti-PrP antibody used was adjusted to obtain maximum OD450 values in the linearly responsive range, whether the maximum OD450 value for a particular antibody was achieved under folded (conformational epitopes) or GdnHCl-treated (linear epitopes) conditions.
Cloning of heavy and light chains of immunoglobulin genes.
Total RNA was extracted from hybridomas using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. The cDNA encoding variable regions of heavy (VH) and light (VL) chains of MAbs 6H10, 106, and 110 (55) were amplified by reverse transcription (RT)-PCR using degenerate primers reported previously (56). The PCR products were cloned into pCRII-TOPO (Invitrogen), and nucleotide sequences were determined using a BigDye Terminator v3.1 cycle-sequencing kit (Applied Biosystems) and ABI-3100 Avant sequencer (Applied Biosystems). After determination of the nucleotide sequences of the cDNAs of the variable regions, 5′ and 3′ cDNA fragments of heavy and light chains of each MAb were amplified by 5′ and 3′ rapid amplification of cDNA ends as previously described (57). Finally, the nucleotide sequences of the Fab regions of heavy and light chains were determined.
Statistical analysis.
Statistical significance was assessed using GraphPad Prism 6 with an unpaired Student t test and Partek Genomics Suite with a one-way analysis of variance (ANOVA) test for multiple comparison, followed by the false-discovery rate (FDR) (Benjamini-Hochberg) method (58). A significance level of 0.05 was used to generate the P values.
RESULTS
Epitopes of most PrP antibodies are hidden in folded conformations of PrPRES.
The epitopes of anti-PrP antibodies used in the study are summarized in Fig. 1. Ten antibodies with linear epitopes spanning residues 95 and 193 were poorly reactive to the folded conformations of the wild-type murine Chandler or 22L PrPRES. However, after chaotropic treatments with 3 or 8 M GdnHCl, these epitopes became more accessible for antibody binding with little evidence of strain-dependent differences (Fig. 2 and 3). This was true not only for antibodies with N-terminal epitopes (Fig. 2), but also for antibodies with epitopes located around the glycosylation sites at residues 180 and 196 (Fig. 3).
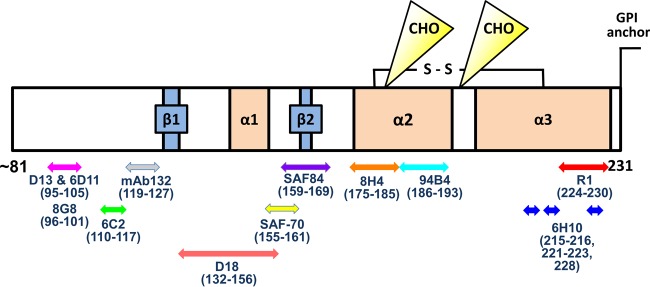
FIG 1.
Epitopes of anti-PrP antibodies used in this study. The schematic representation of PrP primary structure (mouse residues 81 to 231) shows epitopes of anti-PrP antibodies. CHO indicate the N-linked glycosylation sites at residues 180 and 196 in PrP. A disulfide bond is indicated by S-S. Two β-sheets (β1 and β2) and three α-helices (α1, α2, and α3) exist in PrPC; however, the secondary structure of PrPC is not maintained in PrPSc. The C-terminal region is recognized by the PrPSc-specific MAb 6H10 (215 to 216, 221 to 223, and 228 [dark-blue arrows]) in PrPSc and the human Fab anti-PrP antibody R1 (224 to 230 [red arrow]) in both PrPC and PrPSc.
Antibody specificity was verified by examining wells lacking PrPRES and/or the primary or secondary antibodies in every indirect ELISA. The PrPRES dependence of positive signals was also tested by comparisons to wells loaded with “mock PrPRES” preparations from uninfected (PrPRES-free) mouse brains and treated with both primary and secondary antibodies. The low signals (OD450 ≤ 0.2 [data not shown]) in all of the above-mentioned controls verified the specificity of the antibodies and their ability to detect PrPRES, or GdnHCl-treated forms thereof, in each experiment. As described in Materials and Methods, background OD450 readings of the absence of primary antibody were subtracted from OD450 readings of each MAb within the same strain.
Binding of the conformation-dependent C-terminal antibody 6H10 was PrPRES strain dependent.
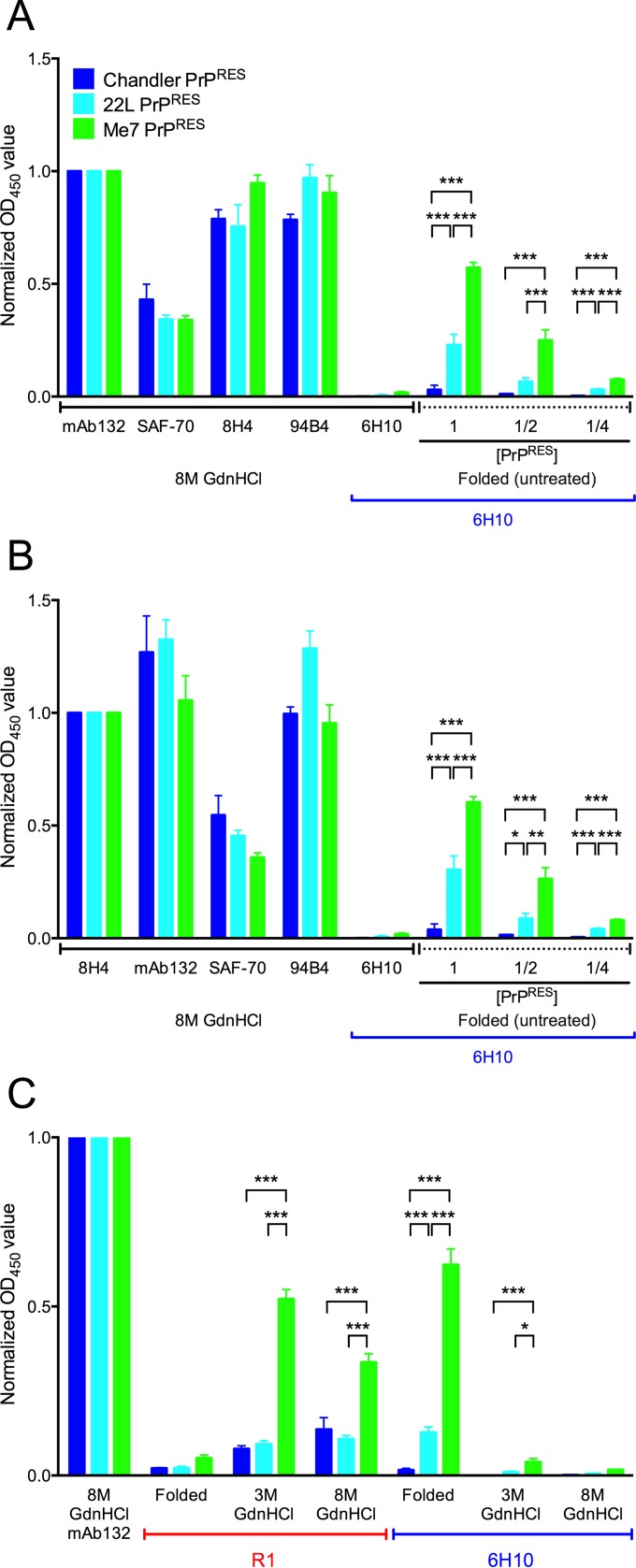
In contrast to the lack of reactivity of the different strains of folded (untreated) PrPRES to multiple antibodies with linear epitopes within residues 95 to 193 (Fig. 2 and 3), we observed strong reactivity of Me7 and 22L PrPRES to antibody 6H10, which has a more C-terminal conformational epitope (Fig. 4). This antibody was generated by immunizing Prnp0/0 mice with PrPSc purified from mice with the Obihiro prion strain (13). To better characterize the antibody, we cloned the cDNA fragments corresponding to the Fabs of heavy and light chains, determined their nucleotide sequences, and compared the deduced amino acid sequences with those of other anti-PrP MAbs (GenBank accession numbers: MAb 6H10 VH, LC110288, and VL, LC110289). Variations were observed in both complementarity-determining region 3 (CDR3) of the VH and CDR1 of the VL. The epitopes of 6H10 are discontinuous (mouse residues 215 to 216, 221 to 223, and 228), conformational, PK resistant, GdnHCl sensitive, and PrPSc/PrPRES specific (13). To compare the reactivities of different PrPRES strains to 6H10, nominally equivalent amounts of PrPRES from each strain were loaded into ELISA wells as described in Materials and Methods. Because the aggregated nature of folded PrPRES could complicate the uniform delivery of PrPRES to wells in each experiment, we also compared the 6H10 ELISA readings for each PrPRES strain with those from several other antibodies with linear epitopes after treatment with 8 M GdnHCl (Fig. 4). When the ELISA readings from the different strains were normalized based on either MAb 132 (Fig. 4A) or MAb 8H4 (Fig. 4B), the signals obtained from antibodies with three other linear epitopes were comparable, providing evidence of nearly equivalent PrPRES loading into the wells. In each case, reduced signals were obtained from wells loaded with smaller amounts of PrPRES, confirming that the 6H10 ELISA conditions were within a responsive range. As expected, 6H10 did not bind to the 8 M GdnHCl-treated wells loaded with Chandler, 22L, or Me7 PrPRES because its epitope is dependent on the folded PrPRES conformation (13). However, without GdnHCl treatment, 6H10 gave responses to folded ME7 and 22L PrPRES that were, respectively, 16- to 20-fold and 6- to 8-fold greater than the corresponding responses to Chandler PrPRES; these differences were apparent regardless of the PrPRES loading level or whether MAb 132 (Fig. 4A) or MAb 8H4 (Fig. 4B) was used for normalization (compare the nine rightmost bars of Fig. 4A and B). We chose these antibodies for normalization because their epitopes are far apart, and they performed similarly to the other MAbs with linear epitopes.
FIG 4.
The PrPSc-specific C-terminal antibody 6H10 recognized the abnormal conformations of Me7 and 22L, but not Chandler, PrPRES. The OD450 readings of MAbs 132, SAF-70, 8H4, and 94B4 after 8 M GdnHCl treatment showed that approximately equivalent amounts of PrPRES were loaded into each well in ELISA plates. (A) MAb 132 was used to normalize the OD450 reading. (B) The same data were normalized to MAb 8H4 readings. Twofold dilutions of PrPRES were used to show that the OD450 readings were in a responsive range (the nine bars on the right in panels A and B). (C) Because PrPRES was treated with PK and several possible PK cleavage sites are at the C terminus of PrP, the C-terminal linear-epitope antibody R1 was used to examine whether the C-terminal epitopes were still present in PrPRES. OD450 readings with background subtracted are indicated as the means and SD from triplicate wells. *, P < 0.05; **, P < 0.01; ***, P < 0.005; one-way ANOVA followed by the FDR (Benjamini-Hochberg) test. Similar results were obtained in at least three independent experiments.
To test whether the strain-dependent reactivity to the 6H10 antibody might be due to differences in PK digestion at the C terminus, another C-terminal antibody, R1, was used to test whether the residues comprising the epitope of 6H10 remained intact at the C terminus in all three strains of PrPRES. The epitope of the R1 antibody is linear and comprised of residues 224 to 230, which extend beyond the C terminus of the 6H10 epitope (Fig. 1). Although reactivity to R1 was weak in folded wild-type Me7, 22L, and Chandler, it increased with GdnHCl treatment (Fig. 4C). This indicated that the residues comprising the R1 epitope, and therefore the more N-terminal residues comprising the 6H10 epitope, remained intact to a detectable extent in each strain of PrPRES after PK treatment. Notably, despite differing severalfold in the binding of 6H10, the denatured 22L and Chandler PrPRES preparations showed equivalent binding to R1. This provided evidence that the difference in 6H10 reactivity between these strains of PrPRES is not solely due to differential PK cleavage. Having said that, greater R1 reactivity was observed with the Me7 PrPRES preparations than with 22L and Chandler (Fig. 4C), suggesting that a greater proportion of the R1 epitope residues were retained after PK treatment of folded Me7. In any case, because PK cleavage of folded PrPRES preparations is conformationally sensitive, like the binding of 6H10, the observed differences in both 6H10 binding and PK cleavage of the R1 epitope indicate strain-dependent differences in the PrPRES conformation near the C terminus.
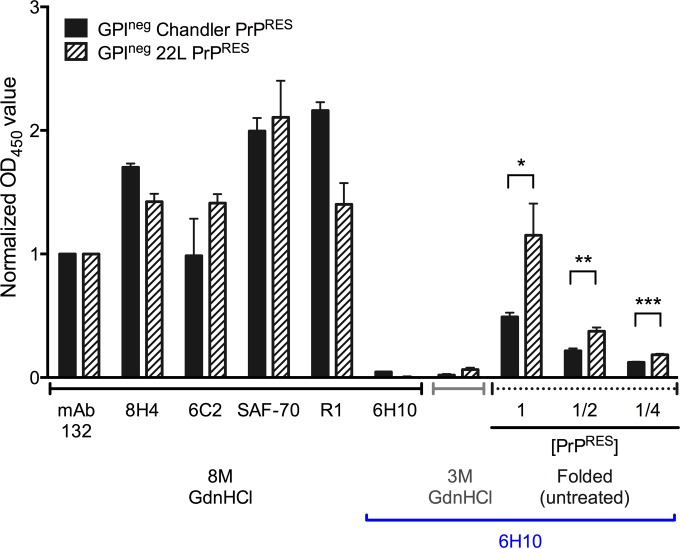
Antibody 6H10 binding differed between PrPRES strains lacking GPI anchors and N-linked glycans.
PrP is a GPI-anchored glycoprotein, and the GPI anchor and glycans may affect the C-terminal conformation of PrPRES. To determine whether the presence of the GPI anchor and N-linked glycans alters MAb 6H10 binding, we purified PrPRES that is inherently mostly unglycosylated from the brains of 22L- and Chandler-affected anchorless-PrP-transgenic (GPIneg) mice (29, 49). These preparations were then tested for MAb 6H10 binding. All normalized OD450 values for wild-type strains were less than 1 when normalized to MAb 132 (Fig. 4A and C), whereas all were between 1 and 2.3 in GPIneg strains, indicating more efficient binding to the latter. The GPIneg Chandler PrPRES had much higher 6H10 reactivity than its wild-type, GPI-anchored counterpart (compare Fig. 5 to Fig. 4). Nonetheless, the reactivity of 6H10 was still ∼2-fold lower with GPIneg Chandler PrPRES than with 22L GPIneg PrPRES, despite the fact that binding of MAbs SAF-70, 8H4, 6C2, 132, and R1 (with linear epitopes) showed that an equivalent or larger amount of the Chandler GPIneg PrPRES was available in the wells after GdnHCl treatment (Fig. 5). As expected, the 6H10 reactivity to both the Chandler and 22L GPIneg PrPRES was lost with GdnHCl treatment. Altogether, the observed differences between these two GPIneg PrPRES strains indicated that differential 6H10 reactivities cannot be attributed solely to differences in the GPI anchor or N-linked glycans, but are also due to C-proximal differences in polypeptide conformation.
FIG 5.
MAb 6H10 differed in its binding to the 22L and Chandler PrPRES lacking GPI anchors and N-linked glycosylations. Purified GPIneg Chandler and 22L PrPRES were tested for epitope exposure of 6H10 using indirect ELISA. The OD450 readings of MAbs 132, SAF-70, 8H4, and 94B4 after 8 M GdnHCl treatment showed that equivalent amounts of PrPRES were loaded in each well in the ELISA plates, and MAb 132 was used to normalize the OD450 readings. Twofold dilutions of PrPRES were used to show that the OD450 readings were in a responsive range. OD450 readings with background subtracted are indicated as the means and SD from triplicate wells. *, P < 0.05; **, P < 0.01; ***, P < 0.005; unpaired two-tailed Student's t test. Similar results were obtained in at least three independent experiments.
DISCUSSION
We have demonstrated that PrPRES preparations from three different mouse strains react differently to antibody 6H10, which recognizes a PrPSc-specific conformational epitope formed by C-proximal residues of molecules packed within infectious multimers. As noted above, the differential binding of 6H10 between the Me7, 22L, and Chandler PrPRES preparations appeared to be due to differences in the 6H10 epitopes themselves and/or the extent to which those epitopes were cleaved by PK, both of which reflect differences in conformation. Another possibility is that strain-dependent differences in PK cleavage might allow conformational rearrangements near the C terminus that affect 6H10 binding. These collective findings are consistent with previous indications from H/D exchange analyses coupled with mass spectrometry (HXMS) (32, 33). Smirnovas and colleagues performed HXMS with the Chandler, 22L, and Me7 GPIneg PrPRES and found that the polypeptide backbone proton H/D exchange rates within residues 217 to 223 were different between the 22L and Chandler GPIneg PrPRES preparations. This suggested that the 22L GPIneg PrPRES has a more extended β-sheet near the C terminus than the Chandler or Me7 strain of GPIneg PrPRES (32). Further, their results strongly suggest that the conformation of the C terminus of PrPRES is substantially different from that of PrPC, in contrast to previous suggestions that the C-terminal α-helices 2 and 3 from PrPC remained intact in PrPRES (59–61). More recent HXMS studies with two distinct strains of human sporadic Creutzfeldt-Jakob disease (sCJD) have shown differing conformations within the near-C-terminal residues 218 to 224 (33). Thus, the previous HXMS studies (32, 33) and our present C-terminal conformation-dependent antibody analyses provide complementary lines of evidence of C-terminal conformational differences between prion strains.
In our experiments, purified PrPRES was immobilized on an ELISA plate by passive adsorption prior to epitope mapping. Therefore, we needed to consider the possibility that different conditions or loading levels might affect the matrix of the PrPRES on the plate, potentially altering exposure of the conformational epitopes. To minimize this issue, each strain of purified PrPRES was sonicated prior to coating in order to promote an even distribution in each well, and the amounts of PrPRES in each well were compared by the ELISA OD450 reading using several anti-PrP antibodies with linear epitopes after epitope exposure by treatment with 8 M GdnHCl. We also tested several different concentrations of PrPRES with two coating-buffer conditions at pH 7.0 and 9.6. Under each condition, MAb 6H10 was able to distinguish the wild-type 22L from Chandler PrPRES. With GPIneg PrPRES, we found that optimal binding required more basic conditions to maximize binding to the wells (data not shown). Further, we established the reproducibility of the data using 2 to 4 separate preparations of purified PrPRES of each strain. Additionally, PrPRES prepared several years ago showed results similar to those obtained with newly purified PrPRES. Therefore, the PrPRES strain-dependent binding of MAb 6H10 was a consistent observation.
The absence of the GPI anchor and N-linked glycans did not eliminate the differential binding of MAb 6H10 to the 22L and Chandler strains, even though enhanced reactivity to the Chandler PrPRES was observed with the anchorless PrPRES (Fig. 5). Fourier transform infrared spectroscopy (FTIR) studies have provided evidence that the polypeptide backbone conformations of the wild-type and anchorless forms of 22L PrPRES are similar to one another (29). The analogous comparisons of wild-type and GPIneg forms of Chandler PrPRES have not been done, but the enhanced recognition of the GPIneg form by the 6H10 antibody suggests at least one of these posttranslational modifications partially obscures or alters the epitope within this strain.
A full understanding of the 6H10 epitope and its variations between strains will require knowledge of the still unknown three-dimensional structures of PrPSc. Although the conformation of PrPSc remains enigmatic, considerable evidence indicates that synthetic amyloid fibrils of PrP that are produced under physiologically compatible conditions in vitro, with (62, 63) or without (64, 65) templating with PrPSc, readily adopt a parallel in-register intermolecular β-sheet (PIRIBS) architecture near the C terminus in the region originally comprising α-helices 2 and 3 of PrPC. Based on these findings, and numerous other empirical constraints, PIRIBS models of PrPSc structure have been built in silico (62). In these hypothetical models, the natural disulfide bond between residues 179 and 214 establishes a hairpin, but the status of more C-terminal residues (215 to 231) is more difficult to predict. Hence, within the confines of the proposed PIRIBS architectures at least, multiple permutations of the C-terminal residues can be envisioned, such as those depicted previously (compare PIRIBS A and B in the study by Groveman et al. [62]). In one case, for example, for Chandler PrPRES, the C terminus may be extended linearly so that the three-dimensional arrangement of the components of the 6H10 epitope is suboptimal for 6H10 binding. In other cases, perhaps for Me7 and 22L PrPRES, there might be folds that bring components of the 6H10 epitope together in space to form conformations to which 6H10 binds more avidly. Therefore, it is tempting to speculate that such conformational differences, or permutations thereof, may distinguish Chandler, Me7, and 22L PrPRES in a manner that affects the exposure and relative orientation of the components of the conformational epitopes of antibody 6H10.
ACKNOWLEDGMENTS
We thank Roger Moore, James Carroll, and Bradley Groveman for providing critical reviews of the manuscript. We thank Stanley Prusiner and Joel Watts for kindly providing antibody R1 and Bruce Chesebro for providing antibodies D13 and D18.
This work was supported by a Japan Society for the Promotion of Science (JSPS) Fellowship for Japanese Biomedical and Behavioral Researchers at NIH to E.S., the Intramural Research Program of the NIAID, the Program for Leading Graduate Schools (F01) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a grant for TSE research (H26-Shokuhin-Ippan-003) from the Ministry of Health, Labor and Welfare of Japan to M.H.
Funding Statement
The Intramural Research Program of the NIAID provided the NIH funding.
REFERENCES
- 1.Prusiner SB. 1998. Prions. Proc Natl Acad Sci U S A 95:13363-13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caughey B. 2001. Interactions between prion protein isoforms: the kiss of death? Trends Biochem Sci 26:235–242. doi: 10.1016/S0968-0004(01)01792-3. [DOI] [PubMed] [Google Scholar]
- 3.Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. 1991. Secondary structure analysis of the scrapie-associated protein PrP 27-30 in water by infrared spectroscopy. Biochemistry 30:7672–7680. doi: 10.1021/bi00245a003. [DOI] [PubMed] [Google Scholar]
- 4.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, Caughey B. 2005. The most infectious prion protein particles. Nature 437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasmezas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D. 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 6.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. 1998. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 7.Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G, Gabizon R, Taraboulos A. 2002. Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41:12868–12875. doi: 10.1021/bi025958g. [DOI] [PubMed] [Google Scholar]
- 8.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, Requena JR. 2006. Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochemistry 45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]
- 9.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. 1997. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 10.Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, Francoeur GP, Papadopoulos M, Haghighat A, Spatz SJ, Head M, Will R, Ironside J, O'Rourke K, Tonelli Q, Ledebur HC, Chakrabartty A, Cashman NR. 2003. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 9:893–899. doi: 10.1038/nm883. [DOI] [PubMed] [Google Scholar]
- 11.Curin Serbec V, Bresjanac M, Popovic M, Pretnar Hartman K, Galvani V, Rupreht R, Cernilec M, Vranac T, Hafner I, Jerala R. 2004. Monoclonal antibody against a peptide of human prion protein discriminates between Creutzfeldt-Jacob's disease-affected and normal brain tissue. J Biol Chem 279:3694–3698. [DOI] [PubMed] [Google Scholar]
- 12.Petsch B, Muller-Schiffmann A, Lehle A, Zirdum E, Prikulis I, Kuhn F, Raeber AJ, Ironside JW, Korth C, Stitz L. 2011. Biological effects and use of PrPSc- and PrP-specific antibodies generated by immunization with purified full-length native mouse prions. J Virol 85:4538–4546. doi: 10.1128/JVI.02467-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horiuchi M, Karino A, Furuoka H, Ishiguro N, Kimura K, Shinagawa M. 2009. Generation of monoclonal antibody that distinguishes PrPSc from PrPC and neutralizes prion infectivity. Virology 394:200–207. doi: 10.1016/j.virol.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Masujin K, Kaku-Ushiki Y, Miwa R, Okada H, Shimizu Y, Kasai K, Matsuura Y, Yokoyama T. 2013. The N-terminal sequence of prion protein consists an epitope specific to the abnormal isoform of prion protein (PrP(Sc)). PLoS One 8:e58013. doi: 10.1371/journal.pone.0058013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ushiki-Kaku Y, Endo R, Iwamaru Y, Shimizu Y, Imamura M, Masujin K, Yamamoto T, Hattori S, Itohara S, Irie S, Yokoyama T. 2010. Tracing conformational transition of abnormal prion proteins during interspecies transmission by using novel antibodies. J Biol Chem 285:11931–11936. doi: 10.1074/jbc.M109.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones M, Wight D, McLoughlin V, Norrby K, Ironside JW, Connolly JG, Farquhar CF, MacGregor IR, Head MW. 2009. An antibody to the aggregated synthetic prion protein peptide (PrP106-126) selectively recognizes disease-associated prion protein (PrP) from human brain specimens. Brain Pathol 19:293–302. doi: 10.1111/j.1750-3639.2008.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou WQ, Zheng J, Gray DM, Gambetti P, Chen SG. 2004. Antibody to DNA detects scrapie but not normal prion protein. Proc Natl Acad Sci U S A 101:1380–1385. doi: 10.1073/pnas.0307825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moroncini G, Kanu N, Solforosi L, Abalos G, Telling GC, Head M, Ironside J, Brockes JP, Burton DR, Williamson RA. 2004. Motif-grafted antibodies containing the replicative interface of cellular PrP are specific for PrPSc. Proc Natl Acad Sci U S A 101:10404–10409. doi: 10.1073/pnas.0403522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moroncini G, Mangieri M, Morbin M, Mazzoleni G, Ghetti B, Gabrielli A, Williamson RA, Giaccone G, Tagliavini F. 2006. Pathologic prion protein is specifically recognized in situ by a novel PrP conformational antibody. Neurobiol Dis 23:717–724. doi: 10.1016/j.nbd.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Solforosi L, Bellon A, Schaller M, Cruite JT, Abalos GC, Williamson RA. 2007. Toward molecular dissection of PrPC-PrPSc interactions. J Biol Chem 282:7465–7471. [DOI] [PubMed] [Google Scholar]
- 21.Tayebi M, Jones DR, Taylor WA, Stileman BF, Chapman C, Zhao D, David M. 2011. PrP(Sc)-specific antibodies with the ability to immunodetect prion oligomers. PLoS One 6:e19998. doi: 10.1371/journal.pone.0019998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biasini E, Seegulam ME, Patti BN, Solforosi L, Medrano AZ, Christensen HM, Senatore A, Chiesa R, Williamson RA, Harris DA. 2008. Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J Neurochem 105:2190–2204. doi: 10.1111/j.1471-4159.2008.05306.x. [DOI] [PubMed] [Google Scholar]
- 23.Peretz D, Williamson RA, Matsunaga Y, Serban H, Pinilla C, Bastidas RB, Rozenshteyn R, James TL, Houghten RA, Cohen FE, Prusiner SB, Burton DR. 1997. A conformational transition at the N terminus of the prion protein features in formation of the scrapie isoform. J Mol Biol 273:614–622. doi: 10.1006/jmbi.1997.1328. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson AG, Stamp JT, Renwick CC, Rennie JC. 1968. Some factors controlling the incidence of scrapie in Cheviot sheep injected with a Cheviot-passaged scrapie agent. J Comp Pathol 78:313–321. doi: 10.1016/0021-9975(68)90007-8. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson AG, Outram GW. 1973. Differences in access into the central nervous system of ME7 scrapie agent from two strains of mice. J Comp Pathol 83:13–18. doi: 10.1016/0021-9975(73)90022-4. [DOI] [PubMed] [Google Scholar]
- 26.Kascsak RJ, Rubenstein R, Merz PA, Carp RI, Robakis NK, Wisniewski HM, Diringer H. 1986. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J Virol 59:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sim VL, Caughey B. 2009. Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol Aging 30:2031–2042. doi: 10.1016/j.neurobiolaging.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Caughey B, Raymond GJ, Bessen RA. 1998. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J Biol Chem 273:32230–32235. doi: 10.1074/jbc.273.48.32230. [DOI] [PubMed] [Google Scholar]
- 29.Baron GS, Hughson AG, Raymond GJ, Offerdahl DK, Barton KA, Raymond LD, Dorward DW, Caughey B. 2011. Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry 50:4479–4490. doi: 10.1021/bi2003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomzig A, Spassov S, Friedrich M, Naumann D, Beekes M. 2004. Discriminating scrapie and bovine spongiform encephalopathy isolates by infrared spectroscopy of pathological prion protein. J Biol Chem 279:33847–33854. doi: 10.1074/jbc.M403730200. [DOI] [PubMed] [Google Scholar]
- 31.Peretz D, Scott MR, Groth D, Williamson RA, Burton DR, Cohen FE, Prusiner SB. 2001. Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10:854–863. doi: 10.1110/ps.39201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, Surewicz WK. 2011. Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 18:504–506. doi: 10.1038/nsmb.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safar JG, Xiao X, Kabir ME, Chen S, Kim C, Haldiman T, Cohen Y, Chen W, Cohen ML, Surewicz WK. 2015. Structural determinants of phenotypic diversity and replication rate of human prions. PLoS Pathog 11:e1004832. doi: 10.1371/journal.ppat.1004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bessen RA, Kocisko DA, Raymond GJ, Nandan S, Lansbury PT, Caughey B. 1995. Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature 375:698–700. doi: 10.1038/375698a0. [DOI] [PubMed] [Google Scholar]
- 35.Saijo E, Kang HE, Bian J, Bowling KG, Browning S, Kim S, Hunter N, Telling GC. 2013. Epigenetic dominance of prion conformers. PLoS Pathog 9:e1003692. doi: 10.1371/journal.ppat.1003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Montalban N, Lee YJ, Makarava N, Savtchenko R, Baskakov IV. 2013. Changes in prion replication environment cause prion strain mutation. FASEB J 27:3702–3710. doi: 10.1096/fj.13-230466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orru CD, Favole A, Corona C, Mazza M, Manca M, Groveman BR, Hughson AG, Acutis PL, Caramelli M, Zanusso G, Casalone C, Caughey B. 2015. Detection and discrimination of classical and atypical L-type bovine spongiform encephalopathy by real-time quaking-induced conversion. J Clin Microbiol 53:1115–1120. doi: 10.1128/JCM.02906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orru CD, Groveman BR, Raymond LD, Hughson AG, Nonno R, Zou W, Ghetti B, Gambetti P, Caughey B. 2015. Correction: bank vole prion protein as an apparently universal substrate for RT-QuIC-based detection and discrimination of prion strains. PLoS Pathog 11:e1005117. doi: 10.1371/journal.ppat.1005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C, Haldiman T, Surewicz K, Cohen Y, Chen W, Blevins J, Sy MS, Cohen M, Kong Q, Telling GC, Surewicz WK, Safar JG. 2012. Small protease sensitive oligomers of PrPSc in distinct human prions determine conversion rate of PrP(C). PLoS Pathog 8:e1002835. doi: 10.1371/journal.ppat.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickinson AG, Meikle VM. 1971. Host-genotype and agent effects in scrapie incubation: change in allelic interaction with different strains of agent. Mol Gen Genet 112:73–79. doi: 10.1007/BF00266934. [DOI] [PubMed] [Google Scholar]
- 41.Crowell J, Hughson A, Caughey B, Bessen RA. 2015. Host determinants of prion strain diversity independent of prion protein genotype. J Virol 89:10427–10441. doi: 10.1128/JVI.01586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessen RA, Marsh RF. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66:2096–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bessen RA, Marsh RF. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 73:329–334. doi: 10.1099/0022-1317-73-2-329. [DOI] [PubMed] [Google Scholar]
- 44.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 45.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. 1996. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 46.Kimberlin RH, Walker CA, Fraser H. 1989. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol 70:2017–2025. doi: 10.1099/0022-1317-70-8-2017. [DOI] [PubMed] [Google Scholar]
- 47.Dickinson AG. 1976. Scrapie in sheep and goats. Front Biol 44:209–241. [PubMed] [Google Scholar]
- 48.Zlotnik I, Rennie JC. 1963. Further observations on the experimental transmission of scrapie from sheep and goats to laboratory mice. J Comp Pathol 73:150–162. doi: 10.1016/S0368-1742(63)80018-1. [DOI] [PubMed] [Google Scholar]
- 49.Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 50.Raymond GJ, Race B, Hollister JR, Offerdahl DK, Moore RA, Kodali R, Raymond LD, Hughson AG, Rosenke R, Long D, Dorward DW, Baron GS. 2012. Isolation of novel synthetic prion strains by amplification in transgenic mice coexpressing wild-type and anchorless prion proteins. J Virol 86:11763–11778. doi: 10.1128/JVI.01353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raymond G, Chabry J. 2004. Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue, p 16–26. In Lehmann S, Grassi J (ed), Techniques in prion research. Birkhäuser Basel, Switzerland. doi: 10.1007/978-3-0348-7949-1_3. [DOI] [Google Scholar]
- 52.Williamson RA, Peretz D, Pinilla C, Ball H, Bastidas RB, Rozenshteyn R, Houghten RA, Prusiner SB, Burton DR. 1998. Mapping the prion protein using recombinant antibodies. J Virol 72:9413–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Supattapone S, Muramoto T, Legname G, Mehlhorn I, Cohen FE, DeArmond SJ, Prusiner SB, Scott MR. 2001. Identification of two prion protein regions that modify scrapie incubation time. J Virol 75:1408–1413. doi: 10.1128/JVI.75.3.1408-1413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shindoh R, Kim CL, Song CH, Hasebe R, Horiuchi M. 2009. The region approximately between amino acids 81 and 137 of proteinase K-resistant PrPSc is critical for the infectivity of the Chandler prion strain. J Virol 83:3852–3860. doi: 10.1128/JVI.01740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim CL, Umetani A, Matsui T, Ishiguro N, Shinagawa M, Horiuchi M. 2004. Antigenic characterization of an abnormal isoform of prion protein using a new diverse panel of monoclonal antibodies. Virology 320:40–51. doi: 10.1016/j.virol.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Kettleborough CA, Saldanha J, Ansell KH, Bendig MM. 1993. Optimization of primers for cloning libraries of mouse immunoglobulin genes using the polymerase chain reaction. Eur J Immunol 23:206–211. doi: 10.1002/eji.1830230132. [DOI] [PubMed] [Google Scholar]
- 57.Horiuchi M, Ishiguro N, Nagasawa H, Toyoda Y, Shinagawa M. 1997. Alternative usage of exon 1 of bovine PrP mRNA. Biochem Biophys Res Commun 233:650–654. doi: 10.1006/bbrc.1997.6511. [DOI] [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300. [Google Scholar]
- 59.DeMarco ML, Daggett V. 2004. From conversion to aggregation: protofibril formation of the prion protein. Proc Natl Acad Sci U S A 101:2293–2298. doi: 10.1073/pnas.0307178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hafner-Bratkovic I, Bester R, Pristovsek P, Gaedtke L, Veranic P, Gaspersic J, Mancek-Keber M, Avbelj M, Polymenidou M, Julius C, Aguzzi A, Vorberg I, Jerala R. 2011. Globular domain of the prion protein needs to be unlocked by domain swapping to support prion protein conversion. J Biol Chem 286:12149–12156. doi: 10.1074/jbc.M110.213926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Govaerts C, Wille H, Prusiner SB, Cohen FE. 2004. Evidence for assembly of prions with left-handed beta-helices into trimers. Proc Natl Acad Sci U S A 101:8342–8347. doi: 10.1073/pnas.0402254101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groveman BR, Dolan MA, Taubner LM, Kraus A, Wickner RB, Caughey B. 2014. Parallel in-register intermolecular beta-sheet architectures for prion-seeded prion protein (PrP) amyloids. J Biol Chem 289:24129–24142. doi: 10.1074/jbc.M114.578344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Groveman BR, Kraus A, Raymond LD, Dolan MA, Anson KJ, Dorward DW, Caughey B. 2015. Charge neutralization of the central lysine cluster in prion protein (PrP) promotes PrP(Sc)-like folding of recombinant PrP amyloids. J Biol Chem 290:1119–1128. doi: 10.1074/jbc.M114.619627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK. 2007. Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proc Natl Acad Sci U S A 104:18946–18951. doi: 10.1073/pnas.0706522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tycko R. 2011. Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem 62:279–299. doi: 10.1146/annurev-physchem-032210-103539. [DOI] [PMC free article] [PubMed] [Google Scholar]