Introduction
Heterotrimeric G protein alpha subunits (Gα), recognized first as regulatory GTPases activated by β adrenergic receptors and rhodopsin, were discovered over forty years ago1–6. Within ten years the archetypal members of the family – Gαs, Gαi and transducin (Gαt) had been purified and enzymatically characterized7–12. More than twenty years ago, the first three-dimensional structures of Gα subunits were described in GTP, GDP and heterotrimeric states13–20. Yet, only recently, with the advent of the crystal structure of the β2-adrenergic receptor:Gs complex21 have we begun to clearly understand how G protein-coupled receptors release GDP from the nucleotide binding site from Gα, leading to its activation.
Among guanine nucleotide binding proteins of the Ras superfamily, heterotrimeric G protein alpha subunits (Gα) constitute a distinct group22. Gα are unique with respect to their tertiary and quaternary structure, mechanisms of activation and signal transduction and in their kinetic properties. Like all members of the Ras superfamily, Gα subunits are composed of a six-stranded parallel β core in which most successive strands are connected by α helices (Figure 1). The guanine nucleotide binding sites of these proteins are similar in structure and, to a lesser extent, in amino acid sequence to those of other members of the Ras superfamily22, 23. Thus, the nucleotide binding sites of Gα proteins are characterized by a guanine recognition motif, a P-loop that envelops the α and β phosphates of GTP and two dynamic structural elements called switch I and switch II that respond to the presence or absence of the GTP γ phosphate (Figure 2, Table 1). Two residues, a serine in the P-loop and a threonine in switch II coordinate Mg2+, which bridges the β and γ phosphates of GTP. In contrast to small G proteins of the Ras family, Mg2+ binds with nanomolar affinity to GTP-bound forms of Gα - it is present in all such complexes described in this review - but only weakly, with affinity in the millimolar range, to the GDP state10, 24. Both switch elements contain catalytic residues that participate in the mechanism of GTP hydrolysis. The switches themselves undergo conformational changes upon conversion of GTP to GDP. Unique to Gα subunits is the insertion, within switch I, of a ~120 residue α-helical domain, and the presence of additional switch regions (III and IV) that participate in effector/regulator binding or show state-dependent conformations25. The helical domain plays a regulatory role in the retention of guanine nucleotide, and contributes to Gα class-specific recognition of effectors and regulators. Roles of the helical domain in the regulatory and catalytic functions of Gα subunits continue to be discovered26.
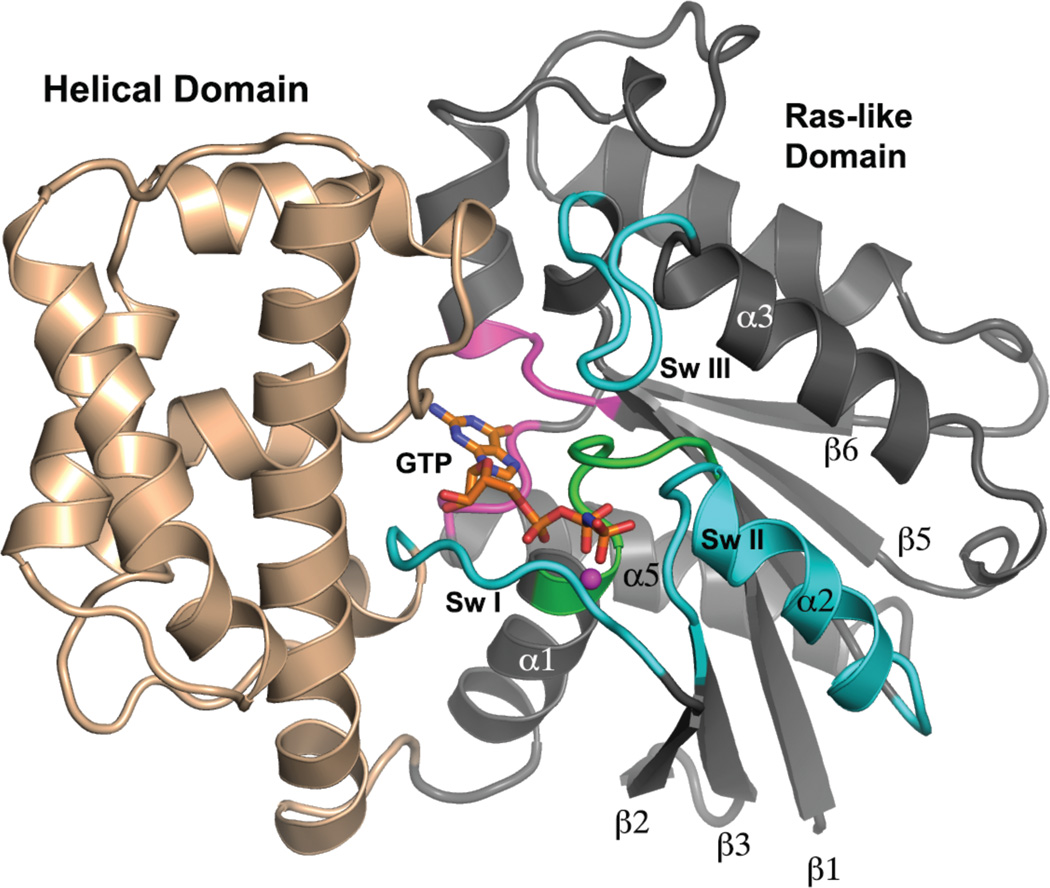
Figure 1.
Tertiary structure of Gα. A model of a Gα subunit bound to GTP and Mg2+ is depicted as a ribbon drawing and is based on the crystal structure of Gαi1•GppNHp (PDB 1CIP). The N-terminal 31, and C-terminal 7 amino acid residues are disordered in this structure, and adopt a variety of conformations in several crystal structures, depending on crystal contacts and binding partners. The Helical domain is colored light brown and the Ras-like domain is rendered in gray. Switch segments involved in effector recognition and GTPase activity are labeled and colored cyan. The P-loop is colored green, and loop regions involved in recognition and binding of the guanine moiety of GDP and GDP are colored pink. GppNHp is shown as a stick figure, and the Mg2+ is represented by magenta sphere. Selected secondary structure elements in the Ras domain are labeled.
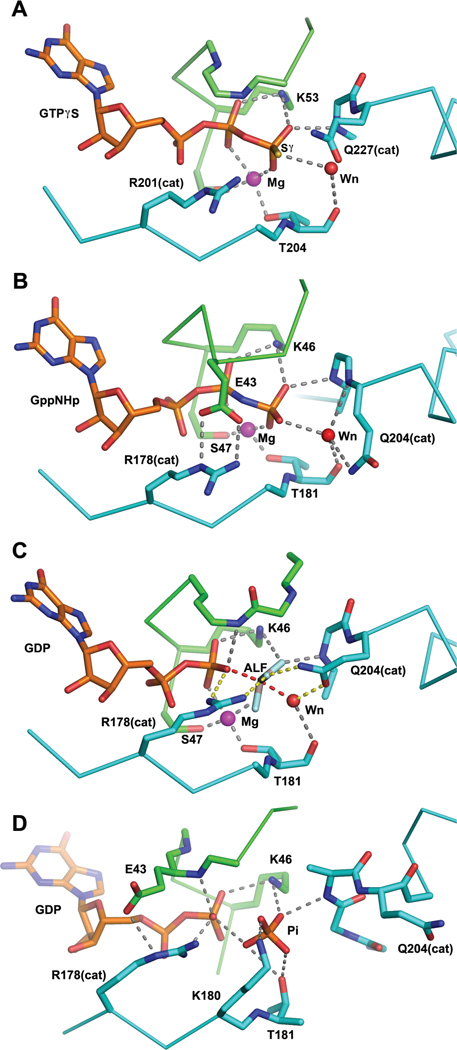
Figure 2.
Snapshots of the Gα catalytic site along the trajectory of GTP hydrolysis, derived from crystal structures. The coloring scheme is the same as that used in Figure 1. Nitrogen, oxygen and phosphorus atoms are colored blue, red and yellow respectively. Sulfer atoms are colored yellow. The magenta and red spheres represent magnesium ion and the water nucleophile, respectively. Residues of interest are labeled. The catalytic Gln and Arg residues are indicated with appropriate residue numbers and “cat” in parentheses. Hydrogen bonds (2.7–3.1Å) and metal-ligand coordination bonds (1.9–2.2Å) are shown as gray dashed lines. A, the structure of Gαs bound to GTPγS and Mg2+ (PDB 1AZT, 2.3Å resolution). Note that neither of the catalytic residues Gln 227 nor Arg 201 form direct contacts with the nucleotide; B, the complex of Gαi1 with GppNHp and Mg2+ (PDB 1CIP, 1.5Å resolution). Here Arg 178 (Argcat) is restrained in a hydrogen—bonded ionic interaction with the P-loop Glu 43. Gln 204 (Glncat) is a hydrogen bond donor to the water nucleophile, thereby orienting its lone pair electrons away from the γ phosphorus. This apparently stable ground-state conformation is expected to be anti-catalytic; C, Gαi1 bound to GDP, Mg2+ and AlF4− (labeled ALF), a model of the pre-organized or pre-transition state (PDB 1GFI, 2.2Å resolution; the AlF4 moiety was not rigidly restrained to planarity during refinement). Argcat is within hydrogen bonding distance of the leaving group β-γ bridge oxygen and Glncat is a hydrogen bond donor to a fluorine (or O−) Al substituent and accepts a hydrogen bond from the presumptive water nucleophile. The hydrogen bond network (yellow dashed lines) involving Argcat, Glncat, Wnuc and the the γ phosphate (modeled by AlF) orient Wnuc for nucleophilic attack and stabilize developing charge at the β-γ bridge leaving group oxygen (note also hydrogen bond to the latter from a P-loop amide, present also in GTP analog-bound structures); D, a model of the GDP, Pi ternary complex of Gα from the crystal structure of the G203A mutant of Gαi1 (PDB 1GIT, 2.6Å resolution). Note that switch II has reoriented and is refolded into an α helix at its N-terminus, forming an electropositive binding site for Pi. Both the β phosphate of GDP and Pi are retained in the catalytic site with multiple hydrogen bonds. The Mg2+ binding site is dismantled due to conformational changes in switches I and II.
Table 1.
Catalytic Residue numbers in G proteins
| Protein | P-loop | switch I * | switch II * |
|---|---|---|---|
| Gαs | 47 GAGESGKS | 201 RVLT | 225 GGQ |
| Gαi1 | 40 GAGESGKS | 178 RVKT | 202 GGQ |
| Gαt | 36 GAGESGKS | 174 RVKT | 198 GGQ |
| Gαz | 40 GTSNSGKS | 179 RVKT | 204 GGQ |
| Gαq | 46 GTGESGKS | 183 RVRT | 207 GGQ |
| Gα13 | 55 GAGESGKS | 200 RRPT | 224 GGQ |
| H-Ras | 10 GAGGVGKS | 32 YPDT | 59 AGQ |
Arg(cat) and Gln(cat) shown in italics and underlined
Gα regulatory activity is tightly integrated with that of heterodimers formed by G protein beta and gamma subunits (Gβγ). The canonical, non-signaling state for both Gα and Gβγ exists in the form of a heterotrimer composed of GDP-bound Gα and Gβγ. As for Ras GTPases, Gα is “activated” for interaction with effectors when bound to GTP, and deactivated by its intrinsic GTPase activity – which, for most Gα proteins, is accelerated by GTPase activating proteins or protein domains (GAPs). Heterotrimeric G proteins are directly activated by integral membrane proteins (G protein-Coupled Receptors: GPCRs) that are stimulated by extracellular agonists27, 28. Cells that express heterotrimeric G proteins thereby monitor external stimuli to direct their metabolic, secretory and transcriptional programs, regulate electrical conductivity and control cellular motility. To a first approximation, the effector specificity and amino acid sequence identity of Gα subunits segregates the family into four distinct classes: s (activation of adenylyl cyclases), i (inhibition of certain adenylyl cyclase isoforms), q/11 (phospholipase β activation) and 12/13 (activation and plasma membrane localization of Rho guanine nucleotide exchange factors (GEFs). The catalog of effectors listed above exemplifies Gα class specificity but is by no means exhaustive. Gβγ heterodimers have their own regulatory targets (e.g., G protein-regulated inward rectifying potassium channels) and in some instances are co-regulators of Gα effectors (e.g., certain isoforms of adenylyl cyclase isoforms and phospholipase β)29–31. Most Gα subunits are reversibly localized at the membrane by palmitoylation at residues near their N-termini, and members of the Gαi subfamily are also N-terminally myristoylated32, 33. Myristoylation increases Gαi1 affinity for adenylyl cyclase, Gβγ subunits34 and the cytosolic GEF/chaperone Ric-8A35, rather than necessarily promoting membrane interaction. Hydrogen-deuterium exchange experiments indicate myristoylation alters secondary structure dynamics of Gαi136.
In this review I am generally concerned with activated, GTP bound Gα subunits: how the energy of GTP binding is utilized, and how its hydrolysis alters the regulatory capacity of Gα. In particular, I focus on the mechanism of Gα− catalyzed GTP hydrolysis, and the means by which the slow intrinsic GTPase activity of Gα is accelerated by GAPs. The forgoing introduction provides only a minimal foundation for our discussion of the role GTP binding and the mechanism of its hydrolysis. While no single review encompasses the complexity of G protein signaling, several provide starting points for more in-depth explorations22, 25, 37–41.
Gα•GTP in effector activation
The energy of GTP binding is used to prevent Gα from interacting with Gβγ in a way that would prevent either species from expressing its regulatory functions. However, GTP does not in all cases cause full dissociation of Gα from Gβγ42, 43. GTP also stabilizes Gα for optimal interaction with effectors. Crystal structures reveal a variety of Gα-effector binding interfaces44–47,48, 49. Central to all, however, is a binding scaffold composed of switch II, an irregular helix, and α3 (Figure 3). Parallel to each other and separated by 12–14Å, the two helices form a spacious groove into which structural elements of the effector penetrate. The stability of this binding surface depends on the conformation of switch II, an inherently dynamic structure, which is disordered in the crystal structures of GDP-bound states of Gαi17 and Gα1250. Yet even in the GDP-bound complex of Gα, switch II can support a productive interaction with effectors. A catalytic domain construct of adenylyl cyclase is weakly activated by Gαs•GDP51, and PDZRhoGEF forms a stable complex with Gα13•GDP, albeit with lower affinity than with Gα13 bound to the slowly hydrolyzing GTP analog, guanosine-5’-O-3-thiophosphate (GTPγS)52. However, in the GTP-bound state, Switch II becomes more rigid, as is evident from several crystal structures15, 16, 53. Even in the GTP-bound state, switch II is a dynamic structure: electron spin resonance studies of Gαi1 harboring a spin-label near the middle of switch II show that it exhibits fast anisotropic motion in solution54. Nevertheless, electrostatic and hydrogen bonding interactions between the γ phosphate of GTP and amide groups at its N-terminus tip the balance from global disorder to dynamic order in Switch II. These hydrogen bonds presumably pay the entropic cost of packing interactions between switch II and side-chains of the underlying β-sheet scaffold, which in turn affords stronger interactions with effectors.
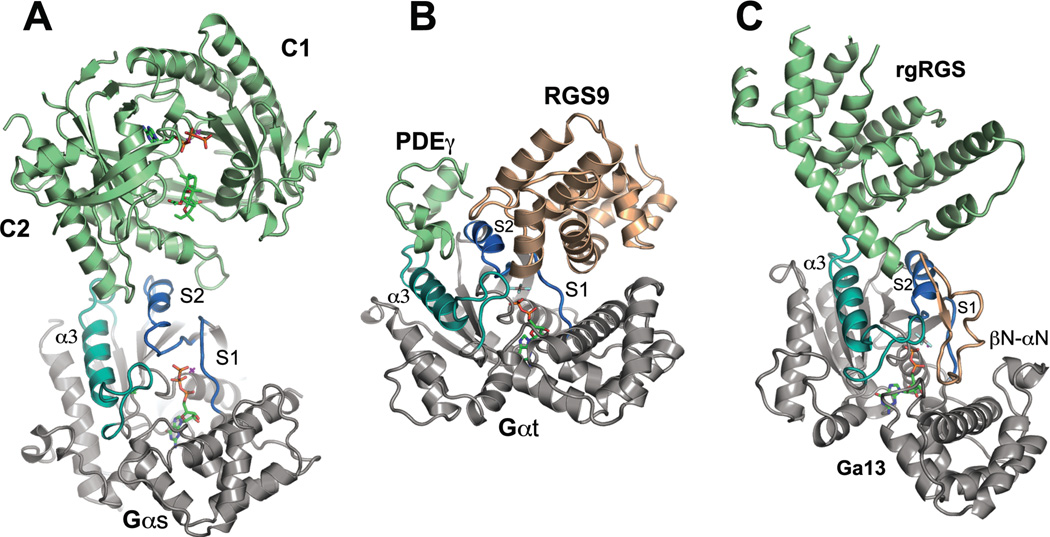
Figure 3.
Structures of Gα bound to effectors and effector-GAPs. In all panels, Gα is rendered in gray except switch I and II, which are colored slate blue, and the α3 helix and switch II, which are rendered in turquoise. Effector domains are colored sage green and GAP domains are rendered in light brown. Ligands and nucleotides are rendered as stick models. A, structure of the catalytic domains (C1 and C2) of adenylyl cyclase bound to the GTPγS complex of Gαs (PDB 1AZS). Two helical segments of adenylyl cyclase and connecting loops engage the middle of switch II and trough between switch II and α3; B, the complex between cyclic GMP phosphodiesterase γ subunit (PDEγ), the RGS domain of RGS9 and Gαt/i1(PDB 1FQK). PDEγ binds at the switch II - α3 interface, while RGS9 occupies a distinct interface between the N-terminal half of switch II and switch I. PDEγ potentiates the GAP activity of RGS9 by stabilizing its interaction with Gα; C, complex of the rgRGS domain of p115RhoGEF with Gα13/i1 (PDB 1SHZ). The RGS-like domain of p115RhoGEF occupies the effector binding region of Gα at the switch II - α3 interface. The βN-αN hairpin domain that conveys GAP activity docks at the interface between the N-terminal half of switch II and switch I.
The mechanism of Gα-catalyzed GTP hydrolysis
The kinetic properties of most Gα subunits were well established nearly thirty years ago5, 22, 39. Single turnover rates at 30 °C are in the range of 2–4 min−1 for most classes of Gα5, but lower for Gαq (0.8 min−1)55 and Gαz (0.1 min−1)56. Yet, these sluggish GTPases are still remarkably efficient, with kcat/KM for some exceeding 105, and comparable to the catalytic efficiencies of “average” enzymes57. This surprising result is the consequence of the micromolar affinity of Gα for its substrate GTP, which is reduced to nanomolar affinity in the presence of the Mg2+ co-factor10, far lower than the physiological concentration of either component58. However, many of the physiological responses – particularly those related to ion channel regulation - require rapid signal termination that far exceeds the intrinsic rate of Gα GTPase activity59. That this catalytic activity can be further stimulated by effectors (PLC-β on Gαq) and “regulators of G protein signaling” (RGS) domains, indicates that the catalytic potential of the Gα GTPase site is not fully realized within the architecture of the protein itself.
The catalytic sites of Ras superfamily proteins, including Gα, are well conserved23 (Table 1) and hence the basic elements of the catalytic mechanism are likely to be the same for both families25. Two amino acids were identified as essential to Gα GTPase activity22 (Figure 2). Near the amino terminus of switch II, a glutamine residue (at position 204 in Gαi1 and 227 in Gαs) hereafter referred to as Glncat, is essential for catalytic activity. The conserved arginine residue in switch I (Arg 201 in Gαs, Arg 178 in Gαi1), hereafter Argcat, is also a major determinant of catalytic activity. The catalytic roles of Glncat and Argcat were not fully appreciated until structures of the complexes of Gαi1 and Gαt with GDP, Mg2+ and AlF4− were determined.
Fluoride ion had long been known to stimulate adenylyl cyclase activity60, and possibly associated with GTP-dependent regulatory activity4, 61, but it was not until experiments were conducted with purified proteins that Gα, in the presence of Mg2+, was confirmed as the target of fluoride activation62. A critical, but cryptic companion of these ions was discovered by neutron activation analysis to be Al3+ - a contaminant in disposable borosilicate glass test tubes and in preparations of ATP used in adenylyl cyclase assays63. Together, these ions increase the intrinsic tryptophan fluorescence of GDP-bound Gα, a property characteristic of the GTP-activated state64. The prescient hypothesis that the activating species is aluminum tetrafluoride, which functions as an analog of a γ phosphate moiety in Gα•GDP65, was shown to be consistent with 19F NMR titration experiments. These indicated a stoichiometry of Mg2+AlFn where n=3 or 4, the latter giving rise to the AlF4− anion and the former, the neutral trifluoride66. Further studies of the pH and [F−] dependence of activation suggested AlF3(OH−) to be the more likely species67. For brevity, I shall henceforth refer to all relevant AlFn species as AlF. The second row element Be, as BeF3− or BeF2 (OH)−, also activates Gα•GDP in the presence of Mg2+ 63, 67. Similarly, at millimolar concentrations, Mg2+ and F− are capable of inducing the activated state of Gα•GDP in the absence of Al3+ 68. Two Mg2+ ions and three to four F− are required for activation. Although the structure of this complex has not been experimentally verified, it is likely that, one Mg2+, like Be2+, forms a trifluoride ion - mimicking a γ phosphate - while the second reprises its role in Gα•GTP complexes by bridging the β phosphate and a fluoride ligand of MgF3−. In all instances, metal (Al3+, Be2+, or Mg2+) fluoride complexes mimic the γ phosphate monoanion.
The crystal structures of GDP•Mg2+•AlF4− (hereafter, GDP•MgAlF)-bound Gαt and Gαi1 are illuminating. AlF appears to be a mimic, not of a γ-phosphate, but rather of a penta-coordinate transition state or intermediate for phosphoryl transfer14, 69. In these complexes, the aluminate has four equatorial fluoride (or possibly three fluoride and one hydroxyl) ligands and two axial oxygen ligands, one being the β phosphorus and the other a water molecule, referred to as Wnuc. This water molecule occupies the position expected for the nucleophile engaged in an in-line attack on the γ phosphate. All of the aluminate ligands, including the axial species, are located 1.9–2.1Å from the metal center. Particularly informative is the reorientation of Argcat and Glncat (Figure 2c) allowing the carboximido moiety of the latter to form hydrogen bonds with a fluoride substituent of AlF that mimics a γ phosphate oxygen atom, and with Wnuc. Argcat forms electrostatic interactions with the pro-S β phosphate oxygen and one of the fluoride substituents of AlF. Although GDP•MgAlF provides a model of the transition state, it does not elucidate the catalytic mechanism for GTP hydrolysis.
It is important to point out that the majority of experimenta, and all of the computational studies of G protein-catalyzed GTP hydrolysis have focused on Ras, or the Ras:Ras-GAP complex. The latter is particularly relevant in the present context, in that it conserves all of the catalytic features found in the catalytic sites of Gα subunits. Dubbed the “arginine finger”, Argcat is provided by Ras-GAP, where it positioned to interact with the β-γ bridging oxygen of the GDP leaving group70. The hypothesis, based on modeling and domain complementation experiments, that Argcat in the catalytic site of Gα is a functional analog of the Ras-GAP “arginine finger” turns out to be exactly correct71. The Ras:Ras-GAP complex has been crystallized with GDP•MgAlF (as the trifluoroaluminate) at the active site of Ras, in which form the complex is most stable72. The catalytic site of the latter is quite similar to that of Gα bound to GDP•MgAlF (as the tetrafluoroaluminate).
At least three events must take place in the course of Gα-catalyzed GTP hydrolysis: 1, the catalytic site must undergo a preorganization step to support the transition state for phosphoryl transfer; 2, depending on the reaction mechanism, an intermediate or transition state for phosphoryl transfer develops; 3, a proton is transferred from the attacking water nucleophile to the γ phosphate leaving group. These steps can, in principle, be stepwise or concerted. A minimal catalytic scheme can be written as:
G•GTP ⇌ G*•GTP ⇌ [G*•GTP]‡ ⇌ G•GDP•Pi ⇌ G•GDP + Pi
where G•GTP represents the Gα Michaelis complex with GTP and Mg2+. For Gα, catalytic site preorganization (transition to G* in the reaction scheme) encounters an activation energy barrier on the order of 3–4 Kcal/mol, as deduced from the rate enhancement provided by Gα GAPs of the Regulator of G protein Signaling (RGS) domain family and discussed in more detail below. In ground state structures of Gα crystallized with GTPγS or guanosine-5’-(βγ-imido)triphosphate (GppNHp) (Figure 2a,b), both Glncat and Argcat exhibit elevated thermal parameters, indicating that they undergo constrained dynamic motion. The average positions of these residues are not always the same in the GTPγS complexes of different Gα proteins15, 53, 69. In particular, the 1.5Å crystal structure of Gαi1•GppNHp is unusual in that both Glncat and Argcat, are highly constrained in conformations that would appear to impede their respective roles in catalysis (Figure 2b)73. In all of these structures, an ordered water molecule is located about 3.8Å from the γ phosphorus and distal to the β−γ bridging oxygen. Occupying a position consistent with its potential role as Wnuc, this ordered water forms a hydrogen bond with a γ-phosphate oxygen, but is offset from the axis of in-line attack (Figure 2a). The structure of the pre-organized state for Gα•GTP is not known, but likely shares features with crystal structures of Gα•GDP•MgAlF, described in a preceding paragraph (Figure 2c). Warshel and coworkers have proposed that the catalytic role of Glncat in Ras is largely allosteric, aiding in the preorganization of the enzyme-substrate complex74, 75. Indeed, the AlF-bound Gα structures clearly show that Glncat, together with the main chain carbonyl oxygen of Thr 181 (Gαi1 numbering, and equivalent to Ras Thr 35) positions Wnuc for in-line attack, thus providing up to two orders of rate acceleration, even for a loose transition state76. As discussed below, an important role of Gα GAPs is to maneuver Glncat into a catalytically functional orientation, as exemplified by the GDP•MgAlF complexes. More global allosteric effects of Glncat upon the conformation of the enzyme active site itself appear to be subtle. Root mean square differences in the positions of main-chain P-loop atoms in the 1.5Å-resolution structure of Gαi1•GppNHp relative to the corresponding 2.2Å-resolution structure of the GDP•MgAlF complex are less than 0.15Å. However, in the GDP•MgAlF complex of Gαi1, switch II shifts slightly away from the nucleotide, such that the amide nitrogen of Gly 203 is displaced by 0.2Á from the γ phosphorus relative to its position in Gαi1•GppNHp. This slight enlargement of the γ phosphate subsite appears to be a consequence of the rotation of the Glncat to its catalytically functional conformation in the AlF complex, and may preorganize the enzyme for orthophosphate formation.
The preponderance of evidence, both experimental and from Quantum Mechanics/Molecular Mechanics (QM/MM) and Electron Valence Bond (EVB) calculations, supports a mechanism in which G protein-catalyzed GTP hydrolysis proceeds through a loose transition state with dissociative character, as is typical for nucleophilic attack on phospho-monoesters76, 77. As such, a catalytic base to deprotonate the nucleophile would not promote catalysis (see Lassila et al. for a comprehensive discussion76). Findings are based for the most part on studies of Ras:Ras-GAP, which, with some caution, may be applied to the intrinsic GTPase activity of Gα, in which the Argcat, that resides in switch I is a built-in component of the active site. Strong evidence for a loose transition state comes from the significant normal kinetic 18O isotope effect (V/K = 1.02) at the bridging β−γ oxygen atom of the leaving group, and secondary isotope effects in the non-bridging β phosphate oxygens78, 79. These are indicative of a redistribution of negative charge towards the β−γ bridge oxygen and to the non-bridging β phosphate oxygen atoms as well (with a concomitant reduction in their bond orders). The magnitude of this kinetic isotope effect (KIE) suggests a low forward commitment to the formation of this transition state and hence, that it is the rate-limiting step of the reaction. In contrast, KIEs at γ phosphate oxygen atoms are near unity, and hence inconsistent with an associative transition state or phosphoryl intermediate. Time-resolved Infrared and Raman spectroscopy of Ras-GAP catalyzed turnover of caged, 18O-labeled GTP after photoexcitation, similarly report accumulation of charge in the β phosphate oxygens80–82, and further, vibrational decoupling of the phosphates resulting from their differential interactions with the Ras active site. QM/MM simulations based on the Ras-GAP complex with GDP• MgAlF indicate a metaphosphate (PO3−) intermediate83, 84. The trend in KIE values with respect to the site of 18O labeling is correctly predicted by this model85. Whether an actual metaphosphate intermediate forms is doubtful. Electron Valence Bond calculations and KIE effects appear to support a more concerted reaction with dissociative character86. In such a mechanism, to accelerate catalysis, the active site of Gα must draw electron density from the bond between the β−γ bridging oxygen and the leaving group, by stabilizing charge at that oxygen and delocalizing charge to the non-bridging oxygen atoms of the β phosphate.
Part of the task of charge redistribution falls to amide groups of the P-loop. For Ras, Maegley et al. saw the amide group of Gly 13 (Glu 43 in Gαi1) as a prime candidate for this role, in view of the short hydrogen bond that it forms to the β−γ bridging oxygen of GTP in several Ras structures87. Indeed, a short, linear 2.7Å hydrogen bond between the corresponding atoms is observed in the crystal structure of the Ras:Ras-GAP complex70. Accordingly, a normal isotope effect is observed for the β-γ bridging oxygen, as well as the Pro-S β oxygen, which accepts hydrogen bonds from P-loop amides at residues 15 and 16, as well as the amine of lysine 16 (Lys 46 in Gαi1)79. These hydrogen bonds are conserved in the GDP•MgAlF-bound structures of Gαi1 and Gαt, as well as in the GTPγS or GppNHp ground states. Thus, there are ample hydrogen bond donors available to stabilize charge on the β-phosphate leaving group. Nevertheless, robust stabilization of charge on the β-γ bridging oxygen requires Argcat, whether it is supplied by an exogenous GAP, or, as in Gα subunits, is resident in switch I. KIE experiments suggest that the Argcat →Ala mutation considerably impairs the GAP activity of NF179 by eliminating the Argcat contribution to charge stabilization at the pro-S β phosphate oxygen. In heterotrimeric G proteins, mutation of the endogenous Argcat results in loss of GTPase activity in Gαs88, 89. The same residue is the target of cholera toxin ADP ribosylation90.
Argcat exerts its catalytic function at the transition state. As noted above, GTP-analog-bound structures of Gα subunits differ in the disposition of Argcat. In Gαt•GTPγS, Argcat is hydrogen bonded to the β-γ bridging oxygen; in Gαi•GTPγS and Gαs•GTPγS, it is partially disordered, and in the “auto-inhibited” conformation observed in Gαi1•GppNHP, Argcat is sequestered from the nucleotide by formation of an ion pair with the side chain of P-loop Glu 43. However, in the GDP•MgAlF complexes of Gαi1, Gαo, Gαt, Gαq, Gα12 and Gα13 (some also bound to effector-GAPs), the conformation of Argcat is invariant, forming in all instances hydrogen bonds to both the bridging oxygen atom and a fluoride substituent of AlF14, 45, 48, 50, 52, 69. In these structures, Glncat accepts a hydrogen bond from Wnuc and donates to a fluoride substituent of AlF (Figure 2c).
Resolution of the loose dissociative transition state is achieved by scission of the bond between Pγ and the β-γ bridging oxygen of the leaving group and formation of a bond from the metaphosphate-like species to the attacking water. This step involves the transfer of a proton from the Wnuc to the γ phosphate, yielding GDP and H2PO4−. Whether concerted with the breakdown of the dissociative transition state or following it, direct transfer of the proton is energetically prohibitive83. A “two-water” 86 proton transfer trajectory, presumably along existing hydrogen bonds, would afford a lower energy route but would require shuttling the proton through an intermediary donor/acceptor. None of the crystal structures of Gα•GDP•MgAlF complexes reveal a water molecule optimally positioned to shuttle a proton from Wnuc to a γ phosphate oxygen. Sondek, et al. proposed such a role for Glncat, wherein the side chain Oδ abstracts a proton from Wnuc while the δ amide donates a proton to the γ phosphate14. This highly unlikely tautomeric shift, in view of the pKas of the groups involved, could be driven by a highly reactive metaphosphate (PO3−) intermediate83. In any case, the absence, in Ras:Ras-GAP-catalyzed GTP hydrolysis, of a KIE on the γ phosphate oxygen atoms suggests that proton transfer to the γ phosphate is not rate limiting79.
Lessons from mutants
Both site-directed mutagenesis and natural sequence variations have provided insight into Gα function. Some outcomes are expected - for example, that mutations of Glncat and Argcat result in constitutive activity, unregulated signaling and associations with pituitary and pancreatic cancer89, 91–94. The X-ray structures of Gαi1•GTPγS harboring these mutations, Q204R and R178C, respectively, show no perturbations of the GTP binding site69. The presumptive Wnuc is observed in the structures of both mutants, as in the structure of wild-type Gαi1•GTPγS, some 3.8Å from the γ phosphate. Thus, neither residue participates significantly in substrate binding, as confirmed by the rates of nucleotide dissociation.
With a turnover rate less than 0.1 min−1 at 30°C, 100-fold slower than Gαi1, Gαz is an exceptionally sluggish GTPase. Gαz harbors threonine and serine residues at positions 41 and 42 of the P-loop, whereas alanine and glycine are found in Gα subunits with typical levels of GTPase activity. Although a modeling experiment suggests that both sequence variations can be easily accommodated in the P-loop, it is possible that, together, they perturb the P-loop amide hydrogen bonds to the GTP β phosphates, and impair the ability of the enzyme to stabilize negative charge at the transition state. Gαq also has a threonine at position 41 (but retains the canonical glycine at 42) and has moderately weak GTPase activity at 0.8 min−1. Thus, evolutionary forces are able to tune intrinsic Gα GTPase activities by P-loop mutations that do not appear to significantly perturb the stereochemistry of the catalytic site.
More surprising are mutations that alter Gα conformation or dynamics in a substrate-dependent manner. Seemingly modest mutagenic perturbations of the P-loop and switch II can result in significant alterations in the GTP, Mg2+ or Gβγ binding properties of Gα, and may be manifested in novel conformations that can be trapped in the solid state. In Gαs, mutation of Gly 226 to alanine results in a serious signaling defect in which receptor engagement of heterotrimeric Gs fails to liberate Gαs•GTP and Gβγ95, 96. Gly 226 is located at the N-terminus of switch II, where its amide group forms a hydrogen bond with a γ phosphate oxygen atom (the equivalent residue in Ras is Gly 60). Because Gly 226 is in van der Waals contact with the P-loop residue Gly 49, substitution with alanine would be expected to introduce a steric clash in that region. Nevertheless, (G226A)Gαs activates adenylyl cyclase. It and its homolog (G203A)Gαi1 have nearly normal GTPase activity, but weaker-than-wild-type affinity for Mg2+ and GTPγS96, 97.
Surprisingly, an attempt to crystallize the GTPγS:Mg2+ complex of (G203A)Gαi1 instead yielded crystals of the Mg2+-free complex of GDP with inorganic phosphate (Pi): a model of the ternary product complex of GTP hydrolysis98 (Figure 2d). Presumably, at the low pH (~5.5) at which the complex was crystallized, the relatively unreactive GTP analog was hydrolyzed during the course of crystallization. The position occupied by GDP and its contacts with the P-loop in the G203A mutant are no different from those observed in wild-type Gαi1. The phosphate, most likely H2PO4−, is within hydrogen bonding distance of the β phosphate, stabilized by the P-loop lysine and switch II Argcat. To accommodate Pi, switch II adopts a more regular helical structure at its N-terminus and becomes kinked near its mid-point, affording its movement away from the catalytic site. Meanwhile, the hydrogen bond between the 203 amide nitrogen and the erstwhile γ phosphate is maintained. Thus, due to a substantial change in the secondary structure of switch II, GαI adopts a conformation that is complementary to GDP•Pi. Yet, Pi binds very weakly to Gαi1•GDP – both to the wild-type and G203A and G42V (see below) mutants - with a Kd of at least 50 mM99. Arguably, by placing steric stress at the N-terminus of switch II A203 stabilized a transitory conformational state, thus trapping the GDP•Pi product complex. Precisely the same GDP•Pi-bound state of the (A42V)Gαi1 mutant can be crystallized99. Like its oncogenic G12A counterpart in Ras100, (A42V)Gαi1 has weak GTP hydrolytic activity, with a turnover rate of 0.13 min−1. GTPase activity may be weakened in this mutant due to steric conflict between the Val 42 side chain and Cβ of Glncat. Accordingly, the steric pressure that induces the reconfiguration of switch II, and affords crystallization of the GDP•Pi complex, originates from an increase in side-chain volume at position 42 in the P-loop.
As we have seen, mutations in switch I and switch II have the potential to drastically alter GTPase activity. Mutation of Gly 202 to alanine, perhaps because it forms a hydrophobic cage that restricts the mobility of Wnuc, causes a 10-fold increase in the intrinsic GTPase rate of Gαi1101. The reverse mutation in Ras, in which the corresponding wild-type residue at position 59 is alanine, results in the loss of GTPase activity102. In the crystal structure of (A59G)Ras•GppNHp, switch II adopts a conformation that is intermediate between the GTP and GDP-bound states. Unfortunately, it was not possible to crystallize (G202A)Gαi1 as a complex with a GTP analog.
Mutations that trap intermediate states have been found elsewhere near the catalytic site. The switch I residue located two positions N-terminal to Argcat is variable among the different Gα classes. In Gi-class Gα subunits, this position is occupied by lysine; in the q/11 Gα class, proline is preferred. This substitution has no effect on the conformation of switch I, because the backbone φ/ψ angles at that position are accessible to proline. However, substitution of lysine for proline resulted in an eight-fold loss of GTPase activity103, whereas a lysine-to-alanine mutation had no effect. However, the impact of this mutation on conformational change rates was startling.
Increase in intrinsic tryptophan fluorescence is a hallmark of Gα activation64, 104 that originates from changes in the solvent accessibility of a tryptophan in switch II upon exchange of GDP for GTP105. Hence, a convenient way to follow GTP hydrolysis has been to monitor the rate at which tryptophan fluorescence is lost as hydrolysis proceeds106. Indeed, the rate of the fluorescence transition is nearly identical to that a which GTP is hydrolyzed as measured by generation of radiolabeled Pi107 or fluorescence quenching of N-methyl-anthranoyl guanine nucleotide derivatives (mGTP)108. For (K180P)Gαi1, the rate of tryptophan fluorescence decay upon addition of Mg2+ to GTP-bound protein exceeded by 60-fold that of Pi or mGDP production. Apparently, switch II began its conformational change before GTP was hydrolyzed, and thus the two events were decoupled. Attempts to crystallize the K180P mutant in the presence of Mg2+ and a hydrolysis-resistant GTP analog were not successful, so the structure of the intermediate from which hydrolysis proceeds remains unknown. The structure of the pre-transition state GDP•MgAlF complex indicated destabilization of the Mg2+ binding site, with switch I constrained by the proline substitution to a conformation similar to the GTP-bound state. The behavior of this mutant suggests the possibility that catalytic pre-organization may involve long-range structural changes that preserve the coupling between GTP hydrolysis and conformational changes in switch II.
Convergent mechanisms of Gα GTPase activating proteins
In the mid-1990s, experiments with yeast and nematodes lead to the discovery of “Regulators of G protein Signaling” (RGS)109 , which were ultimately found to function as GTPase activating proteins (GAPs) for Gα, acting catalytically to increase the rate of Gα-catalyzed GTP hydrolysis by up to 100-fold in vitro110. GAP activity in these proteins is conveyed by a ~120 residue α-helical domain. RGS proteins play complex, integrative roles in cell signaling, acting as “kinetic scaffolds” in conjunction with G protein heterotrimers and GPCRs to maintain high signaling throughput by coupling Gα activation to GTP hydrolysis111–113. Much has been learned about the basis of Gα class specificity exhibited by members of the four major families of RGS GAPs, and their complex roles in cell signaling40. Here we focus on the mechanism by which they accelerate GTP hydrolysis and the remarkable functional convergence of RGS GAPs with certain G protein effectors, which also function as GAPs. Among these are isoforms of PLC-β55, 71 and p115RhoGEF, one of a family of Gα12/13-regulated guanine nucleotide exchange factors for the Rho GTPases114. When co-localized at the plasma membranes with GPCRs and Gβγ, these too, kinetically couple G-protein activation and de-activation, maintaining a high steady-state level of effector activation while agonists are present. This is possible because Gα GAPs and effectors occupy distinct and non-overlapping binding sites on Gα25. In reconstituted vesicles containing Gα, Gβγ, a GPCR and the appropriate RGS protein or effector-GAP, GTP turnover rates can be 1000-fold higher than intrinsic GTPase rates112. The maximal catalytic efficiency for Gα•GTPas a GAP substrate is ~108 M−1s−1, with KM values ranging from ~2 to 600 nM for various RGS proteins, and in the nanomolar range for PLC-β155, 112 and p115RhoGEF115. The effect of GAPs on the KM for GTP at the catalytic site of Gα has not been determined, hence we cannot know how the catalytic efficiency of Gα itself is affected.
RGS GAPs exhibit high affinity for the pre-transition state of Gα as modeled by the complex of Gα•GDP•MgAlF116. The crystal structure of Gαi1•GDP•MgAlF:RGS4 is the prototype for RGS domain-bound Gα complexes that have been subsequently determined45, 117–120 and which currently represent three of the four subfamilies of RGS GAPs bound to α subunits of the i (Gαt, Gαi1, Gαi3) and q (Gαq) classes. As yet, no RGS GAP that recognizes Gαs has been discovered, and it appears that the t1/2 for adenylyl cyclase activation is similar to that of the intrinsic rate of Gαs-catalyzed GTP hydrolysis59. In all of these complexes, we find that RGS, unlike small G protein GAPs that provide Argcat, does not contribute residues that appear to have a direct catalytic function. Rather, RGS sterically restrains the conformation of switch I and switch II, and in particular, Glncat and Argcat, to stabilize the pre-transition state conformation of Gα (Figure 4a). Accordingly, RGS domains form contacts with all three switch regions (I-III), and some engage the helical domain (viz. RGS2 and Gαq120). Most RGS domains provide a conserved asparagine as a hydrogen bonding partner for Glncat, thereby stabilizing its conformation in the pre-transition state. However mutagenesis studies, reviewed by Ross and Wilkie59, and structures that have been determined so far, suggest that considerable variation is tolerated at the RGS:Gα interface. Stabilization of Glncat is crucial. RGS4 cannot restore GTPase activity to (Q204L)Gαi1121 but can rescue the GTPase activity of the Argcat mutant R178C, although not to levels exhibited by wildtype Gα. The active site structure of the (R183C)Gαq•GDP•MgAlF bound to RGS2 is virtually identical to that of RGS domains bound to wild-type Gα subunits120. Thus, the incremental stabilization of charge at the β-γ leaving group oxygen is not essential if Glncat can be conformationally stabilized to effect catalysis.
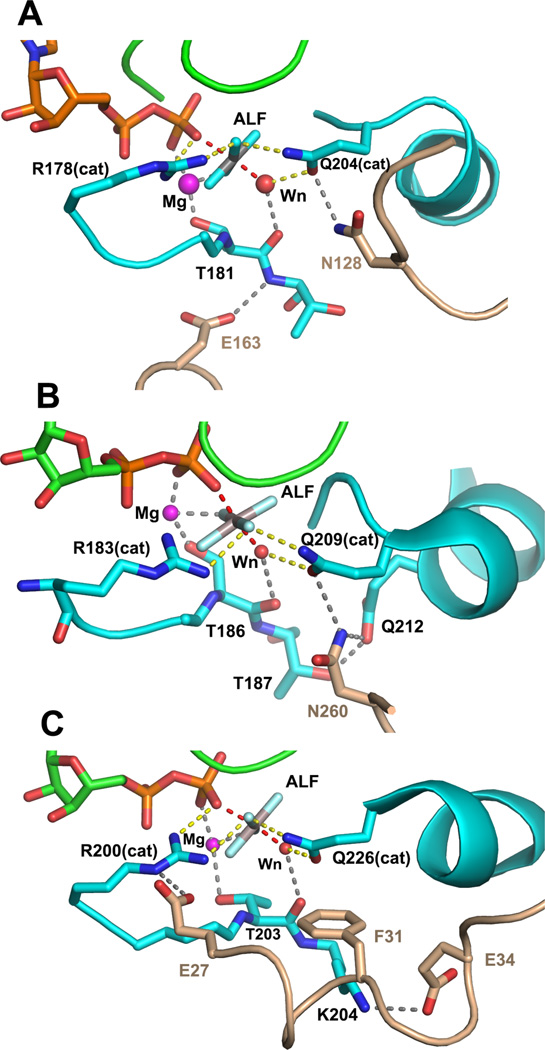
Figure 4.
Interactions between Gα•GDP•MgAlF active site and critical residues of RGS and effector-GAP domains. The coloring scheme used in Figures 1–3 is used. A, contacts between switch I and switch II of Gαi1 and RGS4 (PDB 1AGR, 2.8Å resolution) are shown. RGS residues Asn 128 and Glu163, respectively, constrain the conformation of Gαi1 Q204 (Glncat) to the pre-transition state conformation and stabilize switch I though a hydrogen bond to the backbone amide of Thr 181; B, Asn 260 from the loop between the EF 3 and EF 4 domains of PLC−β3 form a network of hydrogen bonds with residues of switch II at the catalytic site of Gαq (PDB 3OHM, 2.7Å resolution). Interactions between Asn 260 and Gαq mimic that of Asn 128 of RGS4 with Gαi1. The latter are strengthened by hydrogen bond network with residue Gln 212 and Thr 187 of switch I in Gαq. Not shown is the extensive interaction surface of PLC-β3 and the effector-binding surface of Gαq; C, The αN segment of the βN-αN hairpin of p115RhoGEF forms hydrogen bonds with switch I and switch II in Gα13/i1. Acidic residues Glu 27 and Glu 34 form ion pair contacts with Argcat (Arg 200) and Lys 204, respectively, in switch I, stabilizing the interaction between Argcat and the fluoroaluminate, and potentially, the β-γ bridge oxygen of GTP. Phe 31 sterically restrains the position of Glncat as do Asn 128 and Asn 260 in RGS4 and PLC-β3.
The GAP activity of PLC-β3 results from the interaction of an extended loop between the third and fourth EF hand domains with the switch I and switch II regions of Gαq48. At the contact site, PLC-β3 residue Asn 260 is juxtaposed to Glncat in much the same fashion as the essential Asn residue provided by RGS domains (Figure 4b). Here, too, it appears that PLC-β exerts GAP activity by stabilizing the pre-transition state of Glncat. As an effector, PLC-β also has high affinity for the GTP-bound forms of Gαq and accordingly, interactions between the two molecules involve an extensive interface that involve switch I and II as well as the trough between switch II and α3, which is typically reserved for effector binding.
Gα12/13-activated p115RhoGEF affords a 60-fold stimulation of the GTPase activity of Gα13, and a more modest 6-fold acceleration of that for Gα12115. Although p115RhoGEF and its homologs possess RGS-homology (RH or rgRGS) domains, these are not involved in GAP activity. The structure of the complex between the rgRGS domain and a Gα13/Gαi1 chimera revealed that the RGS-like domain binds to Gα13/I in the manner of an effector, with extensive contacts at the switch II - α3 interface, rather than as a GAP49 (Figure 3c). GAP activity was instead conferred by a 20-residue peptide segment (named βN-αN) directly N-terminal to the RGS-like domain (Figures 3c, 4c). The peptide is folded into an antiparallel β-α hairpin. The β segment contains a short hydrophobic sequence that docks against the helical domain of Gα13; a mainchain carbonyl oxygen within this sequence is engaged in a hydrogen bond with an arginine residue in switch III52. The α- helical segment harbors a highly acidic sequence interrupted by a phenylalanine residue (EDEDFE). These residues are critical for GAP activity. The first glutamate residue in this acidic region stabilizes Argcat, and the phenylalanine side chain is positioned analogously to the conserved Asn residue of RGS domains, where it sterically restrains Glncat in its pre-transition state conformation. Mutagenesis of either residue to alanine abolishes GAP activity. The related PDZRhoGEF retains the ability to bind GTPγS-activated Gα12 and Gα13, but has no GAP activity, even though it has affinity for GDP•MgAlF-bound Gα13122. The acidic motif of PDZRhoGEF contains a single deletion in the acidic motif and a tyrosine replaces the phenylalanine (EEDY). Crystal structures show that the misalignment between the shortened acidic motif results in weakened interactions with switch I, reorientation of the tyrosyl residue relative to the position of phenylalanine at the corresponding site in p115RhoGEF, and a loss of order throughout the acidic region52.
RGS GAPs, PLC-β and 1115RhoGEF, though disparate in structure and amino acid sequence, have converged on roughly the same mechanism for GAP activation. Each stabilizes the pre-transition state conformation of Gα by stabilizing catalytic conformations of Argcat and, particularly, Glncat. Overall binding energy derives from interactions with switches I-III, to differing extents with the Gα helical domain, and in the case of effector-GAPs, with Gα effector-binding regions that, at minimum, include switch II and α3. Unlike Ras-family GAPS, Gα GAPs do not participate in the chemistry of GTP hydrolysis. More generally, the structural and kinetic data obtained from Gα mutants suggest that protein dynamics may ultimately determine the rate of GTP hydrolysis by controlling the density of conformational states from which the active site of Gα can access the pre-transition state along a low activation energy pathway. Gα GAPs accelerate hydrolysis by constraining an otherwise mobile switch II, particularly Glncat, forcing it to orient the water nucleophile for in-line attack and stabilizing Argcat through a hydrogen bonding network that includes the γ phosphate. In this way Gα GAPs both promote the pre-organization of the catalytic site and indirectly assist in stabilizing charge at the βγ bridging oxygen – thus lowering the activation energy barrier to release of the leaving group.
Functional consequences of GTP hydrolysis
While GTP hydrolysis modestly diminishes the affinity of Gαi for effectors, it markedly increases affinity for Gβγ10. Gαi1•GDP forms a high affinity, nanomolar Kd complex with Gβγ that sequesters both signaling molecules in an inactive state at the plasma membrane. The release of interactions between the γ-phosphate and the N-terminus of switch II allows the latter to refold, affording new interactions at the Gβγ interface18–20. In the heterotrimer, the β2 strand and the N-terminus of switch II – an extension of β3 - are knit together as in a parallel, hydrogen-bonded network extending to Thr 181 in switch I and Ala 203 in switch II (residues 201 – 204 adopt an unusual 27 helical turn). In this configuration, strands β1 together with β3 and switch II form a platform for the Gβ subunit. Major switch II participants in this interaction are Lys 210 and Glncat, which now plays a structural rather than a catalytic role. Further along in Switch II, the side chain of Lys 210 is buried in the interface with Gβ. The importance of these residues to the affinity of the Gα:Gβ interaction has been noted in computational modeling studies123.
Reactivation of Gα: exchange of GDP for GTP
It is remarkable that a single phosphate moiety at the γ position of GTP is sufficient to effect major rearrangements in switch I and II that liberate both Gαi1 and Gβγ to fulfil their respective roles in GPCR-actuated signaling. In cells, the preponderance of membrane-associated Gα•GDP is bound in a complex with Gβγ. The conformational changes within switch I and II that are necessary to accommodate GTP cannot occur within the heterotrimer, which binds to GDP with 100-fold greater affinity than free Gα subunits10, and within which GDP is inaccessible to solvent18, 20. Rather, GDP must first be released by engagement of the heterotrimer with an agonist-activated GPCR. The extensive conformational changes that result in the ejection of GDP are exemplified in the crystal structure of heterotrimeric Gs bound to the β2 adrenergic receptor21. This structure, together with studies using structure-based mutagenesis124–127, site-directed spin-labeling128–130, molecular dynamics131–133 and other computational approaches134 have arrived at a consistent picture of the receptor-induced conformational transitions that compel GDP release. These and seminal papers reviewed elsewhere (see 135, 136) show that GPCRs engage the C-terminus of G✓ 137, 138, causing it to rotate slightly and translate with respect to the body of the Ras domain. This key perturbation induces conformational changes in the α5-β6 loop at the purine binding site, disrupts interactions with the α1 helix and succeeding P-loop, and destabilizes the nucleotide binding site and contacts between the Ras and helical domains, leading to their separation and facilitating egress of GDP. The cytosolic non-receptor nucleotide exchange factor Ric-8A induces similar and possibly more extensive conformational changes in the structure of Gαi1139.
While the interface between Gαs switch II and Gβγ is largely intact in the complex with the β2 receptor, this interaction is weakened with the disordering of switch I. The P-loop adopts an open conformation, ready to receive the β and γ phosphates of GTP and, with these moieties, to coordinate a magnesium ion123. Awaiting further exploration are the coupled conformational pathways by which the P-loop, switch II and β5-α5 refold around GTP, and thus escape from the complex with Gβγ and the receptor. In aggregate, these rearrangements would eliminate the switch II interface with Gβ, and disrupt that between the receptor and α5.
Conclusions
In the presence of magnesium ion, GTP binds with nanomolar affinity to the α subunits of heterotrimeric G proteins. This extraordinarily high binding energy is used to restrain and stabilize the conformation of otherwise highly dynamic Gα switches I and II. The conformation in which these two structural elements are held is highly complementary to the surfaces of Gα effectors, but incompatible with the Gα binding site on Gβγ. Upon GTP hydrolysis, the energy of these conformational restraints is dissipated and the two switch segments, particularly switch II, become flexible. The GDP-bound state of Gα is easily remodeled for binding to Gβγ. Both signal transducers – Gα and Gβγ - are thereby locked into a nanomolar-affinity complex that can be released only by the catalytic action of agonist-activated G protein-coupled receptors, which allows GTP to disrupt the Gα:Gβγ interface and that with the receptor itself.
The mechanism by which Gα hydrolyzes GTP is likely the same as that used by Ras, with the important difference that Gα possesses a catalytic arginine residue that is absent in Ras, and must be supplied by an exogenous GAP. This provides Gα with about three orders of magnitude in rate enhancement relative to Ras with respect to intrinsic GTPase activity. The intrinsic GTPase rates of different classes of Gα range from ~0.1 min−1 to 4 min−1 at ~20 °C. The differences are likely due in large part to the amino acid sequence of the P-loop, resulting in greater or lesser efficiency in stabilizing charge at the leaving group. Catalytic site pre-organization presents a significant barrier to catalysis possibly due to the richness of non-catalytic states that are accessible to critical residues in the active site of Gα. Some of these states, exemplified by the apparently “anticatalytic” conformation exhibited in the structure of Gαi1•GppNHp (Figure 2b), may actually impede catalytic action. Gα GAPs act by restricting the conformational freedom of Gα active site residues, particularly Glncat and Argcat and enforcing upon them a conformation that is complementary to the transition state for GTP hydrolysis. Glncat in particular, appears to orient and stabilize the γ phosphate and the water nucleophile for an in-line attack. The transition state is probably loose with dissociative character, and phosphoryl transfer may be concerted. Experimental, structural and computational data suggest that electron density from the γ phosphate shifts to the β−γ bridge oxygen and is redistributed to the β non-bridging oxygens. Gα, and more effectively Gα:GAP, catalyzes GTP hydrolysis by promoting this charge redistribution. Along the reaction pathway, possibly in concert with the collapse of the loose transition state, a proton is shuttled from the water nucleophile to the γ phosphate, affording H2PO4−. An ordered water molecule would be an ideal candidate to serve as a shuttle, but it is also is possible that Glncat might act in this capacity. There is still a need for conclusive answers to several questions: does a metaphosphate intermediate occur in the reaction trajectory, or is the reaction concerted, with a loose-transition state? What is the mechanism of proton transfer to the γ phosphate? What, precisely is the role of Glncat? Importantly, why, given the relatively small structural differences between the Gα•GTP “Michaelis” complex and the pre-transition state as modeled by the GDP•MgAlF complex, is the activation energy barrier to GTP hydrolysis so high?
It appears that Gα GAPs have arisen independently on several occasions during the evolution of Gα-regulated signal transduction networks. Incorporation of GAP activity into effectors affords exquisite regulation of GTPase kinetics and effector activation. Co-localization with GPRCs provides additional avenues for steady-state control of G protein signaling. RGS GAPs are structurally well conserved, but several have acquired signaling functions unrelated to GAP activity. For example, the RGS domains present in members of the RGS-RhoGEF family engage Gα in the manner of effectors, whereas GAP activity is conveyed by a short β-α peptide motif. Although they are structurally dissimilar, RGS GAPS and the GAP-domains of RGS-RhoGEFs and PLC-β converge on a common mechanism of action, which is to stabilize the pre-transition state for Gα-catalyzed GTP hydrolysis, acting primarily on the conformation of Argcat and Glncat Arguably, we have a fairly clear understanding of the reaction kinetics and structural transformations involving Gα subunits in the context of the canonical GTPase cycle of activation, effector regulation and signal termination. Considerably less well understood are Gα class-specific modes of signal integration - processes that may involve transient, often membrane-associated, multiprotein complexes that assemble at the plasma membrane and interact with other regulators. Remarkably, many of the proteins that support such signaling agendas harbor RGS domain modules, for example, RGS7, RGS14 and RGS-RhoGEFs140. Unraveling the complex web of G protein regulatory interactions involving these and other signal transducers is our present task.
Acknowledgments
Research conducted in the author’s laboratory has been supported by NIH grants R01-DK046371 and R01-GM105993.
Literature Citations
- 1.Cassel D, Selinger Z. Biochim Biophys Acta. 1976;452:538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- 2.Pfeuffer T, Helmreich EJ. J Biol Chem. 1975;250:867–876. [PubMed] [Google Scholar]
- 3.Ross EM, Howlett AC, Ferguson KM, Gilman AG. J Biol Chem. 1978;253:6401–6412. [PubMed] [Google Scholar]
- 4.Pfeuffer T. J Biol Chem. 1977;252:7224–7234. [PubMed] [Google Scholar]
- 5.Gilman AG. Anual Reveiws in Biochemistry. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 6.Stryer L. Annual review of neuroscience. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 7.Brandt DR, Ross EM. J Biol Chem. 1985;260:266–272. [PubMed] [Google Scholar]
- 8.Ferguson KM, Higashijima T, Smigel MD, Gilman AG. J Biol Chem. 1986;261:7393–7399. [PubMed] [Google Scholar]
- 9.Higashijima T, Ferguson KM, Smigel MD, Gilman AG. J Biol Chem. 1987;262:757–761. [PubMed] [Google Scholar]
- 10.Higashijima T, Ferguson KM, Sternweis PC, Smigel MD, Gilman AG. J Biol Chem. 1987;262:762–766. [PubMed] [Google Scholar]
- 11.Fung BK. J Biol Chem. 1983;258:10495–10502. [PubMed] [Google Scholar]
- 12.Sternweis PC, Northup JK, Smigel MD, Gilman AG. J Biol Chem. 1981;256:11517–11526. [PubMed] [Google Scholar]
- 13.Lambright DG, Noel JP, Hamm HE, Sigler PB. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 14.Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- 15.Noel JP, Hamm HE, Sigler PB. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 16.Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 17.Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 18.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 19.Wall MA, Posner BA, Sprang SR. Structure. 1998;6:1169–1183. doi: 10.1016/s0969-2126(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 20.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm H, Sigler PB. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprang SR. Annual review of biochemistry. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 23.Vetter IR, Wittinghofer A. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 24.Coleman DE, Sprang SR. Biochemistry. 1998;37:14376–14385. doi: 10.1021/bi9810306. [DOI] [PubMed] [Google Scholar]
- 25.Sprang SR, Chen Z, Du X. Advances in protein chemistry. 2007;74:1–65. doi: 10.1016/S0065-3233(07)74001-9. [DOI] [PubMed] [Google Scholar]
- 26.Dohlman HG, Jones JC. Sci Signal. 2012;5:re2. doi: 10.1126/scisignal.2003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce KL, Premont RT, Lefkowitz RJ. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum DM, Rasmussen SG, Kobilka BK. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sternweis PC. Curr Opin Cell Biol. 1994;6:198–203. doi: 10.1016/0955-0674(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 30.Clapham DE, Neer EJ. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 31.Gautam N, Downes GB, Yan K, Kisselev O. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 32.Wedegaertner PB, Wilson PT, Bourne HR. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 33.Smotrys JE, Linder ME. Annual review of biochemistry. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 34.Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 35.Tall GG, Krumins AM, Gilman AG. J Biol Chem. 2003;278:8356–8362. doi: 10.1074/jbc.M211862200. [DOI] [PubMed] [Google Scholar]
- 36.Preininger AM, Kaya AI, Gilbert JA, 3rd, Busenlehner LS, Armstrong RN, Hamm HE. Biochemistry. 2012;51:1911–1924. doi: 10.1021/bi201472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilman AG. Ann Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 38.Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Endocr Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- 39.Oldham WM, Hamm HE. Q Rev Biophys. 2006;39:117–166. doi: 10.1017/S0033583506004306. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard KB, Hepler JR. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Aittaleb M, Boguth CA, Tesmer JJ. Mol Pharmacol. 2010;77:111–125. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunemann M, Frank M, Lohse MJ. Proc Natl Acad Sci U S A. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank M, Thumer L, Lohse MJ, Bunemann M. J Biol Chem. 2005;280:24584–24590. doi: 10.1074/jbc.M414630200. [DOI] [PubMed] [Google Scholar]
- 44.Tesmer JJG, Sunahara RK, Gilman AG, Sprang SR. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 45.Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. Nature. 2001;409:1071–1077. doi: 10.1038/35059138. [DOI] [PubMed] [Google Scholar]
- 46.Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 47.Lyon AM, Tesmer VM, Dhamsania VD, Thal DM, Gutierrez J, Chowdhury S, Suddala KC, Northup JK, Tesmer JJ. Nat Struct Mol Biol. 2011;18:999–1005. doi: 10.1038/nsmb.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Science. 2010;330:974–980. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, Singer WD, Sternweis PC, Sprang SR. Nat Struct Mol Biol. 2005;12:191–197. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 50.Kreutz B, Yau DM, Nance MR, Tanabe S, Tesmer JJ, Kozasa T. Biochemistry. 2006;45:167–174. doi: 10.1021/bi051729t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. J Biol Chem. 1997;272:22265–22271. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Singer WD, Danesh SM, Sternweis PC, Sprang SR. Structure. 2008;16:1532–1543. doi: 10.1016/j.str.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sunahara RK, Tesmer JJG, Gilman AG, Sprang SR. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 54.Van Eps N, Oldham WM, Hamm HE, Hubbell WL. Proc Natl Acad Sci U S A. 2006;103:16194–16199. doi: 10.1073/pnas.0607972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berstein G, Blank JL, Jhon D-Y, Exton JH, Rhee SG, Ross EM. Cell. 1992;70:411–418. doi: 10.1016/0092-8674(92)90165-9. [DOI] [PubMed] [Google Scholar]
- 56.Casey PJ, Fong HK, Simon MI, Gilman AG. J Biol Chem. 1990;265:2383–2390. [PubMed] [Google Scholar]
- 57.Bar-Even A, Noor E, Savir Y, Liebermeister W, Davidi D, Tawfik DS, Milo R. Biochemistry. 2011;50:4402–4410. doi: 10.1021/bi2002289. [DOI] [PubMed] [Google Scholar]
- 58.Traut TW. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 59.Ross EM, Wilkie TM. Annual review of biochemistry. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland EW, Rall TW, Menon T. J Biol Chem. 1962;237:1220–1227. [PubMed] [Google Scholar]
- 61.Ross EM, Gilman AG. J Biol Chem. 1977;252:6966–6969. [PubMed] [Google Scholar]
- 62.Northup J, Sternweis P, Smigel M, Schleifer L, Ross E, Gilman A. Proc Natl Acad Sci U S A. 1980;77:6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sternweis PC, Gilman AG. Proc Natl Acad Sci U S A. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Higashijima T, Ferguson KM, Sternweis PC, Ross EM, Smigel MD, Gilman AG. J Biol Chem. 1987;262:752–756. [PubMed] [Google Scholar]
- 65.Bigay J, Deterre P, Pfister C, Chabre M. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 66.Higashijima T, Graziano MP, Suga H, Kainosho M, Gilman AG. J Biol Chem. 1991;266:3396–3401. [PubMed] [Google Scholar]
- 67.Antonny B, Chabre M. J Biol Chem. 1992;267:6710–6718. [PubMed] [Google Scholar]
- 68.Antonny B, Sukumar M, Bigay J, Chabre M, Higashijima T. J Biol Chem. 1993;268:2393–2402. [PubMed] [Google Scholar]
- 69.Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 70.Scheffzek K, Ahmadian MR, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F, Wittinghofer A. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 71.Biddlecome GH, Berstein G, Ross EM. J Biol Chem. 1996;271:7999–8007. doi: 10.1074/jbc.271.14.7999. [DOI] [PubMed] [Google Scholar]
- 72.Mittal R, Ahmadian MH, Goody RS, Wittinghofer A. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- 73.Coleman DE, Sprang SR. J Biol Chem. 1999;274:16669–16672. doi: 10.1074/jbc.274.24.16669. [DOI] [PubMed] [Google Scholar]
- 74.Glennon TM, Villa J, Warshel A. Biochemistry. 2000;39:9641–9651. doi: 10.1021/bi000640e. [DOI] [PubMed] [Google Scholar]
- 75.Shurki A, Warshel A. Proteins. 2004;55:1–10. doi: 10.1002/prot.20004. [DOI] [PubMed] [Google Scholar]
- 76.Lassila JK, Zalatan JG, Herschlag D. Annual review of biochemistry. 2011;80:669–702. doi: 10.1146/annurev-biochem-060409-092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Admiraal SJ, Herschlag D. Chemistry and Biology. 1995;2:729–739. doi: 10.1016/1074-5521(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 78.Du X, Black GE, Lecchi P, Abramson FP, Sprang SR. Proc Natl Acad Sci U S A. 2004;101:8858–8863. doi: 10.1073/pnas.0401675101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du X, Sprang SR. Biochemistry. 2009;48:4538–4547. doi: 10.1021/bi802359b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allin C, Ahmadian MR, Wittinghofer A, Gerwert K. Proc Natl Acad Sci U S A. 2001;98:7754–7759. doi: 10.1073/pnas.131549798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allin C, Gerwert K. Biochemistry. 2001;40:3037–3046. doi: 10.1021/bi0017024. [DOI] [PubMed] [Google Scholar]
- 82.Cepus V, Scheidig AJ, Goody RS, Gerwert K. Biochemistry. 1998;37:10263–10271. doi: 10.1021/bi973183j. [DOI] [PubMed] [Google Scholar]
- 83.Grigorenko BL, Nemukhin AV, Shadrina MS, Topol IA, Burt SK. Proteins. 2007;66:456–466. doi: 10.1002/prot.21228. [DOI] [PubMed] [Google Scholar]
- 84.Grigorenko BL, Nemukhin AV, Topol IA, Cachau RE, Burt SK. Proteins. 2005;60:495–503. doi: 10.1002/prot.20472. [DOI] [PubMed] [Google Scholar]
- 85.Nemukhin AV, Shadrina MS, Grigorenko BL, Du X. Biochemistry Biokhimiia. 2009;74:1044–1048. doi: 10.1134/s0006297909090132. [DOI] [PubMed] [Google Scholar]
- 86.B RP, Plotnikov NV, Lameira J, Warshel A. Proc Natl Acad Sci U S A. 2013;110:20509–20514. doi: 10.1073/pnas.1319854110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maegley KA, Admiraal SJ, Herschlag D. Proc Natl Acad Sci U S A. 1996;93:8160–8166. doi: 10.1073/pnas.93.16.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freissmuth M, Gilman AG. J Biol Chem. 1989;264:21907–21914. [PubMed] [Google Scholar]
- 89.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 90.Van Dop C, Tsubokawa M, Bourne H, Ramachandran J. J Biol Chem. 1984;259:696–698. [PubMed] [Google Scholar]
- 91.Graziano MP, Gilman AG. J Biol Chem. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 92.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 93.Lyons J, Landis CA, Harsh G, Vallar L, Grünewald K, Feichtinger H, Duh Q-Y, Clark OH, Kawasaki E, Bourne HR, McCormick F. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- 94.O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. Nature reviews Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller RT, Masters SB, Sullivan KA, Beiderman B, Bourne HR. Nature. 1988;334:712–715. doi: 10.1038/334712a0. [DOI] [PubMed] [Google Scholar]
- 96.Lee E, Taussig R, Gilman A. J Biol Chem. 1992;267:1212–1218. [PubMed] [Google Scholar]
- 97.Berghuis AM, Lee E, Raw AS, Gilman AG, Sprang SR. Structure. 1996;4:1277–1290. doi: 10.1016/s0969-2126(96)00136-0. [DOI] [PubMed] [Google Scholar]
- 98.Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 99.Raw AS, Coleman DE, Gilman AG, Sprang SR. Biochemistry. 1997;36:15660–15669. doi: 10.1021/bi971912p. [DOI] [PubMed] [Google Scholar]
- 100.Barbacid M. Ann Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 101.Thomas CJ, Du X, Li P, Wang Y, Ross EM, Sprang SR. Proc Natl Acad Sci U S A. 2004;101:7560–7565. doi: 10.1073/pnas.0304091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hall BE, Bar-Sagi D, Nassar N. Proc Natl Acad Sci U S A. 2002;99:12138–12142. doi: 10.1073/pnas.192453199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Posner BA, Mukhopadhyay S, Tesmer JJ, Gilman AG, Ross EM. Biochemistry. 1999;38:7773–7779. doi: 10.1021/bi9906367. [DOI] [PubMed] [Google Scholar]
- 104.Phillips WJ, Cerione RA. J Biol Chem. 1986;263:15498–15505. [PubMed] [Google Scholar]
- 105.Faurobert E, Otto-Bruc A, Chardin P, Chabre M. Journal of the European Molecular Biology Organization. 1993;12:4191–4198. doi: 10.1002/j.1460-2075.1993.tb06103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guy PM, Koland JG, Cerione RA. Biochemistry. 1990;29:6954–6964. doi: 10.1021/bi00482a003. [DOI] [PubMed] [Google Scholar]
- 107.Lee E, Linder M, Gilman A. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- 108.Remmers AE, Posner R, Neubig RR. J Biol Chem. 1994;269:13771–13778. [PubMed] [Google Scholar]
- 109.Dohlman HG. Progress in molecular biology and translational science. 2009;86:1–14. doi: 10.1016/S1877-1173(09)86001-8. [DOI] [PubMed] [Google Scholar]
- 110.Chidiac P, Ross EM. J Biol Chem. 1999;274:19639–19643. doi: 10.1074/jbc.274.28.19639. [DOI] [PubMed] [Google Scholar]
- 111.Turcotte M, Tang W, Ross EM. PLoS computational biology. 2008;4:e1000148. doi: 10.1371/journal.pcbi.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mukhopadhyay S, Ross EM. Proc Natl Acad Sci U S A. 1999;96:9539–9544. doi: 10.1073/pnas.96.17.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ross EM. Curr Biol. 2008;18:R777–R783. doi: 10.1016/j.cub.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sternweis PC, Carter AM, Chen Z, Danesh SM, Hsiung YF, Singer WD. Advances in protein chemistry. 2007;74:189–228. doi: 10.1016/S0065-3233(07)74006-8. [DOI] [PubMed] [Google Scholar]
- 115.Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 116.Berman DM, Kozasa T, Gilman AG. J Biol Chem. 1996;271:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 117.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 118.Soundararajan M, Willard FS, Kimple AJ, Turnbull AP, Ball LJ, Schoch GA, Gileadi C, Fedorov OY, Dowler EF, Higman VA, Hutsell SQ, Sundstrom M, Doyle DA, Siderovski DP. Proc Natl Acad Sci U S A. 2008;105:6457–6462. doi: 10.1073/pnas.0801508105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slep KC, Kercher MA, Wieland T, Chen CK, Simon MI, Sigler PB. Proc Natl Acad Sci U S A. 2008;105:6243–6248. doi: 10.1073/pnas.0801569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nance MR, Kreutz B, Tesmer VM, Sterne-Marr R, Kozasa T, Tesmer JJ. Structure. 2013;21:438–448. doi: 10.1016/j.str.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berman DM, Wilkie TM, Gilman AG. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 122.Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- 123.Khafizov K, Lattanzi G, Carloni P. Proteins. 2009;75:919–930. doi: 10.1002/prot.22303. [DOI] [PubMed] [Google Scholar]
- 124.Marin EP, Krishna AG, Sakmar TP. J Biol Chem. 2001;276:27400–27405. doi: 10.1074/jbc.C100198200. [DOI] [PubMed] [Google Scholar]
- 125.Kaya AI, Lokits AD, Gilbert JA, Iverson TM, Meiler J, Hamm HE. J Biol Chem. 2014;289:24475–24487. doi: 10.1074/jbc.M114.572875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun D, Flock T, Deupi X, Maeda S, Matkovic M, Mendieta S, Mayer D, Dawson RJ, Schertler GF, Babu MM, Veprintsev DB. Nat Struct Mol Biol. 2015;22:686–694. doi: 10.1038/nsmb.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Posner BA, Mixon MB, Wall MA, Sprang SR, Gilman AG. J Biol Chem. 1998;273:21752–21758. doi: 10.1074/jbc.273.34.21752. [DOI] [PubMed] [Google Scholar]
- 128.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Nat Struct Mol Biol. 2006;13:772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 129.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Proc Natl Acad Sci U S A. 2007;104:7927–7932. doi: 10.1073/pnas.0702623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Eps N, Preininger AM, Alexander N, Kaya AI, Meier S, Meiler J, Hamm HE, Hubbell WL. Proc Natl Acad Sci U S A. 2011;108:9420–9424. doi: 10.1073/pnas.1105810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ceruso MA, Periole X, Weinstein H. J Mol Biol. 2004;338:469–481. doi: 10.1016/j.jmb.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 132.Alexander NS, Preininger AM, Kaya AI, Stein RA, Hamm HE, Meiler J. Nat Struct Mol Biol. 2014;21:56–63. doi: 10.1038/nsmb.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH, Philippsen A, Villanueva N, Yang Z, Lerch MT, Hubbell WL, Kobilka BK, Sunahara RK, Shaw DE. Science. 2015;348:1361–1365. doi: 10.1126/science.aaa5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flock T, Ravarani CN, Sun D, Venkatakrishnan AJ, Kayikci M, Tate CG, Veprintsev DB, Babu MM. Nature. 2015;524:173–179. doi: 10.1038/nature14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manglik A, Kobilka B. Curr Opin Cell Biol. 2014;27:136–143. doi: 10.1016/j.ceb.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thaker TM, Kaya AI, Preininger AM, Hamm HE, Iverson TM. Methods in molecular biology. 2012;796:133–174. doi: 10.1007/978-1-61779-334-9_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hamm HE, Deretic D, Arendt A, Hargrave PA, Koenig B, Hofmann KP. Science. 1988;241:832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- 138.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 139.Van Eps N, Thomas CJ, Hubbell WL, Sprang SR. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1404–1409. doi: 10.1073/pnas.1423878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stewart A, Fisher RA. Progress in molecular biology and translational science. 2015;133:1–11. doi: 10.1016/bs.pmbts.2015.03.002. [DOI] [PubMed] [Google Scholar]