Abstract
Background
The specific causes of recurrent spontaneous abortion (RSA) remain unknown in 37–79% of affected women. The aim of this study was to explore the expression levels of 6 miRNAs in natural killer (NK) cells from the decidua of patients with unexplained RSA (URSA) and to predict the target genes of 3 miRNAs.
Material/Methods
Two groups were examined: URSA (n=20) and controls (n=20). Flow cytometry analysis was used to identify NK cells isolated from the decidua. Transcriptional levels of miRNA were monitored using quantitative real-time reverse transcription-polymerase chain reaction. Prediction and analysis of mRNA targets of differentially expressed miRNAs were performed using bioinformatics methods.
Results
Five miRNAs [miR-34a (+281%, P<0.001), miR-155 (+396%, P<0.001), miR-141 (+142%, P<0.01), miR-125a (+279%, P<0.001), and miR-125b (+185%, P<0.001)] were up-regulated, while miR-24 was down-regulated (−64%, P<0.01) in the URSA group, compared to the control group. This study identified potential miRNA targets: miR-34a-3p/5p, 585/1718 (targets of miR-34a-3p/targets of miR-34a-5p), miR-141-3p/5p, 2270/629 (targets of miR-141-3p/targets of miR-141-5p), and miR-24, 2320 target genes. A total of 140 pathways related to target genes were identified including PI3K-Akt, focal adhesion, MAPK, Wnt, regulation of the actin cytoskeleton, T cell receptor, TGF-β, and estrogen signaling pathways.
Conclusions
This study suggests that miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in decidual NK cells could be associated with URSA. These findings might contribute to the panel of diagnostic and prognostic biomarkers with clinical utility, and facilitate the development of new strategies for targeted therapy against URSA.
MeSH Keywords: Abortion, Induced; Killer Cells, Natural; MicroRNAs
Background
Recurrent spontaneous abortion (RSA) is defined as 3 or more consecutive pregnancy losses before the 22nd gestational week or spontaneous abortion of an embryo/fetus weighing less than 500 g [1]. Diagnosis of RSA requires multiple tests to detect parental chromosomal anomalies and maternal thrombophilic, endocrine, or immunological disorders. However, the specific causes of RSA remain unknown in 37–79% of affected women [1,2]. Over 50% of RSA cases are classified as idiopathic unexplained RSA (URSA) and the mechanisms of URSA are not completely understood [3]. Parental exposure to phenol-based pollutants might play a role in URSA [4], as well as polymorphisms in genes such as methylene tetrahydrofolate reductase and methionine synthase reductase [5]. Nevertheless, the immune system is considered one of the main culprits. Indeed, studies have shown that Th17 cells play a major role in rejecting conceptus antigens due to a Th17/Treg imbalance in URSA [6,7]. In addition, the Th1/Th2 ratio also plays a role in URSA [8–10], as well as the aberrant expression of the human leukocyte antigen (HLA) [11], dysfunction of uterine natural killer (NK) cells [12], and the presence of some auto-antibodies [13]. Immunotherapy with mononuclear cells from the father has been shown to bias the Th17/Treg ratio and to be beneficial for pregnancy.
The decidua and the immune cells residing in it play roles in the maintenance of pregnancy. The decidua invades the uterine mucosa during the first trimester of pregnancy and is thought to be critical for controlling the depth of invasion of trophoblasts. The decidua contains a unique complement of immune cells comprising 70% of NK cells [14]. Decidual NK cells differ from blood NK cells, both phenotypically and functionally. A number of studies have suggested that decidual NK cells play a role in mediating trophoblast invasion and vascular remodeling through their ability to secrete an array of regulatory molecules, chemokines, and cytokines [15]. Therefore, these cells might play a critical role in the occurrence of URSA, but their regulation is still poorly known.
MicroRNAs (miRNAs) are a class of small non-coding regulatory RNAs influencing mRNA translation via direct interaction and/or degradation; they are involved in the control of a variety of cellular functions, including proliferation, stem cell maintenance, differentiation, cell death, and metabolism [16]. Recent studies have indicated involvement of aberrant miRNAs and their target genes in URSA. These studies have shown that miRNA-133a and its target HLA-G, miRNA-125 and its target LIFR/ERBB2, and miRNA-17 were dysregulated, which are involved in immune system regulation and cell proliferation [17–19]. A study has shown that 5 miRNAs were upregulated in the decidua of patients with RSA and that these miRNAs are involved in adhesion, apoptosis, and angiogenesis [20]. Higher expression of miR-486-3p and lower expression of miR-3074-5p in placental villa might also play a role in RSA [21]. In parallel, studies have revealed the ability of some miRNAs to regulate cell proliferation and apoptosis [22,23], which are critical processes of normal embryogenesis [24]. Among candidates miRNAs, members of the miRNA-34 family (miR-34a, miR-34b, and miR-34c) are direct transcriptional targets of the tumor suppressor protein p53, with the potential to regulate both apoptosis and cell proliferation [25]. miR-155 has been shown to regulate apoptosis and was proposed to target caspase 3 and NF-κB signaling [26, 27]. miRNA-125b is suggested to control expression of the tumor necrosis factor α (TNF-α) [28], a powerful activator cytokine of NF-κB [29]. miR-24 has potential target sites in the 3′UTR region of IFN-γ mRNA and negatively regulates IFN-g expression. This miRNA controls a complex network of apoptotic and angiogenic pathways in endothelial cells [30,31]. miR-141, a member of the miR-200 family, is reportedly associated with various human malignancies. For instance, miR-141 is upregulated in ovarian cancer [32], but downregulated in hepatocellular and prostate cancers [33,34].
Therefore, this study aimed to examine the expression of miR-34a, miR-155, miR-141, miR-125a, miR-125b, and miR-24 in URSA. This study identified miRNAs that might be involved in URSA, as well as their target genes. This new knowledge could be used in the design of novel strategies against URSA.
Material and Methods
Patients and samples
All study participants were Chinese and recruited at the Family Planning Department, Beijing Obstetrics and Gynecology Hospital, Capital Medical University (China), between August 2014 and October 2014. The RSA group consisted of 20 childless Chinese women aged 25–35 (31.0±2.87) years with at least 3 or more successive spontaneous abortions at 7–10 (56.4±3.6 days) gestational weeks. Subjects did not suffer from systemic diseases, such as chronic renal failure, heart failure, agglutination disorders, lupus, ovarian polycystic, maternal reproductive anatomical hormonal abnormalities, paternal and maternal chromosomal abnormalities, or infection. RSA was detected using ultrasound. The control group included 20 fertile Chinese women aged 25–35 (29.9±2.0) years at 7–10 (57.9±4.3 days) gestational weeks with 1 or more children and no history of spontaneous abortion. Normal pregnancies were also detected using ultrasound. Decidual tissues were obtained from cases of induced abortion (IA) at about 7 gestational weeks. All patients provided written informed consent, and the study protocol was approved by the Ethics Committee of the Beijing Obstetrics and Gynecology Hospital, Capital Medical University.
Flow cytometry
Decidual tissues were cut into 1-mm pieces and enzymatically digested for 20 min using vigorous shaking and 1.5 mg of type I DNAse and 24 mg of type IV collagenase in 15 mL of RPMI-1640 medium (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA). This procedure was repeated 3 times. After an additional 5 min of incubation at room temperature without shaking, the supernatants were collected and loaded on Ficoll density gradient to purify the lymphocyte population. NK cells were purified using the human NK cell isolation kit (BD Biosciences, Franklin Lake, NJ, USA). The following mouse anti-human monoclonal antibodies (mAbs) conjugated with PC5 and PE were used for cell sorting: anti-CD56 and anti-CD3 (all from BD Biosciences, Franklin Lake, NJ, USA), and matching conjugated isotype controls. For staining and cell sorting, cells were washed in PBS supplemented with 2% FCS and incubated with mAbs on ice for 30 min, followed by washing twice. Cell sorting and fluorescence measurements were performed on a MoFlo high-performance cell sorter (Cytomation, Dako, Glostrup, Denmark). Data from single cell events were collected using a standard FACScalibur™ flow cytometer (Immunocytometry systems; BD Biosciences, Franklin Lake, NJ, USA).
RNA extraction and qRT-PCR
Transcriptional levels of miRNAs were monitored using quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR). Total RNA was extracted using TRIzol total RNA isolation reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. The quality of RNA was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) [35]. Total RNA (1 μg) was used for cDNA synthesis with the First-Strand cDNA Synthesis Kit (Fermentas, Burlington, Canada). Real-time PCR was performed as follows: 95°C for 15 min for hot-start, followed by 95°C for 30 s, and 60°C for 60 s for 40 cycles, using the SYBR Green Quantitative PCR Master Mix (Applied Biosystems, Foster City, CA, USA) on a Bio-Rad IQ5 instrument (Bio-Rad, Hercules, CA, USA). Primers for amplification of microRNA (RiboBio, Guangzhou, China) are listed in Table 1. Each reaction was performed in triplicate and measured using the comparative Ct (2−ΔΔCt) method with U6 snRNA as the normalization control.
Table 1.
Primers for real-time PCR.
| microRNA | Forward primer | Reverse primer |
|---|---|---|
| miRNA34a | 5′-CTCGCTTCGGCAGCACA -3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-24 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-155 | 5′-CTCGCTTCGGCAGCACA -3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-141 | 5′-CTCGCTTCGGCAGCACA -3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-125a/b | 5′-CTCGCTTCGGCAGCACA -3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| U6 snRNA | 5′-CTCGCTTCGGCAGCACA -3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
Prediction and analysis of mRNA targets of 3 differentially expressed miRNAs
Prediction of differentially expressed miRNA target genes was performed using the TargetScan 5.0 database (UK). Data on intersecting predicted target genes of differentially expressed miRNAs were collected. The negative regulatory relationships between miRNAs and genes were highlighted for analysis. Similarly, pathway analysis was used to determine the significant pathways negatively correlated with intersection of target genes according to the Kyoto encyclopedia of genes and genomes (KEGG, Japan), Biocarta (Germany), and Reatome (USA) databases [36,37].
Statistical analysis
SPSS 16.0 (IBM, Armonk, NY, USA), was used for data analysis. Data are presented as means ± standard deviation (SD). Statistical significance was evaluated by independent sample t-test. P<0.05 was considered statistically significant.
For pathway analysis, the Fisher’s exact and the chi-square tests were used to obtain the P and P(k) values to select significant pathways. The false discovery rate (FDR) was used to determine the threshold of the P-value in multiple tests and analyses. Significant pathways were considered as being those with FDR <0.05 and P<0.05.
A post hoc power analysis was performed. miR-141 was selected since it was the one with the smallest difference between the 2 groups. For the comparison of relative miR-141 expression levels between controls and patients with URSA, the power was 100% (n=20 and n=20, respectively, α=5%).
Results
Identification of decidual NK cells
Figure 1 shows that the purity of CD56+ CD3− NK cells in decidua were more than 70%. These cells were sorted using flow cytometric analysis and used for the subsequent experiments.
Figure 1.
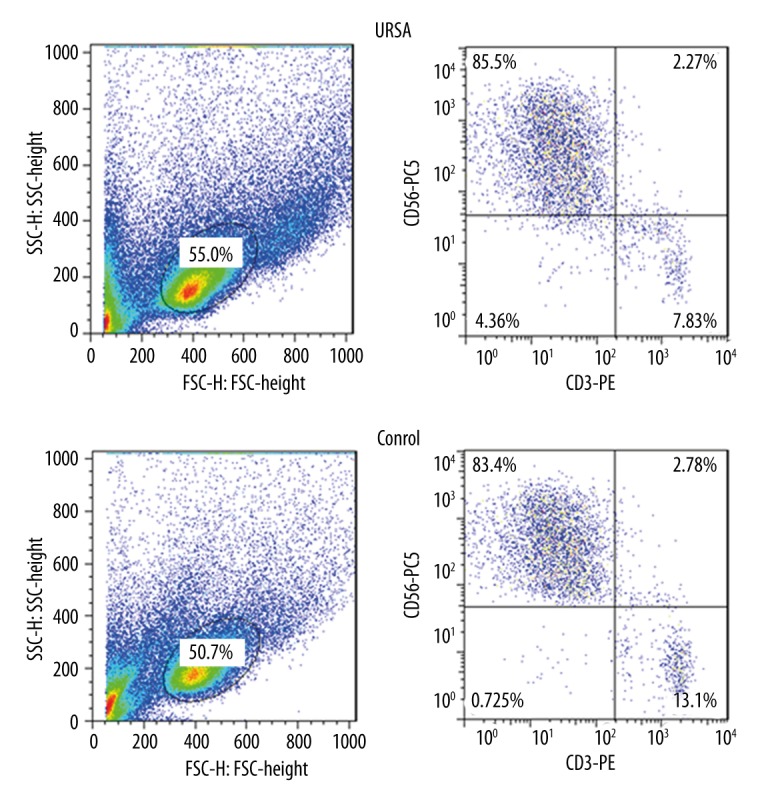
Identification of decidual CD56+ CD3− NK cells from patients with URSA and controls. The purity of CD56+ CD3− NK cells in decidua was more than 70%. These cells were sorted using flow cytometric analysis. URSA – unexplained recurrent spontaneous abortion.
Expression levels of miRNA in decidual NK cells from patients with URSA
The expression levels of the 6 identified miRNAs were evaluated using qRT-PCR. Five of the miRNAs [miR-34a (+281%, P<0.001), miR-155 (+396%, P<0.001), miR-141 (+142%, P<0.01), miR-125a (+279%, P<0.001), and miR-125b (+185%, P<0.001)] were up-regulated, while miR-24 was down-regulated (−64%, P<0.01) in the URSA group, compared to the control group (Figure 2). The data indicate that dysregulation of these specific miRNA expression patterns might be associated with URSA.
Figure 2.

Quantification of miRNA expression levels in the URSA and control groups using real-time RT-PCR. Compared to the control group, 5 miRNAs (miR-34a, miR-155, miR-141, miR-125a, and miR-125b) were up-regulated, while miR-24 was down-regulated. ** P<0.01, *** P<0.001.
Target gene prediction and pathway analysis
Three of the above miRNAs (miR-34a, miR-141, and miR-24) were subjected to further target gene prediction and functional analyses. Available databases were used to compile potential targets for all the miRNA genes, leading to the identification of miR-34a-3p/5p, 585/1718 (number of target genes of miR-34a-3p/number of target genes of miR-34a-5p), miR-141-3p/5p, 2270/629 (number of target genes of miR-141-3p/number of target genes of miR-141-5p), and miR-24, 2320 target genes. KEGG analyses were subsequently performed to determine the biological pathways of the target genes. A total of 140 target gene-related pathways were identified (Supplementary Figure 1), among which the 29 most significant pathways are shown in Figure 3, including PI3K-Akt, focal adhesion, MAPK, Wnt, regulation of the actin cytoskeleton, T cell receptor, TGF-β, and estrogen signaling pathways (Figure 3).
Figure 3.

KEGG pathway analysis of predicted targets of 3 miRNAs. The analysis revealed 140 pathways (see the Supplementary Figure 1). The 29 most significant pathways are presented, including PI3K-Akt, focal adhesion, MAPK, Wnt, regulation of the actin cytoskeleton, T cell receptor, TGF-beta, and estrogen signaling pathways.
Discussion
The specific causes of RSA remain unknown in 37–79% of affected women. Evidence suggests that the immune system is involved in URSA, but the precise mechanisms are poorly understood. Therefore, this study aimed to explore the expression levels of 6 miRNAs in NK cells from the decidua of patients with URSA and to predict the target genes of 3 miRNAs. Results showed that 5 miRNAs (miR-34a, miR-155, miR-141, miR-125a, and miR-125b) were up-regulated, while miR-24 was down-regulated in the URSA group, compared to the control group. This study identified potential miRNA targets, such as miR-34a-3p/5p, 585/1718, miR-141-3p/5p, 2270/629, and miR-24, 2320, and 140 pathways related to target genes were identified including PI3K-Akt, focal adhesion, MAPK, Wnt, regulation of the actin cytoskeleton, T cell receptor, TGF-β, and estrogen signaling pathways. These results suggest some pathways could be involved in URSA. These pathways should be explored because related treatments could be possible.
In this study, decidual CD56+ CD3− NK cells were isolated and flow cytometric analysis revealed a purity of more than 70%. This profile of expression is deemed sufficient to correctly isolate NK cells from the decidua [38,39]. Furthermore, the use of cell sorting allowed the use of a relatively pure population of cells.
As previously shown, members of the miR-34 family are direct regulators of p53 and are involved in the regulation of apoptosis and cell proliferation [25]. No previous study is available showing a possible association between miR-34 and RSA. Nevertheless, previous studies revealed that p53 is involved in URSA. Indeed, Shang et al. [40] showed that the p53-CDKN1A and p53-Bax pathways were activated in RSA, while Wei et al. [41] showed that the placental villi from patients with URSA expressed high levels of p53. A previous study in mice revealed that miR-141 might influence cell proliferation and apoptosis in the endometrium by downregulating the expression of PTEN and that miR-141 plays important roles in embryo implantation [42]. miR-24 is aberrantly expressed in polycystic ovary syndrome, which is in itself associated with pregnancy problems [43]. In addition, miR-24 downregulates MYC, a key proliferative factor involved in embryo implantation [43]. However, the exact relation between these miRNAs and their targets is still poorly understood. Some of the data presented above suggest possible beneficial effects of their miRNAs on pregnancy, but the present study suggests detrimental effects. Additional studies are necessary to address these issues.
Pathway analysis for predicted target genes of miR-34a, miR-141, and miR-24 revealed association with numerous pathways, including PI3K-Akt, MAPK, Wnt, T cell receptor, and TGF-β signaling, all of which regulate discrete cellular functions and interact as a network. The PI3K-Akt signaling pathway plays a significant role in cell survival. Activation of this pathway inhibits apoptosis, allowing cell proliferation [44]. The mitogen-activated protein kinase (MAPK) cascade is a highly conserved module involved in various cellular functions, including proliferation, differentiation, and migration [45].
This study is not without limitations. First, CD56+CD3− NK cells could comprise more than 1 population of cells. Future studies should aim to better characterize these cells. Second, only a few miRNAs were investigated. Third, the targets of the identified miRNAs were not directly assessed. Future studies should examine these target proteins in URSA. Further comprehensive research, such as genome-wide association analyses, may be required to facilitate the identification of more effective targeted treatments and significant markers in URSA.
Conclusions
This study suggests that miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in decidual NK cells are associated with URSA. These findings might contribute to the panel of diagnostic and prognostic biomarkers with clinical utility, and facilitate the development of new strategies for targeted therapy against URSA.
Supplementary materials
A total of 140 target gene-related pathways.
Footnotes
Competing interests
All authors declare that they have no conflict of interest.
Source of support: This work was supported in part by funds from the National Natural Sciences Foundation of China (No. 81270733) and the Beijing Natural Science Foundation (No: 7132097)
References
- 1.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24–29. [PubMed] [Google Scholar]
- 2.Hatasaka HH. Recurrent miscarriage: epidemiologic factors, definitions, and incidence. Clin Obstet Gynecol. 1994;37:625–34. doi: 10.1097/00003081-199409000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Li TC, Makris M, Tomsu M, et al. Recurrent miscarriage: Aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–81. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Chen M, Xu B, et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere. 2013;93:217–22. doi: 10.1016/j.chemosphere.2013.04.067. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L. Polymorphisms in the methylene tetrahydrofolate reductase and methionine synthase reductase genes and their correlation with unexplained recurrent spontaneous abortion susceptibility. Genet Mol Res. 2015;14:8500–8. doi: 10.4238/2015.July.28.19. [DOI] [PubMed] [Google Scholar]
- 6.Liu YS, Wu L, Tong XH, et al. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–11. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu M, Liu P, Cheng L. Galectin-1 reduction and changes in T regulatory cells may play crucial roles in patients with unexplained recurrent spontaneous abortion. Int J Clin Exp Pathol. 2015;8:1973–78. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M, Zhu Y, Zhao J, et al. Soluble costimulatory molecule sTim3 regulates the differentiation of Th1 and Th2 in patients with unexplained recurrent spontaneous abortion. Int J Clin Exp Med. 2015;8:8812–19. [PMC free article] [PubMed] [Google Scholar]
- 9.Szpakowski A, Malinowski A, Glowacka E, et al. [The influence of paternal lymphocyte immunization on the balance of Th1/Th2 type reactivity in women with unexplained recurrent spontaneous abortion]. Ginekol Pol. 2000;71:586–92. [in Polish] [PubMed] [Google Scholar]
- 10.Rezaei A, Dabbagh A. T-helper (1) cytokines increase during early pregnancy in women with a history of recurrent spontaneous abortion. Med Sci Monit. 2002;8(8):CR607–10. [PubMed] [Google Scholar]
- 11.Pfeiffer KA, Fimmers R, Engels G, et al. The HLA-G genotype is potentially associated with idiopathic recurrent spontaneous abortion. Mol Hum Reprod. 2001;7:373–78. doi: 10.1093/molehr/7.4.373. [DOI] [PubMed] [Google Scholar]
- 12.Park DW, Lee HJ, Park CW, et al. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol. 2010;63:173–80. doi: 10.1111/j.1600-0897.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalra S, Tuli A, Choudhry R, Raheja S. Prevalence of anticardiolipin antibody IgG in recurrent first trimester abortions and the role of aspirin in its prevention. Med Sci Monit. 2003;9(6):CR213–16. [PubMed] [Google Scholar]
- 14.King A, Balendran N, Wooding P, et al. CD3– leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol. 1991;1:169–90. doi: 10.1155/1991/83493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalkunte SS, Mselle TF, Norris WE, et al. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–92. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan Q, Chegini N. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med. 2008;26:479–93. doi: 10.1055/s-0028-1096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Li B, Wang J, et al. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod Biomed Online. 2012;25:415–24. doi: 10.1016/j.rbmo.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Liu CM, Qi L, et al. Two common SNPs in pri-miR-125a alter the mature miRNA expression and associate with recurrent pregnancy loss in a Han-Chinese population. RNA Biol. 2011;8:861–72. doi: 10.4161/rna.8.5.16034. [DOI] [PubMed] [Google Scholar]
- 19.Ventura W, Koide K, Hori K, et al. Placental expression of microRNA-17 and -19b is down-regulated in early pregnancy loss. Eur J Obstet Gynecol Reprod Biol. 2013;169:28–32. doi: 10.1016/j.ejogrb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Dong F, Zhang Y, Xia F, et al. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction. 2014;148:33–41. doi: 10.1530/REP-14-0095. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, Zhang X, Yang Q, et al. Aberrant placental villus expression of miR-486-3p and miR-3074-5p in recurrent miscarriage patients and uterine expression of these MicroRNAs during early pregnancy in mice. Gynecol Obstet Invest. 2015 doi: 10.1159/000435879. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle. 2007;6:2127–32. doi: 10.4161/cc.6.17.4641. [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–87. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 24.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–54. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 25.He X, He L, Hannon GJ. The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 26.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Teng G, Papavasiliou FN. Shhh! Silencing by microRNA-155. Philos Trans R Soc Lond B Biol Sci. 2009;364:631–37. doi: 10.1098/rstb.2008.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–89. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 29.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–77. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 30.Fayyad-Kazan H, Hamade E, Rouas R, et al. Downregulation of microRNA-24 and -181 parallels the upregulation of IFN-gamma secreted by activated human CD4 lymphocytes. Hum Immunol. 2014;75:677–85. doi: 10.1016/j.humimm.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Fiedler J, Jazbutyte V, Kirchmaier BC, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–30. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 32.Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Ding Y, Huang J, et al. MiR-141 suppresses the migration and invasion of HCC cells by targeting Tiam1. PLoS One. 2014;9:e88393. doi: 10.1371/journal.pone.0088393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porkka KP, Pfeiffer MJ, Waltering KK, et al. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–35. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 35.Moret I, Sanchez-Izquierdo D, Iborra M, et al. Assessing an improved protocol for plasma microRNA extraction. PLoS One. 2013;8:e82753. doi: 10.1371/journal.pone.0082753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Draghici S, Khatri P, Tarca AL, et al. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–45. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi M, Horton JD, Cohen JC, et al. WholePathwayScope: A comprehensive pathway-based analysis tool for high-throughput data. BMC Bioinformatics. 2006;7:30. doi: 10.1186/1471-2105-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bouteiller P. Human decidual NK cells: Unique and tightly regulated effector functions in healthy and pathogen-infected pregnancies. Front Immunol. 2013;4:404. doi: 10.3389/fimmu.2013.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vacca P, Pietra G, Falco M, et al. Analysis of natural killer cells isolated from human decidua: Evidence that 2B4 (CD244) functions as an inhibitory receptor and blocks NK-cell function. Blood. 2006;108:4078–85. doi: 10.1182/blood-2006-04-017343. [DOI] [PubMed] [Google Scholar]
- 40.Shang W, Shu MM, Liu M, et al. Elevated expressions of p53, CDKNA1, and Bax in placental villi from patients with recurrent spontaneous abortion. Eur Rev Med Pharmacol Sci. 2013;17:3376–80. [PubMed] [Google Scholar]
- 41.Wei D, Wu Q, Shi H. Apoptosis and p53 expression in the placental villi of females with unexplained recurrent spontaneous abortion. Exp Ther Med. 2014;7:191–94. doi: 10.3892/etm.2013.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Gao R, Chen X, et al. Possible roles of mmu-miR-141 in the endometrium of mice in early pregnancy following embryo implantation. PLoS One. 2013;8:e67382. doi: 10.1371/journal.pone.0067382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeon YJ, Kim SY, Rah H, et al. Association of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and miR-499A>G polymorphisms with risk of spontaneously aborted fetuses. Am J Reprod Immunol. 2012;68:408–17. doi: 10.1111/aji.12005. [DOI] [PubMed] [Google Scholar]
- 44.Osaki M, Kase S, Adachi K, et al. Inhibition of the PI3K-Akt signaling pathway enhances the sensitivity of Fas-mediated apoptosis in human gastric carcinoma cell line, MKN-45. J Cancer Res Clin Oncol. 2004;130:8–14. doi: 10.1007/s00432-003-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei YY, Wang WJ, Mei JH, Wang CL. Mitogen-activated protein kinase signal transduction in solid tumors. Asian Pac J Cancer Prev. 2014;15:8539–48. doi: 10.7314/apjcp.2014.15.20.8539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 140 target gene-related pathways.


