Abstract
We previously reported that dietary acrylamide, at doses (10 and 50 mg/kg diet) known to cause rodent tumors, lowered serum total high density lipoprotein and total testosterone, increased serum lipase, and lowered lymphocytes levels together with other hematological parameters in male F344 rats exposed for 10 weeks (doi: 10.1016/j.etap.2014.11.009 [1]). Here we present data related to the role of food-borne acrylamide exposure (at 0, 5, 10 and 50 mg/kg diet) in the presence of low (7% wt/wt) or high (23.9% wt/wt) dietary fat on serum and urinary markers of oxidative stress and inflammation in F344 rats. Briefly, urine and serum samples were collected from the experimental animals a day prior to or at the time of necropsy, respectively and processed for enzyme-linked immunosorbent assay estimations of biochemical markers. Urine samples were analyzed for 8-hydroxydeoxyguanosine and isoprostane, and serum samples for total antioxidant capacity, paraoxonase 1 activity, c-reactive protein, homocysteine, oxidized low-density lipoprotein, intercellular adhesion molecule-1, thromboxin 2, and Nε-(carboxymethyl)lysine.
Keywords: Acrylamide, Food safety, Systemic toxicity, Oxidative stress, Inflammation, 8-Hydroxydeoxyguanosine, Isoprostane, Total antioxidant capacity, Paraoxonase 1, C-reactive protein, Homocysteine, Oxidized low-density lipoprotein, Intercellular adhesion molecule-1, Thromboxin 2, Nε-(carboxymethyl)lysine
Specifications Table
| Subject area | Toxicology |
| More specific subject area | Food toxicology, systemic oxidative stress and inflammation |
| Type of data | Figures, Table. |
| How data was acquired | All data was acquired using the POLARstar Omega multi-mode microplate reader(BMG LABTECH Inc., Cary, NC, USA) |
| Data format | Analyzed data |
| Experimental factors | Urine and serum from rats exposed to dietary acrylamide were processed and individual enzyme-linked immunosorbent assays (ELISAs) were performed to investigate markers of oxidative stress and inflammation. |
| Experimental features | Male F344 rats were fed an AIN−93 G basal diet containing low fat (7% wt/wt) or high fat (23.9% wt/wt) and 0, 5, 10, or 50 mg/kg diet acrylamide for 8 weeks. Rats were placed in metabolic cages 24 h before necropsy for urine sample collection. Blood samples were collected from abdominal aorta at necropsy |
| Data source location | Ottawa, Ontario, Canada |
| Data accessibility | Data is with this article |
Value of the data
-
•
We explored the role of chronic exposure to food-borne acrylamide in modulating markers of systemic oxidative stress and inflammation in rats under low and high fat diet conditions.
-
•
This systemic biochemical data will support previous findings of acrylamide exposure at doses known to cause rodent tumors.
-
•
Our data will be beneficial in updating the existing toxicity information available on food-borne acrylamide for regulatory purposes.
1. Data
The present dataset includes results of biochemical estimations that determined markers of systemic oxidative stress and inflammation in urine and serum samples of F344 rats exposed to dietary acrylamide (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10; Table 2). These results of individual biochemical markers are to be interpreted with the clinical biochemistry, hematology and pathology data previously reported [1].
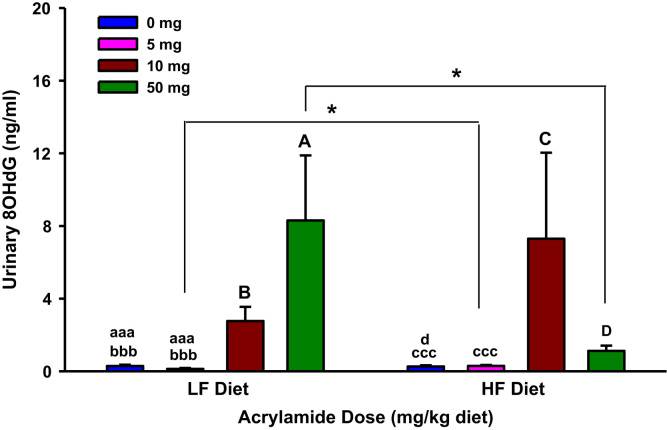
Fig. 1.
Urinary 8-hydroxydeoxyguanosine (8OHdG) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “aaa”, “bbb”, and “ccc” are significantly different from “A”, “B”, and “C” respectively at p<0.001. ”d” is significantly different from “D” at p<0.05. “*” indicates significant difference at p<0.05.
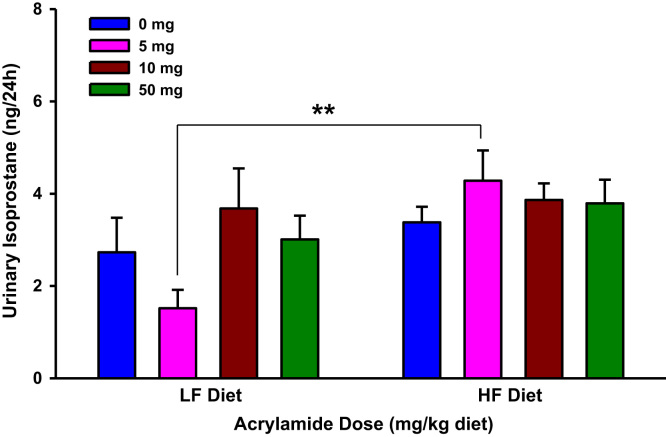
Fig. 2.
Urinary isoprostane levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “*” indicates significant difference at p<0.05.
Fig. 3.
Serum total antioxidant capacity (TAC) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM.
Fig. 4.
Serum paraoxonase 1 (PON1) activity in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “a” is significantly different from “A” at p<0.05. “*” indicates significant difference at p<0.05.
Fig. 5.
Serum c-reactive protein (CRP) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “a” and “b” are significantly different from “A” and “B” at p<0.05, respectively. “aa” is significantly different from “A” at p<0.01. “*” and “**” indicate significant difference at p<0.05 and p<0.01, respectively.
Fig. 6.
Serum homocysteine level in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “aaa” is significantly different from “A” at p<0.001.
Fig. 7.
Serum oxidized low-density lipoprotein (Ox-LDL) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet), n=8/group. The histograms represent mean values±SEM.
Fig. 8.
Serum intercellular adhesion molecule-1 (ICAM-1) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM. “aaa” is significantly different from “A” at p<0.001. “*” and “**” indicate significant difference at p<0.05 and p<0.01, respectively.
Fig. 9.
Serum thromboxin 2 (TBX2) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet, n=8/group. The histograms represent mean values±SEM.
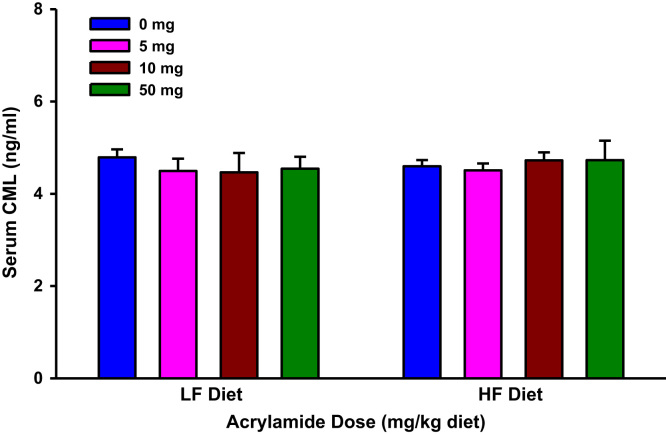
Fig. 10.
Serum Nε-(carboxymethyl)lysine (CML) levels in rats fed low fat (LF) or high fat (HF) diet and treated with acrylamide at 0, 5, 10 or 50 mg/kg diet. The histograms represent mean values±SEM, n=8/group.
Table 2.
Spearman correlation (coefficient) between acrylamide dose and oxidative stress and inflammatory markers.
| Marker | 8OHdG | Isoprostane | TAC | PON1 | CRP | HCy | Ox-LDL | ICAM-1 | TBX2 | CML |
|---|---|---|---|---|---|---|---|---|---|---|
| Low fat diet | ||||||||||
| Acrylamide | 0.681*** | NS | NS | 0.418* | NS | −0.563** | NS | NS | NS | NS |
| 8OHdG | NS | 0.461* | NS | NS | NS | NS | NS | NS | NS | NS |
| Isoprostane | NS | NS | NS | 0.519* | NS | NS | NS | NS | NS | NS |
| TAC | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| PON1 | NS | NS | 0.392* | NS | NS | NS | NS | −0.399* | NS | NS |
| CRP | NS | NS | NS | NS | NS | 0.430* | NS | NS | NS | NS |
| Hcy | NS | NS | NS | NS | NS | NS | NS | 0.443* | NS | NS |
| Ox-LDL | NS | NS | NS | NS | NS | NS | NS | 0.500** | NS | NS |
| ICAM-1 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| TBX2 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| High fat diet | ||||||||||
| Acrylamide | 0.636*** | NS | NS | −0.487** | −0.711*** | NS | NS | −0.615*** | NS | NS |
| 8OHdG | NS | NS | NS | −0.363* | −0.769 | −0.536** | NS | −0.646*** | NS | NS |
| Isoprostane | NS | NS | NS | NS | NS | NS | 0.509* | NS | NS | NS |
| TAC | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| PON1 | NS | NS | NS | NS | 0.408* | NS | NS | 0.409* | NS | NS |
| CRP | NS | NS | NS | NS | NS | 0.451** | NS | 0.623*** | NS | NS |
| Hcy | NS | NS | NS | NS | NS | NS | NS | 0.380* | NS | NS |
| Ox-LDL | NS | NS | 0.383* | NS | NS | NS | NS | NS | NS | NS |
| ICAM-1 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| TBX2 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
“*”,“**” and "***" indicate significant difference at p<0.05, p<0.01 and p<0.001, respectively.
2. Experimental design, materials and methods
2.1. Animals, care and diets
The experimental protocol involving animals was reviewed and approved by the Health Canada Ottawa Animal Care Committee prior to the commencement of the study. Animals were cared for according to the guidelines of the Canadian Council on Animal Care. Six-week-old male F344 rats were procured from Charles River Laboratories Canada (St. Constant, Quebec, Canada) and were pair-housed in laboratory conditions with a 12 h light/12 h dark cycle. Temperature and relative humidity were controlled at 22 °C and 55%, respectively. All animals were acclimatized to the above conditions for 1 week until initiation of the experiment. The rats had free access to either lab chow (during the acclimatization phase) or experimental diets and drinking water ad libitum. The experimental diets were isocaloric and based on the AIN-93G rodent semisynthetic diet formula [2], but containing corn oil instead of soy oil as published earlier [3]. Fat level in the diet was maintained at either low (7%, wt/wt) or high (23.9%, wt/wt). Diets were obtained from Research Diets, Inc. (New Brunswick, NJ, USA) in the form of powder. Acrylamide was mixed with the diets at the required dose using a Hobart mixer, and then made into pellets using a pelleting press. Diets were never exposed to high temperature during processing and were stored in the dark at 4 °C until use. Rats were monitored every day and their body weights and food consumption were recorded twice a week; diets were replenished weekly.
2.2. Experimental design
After the acclimatization phase, animals (n=64) were randomized (2×4 factorial) into eight dietary groups (n=8 rats/group) to receive low or high fat diets without or with acrylamide (0, 5, 10 or 50 mg/kg diet). All animals remained on the experimental diets for a total of 10 weeks. A day before euthanasia, animals (not fasted) were placed in metabolic cages overnight, after which urine was collected (on ice) and urine volume was recorded for assay dilutions and calculations. Following urine collection, all rats were killed by exsanguination under isoflurane anesthesia, and blood was drawn from the abdominal aorta into BD Vacutainer SST™ blood collection tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). Urine was centrifuged at 4000×g and serum was separated by centrifugation at 700×g, and in both cases the supernatant was collected, aliquots prepared, and stored at −80 °C until analysis.
2.3. Enzyme-linked immunosorbent assay (ELISA)
Urine samples were analyzed for 8-hydroxydeoxyguanosine and isoprostane, and serum samples for total antioxidant capacity, paraoxonase 1 activity, c-reactive protein, homocysteine, oxidized low-density lipoproetein, intercellular adhesion molecule-1, thromboxin 2, and Nε-(carboxymethyl)lysine carried out by the ELISA method using commercial kits according to the manufacturer׳s instructions. Details of the kits and test sample dilutions of individual assays are given in Table 1.
Table 1.
Manufacturer details of commercial kits used and test sample dilution factor for individual assays.
| Assay name | Catalog number | Manufacturer (City, Country) | Dilution of urine/blood |
| 8-hydroxydeoxyguanosine | KOG-200 S/E | JaICA Shizuoka, Japan | 1×urine |
| Isoprostane | EA85 | Oxford Biomedical Research, Rochester Hills, MI, USA | 10×urine |
| Total antioxidant capacity | NX 2332 | Randox Laboratiories, Antrim, UK | 1×serum |
| Paraoxonase 1 | E33702 | Thermo Fisher Scientific Inc. Waltham, MA, USA | 170×serum |
| c-Reactive protein (CRP) | 41-CRPRT-E01 | Alpco Salem, NH, USA | 12,000×serum |
| Homocysteine (HCy) | 194-5361 | Bio-Rad Laboratories, Inc. Hercules, CA, USA | 1×serum |
| Oxidized LDL | 10-1158-01 | Mercodia AB Uppsala, Sweden | 21×serum |
| Intercellular adhesion molecule-1 | RIC100 | R&D Systems Minneapolis, MN, USA | 51×serum |
| Thromboxin 2 | 900-002 | Assay Designs, Inc., Ann Arbor, MI, USA | 400×serum |
| Nε-(carboxymethyl)lysine | CY-8066 | CycLex Co., Ltd. Nagano-shi. Japan | 6×serum |
2.4. Statistical analysis
Data was analyzed performed using SigmaPlot 11.0. Statistical comparisons were performed using ANOVA with Tukey׳s post hoc test. For all tests, p<0.05 was considered as statistically significant.
Acknowledgments
The research was supported by funds from the Chemicals Management Plan, Government of Canada. The authors thank Dr. Jin Yan and Mr. Saad Ulhaq for their technical expertise.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.02.024.
Appendix A. Supplementary material
Supplementary material
References
- 1.Raju J., Roberts J., Taylor M., Patry D., Chomyshyn E., Caldwell D., Cooke G., Mehta R. Toxicological effects of short-term dietary acrylamide exposure in male F344 rats. Environ. Toxicol. Pharmacol. 2015;39(1):85–92. doi: 10.1016/j.etap.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN−93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN−76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. PMID: 8229312. [DOI] [PubMed] [Google Scholar]
- 3.Raju J., Sondagar C., Roberts J., Aziz S.A., Caldwell D., Vavasour E., Mehta R. Dietary acrylamide does not increase colon aberrant crypt foci formation in male F344 rats. Food Chem. Toxicol. 2011;49(6):1373–1380. doi: 10.1016/j.fct.2011.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material