Abstract
Botulinum toxin (BoNT) is highly effective in the treatment of cervical dystonia (CD), yet a significant proportion of patients report low levels of satisfaction following treatment and fail to follow-up for repeated treatments. The goal of this study was to determine the reasons some patients have unsatisfactory responses. A total of 35 subjects who came to our center requesting alternative treatments due to unsatisfactory responses following BoNT treatment for CD were evaluated. Included were 26 women and 9 men with an average age of 57.1 years (range, 25–82 years), and an average duration of illness of 12.5 years (range, 1–55 years). Details of unsatisfactory BoNT treatments were methodically collected by a movement specialist using a standardized intake form, including provider subspecialty, product used, number of satisfactory or unsatisfactory trials, doses given, specific muscles treated, use of electromyographic guidance, side effects, and tests of resistance. The specialist then provided repeat treatments if indicated, and followed each case until the reasons for unsatisfactory outcomes could be determined. Multiple reasons for unsatisfactory outcomes were found. They included suboptimal BoNT doses, suboptimal muscle targeting, intolerable side effects, complex movement patterns, discordant perceptions, and incorrect diagnoses. Only 1 patient was functionally resistant to BoNT. Of 32 subjects who received repeat BoNT treatments, 25 (78%) achieved satisfactory responses after revision of the original treatment plan. These results indicate that the majority of unsatisfactory responses to BoNT treatment of CD were caused by correctible factors and imply a need for improved education regarding optimal treatment methods.
Keywords: Botulinum toxin, treatment failure, treatment resistance
INTRODUCTION
Cervical dystonia (CD) is characterized by excessive contraction of muscles of the neck leading to abnormal head movements and neck pain [1, 2]. It is the most common and readily recognized of the adult-onset dystonias. Botulinum toxin (BoNT) is effective in reducing both abnormal movements and pain, and is considered the treatment of choice [3, 4]. Despite its efficacy, only half of treated patients report high levels of satisfaction following treatment [5–7], and 20–40% of patients do not return for repeat treatments [8–13].
Considering the efficacy of BoNT in CD, the reasons for low satisfaction or failure to return for repeat treatments are not entirely clear. Early studies cited a lack of efficacy, although the reasons for poor efficacy were not determined [10, 12, 13]. Current understanding of these reasons is based on anecdotal reports, retrospective chart reviews, or expert consensus opinion [4, 11]. The explanations most often considered include BoNT resistance, incorrect diagnoses, suboptimal doses, incorrect muscle selection, need for electromyographic (EMG) guidance, evolution of the disorder over time, unusually complex muscle patterns, high cost, inconvenience, and unrealistic patient expectations.
There are no prospectively designed studies to provide objective data regarding these many possibilities, so their relative importance remains uncertain. As a result, the optimal management of patients reporting unsatisfactory outcomes remains unclear. The goal of the current study was to methodically assess the reasons for unsatisfactory BoNT treatment results among patients with CD and to develop recommendations for management.
METHODS
Subject Enrollment
This study included 35 patients who had been treated for CD with BoNT and with an unsatisfactory outcome, and presented to a tertiary care movement disorders subspecialty clinic at Emory University over a 2 year period requesting alternative treatment options such as deep brain stimulation surgery. We included all subjects for whom the main clinical problem being treated was CD, regardless of etiology or presence of dystonia and/or tremor in other body regions.
Subject Evaluation
Medical records prior to enrollment were reviewed; and details of prior BoNT treatments were methodically collected using a standardized data intake form that addressed provider subspecialty, product used, number of satisfactory or unsatisfactory trials, doses given, specific muscles treated, use of EMG, side effects, and any tests of resistance.
After reviewing records of prior treatments, an experienced movement disorders specialist reassessed each subject and re-assigned a diagnosis according to currently recommended criteria [14]. All subjects were offered repeat treatment with BoNT, except for those in whom the revised diagnosis suggested BoNT treatment was not indicated. Details of any additional BoNT re-treatments were again methodically collected, and the reasons for unsatisfactory responses were determined by the movement disorders specialist. Because the primary goal of this study was to address reasons and management of BoNT failures in a realistic clinical setting, a fixed protocol was not enforced for any additional re-treatments. Instead, any additional treatments were left to the discretion of the movement disorders specialist including BoNT product, dose, muscle pattern, and use of EMG). Functional resistance was assessed by injecting 10–20 dose equivalents of the most recently used BoNT product into the right or left frontalis, and assessing for asymmetric voluntary forehead wrinkling [15].
Each case was followed to one of the following endpoints: the reasons for BoNT failure were determined, the reasons could not be determined but the subject reached a satisfactory outcome following repeat BoNT treatment, the subject was lost to follow-up. Only 1 case did not return for follow-up assessments, but telephone interview revealed that the cost of treatment was the main reason for not continuing BoNT treatment. The primary goal of this study was to address reasons and management of BoNT failures, so outcomes focused on these reasons and on subject satisfaction with treatments, not on standard clinical rating scales for motor function or disability. Patient satisfaction was assessed as previously described by asking them to estimate their subjective sense of the overall percentage of improvement, with 0% being no improvement and 100% being total relief of CD motor symptoms or neck pain [10]. Subjects also were asked whether outcomes were sufficiently satisfactory to continue treatment, and what percentage improvement they considered to be satisfactory.
It was not the goal of these studies to compare different BoNT products, so dose-equivalents were used to simplify reporting of average dose units across different BoNT formulations as follows: onabotulinumtoxinA and incobotulinumtoxinA, 1 dose-equivalent = 1 unit; abobotulinumtoxinA, 1 dose-equivalent = 3 units; rimabotulinumtoxinB, 1 dose-equivalent = 50 units. When cases had more than one BoNT trial or product, the highest dose-equivalent reached was used for reporting the maximum dose reached.
RESULTS
Subject characteristics at referral
Clinical features for all 35 subjects are shown in Table 1. The average age at evaluation was 57.1 years (range, 25–82 years), with an average duration of illness of 12.5 years (range, 1–55 years). Most (n=26) had a diagnosis of idiopathic isolated CD; in 3 cases the dominant manifestation of CD was head tremor. Among the remaining cases, all had prominent neck involvement that was the focus of treatment. Six had minor dystonia outside of the neck region. One had prior selective peripheral denervation for CD, but none had any other surgeries for CD. One patient had CD with prior exposure to neuroleptics, and therefore presumptively had tardive CD. This heterogeneous population is typical of many larger published series of subjects with CD, except for studies where stricter inclusion and exclusion criteria yielded narrower age ranges or shorter durations of illness.
Table 1.
Patient characteristics
| Age/sex | CD years | Treatments prior to study | Treatments during study | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trials (S/U) | Product/dose | EMG? | Satisfactory response? | Product/dose | EMG | Frontalis test result | Reasons | ||
| 49F | 23 | 8/3 | B/150 | no | yes | B/200 | no | not resistant | dose |
| 64F | 6 | 0/1 | B/NA | yes | yes | X/200 | yes | dose | |
| 62F | 7 | 0/6 | B/355, M/NA | yes | yes | B/400 | no | not resistant | dose |
| 59M | 11 | 0/3 | B/50 | no | yes | D/600 | yes | dose | |
| 53F | 2 | 0/1 | B/140 | no | yes | B/200 | yes | dose | |
| 74M | 6 | 0/1 | M/5000 | no | yes | B/200 | no | dose, muscles | |
| 60F | 2 | 0/8 | B/300, D/1000 | no | yes | B/400 | yes | dose, muscles | |
| 80F | 5 | 0/6 | B/300 | yes | yes | X/400 | yes | not resistant | dose, muscles |
| 58M | 1 | 1/1 | B/NA | NA | yes | D/1000 | yes | dose, muscles, unreliable | |
| 43F | 2 | 0/3 | B/NA | no | yes | X/250 | yes | dose, muscles, iAE | |
| 62F | 6 | 0/4 | B/270 | no | yes | B/210 | yes | dose, muscles, iAE | |
| 44M | 3 | 1/3 | B/300, X/300 | no | yes | X/150 | no | dose, muscles, unreliable, iAE | |
| 71F | 6 | 0/4 | B/300 | yes | yes | D/300 | yes | dose, muscles, iAE | |
| 71F | 21 | 0/3 | B/300 | no | yes | D/900 | no | dose, perception | |
| 25M | 1 | 1/3 | X/345 | yes | yes | X/350 | no | muscles | |
| 31F | 13 | 0/1 | X/200 | no | yes | X/200 | no | muscles | |
| 65M | 6 | 0/8 | B/400, M/7500 | yes | yes | X/400 | yes | muscles | |
| 52F | 2 | 0/6 | B/NA, M/NA | NA | yes | X/200 | no | muscles, iAE | |
| 70F | 1 | 0/1 | B/70 | no | yes | B/100 | yes | muscles, iAE | |
| 82F | 5 | 0/2 | B/400 | yes | NR | NR | NA | diagnosis | |
| 40M | 14 | 0/3 | B/300 | NA | no | NR | no | diagnosis | |
| 46F | 4 | 0/5 | B/600, X/NA | yes | NR | NR | NA | diagnosis | |
| 59F | 14 | 0/4 | B/400 | NA | no | NR | NA | not resistant | perception |
| 59F | 4 | 1/1 | B/250 | yes | yes | X/400 | yes | not resistant | perception |
| 60F | 25 | 0/10 | B/NA | NA | no | NR | NA | perception | |
| 61F | 41 | 0/3 | B/NA | yes | yes | B/300 | no | not resistant | pain |
| 50F | 20 | 0/2 | B/140 | yes | NR | NR | yes | cost | |
| 59M | 5 | 0/3 | B/300 | NA | no | NR | yes | resistant | resistant |
| 50M | 33 | 0/2 | B/NA | NA | NR | NR | NA | undetermined | |
| 59F | 9 | 0/3 | B/200, M/NA | yes | yes | X/200 | yes | undetermined | |
| 68F | 13 | 0/5 | B/175 | no | yes | D/300 | no | undetermined | |
| 62F | 46 | 1/4 | B/165 | no | yes | B/140 | no | undetermined | |
| 45F | 19 | 0/3 | B/NA | NA | no | NR | NA | undetermined | |
| 61F | 55 | 1/2 | B/30 | no | yes | X/300 | no | undetermined | |
| 46F | 5 | >10/NA | B/200 | NA | no | NR | yes | undetermined | |
The main reasons for unsatisfactory responses were improper dose (dose), improper muscle selection (muscles), intolerable adverse events (iAE), improper diagnosis (diagnosis), discordant opinions between patient and provider (perception), financial concerns (cost), and markedly variable responses (unreliable). Other abbreviations: B, onabotulinumtoxinA; D, abobotulinumtoxinA; EMG, electromyographic guidance used; F, female; M, male or rimabotulinumtoxinB; NA, data not available; NR, not relevant; S, satisfactory; U, unsatisfactory; X, incobotulinumtoxinA.
Most cases had been previously treated by a neurologist (n=31), but some were treated instead by specialists in rehabilitation medicine, pain management, orthopedics, or primary care. Most (n=27) reported unsatisfactory outcomes from the start of treatment, even after an average of 3.5 trials (range 1–10 trials) and an average maximum dose-equivalent of 261 units (range 50–600 units). Six reported inconsistent responses, with good responses on at least one occasion and unsatisfactory responses on others. Two had many satisfactory treatments over more than 2 years, followed by loss of responses. Excluding these last 2 cases with waning responses, the remaining cases had a total of 121 trials, 95% of which were unsatisfactory. It was not feasible to summarize the doses applied and individual muscles targeted, because of insufficient detail in the documentation for procedures in many cases. Only 3 had tests for functional resistance to BoNT documented in their records. Two had negative serum antibody tests, and 1 one other had a negative frontalis functional test.
Reasons for BoNT failures
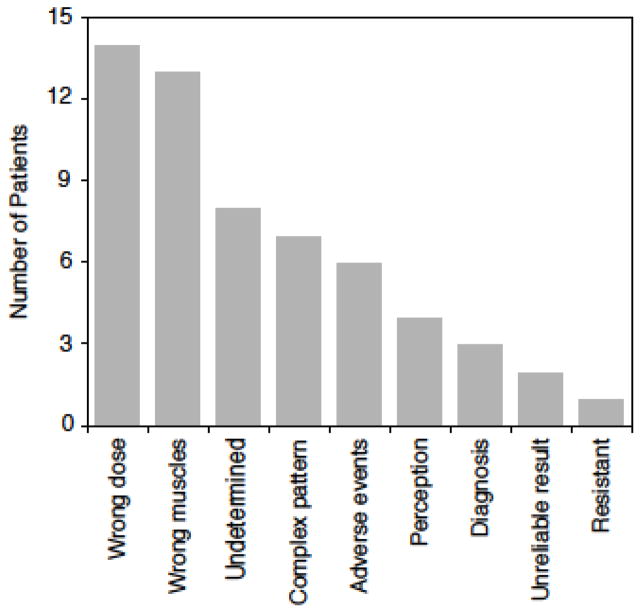
Eleven subjects had more than one reason for unsatisfactory BoNT treatments (Figure 1). The average number of reasons was 1.6 (range 1–3). The most common reason was inadequate dose (n=14), as judged by the movement disorders specialist’s ability to achieve a satisfactory outcome by altering the dose. Six subjects required doses exceeding the maximum dose recommended in the product package insert to reach satisfactory responses. Another common reason was sub-optimal selection of muscles treatment (n=13), as judged by the movement disorders specialist’s ability to achieve a satisfactory outcome by altering the muscles injected. In many cases, inadequate dose was combined with sub-optimal muscle selection. As an example, one subject received 3 unsatisfactory trials, each using a total dose of only 50 units of onabotulinumtoxinA, each time directed exclusively to the sternomastoid. The same subject reported excellent responses with a single trial of 200 units of the same product given across several muscles.
Figure 1.
The number of reasons for unsatisfactory outcomes among 35 patients treated with botulinum toxin for cervical dystonia. The total adds to more than 35, because more than 1 reason applied to many patients. The reasons for 8 cases could not be determined because of inadequate documentation in medical records regarding treatment details for unsatisfactory results, but good results following repeat treatment suggest the main problem was improper dose or muscle selection.
Seven subjects had forms of CD often viewed as more challenging to treat, including 3 with prominent head tremor, 2 with predominant anterocollis, and 2 with sagittal shift. Unsatisfactory outcomes for 6 subjects were due to intolerable side effects. For example, one subject had severe head drop lasting for several weeks and another had severe dysphagia requiring a gastrostomy tube. Two subjects reported severe injection site pain from the injector “digging around” in the neck muscles with the injection needle when searching for good EMG signals. Three subjects had incorrect diagnoses. One diagnosed with idiopathic CD had psychogenic CD. Another thought to have dystonic anterocollis instead had neck extensor weakness, and EMG showed myopathy. The third case had headache with prominent pain and tenseness of neck muscles, with no abnormal movements or posture of the head. Two of the 6 subjects with variable outcomes across different treatment sessions led them to conclude treatment was unreliable.
In 4 subjects, the patient’s opinions regarding benefits were discordant from those of the injector. All 4 reported unsatisfactory results, but the injector recorded >50% improvement in the movement disorder. In 2 of these cases, severe neck pain persisted despite correction of abnormal movements, mostly likely because of secondary orthopedic spine issues. In 2 cases, perceptions of non-response were reversed after treatment was withheld for >4 months. However, one of these latter cases with tremor-dominant CD continued to view the degree of benefit to be unsatisfactory. Only one subject was judged to be truly resistant as determined by the frontalis test.
The reasons for BoNT failure could not be determined for 8 subjects. Seven of these reached satisfactory outcomes using the same or alternative BoNT product, often with doses similar to or lower than those previously reported as not satisfactory. Thus insufficient dose could not provide an explanation. The most likely reason was selection of improper muscles, although verification of this suspected reason was impossible because of limited details in the treatment records for unsatisfactory results. The last subject for whom reasons for failure could not be determined reported she could not return for financial reasons.
Because objective documentation of EMG results was frequently insufficient in records of unsatisfactory treatment trials, the role of EMG in achieving satisfactory outcomes could not be determined. EMG was used in roughly the same proportion of cases reporting satisfactory or unsatisfactory outcomes. EMG appeared to be required for satisfactory outcome in one case who had prior peripheral denervation surgery, because atrophy of many muscles of the posterolateral right neck region obscured unexpected overactivity of deep neck muscles in the same region that could not be identified by examination. EMG also appeared to be required for another case with a very short and thick muscular neck, where the involved muscles were difficult to identify by physical examination alone.
Outcomes after referral
Following re-evaluation, the original diagnosis was revised for 3 cases. All subjects were offered repeat treatment with BoNT, except for one with myopathy and one with tension headaches but no apparent CD. One refused treatment, and reported adequate relief with clonazepam. Of the 32 subjects who received repeat treatments, 25 (78%) reported satisfactory outcomes. Satisfactory outcomes were reached following an average of 2 trials (range 1–8 trials) and an average BoNT dose-equivalent of 249 units (range, 100–400 units).
Subjects who did not reach satisfactory outcomes included one of each of the following: psychogenic dystonia, anterocollic CD, tremor-dominant CD, very short-lived benefits (6–8 weeks), refused follow-up because of cost, refractory neck pain, and true resistance documented by functional testing. Three subjects who did not reach satisfactory outcomes were referred for deep brain stimulation. One refused surgery, one with anterocollic CD continued to have unsatisfactory results, and one with tremor-dominant CD had an excellent result.
DISCUSSION
This prospective study was designed to empirically determine the reasons that patients with CD report unsatisfactory results following BoNT, a treatment that is usually very effective. The majority of unsatisfactory outcomes in this cohort were caused by the selection of improper BoNT doses and/or muscles. Less common explanations included intolerable side effects, perception of benefit, misdiagnosis, unreliable effects, or specific subtypes of CD that are more difficult to treat. True resistance to the pharmacological actions of BoNT was uncommon. The reasons for poor outcomes from this study are similar to those that are often listed in articles based on expert opinion [4]. The results are important for providing empirical evidence and for understanding the relative importance of individual reasons. In addition, the results highlight two reasons that are rarely cited in opinion-based articles, such as the patient’s perception of benefit, and specific subtypes of CD that are more challenging to treat. Overall, these results point to the need for improved education of both physicians and patients regarding the treatment of CD with BoNT.
Prior studies addressing unsatisfactory outcomes have broadly delineated two distinct groups of patients with BoNT non-response, those with primary non-response and those with secondary non-response [9, 16–19]. Patients with primary non-response report poor outcomes starting with initial BoNT treatments. The majority of subjects in the current series reported unsatisfactory outcomes from the start of treatment, and would be considered to be primary non-responders. Only one was truly resistant as documented by the frontalis test. Among the apparent primary non-responders, the tendency of some injectors to begin with a very low initial dose must be considered, especially for subjects who had only a single treatment before concluding treatment was unsatisfactory. However, an inadequate starting dose appeared to account for only 1 of 5 subjects who had only a single trial (Table 1). Overall, these patients continued to have unsatisfactory outcomes despite an average of 3.5 trials (range 1–10) and an average maximum dose equivalent of 261 units (range 50–600 units).
Patients with secondary non-response initially have good responses to BoNT, sometimes for many years, but their responses wane over time. These patients often are thought to have acquired antibody-mediated resistance that blocks the BoNT protein from its normal action [19–22]. This mechanism for non-response was studied extensively with early BoNT preparations, but more recent studies have suggested that antibody formation is uncommon with the currently available highly purified BoNT preparations. In the current series, only 2 cases had multiple successful treatments before responses began to wane. Both might be considered examples of secondary non-response although neither was truly resistant. One was likely due to worsening severity, while the other was due to evolution from torticollis/laterocollis to anterocollis.
Although CD often is viewed as a relatively static disorder that does not progress over time, significant progression occurs over at least 3–5 years [23–27], and the pattern of involved neck muscles may change in response to BoNT [28]. Thus, some patients may appear to be secondary non-responders if the dose and muscle pattern are not adjusted over time [21, 28]. Although BoNT is usually highly effective in CD, some patients are more difficult to treat than others, such as those with predominant anterocollis [29–31]. Cases with forward bending due to dystonic anterocollis must first be distinguished from those with neck extensor weakness and head drop [32, 33]. Dystonic anterocollis can be challenging because the responsible muscles often cannot be identified by physical examination. Some have advocated BoNT treatment of deep pre-vertebral neck muscles with fluoroscopic guidance, when anterior neck muscles such as the sternocleidomastoids or scalene muscles are not overactive [34, 35], although responses are still variable. Patients with pain out of proportion to muscle pulling may also be more difficult to treat, especially if the pain derives from processes that are not directly addressed by BoNT, such as orthopedic issues or radiculomyelopathy. Results from the current study provide empirical confirmation of prior epidemiological evidence that CD patients with prominent tremor [17, 36] or anterior/posterior shifts of the head in the sagittal plane [37] may also be more challenging to treat.
The current study has both strengths and weaknesses. It is the first prospectively designed study aimed to identify reasons for unsatisfactory BoNT responses in CD. It provides empirical validation of several reasons often cited in articles based on expert opinion [4], delineates the relative importance of the various reasons often considered, and highlights some reasons not emphasized in prior reports (patient perception and subtypes that are more difficult to treat). One weakness of this study is that it was conducted at a single center, so a regional or referral bias cannot be excluded. Nonetheless, the population was broadly representative of other published CD cohorts. Another weakness is that this study did not employ a fixed protocol for repeat treatments. Instead, the study was designed to permit flexibility in treatment decisions according to the preferences of the treating physician, which may therefore reflect a more realistic result. Finally, the study was not capable of addressing the value of EMG, which is often claimed to be essential for optimal BoNT results [11]. The main reason the value of EMG could not be determined was a lack of detail regarding EMG results in treatment records, similar to a prior study [11].
The results from the current study point to several recommendations for the management of CD patients who report unsatisfactory responses to BoNT. First, the diagnosis should be reconsidered to rule out disorders that may mimic CD. Second, the dose and muscle pattern should be reconsidered. For proper adjustments to the dose and pattern of muscles treated, precise documentation of doses applied in each muscle is essential for each treatment visit. Such documentation was surprisingly absent from a large number of the cases evaluated in this study, similar to a prior study. The current results neither support nor refute claims that EMG is required to achieve optimal results for CD patients [11], because many of the patients studied here achieved excellent outcomes without EMG. Finally, an assessment of BoNT resistance is helpful. True resistance to BoNT is rare, so it should not be assumed without a direct assessment. Formal tests for BoNT resistance may not be necessary when the patient experiences side effects that demonstrate non-resistance, such as head drop or dysphagia. When formal tests of resistance are indicated, there are two options. The first option involves commercially available tests for antibodies to BoNT, although these have fallen out of favor because of poor clinical correlations [15–17, 20, 22, 38]. The preferred option involves performing a functional test for muscle weakness, such as injection of a small dose of BoNT into the frontalis, corrugator, or extensor digitorum brevis [19]. These tests are simple and easy to apply in the clinic. The results presented here echo prior impressions that these tests are under-utilized [18]. All of these recommendations should be considered before concluding that patients with CD are resistant to BoNT, and before offering more invasive surgical interventions.
Acknowledgments
The authors declare no conflicts of interest relating to the material presented in this article. This work was supported in part by a grant to the Dystonia Coalition (U54 TR001456 and NS065701), a consortium of the Rare Diseases Clinical Research Network (RDCRN) an initiative of the Office of Rare Diseases Research (ORDR) at the National Center for Advancing Clinical and Translational Studies (NCATS) in collaboration with the National Institute for Neurological Diseases and Stroke (NINDS).
Abbreviations
- BoNT
Botulinum neurotoxin
- CD
cervical dystonia
- EMG
electromyography
References
- 1.Jinnah HA, Factor S. Diagnosis and treatment of dystonia. In: Jankovic J, editor. Neurologic Clinics. Elsevier; 2015. pp. 77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. doi: 10.1016/B978-0-444-52014-2.00037-9. [DOI] [PubMed] [Google Scholar]
- 3.Hallett M, Albanese A, Dressler D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. 2013;67:94–114. doi: 10.1016/j.toxicon.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Albanese A, Abbruzzese G, Dressler D, et al. Practical guidance for CD management involving treatment of botulinum toxin: a consensus statement. J Neurol. 2015;262:2201–13. doi: 10.1007/s00415-015-7703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ. 2012;15:419–23. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 6.Skogseid IM, Kerty E. The course of cervical dystonia and patient satisfaction with long-term botulinum toxin A treatment. Eur J Neurol. 2005;12:163–70. doi: 10.1111/j.1468-1331.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 7.Comella C, Bhatia K. An international survey of patients with cervical dystonia. J Neurol. 2015;262:837–48. doi: 10.1007/s00415-014-7586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J, Adler CH, Charles D, et al. Primary results from the Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy (CD PROBE) J Neurol Sci. 2015;349:84–93. doi: 10.1016/j.jns.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Evidente VG, Pappert EJ. Botulinum toxin therapy for cervical dystonia: the science of dosing. Tremor Other Hyperkinet Mov. 2014;4:273. doi: 10.7916/D84X56BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiung GY, Das SK, Ranawaya R, Lafontaine AL, Suchowersky O. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. 2002;17:1288–93. doi: 10.1002/mds.10252. [DOI] [PubMed] [Google Scholar]
- 11.Nijmeijer SW, Koelman JH, Standaar TS, Postma M, Tijssen MA. Cervical dystonia: improved treatment response to botulinum toxin after referral to a tertiary centre and the use of polymyography. Parkinsonism Relat Disord. 2013;19:533–8. doi: 10.1016/j.parkreldis.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Brashear A, Bergan K, Wojcieszek J, Siemers ER, Ambrosius W. Patients’ perception of stopping or continuing treatment of cervical dystonia with botulinum toxin type A. Mov Disord. 2000;15:150–3. doi: 10.1002/1531-8257(200001)15:1<150::aid-mds1024>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Gill CE, Manus ND, Pelster MW, et al. Continuation of long-term care for cervical dystonia at an academic movement disorders clinic. Toxins (Basel) 2013;5:776–83. doi: 10.3390/toxins5040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013;28:863–73. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brin MF, Comella CL, Jankovic J, Lai F, Naumann M. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord. 2008;23:1353–60. doi: 10.1002/mds.22157. [DOI] [PubMed] [Google Scholar]
- 16.Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol. 2009;32:213–8. doi: 10.1097/WNF.0b013e3181914d0a. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira JJ, Colosimo C, Bhidayasiri R, Marti MJ, Maisonobe P, Om S. Factors influencing secondary non-response to botulinum toxin type A injections in cervical dystonia. Parkinsonism Relat Disord. 2015;21:111–5. doi: 10.1016/j.parkreldis.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira JJ, Bhidayasiri R, Colosimo C, Marti MJ, Zakine B, Maisonobe P. Survey of practices employed by neurologists for the definition and management of secondary non-response to botulinum toxin in cervical dystonia. Funct Neurol. 2012;27:225–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm. 2013;120:275–90. doi: 10.1007/s00702-012-0893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman C, Hubble J, Schwab J, Beffy JL, Picaut P, Morte C. Immunoresistance in cervical dystonia patients after treatment with abobotulinumtoxinA. Int J Neurosci. 2012;122:358–62. doi: 10.3109/00207454.2012.668725. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz PJ, Castrillo JC, Burguera JA, et al. Evolution of dose and response to botulinum toxin A in cervical dystonia: a multicenter study. J Neurol. 2011;258:1055–7. doi: 10.1007/s00415-010-5880-1. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and Botulinum toxin therapy: A systematic review and meta-analysis. Neurotox Res. 2015 doi: 10.1007/s12640-015-9565-5. in press. [DOI] [PubMed] [Google Scholar]
- 23.Svetel M, Pekmezovic T, Tomic A, Kresojevic N, Kostic VS. The spread of primary late-onset focal dystonia in a long-term follow up study. Clin Neurol Neurosurg. 2015;132:41–3. doi: 10.1016/j.clineuro.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jahanshahi M, Marion MH, Marsden CD. Natural history of adult-onset idiopathic torticollis. Arch Neurol. 1990;47:548–52. doi: 10.1001/archneur.1990.00530050070014. [DOI] [PubMed] [Google Scholar]
- 25.Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord. 2012;27:1447–50. doi: 10.1002/mds.25088. [DOI] [PubMed] [Google Scholar]
- 26.Svetel M, Pekmezovic T, Jovic J, et al. Spread of primary dystonia in relation to initially affected region. J Neurol. 2007;254:879–83. doi: 10.1007/s00415-006-0457-8. [DOI] [PubMed] [Google Scholar]
- 27.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–81. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- 28.Gelb DJ, Yoshimura DM, Olney RK, Lowenstein DH, Aminoff MJ. Change in pattern of muscle activity following botulinum toxin injections for torticollis. Ann Neurol. 1991;29:370–6. doi: 10.1002/ana.410290407. [DOI] [PubMed] [Google Scholar]
- 29.Waln O, Ledoux MS. Blepharospasm plus cervical dystonia with predominant anterocollis: A distinctive subphenotype of segmental craniocervical dystonia? Tremor Other Hyperkinet Mov. 2011;2011(1) doi: 10.7916/D8SQ8Z4T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapetropoulos S, Tuchman A, Sengun C, Russell A, Mitsi G, Singer C. Anterocollis: clinical features and treatment options. Med Sci Monit. 2008;14:CR427–30. [PubMed] [Google Scholar]
- 31.Jinnah HA, Berardelli A, Comella C, et al. The focal dystonias: Current views and challenges for future research. Mov Disord. 2013;7:926–43. doi: 10.1002/mds.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Revuelta GJ, Montilla J, Benatar M, et al. An (18)F-FDG PET study of cervical muscle in parkinsonian anterocollis. J Neurol Sci. 2014;340:174–7. doi: 10.1016/j.jns.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Revuelta GJ, Benatar M, Freeman A, et al. Clinical subtypes of anterocollis in parkinsonian syndromes. J Neurol Sci. 2011;315:100–03. doi: 10.1016/j.jns.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhidayasiri R. Treatment of complex cervical dystonia with botulinum toxin: involvement of deep-cervical muscles may contribute to suboptimal responses. Parkinsonism Relat Disord. 2011;17(Suppl 1):S20–4. doi: 10.1016/j.parkreldis.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Glass GA, Ku S, Ostrem JL, Heath S, Larson PS. Fluoroscopic, EMG-guided injection of botulinum toxin into the longus colli for the treatment of anterocollis. Parkinsonism Relat Disord. 2009;15:610–3. doi: 10.1016/j.parkreldis.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Misra VP, Ehler E, Zakine B, Maisonobe P, Simonetta-Moreau M group IIC. Factors influencing response to Botulinum toxin type A in patients with idiopathic cervical dystonia: results from an international observational study. BMJ Open. 2012;2(3) doi: 10.1136/bmjopen-2012-000881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flowers JM, Hicklin LA, Marion MH. Anterior and posterior sagittal shift in cervical dystonia: a clinical and electromyographic study, including a new EMG approach of the longus colli muscle. Mov Disord. 2011;26:2409–14. doi: 10.1002/mds.23905. [DOI] [PubMed] [Google Scholar]
- 38.Chinnapongse RB, Lew MF, Ferreira JJ, Gullo KL, Nemeth PR, Zhang Y. Immunogenicity and long-term efficacy of botulinum toxin type B in the treatment of cervical dystonia: report of 4 prospective, multicenter trials. Clin Neuropharmacol. 2012;35:215–23. doi: 10.1097/WNF.0b013e318263163c. [DOI] [PubMed] [Google Scholar]