Abstract
In Drosophila melanogaster, prolonged exposure to males reduces the longevity and fecundity of females. This harm arises from the effects of male courtship behaviours and the toxic side effects of the accessory gland proteins (Acps) in their seminal fluids. Here, we examine the relationship between male exposure and its harmful effect on the lifetime fitness of his mates, and quantify the genetic basis for this variation. We found significant additive genetic variation in the magnitude of harm that males impose on females by exposing females to males from a variety of hemiclonal backgrounds for either a brief or prolonged period of time and measuring their fecundity, a meaningful fitness index. Furthermore, we discovered a strong negative correlation between the magnitude of harm and the short-term effects of male exposure on female fitness. We discuss the evolutionary significance of these results with regards to potential life-history trade-offs in females, and its relationship to male body size.
Keywords: Drosophila melanogaster, male-induced harm, mate choice, sexual conflict, fitness, population genetics
1. Introduction
Selection acting on males to increase their individual reproductive success can sometimes favour the evolution of traits that are ultimately deleterious to the fitness of their mates [1]. This inter-locus sexually antagonistic selection may be manifested through physical harm that males inflict on their mates during courtship and copulation [2,3]. The male-specific benefits associated with harm can be substantial, and are likely to be under strong directional selection [4]. Despite this, we see considerable phenotypic variation in the magnitude of individual male-induced harm within populations (possibly owing to segregating genetic variation) [5,6]. As such, we set out to study the ecological and genetic underpinnings of male-induced harm in order to gain insight into the forces that shape its evolution.
Our study used fruit flies, Drosophila melanogaster, a model species in which the mechanisms of male-induced harm have been extensively investigated. Male flies transfer numerous accessory gland proteins (Acps) in their ejaculate to their mates, some of which have toxic side effects [7,8]. Additionally, males harm females during courtship and copulation [9,10]. This harm is manifested as decreased female fecundity and/or longevity, and thus constitutes an important selective pressure on females in this species [11]. The extent to which this trait is heritable will therefore have important consequences for the rate and/or trajectory of inter-sexual coevolution. Despite its importance, only a handful of attempts have been made to determine if there is additive genetic variance for male-induced harm [12–14]. Additionally, these studies have been limited in several ways. First, they only examined variation present on a single chromosome and/or quantified male harm using females that carried multiple deleterious mutations and were obtained from a different population from the males. Second, harm was quantified by examining female mortality rates, a fitness metric of limited value in laboratory-reared organisms cultured with short, non-overlapping generations. Instead, measuring egg production during key oviposition windows yields more meaningful estimates of fitness [15], and avoids genotype-by-environment interactions for performance under novel test conditions [16].
Here, we set out to determine if phenotypic variation in the magnitude of harm that males inflict on their mates reflects the presence of additive genetic variation. Our assays were conducted using a large, outbred population, and used a protocol that mimicked (as closely as possible) the environment to which fruit flies were adapted [16]. We used hemiclonal analysis techniques ([17]; electronic supplementary material) to quantify the degree of additive genetic variation responsible for male-induced harm to female fecundity, where harm is quantified for each hemiclone line as the difference in fecundity of females that were briefly exposed to males, and those continuously housed with males [18]. Furthermore, we also examined the potential relationship between harm and male body size, as these two traits are often assumed to be positively correlated with each other [19,20].
2. Material and methods
In this experiment, we used fruit flies derived from the large (approx. 3500 adults per generation), wild-type population Ives (hereafter ‘IV’), which has been cultured under standardized conditions for hundreds of generations ([15]; electronic supplementary material). This population harbours considerable genetic variation for many traits, and has been used in a wide range of behavioural ecology and population genetics studies [5,16,21]. The population is cultured on a non-overlapping two-week schedule, in which they are combined en masse every 14 days and distributed onto fresh media. After 2–3 h, adult flies are discarded and the density of eggs in each vial is standardized to approximately 100 apiece. The number of eggs laid by females during this brief period of time is thus of great relevance to their fitness, as it is their only opportunity to directly pass on their genes to the next generation. From this population, we established 26 whole haploid-genome clone lines using cytogenetic cloning techniques [17,22], which we subsequently expressed in a hemiclonal state using established protocols ([17,22]; electronic supplementary material).
To quantify the genetic basis for variation in the magnitude of male-induced harm, 200 hemiclone males were collected from each line and housed in single-sex vials. Simultaneously, 1300 females were collected as virgins (within 8 h of eclosion) from standard IV culture vials. The larvae pupated on acetate inserts [15], which were transferred to holding vials prior to adult eclosion, allowing us to save the ‘natal’ vials (containing spent media) for use as mating chambers. On the morning corresponding to the 11th day of the flies' culture cycle, in each of two replicate blocks, we created two mating chambers for each hemiclonal line, consisting of 25 females and 50 males placed into a ‘natal’ vial. Males and females were left in one of these vials for 180 min (the ‘short-exposure’ treatment), which is typically sufficient to ensure all females will have mated once (T.A.F. Long 2006, personal observation). Following this, males were removed from these vials, and females were retained for an additional 45 h. In the other vial (the ‘long-exposure’ treatment), females and males were housed together for the full 48 h period. At the end of this period, females in both treatments were removed from their vials and individually placed into test tubes (containing 3 ml of fresh media) for 18 h before being discarded and their eggs immediately counted. This protocol attempts to mimic the phenology and developmental conditions historically experienced by this population as closely as possible (see electronic supplementary material). All males were collected, frozen and later dried overnight at 70°C to be weighed on a Sartorius M5 microbalance to the nearest 0.001 mg.
3. Results
For both the long- and short-exposure treatments, male line had a significant effect on the observed phenotypic expression of their mates' fecundity (table 1). Furthermore, prolonged exposure to male flies resulted in a substantial decrease in the fitness of female flies (treatment effect: 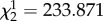 , p < 2.2 × 10−16). However, the male effects on female fecundity that were associated with each of the hemiclonal lines were not homogeneous across exposure treatments (treatment × clone line: p < 0.0001), indicating the presence of significant additive genetic variation in male-induced harm (table 1, figure 1).
, p < 2.2 × 10−16). However, the male effects on female fecundity that were associated with each of the hemiclonal lines were not homogeneous across exposure treatments (treatment × clone line: p < 0.0001), indicating the presence of significant additive genetic variation in male-induced harm (table 1, figure 1).
Table 1.
Variance components estimated using generalized linear mixed models fit by maximum likelihood for phenotypic variation in fecundity (egg production on day 14 of their culture cycle) of female Drosophila melanogaster exposed to male hemiclones for either a short or a long period of time. We created models that included exposure treatment as a fixed effect, and clone line and interaction between exposure treatment and clone line treated as random effects, as well as models for each treatment separately. Variance components and bootstrapped CI values were calculated using lme4 package [27]. The statistical significance of each variance component was determined using a permutation test approach with 10 000 samples (see the electronic supplementary material). A significant interaction between treatment and hemiclone line indicated that the fitness consequence of associating (and mating) with males from a given hemiclone background varies depending on their length of exposure to males.
| fitness | source of variance | variance (s.d.) | bootstrapped upper and lower 95% CI | % of variance explained | p-value |
|---|---|---|---|---|---|
| short-term exposure | clone line | 3.194 (1.787) | 5.524, 1.400 | 10.19 | <0.0001 |
| residual | 28.143 (5.305) | ||||
| long-term exposure | clone line | 2.297 (1.516) | 3.975, 1.047 | 12.84 | <0.0001 |
| residual | 15.589 (3.948) | ||||
| both short and long exposures | treatment × clone line | 2.517 (1.586) | 3.851, 1.104 | 10.23 | <0.0001 |
| clone line | 0.232 (0.481) | 1.155, 0.000 | 0.94 | 0.0257 | |
| residual | 21.853 (4.674) |
Figure 1.

Effect of male genotype and exposure treatment on fecundity (number of eggs laid on day 14 of the culture cycle) of female Drosophila melanogaster. The reaction norm plot at the centre depicts female fecundity for each of the 26 hemiclone lines across the two male-exposure duration treatments, while boxplots illustrate the overall differences between the treatments. The boxes enclose the middle 50% of each distribution (inter-quartile range, IQR), with the horizontal bars indicating the location of the medians. Values > ±1.5 × IQR are outliers, and are indicated by closed circles, and whiskers extend from the margins of the box to the minimum and maximum values that are not outliers.
We found no significant correlation between the number of eggs laid by females mated to hemiclones in the short-exposure treatment, and those laid by females mated to the same hemiclones in the long-exposure treatment. (Spearman's ρ = 0.156, S = 2470, p = 0.446). Similarly, there was no correlation between body mass and the magnitude of harm (the difference in the number of eggs laid between short- and long-exposure treatments [18]) associated with each hemiclone line (Spearman's ρ = 132, S = 2538, p = 0.518). However, a strong negative correlation was detected between the number of eggs laid in the short-exposure treatment and the magnitude of harm (Spearman's ρ = −0.785, S = 5222, p = 4.42 × 10−6, figure 2).
Figure 2.

Scatterplot and regression line illustrating the negative relationship between fecundity of females in the short-term treatment, and the net-cost of prolonged male exposure in female Drosophila melanogaster that had been exposed to 26 different male hemiclone lines. Fecundity is defined as the number of eggs laid by females on day 14 of their culture cycle.
4. Discussion
The outcome of intra- and inter-sexual selection processes in D. melanogaster has important consequences for the variation in individual reproductive success in both sexes. For females, male identity has been associated with a sizeable percentage of the phenotypic variation in female fecundity [23]. Here, we demonstrated that this variation is owing (in part) to the presence of segregating additive genetic variation, which can account for approximately 10% of female fecundity variation. Consistent with previous research [13], we found prolonged exposure to males was associated with decreased female fecundity (i.e. harm), presumably as a side effect of traits that have evolved to benefit male fitness [1,2]. Interestingly, the effect of male hemiclone line on female fitness was not consistent between exposure times (table 1), indicating that variation in the magnitude of harm has a genetic basis. Furthermore, our assay revealed that the impact of a male hemiclone line on a female's fecundity following a brief interaction was negatively correlated with the magnitude of harm associated with their prolonged interaction. This observation may help explain the maintenance of additive genetic variation for harmfulness in this species, if females vary in their preference for males depending on the temporal stability of their social environment and/or their extrinsic mortality risk. When associations are ephemeral, females may prefer mating with males that provide the greatest short-term benefits, but in more stable groups they may prefer to associate and mate with males that are inflict less harm over the long-term. More broadly, these results may prove enlightening to the study of life-history trade-offs between longevity and fecundity [24,25]. In environments where survivorship of females is relatively high, it may be advantageous to mate with males whose harmful effects are less deleterious to later-life fecundity, compared with situations where there are high rates of adult mortality. We look forward to conducting future empirical and theoretical tests of these hypotheses.
A second surprising observation was the lack of any correlation between male body size and variation in short-exposure fitness, long-exposure fitness or magnitude of harm. Interestingly, these findings are at odds with previous studies that have found male body size and harm to be associated with each other [19,20]. However, in these studies body size variation was achieved by manipulating larval densities/nutrition [19,20], while our males were reared under the standardized conditions that the IV population has evolved under for decades. It is possible that phenotypic correlations may have arisen owing to trade-offs resulting from this methodology. While the evolution of Acps is often viewed in the framework of increased post-copulatory success [7,8], there appears to be no genetic correlation between sperm competitive ability and male body size (at least for genes on the 2nd and 3rd chromosomes) [26]. Therefore, the biochemical and morphological traits associated with male-induced harm may actually not scale with male body size. Ultimately, the continued exploration of the relationships between male body size, harm, female preferences and fitness variation under controlled genetic and environmental conditions are promising avenues for future research.
Supplementary Material
Acknowledgements
We thank members of the Long Lab for their ‘fly pushing’.
Ethics
The research conducted in this study did not require approval from an ethics committee.
Data accessibility
The data and codes have been deposited to Dyrad: http://dx.doi.org/10.5061/dryad.6008n.
Authors' contributions
D.C.S.F. carried out the experiments. Both authors designed the experiments, contributed to the data analysis, writing and editing process of the manuscript. Both authors approve of the final manuscript submission and hold accountability for the accuracy and integrity of its contents.
Competing interests
The authors declare no competing interests.
Funding
T.A.F.L. was funded by a Discovery Grant from the Natural Sciences and Engineering Council of Canada (NSERC).
References
- 1.Parker G. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects, pp. 123–166. New York, NY: Academic. [Google Scholar]
- 2.Morrow EH, Arnqvist G, Pitnick S. 2003. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 14, 802–806. ( 10.1093/beheco/arg073) [DOI] [Google Scholar]
- 3.Rice WR. 2000. Dangerous liaisons. Proc. Natl Acad. Sci. USA 97, 12 953–12 955. ( 10.1073/pnas.97.24.12953). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Sawby R, Hughes KA. 2001. Male genotype affects female longevity in Drosophila melanogaster. Evolution 55, 834–839. ( 10.1554/0014-3820(2001)055[0834:MGAFLI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 6.Civetta A, Clark AG. 2000. Correlated effects of sperm competition and postmating female mortality. Proc. Natl Acad. Sci. USA 97, 13 162–13 165. ( 10.1073/pnas.230305397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244. ( 10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 8.Rice WR. 1996. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234. ( 10.1038/381232a0) [DOI] [PubMed] [Google Scholar]
- 9.Partridge L, Fowler K. 1990. Non-mating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 36, 419–425. ( 10.1016/0022-1910(90)90059-O) [DOI] [Google Scholar]
- 10.Kamimura Y. 2007. Twin intromittent organs of Drosophila for traumatic insemination. Biol. Lett. 3, 401–404. ( 10.1098/rsbl.2007.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart AD, Morrow EH, Rice WR. 2005. Assessing putative interlocus sexual conflict in Drosophila melanogaster using experimental evolution. Proc. R. Soc. B 272, 2029–2035. ( 10.1098/rspb.2005.3182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friberg U. 2005. Genetic variation in male and female reproductive characters associated with sexual conflict in Drosophila melanogaster. Behav. Genet. 35, 455–462. ( 10.1007/s10519-004-1246-8) [DOI] [PubMed] [Google Scholar]
- 13.Lew TA, Rice WR. 2005. Natural selection favours harmful male Drosophila melanogaster that reduce the survival of females. Evol. Ecol. Res. 7, 633–641. [Google Scholar]
- 14.Fiumera AC, Dumont BL, Clark AG. 2006. Natural variation in male-induced 'cost-of-mating' and allele-specific association with male reproductive genes in Drosophila melanogaster. Phil. Trans. R. Soc. B 361, 355–361. ( 10.1098/rstb.2005.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long TAF, Montgomerie R, Chippindale AK. 2006. Quantifying the gender load: can population crosses reveal interlocus sexual conflict? Phil. Trans. R. Soc. B 361, 363–374. ( 10.1098/rstb.2005.1786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose MR, Passananti HB, Matos M. 2004. Methuselah flies: a case study in the evolution of aging. Singapore: World Scientific. [Google Scholar]
- 17.Abbott JK, Morrow EH. 2011. Obtaining snapshots of genetic variation using hemiclonal analysis. Trends Ecol. Evolut. 26, 359–368. ( 10.1016/j.tree.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 18.Linder J, Rice WR. 2005. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 18, 568–575. ( 10.1111/j.1420-9101.2004.00872.x) [DOI] [PubMed] [Google Scholar]
- 19.Friberg U, Arnqvist G. 2003. Fitness effects of female mate choice: preferred males are detrimental for Drosophila melanogaster females. J. Evol. Biol. 16, 797–811. ( 10.1046/j.1420-9101.2003.00597.x) [DOI] [PubMed] [Google Scholar]
- 20.Pitnick S, Garcia-Gonzalez F. 2002. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B Lond. B 269, 1821–1828. ( 10.1098/rspb.2002.2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin ES, Long TAF. 2015. Are flies kind to kin? The role of intra- and inter-sexual relatedness in mediating reproductive conflict. Proc. R. Soc. B 282, 20151991 ( 10.1098/rspb.2015.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chippindale AK, Gibson JR, Rice WR. 2001. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA 98, 1671–1675. ( 10.1073/pnas.041378098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tennant HM, Sonser EE, Long TA. 2014. Variation in male effects on female fecundity in Drosophila melanogaster. J. Evol. Biol. 27, 449–454. ( 10.1111/jeb.12305) [DOI] [PubMed] [Google Scholar]
- 24.Travers LM, Garcia-Gonzalez F, Simmons LW. 2015. Live fast die young life history in females: evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci Rep. 5, 15469 ( 10.1038/srep15469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandy B, Gupta V, Sen S, Udaykumar N, Samant MA, Ali SZ, Prasad NG. 2013. Evolution of mate-harm, longevity and behaviour in male fruit flies subjected to different levels of interlocus conflict. BMC Evol. Biol. 13, 212 ( 10.1186/1471-2148-13-212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Amah L, Fiumera AC. 2008. Autosomal variation for male body size and sperm competition phenotypes is uncorrelated in Drosophila melanogaster. Biol. Lett. 4, 500–503. ( 10.1098/rsbl.2008.0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. (http://arxiv.org/abs/1406.5823).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and codes have been deposited to Dyrad: http://dx.doi.org/10.5061/dryad.6008n.


