Abstract
The aim of the study was to investigate the influence of hyperphosphorylation of tau induced by okadaic acid on the expression of nicotinic acetylcholine receptors and the neurotoxicity of β-amyloid peptide. Primary cultures of neurons isolated from the hippocampus of the brains of neonatal rats were exposed to okadaic acid or/and Aβ1–42. Tau phosphorylated at Ser404 and Ser202, and the protein expressions of α7, α4 and α3 nAChR subunits were quantified by Western blotting, and their corresponding mRNAs by real-time PCR. Superoxide dismutase activity was assayed biochemically and malondialdehyde by thiobarbituric acid-reactive substance. As compared to controls, phosphorylations of tau at Ser404 and Ser202 in the neurons were elevated by exposure to 20 nM okadaic acid for 48 h but not by 1 or 2 µM Aβ1–42. Treatment with 20 nM okadaic acid or 1 µM Aβ1–42 for 48 h resulted in the reduced α7, α4 and α3 proteins, and α4 and α3 mRNAs, as well as the decreased activity of superoxide dismutase and the increased malondialdehyde. Okadaic acid and Aβ1–42 together caused more pronounced changes in the expressions of α7 and α4, superoxide dismutase activity and lipid peroxidation than either alone. When pre-treatment with vitamin E or lovastatin, the neurotoxicity induced by okadaic acid was significantly attenuated. These findings indicate that hyperphosphorylation of tau induced by okadaic acid inhibits the expression of nicotinic acetylcholine receptors at both the protein and mRNA levels, as well as enhances the neurotoxicity of β-amyloid peptide.
Keywords: Okadaic acid, tau protein, nicotinic acetylcholine receptor, β-amyloid peptide, neurotoxicity, primary neurons
Introduction
Alzheimer's disease (AD), the most common cause of dementia, is characterized by the presence of extracellular senile plaques in the brain, consisting of aggregated β-amyloid peptide (Aβ) and intracellular neurofibrillary tangles (NFTs) containing hyperphosphorylated tau protein.1 As a neuronal microtubule-associated protein (MAP), tau contains 80 serine/threonine and 5 tyrosine residues that can be potentially phosphorylated.2 Normally, tau in the brain has 2–3 moles of phosphate per mole, and whereas in the brain of patients with AD, this stoichiometry is at least threefold greater than that of normal, in which the hyperphosphorylated protein aggregates into paired helical filaments.2,3
This hyperphosphorylation not only eliminates the ability of tau to promote the assembly of stabilizing microtubules but also causes this abnormal protein to sequester normal tau, as well as MAP1A/MAP1B and MAP2, thereby inhibiting and disrupting microtubules.4 Protein phosphatase 2A (PP2A) is primarily responsible for dephosphorylation of tau in the human brain,5 and the expression and activity of the enzyme are reduced in selected regions of brains with AD.6
Okadaic acid (OA), a polyether C38 fatty acid extracted from the black sponge Hallichondriaokadaii, is a potent selective inhibitor of the serine/threonine (Ser/Thr) protein phosphatase 1 (PP1) and PP2A,7–9 which may have rapid metabolic consequences that lead to cell death by altering rates of phosphorylation/dephosphorylation.10 Moreover, in experimental animals, OA may cause deposition of Aβ and tau hyperphosphorylation, and subsequent neuronal degeneration, loss of synapses and memory impairment, all of which resemble the pathology of AD.10–13 Consequently, OA is a powerful tool for unraveling the various regulatory mechanisms underlying the neurotoxicity associated with AD.11 Although this inhibitor is known to give rise to neurotoxicity by a number of pathways, the exact mechanism remains unclear.
One important feature of AD is loss of cholinergic neurons and alterations in cholinergic receptors.14 For example, expression of nicotinic acetylcholine receptors (nAChRs), which belong to a super-family of ligand-gated ion channels and mediate a variety of important physiological functions, is attenuated in the hippocampus and cerebral cortex, both in patients and animal models with AD.15–17 In the central nervous system, α (α2–α10) and β (β2–β4) subunits combine to form both hetero- and homo-pentameric neuronal nAChRs. OA administration resulted in memory impairment with the decreased cerebral blood flow and ATP level, the increases in mitochondrial (Ca2+)i, neuroinflammation, oxidative-nitrosative stress, Caspase-9 and cholinergic dysfunction in the cerebral cortex and hippocampus of mice brain.18
In the present study, we exposed primary cultures of neurons to OA to determine whether hyperphosphorylation of tau induced by OA influences the expression of nAChRs and thereby potentially enhances the neurotoxicity of Aβ.
Materials and methods
Materials
Lipid Peroxidation Assay Kit for detecting malondialdehyde (MDA), superoxide dismutase (SOD) Determination Kit, poly-L-lysine hydrobromide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), lovastatin, vitamin E and other general chemicals were purchased from Sigma (USA). Rabbit polyclonal anti-α7 nAChR (SC5544) antibody, goat polyclonal anti-α3 (SC1771) and -α4 (SC1772) antibodies, rabbit polyclonal anti-tau phosphorylated at Ser404 antibody (SC12952), mouse monoclonal anti-β-actin antibody, donkey anti-goat IgG (SC2020), anti-mouse IgG (SC2005) and Cruz Marker Molecular Weight Standards (SC2035) conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology Inc. (USA). OA (≥98% pure, as determined by HPLC) was procured from Enzo Life Sciences (USA). Neurobasal-A Medium, Hibernate-E Medium, B-27 Serum-Free Supplement, GlutaMAX Supplement, Dulbecco's MEM medium (DMEM), fetal bovine serum (FBS) and streptomycin–penicillin (Gibco Life Technologies, USA); anti-tau phosphorylated at Ser202 antibody (ab108378) (Abcam, USA); anti-mouse IgG labeled with CY-3, anti-rabbit IgG labeled with 488, a cDNA synthesis kit and TRIzol reagent (Thermo Scientific, USA); SYBR green master (ROCHE, Switzerland); mouse anti-NeuN antibody (Merck Millipore, Germany) and rabbit anti-GFAP antibody (Dako, Denmark) were purchased from the sources indicated.
Cell cultures and treatments
Primary hippocampal neurons were prepared from the brains of neonatal Sprague–Dawley (SD) rats according to a published procedure19,20 with slight modifications. Briefly, the hippocampal regions were separated from the brains of neonatal rats within 2–3 min after sacrifice and maintained in Hibernate A Medium chilled on ice. After removing the meninges, the hippocampus was washed three times with Hank' s buffered saline solution and then digested with 0.25% trypsin for 10 min in 37℃. Subsequently, the incubation medium was discarded and DMEM containing 10% FBS was added to terminate digestion.
After washing twice more with Hank' s buffered saline solution, the digested tissue was resuspended in 2 ml of Neurobasal/B27 complete medium (Neurobasal A Medium with 2% B27, 1% GlutaMAX Supplement, 100 U/ml penicillin and 100 mg/ml streptomycin) and broken apart with fire-polished glass pipettes. The upper single-cell suspension was transferred into a new tube and the cells were counted by trypan blue exclusion, and thereafter placed on 96-, 12- or 6-well on PLL-coated plates at a cell density of approximately 5.0 × 104/cm2. The neurons were maintained in a humidified atmosphere containing 5% CO2 at 37℃, with half-replacement of the medium every three days. The primary neurons isolated in this manner were approximately 90% pure, as determined by immunofluorescent double staining with mouse anti-NeuN antibody (diluted 1:50) and anti-mouse IgG labeled with CY-3 (red), and with rabbit anti-GFAP (glialfibrillary acidic protein) antibody (diluted 1:300) and anti-rabbit IgG labeled with 488 (green). After 10 days of incubation, the medium was replaced with neurobasal medium without B27, and the neurons then were treated with OA or/and Aβ1–42 for 48 or 24 h. To investigate the neuroprotective effect of vitamin E or lovastatin against OA, the cultured neurons were pre-treated with 20 µM vitamin E or 0.1 µM lovastatin for 24 h before treatment with OA for 48 h.
All experiments described here were approved by the regional ethical committee of Guizhou Province of China.
Assay of MTT reduction
Following treatment with OA and/or Aβ1–42 for 48 h, 10 ml of MTT stock (1 mg/ml) was added to each well and the incubation continued for another 4 h. Thereafter, 100 µl of a solution containing 20% SDS and 50% dimethylformamide (pH 4.8) was then added to each well. After incubation overnight, the absorption at a 570-nm was determined.21
Analysis of phosphorylated tau protein by Western blotting
For analysis of tau protein phosphorylated at Ser404 and Ser202, proteins extracted from the neurons22 were separated with 10% SDS-PAGE and blotted onto polyvinylidenedifluoride (PVDF) followed by incubation with primary antibody for 120 min. After washing, the membranes were incubated with HRP conjugated anti-rabbit IgG (1:5000) for 60 min and, finally, with ECL Plus reagent for 5 min. The signals were visualized by exposure to Hyper Performance Chemiluminescence film.
Quantification of α7, α4 and α3 nAChR proteins by Western blotting
The levels of α7, α4 and α3 nAChR proteins were quantitated by Western blotting as described previously.23 Briefly, equal amounts of the solubilized membrane fractions prepared from cultured cells were separated on 10% SDS-PAGE and then blotted onto PVDF membranes. These membranes were then incubated with rabbit polyclonal anti-α7, goat polyclonal anti-α4 or -α3 antibody (0.25 mg/ml in each case) for 120 min, and after washing, with HRP-conjugated anti-goat or anti-rabbit IgG (0.04 mg/ml) for 60 min. Finally, the membranes were incubated in ECL Plus reagent for 5–15 min and signals visualized by exposure to Hyper Performance Chemiluminescence film.
Quantification of mRNA encoding α7, α4 and α3 nAChRs by real-time PCR
Following of extraction of cellular RNA with Trizol Reagent, 1 µg was subjected to reverse transcription with primers specified for α7, α4 or α3 nAChR mRNA (Table 1). Quantitative real-time PCR was carried out in the ABI PRISM 7300 Sequence Detection System (Applied Biosystems, USA) in accordance with the manufacturer's protocol and analyzed with GeneAmp7300 SDS software. In brief, the 10 µl reaction solutions contained 1 µl first-strand cDNA, 5 µl 2×SYBR Green Master (Rox) Mix, 0.5 µl each of the forward and reverse primers (10 µM), 3 µl DNase and RNase-free H2O. The thermal cycling conditions involved 2 min at 50℃ and 10 min at 95℃, followed by 40 cycles at 95℃ for 15 s and 60℃ for 1 min. The levels of α7, α4 and α3 nAChR transcripts were estimated using the formula 2−△△CT, where △△CT (threshold cycle) represents the difference in CT values for the target and housekeeping mRNA.
Table 1.
Primers used for amplification of cDNA encoding for different subunits of rat nAChRs or for cyclophilin
| nAChR subunits or CP | 5′-3′ Nucleotide sequence | Size (bp) | Tm (℃) |
|---|---|---|---|
| α3 | Upper-TTCAGCCGTGCAGACTCCA | 271 | 55 |
| Lower-GGCAACGTACTTCCAATCATC | |||
| α4 | Upper-TAATACGACTCACTATAGGGAGGGCGAGGCCGGCATCTTGAGT | 274 | 60 |
| Lower-GCTGGGCACATGCTGGACAC | |||
| α7 | Upper-TAATACGACTCACTATAGGGAGGAAGAGGCCCGGAGAGGACAA | 207 | 60 |
| Lower-CGCCACATACGACCCCAGAG | |||
| CP | Upper-GACAAGGTCCCAAAGACAGC | 235 | C |
| Lower-GTCCAGCATTTGCCATGGAC |
CP: cyclophilin; Tm: temperature of denaturation.
Analysis of the activity of SOD and content of MDA
SOD was assayed by the SOD determination Kit-WST. In brief, the neurons were homogenized on ice in the lysis buffer and then centrifuged at 13,000 × g for 10 min to remove insoluble material. The supernatant, enzyme assay solution and WST buffer were added in the order indicated by the instructions for the kit. Following incubation at 37℃ for 20 min, the absorbance at 450 nm was determined using a microplate reader and SOD activity calculated.
The content of MDA, a major product of lipid peroxidation, was detected with thiobarbituric acid-reactive substance (TBARS) assay employing the Lipid Peroxidation Assay Kit.24 In brief, 200 µl of the supernatant prepared as described above were placed into a micro-centrifuge tube and 600 µl of the TBARS solution then added. This mixture was incubated at 95℃ for 60 min and cooled to room temperature in an ice bath for 10 min. Finally, 200 µl was pipetted into each well of a 96-well plate and the absorbance at 532 nm measured.
Statistical analysis
The values are expressed as means ± SD for the different groups. These means were examined for statistically significant differences employing analysis of variance (ANOVA), the Student–Newman–Keul's test or the paired-samples t test in the SPSS17.0 software (SPSS Inc., USA).
Results
Purity of the primary cultured neurons
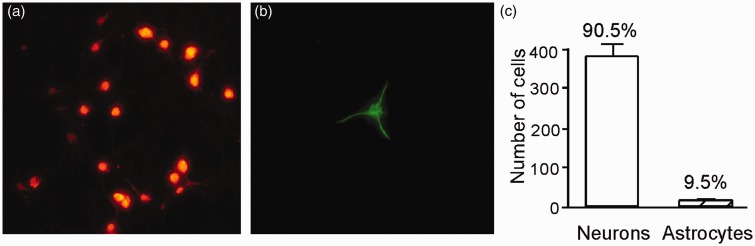
Immunostaining of the primary cultured neurons originating from the hippocampal region of the brains of newborn rats with an antibody directed towards the neuronal marker (NeuN) (Figure 1(a)) and an antibody against GFAP, a marker for astrocytes (Figure 1(b)), revealed that 90.5% of these cells were neurons (Figure 1(c)).
Figure 1.
Immunostaining of primary cultures of neurons originating from the hippocampus of the brains of neonatal rats. The mouse antibody against the NeuN and rabbit antibody against the GFAP were used. NeuN-positive neurons (a) and GFAP-positive astrocytes (b) were shown. (c) The proportions of neurons and astrocytes was shown as bar graph.(A color version of this figure is available in the online journal.)
Cell viability and cytotoxicity
The MTT assay was used to detect cell proliferation and cytotoxicity (Liu et al., 1997). After exposure to various concentrations of OA or Aβ1–42 for 48 h, MTT reduction was attenuated significantly at more than 30 nM or 1.5 µM, respectively (Figure 2).
Figure 2.
MTT levels in the primary neurons treated with OA orAβ1–42. MTT reduction was detected after the primary neurons were treated with 1–40 nM OA (a) or 1–2 µM Aβ1–42 (b) for 48 h. The results represent means ± SD (n = 20). *P < 0.05 and **P < 0.01 in comparison to untreated cells, as determined by analysis of variance (ANOVA), followed by the Student–Newman–Keul's test
Expression of phosphorylated tau
In cells incubated with OA for 24 h, the level of tau phosphorylated at Ser404 or Ser202 did not change significantly (data not shown). However, this levels of tau phosphorylated at Ser404 or Ser202 were increased by 138% and 145%, respectively, upon incubation with 20 nM (but not 10 nM) OA for 48 h (Figure 3(a) and (c)). While, no obvious differences in phosphorylated tau were found when the cultured neurons were treated with 1or 2 µM Aβ1–42 for 48 h (Figure 3(b) and (d)).
Figure 3.
The level of phosphorylation of tau in primary-cultured neurons exposed to OA or Aβ1–42. Tau protein was detected with rabbit polyclonal anti-tau phosphorylated antibody at Ser404 (a and b) or Ser202 (c and d) to OA (10 or 20 nM) and Aβ1–42 (1 or 2 µM), respectively, for 48 h. The results are expressed as means ± SD (n = 8). *P < 0.05 in comparison to untreated cells
Expression of α7, α4 and α3 nAChR subunits at protein and mRNA levels
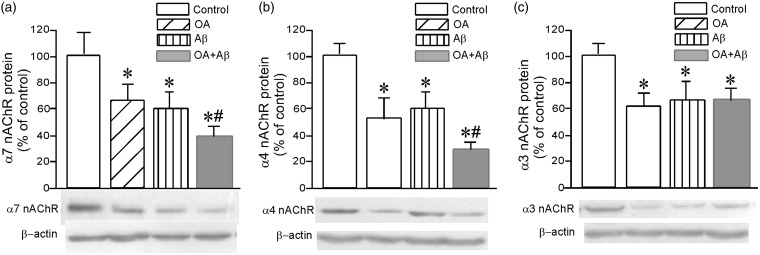
When the primary cultured neurons were treated with 20 nM OA for 48 h, the levels of α7 was reduced by 37% (Figure 4(a)), α4 by 44% (Figure 4(b)) and α3 by 40% (Figure 4(c)). Treatment with 1 µM Aβ1–42 for 48 h resulted in corresponding the significant decreases of 42% (Figure 4(a)), 40% (Figure 4(b)) and 38% (Figure 4(c)), respectively. Interestingly, when the neurons were treated with 20 nM OA and 1 µM Aβ1–42 in combination for 48 h, the level of α7 was lowered by 60% (Figure 4(a)) and that of α4 by 70% (Figure 4(b)) but with no further reduction in the level of α3 (Figure 4(c)).
Figure 4.
The levels of α7 (a), α4 (b) and α3 (c) nAChR proteins in primary-cultured neurons exposed to OA or/and Aβ. Immunoblotting was carried out with rabbit monoclonal anti-α7, and goat polyclonal anti-α4 and -α3 antibodies after primary-cultured neurons were exposed to 20 nM OA or/and 1 µM Aβ1–42 for 48 h. The results are expressed as means ± SD (n = 6). *P < 0.05 in comparison to controls; and #P < 0.05 in comparison to the treatment of OA or Aβ alone. Representative Western blots are depicted beneath the bar graphs
When the cultured neurons were treated with 20 nM OA for 48 h, there was no change in the level of α7 mRNA (Figure 5(a)), whereas the levels of α4 and α3 mRNAs were lowered by 42% (Figure 5(b)) and 45% (Figure 5(c)), respectively. Similarly, incubation with 1 µM Aβ1–42 for 48 h reduced the level of α4 mRNA by 28% (Figure 5(b)) and that of α3 by 50% (Figure 5(c)), without affecting α7 mRNA (Figure 5(a)). Clearly, exposure to OA and Aβ1–42 together caused greater decreases in the levels of α7 mRNA (Figure 5(a)) and α4 mRNA (Figure 5(b)) than either OA or Aβ alone; but no further effect on α3 mRNA (Figure 5(c)).
Figure 5.
The levels of α7 (a), α4 (b) and α3 (c) nAChR mRNA in primary-cultured neurons exposed to OA (20 nM) or/and Aβ1–42 (1 µM) for 48 h. The mRNA levels were determined by real-time PCR. The results are expressed as means ± SD (n = 6). *P < 0.05 in comparison to controls; and #P < 0.05 in comparison to the treatment of OA or Aβ alone
Neurotoxicity induced by OA or Aβ
The activity of SOD was decreased and the content of MDA increased upon exposure of the primary-cultured neurons to 20 nM OA or 1 µM Aβ1–42 for 48 h and even more so upon treatment with a combination of both (Table 2). In addition, when the pre-treatment with vitamin E or lovastatin to primary neurons, the neurotoxicity resulted from OA, including the decreases in activity of SOD, MTT reduction and protein expression of α7 nAChR subunit, was attenuated (Table 3).
Table 2.
SOD activity and MDA content in primary-cultured neurons exposed to OA and Aβ1–42
| Exposure | SOD (U/ mg protein) | MDA (nmol/mg protein) |
|---|---|---|
| Control | 28.87 ± 5.42 | 3.13 ± 0.36 |
| OA | 14.47 ± 3.62* | 3.35 ± 0.44 |
| Aβ | 17.14 ± 4.21* | 4.37 ± 0.89* |
| OA+Aβ | 9.86 ± 3.12*# | 6.25 ± 0.44*# |
n = 8.
P < 0.05 compared to control values.
P < 0.05 as compared to cells exposed to OA or Aβ alone.
Table 3.
Attenuation of Vitamin E and lovastatin on neurotoxicity of primary cultured neurons exposed to OA
| Exposure | SOD (U/mg protein) | MTT reduction (% of control) | nAChR α7 (% of control) |
|---|---|---|---|
| Control | 26.99 ± 3.69 | 100.00 ± 5.12 | 100.00 ± 1.16 |
| OA | 13.22 ± 4.19* | 84.49 ± 4.61* | 78.40 ± 0.72* |
| VE | 27.88 ± ± 1.94 | 102.70 ± 3.87 | 104.8 ± 1.15 |
| Lov | 26.35 ± 2.80 | 98.50 ± 5.48 | 132.7 ± 1.25* |
| OA+VE | 21.52 ± 1.95# | 97.74 ± 5.83# | 99.40 ± 1.06# |
| OA+Lov | 18.45 ± 2.12# | 99.12 ± 6.01# | 97.56 ± 2.34# |
n = 8.
P < 0.05 compared to control values.
P < 0.05 as compared to cells exposed to OA alone.
Discussion
It has been indicated that OA increases tau phosphorylation, β-amyloid cognitive deficiency seen in OA-treated rats without a change in motor function.25 The unilateral micro-infusion of OA into the dorsal hippocampus causes cognitive deficiency, NFTs-like pathological changes (in both the cortex and hippocampus), and oxidative stress (such as enhanced protein carbonylation and lipid peroxidation), which are characteristic features of AD.26 In addition, exposure of rat neurons to OA induced accumulation of autophagosomes, which occurs in AD.27 Thus, OA-induced neurotoxicity may be a novel tool for unraveling the pathology of AD, as well as developing new therapeutic approaches.7
Hyperphosphorylation of tau and subsequent formation of intracellular NFTs are thought to be implicated in the pathogenesis of AD.4 The abnormal phosphorylation of tau observed in AD brains is due to an imbalanced regulation of protein kinases and protein phosphatases. The reduced activities of the tau PP-2A and PP-1 in the AD brain would induce the increased phosphorylation of tau either by diminished dephosphorylation of this protein directly or by tau kinases that are activated by phosphorylation.6
In these investigations in vitro, the exposure of primary cultured neurons or SH-SY5Y cell line to OA induces hyperphosphorylation of tau and neurotoxicity.26,28–30 Here, we found that both of OA and Aβ1–42 at high concentrations are neurotoxic towards the cultured neurons and can attenuate the viability of the cells. The same concentration of OA enhanced phosphorylation of tau at Ser404 or Ser202, in agreement with previous observations.26,30
Although definitive proof is lacking and there is substantial criticism of this hypothesis, accumulating evidence supports involvement of the amyloid cascade and, in particular, changes in Aβ-induced tau-pathology in the development of AD.31,32 However, some reports contradict such involvement. For example, exposure of primary cultures of rat embryonic hippocampal cells to 0.5 µM fibrillary Aβ1–42 for three days caused retraction of dendrites and shrinkage of neuronal cell bodies, with no impact on tau phosphorylation at Ser202, Thr231/Ser235, Ser262 or Ser396/Ser404.33 In the present study, Aβ1–42 did not enhance phosphorylation of tau at Ser404 and Ser202, perhaps because the concentration was too low and/or incubation time too short.
Loss of nAChRs is consistently observed in the brains of patients with AD. Significant reduction in the level of acetylcholine, AChE activity and mRNA, and expression of α7 nAChR in rat brain following injection of OA have been reported.34 In the present study, the levels of α7, α4 and α3 nAChR proteins in neurons exposed to OA were lowered significantly. In addition, significant decreases in the levels of α4 and α3 nAChR mRNA were also detected here. However, the level of α7 mRNA was not changed, which might be a compensation for the loss of the receptors.35,36
Immunohistochemical analysis revealed that the selective α7 nAChR agonist A-582941 enhances phosphorylation of glycogen synthase kinase (GSK)3β in the cingulate cortex and hippocampus, and decreased phosphorylation of tau in hippocampal CA3 Mossy fibers and spinal motoneurons in a hypothermia-induced tau hyperphosphorylation mouse model and double transgenic amyloid precursor peptide (APP)/tau AD mice, respectively, which demonstrates that inactivation of GSK3β may be associated with α7 nAChR-induced signaling, leading to attenuated hyperphosphorylation of tau.37 Moreover, the selective α7 and β2 nAChR agonists PNU 282987 and 5-Iodo-A85380, respectively, in the cultural SH-SY5Y cells could afford neuroprotection against OA-induced neurotoxicity, suggesting that targeting nAChRs might attenuate neurodegeneration secondary to hyperphosphorylation of tau.38 The results documented here indicate that the relationships between OA, nAChRs and hyperphosphorylation of tau are complex.
The discovery, in which certain forms of early onset familial AD might be caused by enhanced production of Aβ, gave rise to the hypothesis, that amyloidogenic Aβ is intimately involved in the pathogenesis of AD.39 Aβ is generally considered to cause synaptic loss and neurodegeneration in AD40 and a belief that has driven the development of drugs for treatment of AD for more than 20 years.41 However, it was indicated that the amyloid hypothesis may be failing therapeutically due to several unresolved issues in the field including the presence of Aβ deposition in cognitively normal individuals, the weak correlation between plaque load and cognition.42 Previously, we found that Aβ induced the inhibited expression of nAChR subunits at both protein and mRNA levels.43,44 Here, we found that exposure of cultured neurons to Aβ1–42 also lowered the levels of α7, α4 and α3 nAChR proteins, as well as α4 and α3 mRNA, which is consistent with our earlier findings.
The α7 nAChR subunit appears to provide neuroprotection in connection with AD, since selective elevation of its level in the brains of transgenic mice carrying the Swedish APP670/671 mutation can compensate for deficits in synaptic plasticity due to an elevated level of soluble β-amyloid peptide (Aβ).35,36 Interestingly, the level of α7 mRNA in cultured neurons was not altered here by exposure to OA or Aβ alone but was lowered significantly by both in combination.
At the same time, such combined treatments did not alter the expression of α3 nAChR protein or mRNA to a greater extent than exposure to OA or Aβ alone. In our earlier reports, α3 nAChR was shown to promote cleavage of APP by α-secretase, enhance antioxidation and inhibit the toxicity of Aβ by reducing the expression of BACE1 (β-site APP cleaving enzyme 1), suggesting that this subunit might also play an important neuroprotective role in connection with the pathogenesis of AD.45,46 Whether α3 nAChR can counteract mixed toxic effects requires, indeed, further investigation. In addition, OA not only inhibits phosphatases acting directly on tau but also elevates oxidative stress and neuroinflammation,7 indicating that OA may play more complex roles in connection with the different subtypes of nAChRs.
Interestingly, upon exposure of cultured neurons to Aβ here, their SOD activity was reduced and MDA content increased, in agreement with earlier observations.14,45,47 The effects were more pronounced upon exposure to OA and Aβ in combination than either alone, indicating that OA may enhance the neurotoxicity of Aβ towards primary cultured neurons or vice-versa.
OA causes neurotoxicity by various pathways. A dramatic increase in the phosphorylation/activation of the extracellular signal-regulated protein kinase (ERK1/2), mitogen/extracellular signal-regulated kinases (MEK1/2), and p70 S6 kinase was observed in OA-treated slices of rat brains, suggesting that in AD brain the decrease in PP-2A activity could cause the activation of ERK1/2, MEK1/2, and p70 S6 kinase, and the abnormal hyperphosphorylation of tau both via an increase in its phosphorylation and a decrease in its dephosphorylation.48 The activation of major kinases, such as mitogen-activated protein kinases, ERK, protein kinase A, c-Jun N-terminal kinase, protein kinase C, Ca2+/calmodulin-dependent protein kinase II, Calpain, and GSK3β, in neurons is associated with AD pathology. These kinases, associated with abnormal hyperphosphorylation of tau, suggest that the cascade of these kinases could exclusively be involved in the pathogenesis of AD.7
Interestingly, here we found that the pre-treatment with vitamin E or lovastatin attenuated the neurotoxicity of OA, including the activity of SOD, cellular viability and expression of α7 nAChR, in the primary-cultured neurons. It indicated that vitamin E completely prevented the lipid peroxidation induced by OA in vitro.49 In our previous study, we have found that lovastatin may play a neuroprotective role without cholesterol dependent and can stimulate α7 nAChR by a mechanism that enhances production of α-secretase during APP processing.50 Further investigations are needed for confirming the neurotoxic effects of OA in molecular mechanism in AD tau pathology.
In conclusion, we show here that OA induces hyperphosphorylation of tau, inhibits expressions of the α7, α4 and α3 nAChR subunits, and results in neurotoxicity; Aβ did not influence the level of tau hyperphosphrylation in the experiment condition; finally, OA may enhance the neurotoxicity of Aβ.
Acknowledgements
This work was supported financially by grants from the Chinese National Natural Science Foundation (81260173); the Foundation of the Ministry of Education of P. R. China (IRT13058); and the Scientific Foundations in Guizhou Province of China ([2014]06, [2014]4010 and [2014]6008).
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. LZ, XY and X-LW conducted most of the experiments and wrote the manuscript; JP advised the design of the research project and guided young researchers; Z-Z G was responsible for the design of the study, controls of data analyses and paper writing. LZ and YX have contributed equally to this paper.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2012; 297: 353–6. [DOI] [PubMed] [Google Scholar]
- 2.Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimer's Dis 2013; 33(Suppl. 1): S123–9. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal K, Gong CX, Liu F. Microtubule-associated protein tau as a therapeutic target in Alzheimer's disease. Expert Opin Ther Targets 2014; 18: 307–18. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I. Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 2005; 1739: 198–210. [DOI] [PubMed] [Google Scholar]
- 5.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 2005; 22: 1942–50. [DOI] [PubMed] [Google Scholar]
- 6.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 1993; 61: 921–7. [DOI] [PubMed] [Google Scholar]
- 7.Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C. Molecular and cellular mechanism of okadaic acid (OKA)-induced neurotoxicity: a novel tool for Alzheimer's disease therapeutic application. Mol Neurobiol 2014; 50: 852–65. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Tung YC, Wang Y, Li XT, Iqbal K, Grundke-Iqbal I. Hyperphosphorylation and accumulation of neurofilament proteins in Alzheimer disease brain and in okadaic acid-treated SY5Y cells. FEBS Lett 2001; 507: 81–7. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci 1990; 15: 98–102. [DOI] [PubMed] [Google Scholar]
- 10.Arias C, Becerra-Garcia F, Arrieta I, Tapia R. The protein phosphatase inhibitor okadaic acid induces heat shock protein expression and neurodegeneration in rat hippocampus in vivo. Exp Neurol 1998; 153: 242–54. [DOI] [PubMed] [Google Scholar]
- 11.Kamat PK, Rai S, Nath C. Okadaic acid induced neurotoxicity: an emerging tool to study Alzheimer's disease pathology. Neurotoxicology 2013; 37: 163–72. [DOI] [PubMed] [Google Scholar]
- 12.Kamat PK, Tota S, Saxena G, Shukla R, Nath C. Okadaic acid (ICV) induced memory impairment in rats: a suitable experimental model to test anti-dementia activity. Brain Res 2010; 1309: 66–74. [DOI] [PubMed] [Google Scholar]
- 13.Yoon SY, Choi JE, Yoon JH, Huh JW, Kim DH. BACE inhibitor reduces APP-beta-C-terminal fragment accumulation in axonal swellings of okadaic acid-induced neurodegeneration. Neurobiol Dis 2006; 22: 435–44. [DOI] [PubMed] [Google Scholar]
- 14.Guan ZZ. Cross-talk between oxidative stress and modifications of cholinergic and glutaminergic receptors in the pathogenesis of Alzheimer's disease. Acta Pharmacol Sin 2008; 29: 773–80. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill MJ, Murray TK, Lakics V, Visanji NP, Duty S. The role of neuronal nicotinic acetylcholine receptors in acute and chronic neurodegeneration. Curr Drug Targets CNS Neurol Disord 2002; 1: 399–411. [DOI] [PubMed] [Google Scholar]
- 16.Nordberg A. Nicotinic receptor abnormalities of Alzheimer's disease: therapeutic implications. Biol Psychiatry 2001; 49: 200–10. [DOI] [PubMed] [Google Scholar]
- 17.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol 2000; 61: 75–111. [DOI] [PubMed] [Google Scholar]
- 18.Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, Shukla R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol 2013; 715: 381–94. [DOI] [PubMed] [Google Scholar]
- 19.Pacifici M, Peruzzi F. Isolation and culture of rat embryonic neural cells: a quick protocol. J Vis Exp 2012; 63: e3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunez J. Primary culture of hippocampal neurons from P0 newborn rats. J Vis Exp 2008; 19: pii 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 1997; 69: 581–93. [DOI] [PubMed] [Google Scholar]
- 22.Otvos LJ, Feiner L, Lang E, Szendrei GI, Goedert M, Lee VM. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 1994; 39: 669–70. [DOI] [PubMed] [Google Scholar]
- 23.Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer's disease. J Neurochem 2000; 74: 237–43. [DOI] [PubMed] [Google Scholar]
- 24.Dong YT, Wang Y, Wei N, Zhang QF, Guan ZZ. Deficit in learning and memory of rats with chronic fluorosis correlates with the decreased expressions of M1 and M3 muscarinic acetylcholine receptors. Arch Toxicol 2015; 89: 1981–91. [DOI] [PubMed] [Google Scholar]
- 25.Cho K, Yoon SY, Choi JE, Kang HJ, Jang HY, Kim DH. CA-074Me, a cathepsin B inhibitor, decreases APP accumulation and protects primary rat cortical neurons treated with okadaic acid. Neurosci Lett 2013; 548: 222–7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Simpkins JW. Anokadaic acid-induced model of tauopathy and cognitive deficiency. Brain Res 2010; 1359: 233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon SY, Choi JE, Kweon HS, Choe H, Kim SW, Hwang O, Lee H, Lee JY, Kim DH. Okadaic acid increases autophagosomes in rat neurons: implications for Alzheimer's disease. J Neurosci Res 2008; 86: 3230–39. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Xu DE, Ma T. Lovastatin suppresses the aberrant tau phosphorylation from FTDP-17 mutation and okadaic acid-induction in rat primary neurons. Neuroscience 2015; 294: 14–20. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Wang D, Li L, Zhao W, Zhang C. Protective effects of humanin on okadaic acid-induced neurotoxicities in cultured cortical neurons. Neuro Chem Res 2014; 39: 2150–9. [DOI] [PubMed] [Google Scholar]
- 30.Vandermeeren M, Lübke U, Six J, Cras P. The phosphatase inhibitor okadaic acid induces a phosphorylated paired helical filament tau epitope in human LA-N-5 neuroblastoma cells. Neurosci Lett 1993; 153: 57–60. [DOI] [PubMed] [Google Scholar]
- 31.Stancu IC, Vasconcelos B, Terwel D, Dewachter I. Models of β-amyloid induced Tau-pathology: the long and ‘folded’ road to understand the mechanism. Mol Neurodegener 2014; 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M. Hyperphosphorylation of Tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway. J Biol Chem 2012; 287: 35222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lain E, Penke B, Delacourte A, Gündisch D, Schröder H, Witter B. Effects of Abeta1-42 fibrils and of the tetrapeptide Pr-IIGL on the phosphorylation state of the tau-protein and on the alpha7 nicotinic acetylcholine receptor in vitro. Eur J Neurosci 2005; 21: 879–88. [DOI] [PubMed] [Google Scholar]
- 34.Kamat PK, Tota S, Rai S, Shukla R, Ali S, Najmi AK, Nath C. Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats. Eur J Pharmacol 2012; 690: 90–8. [DOI] [PubMed] [Google Scholar]
- 35.Yu WF, Guan ZZ, Bogdanovic N, Nordberg A. High selective expression of alpha7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer's disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Exp Neurol 2005; 192: 215–25. [DOI] [PubMed] [Google Scholar]
- 36.Xiu J, Nordberg A, Zhang JT, Guan ZZ. Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1-42). Neurochem Int 2005; 47: 281–90. [DOI] [PubMed] [Google Scholar]
- 37.Bitner RS, Nikkel AL, Markosyan S, Otte S, Puttfarcken P, Gopalakrishnan M. Selective alpha7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3beta and decreases tau phosphorylation in vivo. Brain Res 2009; 1265: 65–74. [DOI] [PubMed] [Google Scholar]
- 38.Del Barrio L, Martín-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, McIntosh JM, Wonnacott S, López MG. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2* nicotinic stimulation. Toxicol Sci 2011; 123: 193–205. [DOI] [PubMed] [Google Scholar]
- 39.Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol 1998; 8: 447–53. [DOI] [PubMed] [Google Scholar]
- 40.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 2008; 14: 837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardy J, Bogdanovic N, Winblad B, Portelius E, Andreasen N, Cedazo-Minguez A, Zetterberg H. Pathways to Alzheimer's disease. J Intern Med 2014; 275: 296–303. [DOI] [PubMed] [Google Scholar]
- 42.Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun 2014; 2: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu RY, Gu R, Qi XL, Zhang T, Zhao Y, He Y, Pei J, Guan ZZ. Decreased nicotinic receptors and cognitive deficit in rats intracerebroventricularly injected with beta-amyloid peptide (1-42) and fed a high-cholesterol diet. J Neurosci Res 2008; 86: 183–93. [DOI] [PubMed] [Google Scholar]
- 44.Guan ZZ, Yu WF, Shan KR, Nordman T, Olsson J, Nordberg A. Loss of nicotinic receptors induced by beta-amyloid peptides in PC12 Cells: possible mechanism involving lipid peroxidation. J Neurosc Res 2003; 71: 397–406. [DOI] [PubMed] [Google Scholar]
- 45.Qi XL, Ou-Yang K, Ren JM, Wu CX, Xiao Y, Li Y, Guan ZZ. Preventing expression of the nicotinic receptor subunit α7 in SH-SY5Y cells with interference RNA indicates that this receptor may protect against the neurotoxicity of Aβ. Neurochem Res 2013; 38: 943–50. [DOI] [PubMed] [Google Scholar]
- 46.Tang Z, An Y, Qi XL, Xiao Y, Shan KR, Guan ZZ. Inhibiting gene expression of alpha3 nicotinic receptor in SHSY5Y cells with the effects on APP metabolism and antioxidation in Alzheimer's disease. Neurochem Int 2008; 53: 112–17. [DOI] [PubMed] [Google Scholar]
- 47.Qi XL, Xiu J, Shan KR, Xiao Y, Gu R, Liu RY, Guan ZZ. Oxidative stress induced by beta-amyloid peptide(1-42) is involved in the altered composition of cellular membrane lipids and the decreased expression of nicotinic receptors in human SH-SY5Y neuroblastoma cells. Neurochem Int 2005; 46: 613–21. [DOI] [PubMed] [Google Scholar]
- 48.Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer's disease. Am J Pathol 2003; 163: 845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matias WG, Traore A, Bonini M, Sanni A, Creppy EE. Oxygen reactive radicals production in cell culture by okadaic acid and their implication in protein synthesis inhibition. Hum Exp Toxicol 1999; 18: 634–9. [DOI] [PubMed] [Google Scholar]
- 50.Xiu J, Nordberg A, Shan KR, Yu WF, Olsson JM, Nordman T, Mousavi M, Guan ZZ. Lovastatin stimulates up-regulation of alpha7 nicotinic receptors in cultured neurons without cholesterol dependency, a mechanism involving production of the alpha-form of secreted amyloid precursor protein. J Neurosci Res 2005; 82: 531–41. [DOI] [PubMed] [Google Scholar]